Abstract
In this post hoc analysis we investigated the effects of insulin degludec/liraglutide fixed‐ratio combination (IDegLira) versus comparators on cardiovascular (CV) risk markers in participants in the DUAL II (vs. insulin degludec), DUAL V (vs. insulin glargine 100 units/mL) and DUAL VII (vs. basal–bolus therapy) trials, grouped by sex, age (<65 years, ≥65 years) and diabetes duration (<10 years, ≥10 years). Treatment contrasts were in favour of IDegLira in many subgroups for changes from baseline in glycated haemoblogin (DUAL II, DUAL V), body weight (all three trials), systolic blood pressure (BP; all three trials), HDL cholesterol (DUAL VII) and LDL cholesterol (DUAL II, DUAL V). Higher heart rates were seen with IDegLira versus comparators (all three trials) plus significantly higher diastolic BP in men (DUAL V). Differences in treatment effect were seen between sexes in waist circumference (DUAL II), systolic BP (DUAL II, DUAL V) and triglycerides (DUAL VII), and between diabetes durations in LDL cholesterol (DUAL V). In conclusion, IDegLira is associated with a general improvement in CV risk markers compared with basal insulin or basal–bolus therapy after 26 weeks of treatment.
Keywords: basal insulin, cardiovascular disease, liraglutide, type 2 diabetes
1. INTRODUCTION
In 2008, the US Food and Drug Administration (FDA) issued guidelines stating that any new antidiabetic therapies should not result in an unacceptable increase in cardiovascular (CV) risk.1 This was mirrored by the 2012 European Medicines Agency (EMA) diabetes guidelines, which state that new antidiabetic therapies should have either beneficial or neutral effects on variables associated with CV risk.2 Although specific considerations for combination therapies are not provided in the FDA guidance,1 the EMA has published a separate guidance document for fixed‐combination therapies.3 No details, however, are provided on specific CV data that need to be assessed.
While no CV outcome trials (CVOTs) have been conducted using insulin degludec/liraglutide fixed‐ratio combination (IDegLira), such trials have been conducted on its monocomponents.4, 5 To evaluate the CV effect of IDegLira, we report the analysis of CV risk markers, split by baseline characteristics, using data collected in three DUAL clinical trials.
2. MATERIALS AND METHODS
This was a post hoc analysis of the previously published DUAL II (NCT01392573),6 DUAL V (NCT01952145)7 and DUAL VII (NCT02420262)8 trials (Table S1 in the Supporting Information), all conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice.
Our analysis compared treatment effects on a number of CV risk markers, grouped by three independent baseline characteristics: sex, age (<65 years of age, ≥65 years of age) and duration of diabetes (<10 years, ≥10 years).
Endpoints from the original trials included in this analysis were changes from baseline to week 26 in glycated haemoglobin (HbA1c; primary), body weight, blood pressure (BP), heart rate, waist circumference, number of hypoglycaemic events and lipid profile, as well as additional CV risk markers collected in DUAL II only (apolipoprotein B, brain natriuretic peptide [BNP] and high‐sensitivity C‐reactive protein [hsCRP]).6, 7, 8
2.1. Statistical analyses
All statistical analyses were performed on the full analysis set (all randomized participants) as per their respective original trials. Full statistical analysis details can be found in the Supporting Information.
3. RESULTS
3.1. Participants
Participants' baseline characteristics were generally well matched between treatment arms across subgroups in the three trials (Table S2 in the Supporting Information). Across the subgroups, end‐of‐trial insulin doses were similar between IDegLira and degludec, but lower for IDegLira compared with insulin glargine 100 units/mL (IGlar U100) or basal–bolus therapy.
3.2. Endpoints
End‐of‐trial values can be found in Table S3 in the Supporting Information. Results were generally consistent with those from the overall populations.6, 7, 8
In DUAL II and DUAL V, there were statistically significant greater reductions in HbA1c with IDegLira compared with basal insulin comparators across all baseline characteristics (Figure 1A,B). In DUAL VII, there were no statistically significant differences between IDegLira and basal–bolus therapy in changes in HbA1c for any baseline characteristic subgroup (Figure 1C).
Figure 1.
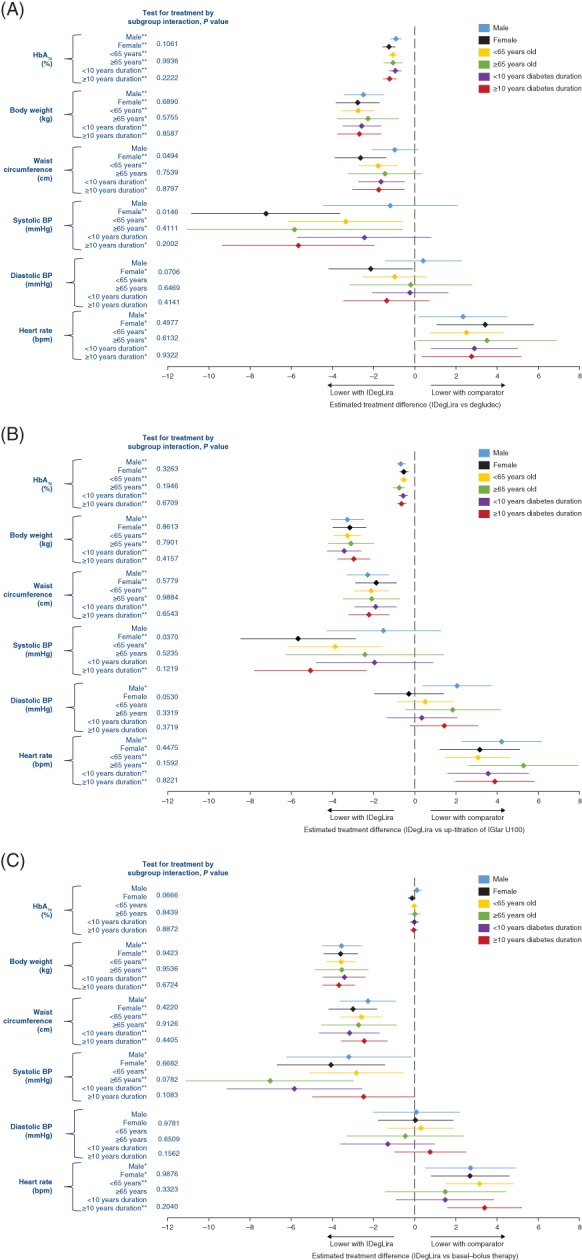
Changes in HbA1c, body weight, waist circumference and vital signs in A, DUAL II (vs insulin degludec), B, DUAL V (vs IGlar U100) and C, DUAL VII (vs basal–bolus insulin), grouped by sex, age and duration of diabetes. *P < 0.05; **P < 0.001. All analyses were based on the full analysis set. In DUAL II and DUAL V the endpoint was analysed using analysis of covariance, with treatment, pre‐trial diabetes treatment (DUAL II only), region, subgroup and interaction between treatment and subgroup as fixed effects and baseline response as covariate. Missing data are imputed using last observation carried forward. In DUAL VII the endpoint was analysed using a mixed model for repeated measures including subgroup, visit, treatment, region and interaction between treatment, and subgroup as fixed factors, and baseline response as covariate. Interactions between visit and all factors and covariates were also included. Analysis of covariance; BP, blood pressure; HbA1c, glycated haemoglobin; IDegLira, insulin degludec/liraglutide fixed‐ratio combination; IGlar U100, insulin glargine 100 units/mL
In all trials there were statistically significant reductions in body weight with IDegLira compared with comparators across all baseline characteristics (Figure 1).
Across all trials, there was a consistently greater change in waist circumference across subgroups favouring IDegLira compared with comparators, with significant differences seen in women, participants aged <65 years and both diabetes duration subgroups in DUAL II (Figure 1A), and in all subgroups in DUAL V (Figure 1B) and DUAL VII (Figure 1C). There was a different treatment effect between the subgroups of men and women in DUAL II (P = 0.0494 for test for treatment interaction), with a larger treatment effect in women.
Overall, IDegLira‐treated participants had a significantly lower mean systolic BP compared with comparators in all subgroups except for men and participants with a diabetes duration <10 years in DUAL II; men, participants aged ≥65 years and with diabetes duration <10 years in DUAL V; and participants with diabetes duration ≥10 years in DUAL VII (Figure 1). There were also different treatment effects between the subgroups of men and women in DUAL II (P = 0.0146 for test for treatment interaction) and DUAL V (P = 0.0370 for test for treatment interaction), with a larger treatment effect seen in women.
In the subgroups, the only statistically significant difference in diastolic BP was a lower BP with IDegLira compared with degludec in women in DUAL II (Figure 1A) and a higher BP with IDegLira compared with IGlar U100 in men in DUAL V (Figure 1B).
In all trials, there were statistically significant increases in heart rate with IDegLira compared with comparators, irrespective of sex, age or diabetes duration, except for participants aged ≥65 years and those with diabetes duration <10 years in DUAL VII (Figure 1).
Levels of LDL cholesterol were consistently lower or equal with IDegLira compared with comparators across subgroups in all trials, with statistical significance achieved in men, participants aged <65 years and both diabetes duration subgroups in DUAL II (Figure 2A) and in all subgroups except in participants with diabetes duration ≥10 years in DUAL V (Figure 2B). In DUAL VII none of the differences were statistically significant (Figure 2C). There was a statistically significant different treatment effect between the diabetes duration subgroups in DUAL V (P = 0.0323 for test for treatment interaction), with a larger treatment effect seen in participants with diabetes duration <10 years.
Figure 2.
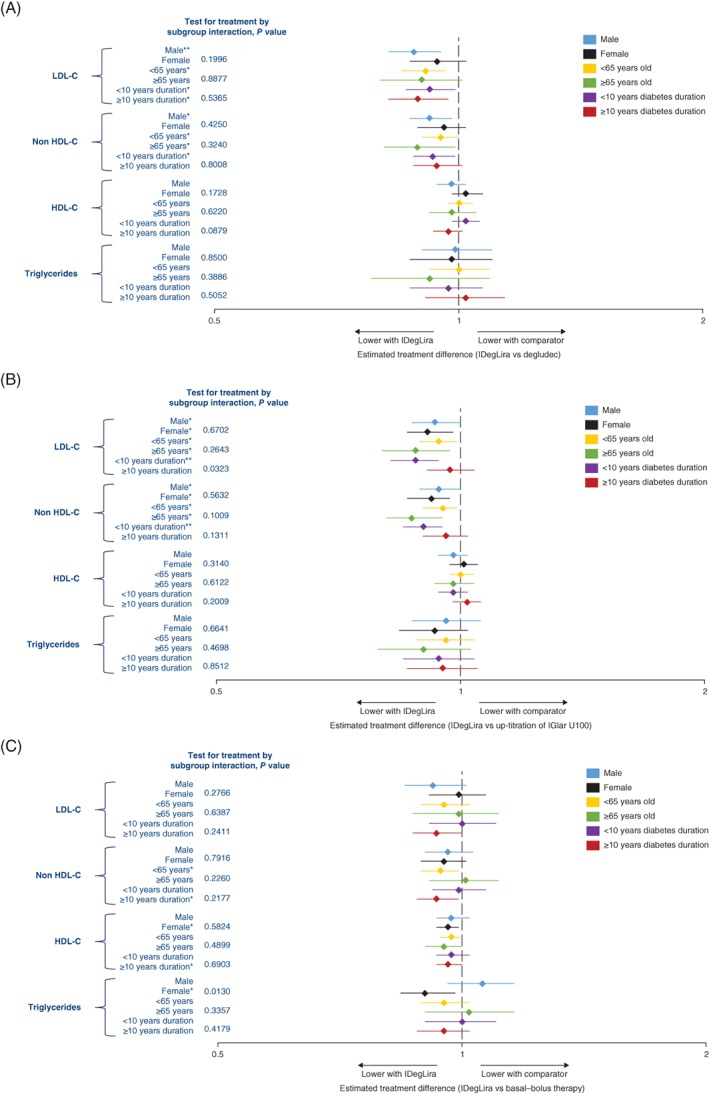
Lipids in A, DUAL II (vs insulin degludec), B, DUAL V (vs IGlar U100) and C, DUAL VII (vs basal–bolus insulin), grouped by sex, age and duration of diabetes. *P < 0.05; **P < 0.001. All analyses were based on the full analysis set. In DUAL II and DUAL V the log‐transformed endpoint was analysed using analysis of covariance, with treatment, pre‐trial diabetes treatment (DUAL II only), region, subgroup and interaction between treatment and subgroup as fixed effects and log‐transformed baseline response as covariate. Missing data were imputed using last observation carried forward. In DUAL VII the log‐transformed endpoint was analysed using a mixed model for repeated measures including subgroup, visit, treatment, region and interaction between treatment, and subgroup as fixed factors, and log‐transformed baseline response as covariate. Interactions between visit and all factors and covariates were also included. HDL‐C, HDL cholesterol; IGlar U100, insulin glargine 100 units/mL; IDegLira, insulin degludec/liraglutide fixed‐ratio combination; IGlar U100, insulin glargine 100 units/mL; LDL‐C, LDL cholesterol
After 26 weeks of treatment, non‐HDL cholesterol levels (geometric means) were consistently lower or equal with IDegLira compared with comparators across the subgroups in all studies. A statistically significant difference in favour of IDegLira was found in all subgroups except for female participants and participants with diabetes duration ≥10 years in DUAL II, participants with diabetes duration ≥10 years in DUAL V and in participants of either sex, aged ≥65 years or with diabetes duration <10 years in DUAL VII (Figure 2).
No statistically significant differences in levels of HDL cholesterol were observed with IDegLira compared with comparators in any of the subgroups in any of the trials except in female participants and participants with diabetes duration ≥10 years in DUAL VII, favouring IDegLira (Figure 2).
The only subgroup that demonstrated a significant difference in triglycerides between the treatment arms was female participants in DUAL VII (Figure 2C), with a statistically significant different treatment effect between female and male participants (P = 0.0130 for test for treatment interaction).
Other lipid characteristics are plotted in Figure S1 in the Supporting Information
Additional CV risk markers were assessed in DUAL II only. BNP levels were statistically significantly lower with IDegLira compared with degludec across all the subgroups, with the exception of participants aged ≥65 years (Figure S2 in the Supporting Information). There were no statistically significant differences in apolipoprotein B between treatments in any of the subgroups. Across all the subgroups, IDegLira‐treated participants had lower levels of hsCRP, but the difference with degludec‐treated participants was not statistically significant (Figure S2 in the Supporting Information).
4. DISCUSSION
The present analysis, overall, showed that IDegLira was associated with a generally consistent improvement in CV risk markers compared with basal insulin or basal–bolus therapy after 26 weeks of treatment across the subgroups.
Treatment effect differences were seen between female and male participants in a few CV markers across the three trials, which could warrant further investigation in larger‐scale and dedicated trials.
In the placebo‐controlled LEADER trial, there were greater reductions in HbA1c, body weight and systolic BP with liraglutide.4 While diastolic BP was reduced with both treatments, but more so with placebo, heart rate was higher with liraglutide compared with placebo, both in addition to standard of care.4 In the DEVOTE trial, there was no mean difference seen between degludec and IGlar U100 in terms of body weight, BP or levels of HDL cholesterol, LDL cholesterol, total cholesterol and triglycerides.5 In this analysis, differences were seen between IDegLira and IGlar U100 in body weight, systolic BP and LDL cholesterol, suggesting that these improvements are likely to be as a result of the liraglutide component of IDegLira; however, the dose of liraglutide administered as part of IDegLira, ranging from an average of 1.4 to 1.7 mg across the subgroups at the end of trial (Table S3 in the Supporting Information), was lower than the dose in LEADER.4 This could imply that the beneficial effect of liraglutide on CV risk may be observed at doses lower than 1.8 mg. Liraglutide is known to result in decreases in intestinal and systemic inflammation and formation of atherosclerotic plaques.9 As a glucagon‐like peptide‐1 receptor‐agonist (GLP‐1RA), liraglutide also acts directly on the atrium of the heart and some blood vessels.9 It has also been postulated that increases in heart rate seen with liraglutide might be attributable to its direct effect on sympathovagal balance.10
Analyses of the effects of other available GLP‐1RAs with respect to CV risk have shown differing results. Data from the ELIXA trial showed that, compared to placebo, the addition of lixisenatide to standard of care did not alter the rate of major adverse CV events (MACE) or other serious adverse events in people with type 2 diabetes and recent acute coronary syndrome.11 Exenatide was shown in the EXSCEL trial to have no significantly different impact on the incidence of MACE compared to placebo12; however, SUSTAIN‐6 showed a significantly lower rate of MACE with once‐weekly semaglutide compared with placebo, confirming the non‐inferiority of semaglutide in patients at high risk of CV events; albiglutide has been shown to be superior to placebo with respect to MACE, and dulaglutide has been shown to reduce CV risk markers such as systolic BP compared with placebo.13, 14, 15
To our knowledge, the present study is the only study to assess CV risk in a basal insulin/GLP‐1RA fixed‐ratio combination. The beneficial effects of IDegLira on CV risk factors are consistent with the results from LEADER.4 The precise mechanism by which liraglutide reduces CV risk, and whether this effect is preserved in IDegLira, remains to be determined.
Interpretation of these results is limited by their post hoc nature and lack of adjustment for multiplicity. Nevertheless, the data originate from randomized controlled trials with large cohorts and large numbers of participants in most categories. We acknowledge that endpoints such as BP were measured during study visits, which may not have represented the true 24‐hour BP value.13 Other limitations include the fact that the trials were not powered to detect treatment differences in subgroups and we were unable to determine whether the improvements seen in the various CV risk factors were secondary to improved glycaemic control or weight loss. In addition, we were unable to examine background anti‐hypertensive and lipid‐lowering therapies across populations and thus were unable to determine if these had any impact on the results. Finally, these data are surrogate risk markers with no MACE evidence from a dedicated CVOT.
In conclusion, analyses of CV risk markers from three comparative trials in participants with type 2 diabetes uncontrolled on basal insulin show that IDegLira is associated with a general improvement across most CV risk markers and baseline subgroups versus basal insulin or basal–bolus therapy after 26 weeks of treatment.
CONFLICT OF INTEREST
T.V. has served on advisory panels for Amgen, Boehringer Ingelheim, Eli Lilly, AstraZeneca, MSD, Sanofi and Novo Nordisk, is a consultant for Amgen, Boehringer Ingelheim, Eli Lilly, AstraZeneca, MSD, Sanofi and Novo Nordisk, has received research support from Eli Lilly and Novo Nordisk, and is on the speakers' bureau for Amgen, Boehringer Ingelheim, Eli Lilly, AstraZeneca, MSD, Sanofi and Novo Nordisk. T.C.B. has received research support from Lilly, Sanofi, Novo, Boehringer Ingelheim and Lexicon; and is on the speakers' bureau for Lilly, Sanofi, Novo, Boehringer Ingelheim, Janssen, Merck, Amgen and AstraZeneca. E.J. has appeared on advisory panels for Amgen, AstraZeneca, Fresenius, Janssen, Lilly, MSD and Novo Nordisk, has received research support from Astra Zeneca, Janssen, Lilly, MSD, Novo Nordisk, Pfizer and Sanofi, and is on the speakers' bureau for Astra‐Zeneca, Boehringer Ingelheim, Janssen, Lilly, MSD, Novartis and Novo Nordisk. N.P. has served on advisory panels for AstraZeneca, Novo Nordisk and Amgen, has received research support from Servier, and is on the speakers' bureau for AstraZeneca, Novo Nordisk, Amgen and Servier. N.T. has appeared on advisory panels for MSD, AstraZeneca, Sanofi, Novo Nordisk, ELPEN, Eli Lilly, Boehringer Ingelheim and Novartis, and has received research support from MSD, Eli Lilly, Novo Nordisk, Sanofi, Pfizer, AstraZeneca, Janssen Cilag, GSK and Novartis. B.F.R.A. is an employee of Novo Nordisk A/S. L.L. is an employee and shareholder of Novo Nordisk A/S. L.A.L. has appeared on advisory panels for Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novo Nordisk, Sanofi and Servier, has received research support from Amgen, AstraZeneca, Boehringer Ingelheim, Esperion, GSK, Janssen, Kowa Pharmaceuticals America, Merck, Novo Nordisk, Sanofi, Servier and The Medicines Company, and has provided Continuing Medical Education on behalf of Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novo Nordisk, Sanofi and Servier.
Author contributions
B.F.R.A. and L.L. made substantial contributions to conception and design, and/or acquisition of data, and/or analysis and interpretation of data. T.V., T.C.B., E.J., N.P., N.T., B.F.R.A., L.L. and L.A.L. participated in drafting the article or revising it critically for important intellectual content. T.V., T.C.B., E.J., N.P., N.T., B.F.R.A., L.L. and L.A.L. gave final approval of the version to be submitted and any revised version. This analysis was funded by Novo Nordisk (A/S). Novo Nordisk was involved in the trial design and protocol development, provided logistical support, and obtained the data, which were evaluated jointly by the authors and the sponsor. As lead author, T.V. takes responsibility for the content of the article and is the guarantor of this work.
Data‐sharing
The datasets generated during and/or analysed during the present study are available from the corresponding author on reasonable request.
Supporting information
Appendix S1. Supplementary Information
ACKNOWLEDGMENTS
Parts of this study were presented as a poster at the 77th American Diabetes Association annual congress, 2017 and an oral presentation at the 53rd annual meeting of the European Association for the Study of Diabetes, 2017. The authors thank the investigators, research coordinators and participants in the trials, as well as Kamilla Begtrup and Randi Grøn (both of Novo Nordisk A/S) for their review and input to the manuscript. The authors also thank Victoria Stone and Germanicus Hansa‐Wilkinson, both of Watermeadow Medical, an Ashfield Company, UK for providing medical writing and editorial support, which was funded by Novo Nordisk A/S, Søborg, Denmark in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Vilsbøll T, Blevins TC, Jodar E, et al. Fixed‐ratio combination of insulin degludec and liraglutide (IDegLira) improves cardiovascular risk markers in patients with type 2 diabetes uncontrolled on basal insulin. Diabetes Obes Metab. 2019;21:1506–1512. 10.1111/dom.13675
Peer Review The peer review history for this article is available at https://publons.com/publon/10.1111/dom.13675.
Funding information Novo Nordisk
REFERENCES
- 1. US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research . Guidance for Industry: Diabetes Mellitus ‐ evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. 2008. https://www.fda.gov/downloads/Drugs/Guidances/ucm071627.pdf. Accessed February 15, 2019.
- 2. European Medicines Agency . Guideline on clinical investigation of medicinal products in the treatment or prevention of diabetes mellitus. 2012. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC500129256.pdf. Accessed February 15, 2019.
- 3. European Medicines Agency . Guideline on clinical development of fixed combination medicinal products. 2017. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2017/03/WC500224836.pdf. Accessed February 15, 2019.
- 4. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marso SP, McGuire DK, Zinman B, et al. Efficacy and safety of degludec versus glargine in type 2 diabetes. N Engl J Med. 2017;377:723‐732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buse JB, Vilsboll T, Thurman J, et al. Contribution of liraglutide in the fixed‐ratio combination of insulin degludec and liraglutide (IDegLira). Diabetes Care. 2014;37:2926‐2933. [DOI] [PubMed] [Google Scholar]
- 7. Lingvay I, Pérez Manghi F, Garcia‐Hernandez P, et al. Effect of insulin glargine up‐titration vs insulin degludec/liraglutide on glycated hemoglobin levels in patients with uncontrolled type 2 diabetes: the DUAL V randomized clinical trial. JAMA. 2016;315:898‐907. [DOI] [PubMed] [Google Scholar]
- 8. Billings LK, Doshi A, Gouet D, et al. Efficacy and safety of IDegLira versus basal‐bolus insulin therapy in patients with type 2 diabetes uncontrolled on metformin and basal insulin: the DUAL VII randomized clinical trial. Diabetes Care. 2018;41:1009‐1016. [DOI] [PubMed] [Google Scholar]
- 9. Drucker D. The cardiovascular biology of glucagon‐like peptide‐1. Cell Metab. 2016;24:15‐30. [DOI] [PubMed] [Google Scholar]
- 10. Kumarathurai P, Anholm C, Larsen BS, et al. Effects of liraglutide on heart rate and heart rate variability: a randomized, double‐blind, placebo‐controlled crossover study. Diabetes Care. 2017;40:117‐124. [DOI] [PubMed] [Google Scholar]
- 11. Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247‐2257. [DOI] [PubMed] [Google Scholar]
- 12. Holman RR, Bethel MA, Mentz RJ, et al. Effects of once‐weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377:1228‐1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ferdinand KC, White WB, Calhoun DA, et al. Effects of the once‐weekly glucagon‐like peptide‐1 receptor agonist dulaglutide on ambulatory blood pressure and heart rate in patients with type 2 diabetes mellitus. Hypertension. 2014;64:731‐737. [DOI] [PubMed] [Google Scholar]
- 14. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834‐1844. [DOI] [PubMed] [Google Scholar]
- 15. Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double‐blind, randomised placebo‐controlled trial. Lancet. 2018;392:1519‐1529. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supplementary Information


