Abstract
Background and purpose
The aim was to evaluate the efficacy of the catechol‐O‐methyltransferase inhibitor opicapone (25 and 50 mg) as adjunct therapy to levodopa in a pooled population of Parkinson's disease patients who participated in the pivotal double‐blind trials of opicapone and their 1‐year open‐label extensions.
Methods
Data (placebo, opicapone 25 mg and opicapone 50 mg) from the BIPARK‐1 and BIPARK‐2 double‐blind and open‐label studies were combined. The studies had similar designs, eligibility criteria and assessment methods. The primary efficacy variable in both double‐blind studies was the change from baseline in absolute OFF time based on patient diaries.
Results
Double‐blind treatment with opicapone (25 and 50 mg) significantly reduced absolute daily OFF time from a baseline of 6.1–6.6 h. The mean (and 95% confidence interval) treatment effect versus placebo was −35.1 (−62.1, −8.2) min (P = 0.0106) for the 25 mg dose and −58.1 (−84.5, −31.7) min (P < 0.0001) for the 50 mg dose. Reductions in OFF time were mirrored by significant increases in ON time without troublesome dyskinesia (P < 0.05 and P < 0.0001 for the 25 and 50 mg doses, respectively). No significant differences were observed for ON time with troublesome dyskinesia. Patient diary results from the open‐label phase indicated a maintenance of effect for patients previously treated with opicapone 50 mg. The group previously treated with the 25 mg dose benefitted with further optimization of therapy during the open‐label phase, whilst switching from placebo to opicapone led to significant reductions in OFF time and increased ON time.
Conclusions
Over at least 1 year of open‐label therapy, opicapone consistently reduced OFF time and increased ON time without increasing the frequency of troublesome dyskinesia.
Keywords: motor fluctuations, OFF time, opicapone, Parkinson's disease
Introduction
Opicapone is a third generation, catechol‐O‐methyltransferase (COMT) inhibitor that has recently been approved in the European Union as adjunctive therapy to preparations of levodopa in adult patients with Parkinson's disease (PD) and end‐of‐dose motor fluctuations 1. The efficacy of opicapone given once daily as an adjunct to levodopa has been established in two large, randomized clinical trials (BIPARK‐1 2 and BIPARK‐2 3) and their associated open‐label extension studies 3, 4.
Similarities in the design of the two double‐blind, randomized, placebo‐controlled studies and their extensions permitted a pooled analysis with increased statistical power to provide further information on the magnitude of the effect of opicapone and were an important component of the approval process in Europe 5. The main aim of this pooled analysis was to evaluate the symptomatic efficacy of opicapone (25 and 50 mg) versus placebo in levodopa‐treated patients experiencing motor fluctuations using the same outcomes as reported in the individual studies. In addition, the long‐term efficacy of opicapone was assessed in patients who participated in the double‐blind and open‐label phases of the trials.
Methods
BIPARK‐1 (NCT01568073) 2 and BIPARK‐2 (NCT01227655) 3 were randomized, double‐blind, placebo‐controlled studies, full details of which have been published previously. Open‐label extension phases of each trial followed patients for up to an additional year of treatment 3, 4. Both studies were conducted in accordance with good clinical practice and the provisions of the International Conference on Harmonization and were sponsored by BIAL – Portela & Ca, S.A. Institutional review boards at the participating sites provided ethics approval and all patients provided written informed consent.
Study designs
Both studies recruited adult (aged 30–83 years) patients with a minimum disease duration of 3 years after diagnosis of PD and a Hoehn and Yahr stage between 1 and 3 (during ON). Eligible patients had to be receiving treatment with levodopa for at least 1 year (3–8 daily doses) and experiencing end‐of‐dose motor deterioration with ≥1.5 h of OFF time per day (excluding pre‐dose morning akinesia). Both studies assessed the efficacy of opicapone 25 mg and 50 mg once daily versus placebo. BIPARK‐1 was an active‐controlled study that included an entacapone arm and a low dose (5 mg) opicapone arm, which are not included in the present analysis.
The open‐label extension began the day after completing double‐blind treatment and continued until patients had completed 52 weeks of open‐label treatment. Open‐label treatment was started with the 25 mg dose but could be titrated up to 50 mg if required to control wearing‐off and if tolerated. In the case of unacceptable dopaminergic adverse events, the levodopa dose was to be adjusted first, followed by opicapone down‐titration in those with persisting adverse events.
Pooled analysis
This pooled analysis was based on integration of individual participant data. Analyses of the double‐blind phase included data from all patients randomized to placebo, opicapone 25 mg or opicapone 50 mg (common treatment arms in both studies), who took one or more doses of study medication and had one or more post‐baseline OFF time assessments (full analysis set). Analyses of open‐label phase data included all patients who received placebo, opicapone 25 mg or opicapone 50 mg in the double‐blind study, received one or more open‐label doses of opicapone and recorded one or more OFF time efficacy assessments.
This pooled analysis followed the same statistical approach as the individual double‐blind studies 2, 3. Reductions in absolute OFF time, increases in ON time and changes in Unified Parkinson's Disease Rating Scale (UPDRS) Parts II and III during randomized treatment were assessed using an analysis of covariance (ancova) with study and geographical area as factors and baseline respective variable as covariate. OFF and ON time responder rates were analysed with a Cochran–Mantel–Haenszel test stratified by study. Clinical (investigator) and Patient Global Impression of Change (CGIC and PGIC) were analysed with a van Elteren test. For the open‐label phase, each of the OFF and ON time variables was analysed versus open‐label baseline using a linear mixed‐effect model for repeated measurements with study and geographical area as factors and double‐blind baseline respective variable as covariate. Patients were considered ‘good’ responders if they were rated as much or very much improved on CGIC and PGIC assessments. All P values are exploratory.
Results
Patient disposition and baseline characteristics
Of the 1027 patients randomized to the two double‐blind studies, 758 patients had one or more post‐baseline efficacy observations (placebo, n = 255; opicapone 25 mg, n = 241; opicapone 50 mg, n = 262) and were included in the pooled analysis. The open‐label analyses included 633 of 764 patients who enrolled in the open‐label extensions. Completion rates for eligible patients were high (Fig. 1).
Figure 1.
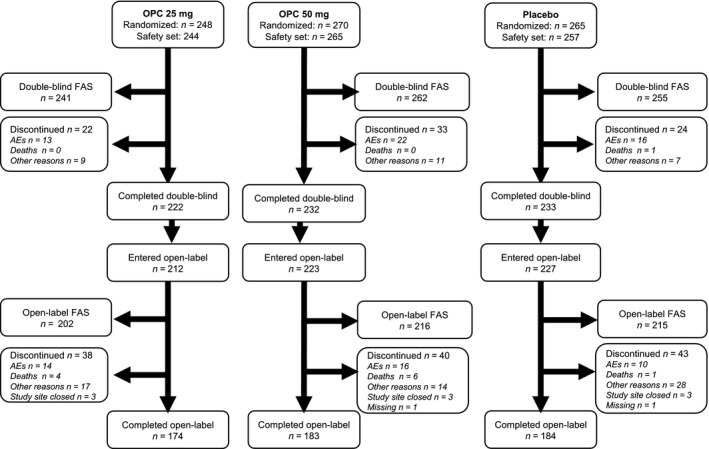
Patient disposition. The double‐blind safety set includes all patients who took at least one dose of study medication. The full analysis set includes all randomized patients who took one or more doses of study medication and had one or more post‐baseline OFF time assessments. AEs, adverse events; FAS, full analysis set; OPC, opicapone.
Baseline characteristics at the start of the double‐blind phase are shown in Table 1. At double‐blind baseline, patients had an average daily OFF time of over 6 h (6.1–6.6 h).
Table 1.
Baseline characteristics
| Characteristics at double‐blind baseline | Placebo n = 257 | Opicapone 25 mg n = 244 | Opicapone 50 mg n = 265 |
|---|---|---|---|
| Sex, male; n (%) | 142 (55.3) | 149 (61.1) | 160 (60.4) |
| Age, years; mean (SD) | 62.8 (9.1) | 63.4 (8.8) | 64.5 (8.8) |
| Race; n (%) | |||
| White | 211 (82.1) | 209 (85.7) | 231 (87.2) |
| Asian | 42 (16.3) | 29 (11.9) | 33 (12.5) |
| Disease duration, years; mean (SD) | 7.8 (3.9) | 7.9 (4.3) | 7.6 (4.3) |
| Time since onset of fluctuations, years; mean (SD) | 2.6 (2.2) | 2.8 (2.7) | 2.7 (2.9) |
| Daily OFF time, h; mean (SD) | 6.1 (2.1) | 6.6 (2.3) | 6.2 (2.0) |
| Daily ON time with troublesome dyskinesia, h; mean (SD) | 0.5 (1.3) | 0.4 (1.1) | 0.4 (1.1) |
| Daily levodopa, mg; mean (SD) | 695 (321) | 732 (370) | 698 (322) |
| Concomitant PD medication, n (%) | |||
| Levodopa/carbidopa | 151 (58.8) | 148 (60.7) | 155 (58.5) |
| Levodopa/benserazide | 127 (49.4) | 106 (43.4) | 124 (46.8) |
| Pramipexole | 95 (37.0) | 79 (32.4) | 96 (36.2) |
| Ropinirole | 72 (28.0) | 65 (26.6) | 69 (26.0) |
| Amantadine | 58 (22.6) | 58 (23.8) | 55 (20.8) |
| Rasagiline | 30 (11.7) | 27 (11.1) | 39 (14.7) |
PD, Parkinson's disease; SD, standard deviation.
Double‐blind phase
Treatment with either opicapone 25 mg or opicapone 50 mg significantly reduced absolute daily OFF time. The mean (95% confidence interval) treatment effect versus placebo was −35.1 (−62.1, −8.2) min (P = 0.0106) for the opicapone 25 mg dose and −58.1 (−84.5, −31.7) min (P < 0.0001) for the 50 mg dose (Fig. 2). Reductions in OFF time were mirrored by significant increases in ON time without troublesome dyskinesia (P < 0.01 and P < 0.0001 for the 25 mg and 50 mg doses, respectively) (Table 2). No significant differences versus placebo were observed for ON time with troublesome dyskinesia (P = 0.622 and P = 0.318 for the 25 mg and 50 mg doses, respectively).
Figure 2.
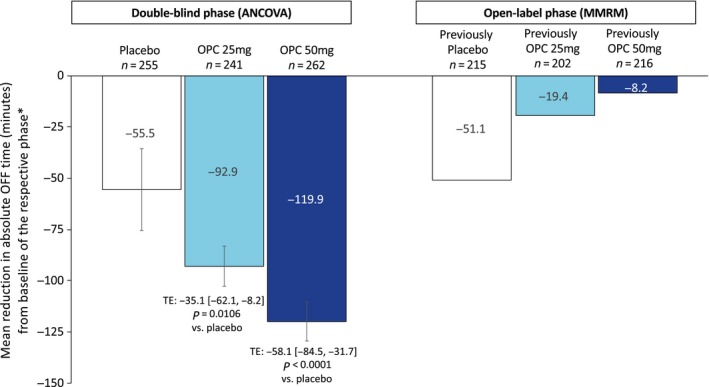
Reductions in OFF time. *Change in OFF time for the double‐blind phase was calculated versus the double‐blind baseline (ancova). Change in OFF time for the open‐label phase is described versus the open‐label baseline. ancova, analysis of covariance; MMRM, mixed‐effect model for repeated measurements; OPC, opicapone; TE, treatment estimate [95% confidence interval]. [Color figure can be viewed at wileyonlinelibrary.com]
Table 2.
Symptomatic efficacy (secondary outcome measures) of opicapone during the double‐blind phase
| Parameter | Placebo (N = 255) | Opicapone 25 mg (N = 241) | Opicapone 50 mg (N = 262) |
|---|---|---|---|
| Total ON time (min) | |||
| Change from baseline; LS mean ± SEM | 50.1 ± 10.0 | 93.1 ± 10.3 | 111.9 ± 10.2 |
| Difference versus placebo; LS mean (95% CI) | 43.0 (16.2, 69.9) | 61.8 (35.5, 88.1) | |
| P value | 0.0017 | <0.0001 | |
| ON time without troublesome dyskinesia (min) | |||
| Change from baseline; LS mean ± SEM | 43.1 ± 11.5 | 85.8 ± 11.3 | 107.8 ± 11.2 |
| Difference versus placebo; LS mean (95% CI) | 42.7 (11.1, 74.4) | 64.7 (33.2, 96.2) | |
| P value | 0.0083 | <0.0001 | |
| ON time with troublesome dyskinesia (min) | |||
| Change from baseline; LS mean ± SEM | 4.5 ± 5.9 | 8.6 ± 5.8 | 12.7 ± 5.8 |
| Difference versus placebo; LS mean (95% CI) | 4.1 (−12.2, 20.4) | 8.3 (−8.0, 24.5) | |
| P value | 0.6220 | 0.3175 | |
| Responder rate OFF time reduction of ≥1 h; n (%) | 125 (49.0) | 148 (61.4) | 177 (67.6) |
| Odds ratio (95% CI) versus placebo | 1.66 (1.16, 2.37) | 2.17 (1.52, 3.09) | |
| P value | 0.0055 | <0.0001 | |
| Responder rate ON time increase of ≥1 h; n (%) | 116 (45.5) | 145 (60.2) | 166 (63.4) |
| Odds ratio (95% CI) versus placebo | 1.81 (1.27, 2.59) | 2.08 (1.46, 2.96) | |
| P value | 0.0011 | <0.0001 | |
| UPDRS Part II (ADL) scores | (n = 249) | (n = 237) | (n = 253) |
| Change from baseline; LS mean ± SEM | −2.2 ± 0·3 | −2.8 ± 0·3 | −2.6 ± 0·3 |
| P value versus placebo | 0.0982 | 0.2180 | |
| UPDRS Part III (motor) scores | (n = 250) | (n = 238) | (n = 257) |
| Change from baseline; LS mean ± SEM | −2.9 ± 0.4 | −4.0 ± 0.4 | −3.2 ± 0.4 |
| P value versus placebo | 0.0482 | 0.5570 | |
| Responder rate CGIC (much or very much improved); n (%) | 48 (18.8) | 68 (28.2) | 66 (25.2) |
| Odds ratio (95% CI) versus placebo | 1.73 (1.13, 2.64) | 1.47 (0.96; 2.24) | |
| P value | 0.0108 | 0.0728 | |
| Responder rate PGIC (much or very much improved); n (%) | 50 (19.6) | 78 (32.4) | 72 (27.5) |
| Odds ratio (95% CI) versus placebo | 2.00 (1.33, 3.02) | 1.57 (1.04; 2.37) | |
| P value | 0.0009 | 0.0314 | |
ADL, activities of daily living; CGIC, Clinical Global Impression of Change; CI, confidence interval; LS, least squares; PGIC, Patient Global Impression of Change; SEM, standard error of the mean; UPDRS, Unified Parkinson's Disease Rating Scale.
Analysis of responder rates showed that significantly more patients receiving either dose of opicapone achieved (i) a ≥ 1 h reduction in OFF time and (ii) a ≥ 1 h increase in ON time compared to placebo (Table 2). PGIC responder rates showed that significantly more patients receiving either dose of opicapone were rated as much or very much improved (P = 0.0009 and P = 0.0314 for opicapone 25 mg and 50 mg, respectively). CGIC responder rates were similar to the PGIC but significantly more patients were rated as much or very much improved with the opicapone 25 mg dose only (P = 0.0108) (Fig. 3, Table 2). There was a small significant difference in UPDRS motor scores for the opicapone 25 mg dose (treatment difference of −1.1 versus placebo, P < 0.005); no other significant differences were observed for UPDRS scores.
Figure 3.
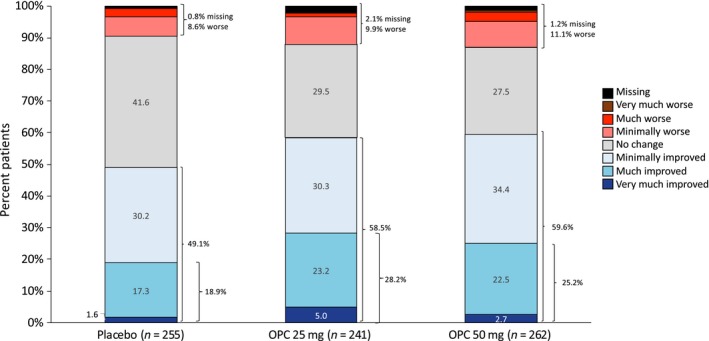
Clinical Global Impression of Change (double‐blind phase). OPC, opicapone. [Color figure can be viewed at wileyonlinelibrary.com]
Open‐label phase
As per protocol, all patients began open‐label treatment with opicapone 25 mg. A total of 341 (53.9%) patients increased their opicapone dose to 50 mg during the study and only 12 (1.9%) patients reduced their opicapone dose to 5 mg. In those patients originally allocated to the placebo group (n = 215), levodopa doses remained stable during the double‐blind phase and decreased slightly during the open‐label phase (mean ± SD levodopa doses were 701 ± 313 mg at double‐blind baseline, 694 ± 301 mg at open‐label baseline and 655 ± 317 mg at the end of the open‐label study). For those originally assigned to active treatment (opicapone 25 mg or 50 mg, n = 418), levodopa doses decreased slightly during the double‐blind phase and remained stable during the open‐label phase (mean ± SD levodopa doses were 718 ± 354 mg at double‐blind baseline, 687 ± 330 mg at open‐label baseline and 679 ± 333 mg at the end of the open‐label study). Use of other PD medications remained stable over the open‐label period; only 3.8% of patients initiated new treatment with another PD agent.
Patient diary results from the open‐label phases of the pivotal trials indicated a maintenance or enhancement of symptomatic effect. For patients previously treated with opicapone 25 and 50 mg during the double‐blind phase, respective mean additional reductions in absolute OFF time during the open‐label phase were −19.4 min and −8.2 min versus the open‐label baseline (Fig. 2). There was an additional mean reduction of −51.1 min versus the open‐label baseline in patients treated with placebo during the double‐blind phase.
The treatment benefit of improvement in ON time without troublesome dyskinesia was also maintained or enhanced during the open‐label phase. For patients previously treated with opicapone 25 and 50 mg during the double‐blind phase, respective mean additional increases in ON time without troublesome dyskinesia during the open‐label phase were +0.6 min and +11.3 min versus the open‐label baseline. There was a mean increase in ON time of +52.4 min versus the open‐label baseline in patients treated with placebo during the double‐blind phase. No relevant changes were observed in the mean ON time with troublesome dyskinesia during the open‐label phase (mean changes versus open‐label baseline of +9.6 min in patients previously allocated to placebo, +15.9 min in patients previously allocated to opicapone 25 mg and −10.6 min in patients previously allocated to opicapone 50 mg).
The long‐term maintenance of clinical effect was confirmed by CGIC and PGIC data. At the end of the open‐label period, investigators rated 32.9%–39.5% of patients as having much or very much improved relative to the double‐blind baseline. For the group of patients treated with opicapone during the double‐blind phase, there was an increase of 3.5%–4.2% of patients rated much or very much improved in relation to the double‐blind end‐point, whilst for those treated with placebo in the double‐blind phase there was an increase of 18.6% of patients rated much or very much improved. Similar results were seen with patient self‐report with 34.7%–40.9% of patients saying they felt much or very much improved relative to when they started the double‐blind study. Among patients treated with opicapone during the double‐blind phase there was an increase of 2.5%–2.8% who reported feeling much or very much improved versus the double‐blind end‐point. By contrast, for patients previously treated with placebo in the double‐blind phase, there was an increase of 18.1% who reported feeling much or very much improved versus the double‐blind end‐point.
Discussion
This pooled analysis combines data from two similar large, randomized, placebo‐controlled studies with their open‐label extensions and confirms the symptomatic efficacy of opicapone as an adjunct to levodopa in fluctuating PD for up to 1 year of treatment. In the double‐blind phase, both the 25 and 50 mg doses of opicapone were associated with significant improvements in motor fluctuations versus placebo, without significantly increasing troublesome dyskinesia.
In the pooled analysis of double‐blind data, both doses of opicapone (25 and 50 mg) were significantly superior to placebo in reducing OFF time and increasing ON time without troublesome dyskinesia. This occurred despite a high placebo response and supports earlier suggestions that the individual studies could have been underpowered to detect treatment effects for the 25 mg dose of opicapone versus placebo 2, 3. As such, these pooled analyses were considered important for European marketing authorization of both opicapone doses 5. The efficacy of the 25 mg dose in some patients is also supported by the relatively high proportion of patients (around 44%) who ended the open‐label phase on this dose.
The magnitude of effects in the pooled double‐blind analyses was similar to those in the separate studies and the incremental differences between the 25 and 50 mg data lend further support for the 50 mg dose as the most effective opicapone dose. Indeed, the group treated with the 25 mg dose in the double‐blind phase benefitted with further optimization of therapy during the open‐label phase. Moreover, the results for increased ON time in this dataset appear more robust compared with a previous meta‐analysis of entacapone data. In the current dataset increases of 61.8 min were observed with the 50 mg dose of opicapone, whereas the reported mean increase for entacapone across clinical studies was 0.6 h (~36 min) 6. Although non‐significant, the BIPARK‐1 study did also find a treatment difference of 19.3 min in total ON time between opicapone 50 mg and entacapone 200 mg 2.
OFF time reductions at the end of the open‐label phase were numerically greater for the patients assigned to opicapone in the double‐blind phase versus those who were originally assigned to placebo. A similar tendency for the earlier (versus 6 months later) initiation of entacapone has previously been shown using data from another pooled analysis of placebo‐controlled trials and open‐label extensions and has led to the suggestion that there may be beneficial effects of earlier versus later initiation of COMT inhibitors in subjects with levodopa‐related fluctuations 7. This concept merits further prospective study. Further support for the benefits of long‐term opicapone treatment comes from ratings of global function as perceived by investigators and patients. Improvements in global function at the end of the double‐blind period were maintained after 1 year of treatment in the open‐label extensions. At the end of the open‐label period, up to 40% of patients were assessed by investigators and self‐assessed as having a good response (i.e. much or very much improved) relative to the double‐blind baseline, which was above the rate observed at the end of double‐blind treatment.
Another commonly used proxy of efficacy is levodopa dosing, where maintenance of dosing reflects a sustained symptomatic effect. For patients treated with opicapone in the double‐blind phase, levodopa doses and use of other antiparkinsonian agents (including dopamine agonists and monoamine oxidase B inhibitors) remained largely stable over the open‐label period, despite investigators having the freedom to adjust medications and dosing according to clinical need, further supporting the long‐term efficacy of this third generation COMT inhibitor.
The main strength of this pooled analysis lies in the similarity of study designs and the analysis of individual patient data. Limitations of these findings include those inherent with retrospective, post hoc analyses, although the data were prospectively collected. The safety and tolerability of opicapone have been extensively reported for the individual studies 4, where opicapone was generally well tolerated and not associated with hepatic toxicity nor increased gastrointestinal problems (e.g. diarrhoea). Across both double‐blind studies, the most common adverse events reported in the opicapone group compared to placebo were the dopaminergic events of dyskinesia, constipation, insomnia, dry mouth and dizziness, as well as increased blood creatine phosphokinase. Dyskinesia was the most frequently reported treatment‐related adverse event with a higher incidence in the combined opicapone groups than placebo (17.7% vs. 6.2%). As demonstrated in the efficacy evaluations of ON time with and without troublesome dyskinesia, patients deemed most dyskinesia as non‐troublesome.
Conclusions
In this pooled analysis of the phase 3 clinical studies, over at least 1 year of continued open‐label therapy opicapone consistently reduced OFF time and increased ON time without increasing the frequency of troublesome dyskinesia.
Disclosure of conflicts of interest
JJF has held consultancy functions with GlaxoSmithKline, Novartis, Teva, Lundbeck, Solvay, Abbott, BIAL, Merck‐Serono, Merz, Ipsen, Biogen; has received lecture fees from Biogen and BIAL; has received grants from GlaxoSmithKline, Grunenthal, MSD, Allergan, Novartis, Fundação MSD (Portugal) and Teva; has been employed by Faculdade de Medicina de Lisboa and CNS – Campus Neurológico Sénior. AL is funded by the Reta Lila Weston Institute of Neurological Studies, University College London, Institute of Neurology and reports consultancies for Britannia Pharmaceuticals, BIAL. He also reports grants and/or research support from the PSP Association, Weston Trust, the Reta Lila Howard Foundation, and honoraria from Britannia, UCB, Roche, Novartis, Boehringer Ingelheim, Lundbeck, GE Healthcare, Teva, GlaxoSmithKline, Ipsen, Allergan, Orion, BIAL, AbbVie, Nordicinfu Care. WP has received consultancy and lecture fees in relation to clinical drug programmes for PD from AbbVie, AstraZeneca, BIAL, Biogen, Britannia, Grünenthal, Intec, Ipsen, Lundbeck, Novartis, Neuroderm, Orion Pharma, Prexton, Roche, Sunovion, Sun Pharma, Takeda, Teva, UCB and Zambon. Royalties: Thieme, Wiley Blackwell, Oxford University Press and Cambridge University Press. OR reports receiving consulting fees from AbbVie, Adamas, Acorda, Addex, AlzProtect, Apopharma, Astrazeneca, Bial, Biogen, Britannia, Clevexel, Cynapsus, INC Research, Lundbeck, Merck, MundiPharma, Neuroderm, Novartis, Osmotica, Oxford Biomedica, Parexel, Pfizer, Prexton Therapeutics, Quintiles, Sanofi, Servier, Takeda, Teva, UCB, XenoPort, Zambon; and grant support from Agence Nationale de la Recherche (ANR), Boehringer Ingelheim, CHU de Toulouse, French Parkinson, GlaxoSmithKline, INSERM‐DHOS, Michael J Fox Foundation, Programme Hospitalier de Recherche Clinique, Recherche Clinique Translationnelle, UCB, Teva and Lundbeck. JFR and PSS are employed by BIAL – Portela & Cª, S.A.
Acknowledgements
The BIPARK‐1 and BIPARK‐2 studies were supported and funded by BIAL – Portela & Ca, S.A. Medical writing assistance was provided by A. Chadha‐Patel and was funded by BIAL – Portela & Ca, S.A.
References
- 1. Ongentys . Summary of Product Characteristics 2018. Available for download at: https://www.ema.europa.eu/documents/product-information/ongentys-epar-product-information_en.pdf (accessed 18/12/2018).
- 2. Ferreira JJ, Lees A, Rocha JF, et al Opicapone as an adjunct to levodopa in patients with Parkinson's disease and end‐of‐dose motor fluctuations: a randomised, double‐blind, controlled trial. Lancet Neurol 2016; 15: 154–165. [DOI] [PubMed] [Google Scholar]
- 3. Lees AJ, Ferreira J, Rascol O, et al Opicapone as adjunct to levodopa therapy in patients with Parkinson disease and motor fluctuations: a randomized clinical trial. JAMA Neurol 2017; 74: 197–206. [DOI] [PubMed] [Google Scholar]
- 4. Ferreira JJ, Lees AJ, Poewe W, et al Effectiveness of opicapone and switching from entacapone in fluctuating Parkinson disease. Neurology 2018; 90: e1849–e1857. [DOI] [PubMed] [Google Scholar]
- 5. European Medicines Agency . Ongentys Assessment Report. 2016. Procedure No. EMEA/H/C/002790/0000. Available for download at: https://www.ema.europa.eu/en/medicines/human/EPAR/ongentys (accessed 18/12/2018).
- 6. Stowe R, Ives N, Clarke CE, et al Meta‐analysis of the comparative efficacy and safety of adjuvant treatment to levodopa in later Parkinson's disease. Mov Disord 2011; 26: 587–598. [DOI] [PubMed] [Google Scholar]
- 7. Nissinen H, Kuoppamaki M, Leinonen M, Schapira AH. Early versus delayed initiation of entacapone in levodopa‐treated patients with Parkinson's disease: a long‐term, retrospective analysis. Eur J Neurol 2009; 16: 1305–1311. [DOI] [PubMed] [Google Scholar]


