Abstract
Background
Melatonin is a multifunctional indolamine and has a cardioprotective role in a variety of cardiovascular processes via antioxidant, anti‐inflammatory, antihypertensive, antithrombotic, and antilipemic effects. It has been reported that lower levels of circulating melatonin are significantly associated with a higher risk of acute myocardial infarction (AMI) and later cardiac remodeling. However, levels of melatonin in patients with dilated cardiomyopathy (DCM) and associations between melatonin levels and cardiac function remain unclear.
Methods and Results
We measured and compared plasma levels of melatonin in 61 control subjects, 81 AMI patients, and 77 DCM patients. Plasma levels of melatonin were progressively decreased from 71.9 pg/mL in the control group to 52.6 pg/mL in the DCM group and 21.9 pg/mL in the AMI group. Next, we examined associations of melatonin levels with parameters of laboratory data, echocardiography, and right‐heart catheterization. In the DCM patients, circulating melatonin showed significant correlations with both high‐sensitivity troponin T (R = −0.422, P < 0.001) and cardiac output (R = 0.431, P = 0.003), but not with B‐type natriuretic peptide (BNP), left ventricular ejection fraction (LVEF), pulmonary artery wedge pressure, or pulmonary artery pressure.
Conclusion
Patients with not only AMI but also DCM had lower circulating melatonin levels. Circulating melatonin levels appear to correlate with myocardial injury and cardiac output in DCM patients.
Keywords: cardiac troponin, dilated cardiomyopathy, echocardiography, hemodynamics, melatonin, natriuretic peptide
1. INTRODUCTION
Melatonin (N‐acetyl‐5‐methoxytryptamine), an endocrine product of the pineal gland, is formed predominantly during the nighttime and regulates circadian rhythms.1, 2, 3 It has been reported that melatonin is a multifunctional indolamine, which plays cardioprotective roles in a variety of cardiovascular processes via antioxidant, anti‐inflammatory, antihypertensive, antithrombotic, and antilipemic effects.1, 2, 3 Lower circulating melatonin levels are significantly associated with coronary artery disease (CAD),4, 5, 6 and a higher occurrence risk of acute myocardial infarction (AMI).7 In a cohort of survivors of AMI, serum melatonin levels measured at admission was an independent predictor of left ventricular (LV) remodeling at 12 months after AMI.8 In addition, experimental studies demonstrated a cardioprotective effect of melatonin against myocardial ischemia reperfusion injury.9, 10, 11 In rats, melatonin has a significant effect on the protection of the heart against isoproterenol‐induced myocardial infarction through maintaining endogenous antioxidant enzyme activities.12 Furthermore, lower serum melatonin levels after AMI were associated with greater adverse cardiovascular events.13 Melatonin supplementation seems to protect CAD or LV reverse remodeling. Although intracoronary and intravenous melatonin did not appear to attenuate reperfusion injury, or myocardial infarct size which was assessed by cardiac magnetic resonance imaging (the myocardial salvage index), after primary percutaneous coronary intervention in AMI patients with ST elevation compared with placebo (MARIA trial),14 the post hoc analysis showed that those presenting within 2.5 hours of symptom onset showed a significant reduction in myocardial infarct size and LV remodeling.15
Dilated cardiomyopathy (DCM) is characterized by ventricular dilation and depressed myocardial performance with genetic or nongenetic background occurring at around 1 in 2500 people.16 DCM patients show the symptoms of heart failure, arrhythmia, and sudden cardiac death with poor outcome, even though the recent advances in the treatment have been developed. Thus, new therapeutic targets and strategies for the DCM are critically needed. Nevertheless, circulating levels of melatonin in patients with cardiomyopathies, especially dilated cardiomyopathy (DCM), have never been reported, associations between circulating melatonin levels, and both cardiac function and other biomarkers remain unclear. Thus, we aimed to (a) measure and compare circulating levels of melatonin in DCM patients, AMI patients, and control subjects and (b) clarify the relationship of melatonin to cardiac function (eg, echocardiography and right‐heart catheterization; RHC) and other biomarkers (eg, natriuretic peptide and cardiac troponin) in DCM patients.
2. METHODS
2.1. Subjects and study protocol
This is a cross‐sectional study with 61 control subjects, 81 AMI patients, and 77 DCM patients who came to Fukushima Medical University Hospital between 2015 and 2017 and were comprehensively diagnosed according to the current guidelines.17, 18, 19 Control subjects were lacking obvious past history of cardiovascular disease (eg, heart failure, AMI) and abnormal structural findings which were assessed by echocardiography. Blood samples were collected from the hospitalized patients in a stable condition at discharge or the outpatients during the light period (between 6:00 and 09:00). We selected the period of the early morning rather than the night period not to disturb regular night sleep in the study subjects. All plasma and serum samples were frozen and stored in aliquots at −80°C. Melatonin levels in plasma samples were measured by using an enzyme‐linked immunoassay (enzyme‐linked immunosorbent assay kit, Cloud‐clone Corp., Houston, TX) according to the manufacturer's protocol.20 Briefly, 50 μL of the plasma samples and the serially diluted standards were applied into the each well, and each reagent was added and incubated and then measured at 450 nm with a microplate reader (SpectraMax i3, Molecular Devices, Sunnyvale, CA). The concentrations of the melatonin were calculated by using the standard curve. Each sample was measured in duplicates. The intra‐assay and inter‐assay coefficients of variations for plasma melatonin were 2.9% and 9.2%, respectively. Serum BNP levels were measured using a specific immunoradiometric assay (Shionoria BNP kit, Shionogi, Osaka, Japan). High‐sensitivity cardiac troponin T levels were measured using an electrochemiluminescence immunoassay (Elecsys Troponin T hs, Roche Diagnostics Ltd., Rotkreuz, Switzerland).21 These assays were performed by experienced laboratory technicians blinded to this study.
Firstly, we compared plasma levels of melatonin in the control subjects, AMI patients, and DCM patients. Secondly, focusing on the DCM patients, we examined the associations of circulating melatonin with BNP and troponin T levels, left ventricular ejection fraction (LVEF) assessed by echocardiography and RHC parameters. Written informed consent was obtained from all study subjects. The study protocol was approved by the Ethics Committee of Fukushima Medical University (No. 823) and was carried out in accordance with the principles outlined in the Declaration of Helsinki. Reporting of the study conforms to STROBE along with references to STROBE and the broader EQUATOR guidelines.22
2.2. Echocardiography and right‐heart catheterization (RHC)
Echocardiography was performed by experienced echocardiographers, who were blinded to this study, using standard techniques.23, 24, 25 The echocardiographic parameters investigated included left ventricular ejection fraction (LVEF), fractional shortening, volume and diameter of left ventricle, left atrial volume, the ratio of early transmitral flow velocity to mitral annular velocity (mitral valve E/e’), right ventricular fractional area change (RV‐FAC), and inferior vena cava diameter. Mitral valve E/e’ was calculated by transmitral Doppler flow and tissue Doppler imaging. The RV‐FAC, defined as (end‐diastolic area ‐ end‐systolic area)/end‐diastolic area × 100, was used as a measure of right ventricular systolic function. All measurements were performed using ultrasound systems (ACUSON Sequoia, Siemens Medical Solutions USA, Inc, Mountain View, CA, USA).23, 24, 25
Right‐heart catheterization was performed in all patients in a stable condition, in a resting supine position under fluoroscopic guidance, and at room air for more than 30 minutes after catheter placement. Pulmonary artery pressure (PAP), pulmonary artery wedge pressure (PAWP), and cardiac output were measured using a 7F Swan‐Ganz catheter (Edwards Lifesciences, Irvine, CA, USA).26 Cardiac output and cardiac index were calculated based on the thermo dilution method.
2.3. Statistical analysis
The categorical variables are expressed as numbers and percentages, and the chi‐square test was used for their comparisons. Normally distributed data are presented as mean ± standard deviation (SD), and non‐normally distributed data (eg, melatonin, BNP, troponin T) are presented as median and interquartile range or log‐transformed. We used analysis of variance followed by Bonferroni's post hoc test. Correlations of plasma melatonin with parameters of laboratory data, echocardiography, and RHC were assessed using Pearson's correlation analysis for parametric variables and Spearman's correlation analysis for nonparametric variables. A value of P < 0.05 was considered statistically significant for all comparisons. These analyses were performed using a statistical software package (SPSS ver. 24.0, IBM, Armonk, NY, USA).
3. RESULTS
Clinical features of the subjects are summarized in Table 1. Prevalence of chronic kidney disease and atrial fibrillation was highest in the DCM group, whereas anemia was highest in the AMI group. In contrast, age, sex, and other comorbidities were comparable among the three groups. Regarding laboratory data and echocardiography, BNP level was highest and LVEF was lowest in the DCM group, whereas troponin T level was highest in the AMI group. Levels of serum sodium were lower in both the DCM and AMI groups than in the control group. As shown in Table 1 and Figure 1, plasma levels of melatonin were highest in the control group followed by the DCM and AMI groups, namely, plasma levels of melatonin in the DCM group were intermediate between those observed in the control and AMI groups. With regard to control subjects and AMI patients, as shown in Figure S1, there was no relationship between melatonin levels with circulating levels of both BNP and troponin T.
Table 1.
Comparisons of clinical characteristics
| Control (n = 61) | DCM (n = 77) | AMI (n = 81) | P‐value | |
|---|---|---|---|---|
| Age (y) | 61.9 ± 13.7 | 62.9 ± 14.3 | 62.2 ± 17.8 | 0.431 |
| Male sex (n, %) | 49 (80.3) | 55 (71.4) | 54 (66.7) | 0.351 |
| NYHA class I/II/III/IV | — | 11/59/7/0 | — | |
| Comorbidity | ||||
| Hypertension (n, %) | 35 (57.4) | 56 (72.7) | 59 (72.8) | 0.117 |
| Diabetes mellitus (n, %) | 28 (45.9) | 33 (42.9) | 28 (34.6) | 0.392 |
| Dyslipidemia (n, %) | 44 (72.1) | 57 (74.0) | 69 (85.2) | 0.122 |
| CKD (n, %) | 16 (26.2) | 43 (55.8) | 28 (34.6) | 0.002 |
| Anemia (n, %) | 9 (14.8) | 29 (37.7) | 37 (45.7) | <0.001 |
| Atrial fibrillation (n, %) | 6 (9.8) | 36 (46.8) | 10 (12.3) | <0.001 |
| Smoking (n, %) | 40 (65.6) | 46 (59.7) | 39 (48.1) | 0.102 |
| Laboratory data | ||||
| BNP (pg/mL)§ | 13.6 (6.0‐25.9) | 413.2 (150.8‐741.0)** | 45.2 (9.8‐139.0)*, †† | <0.001 |
| Troponin T (ng/mL)§ | 0.008 (0.003‐0.022) | 0.027 (0.013‐0.047)* | 0.168 (0.085‐3.628)*, † | 0.026 |
| Total protein (g/dL) | 7.1 ± 0.4 | 7.1 ± 0.7 | 7.1 ± 0.7 | 0.969 |
| Albumin (g/dL) | 4.0 ± 0.4 | 3.8 ± 0.5 | 3.9 ± 0.6 | 0.253 |
| Creatinine (mg/dL) | 0.9 ± 0.8 | 1.1 ± 0.9 | 1.0 ± 0.8 | 0.456 |
| Sodium (mEq/L) | 140.9 ± 2.0 | 138.9 ± 3.0** | 138.7 ± 3.4** | <0.001 |
| CRP (mg/dL)§ | 0.08 (0.04‐0.26) | 0.14 (0.05‐0.43) | 0.16 (0.05‐0.57) | 0.302 |
| Melatonin (pg/mL)§ | 71.9 (37.7‐141.0) | 52.6 (36.8‐76.2) ** | 21.9 (17.0‐39.9)**, †† | <0.001 |
| Echocardiography | ||||
| LVEF (%) | 63.4 ± 8.7 | 31.3 ± 9.7** | 56.2 ± 11.1*, †† | <0.001 |
| LVEDV (mL) | 80.5 ± 41.6 | 140.2 ± 43.1** | 87.0 ± 33.4†† | <0.001 |
| LVESV (mL) | 31.1 ± 26.4 | 98.3 ± 37.9** | 39.8 ± 22.1†† | <0.001 |
| Fractional shortening (%) | 38.6 ± 7.6 | 14.7 ± 7.5** | 33.6 ± 10.5*, †† | <0.001 |
| IVS (mm) | 10.4 ± 2.6 | 9.7 ± 1.5 | 10.1 ± 2.0 | 0.221 |
| LVDd (mm) | 45.5 ± 6.2 | 61.3 ± 8.7** | 47.0 ± 6.0†† | <0.001 |
| LVDs (mm) | 28.0 ± 5.3 | 52.4 ± 9.8** | 31.3 ± 7.1*, †† | <0.001 |
| PW (mm) | 10.6 ± 2.3 | 9.8 ± 1.3 | 10.6 ± 2.7 | 0.065 |
| Left atrial volume (mL) | 49.6 ± 19.8 | 83.9 ± 59.1** | 50.2 ± 34.4†† | <0.001 |
| Mitral valve E/e’ | 9.4 ± 4.4 | 14.6 ± 6.2** | 11.8 ± 5.4†† | <0.001 |
| RV‐FAC (%) | 44.4 ± 9.9 | 38.2 ± 14.3 | 41.3 ± 11.0 | 0.195 |
| IVC (mm) | 12.8 ± 3.8 | 16.0 ± 5.5** | 13.1 ± 3.7†† | <0.001 |
AMI, acute myocardial infarction; BNP, B‐type natriuretic peptide; CKD, chronic kidney disease; CRP, C‐reactive protein; DCM, dilated cardiomyopathy; IVC, inferior vena cava diameter; IVS, interventricular septum wall thickness, LVDd, left ventricular end‐diastolic diameter; LVDs, left ventricular end‐systolic diameter; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end‐systolic volume; NYHA, New York Heart Association; PW, posterior wall thickness; RV‐FAC, right ventricular fractional area change.
P < 0.05 and
P < 0.01 vs control,
P < 0.05 and
P < 0.01 vs DCM.
The data were expressed as mean ± SD or median (interquartile range).
Figure 1.
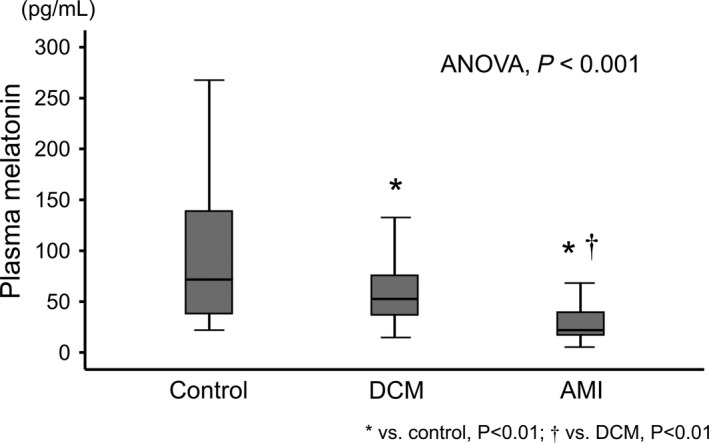
Comparisons of circulating levels of melatonin among the control, dilated cardiomyopathy (DCM), and acute myocardial infarction (AMI) groups. * P < 0.01 vs control; † P < 0.01 vs DCM
Next, we focused on and examined associations of melatonin levels with BNP, troponin T, LVEF, and RHC parameters in the DCM patients (n = 77). As shown in Figures 2 and 3, melatonin was significantly correlated with each of high‐sensitivity cardiac troponin T (R = −0.422, P < 0.001), cardiac output (R = 0.431, P = 0.003), and cardiac index (R = 0.438, P = 0.002), but not with BNP, LVEF, mean PAP, or PAWP in DCM patients. Correlation analysis of plasma melatonin with other echocardiographic parameters in DCM patients are shown in Table S1. In addition, LVEF was not correlated with either troponin T (R = −0.071, P = 0.571), cardiac output (R = 0.131, P = 0.382), or index (R = 0.056, P = 0.710) in DCM patients. Regarding the associations of melatonin levels with New York Heart Association functional class, plasma levels of melatonin in each class did not significantly differ (P = 0.265): class I 40.2 (23.7‐69.6 pg/mL), class II 54.8 (37.8‐77.2 pg/mL), and class III 66.3 (36.6‐96.3 pg/mL). In addition, the associations of melatonin levels with sleep duration were presented in Figure S2 (n = 26). Average sleep duration was assessed by medical interview. There were significant negative correlations between melatonin levels and average sleep duration (R = −0.472, P = 0.015).
Figure 2.
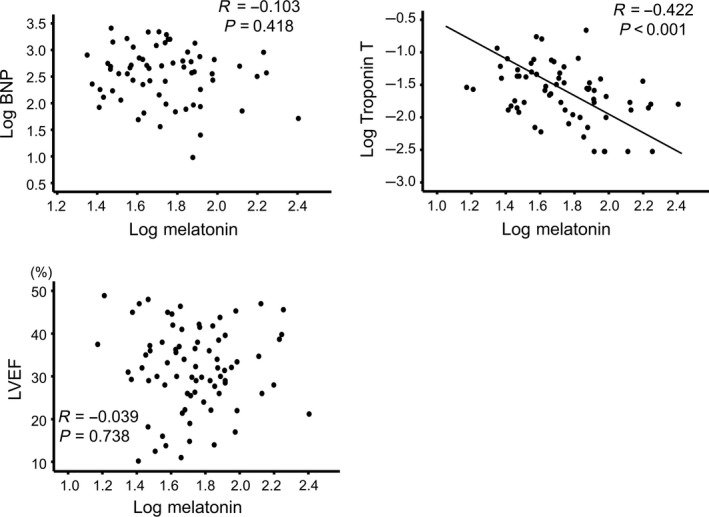
Correlations of melatonin levels with B‐type natriuretic peptide (BNP), troponin T, and left ventricular ejection fraction (LVEF) in DCM patients
Figure 3.
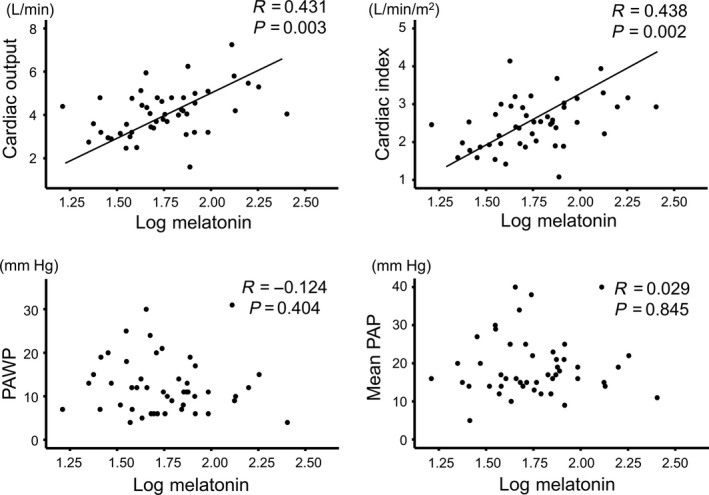
Correlations between melatonin levels with parameters of right‐heart catheterization in DCM patients. PAP, pulmonary artery pressure; PAWP, pulmonary artery wedge pressure.
4. DISCUSSION
To our knowledge, the present study is the first to report that (a) circulating levels of melatonin in the DCM patients were intermediate between the control and AMI subjects, and that (b) in the DCM patients, circulating levels of melatonin were correlated with myocardial damage (troponin T) and cardiac output and index.
Under physiological conditions, melatonin is mainly synthesized in the pineal gland,27 and once synthesized, melatonin is quickly released into the blood. Circulating melatonin levels reflect the total amount of melatonin production. Although age and sex influence the circulating levels of melatonin,28 there were no significant differences in age or sex as clinical background among the three groups in this study. Melatonin signaling functions via two G protein‐coupled melatonin receptors, MT1 and MT2. MT2 is widely abundant in the cardiovascular system including the heart and arteries.29 Regarding the arteries, melatonin ameliorates vascular endothelial dysfunction, inflammation, and atherosclerosis30 and regulates blood pressure31 and lipid metabolism.32 Thus, low levels of circulating melatonin might be related with the risk of CAD,4, 6 which is consistent with our results that the AMI patients had much lower plasma melatonin levels compared to the control subjects.
We firstly found that the circulating levels of melatonin in the DCM patients were significantly lower than in the control subjects and that plasma melatonin was significantly correlated with serum troponin T levels in the DCM patients. These data suggest that the patients with lower circulating levels of melatonin had more myocardial degeneration, because the elevation in circulating cardiac troponin T levels indicates ongoing myocardial injury in the heart failure patients.21, 33 Growing evidence demonstrates that melatonin has a protective effect on cells. Receptor‐dependent cardio‐protection of melatonin was related with anti‐adrenergic actions mediated by nitric oxide synthase and guanylyl cyclase activation10 and with anti‐apoptotic processes by promoting the JAK/STAT pathway.34 Melatonin suppressed opening sensitivity of the mitochondrial permeability transition pore35 and improved calcium handling in the sarcoplasmic reticulum in cardiomyocytes.36 The physiological role of melatonin, and its extraordinary antioxidant and scavenging properties, modulated the levels of inflammatory cytokines, and antifibrotic activity.37 Endogenous melatonin synthesis in the pineal gland is regulated by norepinephrine, which is released from sympathetic nerve fibers and nonadrenergic transmitters.27 The interaction of norepinephrine with adrenergic receptors, β1 and α1, triggers the melatonin synthesis by activation of downstream pathways of the adrenergic receptors in the pinealocytes, and a recent report showed that immune‐competent cells such as dendritic cells and macrophages can induce melatonin production via the activation of adrenoceptors,38 suggesting that there might be a close relationship between sympathetic nerve activity and melatonin generation. Furthermore, previous studies reported that melatonin inhibited sympathetic nervous system activity and attenuated the effects of the renin–angiotensin system.39, 40 Low levels of circulating melatonin were associated with the development of heart failure in patients with hypertensive cardiomyopathy,41 and with LV remodeling in patients after AMI,8 as well as with reverse remodeling in patients with ischemic and nonischemic heart diseases after cardiac resynchronization therapy.42 In this study, the DCM patients showed intermediate levels of plasma melatonin between the control subjects and AMI patients. This suggests that low circulating melatonin levels indicate risk of progression of atherosclerosis in AMI patients, whereas it is related with cardiac remodeling in DCM patients. Circulating levels of melatonin changed during LV remodeling after AMI in rats.43 Melatonin may play a variety of roles in each cardiovascular diseases at different stages. This suggests that melatonin has direct and indirect protective effects on cardiomyocytes and suppresses myocardial injury (troponin), and cardioprotective effects of melatonin might consequently contribute to the improvement of LV remodeling and cardiac output in DCM patients.
On the other hand, we did not find a significant correlation of plasma melatonin levels and BNP, PAWP, or mean PA in DCM patients. Thus, plasma melatonin levels do not seem to be associated with pulmonary congestion. In addition, there was no correlation of plasma melatonin levels and LVEF in the DCM patients. We could not completely explain why plasma levels of melatonin were associated with both cardiac troponin T and cardiac output, but not with LVEF. Probably, LVEF is not a necessary index of pure LV systolic function and is affected by many factors such as response to beta blockers, time course of treatment of heart failure, and genetic backgrounds. In addition, it requires long term to observe the changes in LVEF in DCM patients, while the detection of myocardial injury by measuring circulating troponin T and cardiac output at one point reflects the condition of the disease.44 Indeed, LVEF did not correlate with circulating levels of troponin T in DCM patients in this study and previous report.44 Thus, there might be a time course difference among these parameters. Furthermore, melatonin reduces blood pressure31 and systemic vascular resistance mediated by nitric oxide synthase45; hence, melatonin seemed to have a greater impact on cardiac output rather than LVEF in the DCM patients. Concordant with previous data, melatonin had a significant effect on myocardial remodeling but did not necessarily improve LVEF.8, 42 As this is the cross‐sectional study, the relationship between the melatonin levels and change in LVEF in DCM patients need to be observed during the follow‐up.
The usefulness of melatonin treatment for cardiovascular diseases has been reported.15, 46, 47 Melatonin supplementation protected against pathological cardiac remodeling induced by transverse aortic constriction48 and isoproterenol,46 and melatonin prevented mitochondrial fission and improved cardiac function in mice with diabetes through the SIRT1‐PGC1α pathway.47 Administration of melatonin in AMI patients with ST elevation, who presented early after symptom onset, was associated with a significant reduction in the infarct size and remodeling after primary percutaneous coronary intervention.15 Although the causality between the decrease in melatonin level in the patients and low cardiac output in DCM patients remains to be elucidated, the decreased melatonin production might be causally related to the development of cardiac remodeling in DCM patients. Our study shows that melatonin may be a new therapeutic target for DCM patients to prevent progressive LV remodeling. Studies will be needed to test the exogenous melatonin supplementation would help in cardiac remodeling in DCM patients.
4.1. Study strengths and limitations
Our study has some strengths. This is the first study that demonstrated the comprehensive association of circulating melatonin with BNP, troponin T, echocardiographic, and hemodynamic parameters in DCM patients.
The current study has several limitations. First, as a cross‐sectional study of a single center with a relatively small number of patients, the study may be somewhat underpowered. However, DCM is a relatively rare disease; therefore, the small study population was unavoidable. Second, we did not consider circadian rhythm of melatonin secretion. Third, we used only variables of the patients at one point in this study without considering changes in medical parameters (eg, melatonin). Fourth, although we encouraged catheterizations as much as possible, we were not able to perform these measurements in all patients for various reasons (eg, rejection by the patients, medical reasons, etc). Thus, there may be potential selection bias. Further studies with a larger population are needed.
5. CONCLUSION
Patients with not only AMI but also DCM had lower circulating melatonin levels. Circulating melatonin levels appear to correlate with latent myocardial injury and cardiac output in DCM patients. Melatonin may play a cardioprotective role in DCM patients.
CONFLICT OF INTEREST
Akiomi Yoshihisa and Tomofumi Misaka belong to Department of Advanced Cardiac Therapeutics supported by Fukuda‐Denshi Co, Ltd. This company is not associated with the contents of this study. Koichi Sugimoto and Tetsuro Yokokawa belong to Department of Pulmonary Hypertension supported by Actelion Pharmaceuticals Japan Co, Ltd. This company is not associated with the contents of this study.
Supporting information
ACKNOWLEDGEMENTS
The authors acknowledge Ms. Tomiko Miura, Ms. Kumiko Watanabe and Ms. Hitomi Kobayashi for their outstanding technical assistance.
Misaka T, Yoshihisa A, Yokokawa T, et al. Plasma levels of melatonin in dilated cardiomyopathy. J Pineal Res. 2019;66:e12564 10.1111/jpi.12564
These authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Funding information
This work was supported in part by a grant in aid for Scientific Research (No. 16K09447) from the Japan Society for the Promotion of Science, Tokyo, Japan.
REFERENCES
- 1. Dominguez‐Rodriguez A, Abreu‐Gonzalez P, Reiter RJ. Melatonin for cardioprotection in ST elevation myocardial infarction: are we ready for the challenge? Heart. 2017;103(9):647‐648. [DOI] [PubMed] [Google Scholar]
- 2. Dominguez‐Rodriguez A, Abreu‐Gonzalez P, Sanchez‐Sanchez JJ, Kaski JC, Reiter RJ. Melatonin and circadian biology in human cardiovascular disease. J Pineal Res. 2010;49(1):14‐22. [DOI] [PubMed] [Google Scholar]
- 3. Tengattini S, Reiter RJ, Tan DX, Terron MP, Rodella LF, Rezzani R. Cardiovascular diseases: protective effects of melatonin. J Pineal Res. 2008;44(1):16‐25. [DOI] [PubMed] [Google Scholar]
- 4. Sakotnik A, Liebmann PM, Stoschitzky K, et al. Decreased melatonin synthesis in patients with coronary artery disease. Eur Heart J. 1999;20(18):1314‐1317. [DOI] [PubMed] [Google Scholar]
- 5. Dominguez‐Rodriguez A, Abreu‐Gonzalez P, Avanzas P. The role of melatonin in acute myocardial infarction. Front Biosci (Landmark Ed). 2012;17:2433‐2441. [DOI] [PubMed] [Google Scholar]
- 6. Yaprak M, Altun A, Vardar A, Aktoz M, Ciftci S, Ozbay G. Decreased nocturnal synthesis of melatonin in patients with coronary artery disease. Int J Cardiol. 2003;89(1):103‐107. [DOI] [PubMed] [Google Scholar]
- 7. McMullan CJ, Rimm EB, Schernhammer ES, Forman JP. A nested case‐control study of the association between melatonin secretion and incident myocardial infarction. Heart. 2017;103(9):694‐701. [DOI] [PubMed] [Google Scholar]
- 8. Dominguez‐Rodriguez A, Abreu‐Gonzalez P, Arroyo‐Ucar E, Reiter RJ. Decreased level of melatonin in serum predicts left ventricular remodelling after acute myocardial infarction. J Pineal Res. 2012;53(3):319‐323. [DOI] [PubMed] [Google Scholar]
- 9. Reiter RJ, Tan DX, Paredes SD, Fuentes‐Broto L. Beneficial effects of melatonin in cardiovascular disease. Ann Med. 2010;42(4):276‐285. [DOI] [PubMed] [Google Scholar]
- 10. Genade S, Genis A, Ytrehus K, Huisamen B, Lochner A. Melatonin receptor‐mediated protection against myocardial ischaemia/reperfusion injury: role of its anti‐adrenergic actions. J Pineal Res. 2008;45(4):449‐458. [DOI] [PubMed] [Google Scholar]
- 11. Yang Y, Sun Y, Yi W, et al. A review of melatonin as a suitable antioxidant against myocardial ischemia‐reperfusion injury and clinical heart diseases. J Pineal Res. 2014;57(4):357‐366. [DOI] [PubMed] [Google Scholar]
- 12. Patel V, Upaganlawar A, Zalawadia R, Balaraman R. Cardioprotective effect of melatonin against isoproterenol induced myocardial infarction in rats: A biochemical, electrocardiographic and histoarchitectural evaluation. Eur J Pharmacol. 2010;644(1‐3):160‐168. [DOI] [PubMed] [Google Scholar]
- 13. Dominguez‐Rodriguez A, Abreu‐Gonzalez P, Garcia‐Gonzalez M, Reiter RJ. Prognostic value of nocturnal melatonin levels as a novel marker in patients with ST‐segment elevation myocardial infarction. Am J Cardiol. 2006;97(8):1162‐1164. [DOI] [PubMed] [Google Scholar]
- 14. Ekeloef S, Halladin N, Fonnes S, et al. Effect of intracoronary and intravenous melatonin on myocardial salvage index in patients with ST‐elevation myocardial infarction: a randomized placebo controlled trial. J Cardiovasc Transl Res. 2017;10(5‐6):470‐479. [DOI] [PubMed] [Google Scholar]
- 15. Dominguez‐Rodriguez A, Abreu‐Gonzalez P, de la Torre‐Hernandez JM, et al. Usefulness of early treatment with melatonin to reduce infarct size in patients with ST‐segment elevation myocardial infarction receiving percutaneous coronary intervention (from the melatonin adjunct in the acute myocardial infarction treated with angioplasty trial). Am J Cardiol. 2017;120(4):522‐526. [DOI] [PubMed] [Google Scholar]
- 16. Bozkurt B, Colvin M, Cook J, et al. Current diagnostic and treatment strategies for specific dilated cardiomyopathies: a scientific statement from the American heart association. Circulation. 2016;134(23):e579‐e646. [DOI] [PubMed] [Google Scholar]
- 17. Elliott P, Andersson B, Arbustini E, et al. Classification of the cardiomyopathies: a position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2008;29(2):270‐276. [DOI] [PubMed] [Google Scholar]
- 18. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129‐2200. [DOI] [PubMed] [Google Scholar]
- 19. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147‐239. [DOI] [PubMed] [Google Scholar]
- 20. Budkowska M, Ostrycharz E, Wojtowicz A, et al. A Circadian Rhythm in both Complement Cascade (ComC) activation and Sphingosine‐1‐Phosphate (S1P) levels in human peripheral blood supports a role for the ComC‐S1P axis in circadian changes in the number of stem cells circulating in peripheral blood. Stem Cell Rev. 2018;14(5):677‐685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nakamura Y, Yoshihisa A, Takiguchi M, et al. High‐sensitivity cardiac troponin T predicts non‐cardiac mortality in heart failure. Circ J. 2014;78(4):890‐895. [DOI] [PubMed] [Google Scholar]
- 22. von Elm E, Altman DG, Egger M, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806‐808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7(2):79‐108. [DOI] [PubMed] [Google Scholar]
- 24. Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685‐713. quiz 786‐688 [DOI] [PubMed] [Google Scholar]
- 25. Yoshihisa A, Takiguchi M, Shimizu T, et al. Cardiovascular function and prognosis of patients with heart failure coexistent with chronic obstructive pulmonary disease. J Cardiol. 2014;64(4):256‐264. [DOI] [PubMed] [Google Scholar]
- 26. Yoshihisa A, Kimishima Y, Kiko T, et al. Liver fibrosis marker, 7S domain of collagen type IV, in patients with pre‐capillary pulmonary hypertension. Int J Cardiol. 2018;258:269‐274. [DOI] [PubMed] [Google Scholar]
- 27. Simonneaux V, Ribelayga C. Generation of the melatonin endocrine message in mammals: a review of the complex regulation of melatonin synthesis by norepinephrine, peptides, and other pineal transmitters. Pharmacol Rev. 2003;55(2):325‐395. [DOI] [PubMed] [Google Scholar]
- 28. Obayashi K, Saeki K, Tone N, et al. Lower melatonin secretion in older females: gender differences independent of light exposure profiles. J Epidemiol. 2015;25(1):38‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ekmekcioglu C, Thalhammer T, Humpeler S, et al. The melatonin receptor subtype MT2 is present in the human cardiovascular system. J Pineal Res. 2003;35(1):40‐44. [DOI] [PubMed] [Google Scholar]
- 30. Hu ZP, Fang XL, Fang N, et al. Melatonin ameliorates vascular endothelial dysfunction, inflammation, and atherosclerosis by suppressing the TLR4/NF‐kappaB system in high‐fat‐fed rabbits. J Pineal Res. 2013;55(4):388‐398. [DOI] [PubMed] [Google Scholar]
- 31. Baker J, Kimpinski K. Role of melatonin in blood pressure regulation: An adjunct anti‐hypertensive agent. Clin Exp Pharmacol Physiol. 2018;45(8):755‐766. [DOI] [PubMed] [Google Scholar]
- 32. Dominguez‐Rodriguez A, Abreu‐Gonzalez P, Garcia‐Gonzalez M, Ferrer‐Hita J, Vargas M, Reiter RJ. Elevated levels of oxidized low‐density lipoprotein and impaired nocturnal synthesis of melatonin in patients with myocardial infarction. Atherosclerosis. 2005;180(1):101‐105. [DOI] [PubMed] [Google Scholar]
- 33. Sato Y, Yamada T, Taniguchi R, et al. Persistently increased serum concentrations of cardiac troponin t in patients with idiopathic dilated cardiomyopathy are predictive of adverse outcomes. Circulation. 2001;103(3):369‐374. [DOI] [PubMed] [Google Scholar]
- 34. Lau WW, Ng JK, Lee MM, Chan AS, Wong YH. Interleukin‐6 autocrine signaling mediates melatonin MT(1/2) receptor‐induced STAT3 Tyr(705) phosphorylation. J Pineal Res. 2012;52(4):477‐489. [DOI] [PubMed] [Google Scholar]
- 35. Petrosillo G, Colantuono G, Moro N, et al. Melatonin protects against heart ischemia‐reperfusion injury by inhibiting mitochondrial permeability transition pore opening. Am J Physiol Heart Circ Physiol. 2009;297(4):H1487‐H1493. [DOI] [PubMed] [Google Scholar]
- 36. Yeung HM, Hung MW, Fung ML. Melatonin ameliorates calcium homeostasis in myocardial and ischemia‐reperfusion injury in chronically hypoxic rats. J Pineal Res. 2008;45(4):373‐382. [DOI] [PubMed] [Google Scholar]
- 37. Dominguez‐Rodriguez A, Abreu‐Gonzalez P, Garcia M, et al. Light/dark patterns of interleukin‐6 in relation to the pineal hormone melatonin in patients with acute myocardial infarction. Cytokine. 2004;26(2):89‐93. [DOI] [PubMed] [Google Scholar]
- 38. Pires‐Lapa MA, Carvalho‐Sousa CE, Cecon E, Fernandes PA, Markus RP. beta‐Adrenoceptors trigger melatonin synthesis in phagocytes. Int J Mol Sci. 2018;19(8):E2182 10.3390/ijms19082182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dominguez‐Rodriguez A, Abreu‐Gonzalez P. Relationship between nighttime blood pressure, the renin‐angiotensin system, and melatonin. Rev Esp Cardiol. 2013;66(10):831‐832. [DOI] [PubMed] [Google Scholar]
- 40. Campos LA, Cipolla‐Neto J, Amaral FG, Michelini LC, Bader M, Baltatu OC. The Angiotensin‐melatonin axis. Int J Hypertens. 2013;2013:521783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dominguez‐Rodriguez A, Abreu‐Gonzalez P, Reiter RJ. The potential usefulness of serum melatonin level to predict heart failure in patients with hypertensive cardiomyopathy. Int J Cardiol. 2014;174(2):415‐417. [DOI] [PubMed] [Google Scholar]
- 42. Dominguez‐Rodriguez A, Abreu‐Gonzalez P, Piccolo R, Galasso G, Reiter RJ. Melatonin is associated with reverse remodeling after cardiac resynchronization therapy in patients with heart failure and ventricular dyssynchrony. Int J Cardiol. 2016;221:359‐363. [DOI] [PubMed] [Google Scholar]
- 43. Sallinen P, Manttari S, Leskinen H, et al. The effect of myocardial infarction on the synthesis, concentration and receptor expression of endogenous melatonin. J Pineal Res. 2007;42(3):254‐260. [DOI] [PubMed] [Google Scholar]
- 44. Kawahara C, Tsutamoto T, Nishiyama K, et al. Prognostic role of high‐sensitivity cardiac troponin T in patients with nonischemic dilated cardiomyopathy. Circ J. 2011;75(3):656‐661. [DOI] [PubMed] [Google Scholar]
- 45. Blanchard B, Pompon D, Ducrocq C. Nitrosation of melatonin by nitric oxide and peroxynitrite. J Pineal Res. 2000;29(3):184‐192. [DOI] [PubMed] [Google Scholar]
- 46. Simko F, Bednarova KR, Krajcirovicova K, et al. Melatonin reduces cardiac remodeling and improves survival in rats with isoproterenol‐induced heart failure. J Pineal Res. 2014;57(2):177‐184. [DOI] [PubMed] [Google Scholar]
- 47. Ding M, Feng N, Tang D, et al. Melatonin prevents Drp1‐mediated mitochondrial fission in diabetic hearts through SIRT1‐PGC1alpha pathway. J Pineal Res. 2018;65(2):e12491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhai M, Liu Z, Zhang B, et al. Melatonin protects against the pathological cardiac hypertrophy induced by transverse aortic constriction through activating PGC‐1beta: In vivo and in vitro studies. J Pineal Res. 2017;63(3). 10.1111/jpi.12433. Epub 2017 Aug 16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


