Abstract
Background
Data on chronic pain after kidney donation are sparse. The aim of this study was to assess the incidence of chronic pain after hand‐assisted laparoscopic nephrectomy.
Methods
Living kidney donors who donated between 2011 and 2017 at the University Medical Centre Groningen were included. All patients underwent hand‐assisted laparoscopic donor nephrectomy. Postdonation pain and movement disabilities were assessed using the Carolinas Comfort Scale (CCS) and a visual analogue scale (VAS). The prevalence, severity of pain and the need for analgesics were reported.
Results
Some 333 living kidney donors with a mean age of 56 years were included. At a median of 19 (i.q.r. 10–33) months after donation, 82 donors (24·6 per cent) had a CCS score above 0, of which 58 (71 per cent) had a CCS score of at least 2 and 57 (70 per cent) reported movement limitations. Some 110 donors (33·0 per cent) had a VAS score of more than 0. Complaints mainly occurred during bending over (12·3 per cent) and exercising (12·4 per cent). Thirty‐two donors (9·7 per cent) required analgesics during follow‐up between donation and the time of measurement, and six of 82 (7 per cent) reported chronic inguinal pain. In multivariable analysis, donor age (odds ratio (OR) 0·97, 95 per cent c.i. 0·95 to 0·99; P = 0·020) and length of hospital stay (OR 1·21, 1·01 to 1·51; P = 0·041) were independently associated with chronic pain.
Conclusion
One‐quarter of donors experienced chronic postdonation pain or discomfort, most of which was bothersome. Younger donors and those with a longer postoperative hospital stay had more symptoms.
Introduction
Transplantation with a kidney from a living donor is the best treatment for patients with end‐stage renal disease, and leads to a better survival rate and quality of life than transplantation with a kidney from a deceased donor1, 2. Previous studies3, 4 have shown that hand‐assisted laparoscopic (HAL) donor nephrectomy is a safe procedure with good outcomes in terms of quality of life and quick recovery. Outcomes after laparoscopic nephrectomy are superior to those of open procurement in terms of blood loss, length of hospital stay and postoperative pain5. However, a number of donor patients suffer from chronic pain and this poses an important barrier to living kidney donor programmes6, 7, 8, 9. Generally, kidney donation leads to an increase in perceived quality of life by living kidney donors, but chronic postoperative pain can, importantly, impair quality of life in these otherwise healthy people10, 11.
Studies12, 13 investigating pain after donation have focused mainly on short‐term postoperative pain. The aim of this study was to assess the incidence, location and precipitating causes of chronic pain lasting at least 3 months after HAL donor nephrectomy.
Methods
Patients who underwent a HAL donor nephrectomy at the University Medical Centre Groningen were asked to complete questionnaires regarding chronic pain. In accordance with donor selection criteria, potential donors with a history of diabetes, kidney disease or cardiovascular events were excluded from the donation programme. Those with hypertension were included if BP was controlled adequately with a maximum of two antihypertensive drugs. Exclusion criteria for the present study were: age less than 18 years; inability to fill out the questionnaire (for example owing to foreign language); and no longer alive at time of inquiry. Informed consent was obtained from all participants. The study was approved by the institutional ethical review board (METc 2014/077). All procedures were conducted in accordance with the Declarations of Helsinki and Istanbul.
Donor nephrectomy procedure
All patients underwent hand‐assisted laparoscopic donor nephrectomy, either transperitoneal (HAL) or retroperitoneal (hand‐assisted retroperitoneal nephrectomy, HARN)14. The operation was converted to an open procedure where necessary. The wound area was infiltrated before surgery with 10 ml 0·25 per cent bupivacaine in all patients. A suprapubic transverse incision was made for access of the Gelport® (Applied Medical, Rancho Santa Margarita, California, USA) in both surgical procedures. All trocars (Applied Medical) were positioned in view of the camera. One 10‐mm trocar was placed in the iliac fossa (left or right depending on the kidney to be donated), one 10‐mm periumbilical trocar for the 30° video port, and one 5‐mm subcostal/epigastric trocar (left or right depending on the kidney to be donated). The kidney was dissected from surrounding tissues, and the ureter and hilar vessels identified. The ureter and artery were clipped (Ligaclip®; Ethicon, Johnson & Johnson, Somerville, New Jersey, USA) and cut, and the vein was stapled and cut (Endo GIA™ 30–2·5; Medtronic Covidien, Minneapolis, Minnesota, USA). After removal of the kidney via a suprapubic incision, the suprapubic fascia was closed using a running absorbable suture. The 12‐mm trocar port sites were closed at the fascia level by means of interrupted absorbable sutures.
Before surgery all patients were given 1000 mg paracetamol. Intraoperative analgesia was managed with remifentanil or sufentanil using manually controlled infusion or a target‐controlled infusion system15, 16. Patients received 0·1 mg/kg piritramide/morphine 30–45 min before the end of surgery. Postoperative pain management consisted of 1000 mg paracetamol four times daily and piritramide or morphine using a patient‐controlled analgesia (PCA) technique. Non‐steroidal anti‐inflammatory drugs were not used. The use of PCA on the ward was managed by the acute pain service of the Department of Anaesthesia.
Clinical data collection
Data collected were: age; sex; BMI; glomerular filtration rate measured before donation using [125I]iothalamate; surgical complications according to the Clavien–Dindo classification17; type of donor (related, unrelated or altruistic); type of nephrectomy (HAL or HARN); site of nephrectomy; duration of surgery; conversion (to open surgery, or from HARN to HAL); blood loss; need for reintervention; and length of hospital stay (LOS). Data regarding pain medication at hospital discharge were obtained from patient medical records.
Questionnaire
The questionnaire consisted of the modified Carolinas Comfort Scale (CCS) (Appendix S1, supporting information)18, 19, a visual analogue scale (VAS) with scores ranging from 0 to 100 on a 100‐mm scale20, and questions regarding the medical history and use of analgesics. The CCS was originally validated for pain assessment after inguinal hernia repair with use of a mesh, and was modified in a previous study on chronic pain in kidney recipients12. The questionnaires were sent by mail and all those who did not reply were contacted by telephone after 4 weeks. General questions were added to the enquiry to allow assessment of potential confounding factors.
The CCS comprises eight subcategories in which patients could indicate to what extent they experienced pain and movement impairment on a scale from 0 (no complaints) to 5 (disabling complaints). The subcategories were: lying down, bending over, sitting up, activities of daily living, coughing, walking, walking up stairs and exercise. The patients were asked to indicate the severity of ongoing complaints at the time of filling in the questionnaire, under the condition that these complaints lasted at least 3 months from nephrectomy to the date of enquiry. Chronic pain was defined by complaints or pain in the inguinal region or flank at the time of survey, which was assessed at least 3 months after surgery. Patients with a CCS score above 0 were scored as positive for pain (referred to as ‘patients with complaints’ in the CCS). Patients with a CCS of at least 2 in one of the subcategories were considered to have ‘significant and bothersome complaints’, according to the CCS classification and this was considered clinically significant.
In a subgroup of donors who also participated in the TransplantLines study (ClinicalTrials.gov NCT03272841), additional data regarding pain were collected along with the CCS scores at clinical follow‐up visits after nephrectomy. These additional data consisted of a detailed pain history, taken by a trained researcher, and involved questions about the type of pain, intensity of pain measured on a numerical rating scale (NRS)20, 21, location, referred pain, signs of sympathetic nerve activation, and the influence of perceived pain on sleep and activities of daily living.
Statistical analysis
Categorical variables are presented as numbers with percentages, and were analysed using χ2 test or Fisher's exact test. Continuous variables with a normal distribution are presented as mean(s.d.) and those with a skewed distribution as median (i.q.r.), with analysis by means of Student t test and Mann–Whitney U test respectively.
Univariable and multivariable analyses of factors associated with a higher CCS score and sensitivity analyses were performed using logistic regression. Variables included in the analysis were: sex, age, BMI, systolic BP, glomerular filtration rate, side of nephrectomy, type of donor procurement (altruistic or related), blood loss, length of hospital stay and complications. Characteristics that were univariably associated with CCS score, or had a univariable P value below 0·200, were added to the multivariable model. Multiple imputation using the fully conditional specification method was undertaken to correct for missing data, as described previously22. Two‐tailed P values were used throughout and significance was set at P < 0·050. The statistical analyses were done using SPSS® version 23 (IBM, Armonk, New York, USA).
Results
Consecutive patients who underwent a HAL donor nephrectomy between January 2011 and December 2016 were asked to complete questionnaires regarding chronic pain. Some 419 donors were approached at a median of 19 (i.q.r. 10–33) months after nephrectomy. A total of 333 donors completed the study questionnaires (response rate 79·5 per cent), and formed the basis for this analysis. Mean(s.d.) age at time of donation was 56(11) years and 48·6 per cent of the donors were men. Mean BMI at donor screening was 26·4(3·3) kg/m2 (Table 1).
Table 1.
Donor characteristics
| All donors (n = 333) | Donors with no pain (n = 251) | Donors with pain (n = 82) | P § | |
|---|---|---|---|---|
| Age (years) * | 56(11) | 57(11) | 53(11) | 0·011¶ |
| Sex ratio (M : F) | 162 : 171 | 121 : 130 | 41 : 41 | 0·800 |
| Height (cm) * | 175(9) | 175(9) | 175(10) | 0·692¶ |
| Weight (kg) * | 801(13) | 81(12) | 82(14) | 0·450¶ |
| BMI (kg/m 2 ) * | 26·4(3·3) | 26·4(3·2) | 26·6(3·5) | 0·616¶ |
| BP (mmHg) * | ||||
| Systolic | 127(12) | 128(12) | 125(11) | 0·132¶ |
| Diastolic | 76(9) | 76(9) | 76(8) | 0·974¶ |
| GFR (ml/min) * | 111(22·2) | 110(20·8) | 114(26·2) | 0·199¶ |
| Type of donor | 0·084# | |||
| Living related | 139 (41·7) | 98 (39·0) | 41 (50) | |
| Living unrelated | 166 (49·8) | 128 (51·0) | 38 (46) | |
| Altruistic | 28 (8·4) | 25 (10·0) | 3 (4) | |
| Side of donor nephrectomy | 0·460 | |||
| Right | 80 (24·0) | 63 (25·1) | 17 (21) | |
| Left | 253 (76·0) | 188 (74·9) | 65 (79) | |
| Intended surgical technique | 0·426 | |||
| HAL | 314 (94·3) | 238 (94·8) | 76 (93) | |
| HARN | 19 (5·7) | 13 (5·2) | 6 (7) | |
| Blood loss (ml) † | 0 (0–100) | 0 (0–100) | 0 (0–100) | 0·674** |
| Duration of surgery (min) * | 187(42) | 186(42) | 187(43) | 0·880¶ |
| Length of hospital stay (days) * | 4·6(1·2) | 4·5(1·1) | 4·8(1·4) | 0·033¶ |
| Grade III–IV complications < 30 days ‡ | 8 (2·4) | 6 (2·4) | 2 (2) | 1·000 |
| Conversion rate | 0·338# | |||
| No conversion, primary HAL | 313 (94·0) | 237 (94·4) | 76 (93) | |
| No conversion, primary HARN | 11 (3·3) | 9 (3·6) | 2 (2) | |
| Conversion of HARN to HAL | 8 (2·4) | 4 (1·6) | 4 (5) | |
| Conversion of HAL to open | 1 (0·3) | 1 (0·4) | 0 (0) | |
| Need for reoperation | 2 (0·6) | 2 (0·8) | 0 (0) | 1·000 |
| Follow‐up (months) † | 19 (10–33) | 20 (12–33) | 16 (8–32) | 0·080** |
Values in parentheses are percentages unless indicated otherwise; values are
mean(s.d.) and
median (i.q.r.).
Clavien–Dindo grade III–IV: any complication that requires surgical, endoscopic or radiological intervention. GFR, glomerular filtration rate; HAL, hand‐assisted laparoscopy; HARN, hand‐assisted retroperitoneal nephrectomy.
Fisher's exact test, except
Student's t test,
χ2 test and
Mann–Whitney U test.
Carolinas Comfort Scale and visual analogue scale scores
Eighty‐two donors (24·6 per cent) reported a CCS score above 0, indicating the presence of pain symptoms at the time of completing the surveys. Fifty‐eight patients (17·4 per cent) experienced bothersome complaints in at least one of the subcategories (CCS score at least 2). The median CCS score for all 333 donors was 6 (4–12). Patients with a CCS score of 2 or more had a median VAS score of 18 (3–33), whereas among those with a CCS score below 2 it was 1 (0–0). Pain was most often reported while bending over (12·3 per cent) and during exercise (12·4 per cent) (Table 2, Fig. 1). Fifty‐seven donors (17·1 per cent) reported movement limitations in the questionnaire. These also occurred most frequently during bending over (10·0 per cent) and exercising (10·9 per cent). Total scores for each individual are shown in Fig. 2.
Table 2.
Analysis of pain and complaints in living kidney donors
| Prevalence | |
|---|---|
| Complaints (n = 333) | |
| CCS > 0 | 82 (24·6) |
| VAS > 0 | 110 (33·0) |
| Complaints ≥ 1 year after donation (n = 230) | |
| CCS > 0 | 47 (20·4) |
| VAS > 0 | 67 (29·1) |
| Pain (n = 333) | 82 (24·6) |
| Lying down | 25 (7·5) |
| Bending over | 41 (12·3) |
| Sitting up | 25 (7·5) |
| Activities of daily living | 29 (8·7) |
| Coughing or deep breathing | 21 of 332 (6·3) |
| Walking | 31 (9·3) |
| Walking up stairs | 30 (9·0) |
| Exercising | 40 of 322 (12·4) |
| Movement limitations (n = 333) | 57 (17·1) |
| Lying down | n.a. |
| Bending over | 33 of 331 (10·0) |
| Sitting up | 22 of 331 (6·6) |
| Activities of daily living | 24 of 330 (7·3) |
| Coughing or deep breathing | 11 of 331 (3·3) |
| Walking | 20 of 331 (6·0) |
| Walking up stairs | 25 of 331 (7·6) |
| Exercising | 35 of 320 (10·9) |
| Pain ≥ 1 year after donation (n = 230) | 47 (20·4) |
| Lying down | 14 (6·1) |
| Bending over | 20 (8·7) |
| Sitting up | 16 (7·0) |
| Activities of daily living | 18 (7·8) |
| Coughing or deep breathing | 14 of 229 (6·1) |
| Walking | 22 (9·6) |
| Walking up stairs | 19 (8·3) |
| Exercising | 25 of 228 (11·0) |
| Movement limitations ≥ 1 year after donation (n = 230) | 34 (14·8) |
| Bending over | 13 of 228 (5·7) |
| Sitting up | 10 of 228 (4·4) |
| Activities of daily living | 12 of 228 (5·3) |
| Coughing or deep breathing | 6 of 228 (2·6) |
| Walking | 14 of 228 (6·1) |
| Walking up stairs | 16 of 228 (7·0) |
| Exercising | 24 of 226 (10·6) |
| Analgesic use for any indication (n = 333) | 32 of 330 (9·7) |
| Location of pain (n = 82) | |
| Inguinal | 6 (7) |
| At site of wound | 10 (12) |
| Abdomen other | 26 (32) |
| Extra‐abdominal | 8 (10) |
| Not specified | 32 (39) |
Values in parentheses are percentages. The Carolinas Comfort Scale (CCS) measures the severity of pain and movement limitations, on a scale from 0 (no pain/movement limitations) to 5 (severe pain/movement limitations). The visual analogue scale (VAS) measures pain on a continuous scale, represented by a horizontal line with scores ranging from 0 (no pain) to 100 (severe pain) on a linear scale. n.a., Not applicable.
Figure 1.
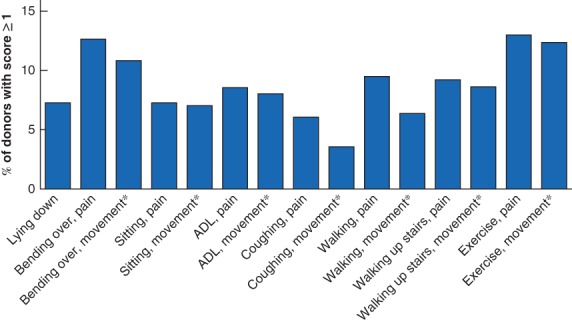
Carolinas Comfort Scale subcategory scores of living kidney donorsPercentage of living donors with a Carolinas Comfort Scale score of at least 1 for each subcategory are shown. *Movement limitations. ADL, activities of daily living.
Figure 2.
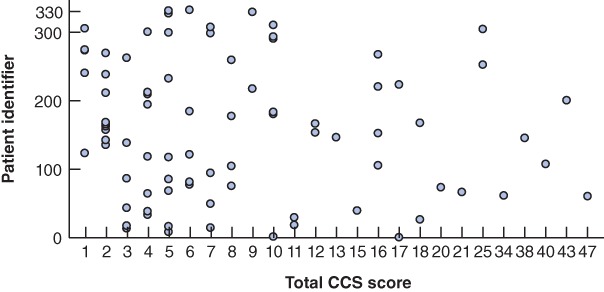
Scatterplot showing individual Carolinas Comfort Scale scoresScores are plotted for 82 patients who had a Carolinas Comfort Scale (CCS) score above zero.
A total of 110 donors (33·0 per cent) reported a VAS score of more than 0, with a median of 4(1–16). Donors experiencing pain were younger (mean 53(11) versus 57(11) years; P = 0·011) and had a longer hospital stay (mean 4·8(1·4) versus 4·5(1·1) days; P = 0·033), but otherwise did not differ significantly from those without pain symptoms (Table 1). The abdominal region (32 per cent), the site of nephrectomy (12 per cent) and the inguinal region (7 per cent) were the most frequently reported pain locations. Most patients (94·3 per cent) underwent transperitoneal donor procurement instead of a retroperitoneal approach. One patient required conversion to open surgery.
Determinants of chronic pain
In univariable logistic regression analysis, age was a significant determinant of pain, with younger donors more frequently experiencing pain (OR per year 0·97, 95 per cent c.i. 0·95 to 0·99; P = 0·012) (Table S1, supporting information). A longer hospital stay was also associated with a higher risk of developing chronic pain during follow‐up (OR 1·24, 1·01 to 1·52; P = 0·036+). Altruistic donors showed a trend towards a lower incidence of pain (OR 0·33, 0·10 to 1·13; P = 0·075) and living related donors showed a trend towards a higher incidence (OR 1·57, 0·95 to 2·60; P = 0·078).
Secondary analysis
In univariable analysis, younger age was associated with increased pain in the following subdomains: lying down (OR 0·95, 0·61 to 0·98; P = 0·003), bending over (OR 0·96, 0·93 to 0·99; P = 0·007), sitting (OR 0·95, 0·91 to 0·98; P = 0·002), activities of daily living (OR 0·96, 0·93 to 0·99; P = 0·014), coughing (OR 0·95, 0·91 to 0·99; P = 0·006) and climbing stairs (OR 0·96, 0·93 to 0·99; P = 0·014) (Table S1, supporting information). In linear regression, donor age (β = –0·06, P = 0·293), donor type (β = –0·94, P = 0·348) and LOS were not associated with the donors' VAS score (β = –0·05, P = 0·958). Opioids were prescribed more frequently at discharge from hospital after nephrectomy among donors experiencing pain than among those without pain (Table S2, supporting information). In multivariable analysis, donor age (OR 0·97, 0·95 to 0·99; P = 0·020) and LOS (OR 1·21, 1·01 to 1·51; P = 0·041) remained independently associated with pain (Table 3).
Table 3.
Multivariable logistic regression analysis of variables associated with different subcategories of the Carolinas Comfort Scale
| CCS domain | Odds ratio for CCS score > 0 | ||||
|---|---|---|---|---|---|
| Age | Systolic BP | Altruistic donor | Related donor | LOS | |
| Total | 0·97 (0·95, 0·99)* | 0·99 (0·98, 1·02) | 0·43 (0·11, 1·63) | 1·44 (0·84, 2·44) | 1·21 (1·01, 1·51)* |
| Lying | 0·95 (0·91, 0·98)† | 0·99 (0·97, 1·02) | 0·49 (0·03, 4·29) | 0·67 (0·28, 1·62) | 1·20 (0·88, 1·64) |
| Bending | 0·96 (0·93, 0·99)† | 0·99 (0·97, 1·01) | 0·70 (0·14, 3·47) | 1·28 (0·64, 2·58) | 1·21 (0·93, 1·56) |
| Sitting | 0·95 (0·91, 0·98)† | 0·98 (0·94, 1·02) | 0·49 (0·05, 4·96) | 0·77 (0·32, 1·87) | 1·16 (0·84, 1·60) |
| Activities of daily living | 0·96 (0·93, 0·99)* | 0·99 (0·96, 1·01) | 1·09 (0·20, 5·89) | 1·27 (0·56, 2·86) | 1·18 (0·88, 1·59) |
| Coughing | 0·95 (0·91, 0·99)* | 0·97 (0·93, 1·00) | 0·79 (0·70, 9·93) | 2·01 (0·76, 5·30) | 1·28 (0·91, 1·80) |
| Walking | 0·98 (0·94, 1·01) | 1·00 (0·97, 1·02) | 0·37 (0·05, 3·11) | 1·06 (0·49, 2·27) | 1·22 (0·92, 1·61) |
| Walking up stairs | 0·96 (0·93, 0·99)* | 1·00 (0·97, 1·02) | 0·50 (0·06, 4·02) | 1·31 (0·60, 2·87) | 1·19 (0·89, 1·59) |
| Exercise | 0·98 (0·95, 1·01) | 0·99 (0·97, 1·01) | 0·58 (0·14, 2·35) | 0·86 (0·45, 1·64) | 1·26 (0·99, 1·59) |
| Bothersome | 0·97 (0·95, 0·99)* | 0·99 (0·98, 1·02) | 0·45 (0·12, 1·70) | 1·29 (0·75, 2·21) | 1·25 (1·02, 1·54)* |
Values in parentheses are 95 per cent confidence intervals. CCS, Carolinas Comfort Scale; LOS, length of hospital stay.
P < 0·050,
P < 0·010.
Detailed analysis of pain
A more detailed analysis of the pain symptoms was performed in a subgroup of 218 donors who also participated in the TransplantLines study. Some 137 (62·8 per cent) of these donors had follow‐up of more than 5 years after donation. Of the 218 donors, 46 (21·1 per cent) experienced pain in the lower thorax/abdominal region, with emphasis on hypogastric (37 of 46) or umbilical (6 of 46) areas, or lower left (5 of 46) or right (5 of 46) abdominal quadrants. Twenty‐seven of 46 donors ascribed their pain to the nephrectomy. The pain was most often described as stabbing (37 per cent), deep/nagging (32 per cent) or burning (12 per cent). The median intensity of pain on a NRS ranging from 0 to 10 was 4 (i.q.r. 2–6), and referred pain in the stomach or back was described in 17 per cent. Eight donors (20 per cent) described continuous pain symptoms. Three donors (8 per cent) experienced generalized symptoms such as sweating, nausea and paleness during pain episodes, and 12 (27 per cent) reported interference with sleep. As with the CCS score, donors most often described the pain during bending over (20 per cent). Most donors (54 per cent) experiencing pain reported no limitations in daily activities, but six (13 per cent) experienced medium impairment and three (7 per cent) severe impairment in their daily life.
Discussion
In this study, 24·6 per cent of donors experienced chronic pain or discomfort after HAL donor nephrectomy, of whom 71 per cent had bothersome complaints (CCS score at least 2). Complaints occurred most often during bending over and exercising, and required analgesia in a minority of patients. These data can be used to help raise awareness and develop individualized interventions for pain reduction in living kidney donors that focus on specific activities provoking these symptoms.
The incidence of chronic pain was highest in younger patients. Previous studies reported similar results after inguinal hernia repair23, 24 and living liver donation25. There is no clear explanation why younger patients, in a relatively healthy population, are prone to experience more pain, although the elderly appear to have a higher threshold for low‐intensity pain26. The complaints during bending over are probably explained by the fact that younger patients have a more active lifestyle. However, differences in immune response can also contribute to increased nociceptor activation which then can lead to hyperalgesic priming and/or wind‐up, and eventually to central sensitization through long‐term potentiation in the central nervous system27.
Altruistic donors may be at lower risk of developing chronic pain, although the results were not statistically significant. It has been suggested that a specific neurocognitive pathway is activated in altruistic donors28, which may involve enhanced volume and function of the amygdala, thereby dampening the biological response to pain. Another possibility is that, because altruistic donors in the Netherlands donate anonymously, a poor graft or recipient outcome does not affect the donor psychologically4, 11.
The hospital stay was longer in patients reporting chronic pain. Because no difference in complication rate was found, it might be possible that donors experiencing chronic pain had a longer hospital stay because of a higher pain intensity directly after surgery29, 30. Owing to the retrospective nature of this study, reliable estimation or collection of pain data during the hospital admission was not possible. However, opiates were prescribed more frequently at hospital discharge in donors with chronic pain.
Although the incidence of inguinal pain was relatively low, the CCS scores were comparable with VAS scores, which are frequently used in studies assessing chronic pain after donor nephrectomy31, 32, 33, 34.
There was no difference in chronic pain after retroperitoneal nephrectomy compared with transperitoneal dissection. However, the conversion rate from retroperitoneal to transperitoneal nephrectomy was rather high (8 of 19), which could have biased these results. No difference in incidence of chronic pain between donors with and without the need for conversion was demonstrated.
The results of this study may be used for the counselling of future living kidney donors and their recipients. Although kidney donation generally tends to increase donor quality of life10, 11, the incidence of pain may have a reducing effect. Even though HAL nephrectomy greatly reduces the incidence of pain compared with an open procedure31, 32, 33, the present findings emphasize that pain is still an important problem affecting one‐quarter of donors. Although chronic pain may not be entirely unexpected after major surgery, an important difference in the case of living donors should be pointed out. Living kidney donors differ from the normal surgical population because they undergo surgery voluntarily for the benefit of others, greatly affecting the way they should be informed about expectations regarding the postoperative course. In this respect, there are important parallels with living liver donors in whom comparable rates of chronic pain have been reported (27 per cent)25. The well‐being of donors does not only reflect their own quality of life, but has extensive psychosocial effects on the recipient and other family relatives in terms of reciprocity, anxiety and feelings of guilt25, 35, 36. This underlines that in these specific populations every measure should be taken to prevent chronic pain, but at least provide enough information to come to a well considered decision.
There is a strong relationship between acute postoperative pain and development of chronic pain, and this transition is likely to be caused by sensitization to pain owing to a complex process of psychosocial and biological factors37, 38, 39, 40. Various interventions to prevent acute pain after laparoscopic donor nephrectomy have been described, but their effect on chronic pain has yet to be clarified fully. Perioperative administration of an α2 agonist (such as clonidine), lidocaine infusion, pregabalin or ketamine have all been described as effective methods for reducing acute postoperative pain and the need for opioid medication28, 41, 42. Effective intraoperative interventions are subfascial administration of bupivacaine, transversus abdominis plane block, low‐pressure pneumoperitoneum and deep neuromuscular blockade43, 44. Future studies are needed to determine which approach is most appropriate and effective in living kidney donors.
This study has a few limitations that need to be addressed. The CCS score was primarily validated in studies reporting chronic pain after inguinal herniorrhaphy19 and donor nephrectomy is performed in different anatomical planes. For example, the peritoneal cavity is opened during HAL nephrectomy, which could lead to a different aetiology of pain, rather than chronic pain induced by inguinal nerve injury, even though the same inguinal nerves are encountered at a more proximal location during donor nephrectomy. This is further underlined by a subanalysis in which the location of pain was evaluated in 46 patients, only three of whom reported specific inguinal pain. Along with peritoneal visceral pain, more localized pain in the abdomen or flank in donors could be explained by the dissection of perforating nerves around the kidney during procurement. These nerves are innervated by splanchnic nerve roots that originate in the aorticorenal ganglia located at the origin of the renal artery. This could suggest that local anaesthetic infiltration or spraying at this level may be beneficial in reducing the incidence or severity of postoperative pain.
Use of the CCS was preferred in this study because it generated detailed information about complaints and pain during activities of daily living. Because the CCS subcategories do not focus fully on inguinal pain, it appears applicable to other surgical populations. Kidney donors and patients after hernia repair are similar populations, comprising healthy individuals with a very low burden of co‐morbidity. The VAS method and Short Form 36 (SF‐36) are used most in these populations, but either have low specificity or are too extensive. A previous study45 compared the CCS with the SF‐36 quality‐of‐life survey and showed that 75 per cent of patients preferred the CCS over the SF‐36, mostly because it was easier to understand. Future studies are needed to validate this short but effective method, in which the questionnaire should be completed before and after operation to enable an analysis relative to baseline.
In this study, a CCS score of at least 2 was considered clinically significant. This threshold is debatable because no previous study has assessed chronic pain in living kidney donors using the CCS survey. Furthermore, there is significant heterogeneity among RCTs in use of definitions for various degrees of pain46. This is partly because pain is a highly subjective experience. When comparing two groups, a difference of 10–30 mm on a VAS scale is generally considered significant46. Patients in the present cohort with a CCS score of 2 or more suffered from bothersome pain and had a median VAS score of 18 (i.q.r. 3–33), in contrast to a VAS score of 1 (0–0) among those with a CCS score below 2. However, owing to the retrospective nature of this study, no comparisons could be made with preoperative CCS scores.
Another limitation is the retrospective design of this study and relatively limited follow‐up (maximum 6 years). One previous study47 reported an increased incidence of pain 10 years after donation compared to the general population. However the other domains concerning quality of life between groups were comparable. There were some missing data in the present study as 7·0 per cent of patients did not fill in the questionnaire completely. Multiple imputation was used to account for the missing data22. Because pain and movement disability are highly inter‐related, the bias from the missing answers was probably limited. In addition, bias was further reduced by using a binary outcome (pain or no pain) in the analyses. Data on opiate use at discharge after nephrectomy were collected, which may have been subject to under‐reporting in the patient files. Finally, the questionnaire was not completed at a set time after surgery, which could also have led to bias. However, no relationship between the duration of follow‐up and incidence of chronic pain was found, suggesting that this effect was negligible.
Supporting information
Appendix S1. Modified Carolinas Comfort Scale
Table S1. Univariable analysis of variables associated with the different categories of the Carolinas Comfort Scale (CCS)
Table S2. Opiate prescription at discharge after nephrectomy
Acknowledgements
This study is based on data from the TransplantLines Biobank and Data Repository.
Disclosure: The authors declare no conflict of interest.
References
- 1. Terasaki PI, Cecka JM, Gjertson DW, Takemoto S. High survival rates of kidney transplants from spousal and living unrelated donors. N Engl J Med 1995; 333: 333–336. [DOI] [PubMed] [Google Scholar]
- 2. Cecka JM. The UNOS renal transplant registry. Clin Transpl 2001: 1–18. [PubMed] [Google Scholar]
- 3. Yuan H, Liu L, Zheng S, Yang L, Pu C, Wei Q et al. The safety and efficacy of laparoscopic donor nephrectomy for renal transplantation: an updated meta‐analysis. Transplant Proc 2013; 45: 65–76. [DOI] [PubMed] [Google Scholar]
- 4. Wirken L, van Middendorp H, Hooghof CW, Rovers MM, Hoitsma AJ, Hilbrands LB et al. The course and predictors of health‐related quality of life in living kidney donors: a systematic review and meta‐analysis. Am J Transplant 2015; 15: 3041–3054. [DOI] [PubMed] [Google Scholar]
- 5. Greco F, Hoda MR, Alcaraz A, Bachmann A, Hakenberg OW, Fornara P. Laparoscopic living‐donor nephrectomy: analysis of the existing literature. Eur Urol 2010; 58: 498–509. [DOI] [PubMed] [Google Scholar]
- 6. Owen M, Lorgelly P, Serpell M. Chronic pain following donor nephrectomy – a study of the incidence, nature and impact of chronic post‐nephrectomy pain. Eur J Pain 2010; 14: 732–734. [DOI] [PubMed] [Google Scholar]
- 7. Alper I, Yüksel E. Comparison of acute and chronic pain after open nephrectomy versus laparoscopic nephrectomy: a prospective clinical trial. Medicine (Baltimore) 2016; 95: e3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reese PP, Boudville N, Garg AX. Living kidney donation: outcomes, ethics, and uncertainty. Lancet 2015; 385: 2003–2013. [DOI] [PubMed] [Google Scholar]
- 9. Tong A, Chapman JR, Wong G, Kanellis J, McCarthy G, Craig JC. The motivations and experiences of living kidney donors: a thematic synthesis. Am J Kidney Dis 2012; 60: 15–26. [DOI] [PubMed] [Google Scholar]
- 10. Johnson EM, Anderson JK, Jacobs C, Suh G, Humar A, Suhr BD et al. Long‐term follow‐up of living kidney donors: quality of life after donation. Transplantation 1999; 67: 717–721. [DOI] [PubMed] [Google Scholar]
- 11. Glotzer OS, Singh TP, Gallichio MH, Conti DJ, Siparsky NF. Long‐term quality of life after living kidney donation. Transplant Proc 2013; 45: 3225–3228. [DOI] [PubMed] [Google Scholar]
- 12. Dolce CJ, Keller JE, Walters KC, Griffin D, Norton HJ, Heniford BT et al. Laparoscopic versus open live donor nephrectomy: outcomes analysis of 266 consecutive patients. Surg Endosc 2009; 23: 1564–1568. [DOI] [PubMed] [Google Scholar]
- 13. Aguiar WF, Passerotti CC, Claro JF, Almeida CJ, Gattas N, Cedenho AP et al. Mini‐incisions by lombotomy or subcostal access in living kidney donors: a randomized trial comparing pain, safety, and quality of life. Clin Transplant 2007; 21: 269–276. [DOI] [PubMed] [Google Scholar]
- 14. Dols LFC, Kok NFM, D'Ancona FCH, Klop KW, Tran TC, Langenhuijsen JF et al. Randomized controlled trial comparing hand‐assisted retroperitoneoscopic versus standard laparoscopic donor nephrectomy. Transplantation 2014; 97: 161–167. [DOI] [PubMed] [Google Scholar]
- 15. Minto CF, Schnider TW, Shafer SL. Pharmacokinetics and pharmacodynamics of remifentanil. II. Model application. Anesthesiology 1997; 86: 24–33. [DOI] [PubMed] [Google Scholar]
- 16. Gepts E, Shafer SL, Camu F, Stanski DR, Woestenborghs R, Van Peer A et al. Linearity of pharmacokinetics and model estimation of sufentanil. Anesthesiology 1995; 83: 1194–1204. [DOI] [PubMed] [Google Scholar]
- 17. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD et al. The Clavien–Dindo classification of surgical complications: five‐year experience. Ann Surg 2009; 250: 187–196. [DOI] [PubMed] [Google Scholar]
- 18. Zorgdrager M, Lange JF, Krikke C, Nieuwenhuijs GJ, Hofker SH, Leuvenink HG et al. Chronic inguinal pain after kidney transplantation, a common and underexposed problem. World J Surg 2017; 41: 630–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nielsen K, Poelman MM, den Bakker FM, van der Ploeg T, Bonjer HJ, Schreurs WH. Comparison of the Dutch and English versions of the Carolinas Comfort Scale: a specific quality‐of‐life questionnaire for abdominal hernia repairs with mesh. Hernia 2014; 18: 459–464. [DOI] [PubMed] [Google Scholar]
- 20. Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short‐Form McGill Pain Questionnaire (SF‐MPQ), Chronic Pain Grade Scale (CPGS), Short Form‐36 Bodily Pain Scale (SF‐36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res (Hoboken) 2011; 63(Suppl 11): S240–S252. [DOI] [PubMed] [Google Scholar]
- 21. Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs 2005; 14: 798–804. [DOI] [PubMed] [Google Scholar]
- 22. Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Methods 2002; 7: 147–177. [PubMed] [Google Scholar]
- 23. Aasvang E, Kehlet H. Chronic postoperative pain: the case of inguinal herniorrhaphy. Br J Anaesth 2005; 95: 69–76. [DOI] [PubMed] [Google Scholar]
- 24. Poobalan AS, Bruce J, Smith WC, King PM, Krukowski ZH, Chambers WA. A review of chronic pain after inguinal herniorrhaphy. Clin J Pain 2003; 19: 48–54. [DOI] [PubMed] [Google Scholar]
- 25. Holtzman S, Clarke HA, McCluskey SA, Turcotte K, Grant D, Katz J. Acute and chronic postsurgical pain after living liver donation: incidence and predictors. Liver Transpl 2014; 20: 1336–1346. [DOI] [PubMed] [Google Scholar]
- 26. Lautenbacher S, Peters JH, Heesen M, Scheel J, Kunz M. Age changes in pain perception: a systematic‐review and meta‐analysis of age effects on pain and tolerance thresholds. Neurosci Biobehav Rev 2017; 75: 104–113. [DOI] [PubMed] [Google Scholar]
- 27. Eller‐Smith OC, Nicol AL, Christianson JA. Potential mechanisms underlying centralized pain and emerging therapeutic interventions. Front Cell Neurosci 2018; 12: 35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marsh AA, Stoycos SA, Brethel‐Haurwitz KM, Robinson P, VanMeter JW, Cardinale EM. Neural and cognitive characteristics of extraordinary altruists. Proc Natl Acad Sci U S A 2014; 111: 15036–15041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mathuram Thiyagarajan U, Bagul A, Nicholson ML. Pain management in laparoscopic donor nephrectomy: a review. Pain Res Treat 2012; 2012: 201852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gerbershagen HJ, Dagtekin O, Rothe T, Heidenreich A, Gerbershagen K, Sabatowski R et al. Risk factors for acute and chronic postoperative pain in patients with benign and malignant renal disease after nephrectomy. Eur J Pain 2009; 13: 853–860. [DOI] [PubMed] [Google Scholar]
- 31. Wolf JS Jr, Moon TD, Nakada SY. Hand assisted laparoscopic nephrectomy: comparison to standard laparoscopic nephrectomy. J Urol 1998; 160: 22–27. [PubMed] [Google Scholar]
- 32. Andersen MH, Mathisen L, Oyen O, Edwin B, Digernes R, Kvarstein G et al. Postoperative pain and convalescence in living kidney donors – laparoscopic versus open donor nephrectomy: a randomized study. Am J Transplant 2006; 6: 1438–1443. [DOI] [PubMed] [Google Scholar]
- 33. Raman JD, Bagrodia A, Cadeddu JA. Single‐incision, umbilical laparoscopic versus conventional laparoscopic nephrectomy: a comparison of perioperative outcomes and short‐term measures of convalescence. Eur Urol 2009; 55: 1198–1206. [DOI] [PubMed] [Google Scholar]
- 34. Perry KT, Freedland SJ, Hu JC, Phelan MW, Kristo B, Gritsch AH et al. Quality of life, pain and return to normal activities following laparoscopic donor nephrectomy versus open mini‐incision donor nephrectomy. J Urol 2003; 169: 2018–2021. [DOI] [PubMed] [Google Scholar]
- 35. Thys K, Schwering KL, Siebelink M, Dobbels F, Borry P, Schotsmans P et al.; ELPAT Pediatric Organ Donation and Transplantation Working Group. Psychosocial impact of pediatric living‐donor kidney and liver transplantation on recipients, donors, and the family: a systematic review. Transpl Int 2015; 28: 270–280. [DOI] [PubMed] [Google Scholar]
- 36. Ralph AF, Butow P, Hanson CS, Chadban SJ, Chapman JR, Craig JC et al. Donor and recipient views on their relationship in living kidney donation: thematic synthesis of qualitative studies. Am J Kidney Dis 2017; 69: 602–616. [DOI] [PubMed] [Google Scholar]
- 37. Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain 2011; 152(Suppl): S2–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Katz J, Seltzer Z. Transition from acute to chronic postsurgical pain: risk factors and protective factors. Expert Rev Neurother 2009; 9: 723–744. [DOI] [PubMed] [Google Scholar]
- 39. Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006; 367: 1618–1625. [DOI] [PubMed] [Google Scholar]
- 40. Hruschak V, Cochran G. Psychosocial predictors in the transition from acute to chronic pain: a systematic review. Psychol Health Med 2018; 23: 1151–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bornemann‐Cimenti H, Lederer AJ, Wejbora M, Michaeli K, Kern‐Pirsch C, Archan S et al. Preoperative pregabalin administration significantly reduces postoperative opioid consumption and mechanical hyperalgesia after transperitoneal nephrectomy. Br J Anaesth 2012; 108: 845–849. [DOI] [PubMed] [Google Scholar]
- 42. Nasrallah G, Souki FG. Perianesthetic management of laparoscopic kidney surgery. Curr Urol Rep 2018; 19(1): 1. [DOI] [PubMed] [Google Scholar]
- 43. Özdemir‐van Brunschot DMD, Scheffer GJ, van der Jagt M, Langenhuijsen H, Dahan A, Mulder JEEA et al. Quality of recovery after low‐pressure laparoscopic donor nephrectomy facilitated by deep neuromuscular blockade: a randomized controlled study. World J Surg 2017; 41: 2950–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hosgood SA, Thiyagarajan UM, Nicholson HF, Jeyapalan I, Nicholson ML. Randomized clinical trial of transversus abdominis plane block versus placebo control in live‐donor nephrectomy. Transplantation 2012; 94: 520–525. [DOI] [PubMed] [Google Scholar]
- 45. Heniford BT, Walters AL, Lincourt AE, Novitsky YW, Hope WW, Kercher KW. Comparison of generic versus specific quality‐of‐life scales for mesh hernia repairs. J Am Coll Surg 2008; 206: 638–644. [DOI] [PubMed] [Google Scholar]
- 46. Ruyssen‐Witrand A, Tubach F, Ravaud P. Systematic review reveals heterogeneity in definition of a clinically relevant difference in pain. J Clin Epidemiol 2011; 64: 463–470. [DOI] [PubMed] [Google Scholar]
- 47. Janki S, Klop KW, Dooper IM, Weimar W, Ijzermans JN, Kok NF. More than a decade after live donor nephrectomy: a prospective cohort study. Transpl Int 2015; 28: 1268–1275. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Modified Carolinas Comfort Scale
Table S1. Univariable analysis of variables associated with the different categories of the Carolinas Comfort Scale (CCS)
Table S2. Opiate prescription at discharge after nephrectomy


