Abstract
Background
Nasal continuous‐positive airway pressure (nCPAP) with the INSURE (INtubation‐SURfactant‐Extubation) or LISA (Less‐Invasive Surfactant Administration) procedures are increasingly being chosen as the initial treatment for neonates with surfactant deficiency. Our objective was to compare the effects on cerebral oxygenation of different methods for surfactant administration: INSURE and LISA, using a nasogastric tube (NT) or a LISAcath® catheter, in spontaneously breathing SF‐deficient newborn piglets.
Methods
Eighteen newborn piglets with SF‐deficient lung injury produced by repetitive bronchoalveolar lavages were randomly assigned to INSURE, LISA‐NT, or LISAcath® groups. We assessed pulmonary (gas exchange, lung mechanics, lung histology) and hemodynamic (mean arterial blood pressure, heart rate) changes, cerebral oxygenation (cTOI) and cerebral fractional tissue extraction (cFTOE), with near‐infrared spectroscopy, carotid blood flow and brain histology.
Results
SF‐deficient piglets developed respiratory distress (FiO2 = 1, pH <7.2, PaCO2 >70 mmHg, PaO2 <70 mmHg, Cdyn <0.5 mL/cmH2O/kg). Rapid improvements in pulmonary status were observed in all surfactant‐treated groups without hemodynamic alterations. In the INSURE group, a transient decrease in cTOI occurred during and immediately after surfactant administration, while cTOI only decreased during surfactant administration in the LISA‐NT group and did not change significantly in the LISAcath® group. Brain injury scores were low in all surfactant‐treated groups.
Conclusion
In spontaneously breathing SF‐deficient newborn piglets, short‐lasting decreases in cerebral oxygenation are associated with surfactant administration by the INSURE method or LISA using an NT, while no cerebral oxygenation changes occurred with LISA using a LISAcath®. Notably, none of treatments studied seems to have a negative impact on the neonatal brain.
Keywords: cerebral oxygenation, INSURE, LISAcath®, NIRS, respiratory distress syndrome, surfactant
1. INTRODUCTION
When surfactant (SF) treatment of neonatal respiratory distress syndrome (RDS) was introduced during the 1990s, it was exclusively administered as a bolus into intubated babies who required mechanical ventilation. In recent decades, there have been important developments in RDS treatment including the use of non‐invasive ventilation such as nasal continuous positive airway pressure (nCPAP),1 and the development of different strategies for SF replacement.2, 3, 4, 5 Early nCPAP is associated with reduced pulmonary morbidity and lower rates of bronchopulmonary dysplasia (BPD).6 Moreover, early SF administration has been shown to be more efficient than late treatment and is associated with decreases neonatal mortality, incidence of air leak syndromes and BPD.7 Hence, combining early nCPAP with early SF administration during spontaneously breathing seemed logical.
The administration of SF during nCPAP has been investigated considering two main options. On the one hand, the INSURE (INtubation‐SUrfactant‐Extubation) procedure combined with nCPAP reduces the need for and duration of mechanical ventilation, and length of oxygen therapy, as well as the need for additional SF doses.8, 9 Nevertheless, the INSURE procedure carries its own risks, as a brief period of endotracheal intubation and mechanical ventilation is still required,10 and it can also be difficult to extubate infants following the procedure.11 On the other hand, some authors have suggested using a less invasive SF administration (LISA) method, which involves the insertion of a nasogastric tube (NT), vascular catheter or a new catheter purpose‐built for LISA, the LISAcath®, into the trachea and its removal immediately after SF instillation, while the patient is spontaneously breathing on nCPAP. Use of the LISA method has been shown to have beneficial effects on mortality, BPD, need for mechanical ventilation and survival in preterm infants.12, 13, 14 Nonetheless, both the LISA and INSURE procedures have been associated with side effects such as transient hypoxemia, bradycardia, and desaturation.12, 13, 14 Such alterations may produce changes in cerebral hemodynamics, oxygenation and electrical brain activity15, 16, 17, 18, 19 which could negatively influence long‐term neurodevelopment.20 To date, a relatively small number of studies have assessed the impact on the brain of different methods of SF administration (INSURE vs LISA).15, 16, 17, 18, 19 Moreover, no one has evaluated the effects on the lung and brain of using the new purpose‐built catheter, LISAcath®, for SF administration.
In this context of uncertainty about the impact of different SF administration strategies on the brain, the hypothesis of the study was that the LISA procedure using an NT or LISAcath® catheter may potentially be as efficient and safe as the INSURE procedure. Our main objective was to compare these methods of SF administration evaluating their impact on the brain in development. In addition, the effect of SF administration on gas exchange, hemodynamic parameters and lung injury scores were assessed using each of these SF administration methods in spontaneously breathing SF‐deficient newborn piglets.
2. MATERIAL AND METHODS
2.1. Animal preparation
The experimental protocol meets European and Spanish regulations for the protection of experimental animals (UE2010/63‐RD53/2013) and was approved by the Ethics Committee for Animal Welfare of Biocruces Bizkaia Health Research Institute.
As previously described,21 2‐ to 4‐day‐old newborn piglets were sedated with an i.m. ketamine‐diazepam‐atropine injection and anesthetized with sevofluorane (2‐3%). A cuffed‐tracheal tube was inserted and connected to a positive pressure ventilator (Carescape R860, Datex‐Ohmeda Inc, Madison, WI) with the following initial settings: FiO2 = 0.21‐0.28, respiratory rate (RR) = 28 breaths/min, positive end‐expiratory pressure (PEEP) = 3 cmH2O and positive inspiratory pressure (PIP) = 9‐11 cmH2O.
An arterial catheter was inserted into the femoral artery to monitor mean arterial blood pressure (MABP) and heart rate (HR) and obtain blood samples for gas analysis. Moreover, pulse oximetry oxygen saturation (SpO2) was continuously monitored.
The right common carotid blood flow was measured, with an ultrasonic flow probe (Transonic Systems Inc., NY), as a proxy for cerebral blood flow. Rectal temperature was maintained between 38 and 39°C with heating lamps.
2.2. Lung injury and study design
SF‐deficient lung injury was achieved by repetitive saline lavage (30 mL/kg; 37°C with FiO2 = 1).21, 22 After the first bronchoalveolar lavage (BAL), the PEEP was increased to 5 cmH2O, and PIP and RR were adjusted to a maximum of 25 cmH2O and 45 bpm, respectively. Lavage procedures were repeated at 5 min intervals until PaO2 < 100 mmHg was obtained. After 30 min of stabilization, all newborn piglets received a bolus dose of 20 mg/kg of caffeine citrate (Peyona® 20 mg/mL; Chiesi Farmaceutici, Parma, Italy) before extubation. Customized tightly‐fitting short binasal prongs were fitted to all animals. Once spontaneous breathing had been established, piglets were randomly assigned to one of the following groups:
INSURE group (n = 6): newborn piglets with SF‐deficient lung injury that received 200 mg/kg of SF (Poractant‐alfa, Curosurf®, Chiesi Farmaceutici, Parma, Italy) by the INSURE method.19 With the animal in a supine position, the SF bolus was administered over 30‐60 s. After SF administration, mechanical ventilation was delivered for 1 min set at 18/5 cmH2O. Then the piglets were immediately extubated and placed on nCPAP for 180 min.
LISA‐NT group (n = 6): newborn piglets with SF‐deficient lung injury that received 200 mg/kg of SF by the LISA method.19 With the animal maintained on nCPAP, the LISA procedure was performed using 5Fr flexible NT that had been appropriately shortened. It was placed in the trachea with direct visualization of the vocal cords with a laryngoscope and using Magill forceps.
LISAcath® group (n = 6): newborn piglets with SF‐deficient lung injury that received 200 mg/kg of SF by the LISA method. With the animal maintained on nCPAP, the LISA procedure was performed using a LISAcath® catheter (Chiesi Farmaceutici, Parma, Italy). It was placed in the trachea with direct visualization of the vocal cords with a laryngoscope (without the use of Magill forceps).
After NT or LISAcath® placement, the laryngoscope was removed, and 200 mg/kg of SF was intratracheally administered over 30‐60 s. The piglet́s mouth was closed during the procedure, in order to avoid loss of pressure in the airways during SF administration. After instillation, the NT or LISAcath® was immediately removed. The nCPAP support was maintained for 180 min, at a level of 5 cmH2O with a flow of 3 L/min.
2.3. Lung measurements
Gas exchange and cardiovascular parameters
-
–
Arterial pH, PaO2 and PaCO2 were measured.
-
–
Alveolar‐arterial (A‐a) oxygen tension difference (A‐aDO2), arterial/alveolar (a/A) ratio and PaO2/FiO2 parameters were calculated.
-
–
Intrapulmonary intrapulmonary‐shunt (Qs/Qt) and hemodynamic parameters, namely, HR and MABP (Intellivue MP70, Philips‐Medical, Eindhoven, The Netherlands) were measured.
All these parameters were obtained at baseline, after the BAL procedure, during the 30 min of stabilization under conventional mechanical ventilation and, after extubation, during nCPAP every 30 min until the end of experiment, at 180 min.
2.3.1. Lung mechanics
Lung mechanics were measured with a computerized system (M1014A, Philips Medical, Eindhoven, The Netherlands). The system reported values for dynamic compliance (Cdyn), tidal volume and airway resistance at baseline, after the BAL procedure, after 30 min of stabilization, and at the end of the experiment (animals being intubated and lung mechanics measured).
2.3.2. Lung tissue analysis
Postmortem, the lungs were fixed in formalin 4% at 15 cmH2O for histological analysis. Lung injury was scored by a pathologist blinded to treatment group using a semi‐quantitative scoring system. Pathological signs of lung injury (atelectasis, alveolar and interstitial inflammation, alveolar and interstitial hemorrhage, edema, and necrosis) were each scored on a 0‐4 point scale: 0 corresponding to no injury; 1, 2, and 3 to injury to 25%, 50%, and 75% of the field, respectively; and four to injury across the field.21, 23 Moreover, all seven lung injury scores were summed in order to obtain a mean total lung injury score for each group, with a minimum value of 0 and maximum of 28, values higher than 12 corresponding to quite severe lung injury.23
2.4. Brain measurements
As for the aforementioned physiological parameters, brain measurements were taken at baseline, after the BAL procedure, during the 30 min of stabilization and, after extubation, every 30 min until the end of experiment, at 180 min. Moreover, in order to assess changes induced during the period of SF administration, the brain measurements were also taken: 2 min before SF administration (−2 min), during its administration (0 min), and 1 and 5 min after its administration.
2.4.1. Carotid blood flow and near‐infrared spectroscopy (NIRS) measurements
Carotid blood flow (CBF) was recorded to assess changes in cerebral blood flow. Changes in cerebral perfusion‐oxygenation were assessed using a near‐infrared‐spectroscopy NIRS system (NIRO‐200, Hamamatsu Photonics, Joko Cho, Japan). The sensor was placed on and fixed to the frontoparietal region of the piglet's head. The cerebral tissue oxygen index (cTOI) was continuously monitored. This index represents the cerebral oxygen saturation expressed as a percentage and was also used to calculate the cerebral fractional tissue oxygen extraction (cFTOE): cFTOE = (SpO2‐cTOI)/SpO2.
2.4.2. Brain tissue analysis
To perform histological analysis, the brain was fixed (formalin 4%) and divided into cortex, inner regions (striatum, thalamus and hippocampus), and cerebellum and brain stem. A total of 20 fields were analyzed with light microscopy. Pathological features of brain injury (necrosis, inflammation, hemorrhage, edema and infarction) were each scored on a 0‐ to 3‐point scale: 0 corresponding to no injury; and 1, 2, and 3 to injury to mild, moderate, and severe injury across the field, respectively. The presence of more than five necrotic cells/field was considered to indicate neuronal necrosis (score range: 0‐20).21
2.5. Statistical analysis
Results are expressed as mean ± standard deviation (SD). Data were assessed using Levene's test to confirm the homogeneity of variance between the treatments and the Kolmogorov‐Smirnoff test for normality (JMP8, Statistical Discovery, SAS, NC). Intragroup comparisons were performed with one‐factor analysis of variance (ANOVA). Short‐term cerebral effects, and lung and brain injury scores were analysed using the Wilcoxon nonparametric test. A P < 0.05 was considered significant.
3. RESULTS
Animals in each group were similar in age (4 ± 1 days) and size (INSURE group: 2.1 ± 0.2 kg; LISA‐NT group: 2.3 ± 0.3 kg; LISAcath group: 2.3 ± 0.3 kg). Multiple BALs (INSURE group: 12 ± 4; LISA‐NT group: 12 ± 3; LISAcath® group: 11 ± 3) were needed to induce the lung injury, no significant differences being observed between groups in the number required. The procedure was well tolerated.
3.1. Pulmonary outcomes
All animals had similar pH, PaO2/FiO2, PaCO2, A‐aDO2, and Cdyn parameters at baseline, after induction of SF‐deficient lung injury and after 30 min of stabilization (Table 1 and Figure 1). BAL produced an abrupt decrease in PaO2/FiO2 (Figure 1A), Cdyn (Figure 1C), and pH (Table 1), with a significant increase in A‐aDO2 and PaCO2 Levels (Table 1 and Figure 1B), without significant differences between groups.
Table 1.
Gas exchange and ventilatory parameters in surfactant deficiency newborn piglets treated with SF administered using INSURE or LISA technique (by nasogastric tube (NT) or by LISAcath®)
| Groups | Basal | BAL | 30 ST | 15 min | 30 min | 60 min | 90 min | 120 min | 150 min | 180 min |
|---|---|---|---|---|---|---|---|---|---|---|
| pH | ||||||||||
| INSURE | 7.38 ± 0.04 | 7.12 ± 0.09& | 7.10 ± 0.12& | 7.29 ± 0.05 | 7.36 ± 0.06 | 7.39 ± 0.07 | 7.42 ± 0.08 | 7.46 ± 0.08 | 7.46 ± 0.07 | 7.44 ± 0.08 |
| LISA‐NT | 7.38 ± 0.03 | 7.09 ± 0.08& | 7.07 ± 0.13& | 7.31 ± 0.02 | 7.33 ± 0.08 | 7.42 ± 0.05 | 7.43 ± 0.05 | 7.43 ± 0.04 | 7.46 ± 0.04 | 7.46 ± 0.03 |
| LISAcath | 7.37 ± 0.05 | 7.06 ± 0.05& | 7.10 ± 0.10& | 7.27 ± 0.06 | 7.30 ± 0.06 | 7.34 ± 0.06* | 7.42 ± 0.02 | 7.42 ± 0.05 | 7.40 ± 0.05 | 7.42 ± 0.04 |
| A‐aDO2 (mmHg) | ||||||||||
| INSURE | 77 ± 11 | 744 ± 17& | 728 ± 27& | 507 ± 102 | 338 ± 89 | 247 ± 98 | 193 ± 91 | 141 ± 61 | 111 ± 36 | 91 ± 23 |
| LISA‐NT | 79 ± 7 | 731 ± 23& | 716 ± 38& | 554 ± 125 | 389 ± 69 | 267 ± 87 | 206 ± 67 | 163 ± 47 | 117 ± 13 | 103 ± 10 |
| LISAcath | 79 ± 11 | 740 ± 18& | 730 ± 22& | 547 ± 119 | 406 ± 115 | 292 ± 60 | 226 ± 66 | 160 ± 36 | 119 ± 20 | 110 ± 18 |
| FiO2 | ||||||||||
| INSURE | 0.28 ± 0.01 | 1.00 ± 0.00& | 1.00 ± 0.00& | 0.83 ± 0.15 | 0.62 ± 0.12 | 0.47 ± 0.11 | 0.41 ± 0.12 | 0.34 ± 0.07 | 0.31 ± 0.05 | 0.29 ± 0.01 |
| LISA‐NT | 0.28 ± 0.01 | 1.00 ± 0.00& | 1.00 ± 0.00& | 0.89 ± 0.09 | 0.65 ± 0.21 | 0.50 ± 0.11 | 0.41 ± 0.08 | 0.37 ± 0.07 | 0.31 ± 0.02 | 0.29 ± 0.01 |
| LISAcath | 0.28 ± 0.01 | 1.00 ± 0.00& | 1.00 ± 0.00& | 0.85 ± 0.13 | 0.66 ± 0.14 | 0.51 ± 0.08 | 0.44 ± 0.07 | 0.36 ± 0.08 | 0.31 ± 0.03 | 0.30 ± 0.02 |
| RR (breath/min) | ||||||||||
| INSURE | 27 ± 1 | 42 ± 1& | 42 ± 1& | 50 ± 26 | 50 ± 12 | 43 ± 10 | 42 ± 14 | 44 ± 14 | 42 ± 12 | 40 ± 13 |
| LISA‐NT | 27 ± 1 | 42 ± 1& | 42 ± 1& | 64 ± 27 | 57 ± 20 | 63 ± 18* | 55 ± 13 | 46 ± 11 | 46 ± 10 | 45 ± 17 |
| LISAcath | 28 ± 1 | 42 ± 1& | 42 ± 1& | 68 ± 22 | 65 ± 16* | 59 ± 22 | 51 ± 12 | 46 ± 9 | 41 ± 4 | 36 ± 6 |
Statistical differences (&)P < 0.05 versus basal point; (*)P < 0.05 versus INSURE group. Values are expressed as mean ± SD.
Alveolar‐arterial oxygen tension difference (A‐aDO2); inspiratory oxygen fraction (FiO2); respiratory rate (RR)
Figure 1.
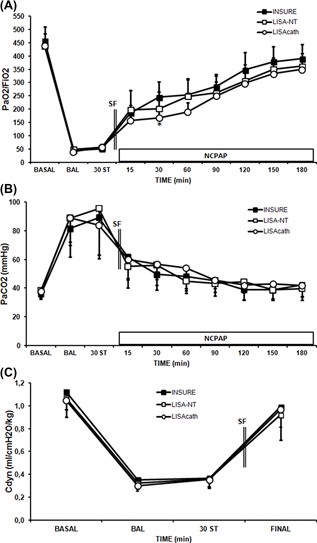
Changes in PaO2/FIO2 ratio, PaCO2 and dynamic compliance (Cdyn) in surfactant (SF)‐deficient newborn piglets treated with SF administered by the INSURE method or LISA using a nasogastric tube (NT) or LISAcath®. PaO2/FIO2 (A), PaCO2 (B), and Cdyn (C) values in the INSURE (black square), LISA‐NT (white square) and LISAcath® (white circle) groups. (*)P < 0.05 versus INSURE group (one‐way ANOVA). Values are mean ± SD. BAL, bronchoalveolar lavage; ST, stabilization
The time course of response to SF administration was similar in INSURE, LISA‐NT, and LISAcath® groups, with rapid improvement in the PaO2/FiO2 ratio, PaCO2, pH and A‐aDO2 values in all SF‐treated groups (Figure 1 and Table 1), while the FiO2 ratio rapidly reduced in all groups (Table 1). These improvements were maintained over time, and were similar in all SF‐treated groups.
Regarding Cdyn, it recovered close to baseline levels with all methods of SF administration (Figure 1C). Moreover, no significant differences were observed between groups in tidal volume or resistance parameters (data not shown).
The LISAcath® group obtained significantly higher scores for atelectasis, alveolar inflammation and total lung injury than INSURE and LISA‐NT groups (Table 2). Nevertheless, the scores for all parameters studied were low, none indicating relevant lung injury (Figure 2).
Table 2.
Total lung injury scores in surfactant deficiency newborn piglets treated with SF administered using INSURE or LISA technique (by nasogastric tube [NT] or by LISAcath®)
| Lung region | Groups | Atelectasis | Necrosis | Edema | Alveolar inflammation | Interstitial inflammation | Alveolar hemorrhage | Interstitial hemorrhage | Total |
|---|---|---|---|---|---|---|---|---|---|
| UPPER | INSURE | 0.17 ± 0.41 | 0 | 0 | 0.17 ± 0.41 | 0.67 ± 0.52 | 0 | 0.17 ± 0.41 | 1.17 ± 1.17 |
| LISA‐NT | 0.17 ± 0.41 | 0 | 0 | 0.50 ± 0.54 | 1.16 ± 1.16 | 0 | 0 | 1.83 ± 1.17 | |
| LISAcath | 1.00 ± 1.10 | 0 | 0 | 0.83 ± 0.54* | 1.33 ± 0.81 | 0 | 0 | 3.16 ± 2.04 | |
| MIDDLE | INSURE | 0.33 ± 0.51 | 0 | 0 | 0.33 ± 0.51 | 1.00 ± 0.89 | 0 | 0 | 1.67 ± 1.51 |
| LISA‐NT | 0.83 ± 0.75 | 0 | 0.17 ± 0.40 | 0.33 ± 0.51 | 1.33 ± 1.03 | 0 | 0 | 2.67 ± 1.75 | |
| LISAcath | 0.83 ± 1.69 | 0 | 0 | 0.83 ± 0.40 | 1.33 ± 1.03 | 0 | 0 | 3.00 ± 2.19 | |
| LOWER | INSURE | 0.33 ± 0.52 | 0 | 0 | 0.33 ± 0.52 | 1.00 ± 0.89 | 0.17 ± 0.41 | 0 | 1.83 ± 1.47 |
| LISA‐NT | 0.66 ± 0.81 | 0 | 0 | 0.33 ± 0.52 | 0.83 ± 0.75 | 0 | 0 | 1.83 ± 1.72 | |
| LISAcath | 1.33 ± 1.02 | 0 | 0.17 ± 0.40 | 1.33 ± 0.52*$ | 1.50 ± 0.83 | 0 | 0.50 ± 0.54 | 3.70 ± 2.50*$ | |
| TOTAL | INSURE | 0.28 ± 0.46 | 0 | 0 | 0.28 ± 0.46 | 0.89 ± 0.76 | 0.06 ± 0.24 | 0.06 ± 0.24 | 1.56 ± 1.34 |
| LISA‐NT | 0.56 ± 0.70 | 0 | 0.05 ± 0.23 | 0.38 ± 0.50 | 1.11 ± 0.96 | 0 | 0 | 2.10 ± 1.53 | |
| LISAcath | 1.00 ± 1.00* | 0 | 0.05 ± 0.23 | 1.00 ± 0.48*$ | 1.38 ± 0.85 | 0 | 0.16 ± 0.38 | 3.6 ± 2.25*$ |
Statistical differences were assessed using Wilcoxon non‐parametric test; (*)P < 0.05 versus Insure group, ($)P < 0.05 versus LISA‐NT group. Values are expressed as mean ± SD.
Figure 2.
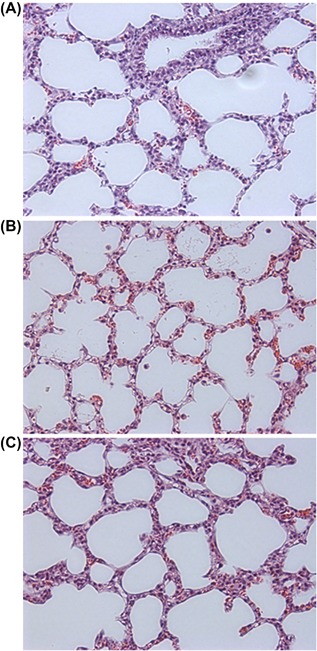
Photomicrographs (200× magnification) of representative lung sections from INSURE (A), LISA‐NT (B) and LISAcath® (C) groups. Panels obtained from the middle region of the lung
3.2. Intrapulmonary shunt and hemodynamic assessment
BAL produced an abrupt increase in Qs/Qt (Figure 3A). Subsequently, rapid improvements in Qs/Qt were observed with all SF treatment strategies, levels reaching baseline 2 h after SF administration (Figure 3A).
Figure 3.
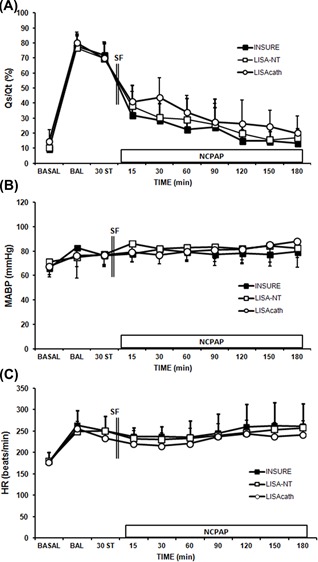
Changes in intrapulmonary shunt (Qs/Qt), arterial blood pressure (MABP), and heart rate (HR) in surfactant (SF)‐deficient newborn piglets treated with SF administered by the INSURE method or LISA using a nasogastric tube (NT) or LISAcath®. Mean Qs/Qt (A), MABP (B), and HR (C) values in the INSURE (black square), LISA‐NT (white square) and LISAcath® (white circle) groups. (*)P < 0.05 versus INSURE group (one‐way ANOVA). Values are mean ± SD. BAL, bronchoalveolar lavage; ST, stabilization
At baseline, there were no differences between groups in the hemodynamic parameters studied. No significant changes in MABP were observed after BAL (Figure 3B), while the HR rose (Figure 3C). MABP and HR did not change significantly during the experimental period and did not differ significantly between the groups at any point.
3.3. Cerebral assessment
3.3.1. Overall cerebral effect
Carotid blood flow increased in all groups after the BAL procedure, while cTOI, cFTOE, and systemic oxygenation (SpO2) decreased (Figure 4). SF administration was followed by a continuous decrease in carotid blood flow, this reaching baseline levels after 60 min in all SF‐treated groups (Figure 4A). On the other hand, cTOI, cFTOE, and SpO2 increased after SF treatment, reaching baseline levels after 15‐30 min of treatment, with no significant differences between groups (Figures 4B‐4D).
Figure 4.
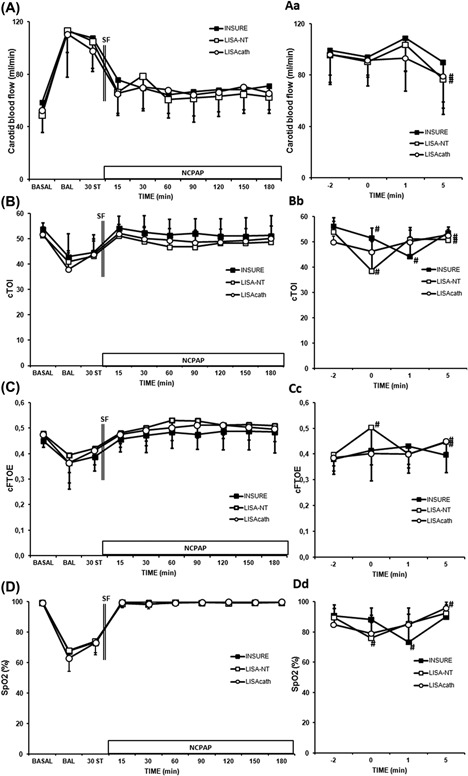
Changes in carotid blood flow, cerebral oxygenation (cTOI), cerebral fractional tissue oxygen fraction (cFTOE) and pulse‐oximetry saturation (SpO2) in SF‐deficient newborn piglets treated with SF administered by the INSURE method or LISA using a nasogastric tube (NT) or LISAcath®. Cerebral effect on carotid blood flow (A), cTOI (B), cFTOE (C) and SpO2 (D) values; SF administration interval effect on carotid blood flow (Aa), cTOI (Bb), cFTOE (Cc) and SpO2 (Dd) values in the INSURE (black square), LISA‐NT (white square) and LISAcath® (white circle) groups. (*)P < 0.05 versus INSURE group (one‐way ANOVA); (#)P < 0.05 versus −2 min time point (Wilcoxon non‐parametric test). Values are mean ± SD. BAL, bronchoalveolar lavage; ST, stabilization
3.3.2. Cerebral effects around the time of SF administration
Assessing the impact of SF during and immediately after its administration, we observed transient decreases in SpO2 and cTOI in INSURE and LISA‐NT groups (Figures 4Bb and 4Dd). The effect of the INSURE procedure on SpO2 was observed 1 min after SF administration, while cTOI showed a continuous decrease immediately on administration of SF and during the first minute following its administration and in the LISA‐NT group, a significant decrease in both these variables was observed immediately on administration of SF (Figures 4Bb and 4Dd). In contrast, SpO2 and cTOI values did not significantly decrease during SF administration in the LISAcath® group. By 5 min after administering SF, SpO2, and cTOI had recovered to some extent in the INSURE group and completely (to baseline levels) in the LISA‐NT group (Figures 4Bb and 4Dd).
Cerebral FTOE values increased in the LISA‐NT group immediately on administration of SF and decreased thereafter, while no changes were observed in the INSURE or LISAcath® groups (Figure 4Cc). Progressive increases in cFTOE were observed in both LISA‐NT and LISAcath® groups, reaching baseline levels by 5 min after SF administration (Figure 4Cc).
3.3.3. Brain injury
All groups studied obtained low brain injury scores, with similar necrosis, edema, hemorrhage, inflammation, and infarction scores in all regions studied (Table 3 and Figure 5).
Table 3.
Total brain injury scores in surfactant deficiency newborn piglets treated with SF administered using INSURE or LISA technique (by nasogastric tube (NT) or by LISAcath®)
| Necrosis | Edema | Inflammation | Hemorrhage | Infarct | |
|---|---|---|---|---|---|
| CORTEX | |||||
| INSURE | 8 (2‐15) | 0 | 0.7 (0‐1) | 0 | 0 |
| LISA‐NT | 9 (4‐14) | 0.2 (0‐1) | 0.6 (0‐1) | 0 | 0 |
| LISAcath | 8 (4‐14)$ | 0.4 (0‐1)* | 0.6 (0‐1) | 0 | 0 |
| INNER | |||||
| INSURE | 7 (2‐14) | 0 | 0.4 (0‐1) | 0 | 0 |
| LISA‐NT | 7 (2‐14) | 0.1 (0‐1) | 0.4 (0‐1) | 0 | 0 |
| LISAcath | 8 (0‐12) | 0.2 (0‐1) | 0.4 (0‐1) | 0 | 0 |
| CB+B | |||||
| INSURE | 9 (3‐16) | 0 | 0 | 0 | 0 |
| LISA‐NT | 7 (3‐16) | 0 | 0 | 0 | 0 |
| LISAcath | 8 (4‐16) | 0 | 0 | 0 | 0 |
Statistical differences were observed between groups using Wilcoxon non‐parametric test; (*)P < 0.05 versus INSURE group; ($)P < 0.05 versus LISA‐NT group. Values are expressed as mean ± range. CB + B: cerebellum + brainsterm.
Figure 5.
Photomicrographs (200× magnification) of representative brain sections from INSURE (A), LISA‐NT (B) and LISAcath® (C) groups. Panels obtained from the striatum region of the brain
4. DISCUSSION
We have been able to demonstrate in our spontaneously breathing newborn piglet model of SF‐deficient lung injury that two different methods of SF administration (INSURE and LISA) are both associated with rapid and significant improvements in pulmonary status (gas exchange, respiratory parameters, and lung mechanics) together with low lung injury scores. Moreover, this is the first study that investigates the effect of two different LISA procedures (using an NT or a LISAcath® catheter) on cTOI and cFTOE and compares them to the INSURE procedure. The INSURE procedure and LISA using an NT were associated with brief transient decreases in SpO2 and cTOI immediately after and during SF treatment, respectively, while LISA using a LISAcath® catheter was not associated with any changes in SpO2 or cerebral oxygenation as measured by NIRS. The brain injury scores were similar across groups, implying that none of these SF administration procedures has any significant clinical effects on the brain in development.
Endotracheal intubation, SF bolus instillation and prolonged mechanical ventilation have been the standard treatment for neonatal RDS, but this approach carries a risk of acute lung injury in preterm infants.24 Therefore, noninvasive ventilation such as nCPAP has been introduced as a primary treatment for preterm infants with spontaneous breathing after birth seeking to reduce acute lung injury. Moreover, the INSURE procedure was the first method used to combine nCPAP with SF administration,8 this approach being the most efficient to treat RDS. Nevertheless, it still requires a brief period of tracheal intubation and sedation, before the SF administration and rapid extubation, and the latter is not always possible. On the other hand, many researchers are investigating minimally invasive methods for SF administration,2, 3, 4, 5 to avoid tracheal intubation and sedation altogether. With LISA, less invasive SF administration, SF is administered into the trachea by direct laryngoscopy, via a thin tube (NT, vascular catheter, or LISAcath®), possibly with the aid of Magill forceps, while the infant is supported with nCPAP.13, 25, 26 After SF instillation, the thin tube is immediately removed, avoiding the need for sedation and tracheal intubation.
It has previously been demonstrated that the process of SF administration itself may induce rapid changes in systemic and cerebral hemodynamic (blood pressure, cerebral blood flow velocities, cerebral blood volume), PaCO2 and oxygenation values.27, 28, 29 Nevertheless, it is not well known how the new ways of SF administration affect the brain in development, this issue remaining relatively unexplored. This is of great importance, however, given that preterm infants, in whom RDS is relevant and common, have a significantly higher risk of acute and chronic brain injury than term infants.30 Cerebral NIRS enables continuous non‐invasive estimation of cerebral oxygenation, providing clinicians with important information concerning how and how long different SF administration procedures may affect the brain in development.
In our study, as expected, SF administration by the INSURE method rapidly improved pulmonary outcomes without significant changes in MABP, HR or carotid blood flow. On the other hand, short‐lasting hypoxemia (as demonstrated by pulse oximetry) and a brief transient decrease in cTOI (not associated with an increase in cFTOE), without changes in carotid blood flow were observed during and after SF administration, though levels recovered thereafter, as described previously.18, 19, 31 In contrast, van der Berg et al16 did not observe a perturbation of cerebral oxygen delivery or extraction, whereas electrical brain activity decreased for a prolonged period of time. Moreover, Li et al18 suggest that cerebral autoregulation may be affected transiently by SF administration using the INSURE technique, the impact lasting for 5‐10 min. The effect of the INSURE procedure on cerebral oxygenation and perfusion observed in our study may be explained by two main factors. Firstly, in our study, 200 mg/kg of SF was used, while van der Berg et al and Li et al used low doses (100 mg/kg and 120 mg/kg, respectively) and thus low volumes of SF, and it is known that the amount of fluid instilled endotracheally plays an important role in determining changes in cerebral oxygenation and cerebral blood flow due to SF treatment.27 Secondly, mechanical ventilation was maintained during and 1 min after SF administration, which may in some way help SF to spread and increase lung recruitment, as reflected in the gradual decrease in cTOI observed in our study.
SF administration by LISA using an NT or a LISAcath®, like the INSURE procedure, rapidly improved pulmonary outcomes without significant changes in hemodynamic parameters. Currently, there is not a clear consensus on the best catheter to use for LISA or the need for Magill forceps, among other options. Notably, based on our data, the NT and LISAcath® catheter seem to be associated with slightly different effects on cerebral oxygenation. On the one hand, when SF was administered through an NT, we observed transient decreases in SpO2 and decrease in cTOI with a significant increase in cFTOE during SF administration, these alterations lasting for less than 1 min. The short increase in cFTOE may represent a compensatory mechanism for maintaining adequate cerebral oxygenation,19 as this SF administration method requires appropriate placement of the NT in the trachea using Magill forceps and the SF is administered to a spontaneously breathing patient maintained on nCPAP. On the other hand, if SF was administered using a LISAcath® catheter no significant alteration was observed in any of aforementioned parameters. In accordance with this, Bao et al17 observed less fluctuation in FiO2 and SpO2 in infants receiving LISA than those in the INSURE group, during the whole SF administration procedure, the improvement in SpO2 being faster during the INSURE procedure.
The LISAcath® catheter has been specifically designed to be placed in the trachea without the need for Magill forceps, the handling and insertion of the catheter being straightforward due to its greater thickness and the ease of double checking the position, thanks to two sets of marks, and its placement in the trachea has been perceived to be less traumatic.26 The present study is the first to evaluate the cerebral effect of the LISA procedure using a LISAcath® catheter, and hence, these results cannot be compared to previous studies. In looking for an explanation of why an effect on cerebral oxygenation was observed when LISA was applied using an NT but not a LISAcath®, we hypothesize that the maneuver requiring NT placement in the trachea with Magill forceps may in some way disturb cerebral oxygenation, as the NT and LISAcath® catheter size (5Fr), medication used, animal weight and SF dose and duration of administration were similar in the two LISA groups.
The INSURE and LISA techniques require the use of a laryngoscope to visualize the vocal cords. Hence, both involve procedures that can be difficult and traumatic, especially if performed by untrained individuals. Previous articles have indicated that LISA and INSURE procedures need well‐developed skills and it is really important that they are performed after adequate training by clinicians with sufficient expertise.26, 32 As previously reported, longer training periods lead to better results in terms of avoiding intubation, and mechanical ventilation, changes in hemodynamic parameters, among other factors, possibly because the technique is better executed.33
Using our pathological injury score, the LISAcath procedure seems to result in higher values of atelectasis, alveolar inflammation and total lung injury than INSURE and LISA‐NT procedures. Nevertheless, as occurs when physiological variables are analyzed, it should be borne in mind that there is a range of values between which it cannot be considered that there is relevant lung damage. Zimmermann et al23 described that total lung injury scores of 12 can considered quite severe lung injury. Unpublished data from our group using this animal model suggest that mean values higher than eight may be related to mild lung injury, and hence, irrespective of whether SF was administered, in the current study, it was not associated with poor histological lung injury outcomes.
In brief, the INSURE method and LISA, using either an NT or a LISAcath® catheter, are different ways of delivering SF that all provide effective therapies. Further, although a one‐off perturbation of cerebral oxygenation was observed in INSURE and LISA groups during SF administration, it was short, and no significant changes in the brain were detected in histological analysis.34, 35
4.1. Limitations
Limitations of this study include the use of newborn piglets (2‐4 days old), which require SF washout lavage to induce lung injury. The SF washout lavage model has been frequently used in adult and juvenile animals to implement successful animal models of acute pulmonary failure in the context of RDS.21, 22, 36, 37 The newborn piglet model was chosen because the brain maturation, lung volume, and birth weight resemble those of newborn infants. Moreover, the feasibility of using nCPAP and SF strategies in this model of SF‐deficient lung injury has been previously demonstrated.21, 36, 37 Nevertheless, 2‐ to 4‐day‐old piglets have a greater level of brain development than a 32‐week human fetus,38 The effects of INSURE and LISA procedures on the brain need to be interpreted with caution, as effects on the term piglet brain may not be the same as those on the vulnerable premature brain of newborn infants.
Another limitation is that we did not have a control group that was not administered SF in this study. Only two studies have evaluated the impact of spontaneous breathing during39 or after SF administration40 on SF distribution, with somewhat conflicting and inconclusive results. It remains uncertain how much SF is the ideal amount that should be deposited and how much SF actually reaches the lung with the use of INSURE and LISA techniques. Factors influencing SF distribution, including the optimal dose, rate of administration and/or nCPAP level, need to be explored.
5. CONCLUSION
In spontaneously breathing SF‐deficient newborn piglets, INSURE and LISA procedures improved pulmonary outcomes without changes in hemodynamic parameters. On the other hand, short‐lasting decreases in cerebral oxygenation are associated with SF administration by the INSURE method or LISA using a nasogastric tube, while the LISA procedure using a LISAcath® was not associated with any changes in cerebral oxygenation. Notably, none of treatments studied seem to have a negative impact on the neonatal brain.
STATEMENT OF FINANCIAL SUPPORT
This study has been funded by Carlos III Health Institute through project PI14/00024 (co‐financed by the European Regional Development Fund (ERDS) “A way to make Europe”) and Chiesi Farmaceutici.
DISCLOSURE STATEMENT
Fabrizio Salomone is an employee at Chiesi Farmaceutici.
ACKNOWLEDGMENTS
The authors thank Alfredo Alonso Digon for excellent technical assistance and for his valuable support in the experimental handling of the animals.
Rey‐Santano C, Mielgo VE, Gomez‐Solaetxe MA, Salomone F, Gastiasoro E, Loureiro B. Cerebral oxygenation associated with INSURE versus LISA procedures in surfactant‐deficient newborn piglet RDS model. Pediatric Pulmonology. 2019;54:644–654. 10.1002/ppul.24277
Carmen Rey‐Santano and Victoria E Mielgo contributed equally to this work.
REFERENCES
- 1. Gregory GA, Kitterman JA, Phibbs RH, Tooley WH, Hamilton WK. Treatment of the idiopathic respiratory distress syndrome with continuous positive airway pressure. N Engl J Med. 1971;284:1333–1340. [DOI] [PubMed] [Google Scholar]
- 2. More K, Sakhuja P, Shah PS. Minimally invasive surfactant administration in preterm infants: a meta‐narrative review. JAMA Pediatr. 2014;168:901–908. [DOI] [PubMed] [Google Scholar]
- 3. Kattwinkel J, Robinson M, Bloom BT, Delmore P, Ferguson JE. Technique for intrapartum administration of surfactant without requirement for an endotracheal tube. J Perinatol. 2004;24:360–365. [DOI] [PubMed] [Google Scholar]
- 4. Attridge JT, Stewart C, Stukenborg GJ, Kattwinkel J. Administration of rescue surfactant by laryngeal mask airway: lessons from a pilot trial. Am J Perinatol. 2013;30:201–206. [DOI] [PubMed] [Google Scholar]
- 5. Jorch G, Hartl H, Roth B, et al. To the editor: surfactant aerosol treatment of respiratory distress syndrome in spontaneously breathing premature infants. Pediatr Pulmonol. 1997;24:222–224. [DOI] [PubMed] [Google Scholar]
- 6. Kamper J, Wulff K, Larsen C, Lindequist S. Early treatment with nasal continuous positive airway pressure in very low‐birth‐weight infants. Acta Paediatr. 1993;82:193–197. [DOI] [PubMed] [Google Scholar]
- 7. Stevens TP, Harrington EW, Blennow M, Soll RF. Early surfactant administration with brief ventilation vs. selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome. Cochrane Database Syst Rev. 2007;CD003063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Verder H, Robertson B, Greisen G, et al. Surfactant therapy and nasal continuous positive airway pressure for newborns with respiratory distress syndrome. Danish‐Swedish Multicenter Study Group. N Engl J Med. 1994;331:1051–1055. [DOI] [PubMed] [Google Scholar]
- 9. Dani C, Corsini I, Bertini G, Fontanelli G, Pratesi S, Rubaltelli FF. The INSURE method in preterm infants of less than 30 weeks' gestation. J Matern Fetal Neonatal Med. 2010;23:1024–1029. [DOI] [PubMed] [Google Scholar]
- 10. Björklund LJ, Ingimarsson J, Curstedt T, et al. Manual ventilation with a few large breaths at birth compromises the therapeutic effect of subsequent surfactant replacement in immature lambs. Pediatr Res. 1997;42:348–355. [DOI] [PubMed] [Google Scholar]
- 11. Venkatesh V, Ponnusamy V, Anandaraj J, et al. Endotracheal intubation in a neonatal population remains associated with a high risk of adverse events. Eur J Pediatr. 2011;170:223–227. [DOI] [PubMed] [Google Scholar]
- 12. Kribs A, Roll C, Göpel W, et al. Nonintubated surfactant application vs conventional therapy in extremely preterm infants: a randomized clinical trial. JAMA Pediatr. 2015;169:723–730. [DOI] [PubMed] [Google Scholar]
- 13. Dargaville PA, Aiyappan A, De Paoli AG, et al. Minimally‐invasive surfactant therapy in preterm infants on continuous positive airway pressure. Arch Dis Child Fetal Neonatal Ed. 2013;98:F122–F126. [DOI] [PubMed] [Google Scholar]
- 14. Göpel W, Kribs A, Ziegler A, et al. Avoidance of mechanical ventilation by surfactant treatment of spontaneously breathing preterm infants (AMV): an open‐label, randomised, controlled trial. Lancet. 2011;378:1627–1634. [DOI] [PubMed] [Google Scholar]
- 15. Roll C, Knief J, Horsch S, Hanssler L. Effect of surfactant administration on cerebral haemodynamic and oxygenation in premature infants. A near infrared spectroscopy study. Neuropediatrics. 2000;31:16–23. [DOI] [PubMed] [Google Scholar]
- 16. van den Berg E, Lemmers PM, Toet MC, Klaessens JH, van Bel F. Effect of the “InSurE“ procedure on cerebral oxygenation and electrical brain activity of the preterm infant. Arch Dis Child Fetal Neonatal Ed. 2010;95:F53–F58. [DOI] [PubMed] [Google Scholar]
- 17. Bao Y, Zhang G, Wu M, Ma L, Zhu J. A pilot study of less invasive surfactant administration in very preterm infants in a Chinese tertiary center. BMC Pediatr. 2015;15:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li XF, Cheng TT, Guan RL, et al. Effects of different surfactant administrations on cerebral autoregulation in preterm infants with respiratory distress syndrome. J Huazhong Univ Sci Technolog Med Sci. 2016;36:801–805. [DOI] [PubMed] [Google Scholar]
- 19. Bertini G, Coviello C, Gozzini E, et al. Change of cerebral oxygenation during surfactant treatment in preterm infants: “LISA“ versus “InSurE“ procedures. Neuropediatrics. 2017;48:98–103. [DOI] [PubMed] [Google Scholar]
- 20. Poets CF, Roberts RS, Schmidt B, et al. Association between intermittent hypoxemia or bradycardia and late death or disability in extremely preterm infants. JAMA. 2015;314:595–603. [DOI] [PubMed] [Google Scholar]
- 21. Rey‐Santano C, Mielgo VE, Gomez‐Solaetxe MA, et al. Non‐invasive ventilation and surfactant treatment as the primary mode of respiratory support in surfactant‐deficient newborn piglets. Pediatr Res. 2018;83:904–914. [DOI] [PubMed] [Google Scholar]
- 22. Lachmann B, Robertson B, Vogel J. In vivo lung lavage as an experimental model of the respiratory distress syndrome. Acta Anaesthesiol Scand. 1980;24:231–236. [DOI] [PubMed] [Google Scholar]
- 23. Zimmermann AM, Roberts KD, Lampland AL, et al. Improved gas exchange and survival after KL‐4 surfactant in newborn pigs with severe acute lung injury. Pediatr Pulmonol. 2010;45:782–788. [DOI] [PubMed] [Google Scholar]
- 24. Iliodromiti Z, Zygouris D, Sifakis S, et al. Acute lung injury in preterm fetuses and neonates: mechanisms and molecular pathways. J Matern Fetal Neonatal Med. 2013;26:1696–1704. [DOI] [PubMed] [Google Scholar]
- 25. Kribs A, Pillekamp F, H?nseler C, Vierzig A, Roth B. Early administration of surfactant in spontaneous breathing with nCPAP: feasibility and outcome in extremely premature infants (postmenstrual age <27 weeks). Pediatri Anesth. 2007;17:364–369. [DOI] [PubMed] [Google Scholar]
- 26. Lista G, Bresesti I, Fabbri L. Is less invasive surfactant administration necessary or “Only“ helpful or just a fashion? Am J Perinatol. 2018;35:530–533. [DOI] [PubMed] [Google Scholar]
- 27. Dorrepaal CA, Benders MJ, Steendijk P, van de Bor M, van Bel F. Cerebral hemodynamics and oxygenation in preterm infants after low‐vs. high‐dose surfactant replacement therapy. BiolNeonate. 1993;64:193–200. [DOI] [PubMed] [Google Scholar]
- 28. Kaiser JR, Gauss CH, Williams DK. Surfactant administration acutely affects cerebral and systemic hemodynamics and gas exchange in very low birth weight infants. JPediatr. 2004;144:809–814. [DOI] [PubMed] [Google Scholar]
- 29. Fujii AM, Bailey J, Doros G, et al. Effects of beractant and poractant administration on cerebral hemodynamics. J Neonatal Perinatal Med. 2009;2:27–34. [Google Scholar]
- 30. Beck S, Wojdyla D, Say L, et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ. 2010;88:31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Skov L, Hellstr”m‐Westas L, Jacobsen T, Greisen G, Svenningsen NW. Acute changes in cerebral oxygenation and cerebral blood volume in preterm infants during surfactant treatment. Neuropediatrics. 1992;23:126–130. [DOI] [PubMed] [Google Scholar]
- 32. Aguar M, Cernada M, Brugada M, Gimeno A, Gutierrez A, Vento M. Minimally invasive surfactant therapy with a gastric tube is as effective as the intubation, surfactant, and extubation technique in preterm babies. Acta Paediatr. 2014;103:e229–e233. [DOI] [PubMed] [Google Scholar]
- 33. Kribs A, Vierzig A, Hünseler C, et al. Early surfactant in spontaneosly breathing with nCPAP in ELBW infants‐ a single centre four year experience. Acta Paediatr. 2008;97:293–298. [DOI] [PubMed] [Google Scholar]
- 34. Porath M, Korp L, Wendrich D, Dlugay V, Roth B, Kribs A. Surfactant in spontaneous breathing with nCPAP: neurodevelopmental outcome at early school age of infants ≤27 weeks. Acta Paediatr. 2011;100:352–359. [DOI] [PubMed] [Google Scholar]
- 35. Teig N, Weitkämper A, Rothermel J, et al. Observational study on less invasive surfactant administration (LISA) in preterm infants <29 Weeks–Short and long‐term outcomes. Z Geburtshilfe Neonatol. 2015;219:266–273. [DOI] [PubMed] [Google Scholar]
- 36. Nold JL, Meyers PA, Worwa CT, et al. Decreased lung injury after surfactant in piglets treated with continuous positive airway pressure or synchronized intermittent mandatory ventilation. Neonatology. 2007;92:19–25. [DOI] [PubMed] [Google Scholar]
- 37. Lampland AL, Meyers PA, Worwa CT, Swanson EC, Mammel MC. Gas exchange and lung inflammation using nasal intermittent positive‐pressure ventilation versus synchronized intermittent mandatory ventilation in piglets with saline lavage‐induced lung injury: an observational study. Crit Care Med. 2008;36:183–187. [DOI] [PubMed] [Google Scholar]
- 38. Eiby YA, Wright LL, Kalanjati VP, et al. A pig model of the preterm neonate: anthropometric and physiological characteristics. PLoS One. 2013;8:e68763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Niemarkt HJ, Kuypers E, Jellema R, et al. Effects of less‐invasive surfactant administration on oxygenation, pulmonary surfactant distribution, and lung compliance in spontaneously breathing preterm lambs. Pediatr Res. 2014;76:166–170. [DOI] [PubMed] [Google Scholar]
- 40. Bohlin K, Bouhafs RK, Jarstrand C, Curstedt T, Blennow M, Robertson B. Spontaneous breathing or mechanical ventilation alters lung compliance and tissue association of exogenous surfactant in preterm newborn rabbits. Pediatr Res. 2005;57:624–630. [DOI] [PubMed] [Google Scholar]


