Abstract
Previous studies revealed the increasing risk of tubal pregnancy following failure of levonorgestrel (LNG)‐induced emergency contraception, which was attributed to the reduced ciliary motility in response to LNG. However, understanding of the mechanism of LNG‐induced reduction in the ciliary beat frequency (CBF) is limited. The transient receptor potential vanilloid (TRPV) 4 channel is located widely in the female reproductive tract and generates an influx of Ca2+ following its activation under normal physiological conditions, which regulates the CBF. The present study aimed to explore whether LNG reduced the CBF in the Fallopian tubes by modulating TRPV4 channels, leading to embryo retention in the Fallopian tubes and subsequent tubal pregnancy. The study provided evidence that the expression of TRPV4 was downregulated in the Fallopian tubes among patients with tubal pregnancy and negatively correlated with the serum level of progesterone. LNG downregulated the expression of TRPV4, limiting the calcium influx to reduce the CBF in mouse oviducts. Furthermore, the distribution of ciliated cells and the morphology of cilia did not change following the administration of LNG. LNG‐induced reduction in the CBF and embryo retention in the Fallopian tubes and in mouse oviducts were partially reversed by the progesterone receptor antagonist RU486 or the TRPV4 agonist 4α‐phorbol 12,13‐didecanoate (4α‐PDD). The results indicated that LNG could downregulate the expression of TRPV4 to reduce the CBF in both humans and mice, suggesting the possible mechanism of tubal pregnancy. © 2019 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of Pathological Society of Great Britain and Ireland.
Keywords: ciliary beat, levonorgestrel, TRPV4, calcium, oviduct, tubal pregnancy
Introduction
Ectopic pregnancy is a major cause of maternal morbidity and even mortality, and occurs in approximately 1.4% of pregnancies 1. A previous epidemiological study revealed the increased risk of ectopic pregnancy following levonorgestrel (LNG)‐induced emergency contraceptive failure 2. Tubal pregnancy (TP) accounts for approximately 95% of all cases of ectopic pregnancy 3. The Fallopian tube is an important reproductive organ involved in physiological processes including oocyte pickup, ovum maturation, fertilization, embryo development, and embryo transport. TP is a consequence of ectopic implantation in the Fallopian tubes, which might be attributed to impaired embryo transport in the Fallopian tubes and/or alterations in the tubal microenvironment 4, 5. Ciliary motility in the Fallopian tubes is principally capable of transporting embryos 6. Previous findings indicated that LNG and progesterone did not affect the tubal morphological structure or its epithelial receptivity in humans, but rapidly reduced the ciliary beat frequency (CBF) 7, leading to embryo retention in the Fallopian tubes and allowing early implantation 4. Although many studies have demonstrated reduced CBF at high progesterone levels in mammals 8, 9, 10, its underlying mechanism is still unclear.
A motile cilium is a microtubule‐based cytoskeleton characterized by a typical ‘9 + 2’ architecture, with nine outer microtubule doublets and a central pair of microtubules. The ciliary beat is produced by dynein, which can be affected by several intracellular factors, including progesterone, cAMP, prostaglandins, adrenomedullin, and Ca2+ 11, 12, 13, 14. Calcium was found to be involved in the regulation of CBF in response to mechanical and chemical stimuli, including progesterone 15. Transient receptor potential vanilloid (TRPV) is a family of transient receptor potential cation channels including six members in mammals, TRPV1–6. Among the six TRPVs, TRPV1–4 are moderately calcium‐selective 16. The activation of TRPV4 especially can lead to the influx of calcium, subsequently increase cellular excitability, and regulate microtubule‐based motor proteins 11, 17, because an increase in cytosolic Ca2+ is associated with an increase in CBF.
Recently, TRPV4 was reported as a calcium‐permeable ion channel expressed in ciliated epithelia which accelerated Ca2+ influx, leading to an increase in intracellular Ca2+ concentration and the consequent enhancement of CBF 18, 19. Furthermore, a study by Jung et al indicated that progesterone could repress the transcription of TRPV4 via its receptor, leading to a reduction in intracellular Ca2+ concentration and depolarization of the membrane potential. This effect could be reversed by the progesterone receptor antagonist RU486 20. The alteration in the expression of TRPV4 was believed to be associated with the regulation of CBF 17. However, only two studies have explored TRPV4 and CBF in the Fallopian tube 11, 21 and neither study focused on human diseases related to tubal pregnancy.
Considering the involvement of TRPV4 in the regulation of CBF and progesterone‐induced reduction in the CBF, the present study was designed to investigate the effect of LNG, a synthetic analog of progesterone, on TP via TRPV4 channels and the mechanism underlying this effect.
Materials and methods
Collection of clinical information and human Fallopian tube samples
Women who underwent surgical treatment for TP at the International Peace Maternity and Child Health Hospital between September 2014 and October 2015 were included as the study group (n = 34). Among these women were 12 patients with TP following failure of LNG‐induced contraception and 22 patients with TP exposed to the traditional risk factors for TP. Patients undergoing hysterectomies for benign conditions (uterine leiomyoma) in the luteal phase without using any hormonal medication within 3 months were included as the control group (n = 24). The Fallopian tube samples were collected for the study after obtaining written informed consent from the patients and approval of the local ethical committee (GKLW No 201630). Blood samples (5 ml) were also collected on the day of surgery for progesterone assay.
Reagents and chemicals
Crystalline LNG (Y0001379; Sigma‐Aldrich, St Louis, MO, USA) and progesterone receptor antagonist (M8046; Sigma‐Aldrich) were dissolved in dimethyl sulfoxide (DMSO; D2650; Sigma‐Aldrich) for experiments. The TRPV4 agonist 4α‐phorbol 12,13‐didecanoate (4α‐PDD) was purchased from Sigma‐Aldrich (P8014). The antibodies used in this study are listed in the supplementary material, Table S1.
Assessment of serum progesterone levels
Serum samples were collected in 10 ml vacutainer tubes, centrifuged, and the progesterone concentration was measured using the Access Progesterone Reagent Pack (33550; Beckman Coulter, CA, USA) and a chemiluminescence immunoassay system (UniCel DxI 800; Beckman Coulter).
Cell line culture, isolation of primary ciliated cells, and transfection
OE‐E6/E7, a human Fallopian tubal epithelial cell line expressing progesterone receptor 22, was obtained from Dr Kai‐Fai Lee, University of Hong Kong. Epithelial ciliated cells were isolated from collected human Fallopian tubal tissues according to the protocol from Chan et al 23. siRNA‐mediated knockdown and TRPV4 reconstitution were performed 48 h before functional experiments. Details of cell line culture, isolation of primary ciliated cells, and transfection are given in the supplementary material, Supplementary materials and methods.
Gene expression analysis
Details of RNA isolation, cDNA synthesis, and RT‐qPCR analysis are given in the supplementary material, Supplementary materials and methods.
Western blotting analysis
Fallopian tubal tissues or cells were homogenized in RIPA buffer. The homogenate was incubated on ice for 40 min and centrifuged at 15 000 × g for 15 min. The protein concentration of supernatants was determined using the Bradford assay (Bio‐Rad Laboratories, Hercules, CA, USA). Samples (30 μg per lane) were separated on a 12% SDS–polyacrylamide gel. The separated samples were transferred to nitrocellulose membranes; exposed to primary antibody (listed in the supplementary material, Table S1) 4 °C overnight, followed by horseradish peroxidase‐conjugated goat anti‐rabbit immunoglobulin G for 1 h at room temperature; and visualized using an enhanced chemiluminescence detection reagent.
Immunohistochemistry and immunofluorescence
For immunohistochemical staining, the Fallopian tubal tissues were formalin‐fixed, paraffin‐embedded, sectioned, and mounted onto slides. Tissue sections were deparaffinized and rehydrated. The slides were then incubated in a 3% H2O2 solution to block endogenous peroxidase and rinsed with PBS. The sections were treated with heated antigen retrieval solution containing EDTA and then incubated with 5% bovine serum albumin for 30 min and then with the primary antibody rabbit anti‐TRPV4 at 4 °C for 12 h. After incubation with goat anti‐rabbit secondary antibody for 60 min, the sections were treated with diaminobenzidine (K3467; DakoCytomation, Glostrup, Denmark), counterstained with hematoxylin, dehydrated, and mounted in Distrene dibutyl phthalate xylene (DPX). For immunofluorescence, the tissues were paraformaldehyde‐fixed and then embedded in optimal cutting temperature compound; a 10 μm section was then cut and mounted. The sections were blocked for 1 h at room temperature and incubated with the primary antibody (listed in the supplementary material, Table S1) at 4 °C for 12 h. A secondary fluorescent antibody was used after the sections were washed three times with PBS. The sections were mounted with Antifade Mounting Medium containing 4,6‐diamidino‐2‐phenylindole (DAPI; VECTASHIELD, Vector Laboratories, Burlingame, CA, USA).
Scanning electron microscopy
The length of cilia was measured using scanning electron microscopy (SEM). Primary ciliated cells were fixed with 2% paraformaldehyde and 2.5% glutaraldehyde. After washing with phosphate buffer (PB), the samples were post‐fixed with 1% osmium tetroxide in PB at 4°C for 2 h; dehydrated through a graded ethanol–water series, followed by t‐butyl alcohol; and freeze‐dried overnight under vacuum. The samples were then mounted onto an aluminum stub with a sticky carbon tab, sputter‐coated with an ion sputter coater, and observed using a Hitachi SU1080 scanning electron microscope (Hitachi, Ltd, Tokyo, Japan).
Ciliary length measurement
Images were taken with a Leica DMi8 microscope and scanning electron microscope. Scale bars were automatically embedded into all images for further ciliary length analysis. To calibrate the length of 10‐μm scale bars, the ‘Straight‐line’ tool was used to draw a line and set to a known distance of 10 μm using the ‘Set Scale’ tool. Then the ciliary length of each ciliated epithelial cell was measured from the apical membrane (base of the cilia) to the tip of the cilia using the ‘Straight‐line’ tool, as previously described 24.
Measurement of CBF
The CBF was measured as previously described at 37 °C 25. An inverted bright‐field microscope equipped with a 12‐bit high‐speed camera and a temperature controller (Leica DMi8 Microsystems, Wetzlar, Germany) was used to record a video sequence of moving cilia at a rate of 120 frames per second for 20 s. The CBF was calculated using the ciliaFA software system. A plugin for ImageJ (software version 1.49t; NIH, Bethesda, MD, USA) was developed that extracted pixel intensities and performed fast Fourier transformation using Microsoft Excel.
Calcium influx measurement
Calcium influx was analyzed by measuring the increase of intracellular calcium indicated by Fluo‐4 AM (F14201; Thermo Fisher, Waltham, MA, USA). The mean fluorescence intensity (MFI) of each 30 s histogram was normalized against the basal MFI before adding the TRPV4 agonist 4α‐PDD, and plotted as a time course. Details of the calcium influx measurement are given in the supplementary material, Supplementary materials and methods.
Animal experiments and embryo‐tube transport
All animal experiments were approved by the Medical Ethics Committee of Shanghai Research Center for Model Organisms. After being injected intraperitoneally with saline, LNG, LNG + RU486, or LNG + TRPV4 agonist 4α‐PDD, mice were sacrificed for the experiments of oviductal TRPV4 expression and localization detection, CBF measurements, and the embryo‐tube transport assay as described by Ning et al 26. Details of the animal experiments are given in the supplementary material, Supplementary material and methods.
Statistical analysis
The results were expressed as means ± standard error of the mean (SEM). Student's t‐test or one‐way analysis of variance and Tukey–Kramer multiple comparison tests were performed to compare the relative efficiency of groups where appropriate (PRISM software version 6.0; GraphPad, Inc, San Diego, CA, USA) and hence to determine the statistical significance of each treatment. The correlation coefficient of the association between TRPV4 levels and serum progesterone level was calculated using Pearson correlation analysis. All P values were calculated using two‐sided tests and differences were considered significant if the P value was less than 0.05.
Results
LNG downregulated the expression of TRPV4 in human Fallopian tubal epithelial cell line and mouse oviduct
TRPV1–4 were detected at the transcript level following the administration of 1 μm LNG for 24 h in OE‐E6/E7 cell lines to determine the expression of TRPV1–4, whose proteins are moderately calcium‐selective, in response to LNG exposure. Only the transcript level of TRPV4 was significantly downregulated (Figure 1A). The protein level of TRPV4 was decreased after LNG treatment (Figure 1B), which was partially reversed in the presence of the progesterone receptor antagonist RU486 (Figure 1C). Figure 1D–F shows the histologic localization and quantitative expression of TRPV4 in mouse oviducts after intraperitoneal injection using saline, LNG, and LNG + RU486. TRPV4 was localized to cilia and apical membranes, and the localization of TRPV4 did not change with LNG treatment. The expression of TRPV4 in mouse oviducts was significantly reduced after LNG treatment, and was reversed by the progesterone receptor antagonist RU486.
Figure 1.
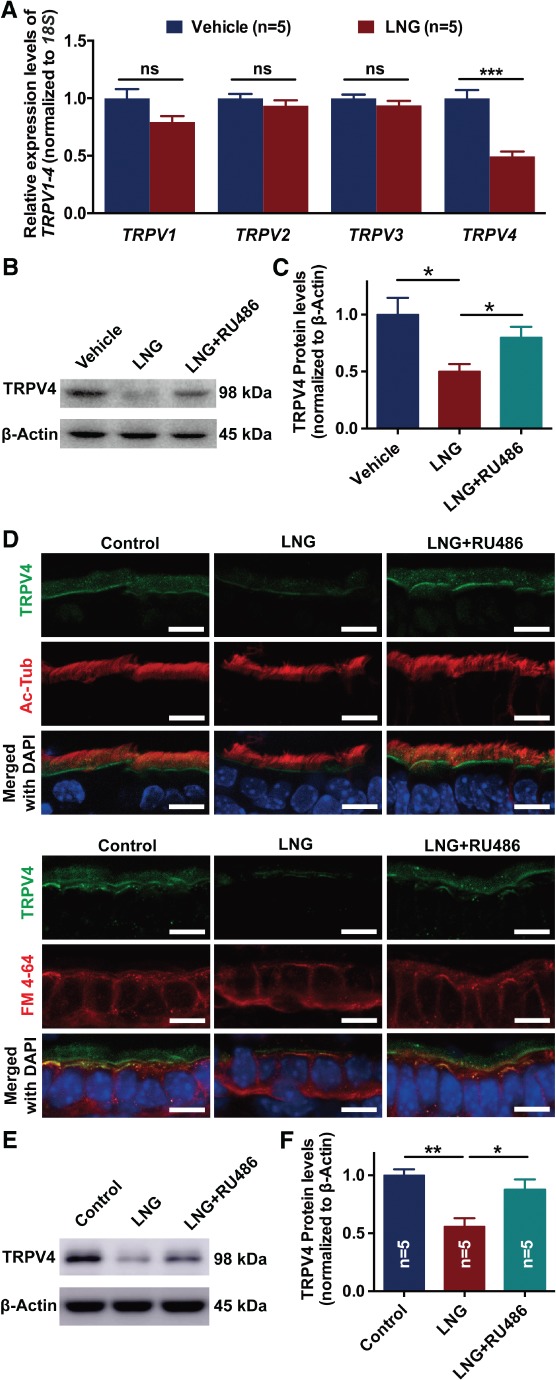
Effects of LNG on the expression of TRPV4 in human Fallopian tubal epithelial cell line and mouse oviduct. (A) mRNA expression of TRPV1–4 in OE‐E6/E7 by RT‐qPCR following treatment with vehicle and LNG (n = 5 per group; ns, not significant; *p < 0.05; Student's t‐test for each gene). (B, C) Protein expression of TRPV4 in OE‐E6/E7 following treatment with LNG and RU486 (n = 5 per group; *p < 0.05; one‐way ANOVA followed by a Tukey post hoc test). (D) Immunofluorescence analysis of the localization and expression of TRPV4 in mouse oviducts following intraperitoneal injection with saline controls, LNG, and LNG plus blocking progesterone receptor with RU486. Ac‐Tub, acetyl‐α‐tubulin, marker of cilia; DAPI, nucleus; FM 4‐64, cell membrane. Scale bar = 10 μm. (E, F) Protein expression of TRPV4 in mouse oviducts following intraperitoneal injection with saline, LNG, and LNG + RU486 (n = 5 per group; ns, not significant; *p < 0.05; **p < 0.01; one‐way ANOVA followed by a Tukey post hoc test). TRPV, transient receptor potential vanilloid; LNG, levonorgestrel; RU486, progesterone receptor antagonist.
Downregulation of TRPV4 was found in human Fallopian tubes for patients with traditional tubal pregnancies and patients with tubal pregnancy following failure of LNG‐induced contraception
The Fallopian tubal tissues partly obtained from non‐pregnant controls and patients with TP were used to detect the expression and localization of TRPV4, specifically in tubal epithelium. TRPV4 was expressed in human Fallopian tubes and was especially localized in the epithelial cells rather than stromal cells or smooth muscles (Figure 2A). Notably, a significant decrease in the expression of TRPV4 was found in the Fallopian tube epithelium in patients with tubal pregnancies compared with non‐pregnant controls (Figure 2A–C). Interestingly, even lower expression of TRPV4 was observed in 12 patients with TP following LNG‐induced contraceptive failure compared with 22 patients with TP who were exposed to the traditional risk factors for TP, including tubal inflammatory diseases (Figure 2B,C). The association between expression of TRPV4 and serum progesterone level was further assayed in 34 patients with TP and 24 controls. A similar trend toward decreased expression of TRPV4 in the Fallopian tube was noted with the increase of serum progesterone level in both patients with TP and controls (Figure 2D).
Figure 2.
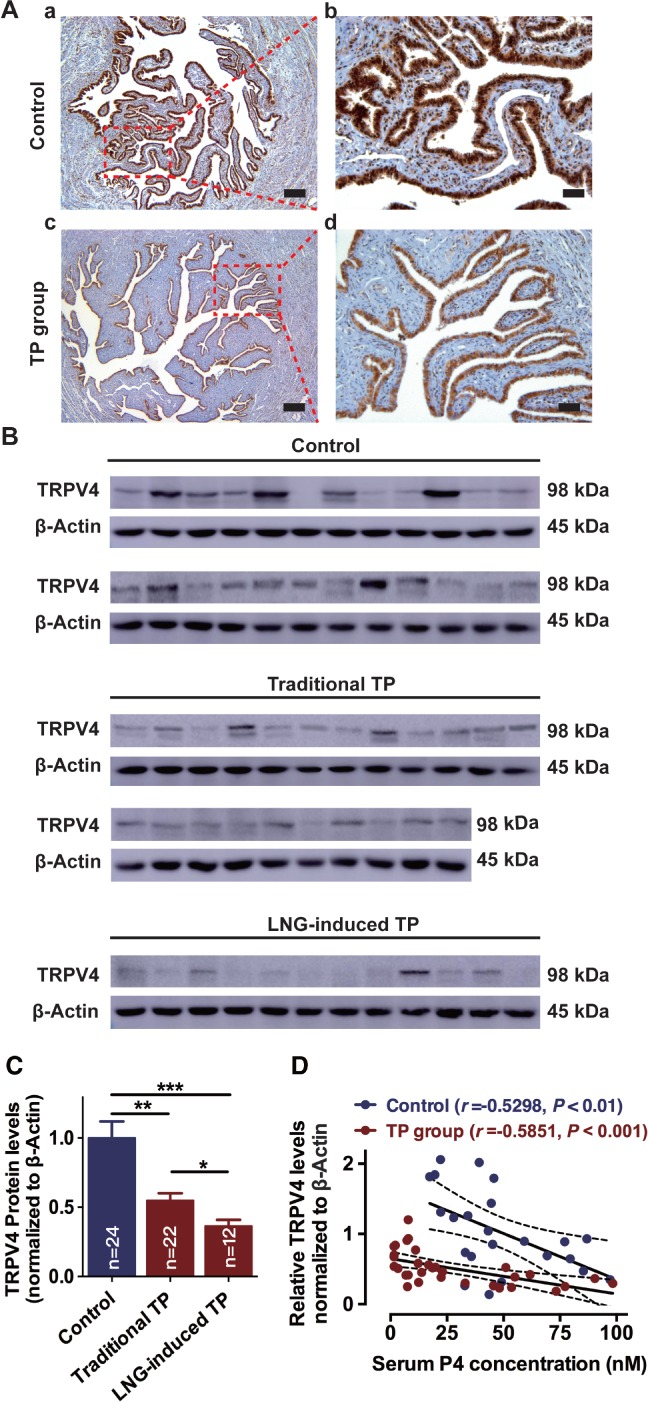
Clinical evidence of the association between expression of TRPV4 and tubal pregnancy. (A) Immunohistochemical analysis of the expression and localization of TRPV4 in the ampulla of human Fallopian tubes in non‐pregnant control and patients with TP (scale bar = 200 μm for A,a and A,c; and 50 μm for A,b and A,d). (B, C) Expression of TRPV4 protein in human Fallopian tubes for patients who were stratified into non‐pregnant controls (n = 24), traditional TP (n = 22), and LNG‐induced TP (n = 12) subgroups (*p < 0.05; **p < 0.01; ***p < 0.001; one‐way ANOVA followed by a Tukey post hoc test). (D) Negative relationship between TRPV4 protein level and serum progesterone level among patients with TP (n = 34, r = −0.5851, p < 0.001; Pearson correlation test) and controls (n = 24, r = −0.5298, p < 0.01; Pearson correlation test). LNG, levonorgestrel; TP, tubal pregnancy; TRPV4, transient receptor potential vanilloid 4; P4, progesterone.
LNG inhibited TRPV4‐mediated Ca2+ influx via progesterone receptor in OE‐E6/E7 cells
As the progesterone analog LNG performs its biological functions via the progesterone receptor, we propose that LNG decreases the expression of TRPV4 through progesterone receptor signaling, and the progesterone receptor antagonist RU486 can block this effect. Flow cytometry analysis was conducted in the OE‐E6/E7 cell line with the expression of progesterone receptor and TRPV4, to assess whether LNG reduced Ca2+ influx by modulating TRPV4. Basal intracellular Ca2+ was significantly reduced following the LNG treatment, and was reversed after blocking progesterone receptor with RU486 (Figure 3A,B). After stimulation using the TRPV4 agonist 4α‐PDD, intracellular Ca2+ increased almost 1.7‐fold. However, LNG markedly inhibited the 4α‐PDD‐evoked intracellular Ca2+ increase. Intriguingly, when the cells were pretreated with LNG in the presence of progesterone receptor antagonist RU486, the intracellular Ca2+ concentration was partially recovered after stimulating with 4α‐PDD (Figure 3C–E).
Figure 3.
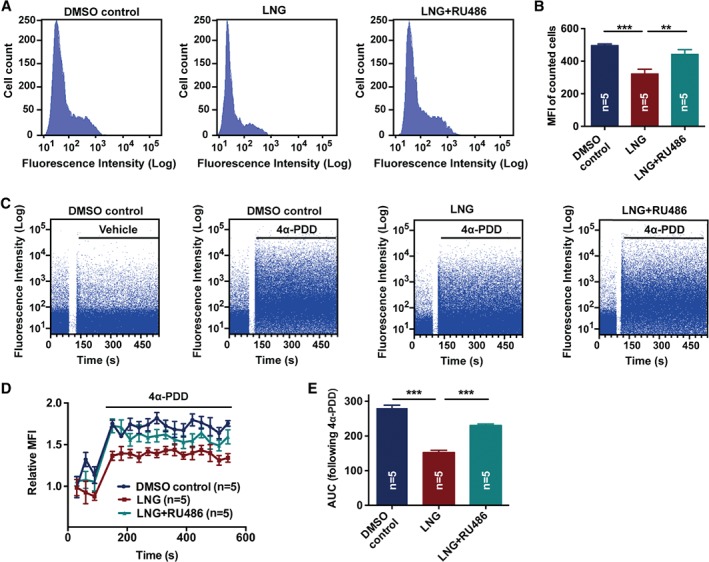
Effect of LNG on TRPV4‐mediated Ca2+ influx by binding with progesterone receptor in human Fallopian tube cells. (A, B) Basal cytosolic free Ca2+ measurement in Fallopian tubal cells pretreated with DMSO control, LNG, and LNG + RU486 for 24 h. Fluorescence was recorded from single cells loaded with Fluo‐4‐AM dye (n = 5 per group; **p < 0.01; ***p < 0.001; one‐way ANOVA followed by a Tukey post hoc test). (C) The transient influx of Ca2+ was measured as the increase in fluorescence intensity following 4α‐PDD stimuli in Fallopian tubal cells pretreated with DMSO control, LNG, or LNG + RU486 for 24 h. The blank space in the graphs is the pause time during which 4α‐PDD was added to the cuvette. The plots in the graphs indicate cells detected and the intensity. (D, E) MFI and the AUC for each group (n = 5 per group; ***p < 0.001; one‐way ANOVA followed by a Tukey post hoc test). 4α‐PDD, 4α‐phorbol 12,13‐didecanoate; DMSO, dimethyl sulfoxide; LNG, levonorgestrel; RU486, progesterone receptor antagonist; MFI, mean fluorescence intensity; AUC, area under the curve.
TRPV4 was required for LNG‐evoked CBF reduction in cultured primary ciliated cells of human Fallopian tube
Primary cultured human Fallopian tubal ciliated cells were identified by specific markers of cilia and basal body, acetyl‐α‐tubulin, and ninein. TRPV4 was also found to co‐localized with cilia (Figure 4A). An siRNA was designed to silence TRPV4 by reducing its protein level in primary ciliated cells compared with scrambled siRNA‐transfected cells, and reconstitution with TRPV4 was also used to confirm the specificity of TRPV4 siRNA (Figure 4B).
Figure 4.
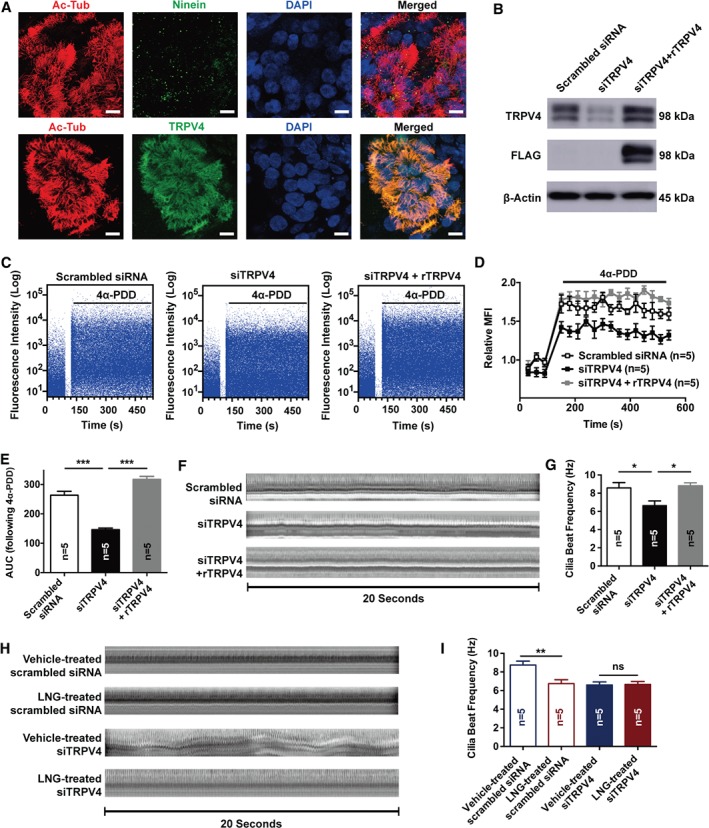
Identification of TRPV4 siRNA on the inhibition of TRPV4‐mediated Ca2+ influx and CBF in human Fallopian tubal ciliated cells. (A) Identification of primary tubal ciliated cells. Ac‐Tub, acetyl‐α‐tubulin, marker of cilia; ninein, marker of the basal body; DAPI, nucleus. Scale bar = 10 μm. (B) Protein level of TRPV4 in ciliated cells treated with scrambled siRNA, targeted specific siRNA TRPV4 (siTRPV4), or siRNA TRPV4 with rescuing TRPV4 reconstitution. (C) The transient influx of Ca2+ was measured as the increase in fluorescence intensity following 4α‐PDD stimuli in three cells. (D, E) Relative MFI and AUC of three groups (n = 5 per group; ***p < 0.001; one‐way ANOVA followed by a Tukey post hoc test). (F) Orthographic views of cilia beat within 20 s in three cells (one cilia beat is represented as one shift of bright or dark in the timeline). (G) TRPV4 siRNA‐treated ciliated cells showed a lower CBF in vitro compared with scrambled siRNA and siTRPV4 + rTRPV4‐treated cells (n = 5 per group; *p < 0.05; one‐way ANOVA followed by a Tukey post hoc test). (H) Orthographic views of cilia beat within 20 s in scrambled siRNA‐ or siTRPV4‐treated ciliated cells following the administration of vehicle and LNG, respectively (one cilia beat is represented as one shift of bright or dark in the timeline). (I) LNG reduced CBF in scrambled siRNA‐treated ciliated cells compared with DMSO vehicle control, and siTRPV4‐treated ciliated cells showed no difference in the CBF following LNG treatment. (n = 5 per group; **p < 0.01; ns, not significant; one‐way ANOVA followed by a Tukey post hoc test). TRPV4, transient receptor potential vanilloid 4; 4α‐PDD, 4α‐phorbol 12,13‐didecanoate; MFI, mean fluorescence intensity; AUC, area under the curve; LNG, levonorgestrel.
As TRPV4 is a thermosensitive channel, and the CBF was greatly varied by temperature, we first tested the effect of a warm temperature on the generation of Ca2+ signals and modulation of CBF in scrambled siRNA, siTRPV4, and siTRPV4 + rTRPV4‐treated ciliated cells. Switching the temperature from 24 °C to 37 °C triggered a Ca2+ response indicated by an increased fluorescence intensity toward the baseline. Compared with scrambled siRNA control cells, cells in which we interfered with the expression of TRPV4 responded with a smaller increase in Ca2+ influx when exposed to warm temperatures (supplementary material, Figure S1A–C). Accordingly, siTRPV4‐treated cells exposed to warm temperatures responded with a smaller increase in CBF (supplementary material, Figure S1D,E).
The function of TRPV4 on calcium and CBF was detected at a temperature of 37°C. After interfering with the expression of TRPV4, the 4α‐PDD‐induced Ca2+ influx was significantly reduced (Figure 4C–E). Notably, interfering with the expression of TRPV4 in tubal ciliated cells significantly reduced the CBF to 6.66 ± 0.49 Hz, as opposed to 8.58 ± 0.59 Hz in the scrambled siRNA control group (Figure 4F,G). Importantly, LNG largely reduced the CBF (Figure 4H,I; 8.75 ± 0.42 Hz in vehicle‐treated scrambled siRNA versus 6.75 ± 0.41 in LNG‐treated scrambled siRNA, p < 0.01), which was abolished via TRPV4 knockdown (Figure 4H,I; 6.60 ± 0.34 Hz in vehicle‐treated TRPV4 siRNA versus 6.65 ± 0.32 Hz in LNG‐treated TRPV4 siRNA). These results suggested that TRPV4 is involved in LNG‐induced CBF reduction.
LNG did not affect the distribution of cilia in mice oviducts or cilia length in human primary ciliated cells of human Fallopian tubes
CBF in female oviducts largely depends on the percentage of ciliated cells and their length. Full length oviducts were obtained from mice following intraperitoneal injection of saline, LNG, or LNG + RU486 to assess whether the ciliation frequency of each part in the oviduct was influenced by LNG. Although the distribution of ciliated cells decreased from the ampulla to the isthmus of oviducts, it showed no difference between the saline control, LNG or LNG + RU486 (Figure 5A,B). Furthermore, the length of cilia of primary human Fallopian tubal cells was also not influenced by in vitro treatments of the DMSO control, LNG, or LNG + RU486 for 24 h, as indicated by immunofluorescence (Figure 5C,D) and SEM (Figure 5E,F).
Figure 5.
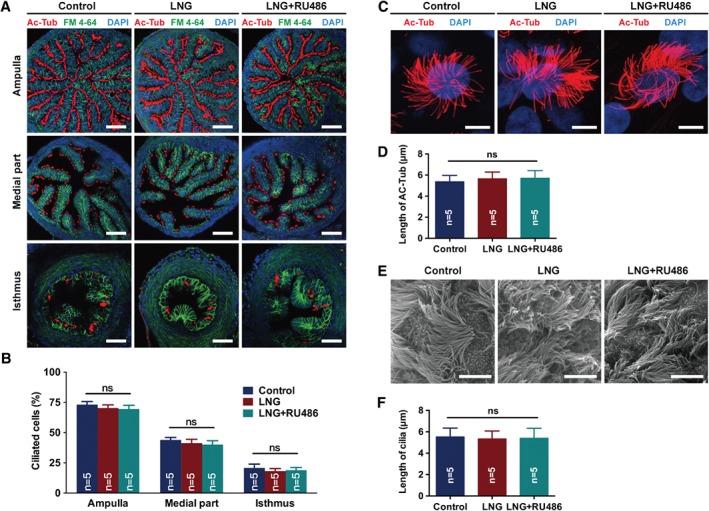
Effect of LNG on the distribution of cilia in mouse oviduct and cilia length in human Fallopian tubal ciliated cells. (A, B) Immunofluorescence analysis of the percentage of ciliated cells in the ampulla, middle part, and isthmus of mouse oviducts following intraperitoneal injection of saline control, LNG, or LNG + RU486 for 24 h. Ac‐Tub, acetyl‐α‐tubulin, marker of cilia; FM 4‐64, cell membrane; DAPI, nucleus; scale bar = 50 μm; n = 5 per group; ns, not significant; one‐way ANOVA for each part. (C, D) Immunofluorescence analysis of the length of ciliated cells following administration of DMSO control, LNG, or LNG + RU486 for 24 h. Ac‐Tub, acetyl‐α‐tubulin, marker of cilia; DAPI, nucleus; scale bar = 10 μm; n = 5 per group; ns, not significant; one‐way ANOVA). (E, F) Scanning electron microscopy of the length of ciliated cells following administration of DMSO control, LNG, or LNG + RU486 for 24 h (scale bar = 5 μm; n = 5 per group; ns, not significant; one‐way ANOVA). LNG, levonorgestrel; RU486, progesterone receptor antagonist.
TRPV4 played a critical role in LNG‐induced retention of embryo‐tube transport
Retention of embryos in the oviduct is a critical role of the CBF in embryo transport in the oviduct. The CBF in mouse oviduct was significantly reduced following LNG treatment compared with saline control (Figure 6A,B; 11.94 ± 0.32 Hz in saline control versus 8.77 ± 0.53 Hz in LNG, p < 0.001). The LNG‐induced reduction in the CBF was reversed by either the progesterone receptor antagonist RU486 (11.89 ± 0.52 Hz versus 8.77 ± 0.53 Hz, p < 0.001) or the TRPV4 agonist 4α‐PDD (11.56 ± 0.46 Hz versus 8.77 ± 0.53 Hz, p < 0.01) (Figure 6A,B). As expected, none of the mice yielded any embryos within the oviducts of the saline‐treated control. However, after administration of LNG, nine of ten mice were found to suffer from embryo retention in the oviduct (Figure 6C). The number of mice experiencing embryo retention in the oviduct was reduced after administration of progesterone receptor antagonist RU486 or TRPV4 agonist 4α‐PDD (Figure 6C, 6/10 in the LNG + RU486 group; 7/10 in the LNG + 4α‐PDD group). In addition, the percentage of embryos recovered from oviducts or the uterus was calculated. No embryos were retained in the oviducts in the saline‐treated control. However, 12.7% of embryos were not transported to the uterus after administering LNG. The percentage of embryos retained in the oviducts was partially reversed by either progesterone receptor antagonist RU486 (5.6%) or TRPV4 agonist 4α‐PDD (6.1%) (Figure 6D).
Figure 6.
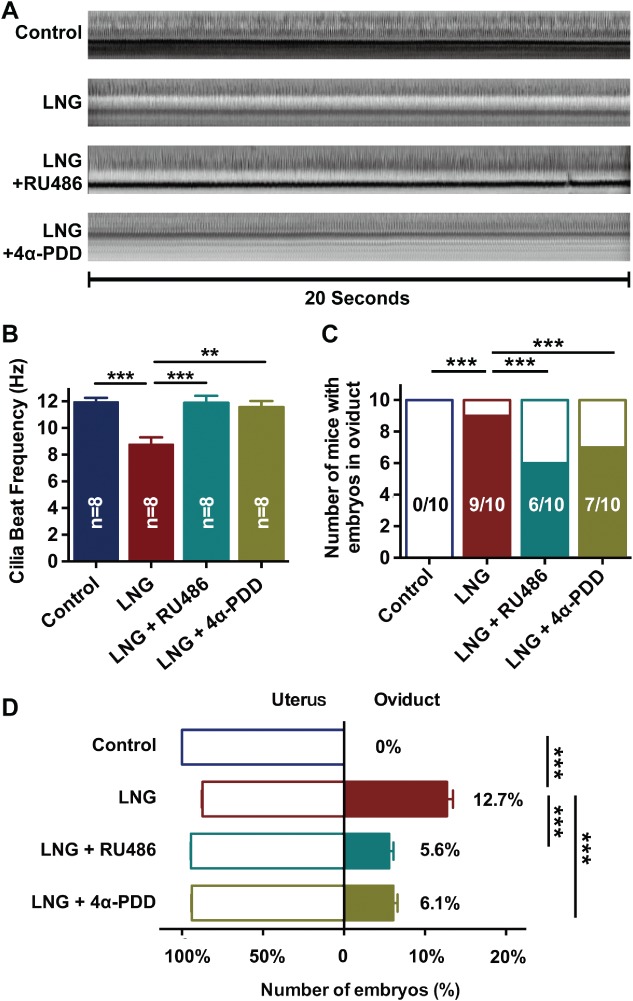
Effects of TRPV4 on embryo‐tube transport in mice. (A) Orthographic views of cilia beat within 20 s in mouse oviducts. (B) In vivo CBF measurement of mouse oviducts following intraperitoneal injection with saline, LNG, LNG + 4α‐PDD, or LNG + RU486 (n = 8 per group; *p < 0.05; one‐way ANOVA followed by a Tukey post hoc test). (C) Number of mice with oviductal retention of embryos/total number of mice examined at 74 h following observation of the vaginal plug, and with saline control, LNG, LNG + 4α‐PDD, or LNG + RU486 treatment (n = 10 per group; ***p < 0.001; Pearson χ2 test). (D) Embryos counted in both oviducts and uterus expressed as a percentage (n = 10 per group; *** p < 0.001; one‐way ANOVA followed by a Tukey post hoc test). LNG, levonorgestrel; RU486, progesterone receptor antagonist; 4α‐PDD, 4α‐phorbol 12,13‐didecanoate.
Discussion
Our novel study provided evidence that the downregulation of the expression of TRPV4 in Fallopian tubes was negatively correlated with the level of progesterone. Furthermore, LNG could inhibit the transcriptional level of TRPV4, and result in a downregulation of TRPV4 of oviduct ciliated cells, limiting the calcium influx to reduce the CBF in oviducts and subsequently leading to impairment of embryo‐tube transport; this effect could be reversed by progesterone receptor antagonist (supplementary material, Figure S2). The findings of the present study might reveal the possible mechanism of LNG‐induced tubal pregnancy.
Motile ciliated cells are distributed mainly in the airway, central nervous system, and Fallopian tubes. In the Fallopian tubes, the CBF is the principal driver of embryo transport toward the uterine cavity 6. Although modest deciliation has been described in some parts of the tubal epithelium during the secretory phase, most ciliated cells remain intact in the Fallopian tubes in the course of the ovarian cycle 27, 28 Moreover, the distribution of ciliated cells varies in different parts of the Fallopian tubes, from almost 75% of the cells in the ampulla to 30% in the isthmus 29. Additionally, approximately 95% of ectopic pregnancies occur in the Fallopian tubes, and most are located in the ampulla. Therefore, this study focused on the regulation of CBF in the ampulla of the Fallopian tubes.
The available literature reported that the percentage of ciliated cells decreased during pregnancy and with steroid contraception early in 1976 30. However, a previous study indicated that LNG‐induced emergency contraception would only affect the Fallopian tubal physiology, including a reduction in the CBF, and not the tubal morphology 7. LNG, as a second‐generation synthetic progesterone, differs from progesterone in its structure and pharmacological properties, and has now been used as emergency contraception. Since LNG‐only pills for emergency contraception are available in an over‐the‐counter form in many countries with a high efficacy in protecting unwanted pregnancy, the problems including ectopic pregnancy following its subsequent contraceptive failure have been confirmed. Compared with the steroid contraception used decades ago, LNG showed a high affinity to its receptor and reduced the CBF in the rat oviduct 7. Despite recent findings from Jung et al which revealed that progesterone had the effect (from seconds to minutes) of rapidly increasing CBF as well as the long‐term effect (hours) of reducing CBF 31, a lower CBF in the Fallopian tube following long‐term effects of LNG was still regarded as one of the critical factors associated with tubal pregnancy, especially the one following LNG‐induced emergency contraceptive failure, as it took several hours for embryo transport through the Fallopian tubes.
Embryo transport in the Fallopian tube involves two main factors: ciliary motility and muscle contractility. A study by Halbert et al showed that the embryo transport rate in the tube was directly determined by the CBF rather than by muscle contractility 32. The physiological function of tubal ciliated cells includes picking up the oocytes, transporting them to the ampulla for fertilization, and then transporting the embryos to the uterus at a precisely regulated rate for implantation 33, 34. However, retention of embryo transport in the Fallopian tube leads to desynchronization between embryo development and the endometrium, leading to embryo implantation failure or ectopic implantation. Calcium is widely regarded as a critical factor in regulating the CBF 18. As a nonselective ion channel, TRPV4 is widely located in ciliated cells, including bronchi, brain ventricles, and the female reproductive tract, and generates an influx of Ca2+ following its activation under normal physiological conditions and alternation of osmotic pressure 35. Furthermore, the modulation of tubulin dynamics by TRPV4 activity may help to explain the mechanism of ciliary dysfunction 36. Although many researchers have reported the phenotype of Trpv4 knockout mice, and indicated the cilia beat reduction in the respiratory system 19, there have only been a limited number of studies on the phenotype of oviducts in Trpv4 knockout mice until a recent study reported the reduced basal CBF in oviductal ciliated cells in Trpv4 knockout mice 31, consistent with the findings of our study. Furthermore, compared with the vast knowledge about TRPV4 channel regulation, little is known about the control of TRPV4 transcription, especially in the ciliated cells of the human Fallopian tube 37. Jung et al reported that TRPV4 promoter activity was reduced by co‐expression of the progesterone receptor in the nucleus and this further affected the transcription of TRPV4 in the respiratory system 20. In accordance with the findings in the respiratory system, the present study also demonstrated the long‐term effect (hours) of LNG in reducing the expression of TRPV4 in cell culture and mice, further reducing the calcium influx in tubal ciliated cells and leading to a reduced CBF.
It is believed that embryo retention in the Fallopian tube as a result of decreased ciliary motility is one cause of ectopic pregnancy. However, due to the different placentation mechanisms in humans, ectopic implantation was rarely observed in animals, increasing the difficulty of studying the mechanism of tubal implantation in vivo. In 2014, Ning et al established an indirect assay to evaluate the ability of embryo‐tube transport by counting the number of embryos retained in the oviduct 74 h after the discovery of the vaginal plug in mice 26. Using this in vivo assay, the present study confirmed that the reduced CBF by LNG influenced embryo‐tube transport and could be partially reversed by the TRPV4 agonist 4α‐PDD. TRPV4 is a mediator of tubal ciliary motility. Downregulation of TRPV4 by LNG has also been found in the human Fallopian tube with an ectopic pregnancy, especially in patients with LNG‐induced contraceptive failure. The present study demonstrated that under normal physiological conditions, TRPV4 was crucial for embryo transport. Conversely, exposure to LNG could downregulate TRPV4 and was involved in reducing ciliary motility, which is essential for embryo transport in the Fallopian tube during early pregnancy. LNG was originally used in contraceptive pills for it can inhibit or delay ovulation. However, the acute effect of LNG in downregulating TRPV4 might lead to reduced ciliary motility and a subsequent tubal pregnancy. The activation of TRPV4 might be a potential target to prevent women from tubal implantation when they have experienced LNG contraceptive failure.
SUPPLEMENTARY MATERIAL ONLINE.
Supplementary materials and methods
Supplementary figure legends
Figure S1. Effect of temperature on the generation of Ca2+ signals and modulation of CBF
Figure S2. Proposed model of TRPV4 action in regulating the cilia beat frequency in the Fallopian tube exposed to levonorgestrel
Figure S3. Expression of PR and TRPV4 in OE‐E6/E7 cell line (mentioned in the supplementary material, Supplementary materials and methods)
Figure S4. Overview of embryo‐tube transport assay in mice (mentioned in the supplementary material, Supplementary materials and methods)
Table S1. Antibody and stain list
Table S2. Oligonucleotide sequence (mentioned in the supplementary material, Supplementary materials and methods)
Supporting information
Supplementary materials and methods
Supplementary figure legends
Figure S1. Effect of temperature on the generation of Ca 2+ signals and modulation of CBF.
(A) Transient influx of Ca2+ was measured as the increase in fluorescence intensity in response to a change in temperature from 24 °C to 37 °C among three cells. (B,C) Relative MFI and AUC of three groups (n = 5 per group; * P < 0.05; ** P < 0.01; one‐way ANOVA followed by a Tukey post hoc test for each temperature). (D‐E) Orthographic views of cilia beat within 20 s in scrambled siRNA or TRPV4 siRNA treated ciliated cells from 24 to 37 °C (one cilia beat represented as one shift of bright or dark in the timeline) (n = 5 per group; *P < 0.05; one‐way ANOVA followed by a Tukey post hoc test for each temperature).
Figure S2. Proposed model of TRPV4 action in regulating the cilia beat frequency in the Fallopian tube exposed to levonorgestrel.
The data presented here indicate that TRPV4 was localized to cilia and apical membrane of oviduct ciliated cells. It was hypothesized that exposure to LNG could reduce promotor activity of TRPV4 via progesterone receptor action in the nucleus, and cause the inhibition of TRPV4 transcription, which leads to a decreased protein level of TRPV4. The inhibition of TRPV4 in ciliated cells reduced the calcium influx. Furthermore, the data indicate that the alternation of intracellular Ca2+ by the TRPV4‐modified cilia beat frequency in response to LNG, which might be the reason for embryo retention in Fallopian tubes. LNG, levonorgestrel; TRPV4, transient receptor potential vanilloid 4; PR, progesterone receptor.
Figure S3. Expression of PR and TRPV4 in OE‐E6/E7 cell line.
(A) Protein expression of PR‐A and PR‐B in 293FT, Ishikawa, and OE‐E6/E7 cell lines. (B) Protein expression of TRPV4 in 293FT, Ishikawa, and OE‐E6/E7 cell lines.
Figure S4. Overview of embryo‐tube transport assay in mice.
Female mice were mated randomly, and vaginal plug‐positive mice were randomly intraperitoneally injected with saline, LNG, LNG + RU486, or LNG + TRPV4 agonist 4α‐PDD immediately. Seventy‐four hours after observing the vaginal plug, the oviducts and uterus were ligated. The embryos were flushed from the oviducts or uteri to count the percentage of embryos retained in the oviducts.
Table S1. Antibody and stain list
Table S2. Oligonucleotide sequence (mentioned in the supplementary material, Supplementary materials and methods)
Acknowledgements
This study was supported by the National Natural Science Foundation of China (grant numbers 81671482 and 81671412). We thank Professor Xiuming Yan and Dr Lei Zhu (State Key Laboratory of Cell Biology, Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences) for their help with the primary culture of ciliated cells. We also thank Dr Chuan‐Jin Yu for providing the cell membrane stain (FM™ 4‐64FX) and for help with scanning electron microscopy.
Author contributions statement
JZ and H‐FH conceived the study and participated in its design, as well as supervising the study and critically revising the manuscript. CL and Y‐TW were responsible for the animal study and writing the manuscript. QZ and H‐YZ participated in the cell culture experiments. ZH, DZ, HQ, X‐QH, and X‐FW participated in the sample collection, and in writing and revising the manuscript. CL, Y‐TW, G‐LL, and XT contributed to the statistical analysis. All the authors contributed substantially to the revision of the manuscript.
No conflicts of interest were declared.
Contributor Information
He‐Feng Huang, Email: huanghefg@sjtu.edu.cn.
Jian Zhang, Email: zhangjian_ipmch@sjtu.edu.cn.
References
- 1. Stulberg DB, Cain LR, Dahlquist I, et al. Ectopic pregnancy rates and racial disparities in the Medicaid population, 2004–2008. Fertil Steril 2014; 102: 1671–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang J, Li C, Zhao WH, et al. Association between levonorgestrel emergency contraception and the risk of ectopic pregnancy: a multicenter case–control study. Sci Rep 2015; 5: 8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Farquhar CM. Ectopic pregnancy. Lancet 2005; 366: 583–591. [DOI] [PubMed] [Google Scholar]
- 4. Shaw JL, Dey SK, Critchley HO, et al. Current knowledge of the aetiology of human tubal ectopic pregnancy. Hum Reprod Update 2010; 16: 432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Horne AW, Critchley HO. Mechanisms of disease: the endocrinology of ectopic pregnancy. Expert Rev Mol Med 2012; 14: e7. [DOI] [PubMed] [Google Scholar]
- 6. Brooks ER, Wallingford JB. Multiciliated cells. Curr Biol 2014; 24: R973–R982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhao W, Zhu Q, Yan M, et al. Levonorgestrel decreases cilia beat frequency of human fallopian tubes and rat oviducts without changing morphological structure. Clin Exp Pharmacol Physiol 2015; 42: 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li HW, Liao SB, Chiu PC, et al. Expression of adrenomedullin in human oviduct, its regulation by the hormonal cycle and contact with spermatozoa, and its effect on ciliary beat frequency of the oviductal epithelium. J Clin Endocrinol Metab 2010; 95: E18–E25. [DOI] [PubMed] [Google Scholar]
- 9. Paltieli Y, Eibschitz I, Ziskind G, et al. High progesterone levels and ciliary dysfunction – a possible cause of ectopic pregnancy. J Assist Reprod Genet 2000; 17: 103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bylander A, Lind K, Goksor M, et al. The classical progesterone receptor mediates the rapid reduction of fallopian tube ciliary beat frequency by progesterone. Reprod Biol Endocrinol 2013; 11: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Andrade YN, Fernandes J, Vazquez E, et al. TRPV4 channel is involved in the coupling of fluid viscosity changes to epithelial ciliary activity. J Cell Biol 2005; 168: 869–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liao SB, Li HW, Ho JC, et al. Possible role of adrenomedullin in the pathogenesis of tubal ectopic pregnancy. J Clin Endocrinol Metab 2012; 97: 2105–2112. [DOI] [PubMed] [Google Scholar]
- 13. Schmid A, Sutto Z, Schmid N, et al. Decreased soluble adenylyl cyclase activity in cystic fibrosis is related to defective apical bicarbonate exchange and affects ciliary beat frequency regulation. J Biol Chem 2010; 285: 29998–30007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lyons RA, Saridogan E, Djahanbakhch O. The reproductive significance of human fallopian tube cilia. Hum Reprod Update 2006; 12: 363–372. [DOI] [PubMed] [Google Scholar]
- 15. Verdugo P. Ca2+‐dependent hormonal stimulation of ciliary activity. Nature 1980; 283: 764–765. [DOI] [PubMed] [Google Scholar]
- 16. Clapham DE. TRP channels as cellular sensors. Nature 2003; 426: 517–524. [DOI] [PubMed] [Google Scholar]
- 17. Voets T, Prenen J, Vriens J, et al. Molecular determinants of permeation through the cation channel TRPV4. J Biol Chem 2002; 277: 33704–33710. [DOI] [PubMed] [Google Scholar]
- 18. Alenmyr L, Uller L, Greiff L, et al. TRPV4‐mediated calcium influx and ciliary activity in human native airway epithelial cells. Basic Clin Pharmacol Toxicol 2014; 114: 210–216. [DOI] [PubMed] [Google Scholar]
- 19. Lorenzo IM, Liedtke W, Sanderson MJ, et al. TRPV4 channel participates in receptor‐operated calcium entry and ciliary beat frequency regulation in mouse airway epithelial cells. Proc Natl Acad Sci U S A 2008; 105: 12611–12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jung C, Fandos C, Lorenzo IM, et al. The progesterone receptor regulates the expression of TRPV4 channel. Pflügers Arch 2009; 459: 105–113. [DOI] [PubMed] [Google Scholar]
- 21. Teilmann SC, Byskov AG, Pedersen PA, et al. Localization of transient receptor potential ion channels in primary and motile cilia of the female murine reproductive organs. Mol Reprod Dev 2005; 71: 444–452. [DOI] [PubMed] [Google Scholar]
- 22. Mönkkönen KS, Aflatoonian R, Lee KF, et al. Hormonal regulation of Gαi2 and mPRα in immortalized human oviductal cell line OE‐E6/E7. Mol Hum Reprod 2007; 13: 845–851. [DOI] [PubMed] [Google Scholar]
- 23. Chan RW, Mak AS, Yeung WS, et al. Human female reproductive tract epithelial cell culture. Methods Mol Biol 2013; 945: 347–363. [DOI] [PubMed] [Google Scholar]
- 24. Li S, O'Neill SR, Zhang Y, et al. Estrogen receptor alpha is required for oviductal transport of embryos. FASEB J 2017; 31: 1595–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yuan J, Zhao W, Yan M, et al. Ulipristal acetate antagonizes the inhibitory effect of progesterone on ciliary beat frequency and upregulates steroid receptor expression levels in human fallopian tubes. Reprod Sci 2015; 22: 1516–1523. [DOI] [PubMed] [Google Scholar]
- 26. Ning N, Zhu J, Du Y, et al. Dysregulation of hydrogen sulphide metabolism impairs oviductal transport of embryos. Nat Commun 2014; 5: 4107. [DOI] [PubMed] [Google Scholar]
- 27. Verhage HG, Bareither ML, Jaffe RC, et al. Cyclic changes in ciliation, secretion and cell height of the oviductal epithelium in women. Am J Anat 1979; 156: 505–521. [DOI] [PubMed] [Google Scholar]
- 28. Lyons RA, Djahanbakhch O, Mahmood T, et al. Fallopian tube ciliary beat frequency in relation to the stage of menstrual cycle and anatomical site. Hum Reprod 2002; 17: 584–588. [DOI] [PubMed] [Google Scholar]
- 29. Noreikat K, Wolff M, Kummer W, et al. Ciliary activity in the oviduct of cycling, pregnant, and muscarinic receptor knockout mice. Biol Reprod 2012; 86: 120. [DOI] [PubMed] [Google Scholar]
- 30. Brosens IA, Vasquez G. Fimbrial microbiopsy. J Reprod Med 1976; 16: 171–178. [PubMed] [Google Scholar]
- 31. Jung C, Fernández‐Dueñas V, Plata C, et al. Functional coupling of GABAA/B receptors and the channel TRPV4 mediates rapid progesterone signaling in the oviduct. Sci Signal 2018; 11: eaam6558. [DOI] [PubMed] [Google Scholar]
- 32. Halbert SA, Tam PY, Blandau RJ. Egg transport in the rabbit oviduct: the roles of cilia and muscle. Science 1976; 191: 1052–1053. [DOI] [PubMed] [Google Scholar]
- 33. Ishikawa T. Axoneme structure from motile cilia. Cold Spring Harb Perspect Biol 2017; 9: a028076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li S, Winuthayanon W. Oviduct: roles in fertilization and early embryo development. J Endocrinol 2017; 232: R1–R26. [DOI] [PubMed] [Google Scholar]
- 35. Zhang ZR, Chu WF, Song B, et al. TRPP2 and TRPV4 form an EGF‐activated calcium permeable channel at the apical membrane of renal collecting duct cells. PLoS One 2013; 8: e73424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Goswami C, Kuhn J, Heppenstall PA, et al. Importance of non‐selective cation channel TRPV4 interaction with cytoskeleton and their reciprocal regulations in cultured cells. PLoS One 2010; 5: e11654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Garcia‐Elias A, Mrkonjić S, Jung C, et al. The TRPV4 channel. Handb Exp Pharmacol 2014; 222: 293–319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials and methods
Supplementary figure legends
Figure S1. Effect of temperature on the generation of Ca 2+ signals and modulation of CBF.
(A) Transient influx of Ca2+ was measured as the increase in fluorescence intensity in response to a change in temperature from 24 °C to 37 °C among three cells. (B,C) Relative MFI and AUC of three groups (n = 5 per group; * P < 0.05; ** P < 0.01; one‐way ANOVA followed by a Tukey post hoc test for each temperature). (D‐E) Orthographic views of cilia beat within 20 s in scrambled siRNA or TRPV4 siRNA treated ciliated cells from 24 to 37 °C (one cilia beat represented as one shift of bright or dark in the timeline) (n = 5 per group; *P < 0.05; one‐way ANOVA followed by a Tukey post hoc test for each temperature).
Figure S2. Proposed model of TRPV4 action in regulating the cilia beat frequency in the Fallopian tube exposed to levonorgestrel.
The data presented here indicate that TRPV4 was localized to cilia and apical membrane of oviduct ciliated cells. It was hypothesized that exposure to LNG could reduce promotor activity of TRPV4 via progesterone receptor action in the nucleus, and cause the inhibition of TRPV4 transcription, which leads to a decreased protein level of TRPV4. The inhibition of TRPV4 in ciliated cells reduced the calcium influx. Furthermore, the data indicate that the alternation of intracellular Ca2+ by the TRPV4‐modified cilia beat frequency in response to LNG, which might be the reason for embryo retention in Fallopian tubes. LNG, levonorgestrel; TRPV4, transient receptor potential vanilloid 4; PR, progesterone receptor.
Figure S3. Expression of PR and TRPV4 in OE‐E6/E7 cell line.
(A) Protein expression of PR‐A and PR‐B in 293FT, Ishikawa, and OE‐E6/E7 cell lines. (B) Protein expression of TRPV4 in 293FT, Ishikawa, and OE‐E6/E7 cell lines.
Figure S4. Overview of embryo‐tube transport assay in mice.
Female mice were mated randomly, and vaginal plug‐positive mice were randomly intraperitoneally injected with saline, LNG, LNG + RU486, or LNG + TRPV4 agonist 4α‐PDD immediately. Seventy‐four hours after observing the vaginal plug, the oviducts and uterus were ligated. The embryos were flushed from the oviducts or uteri to count the percentage of embryos retained in the oviducts.
Table S1. Antibody and stain list
Table S2. Oligonucleotide sequence (mentioned in the supplementary material, Supplementary materials and methods)


