Abstract
Background
Opioid‐primed relapse is a global burden. Although current strategies have improved, optimal therapy is urgently needed.
Methods
A recombinant adenovirus (Ad‐NEP) expressing β‐endorphin (β‐EP) was designed and injected intracerebroventricularly (icv) into the right lateral ventricle in rats. Spatial and temporal β‐EP expression in the lateral ventricle wall, subventricular zone and adjacent choroid plexus and the β‐EP concentration in the cerebrospinal fluid (CSF) were observed during a 21‐day period. A morphine priming‐induced conditioned place preference (CPP) rat model was established. The β‐EP‐ir neuron counts, CSF β‐EP concentration, and CPP score, which were used to evaluate morphine‐primed reinstatement following extinction, were recorded 7 days after the icv injection. Additionally, the rats were pretreated with the irreversible μ opioid receptor antagonist β‐funaltrexamine (β‐FNA) and the selective κ opioid receptor antagonist nor‐binaltorphimine (nor‐BNI) to identify the receptor‐dependent mechanism.
Results
Both peak β‐EP expression in target neurons and the peak CSF β‐EP concentration occurred 7 to 8 days after Ad‐NEP icv injection. The sustainable increase in the CSF β‐EP concentration was correlated with a decrease in the CPP score 7 days after the Ad‐NEP icv injection. Furthermore, reinstatement was almost reversed by β‐FNA pretreatment 24 hours before the behavioral test, but nor‐BNI had little effect.
Conclusion
The increasing cerebrospinal fluid β‐endorphin concentrations showed that the therapeutic effect on opioid relapse occurred predominantly through a μ opioid receptor‐dependent mechanism. The Ad‐NEP adenovirus can be considered an alternative therapy for opioid relapse.
Keywords: adenovirus, β‐endorphin, opioid addiction, reinstatement, relapse
Highlights
The sustainable increase β‐EP in the CSF was induced by the recombinant adenovirus expressing β‐EP via icv. injection.
Morphine‐induced reinstatement behavior can be reversed by the increase β‐EP in CSF.
The therapeutic effect of β‐EP on opioid relapse was reversed by μ opioid receptor antagonist β‐FNA pretreatment.
1. INTRODUCTION
Ordinarily, typical opioid addiction and new psychoactive substance abuse lead to a chronic and relapsing disease that is characterized by a strong desire for opioid usage, increased tolerance, and withdrawal syndrome. The latest global report 1, 2 showed that opioid and new psychoactive substance dependence was one of the most common types of illicit drug dependence, affecting more than 30 million adults aged 15 to 64 years globally. Opioid dependence is directly associated with an increase in major public health issues, including infectious diseases, lack of social duty, economic functioning, and drug‐related crimes.2, 3, 4 Continual relapse due to physical and psychological dependence 5, 6 is the primary cause of detoxification failure. Thus, exploring optimal therapies for opioid relapse is a worldwide challenge.7, 8 Although current strategies to prevent opioid relapse have improved through multidisciplinary endeavors, the relapse rate after opioid detoxification is still high and ranges from 72% to 88% after 12 to 36 months, as indicated by an epidemiological investigation.9
Prolonged neuroendocrine system dysfunctions in opioid addicts play a role in protracted withdrawal symptoms and contribute to relapse vulnerability. Evidence from both clinical trials and animal studies has revealed that endorphins (EPs), including β‐EP and its endogenous receptor, undergo a fundamental change in vivo during the opioid addiction process.10, 11 A previous observational study investigated the decrease in β‐EP in opioid addiction models, especially in the blood, spinal cord, and brain nuclei.12, 13 Our previous work and that of others confirmed that upregulation of exogenous β‐EP could attenuate withdrawal syndrome in opioid‐dependent rats.14, 15, 16, 17 Moreover, studies have shown that glycyl‐glutamine, DTgammaE, and DEgammaE, which are the active peptide and fragments derived from β‐EP, respectively, present favorable effects on attenuation of opioid withdrawal symptoms.11, 18 This evidence strongly indicates that β‐EP plays an important role in preventing opioid addiction. However, inducing a sustained and increased β‐EP level in vivo takes effort since the routes capable of increasing β‐EP require an exogenous injection. Inspiringly, our previous study successfully constructed an adenovirus vector (Ad‐NEP) that expressed exogenous β‐EP, and the preliminary observational study showed a sustainable increase in β‐EP in the cerebrospinal fluid (CSF),17 which might provide an innovative treatment method for physical dependence.19
Drug primed‐induced reinstatement in conditioned place preference (CPP) is the rapid reacquisition of an extinct behavior caused by the presentation of an unconditioned stimulus in response to certain drugs. The CPP is widely used to test the ability of certain drugs to inhibit different types of reinstatement behavior and to study relapse of drug‐seeking behavior and its intensity for estimation of psychological dependence.20, 21 To determine the feasibility of gene therapy with Ad‐NEP for the prevention of psychological dependence after physical dependence, the current study observed temporary β‐EP expression in the lateral ventricle wall, subventricular zone and adjacent choroid plexus and the CSF β‐EP concentration during a 21‐day period after intracerebroventricular (icv) injection of a recombinant adenovirus (Ad‐NEP) expressing β‐EP. Thereafter, we demonstrated the therapeutic effect of Ad‐NEP for preventing relapses in a morphine priming‐induced CPP rat model. Finally, the potential receptor‐dependent mechanism was identified by pretreating the rats with the irreversible μ opioid receptor antagonist β‐funaltrexamine (β‐FNA) and selective κ opioid receptor antagonist nor‐binaltorphimine (nor‐BNI) 24 hours before the behavioral test. Taken together, our results demonstrate a previously undefined role for increasing β‐EP with gene therapy and preventing morphine‐primed relapse behavior predominantly in a μ opioid receptor‐dependent manner.
2. MATERIALS AND METHODS
2.1. Animals
Male Sprague‐Dawley rats (Shanghai Experimental Animal Center, China) weighing 250 to 300 g were housed in groups of four in polypropylene cages with a 12 hours light/dark cycle and water and food provided ad libitum. The room and cage conditions were monitored twice daily. Before the experiment, the rats were allowed to adapt to the environment and were acclimated to handling for 3 days. Monitoring for health problems was performed three times per day. All animal experiments were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The protocols were approved by the Shanghai Animal Care and Use Committee.
2.2. Propagation and purification of adenoviral vectors
As described previously,17 the adenoviral vectors were propagated in HEK293 cells and purified by double cesium chloride gradient centrifugation. An empty E1/E3 deletion type 5 adenovirus (Ad‐Null) stored in our lab was used as the control. The final titers of Ad‐NEP and Ad‐Null were determined as plaque‐forming units (PFUs) and adjusted to 5 × 1010 PFUs.
2.3. CPP model
Apparatus: The CPP was performed in an apparatus (JL Behv‐CPPG, Shanghai Jiliang Software Technology Co Ltd, Shanghai, China) consisting of two compartments with equal sizes (30 cm × 30 cm × 40 cm) connected by a cuboid corridor (7 cm × 7 cm × 10 cm) with a 7 cm × 7 cm sluice gate in the center. The two compartments were different colors (black or white) inside and had two types of floor textures (mesh or grid). The time spent on each side and the numbers of shuttling events between the compartments during these periods were recorded by video and analyzed with the DigBehv‐CPP Video Analysis System (Shanghai Jiliang Software Technology Co Ltd). The time spent in the drug‐paired compartment was recorded as the CPP score.
CPP procedure schedule and phases: The whole CPP procedure schedule lasted 30 days, including four CPP tests for gained CPP scores in four successive phases (preconditioning, acquisition, extinction, and reinstatement). Then, four CPP scores were gained during the four successive phases.
Preconditioning phase: This procedure was similar to a previously described procedure.22, 23 Before morphine administration for conditional training, the rats were placed into compartments and allowed to move freely for at least 30 minutes for habituation before the experiment. Then, their baseline preferences for the white or black compartment were determined over a 15‐minute period. Rats that presented initial bias behavior by spending ≥70% of the total test time in each compartment were removed from the subsequent experiment.
Conditioning phase: The conditioning phase closely followed the preconditioning phase and lasted 8 days. In this phase, the rats were treated daily for 8 days with a consecutive schedule with four alternate cycles of subcutaneous morphine injection (10 mg/kg) in the white compartment (drug‐paired compartment) and normal saline injection (same volume as morphine, as a placebo) in the black compartment. Rats receiving morphine were confined in the white compartment and those receiving saline were confined in the black compartment for 45 minutes after the injection. After the conditioning phase, tests were performed to obtain the acquisition scores. The rats were allowed to access both compartments freely for 15 minutes, and the time the rats lingered in the white compartment was recorded as the acquisition score.
Extinction phase: The extinction phase was different due to individual variation. During the phase, all rats received a normal saline injection without reinforcement by morphine in both compartments and training for 45 minutes with the center door closed. After daily training, the rats were allowed to access both compartments freely for 15 minutes, and the time in the white compartment was recorded as the extinction score. If the extinction scores returned to baseline for three consecutive days, we considered the CPP behavior successfully extinct. The 3‐day average score was used as the extinction scores for the individuals who received brain stereotaxic surgery for icv injection the next day.
Reinstatement phase: The time following the extinction phase was the reinstatement phase. After stereotaxic surgery for icv injection, morphine (inefficient dose, 2 mg/kg) was administered for priming‐induced reinstatement CPP. After priming‐inducing, the rats were placed in the apparatus with the central gate open for 15 minutes, and the time spent in the white compartment was recorded as the reinstatement score.
2.4. Groups and intervention
Experiment 1: To observe spatial and temporal β‐EP expression in target neurons and the β‐EP concentration in the CSF during a 21‐day period after Ad‐NEP icv injection. All eligible rats were divided randomly into the following three groups: Sham group (n = 39, icv normal saline (NS) with stereotaxic surgery); Ad‐Null group (n = 39, icv Ad‐Null); and Ad‐NEP group (n = 39, icv Ad‐NEP). CSF was collected from three rats per group at each given time point guided by ultrasound for detection of the β‐EP concentration. Then, the rats were killed to measure temporary β‐EP expression in the target neurons before and on days 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 14, and 21 after Ad‐NEP icv injection by immunohistochemical staining.
Experiment 2: To observe the therapeutic effect of Ad‐NEP on prevention of morphine‐primed relapse in CPP rats. All eligible rats were divided randomly into the following four groups: Sham group (n = 8, no administration and received only stereotaxic surgery), NS group (n = 8, morphine + NS), Ad‐Null group (n = 8, morphine + Ad‐Null), and Ad‐NEP group (n = 8, morphine + Ad‐NEP).
The Sham group was not administered morphine or NS and received only stereotaxic surgery but underwent the complete CPP procedure. The other three groups received morphine administration (10 mg/kg, once a day, tertian) during the conditioning phase and then were placed into the drug‐paired white compartment for 45 minutes according to the schedule. The Ad‐Null and Ad‐NEP groups were administered Ad‐Null and Ad‐NEP (5 × 1010 PFU; 10 μL, for at least 10 minutes), respectively, through icv injection after CPP extinction was obtained, and the NS group was administered normal saline with the same volume. During the reinstatement phase, all groups (except the Sham group) received a subcutaneous morphine injection (inefficient dose, 2 mg/kg) to ignite priming‐induced reinstatement CPP.
Experiment 3: To explore the potential receptor‐dependent mechanism. All eligible rats were divided randomly into the following six groups: Ad‐Null group (n = 8, NS + morphine + Ad‐Null), Ad‐NEP group (n = 8, NS + morphine + Ad‐NEP), β‐FNA + Ad‐Null group (n = 8, β‐FNA + morphine + Ad‐Null), β‐FNA + Ad‐NEP group (n = 8, β‐FNA + morphine + Ad‐NEP), nor‐BNI + Ad‐Null group (n = 8, nor‐BNI A + morphine + Ad‐Null), and nor‐BNI + Ad‐NEP group (n = 8, nor‐BNI + morphine + Ad‐NEP).
The CPP rat model was established following the protocol described in Experiment 2. The rats were pretreated with β‐FNA and nor‐BNI by icv injection 24 hours before the reinstatement behavioral test in the related groups.
2.5. The icv injections
All groups underwent stereotaxic surgery after the extinction phase. After anesthetization with pentobarbital (50 mg/kg, ip, Sigma, St. Louis, MO), the rats were fixed in a stereotaxic frame (Model 51600; Stoelting Co., Wood Dale, IL). As shown in Figure 2, a stainless steel injection cannula (28G) was inserted into the right lateral ventricle (coordinates, 1.5 mm lateral to the midline, − 0.8 mm posterior to the bregma, and −4.6 mm ventral to the skull surface).24 Then, a single icv dose of adenovirus or normal saline was administered (volume, 10 μL; rate, 0.5 μL/minute) using a microinfusion pump (Bioanalytical Systems, Inc., West Lafayette, IN) and a 10‐μL microsyringe (Hamilton, Bonaduz, Switzerland). The cannula was left in place for 5 minutes after the injection was finished and pulled out intermittently. A new cannula filled with saline implants was secured and affixed with TitanBond and dental cement thereafter. The rats were given postoperative care for 7 days. β‐FNA (CAS 72786‐10‐8; Santa Cruz, CA), which is a κ opioid receptor agonist and an irreversible μ opioid receptor antagonist (10 mg/kg diluted in 5 μL of sterile saline) was icv injected via cannula 24 hours before the behavioral test under isoflurane anesthesia in the β‐FNA + Ad‐Null and β‐FNA + Ad‐NEP groups.25, 26 Additionally, nor‐BNI (CAS 105618‐26‐6; Abcam, Cambridge, UK), which is a selective κ opioid receptor antagonist (5 mg/kg diluted in 5 μL of sterile saline) was icv injected via cannula 24 hours before the behavioral test under isoflurane anesthesia in the nor‐BNI + Ad‐Null and nor‐BNI + Ad‐NEP groups.27, 28 At the end of the experiments, the rats were administered an icv injection of 5% Evans Blue (5 μL) and then killed by cervical dislocation for confirmation of the cannula location. The behavioral result from that particular animal was excluded if the cannula was incorrectly positioned.
Figure 2.
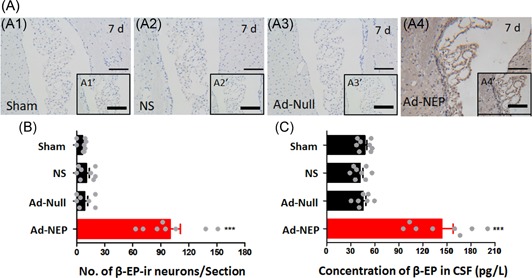
The peak of β‐EP expression in targeted neurons and the CSF β‐EP concentration after Ad‐NEP icv injection in morphine‐primed relapse rats. (A) β‐EP‐ir neurons in the lateral ventricle wall, subventricular zone and adjacent choroid plexus in the Sham (A1), NS (A2), Ad‐Null (A3), and Ad‐NEP (A4) groups on day 7 after icv injection. (B) Statistical analysis of the number of β‐EP‐ir neurons on day 7 after icv injection in all groups. (C) Statistical analysis of the β‐EP concentration in the CSF on day 7 after icv injection in all groups. ***Compared with the Sham group; P < 0.001. Ad‐NEP, adenovirus vector; β‐EP, β‐endorphin; CSF, cerebrospinal fluid; icv, intracerebroventricular
2.6. Immunohistochemistry (IHC)
Paraffin‐embedded rat brain tissue was cut into 4‐μm‐thick sections. IHC was performed according to the protocol provided by the manufacturer. Briefly, the sections were dewaxed in xylene and rehydrated using a gradient ethanol solution. The endogenous peroxidase activity was blocked with 3% H2O2 for 20 minutes. Then, antigens were recovered with Na‐Citrate buffer (10 mM; pH 6.5) for 15 minutes in the microwave. After incubation with 10% fetal bovine serum for 30 minutes, the primary rabbit antiendorphin antibody (1:500 dilution; Phoenix Pharmaceuticals, Belmont, CA) was added to the slides and incubated overnight at 4°C, followed by a biotinylated secondary antibody (Maxin Biotechnology Inc, Fujian, China). The immunoreaction was visualized with the chromogen diaminobenzidine (Maxin Biotechnology Inc). Before the IHC results were quantitatively analyzed, the slides were scanned using the ScanScope system (Aperio, Vista, CA). Thereafter, the numbers of β‐EP‐ir neurons in the IHC photographs were counted from 3 to 4 sections. Only neurons with crossing nuclei were selected for counting using the free NIH software “ImageJ” (2x V2.1.).
2.7. β‐EP concentration in the CSF
Ultrasound‐guided CSF collection was performed on day 7 after icv injection as described previously.29 The samples were assayed using an RIA kit (RK‐022‐33; Phoenix Pharmaceuticals, Inc, Phoenix, AZ) according to the manufacturer’s instructions. Briefly, the antiserum (rabbit anti‐β‐EP) was added to the assay tubes and incubated with CSF or standard samples for 24 hours at 4°C. The 125I‐peptide (10 000 cpm/100 μL) and radioimmunoassay (RIA) buffer were mixed well to make the tracer solution, which was added to each tube (100 μL). All tubes were incubated for 24 hours at 4°C. Goat anti‐rabbit IgG and normal goat sera were added successively. All tubes were incubated at room temperature for 90 minutes. Finally, RIA buffer (500 μL) was added, and each tube was centrifuged (3000 rpm × 20 minutes at 4°C). The supernatants were aspirated. A γ‐counter (SN‐695, Shanghai Hesuo Rihuan Photoelectric Instrument Co, Ltd, Shanghai, China) was applied to count the cpm of the pellets containing the radioligand fraction.
2.8. Statistical analysis
The data are presented as the mean ± standard error of the mean (SEM). The data were processed using the commercially available software GraphPad Prism version 8.0 for Windows (Graph Pad Software, San Diego, CA; www.graphpad.com). An independent sample/paired t test or repeated measures/block randomized one‐way analysis of variance (ANOVA) followed by post hoc analysis (Dunnett’s or Newman‐Keuls’ test) was used to compare the CPP scores obtained from two or more control and experimental groups. P values less than 0.05 (P < 0.05) were considered statistically significant differences.
3. RESULTS
3.1. The icv injection of AD‐NEP induced sustained β‐EP expression and increased β‐EP in the rat CSF
To examine spatial and temporal β‐EP expression in target neurons and the β‐EP concentration in the CSF, a 21‐day observational study was conducted after Ad‐NEP icv injection. The lateral ventricle wall, subventricular zone and adjacent choroid plexus were the observed target domains (Figure 1A).17 In the Ad‐NEP group, β‐EP‐ir neurons (brown) were obviously observed in the target domains (indicated by red arrows), especially on day 7 after icv injection, whereas very little β‐EP was detected in the other groups (Figure 1C). These results illustrated that Ad‐NEP was transfected into epithelial cells and expressed β‐EP successfully in the Ad‐NEP group but not in the other three groups. Concurrently, when β‐EP expression in the target neurons reached its peak, the CSF β‐EP concentration in the Ad‐NEP group was significantly higher than that in the other three groups on day 7 after the icv, Ad‐NEP injection (P < 0.01, one‐way ANOVA) (Figure 1D and 1E).
Figure 1.
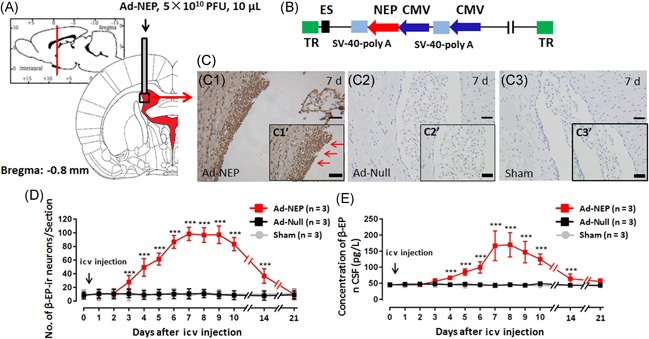
Spatial and temporal β‐EP expression in the targeted neurons and the CSF β‐EP concentration after Ad‐NEP icv injection. (A) Cartoon of icv injection. (B) Cartoon of the Ad‐NEP structure. (C) β‐EP‐ir neurons in the lateral ventricle wall, subventricular zone and adjacent choroid plexus in the Ad‐NEP (C1), Ad‐Null (C2), and Sham (C3) groups on day 7 after icv injection. (D) Statistical analysis of the number of β‐EP‐ir neurons for 21 consecutive days after the icv injection. (E) Statistical analysis of the CSF β‐EP concentration for 21 consecutive days after the icv injection. ***Compared with the Sham group, P < 0.001. Ad‐NEP, adenovirus vector; β‐EP, β‐endorphin; CSF, cerebrospinal fluid; icv, intracerebroventricular
3.2. Therapeutic effect of increasing CSF β‐EP in morphine priming‐induced CPP rats
To demonstrate the therapeutic effect of increasing β‐EP in the CSF for the prevention of relapses in morphine priming‐induced CPP rats, we focused on the peak β‐EP expression on day 7 after the icv Ad‐NEP injection. Both the IHC and RIA results showed significantly higher β‐EP‐ir‐expressing neurons in the lateral ventricle wall, subventricular zone and adjacent choroid plexus as well as an increased β‐EP concentration in the CSF in the Ad‐NEP group than in the Sham, NS, and Ad‐Null groups (Figure 2A, 2B, and 2C; P < 0.01).
Morphine prime‐induced reinstatement behavior was confirmed by the CPP test on day 7 after the icv Ad‐NEP injection. During postoperative care, one rat in the Ad‐Null group died from an intracranial infection. The protocol used for the morphine‐primed relapse in the rats is presented in Figure 3A. The CPP procedure is presented in Figure 3B and 3C. No significant differences were observed in the CPP scores in all groups during the preconditioning phase (Figure 3D1; P > 0.05), indicating that rats with natural CPP were used in this study. During the conditioning phase, the CPP scores were significantly higher in the NS, Ad‐Null, and Ad‐NEP groups than in the Sham group (Figure 3D2; P < 0.05), indicating that the morphine addiction rat model was successfully constructed. However, during the extinction phase, the CPP scores in all groups returned to baseline (Figure 3D3; P > 0.05). Furthermore, an inefficient dose of morphine could induce seeking behavior indicated by significantly increased CPP scores in both the NS and Ad‐Null groups compared to those of the Ad‐NEP group (Figure 3D4; P < 0.05). Furthermore, no significant differences were observed in the total distance and shuttle times among the groups, indicating similar locomotion ability (Figure 3E1 and 3E2; P > 0.05).
Figure 3.
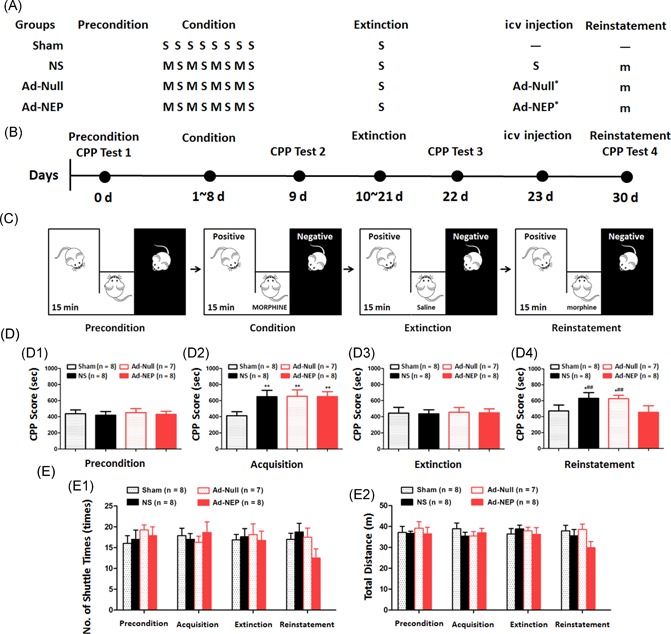
Effect of an increased CSF β‐EP concentration in the morphine‐primed relapse rats. (A) The protocol for morphine‐primed relapse in the rats. (B) The time schedule of the CPP behavioral test in each group. (C) Cartoon of the CPP behavioral test. (D) Statistical analysis of the CPP scores during the preconditioning phase (C1), conditioning phase (C2), the extinction phase (C3), and reinstatement phase (C4). E: Statistical analysis of the locomotion ability indicated by the number of shuttle times (E1) and the total distance (E2). S, saline; M, morphine 10 mg/kg; m, morphine 2 mg/kg; Priming: morphine priming‐induced reinstatement; Ad‐Null *empty type 5 adenovirus with E1/E3 deletion; Ad‐NEP *adenoviral vector expressing β‐EP. *Compared with the Sham group, P < 0.05; **Compared with the Sham group, P < 0.01; ##Compared with the Ad‐NEP group, P < 0.01. Ad‐NEP, adenovirus vector; β‐EP, β‐endorphin; CPP, conditioned place preference; icv, intracerebroventricular
3.3. Increasing β‐EP in the CSF alleviates morphine‐primed relapse behavior through the μ opioid receptor
To identify the specific receptor‐dependent mechanism, we applied β‐FNA, which is a κ opioid receptor agonist and an irreversible μ opioid receptor antagonist, and nor‐BNI, which is a selective κ opioid receptor antagonist. The morphine‐primed relapse protocol in the rats is presented in Figure 4A. The CPP procedure and the β‐FNA and nor‐BNI pretreatment protocols are presented in Figure 4B and 4C. Our results showed a significant reversal in the CPP scores in the β‐FNA + Ad‐NEP group compared with those of the Ad‐NEP group (Figure 4D4; P < 0.05) in the successful morphine addiction rat model (Figure 4D1‐3) with similar locomotion ability (Figure 4E1 and 4E2; P > 0.05). However, little reversal of the effect was observed in the nor‐BNI pretreated rats (Figure 4D4; P < 0.05). Taken together, our results demonstrated the therapeutic effect of increasing β‐EP in the CSF with gene therapy for the prevention of morphine‐primed relapse behavior, which occurred predominantly through a μ opioid receptor‐dependent mechanism.
Figure 4.
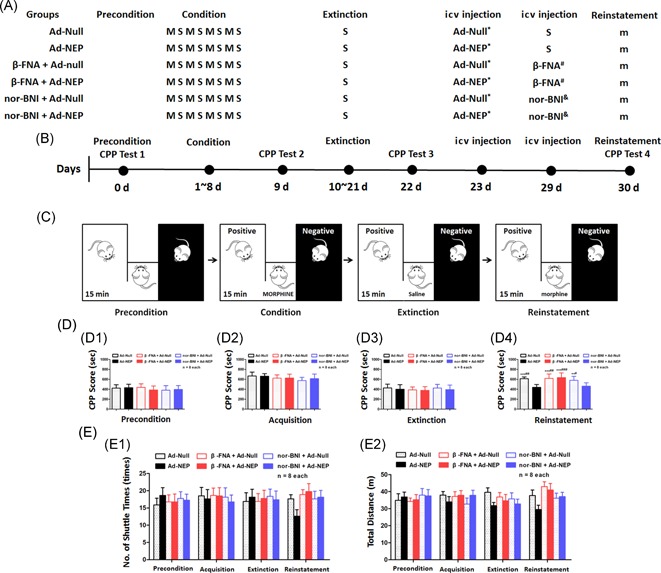
The role of μ and κ opioid receptors in the therapeutic effect of Ad‐NEP icv injection in morphine‐primed relapsed rats. (A) The protocol for morphine‐primed relapse in the rats. (B) The time schedule of the CPP behavioral test in each group. (C) Cartoon of the CPP behavioral test. (D) Statistical analysis of the CPP scores during the preconditioning phase (C1), conditioning phase (C2), the extinction phase (C3), and reinstatement phase (C4). (E) Statistical analysis of the locomotion ability indicated by the number of shuttle times (E1) and the total distance (E2). S, saline; M, morphine 10 mg/kg; m, morphine 2 mg/kg; Priming, morphine priming‐induced reinstatement; Ad‐Null *empty type 5 adenovirus with E1/E3 deletion; Ad‐NEP *adenoviral vector expressing β‐EP; β‐FNA #β‐FNA pretreatment via the icv route 24 hours before the CPP test 4; nor‐BNI &nor‐BNI pretreatment via the icv route 24 hours before the CPP test 4.*Compared with the Sham group, P < 0.05; **Compared with the Ad‐NEP group, P < 0.01; ***Compared with the Ad‐NEP group, P < 0.001; #Compared with the nor‐BNI + Ad‐NEP group, P < 0.01; ##Compared with the nor‐BNI + Ad‐NEP group, P < 0.01; ###Compared with the nor‐BNI + Ad‐NEP group, P < 0.001. Ad‐NEP, adenovirus vector; β‐EP, β‐endorphin; β‐FNA, β‐funaltrexamine; CPP, conditioned place preference; icv, intracerebroventricular; nor‐BNI, nor‐binaltorphimine
4. DISCUSSION
In the current study, the recombinant virus Ad‐NEP was introduced into the central nervous system via stereotaxic surgery, resulting in a sustainable increase in β‐EP in the CSF by transfecting epithelial cells of the lateral ventricles. Concurrent with the increase in β‐EP in the CSF, morphine priming‐induced reinstatement behavior was obviously attenuated. Furthermore, the rescue behavior was reversed by icv injection of the irreversible μ opioid receptor antagonist β‐FNA but not the κ opioid antagonist nor‐BNI 24 hours before the behavioral test. This evidence indicates that an increasing CSF β‐EP concentration plays a role in alleviating morphine relapse in a μ opioid receptor‐dependent manner, suggesting that the recombinant virus can be considered as an alternative approach for preventing opioid relapse.
Relapse treatment is still a source of controversy because it is regarded as treatment failure for drug addiction that causes an increasing public health burden. Many researchers have focused on finding novel methods to reduce drug relapse through multidisciplinary endeavors, such as pharmacotherapy, cognitive behavioral techniques, and contingency management.30 Previous studies have reported that pharmacotherapy, such as pioglitazone and levotetrahydropalmatine, can successfully reduce reinstatement of opioid‐seeking and opioid‐induced behavior. However, other studies have shown inadequate efficacy of current opioid dependence treatments, mostly due to failure in the prevention and/or treatment of relapse.31, 32, 33 Our study here and results reported elsewhere demonstrated a new genetic method based on β‐EP expression by the recombinant adenovirus Ad‐NEP, which alleviated morphine addiction and relapse behavioral symptoms during both acute morphine addiction 17 and in the morphine‐primed reinstatement rat model. This approach represents a potentially efficacious method for both detoxification and prevention of opioid addiction relapse.
Previous studies have shown that some special neurons that sequentially project from the ventral tegmental area into the nucleus accumbens and then into the mesolimbic reward system (consisting mainly of the prefrontal cortex, anterior cingulate cortex, hippocampus, and amygdala) 34, 35 play crucial roles in opioid addiction and relapse.36 Activation of µ opioid receptors, which are distributed broadly in these neurons, is critical for opioid reinforcement, and activation of opioid receptors by opioid priming is a trigger of relapse. Moreover, some studies have confirmed that the pathophysiology of opioid addiction can be altered by up‐ or downregulation of endogenous opioids. The Sarah team demonstrated a strong negative correlation between plasma β‐EP and reinstatement.37 Our results further showed specific β‐EP‐positive cells located in the lateral ventricle wall and paraplexus. Similar results from our previous work showed that ependymal cells transfected with the recombinant adenovirus Ad‐NEP lined the lateral ventricle after icv injection.38 Thereafter, the transfected cells would sustainably secrete β‐EP into the CSF and activate μ opioid receptors.
To identify the specific receptor‐dependent mechanism, the rats were pretreated with the κ opioid receptor agonist and irreversible μ opioid receptor antagonist β‐FNA and the selective κ opioid receptor antagonist nor‐BNI. A previous study focused on the pharmacological characteristics of β‐FNA showed that the κ opioid agonist property was maintained for approximate 4 to 6 hours after icv β‐FNA injection, whereas the irreversible μ opioid receptor antagonism property lasted for more than 24 to 72 hours.39 Therefore, the morphine‐primed relapse behavior was examined 24 hours after the β‐FNA icv injection to exclude the possible influence of the κ opioid agonist. Additionally, the selective κ opioid receptor antagonist nor‐BNI was applied to evaluate the possible role of the κ opioid receptor in morphine‐primed relapse behavior. Since our previous study showed that nor‐BNI was a slow‐onset, long‐lasting, selective κ antagonist in vivo, we pretreated the rats with nor‐BNI 24 hours before the behavioral test to reach the plateau of κ antagonism.27, 28 Significantly increased CPP scores were observed in the β‐FNA pretreated rats. Conversely, a limited reversed effect was observed in the nor‐BNI pretreated rats. Robust evidence from our data and other studies 16 showed that μ, but not κ, opioid receptors played a key role in the alleviation of the effect of β‐EP on morphine‐primed reinstatement. Additionally, we analyzed the potential influence on impaired locomotion ability indicated by the number of shuttle times and total distance in the CPP test. Our results showed a slight but nonsignificant decrease in locomotion in the Ad‐NEP group, which might contribute to the sedative characteristic of μ opioid receptor activation.
The biological characteristic of β‐EP in specific brain regions, such as the lateral cerebral ventricle and nucleus arcuatus hypothalamus, has been confirmed to be involved in the addiction process.17 Wu et al40 found that reversal of acute morphine withdrawal syndrome behaviors contributed to a sustainable increase in endomorphin‐2 in the CSF by intrathecal injection of an adenovirus engineered to express the endomorphin‐2 gene. These results supported the hypothesis that opioid addiction and relapse could be improved by external secretion of the endogenous opioid peptide in targeted brain regions via CSF circulation. However, great efforts should still be undertaken to identify the specific brain regions and/or nuclei involved in this process.
The CPP induced by morphine as a conditioned stimulus is a stable and reliable behavior model for studies of drug‐seeking behavior and psychological dependence in rats.41 In the CPP model procedure, euphoria and rewarding effects that occur via a conditioned stimulus stir up psychological dependence. Due solely to this psychological dependence, reinstatement of CPP, which represents drug‐seeking behavior, arises in response to a priming‐induced, conditioned stimulus, context, relative cues or a stressor following natural extinction.42 In the current study, the time during which the rats lingered in the drug‐paired compartment was considered the CPP score and was used as an indicator to evaluate the intensity of drug‐seeking behavior and relapse.43 The decrease in the CPP score induced by Ad‐NEP represented the alleviation of reinstatement, which indicated that this peptide might have a therapeutic effect on psychological dependence.
The recombinant adenovirus Ad‐NEP applied in our study was a defective‐duplicated virus that possessed significant immunogenicity and a strong infection capability in mammalian cells. The immunogenicity of adenovirus can activate the innate immune system and ultimately clear the adenovirus. The infection characteristic for the nervous system makes adenovirus a useful gene therapy tool for neurological disorders.44 Many studies have revealed that adenovirus can be maintained in the nervous system for a certain period before activation of T lymphocytes.45 During this period, β‐EP in the CSF should reach its peak concentration and then decline gradually along with the elimination of the adenovirus according to our previous publication.17 Based on the strategy of substitutional and decremental methadone treatment,46 this gradual decline in the β‐EP concentration could be considered an advantage of gene therapy for relapse using an adenovirus. In this case, this delivery method for β‐EP could be a potential solution to manage opioid relapse. Other vehicles, such as a lentiviral system, need to be considered as a delivery tool for the nervous system due to their natural neurotropism. For future clinical practice, an inducible gene regulation system, such as a tetracycline regulating system, is required for precise regulation of the levels and timing of target gene expression, including β‐EP. A nanoparticle‐modified adenovirus system with better permeation will become our research focus in the future.
In summary, this study confirmed that β‐EP expressed by the recombinant adenovirus Ad‐NEP could alleviate reinstatement CPP after icv administration in rats in a μ opioid receptor‐dependent manner. An adenovirus carrying the β‐EP transgene may be used as a favorable solution to prevent opioid relapse.
ACKNOWLEDGMENTS
The authors appreciate the support with vector construction from Xuewu Xu, MD, PhD, at the Department of Anesthesiology, Hospital 306 of PLA, Beijing, China. This study is supported by the National Natural Science Foundation of China (No. 30901403) and the Natural Science Foundation of Shanghai, China (Grant No. 11ZR1449200).
He Y, Lu Y, Shen Y, et al. Transgenic increase in the β‐endorphin concentration in cerebrospinal fluid alleviates morphine‐primed relapse behavior through the μ opioid receptor in rats. J Med Virol. 2019;91:1158‐1167. 10.1002/jmv.25415
He, Lu, and Shen have contributed equally to this project.
Contributor Information
Yuming Sun, Email: 13301836930@163.com.
Weifeng Yu, Email: ywf808@sohu.com.
References
REFERENCES
- 1. Peacock A, Leung J, Larney S, et al. Global statistics on alcohol, tobacco and illicit drug use: 2017 status report. Addiction. 2018;113(10):1905‐1926. [DOI] [PubMed] [Google Scholar]
- 2. Degenhardt L, Peacock A, Colledge S, et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health. 2017;5(12):e1192‐e1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Manzoni P, Leonessa ML, Monetti C, Farina D, Gomirato G. Prevention strategies in patients at high‐risk for Candida infections: data from a neonatal intensive care setting. Intensive Care Med. 2006;32(6):936‐936. author reply 935 [DOI] [PubMed] [Google Scholar]
- 4. Miller MC. Is addiction hereditary? Harv Ment Health Lett. 2006;22(8):8. [PubMed] [Google Scholar]
- 5. Polunina AG, Davydov DM, Briun EA. [Psychic dependence in drug addiction: a role of the mesocorticolimbic dopaminergic system]. Zh Nevrol Psikhiatr Im SS Korsakova. 2007;107(2):70‐75. [PubMed] [Google Scholar]
- 6. Saika F, Kiguchi N, Kishioka S. [The role of CC‐chemokine ligand 2 in the development of psychic dependence on methamphetamine]. Nihon Arukoru Yakubutsu Igakkai Zasshi. 2015;50(5):189‐195. [PubMed] [Google Scholar]
- 7. Hirchak KA, Murphy SM. Assessing differences in the availability of opioid addiction therapy options: rural versus urban and american indian reservation versus nonreservation. J Rural Health. 2017;33(1):102‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wakeman SE, Rich JD. Barriers to post‐acute care for patients on opioid agonist therapy; an example of systematic stigmatization of addiction. J Gen Intern Med. 2017;32(1):17‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chalana H, Kundal T, Gupta V, Malhari AS. Predictors of relapse after inpatient opioid detoxification during 1‐year follow‐up. J Addict. 2016;2016:7620860‐7620867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shi J, Li S, Zhang X, et al. Time‐dependent neuroendocrine alterations and drug craving during the first month of abstinence in heroin addicts. Am J Drug Alcohol Abuse. 2009;35(5):267‐272. [DOI] [PubMed] [Google Scholar]
- 11. Capasso A. Beta‐endorphin fragments DTgammaE and DEgammaE reduced morphine inhibition of electrically‐induced contractions and opiate withdrawal. Med Chem. 2009;5(2):165‐170. [DOI] [PubMed] [Google Scholar]
- 12. Niikura K, Narita M, Narita M, et al. Direct evidence for the involvement of endogenous beta‐endorphin in the suppression of the morphine‐induced rewarding effect under a neuropathic pain‐like state. Neurosci Lett. 2008;435(3):257‐262. [DOI] [PubMed] [Google Scholar]
- 13. Gudehithlu KP, Tehwani GA, Bhargava HN. Beta‐endorphin and methionine‐enkephalin levels in discrete brain regions, spinal cord, pituitary gland and plasma of morphine tolerant‐dependent and abstinent rats. Brain Res. 1991;553(2):284‐290. [DOI] [PubMed] [Google Scholar]
- 14. Smyth DG. 60 Years of POMC: lipotropin and beta‐endorphin: a perspective. J Mol Endocrinol. 2016;56(4):T13‐T25. [DOI] [PubMed] [Google Scholar]
- 15. Fell GL, Robinson KC, Mao J, Woolf CJ, Fisher DE. Skin beta‐endorphin mediates addiction to UV light. Cell. 2014;157(7):1527‐1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bond C, LaForge KS, Tian M, et al. Single‐nucleotide polymorphism in the human mu opioid receptor gene alters beta‐endorphin binding and activity: possible implications for opiate addiction. Proc Natl Acad Sci USA. 1998;95(16):9608‐9613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. He Y, Wu FX, Miao XR, et al. Suppression of acute morphine withdrawal syndrome by adenovirus‐mediated beta‐endorphin in rats. Brain Res. 2011;1422:13‐19. [DOI] [PubMed] [Google Scholar]
- 18. Cavun S, Goktalay G, Millington WR. Glycyl‐glutamine, an endogenous beta‐endorphin‐derived peptide, inhibits morphine‐induced conditioned place preference, tolerance, dependence, and withdrawal. J Pharmacol Exp Ther. 2005;315(2):949‐958. [DOI] [PubMed] [Google Scholar]
- 19. Beine A, de Timary P, Hermans E. [Neurobiology and psychology of addictions]. J Pharm Belg. 2006;61(1):15‐25. [PubMed] [Google Scholar]
- 20. Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168(1‐2):3‐20. [DOI] [PubMed] [Google Scholar]
- 21. Mueller D, Stewart J. Cocaine‐induced conditioned place preference: reinstatement by priming injections of cocaine after extinction. Behav Brain Res. 2000;115(1):39‐47. [DOI] [PubMed] [Google Scholar]
- 22. Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56(6):613‐672. [DOI] [PubMed] [Google Scholar]
- 23. Valjent E, Corbille AG, Bertran‐Gonzalez J, Herve D, Girault JA. Inhibition of ERK pathway or protein synthesis during reexposure to drugs of abuse erases previously learned place preference. Proc Natl Acad Sci USA. 2006;103(8):2932‐2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Paxinos G, Watson C, Pennisi M, Topple A. Bregma, lambda and the interaural midpoint in stereotaxic surgery with rats of different sex, strain and weight. J Neurosci Methods. 1985;13(2):139‐143. [DOI] [PubMed] [Google Scholar]
- 25. Rezende RM, Paiva‐Lima P, Dos Reis WGP, et al. Endogenous opioid and cannabinoid mechanisms are involved in the analgesic effects of celecoxib in the central nervous system. Pharmacology. 2012;89(3‐4):127‐136. [DOI] [PubMed] [Google Scholar]
- 26. Morales‐Mulia M, Estrada‐Camarena E, Amaya MI, et al. Anxiolytic effects of ethanol are partially related to a reduced expression of adenylyl cyclase 5 but not to mu‐opioid receptor activation in rat nucleus accumbens. Behav Brain Res. 2012;235(2):189‐194. [DOI] [PubMed] [Google Scholar]
- 27. Takemori AE, Ho BY, Naeseth JS, Portoghese PS. a highly selective kappa‐opioid antagonist in analgesic and receptor binding assays. J Pharmacol Exp Ther. 1988;246(1):255‐258. [PubMed] [Google Scholar]
- 28. Endoh T, Matsuura H, Tanaka C, Nagase H. Nor‐binaltorphimine: a potent and selective kappa‐opioid receptor antagonist with long‐lasting activity in vivo. Arch Int Pharmacodyn Ther. 1992;316:30‐42. [PubMed] [Google Scholar]
- 29. Lu Y, Wei W, Wang L, et al. Ultrasound‐guided cerebrospinal fluid collection from rats. J Neurosci Methods. 2013;215(2):218‐223. [DOI] [PubMed] [Google Scholar]
- 30. Crombag HS, Bossert JM, Koya E, Shaham Y. Review. Context‐induced relapse to drug seeking: a review. Philos Trans R Soc London Ser B. 2008;363(1507):3233‐3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ramli M, Zafri AB, Junid MR, Hatta S. Associated risk factors to non‐compliance to methadone maintenance therapy. Med J Malaysia. 2012;67(6):560‐564. [PubMed] [Google Scholar]
- 32. Sharma V, Chamroonswasdi K, Srisorrachatr S. Rate of adherence to and factors associated with methadone maintenance treatment program (Mmtp) compliance among injecting drug use patients in Nepal. Southeast Asian J Trop Med Public Health. 2016;47(2):287‐298. [PubMed] [Google Scholar]
- 33. Bradshaw SD, Shumway ST, Dsauza CM, Morris N, Hayes ND. Hope, coping skills, and the prefrontal cortex in alcohol use disorder recovery. Am J Drug Alcohol Abuse. 2017;43(5):591‐601. [DOI] [PubMed] [Google Scholar]
- 34. Arjmand S, Behzadi M, Stephens GJ, et al. A brain on a roller coaster: can the dopamine reward system act as a protagonist to subdue the ups and downs of bipolar disorder? Neuroscientist. 2018;24(5):423‐439. [DOI] [PubMed] [Google Scholar]
- 35. Colasanti A, Searle GE, Long CJ, et al. Endogenous opioid release in the human brain reward system induced by acute amphetamine administration. Biol Psychiatry. 2012;72(5):371‐377. [DOI] [PubMed] [Google Scholar]
- 36. Dichiara G, Bassareo V. Reward system and addiction: what dopamine does and doesn't do. Curr Opin Pharmacol. 2007;7(1):69‐76. [DOI] [PubMed] [Google Scholar]
- 37. Sushchyk S, Xi ZX, Wang JB. Combination of levo‐tetrahydropalmatine and low dose naltrexone: a promising treatment for prevention of cocaine relapse. J Pharmacol Exp Ther. 2016;357(2):248‐257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xu X, Pei S, Miao X, Yu W. Human signal peptide had advantage over mouse in secretory expression. Histochem Cell Biol. 2009;132(2):239‐246. [DOI] [PubMed] [Google Scholar]
- 39. Qi JA, Heyman JS, Sheldon RJ, Koslo RJ, Porreca F. Mu antagonist and kappa agonist properties of beta‐funaltrexamine (beta‐FNA) in vivo: long‐lasting spinal analgesia in mice. J Pharmacol Exp Ther. 1990;252(3):1006‐1011. [PubMed] [Google Scholar]
- 40. Wu F, He Y, Di H, et al. An engineered endomorphin‐2 gene for morphine withdrawal syndrome. PLOS One. 2016;11(3):e0149877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gupta R, Prakash V. CPP‐ACP complex as a new adjunctive agent for remineralisation: a review. Oral Health Prev Dent. 2011;9(2):151‐165. [PubMed] [Google Scholar]
- 42. McCabe SE, West BT. The 3‐year course of multiple substance use disorders in the United States: a national longitudinal study. J Clin Psychiatry. 2017;78(5):e537‐e544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schmidt KT, Schroeder JP, Foster SL, et al. Norepinephrine regulates cocaine‐primed reinstatement via alpha1‐adrenergic receptors in the medial prefrontal cortex. Neuropharmacology. 2017;119:134‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kamran N, Candolfi M, Baker GJ, et al. Gene therapy for the treatment of neurological disorders: central nervous system neoplasms. Methods Mol Biol. 2016;1382:467‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Riß GL, Chang DI, Wevers C, et al. Opioid maintenance therapy restores CD4 + T cell function by normalizing CD4 + CD25(high) regulatory T cell frequencies in heroin user. Brain Behav Immun. 2012;26(6):972‐978. [DOI] [PubMed] [Google Scholar]
- 46. Robles E, Gilmore‐Thomas KK, Miller FB, McMillan DE. Sensitivity to acute methadone dose changes in maintenance patients. J Subst Abuse Treat. 2002;23(4):409‐413. [DOI] [PubMed] [Google Scholar]


