Abstract
Objective
To investigate the value of repeated magnetic resonance imaging (MRI) of the sacroiliac (SI) joints in diagnosing chronic back pain patients in whom axial spondyloarthritis (SpA) is suspected and to examine determinants of positive MRI findings in SI joints.
Methods
Patients with chronic back pain (duration 3 months–2 years, age ≥16 years, age at onset <45 years) with ≥1 SpA feature who were included in the Spondyloarthritis Caught Early cohort underwent visits at baseline, at 3 months, and at 1 year. Visits included an evaluation of all SpA features and repeated MRI of SI joints. MRI‐detected axial SpA positivity (according to the definition from the Assessment of SpondyloArthritis international Society) was evaluated by 2 or 3 well‐trained readers who were blinded with regard to clinical information. The likelihood of a positive MRI finding at follow‐up visits (taking into consideration contributing factors) was calculated by generalized estimating equation analysis.
Results
Of the 188 patients, 38.3% were male, the mean ± SD age was 31.0 ± 8.2 years, and the mean ± SD symptom duration was 13.2 ± 7.1 months. Thirty‐one patients (16.5%) had positive MRI findings in the SI joints at baseline. After 3 months and after 1 year, the MRI results had changed from positive to negative in 3 of 27 patients (11.1%) and 11 of 29 patients (37.9%), respectively, which was attributable in part to the initiation of anti–tumor necrosis factor therapy. Status changes from negative to positive were seen in 5 of 116 patients (4.3%) after 3 months and in 10 of 138 patients (7.2%) after 1 year. HLA–B27 positivity and male sex were independent determinants of the likelihood of a positive MRI scan at any time point (42% in HLA–B27+ men and 6% in HLA–B27− women). If the baseline results were negative, the likelihood of a positive scan at follow‐up was very low (≤7%).
Conclusion
MRI‐detected status changes in the SI joints were seen in a minority of the patients, and both male sex and HLA–B27 positivity were important predictors of MRI positivity. Our findings indicate that conducting MRI scans after 3 months or after 1 year in patients with suspected early axial SpA is not diagnostically useful.
Introduction
Sacroiliac (SI) joint imaging plays a pivotal role in the process of diagnosing axial spondyloarthritis (SpA) 1. Conventional radiography has been and remains the most commonly used method to detect sacroiliitis. However, radiographic abnormalities evolve over several years, which contributes to a reported delay in diagnosis of 8–9 years 2, 3. This substantial delay is problematic, because effective treatments are available for patients with axial SpA 4, 5, 6. Over the last decade, magnetic resonance imaging (MRI) rapidly gained ground and proved to be an important imaging technique in the diagnosis of (nonradiographic) axial SpA 7. MRI enables the detection of inflammatory sacroiliitis at an early stage, months to years before structural damage can be detected by conventional radiography 8, 9. Besides the fact that MRI has substantial advantages over radiography in terms of sensitivity, it has the benefit of providing information on both activity and structural damage through the use of only one imaging modality 10, 11, 12.
Axial SpA should be considered in patients with chronic back pain who have an age at onset of <45 years. Regrettably, no formal diagnostic criteria exist, and there is no single SpA feature with sufficient specificity to establish the diagnosis. The modified Berlin algorithm 1 is a helpful tool for rheumatologists in establishing an early diagnosis of axial SpA with greater confidence. According to this algorithm, MRI of the SI joints should be performed in some patients after obtaining conventional radiographs and HLA–B27 testing 13. The most recently published European League Against Rheumatism recommendations on imaging in SpA even state that for certain patients, such as young patients and those with short symptom duration periods, MRI of the SI joints is an alternative primary imaging method 14.
Although inflammation detected by MRI is now widely considered to be an important manifestation in early axial SpA, not much evidence is available on how inflammatory lesions develop over time (outside of clinical trials) 15, 16. However, with the increased interest in MRI in the early diagnosis of axial SpA, examining this manifestation is important. We know that inflammatory lesions (e.g., bone marrow edema [BME]) can change over relatively short periods of time in patients diagnosed as having SpA, but in patients with chronic back pain and in whom axial SpA is suspected, it is unclear if BME lesions newly develop or fluctuate both in number and in size over time. Therefore, a relevant clinical question is: if an MRI scan is normal but there is still a clinical suspicion of axial SpA, should the MRI be repeated, and if so, after what period of follow‐up? Or does this not impact the diagnostic process? The Spondyloarthritis Caught Early (SPACE) cohort is an ideal cohort to investigate this research question, as it includes a population of patients with back pain of short duration who were referred to rheumatologists with a suspicion of SpA, but without the mandatory presence of single or multiple SpA features. In the present study, we aimed to investigate the evolution of MRI‐detected lesions at 3‐month and 1‐year time points in the SPACE cohort.
Patients and methods
Study design and patient population
SPACE is a multinational ongoing cohort study that began in January 2009. Across 5 participating centers in Europe, patients with chronic back pain (duration 3 months–2 years, age ≥16 years, age at onset <45 years) are included. Before the start of the study, approval was obtained by local medical ethics committees. Before inclusion, written informed consent was obtained from all patients in accordance with the Declaration of Helsinki. A detailed description of the SPACE cohort has been published elsewhere 17. All patients underwent a diagnostic evaluation at baseline, which included a physical examination, MRI and radiographs of the SI joints, HLA–B27 testing, and an examination for all other SpA features 13, 18. Patients fulfilling the Assessment of SpondyloArthritis international Society (ASAS) axial SpA criteria at baseline or patients with possible axial SpA (i.e., the evidence of SpA features [though not to a sufficient degree to be classified as axial SpA, defined as the presence of ≥1 SpA feature]) were included for follow‐up visits. At the 3‐month and 1‐year follow‐up visits, clinical and laboratory data were collected, and another MRI of the SI joints was performed. Three‐month MRI data on patients enrolled in the cohort after July 2012 were not included, because it was decided that 3‐month data on ~150 patients were sufficient to make valid conclusions regarding lesion change.
Imaging and scoring methodology
MRI of the SI joints was performed using a 1.5T scanner at baseline and follow‐up. Coronal oblique MRI images were obtained, with a slice thickness of 4 mm. Both short tau inversion recovery (STIR) and T1‐weighted turbo spin‐echo (T1WSE) sequences were acquired and evaluated in the scoring process. At baseline, conventional radiography of the pelvis (i.e., SI joints) in anteroposterior view was performed. MRI scans and radiographs of the SI joints were scored independently by 2 trained and well‐calibrated readers who were blinded with regard to patient characteristics, clinical data, time sequence, and data from the other imaging modality. In the case of score discrepancies, a third reader scored the images.
Radiographs were marked positive for sacroiliitis according to the modified New York criteria 19 (i.e., the presence of bilateral grade 2–4 sacroiliitis and/or unilateral grade 3–4 sacroiliitis). According to the ASAS definition, an MRI scan of the SI joints was recorded as positive if ≥1 BME lesion highly suggestive of SpA was present on ≥2 consecutive slices or if several BME lesions highly suggestive of SpA were visible on a single slice 7, 18. MRI scans were also scored according to the Spondyloarthritis Research Consortium of Canada (SPARCC) system, which measures inflammation on a continuous scale (0–72) 20. According to the SPARCC score, the presence of an increased signal corresponding to BME lesions is marked on 6 consecutive slices in an SI joint MRI scan. The maximum score for 2 SI joints on each slice is 8. In addition to these 8 points, a score for intensity (additional 1 point) may be assigned to each SI joint if an “intense” signal 20 is detected in any quadrant within the slice. The signal from presacral blood vessels defined a lesion that is scored as intense. Furthermore, a score for depth (additional 1 point) may be assigned to each SI joint if a homogeneous and unequivocal increase in signal extends over a depth of ≥1 cm from the articular surface on each slice, resulting in a maximum score of 12 points per slice. For the assessment using both the ASAS definition and the SPARCC score on the STIR sequence, readers took into account the findings on the T1WSE sequences, looking at both sequences simultaneously.
In the case of disagreement between the 2 readers regarding the presence of radiographic sacroiliitis (based on the modified New York criteria) or a positive MRI result (based on the ASAS definition), a third reader served as adjudicator, and the 2 scores in agreement were considered to be the final score. The positive MRI SPARCC scores from the 2 in agreement were used for further analysis.
Statistical analysis
Disease characteristics of patients were recorded using descriptive statistics. We described the MRI scans of SI joints (using the ASAS definition of axial SpA) in different ways. First, we depicted the course of MRI‐detected status (positive or negative readings in the SI joints) at the (2 or 3) available time points, and second, we used a 2 × 2 table to reflect changes in MRI‐detected status. Agreement on the absence or presence of MRI‐detected inflammation was assessed by cross‐tabulation and expressed as Cohen's kappa. Cumulative probability plots were used to visualize baseline and 1‐year SPARCC scores, in which patients were grouped according to either positivity or negativity according to the ASAS definition. Subsequently, patients of special interest (i.e., those who had MRI scans that reflected a change in axial SpA status at the 3‐month or 1‐year follow‐up) were described phenotypically (according to the presence of SpA features and other disease characteristics).
Thereafter, we investigated the likelihood of having a positive MRI result at any time point during follow‐up and identified which factors determine ASAS‐defined and MRI‐detected axial SpA positivity in the SI joints. After the analysis of the whole patient group, we repeated this analysis in the subset of patients with inflammatory back pain (IBP) according to the ASAS definition 21. We then looked at the likelihood of having a positive MRI at the 2 follow‐up time points (in all patients and in the subgroup of patients with IBP), taking into account the baseline MRI‐detected status. This was done by using generalized estimating equation (GEE) analysis for binomial outcome variables; MRI‐detected axial SpA was used as the dependent variable, and HLA–B27 status and sex were used as independent explanatory variables. C‐reactive protein (CRP) level was added to the model as a covariate in order to assess the contribution of CRP levels in explaining a positive MRI. The likelihood of a positive MRI result (at 3‐month or 1‐year time points) if the baseline scan was either positive or negative was calculated, taking into account HLA–B27 status and sex. Odds ratios (ORs) from the model were converted into probabilities (likelihood) 22, and 95% confidence intervals (95% CIs) were calculated. Data analysis was performed using Stata version 14 software (StataCorp).
Results
Patient characteristics
In total, 188 patients were included in the current study. Baseline characteristics are described in Table 1. The mean ± SD age of the patients was 31.0 ± 8.2 years, and 38.3% were male. The mean ± SD duration of back pain was 13.2 ± 7.1 months, and 139 patients (74.3%) had ASAS‐defined IBP. Almost half of the patients (48.4%) were HLA–B27 positive.
Table 1.
Baseline patient characteristicsa
| Age at enrollment, mean ± SD years | 31.0 ± 8.2 |
| Male sex | 72 (38.3) |
| Symptom duration at first visit, mean ± SD months | 13.2 ± 7.1 |
| Good response to NSAIDs | 76 (41.3) |
| IBP | 139 (74.3) |
| Family history of SpA | 96 (51.3) |
| Peripheral arthritis | 34 (18.2) |
| Dactylitis | 15 (8.0) |
| Enthesitis | 41 (21.9) |
| Uveitis | 16 (8.6) |
| IBD | 17 (9.1) |
| Psoriasis | 25 (13.4) |
| Elevated CRP level | 35 (18.9) |
| HLA–B27 positive | 91 (48.4) |
| Radiographically detected sacroiliitis | 19 (11.1) |
| MRI‐detected positive result for axial SpAb | 31 (16.5) |
| Axial SpA diagnosisc | 74 (39.6) |
Except where indicated otherwise, values are the number (%) of patients. NSAIDs = nonsteroidal antiinflammatory drugs; IBP = inflammatory back pain; SpA = spondyloarthritis; IBD = inflammatory bowel disease; CRP = C‐reactive protein; MRI = magnetic resonance imaging.
Based on Assessment of SpondyloArthritis international Society axial SpA criteria.
According to rheumatologist, with a confidence level of ≥7 (rating scale 0–10).
MRI findings
Agreement between the 2 readers regarding the designation of an ASAS‐defined, axial SpA–positive MRI finding was good (κ = 0.85). In 8 of 188 cases (4.3%), adjudication from a third reader was needed, as reader 1 and reader 2 were in disagreement on axial SpA status.
Table 2 describes the course of MRI‐detected axial SpA positivity over time. For 122 of the 188 patients (65%), MRI data from all 3 time points were available. For 66 of the 188 patients (35%), MRI was performed at only 2 time points: at baseline and at 3 months for 21 of the 66 patients (32%), and at baseline and at 1 year for 45 patients (68%). In these 3 scenarios, the vast majority of patients (77.1%) had a negative MRI finding at baseline, which had not changed at the follow‐up time point(s). Of the 122 patients who had MRI data available from all 3 time points, persistence of a positive MRI finding was seen in 15 (12.3%), and MRI status fluctuations were seen in 21 (17.2%) over time (i.e., negative–negative–positive results, or positive–positive–negative results, etc.).
Table 2.
Course of positive MRI results in sacroiliac joints over 1 yeara
| MRI results over time | No. of patients |
|---|---|
| Patients with available MRI results at baseline/3 months/1 year | 122 |
| −/−/− | 86 |
| +/+/+ | 15 |
| −/−/+ | 7 |
| +/+/− | 7 |
| −/+/+ | 3 |
| +/−/− | 2 |
| +/−/+ | 1 |
| −/+/− | 1 |
| Patients with available MRI results at baseline/3 months | 21 |
| −/− | 18 |
| +/+ | 2 |
| −/+ | 1 |
| Patients with available MRI results at baseline/1 year | 45 |
| −/− | 41 |
| +/+ | 2 |
| +/− | 2 |
Positive magnetic resonance imaging (MRI) results for axial spondyloarthritis are represented by +, and negative results by −.
Changes in MRI‐detected axial SpA positivity over time are depicted in Table 3. In contrast to Table 2, data are clustered and shown independently of whether there were available data from a third time point. In 8 of 143 patients (5.6%), a change in axial SpA status was seen at the 3‐month follow‐up. At the 1‐year follow‐up, this proportion was slightly higher at 12.6%. The MRI results in 10 of 138 patients (7.2%) changed from negative at baseline to positive at the 1‐year follow‐up (compared to 5 of 116 patients [4.3%] who had a positive result at the 3‐month follow‐up), and 11 of 29 patients (37.9%) with a positive MRI finding at baseline had a negative finding at the 1‐year follow‐up (compared to 3 of 27 patients [11.1%] who had a negative result at the 3‐month follow‐up). Thus, patients were more likely to have a negative MRI finding following a positive baseline finding than they were to have a positive finding following a negative baseline finding. Cumulative probability plots (for each of the readers) of baseline and 1‐year SPARCC scores, in which patients were grouped according to either ASAS‐defined axial SpA positivity or negativity, were completed (see Supplementary Figure 1, on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.40718/abstract).
Table 3.
Changes in MRI‐detected axial spondyloarthritis status over 3 months and 1 yeara
| MRI ASAS positive | MRI ASAS negative | Total | |
|---|---|---|---|
| 3‐month status | |||
| MRI baseline positive | 24 | 3 | 27 |
| MRI baseline negative | 5 | 111 | 116 |
| Total | 29 | 114 | 143 |
| 1‐year status | |||
| MRI baseline positive | 18 | 11 | 29 |
| MRI baseline negative | 10 | 128 | 138 |
| Total | 28 | 139 | 167 |
MRI = magnetic resonance imaging; ASAS = Assessment of SpondyloArthritis international Society.
We reviewed with special interest the disease characteristics of patients who showed changes in MRI‐detected axial SpA status (Table 4). Of the 5 patients whose SpA status changed from negative at baseline to positive at 3 months, 3 patients (60%) were male, and 3 patients (60%) were HLA–B27 positive. One of these patients had radiographically detected sacroiliitis (using the modified New York criteria) at baseline. At 3 months, 2 patients developed a new SpA feature, namely responsiveness to NSAIDs. One patient took NSAIDs at baseline, while 3 patients (60%) took them at 3 months. The 3 patients who had an axial SpA–positive MRI at baseline but had a negative result at the 3‐month follow‐up were HLA–B27–positive men who had been receiving NSAID treatment since baseline (Table 4).
Table 4.
Disease characteristics of patients with a change in MRI‐detected axial SpA status at 3‐month or 1‐year follow‐upsa
| MRI‐detected axial SpA status | Radiographically detected sacroliitisb | HLA–B27 positive | Age/sex | SpA features at baselinec | SpA features at follow‐upc | CRP level at baseline | CRP level at follow‐up | ASDAS at baseline | ASDAS at follow‐up | Medication at baseline | Medication at follow‐up | SPARCC score at baseline (reader 1/ reader 2) | SPARCC score at follow‐up (reader 1/ reader 2) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline negative/ 3‐month positive | |||||||||||||
| Patient 1 | Yes | Yes | 19/M | 1, 2 | Idem | 3 | 5 | 0.8 | – | – | – | 0/0 | 5/6 |
| Patient 2 | No | No | 38/F | 1, 3 | Idem | 3 | 5 | – | – | NSAID | NSAID | 1/1 | 2/2 |
| Patient 3 | No | Yes | 25/M | 2, 8 | Idem + 9 | 6 | 4 | 1.6 | – | – | NSAID | 0/0 | 10/7 |
| Patient 4 | No | No | 29/F | 1 | Idem | 3 | 1 | 0.9 | – | – | – | 0/0 | 9/12 |
| Patient 5 | No | Yes | 26/M | 1, 2 | Idem + 9 | 3 | 3 | 1.6 | – | – | NSAID | 0/0 | 5/5 |
| Baseline positive/ 3‐month negative | |||||||||||||
| Patient 6 | Yes | Yes | 21/M | 1, 9 | Idem | 6 | 14 | 1.4 | – | NSAID | NSAID | 3/5 | 0/3 |
| Patient 7 | No | Yes | 32/M | 1, 6, 9 | Idem | 3 | 4 | 3.8 | – | NSAID | NSAID | 2/4 | 0/0 |
| Patient 8 | No | Yes | 31/M | 1, 6, 8, 9 | Idem | 9 | 2 | 3.4 | – | NSAID | NSAID | 2/2 | 0/0 |
| Baseline negative/ 1‐year positive | |||||||||||||
| Patient 9 | No | Yes | 21/F | 1, 2, 5, 9 | Idem | 3 | 3 | 2.2 | 2.9 | NSAID | NSAID | 0/0 | 4/3 |
| Patient 10 | Yes | Yes | 33/M | 1, 4 | Idem + 9 | 3 | 3 | 0.9 | 2.3 | – | NSAID | 0/0 | 5/6 |
| Patient 11 | Yes | Yes | 19/M | 1, 2 | Idem | 3 | 1 | 0.8 | 0.4 | – | NSAID | 0/0 | 16/18 |
| Patient 12 | No | Yes | 31/M | 1,2 | Idem | 3 | 3 | 2.4 | 2.4 | NSAID | NSAID | 0/2 | 2/3 |
| Patient 13 | No | Yes | 32/M | 1 | Idem | 13 | 9 | 3.0 | 3.2 | NSAID | NSAID | 0/0 | 3/2 |
| Patient 14 | No | Yes | 19/F | 2 | Idem | 4 | 1 | 3.3 | 1.2 | NSAID | NSAID | 0/0 | 2/2 |
| Patient 15 | No | No | 32/F | 1, 2, 6, 9 | Idem | 1 | 1 | 2.7 | – | NSAID | NSAID | 1/0 | 5/5 |
| Patient 16 | No | Yes | 25/M | 2, 8 | Idem + 1, 7, 9 | 6 | 3 | 1.6 | – | – | NSAID | 0/0 | 10/7 |
| Patient 17 | Yes | Yes | 35/F | 9 | Idem | 3 | 10 | 2.5 | – | – | NSAID | 1/0 | 14/11 |
| Patient 18 | No | No | 29/F | 1 | Idem | 3 | 1 | 0.9 | 1.0 | – | NSAID | 0/0 | 13/16 |
| Baseline positive/ 1‐year negative | |||||||||||||
| Patient 19 | No | No | 43/F | 1, 6 | Idem + 5 | 1 | 1 | 1.4 | 1.1 | – | – | 3/2 | 0/0 |
| Patient 20 | Yes | Yes | 29/F | 1 | Idem + 9 | 1 | 1 | 1.6 | 1.4 | NSAID | NSAID + anti‐TNF | 14/14 | 0/0 |
| Patient 21 | No | Yes | 31/M | 1, 6, 8, 9 | Idem + 7 | 9 | 1 | 3.4 | 0.8 | NSAID | NSAID | 2/2 | 0/0 |
| Patient 22 | No | No | 40/F | 1 | Idem + 4, 9 | 4 | 4 | 2.1 | 3.7 | – | Anti‐TNF | 8/8 | 0/0 |
| Patient 23 | Yes | Yes | 22/M | ‐ | 1, 2, 9 | 3 | 3 | 3.1 | 2.7 | NSAID | NSAID | 9/13 | 1/0 |
| Patient 24 | No | Yes | 32/M | 1, 6, 9 | Idem + 2 | 3 | 3 | 3.8 | ‐ | NSAID | NSAID | 2/4 | 0/0 |
| Patient 25 | No | Yes | 33/M | 1, 8, 9 | Idem | 23 | 1 | 2.9 | 1.0 | NSAID | Anti‐TNF | 7/5 | 0/0 |
| Patient 26 | No | No | 46/M | 1, 9 | Idem | 4 | 4 | 2.7 | 1.6 | NSAID | NSAID | 4/2 | 1/0 |
| Patient 27 | Yes | Yes | 24/F | 2, 5, 6 | Idem + 1, 9 | 3 | 3 | 3.0 | 1.0 | NSAID | NSAID + anti‐TNF | 46/44 | 0/0 |
| Patient 28 | Yes | Yes | 42/M | 6, 9 | Idem | 1 | 1 | 3.0 | 1.0 | NSAID | NSAID | 10/9 | 0/0 |
| Patient 29 | Yes | Yes | 26/M | 1, 2 | Idem | 2 | 2 | 2.0 | 2.3 | – | NSAID | 18/16 | 2/0 |
Except where indicated otherwise, characteristics are recorded at baseline. Follow‐up data were obtained at either 3 months (patients 1–8) or 1 year (patients 9–29). MRI = magnetic resonance imaging; CRP = C‐reactive protein; ASDAS = ankylosing spondylitis disease activity score; SPARCC = Spondyloarthritis Research Consortium of Canada system.
Based on the modified New York criteria.
Spondyloarthritis (SpA) characteristics are numerically represented. 1 = inflammatory back pain; 2 = family history of SpA; 3 = uveitis; 4 = irritable bowel disease; 5 = psoriasis; 6 = enthesitis; 7 = dactylitis; 8 = peripheral arthritis; 9 = nonsteroidal antiinflammatory drug (NSAID) responsiveness; idem = same features as at baseline.
Of the 10 patients who were newly MRI‐positive for axial SpA at the 1‐year follow‐up, 5 (50%) were male, and 8 (80%) were HLA–B27 positive. Radiographically detected sacroiliitis was present in 3 patients (30%). At the 1‐year time point, 2 of these 10 patients had developed new SpA features that were not present at baseline (patient 10: NSAID responsiveness; patient 16: NSAID responsiveness, inflammatory back pain, and dactylitis) (Table 4). All 10 patients were taking NSAIDs at the 1‐year follow‐up (versus 50% at baseline), and there were no patients receiving anti‐TNF treatment in this group. Of the 11 patients that had a positive baseline MRI result followed by a negative result at the 1‐year follow‐up, the majority were male (64%), HLA–B27 positive (73%), and had developed new SpA features (55%). In this group, between baseline and the 1‐year follow‐up, anti‐TNF therapy had been added to the treatment regimen of 4 patients, and an NSAID had been added for 1 patient.
Overall, when comparing SPARCC scores assigned by the 2 readers, only modest differences were observed, suggesting a high level of agreement (Table 4). Approximately half of the patients who had negative MRI results for axial SpA and later had positive results (either at 3 months or 1 year) also had marginally positive SPARCC scores at the same time point, while the remaining patients had evidently positive SPARCC scores (as high as 18 in 1 patient). Patients who were initially ASAS positive but became negative after 1 year had mostly low SPARCC scores at baseline, with a few exceptions. Four patients from this group had started receiving anti‐TNF therapy by the 1‐year follow‐up, at which time all 4 showed a notable decrease in SPARCC scores.
Factors determining a positive MRI
According to the GEE analysis, both HLA–B27 positivity (OR 2.36 [95% CI 1.09–5.12], P = 0.029) and male sex (OR 5.63 [95% CI 2.58–12.27], P < 0.001) were independent determinants of the likelihood of a positive MRI at any time point. Figure 1 displays the effects of HLA–B27 and sex in an absolute manner. The likelihood of an axial SpA–positive MRI finding in HLA–B27–negative women with chronic back pain was only 7%, whereas in HLA–B27–positive men it was 43% (HLA–B27–positive women 6%, HLA–B27–negative men 14%). In men, HLA–B27 status had a significant impact on the likelihood of having a positive MRI at any time point (OR 4.54 [95% CI 1.50–13.79], P = 0.008), whereas this impact was not observed in women (OR 0.84 [95% CI 0.23–3.12], P = 0.800). The influence of CRP levels on axial SpA status was investigated in all models, but correcting for CRP levels produced only minor changes, and therefore only uncorrected data are shown. Only minor differences were seen between patients with chronic back pain and those with IBP according to the ASAS definition (data not shown).
Figure 1.
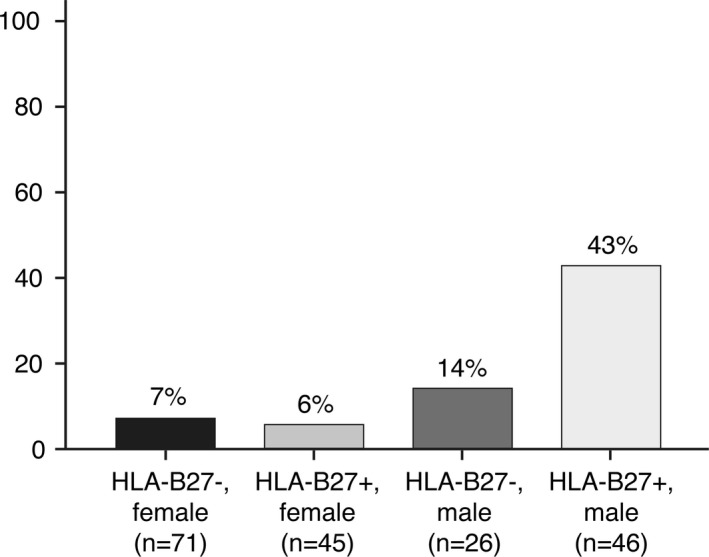
Likelihood of a positive magnetic resonance imaging result at any time point (baseline, 3 months, and 1 year) in chronic back pain patients investigated for axial spondyloarthritis. HLA–B27 status and sex were used as variables.
Likelihood of a positive MRI during follow‐up
The likelihood of an MRI‐detected axial SpA positive result at 3 months or 1 year, according to the baseline MRI status, was considered. Both HLA–B27 status (OR 2.41 [95% CI 0.94–6.18], P = 0.067) and baseline MRI status (OR 43.89 [95% CI 17.59–109.52], P < 0.001) independently contributed to the likelihood of a positive MRI at follow‐up. Other factors contributing to a positive MRI over time included male sex (OR 2.54 [95% CI 1.01–6.39], P = 0.048) and baseline MRI positivity (OR 36.04 [95% CI 14.42–90.08], P < 0.001). Once again, only minor (not significant) differences were observed between chronic back pain patients and IBP patients (data not shown).
In Figure 2, the likelihood of a positive MRI in relation to baseline MRI and HLA–B27 status and sex is depicted. In an HLA–B27–negative patient with a negative baseline MRI, the likelihood of a positive MRI at follow‐up was negligible (1.5%) (Figure 2A). On the contrary, in an HLA–B27–positive patient with a positive baseline MRI, the likelihood was 73%. In patients with a positive baseline MRI, HLA–B27 status did not influence the likelihood of a positive MRI at any follow‐up time point (OR 0.65 [95% CI 0.14–2.96], P = 0.582). However, in patients with a negative baseline MRI, HLA–B27 positivity had a significant effect on the likelihood of a positive MRI at follow‐up (OR 8.12 [95% CI 1.65–40.11], P = 0.010).
Figure 2.
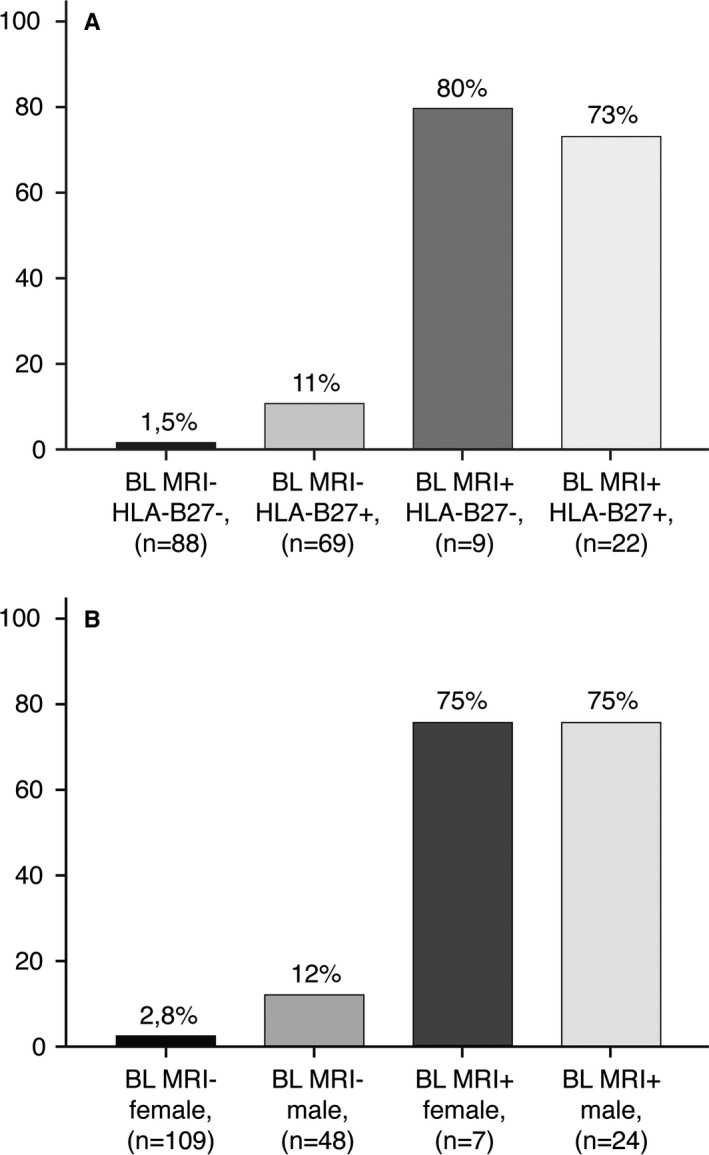
Likelihood of a positive magnetic resonance imaging result at 3‐month or 1‐year follow‐up in chronic back pain patients investigated for axial spondyloarthritis. A, Baseline MRI result and HLA–B27 status were used as variables. B, Baseline MRI result and sex were used as variables.
For a male or female patient with a positive baseline MRI, the likelihood of having a positive MRI at 3 months or 1 year was 75%; however, the likelihood was only 2.8% for a female patient with a negative baseline MRI and 12% for a male patient with a negative baseline MRI (Figure 2B). In patients with a negative baseline MRI, sex significantly affected the likelihood of having a positive MRI at follow‐up (OR 4.67 [95% CI 1.41–15.44], P = 0.01), though this was not true for patients with a positive baseline MRI (OR 0.96 [95% CI 0.20–4.54], P = 0.959). MRI scans of SI joints in patients with axial SpA status changes over 1 year are shown in Supplementary Figure 2 (available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.40718/abstract).
Discussion
In the present study, 83.5% of patients (157 of 188) with chronic back pain and in whom axial SpA was suspected had a negative MRI at baseline. Only 7.6% of these patients (12 of 157) had a positive MRI at either follow‐up time point. Twelve of the 31 patients (38.7%) with a positive MRI at baseline had a negative MRI at follow‐up. Although reversals in axial SpA status are apparent in both directions, relatively more patients who had a positive baseline MRI result later had a negative MRI result (11.1% at 3 months and 37.9% at 1 year) than those who had a negative baseline MRI result and later had a positive MRI result (4.3% at 3 months and 7.2% at 1 year). Nevertheless, it is important to note that 36% of the patients who had negative findings after 1 year had begun anti‐TNF therapy, which is known to decrease visible inflammation in the SI joints 16.
This study showed that MRI‐detected axial SpA positivity at baseline appears to strongly influence the chance of positive SI joint MRI results in the future. If the baseline MRI result was positive, the likelihood that the MRI result would be positive again at 3 months or 1 year was very high (75%). The usefulness of repeated negative MRI in terms of diagnostic yield is low, but there are different risks related to sex and HLA–B27 status.
In patients who had a negative baseline MRI result, HLA–B27 status had a significant effect on the likelihood of a positive MRI at follow‐up. In HLA–B27–negative patients with a negative MRI result at baseline, sacroiliitis at follow‐up could be excluded with a high level of confidence: the likelihood of a positive MRI result at follow‐up was only 1.5%. In HLA–B27–positive patients with a negative MRI result at baseline, the likelihood of a positive MRI result at 3 months or 1 year was still low, though somewhat higher (11%). Of course, we can debate the clinical relevance of this small difference in terms of percentage and, in general, the chances of MRI positivity at follow‐up were very low when the baseline MRI result was negative. However, if a clinical suspicion of axial SpA remains (i.e., if a patient develops other SpA features), it may be worthwhile to consider repeating an MRI scan in HLA–B27–positive patients. Likewise, there was a statistically significant difference between male and female patients who had a negative baseline MRI result, specifically that male patients more frequently had a positive MRI result at follow‐up (12% of men versus 3% of women). Interpretation of MRI findings should always be made in the context of all clinical and laboratory results (e.g., other SpA features that enhance diagnostic confidence) and other available imaging parameters. Additionally, other MRI findings (e.g., the presence of structural lesions) can be supportive in the diagnostic process. However, in this group of patients with short symptom duration, the frequency of structural changes in SI joints was relatively low and discriminated between patients with and without axial SpA only if ≥5 structural lesions (especially erosions and fatty lesions) were present 11. This suggests that at this phase of the disease, BME is the feature with the best predictive value.
Van Onna et al performed a 2‐year follow‐up study 23, in which they recorded MRI status changes in 15% of their patients with recent‐onset IBP. These findings were similar to our own, although follow‐up time was considerably shorter in our study. On the other hand, our study included substantially more patients, and we used 2 validated scoring methods. Also in accordance with our results, their data showed that more patients had a negative follow‐up result after a positive baseline result, compared to patients who developed a positive result after a negative baseline result (30% versus 15%, respectively, based on 1‐year or 2‐year follow‐up MRI). Additionally, they found that male sex and HLA–B27 positivity were predictive of a positive MRI result at follow‐up, which is consistent with our finding that these factors independently determine the likelihood of a positive MRI result at any time point. HLA–B27–positive male patients with chronic back pain have the highest chance of a positive MRI result at any time.
Other studies have investigated the natural history of MRI‐detected BME in individuals with suspected axial SpA. Sengupta et al concluded that repeated MRI scans within a 12‐week period should be considered only for HLA–B27–positive men who fulfill the ASAS IBP criteria, since there were no HLA–B27–negative patients in their study who had a negative MRI result followed by a positive MRI result 24. Although their study included a considerably smaller group of patients, the data are consistent with our findings that HLA–B27 positivity determines the likelihood of a positive MRI result. Marzo‐Ortega et al also reported a higher chance of a positive MRI result at 1 year in untreated patients with early IBP who were HLA–B27 positive 25.
Regarding sex differences, ankylosing spondylitis (AS) has historically been considered a predominantly male disease, but it has been reported that 46% of patients diagnosed since 1990 were female, compared to 10% in 1960 26. This suggests that perceived male predominance in AS and axial SpA may be caused (at least in part) by a missed AS diagnosis among women (especially prior to 1990); data now suggest that the percentage of female patients with nonradiographic axial SpA and AS is substantial. Another reason for the higher male:female ratio in AS may be that men develop radiographic sacroiliitis at a higher rate than women. This is also in accordance with our findings that male patients are more likely to have a positive MRI result at any time point, which is a predictor of development of radiographic sacroiliitis 9.
Another issue we considered in our study is timing, specifically at what time point to repeat an MRI scan in the case of persistent suspected axial SpA. We looked at the 3‐month and 1‐year follow‐ups, and at both time points the additional value is very limited. Given the low diagnostic yield (and taking into account costs and feasibility), MRI should not be routinely repeated after 3 months or 1 year. Two‐year data on the SPACE cohort will be available in the future and will provide information on a longer period of time.
In general, MRI has become an important tool in the evaluation of patients who have (or may have) axial SpA. Relevant improvements in the field have taken place as a result, such as the standardization of imaging protocols and the development and validation of standardized descriptions of lesions. These descriptions pertain not only to inflammatory lesions but to structural lesions (e.g., fatty lesions, erosions, sclerosis, and ankylosis). MRI has the unique benefit of providing visualization of both inflammatory and structural lesions via one imaging technique. Additionally, it is hypothesized that assessment for structural lesions could enhance sensitivity and/or specificity, which might be helpful when a diagnosis is initially unclear. Research on the incremental value of including structural lesions is ongoing.
In terms of methodology, the fact that we repeated MRI in all patients irrespective of diagnosis is an important strength of our study, compared to studies that conducted scans in a select population within their patient groups. Moreover, our follow‐up was quite comprehensive and avoided the unintentional bias that could occur by excluding patients with a low likelihood of axial SpA. Another strength of our study is our scoring process that involved 2 readers and adjudication from a third reader in the case of discrepancy, which adds to the credibility of the findings. Moreover, the fact that we used 2 well‐validated scoring methods (ASAS criteria and SPARCC scoring system) provides additional insight. On the other hand, limitations of the current study include the relatively short duration of follow‐up and the fact that we could not compare these findings to an external standard. Diagnosis of axial SpA was influenced by MRI findings and could lead to circular reasoning. Moreover, we lacked validation from another imaging technique such as low‐dose computed tomography or from histology. Prospective evaluation over a sufficient time frame with a longer follow‐up should enhance confidence in the diagnosis of this sometimes slow‐to‐evolve disease.
In conclusion, ASAS‐defined, MRI‐detected axial SpA status changes were seen in a minority of the patients in the SPACE cohort, and both changes from negative to positive and from positive to negative occurred. A very small percentage of patients had a positive MRI result at follow‐up after having a negative result at baseline (4.3% at 3 months and 7.2% at 1 year), which indicates that the diagnostic usefulness of repeating an MRI of the SI joints at 3 months or 1 year is very limited. Relatively more patients had a negative MRI result after having a positive result at baseline (37.9% after 1 year), and the resolution of inflammation was partly caused by the use of anti‐TNF therapy. Male sex and HLA–B27 positivity independently determined the likelihood of a positive MRI result at any time point, while MRI‐detected axial SpA status at baseline strongly predicted status at follow‐up.
Author contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Bakker had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Van der Heijde.
Acquisition of data
Bakker, Ez‐Zaitouni, van Lunteren, Berg, Landewé, Ramonda, van Oosterhout, Reijnierse, van Gaalen, van der Heijde.
Analysis and interpretation of data
Bakker, Ramiro, van der Heijde.
Supporting information
References
- 1. Van den Berg R, de Hooge M, Rudwaleit M, Sieper J, van Gaalen F , Reijnierse M, et al. ASAS modification of the Berlin algorithm for diagnosing axial spondyloarthritis: results from the SPondyloArthritis Caught Early (SPACE)‐cohort and from the Assessment of SpondyloArthritis international Society (ASAS)‐cohort. Ann Rheum Dis 2013;72:1646–53. [DOI] [PubMed] [Google Scholar]
- 2. Feldtkeller E, Bruckel J, Khan MA. Scientific contributions of ankylosing spondylitis patient advocacy groups. Curr Opin Rheumatol 2000;12:239–47. [DOI] [PubMed] [Google Scholar]
- 3. Rudwaleit M, Khan MA, Sieper J. The challenge of diagnosis and classification in early ankylosing spondylitis: do we need new criteria? Arthritis Rheum 2005;52:1000–8. [DOI] [PubMed] [Google Scholar]
- 4. Kroon FP, van der Burg LR, Ramiro S, Landewe RB, Buchbinder R, Falzon L, et al. Nonsteroidal antiinflammatory drugs for axial spondyloarthritis: a Cochrane review. J Rheumatol 2016;43:607–17. [DOI] [PubMed] [Google Scholar]
- 5. Van der Heijde D, Ramiro S, Landewe R, Baraliakos X, van den Bosch F, Sepriano A, et al. 2016 update of the ASAS‐EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis 2017;76:978–91. [DOI] [PubMed] [Google Scholar]
- 6. Smolen JS, Schols M, Braun J, Dougados M, FitzGerald O, Gladman DD, et al. Treating axial spondyloarthritis and peripheral spondyloarthritis, especially psoriatic arthritis, to target: 2017 update of recommendations by an international task force. Ann Rheum Dis 2018;77:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lambert RG, Bakker PA, van der Heijde D, Weber U, Rudwaleit M, Hermann KG, et al. Defining active sacroiliitis on MRI for classification of axial spondyloarthritis: update by the ASAS MRI working group. Ann Rheum Dis 2016;75:1958–63. [DOI] [PubMed] [Google Scholar]
- 8. Oostveen J, Prevo R, den Boer J, van de Laar M. Early detection of sacroiliitis on magnetic resonance imaging and subsequent development of sacroiliitis on plain radiography: a prospective, longitudinal study. J Rheumatol 1999;26:1953–8. [PubMed] [Google Scholar]
- 9. Dougados M, Sepriano A, Molto A, van Lunteren M, Ramiro S, de Hooge M, et al. Sacroiliac radiographic progression in recent onset axial spondyloarthritis: the 5‐year data of the DESIR cohort. Ann Rheum Dis 2017;76:1823–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weber U, Lambert RG, Pedersen SJ, Hodler J, Ostergaard M, Maksymowych WP. Assessment of structural lesions in sacroiliac joints enhances diagnostic utility of magnetic resonance imaging in early spondylarthritis. Arthritis Care Res (Hoboken) 2010;62:1763–71. [DOI] [PubMed] [Google Scholar]
- 11. De Hooge M, van den Berg R, Navarro‐Compan V, Reijnierse M, van GF , Fagerli K, et al. Patients with chronic back pain of short duration from the SPACE cohort: which MRI structural lesions in the sacroiliac joints and inflammatory and structural lesions in the spine are most specific for axial spondyloarthritis? Ann Rheum Dis 2016;75:1308–14. [DOI] [PubMed] [Google Scholar]
- 12. Bakker PA, van den Berg R, Lenczner G, Thevenin F, Reijnierse M, Claudepierre P, et al. Can we use structural lesions seen on MRI of the sacroiliac joints reliably for the classification of patients according to the ASAS axial spondyloarthritis criteria? Data from the DESIR cohort. Ann Rheum Dis 2017;76:392–8. [DOI] [PubMed] [Google Scholar]
- 13. Rudwaleit M, van der Heijde D, Khan MA, Braun J, Sieper J. How to diagnose axial spondyloarthritis early. Ann Rheum Dis 2004;63:535–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mandl P, Navarro‐Compan V, Terslev L, Aegerter P, van der Heijde D, D'Agostino MA, et al. EULAR recommendations for the use of imaging in the diagnosis and management of spondyloarthritis in clinical practice. Ann Rheum Dis 2015;74:1327–39. [DOI] [PubMed] [Google Scholar]
- 15. Pedersen SJ, Poddubnyy D, Sorensen IJ, Loft AG, Hindrup JS, Thamsborg G, et al. Course of magnetic resonance imaging‐detected inflammation and structural lesions in the sacroiliac joints of patients in the randomized, double‐blind, placebo‐controlled Danish multicenter study of adalimumab in spondyloarthritis, as assessed by the Berlin and Spondyloarthritis Research Consortium of Canada methods. Arthritis Rheumatol 2016;68:418–29. [DOI] [PubMed] [Google Scholar]
- 16. Maksymowych WP, Dougados M, van der Heijde D, Sieper J, Braun J, Citera G, et al. Clinical and MRI responses to etanercept in early non‐radiographic axial spondyloarthritis: 48‐week results from the EMBARK study. Ann Rheum Dis 2016;75:1328–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Van den Berg R, de Hooge M, van GF , Reijnierse M, Huizinga T, van der Heijde D. Percentage of patients with spondyloarthritis in patients referred because of chronic back pain and performance of classification criteria: experience from the Spondyloarthritis Caught Early (SPACE) cohort. Rheumatology (Oxford) 2013;52:1492–9. [DOI] [PubMed] [Google Scholar]
- 18. Rudwaleit M, Jurik AG, Hermann KG, Landewe R, van der Heijde D, Baraliakos X, et al. Defining active sacroiliitis on magnetic resonance imaging (MRI) for classification of axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI group. Ann Rheum Dis 2009;68:1520–7. [DOI] [PubMed] [Google Scholar]
- 19. Van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis: a proposal for modification of the New York criteria. Arthritis Rheum 1984;27:361–8. [DOI] [PubMed] [Google Scholar]
- 20. Maksymowych WP, Inman RD, Salonen D, Dhillon SS, Williams M, Stone M, et al. Spondyloarthritis Research Consortium of Canada magnetic resonance imaging index for assessment of sacroiliac joint inflammation in ankylosing spondylitis. Arthritis Rheum 2005;53:703–9. [DOI] [PubMed] [Google Scholar]
- 21. Sieper J, van der Heijde D, Landewe R, Brandt J, Burgos‐Vagas R, Collantes‐Estevez E, et al. New criteria for inflammatory back pain in patients with chronic back pain: a real patient exercise by experts from the Assessment of SpondyloArthritis international Society (ASAS). Ann Rheum Dis 2009;68:784–8. [DOI] [PubMed] [Google Scholar]
- 22. Mean P.. Calculating predicted probabilities from a logistic regression model. 2013. URL: http://www.pmean.com/13/predicted.html.
- 23. Van Onna M, Jurik AG, van der Heijde D, van Tubergen A, Heuft‐Dorenbosch L, Landewe R. HLA‐B27 and gender independently determine the likelihood of a positive MRI of the sacroiliac joints in patients with early inflammatory back pain: a 2‐year MRI follow‐up study. Ann Rheum Dis 2011;70:1981–5. [DOI] [PubMed] [Google Scholar]
- 24. Sengupta R, Marzo‐Ortega H, McGonagle D, Wadeley A, Bennett AN. Short‐term repeat magnetic resonance imaging scans in suspected early axial spondyloarthritis are clinically relevant only in HLA‐B27‐positive male subjects. J Rheumatol 2018;45:202–5. [DOI] [PubMed] [Google Scholar]
- 25. Marzo‐Ortega H, McGonagle D, O'Connor P, Hensor EM, Bennett AN, Green MJ, et al. Baseline and 1‐year magnetic resonance imaging of the sacroiliac joint and lumbar spine in very early inflammatory back pain: relationship between symptoms, HLA‐B27 and disease extent and persistence. Ann Rheum Dis 2009;68:1721–7. [DOI] [PubMed] [Google Scholar]
- 26. Stolwijk C, van Onna M, Boonen A, van Tubergen A. Global prevalence of spondyloarthritis: a systematic review and meta‐regression analysis. Arthritis Care Res (Hoboken) 2016;68:1320–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


