Abstract
The pioneer forkhead box (FOX)A2 transcription factor is specifically expressed in the glands of the uterus, which are central to endometrial function and fertility. In mice, FOXA2 is a critical regulator of uterine gland development in the neonate and gland function in the adult. An integrative approach was used here to define the FOXA2 cistrome in the human endometrium. Genome-wide mapping of FOXA2 binding intervals by chromatin immunoprecipitation sequencing was performed using proliferative (P)- and midsecretory (MS)-phase endometrium and integrated with the transcriptome determined by RNA sequencing. Distinctive FOXA2 binding intervals, enriched for different transcription factor binding site motifs, were detected in the P and MS endometrium. Pathway analysis revealed different biologic processes regulated by genes with FOXA2 binding intervals in the P and MS endometrium. Thus, FOXA2 is postulated to regulate gene expression in concert with other transcription factors and impact uterine gland development and function in a cycle phase–dependent manner. Analyses also identified potential FOXA2-regulated genes that influence uterine receptivity, blastocyst implantation, and stromal cell decidualization, which are key events in pregnancy establishment.—Kelleher, A. M., Behura, S. K., Burns, G. W., Young, S. L., DeMayo, F. J., Spencer, T. E. Integrative analysis of the forkhead box A2 (FOXA2) cistrome for the human endometrium.
Keywords: uterus, ChIP-seq, transcription factor
Uterine glands are a prominent feature of the endometrium and essential for pregnancy success. Mice that lack uterine glands are infertile because of defects in uterine receptivity and blastocyst implantation (1–6). In women, glands of the functionalis endometrium exhibit marked growth during the proliferative (P) phase and differentiation during the midsecretory (MS) phase of the menstrual cycle (7). Available evidence in humans supports a primary role for uterine glands and their secretions and products in blastocyst implantation, pregnancy establishment, and recurrent pregnancy loss (8–10). Uterine glands are also postulated to impact stromal cell decidualization (2), which is essential for pregnancy establishment in humans and rodents (11, 12). Recently, defective stromal cell decidualization in early pregnancy was linked to later pregnancy complications such as preeclampsia and miscarriage (13, 14). Thus, uterine glands and, by inference, their secretions and products are essential determinants of the embryotrophic potential and functional capacity of the uterus for pregnancy.
Forkhead box (FOX)A2 is a pioneer transcription factor that belongs to a family of 3 forkhead transcription factors encoded by different genes (15) with essential roles in development, function, and disease in many organs (16, 17). In neonatal and adult mice, FOXA2 is the only FOXA member expressed in the endometrium and exclusively detected in the glandular epithelium (GE) cells (1, 3, 4). Conditional-deletion studies in mice found that Foxa2 is a critical regulator of uterine gland development in the neonate and differentiated gland function for pregnancy establishment in the adult (1, 2, 4). In the glands of the mouse uterus, Foxa2 is critical for estrogen induction of leukemia inhibitory factor and progesterone induction of many genes encoding secreted proteins such as Prss28 protease, Ser28 and protease, Ser29 (1, 2, 18, 19). Of note, leukemia inhibitory factor is a critical GE-derived factor essential for uterine receptivity and blastocyst implantation (1, 20). In humans, FOXA2 is also expressed in GE of the uterus and is mutated or down-regulated in several of the most prevalent uterine diseases, including complex atypical endometrial hyperplasia (21), uterine carcinosarcomas and carcinomas (22, 23), and endometriosis (24). However, little is known about the biology of FOXA2 in the human uterus.
Available evidence supports the hypothesis that FOXA2 is involved in uterine gland development, function, and disease in humans. Here, a genome-wide investigation of in vivo FOXA2 binding was conducted in P- and MS-phase human endometrium using chromatin immunoprecipitation (ChIP) coupled with massively parallel ChIP sequencing (ChIP-Seq). Those data were then integrated with the transcriptomes of P and MS endometrium determined by RNA sequencing (RNA-Seq). This integrative approach provides important insights into the genes and biologic pathways regulated by FOXA2 and provides a foundation for mechanistic studies into FOXA2 function in the human endometrium.
MATERIALS AND METHODS
Human endometrial samples
Healthy women ages 19–37 with a regular intermenstrual interval between 25 and 35 d and no history of infertility or pelvic disease were recruited to participate in the study. Subjects were excluded if they met any of the following criteria: 1) an intermenstrual interval that varied by more than 3 d; 2) use of any medication known to affect reproductive hormones or fertility within the 60 d prior to enrollment; 3) chronic disease (endometriosis, endometrial cancer, polycystic ovaries); 4) a body mass index >37.9 or <19.5; or 5) history of infertility (defined as a failure to conceive for ≥1 yr despite regular intercourse without contraception). The study was reviewed and approved by the Committee for the Protection of Human Subjects (Institutional Review Board) at the University of North Carolina–Chapel Hill.
Endometrial specimens were collected from women using a Pipelle suction device (CooperSurgical, Trumbull, CT, USA) in an outpatient clinic setting. Samples were immediately flash frozen in liquid nitrogen, and a portion of the sample was fixed in formalin and embedded in paraffin for histology. Samples of P endometrium were collected between 8 and 12 d after onset of menses, and MS samples were collected 6–10 d after urinary detection of the LH surge. Sections of tissue stained with hematoxylin and eosin were examined to confirm cycle phase using the Noyes criteria to exclude unexpected abnormalities (25).
Immunolocalization of FOXA2
Fixed endometrial biopsies (n = 4/phase) were sectioned (5 µm), mounted on slides, deparaffinized in xylene, and rehydrated in a graded alcohol series. Antigen retrieval was performed by incubating sections in boiling 10 mM citrate buffer (pH 6.0) for 10 min. Sections were then blocked with 5% (v/v) normal goat serum (50062Z; Thermo Fisher Scientific, Waltham, MA, USA) in PBS (pH 7.2) at room temperature for 1 h and then incubated with rabbit FOXA2 mAb [1:1000; ab108422; Research Resource Identifier (RRID): AB_11157157; Abcam, Cambridge, United Kingdom] or rabbit cadherin 1 (E-cadherin) mAb (1:1000; 24E10; RRID: AB_10694492; Cell Signaling Technology, Danvers, MA, USA) overnight at 4°C in 1% bovine serum albumin in PBS. Sections were washed in PBS with 0.05% Tween 20, and immunofluorescence detection was performed using secondary Alexa Fluor 555–conjugated donkey anti-rabbit IgG (H+L) pAb (1:400; A-31572; RRID: AB_162543; Thermo Fisher Scientific) incubated for 90 min at room temperature. Sections were stained with Hoechst 33342 (2 μg/ml; H3570; Thermo Fisher Scientific) before coverslips were affixed with ProLong Gold Antifade Mountant (Thermo Fisher Scientific). Fluorescent images were procured using a Leica DM5500 B upright microscope and Leica DFC450 C camera using Leica Application Suite X (Leica Microsystems, Wetzlar, Germany).
RNA extraction, sequencing, and analyses
Total RNA was isolated from frozen endometrial biopsies (n = 6 P phase and n = 5 MS phase) using a standard Trizol-based protocol (Thermo Fisher Scientific). Extracted RNA was treated with DNase I and purified using an RNeasy MinElute Cleanup Kit (Qiagen, Hilden, Germany) to eliminate genomic DNA contamination. Quality and concentration of RNA were determined using a Fragment Analyzer (Agilent Technologies, Santa Clara, CA, USA). Libraries were prepared by the University of Missouri DNA Core Facility (Columbia, MO, USA) using an Illumina TruSeq mRNA kit (Illumina, San Diego, CA, USA) and sequenced (2 × 75 bp paired-end to a depth of 40–50 million reads) using an Illumina NextSeq 500 (Illumina). Adapters were trimmed from reads using cutadapt (v.1.11; https://cutadapt.readthedocs.io/en/stable/index.html) and quality trimmed to a sliding window quality score of 30 and minimum length of 20 bp with fqtrim software (https://ccb.jhu.edu/software/fqtrim/). Reads were mapped to the Homo sapiens genome assembly (GRCh38/hg38) using Hierarchical Indexing for Spliced Alignment of Transcripts 2 (v.2.0.3) (26). Reads overlapping Ensembl annotations were quantified with featureCounts (v.1.5.0) (27). Genes with evidence of expression (counts per million; rowSum >0) were used for model-based differential expression analysis using the edgeR robust method (28). Differentially expressed gene (DEG) list [false discovery rate (FDR); P < 0.05] enrichment analysis was conducted using ToppFun (https://toppgene.cchmc.org/) with default settings (29). The Bonferroni procedure was used to control FDR for gene ontology term and pathway enrichment analyses. Raw FASTQ files were deposited in the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) under GSE119209.
ChIP, sequencing, and analyses
Frozen human endometrium biopsy tissue (P phase, n = 2; MS phase, n = 2) was submerged in PBS containing 1% formaldehyde, cut into small pieces, and incubated at room temperature for 15 min. Fixation was stopped by the addition of 0.125 M glycine (final). The tissue pieces were then homogenized with a Tissue-Tearor (BioSpec, Bartlesville, OK, USA), centrifuged, and washed twice in PBS. Chromatin was isolated by the addition of lysis buffer followed by disruption with a Dounce homogenizer. Lysates were sonicated, and DNA was sheared to a mean length of 300–500 bp. Genomic DNA (input) was prepared by treating aliquots of chromatin with RNase, proteinase K, and heat for de–cross-linking, followed by ethanol precipitation. Pellets were resuspended, and the resulting DNA was quantified on a NanoDrop spectrophotometer (Thermo Fisher Scientific). Extrapolation to the original chromatin volume allowed quantitation of the total chromatin yield.
An aliquot of chromatin (30 μg) was precleared with protein G agarose beads (Thermo Fisher Scientific). Genomic DNA regions of interest were isolated using 4 μg of goat anti-human FOXA2 pAb (sc-6554, Lot I1715; RRID: AB_2262810; Santa Cruz Biotechnology, Dallas, TX, USA). Complexes were washed, eluted from the beads with SDS buffer, and subjected to RNase and proteinase K treatment. Cross-links were reversed by incubation overnight at 65°C, and ChIP DNA was purified by phenol-chloroform extraction and ethanol precipitation.
Illumina sequencing libraries were prepared from the ChIP and input DNAs by the standard consecutive enzymatic steps of end polishing, dA addition, and adaptor ligation. After a final PCR amplification step, the resulting DNA libraries were quantified and sequenced on an Illumina NextSeq 500 (75 nt reads, single end). Raw sequences were assessed by the FastQC tool (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/) for data quality. The program fqtrim was used to perform quality trimming (phred score >30) by a sliding window scan (6 nt) and to remove reads that were <20 nt in length. Reads were aligned to the human genome (hg38) using the Burrows-Wheeler Aligner algorithm (default settings). The postalignment quality control steps, including removing PCR duplications, number of mismatches, and uniquely mapped reads, were performed using the binary alignment files by Picard (https://broadinstitute.github.io/picard/) per Encyclopedia of DNA Elements guidelines. Peak locations were determined using the Model-Based Analysis of ChIP-Seq algorithm (v.2.1.0) with a cutoff of P = 0.01. Bedtools and custom awk scripts were used to generate detailed information on peak metrics, peak locations, and distance to closest genes as well as determine common or specific peaks to the sample groups. ChIP-Seq data were deposited into the NCBI GEO (GSE121586).
Transcription factor binding site (TFBS) motifs in FOXA2 binding intervals from P and MS endometrium were determined and compared using the Hypergeometric Optimization of Motif Enrichment (HOMER) de novo motif discovery suite of tools (http://homer.ucsd.edu/homer/) (30).
Receptor ligand and network analysis methods
Receptor-ligand interactions were predicted using the Functional Annotation of the Mammalian Genome (FANTOM5) database (http://fantom.gsc.riken.jp/5/) (31). Genes with FOXA2 binding intervals near or within them and expressed in the P or MS phases were used as the query to search ligand molecules in the database. Expression of their corresponding receptors was determined from the RNA-Seq data of human endometrial stromal cells (ESCs) (NCBI GEO: GSE112362) (32).
Statistical analyses
All statistical tests, including hypergeometric tests, were conducted in R (https://www.r-project.org/). A binary logit regression analysis was conducted, in which change in gene expression in the MS phase relative to the P phase was used a dependent variable, and binding to MS, P, or both phases was used as the predictor variable. In this analysis, a gene significantly differentially expressed (as determined by edgeR robust) was assigned 1; otherwise it was assigned 0. The genomic position of FOXA2 binding sites (as determined by Model-Based Analysis of ChIP-Seq 2) along with gene coordinates were analyzed by bedtools (https://bedtools.readthedocs.io/en/latest/#) to find the peak closest to each gene. The pattern of FOXA2 binding sites relative to the closest genes was used as a predictor variable in the binary regression model. Model fitting was performed using a generalized linear model (family = binary) to determine the regression coefficients and significance of the association between FOXA2 binding and differential gene expression between P and MS phases. For expression network analysis, the R package minet (http://minet.meyerp.com/) was used to infer mutual information (MI) of expression variation between genes. The MI analysis measures the information content between 2 variables (genes in this case) and determines how much knowing 1 variable would predict variability of the other. The MI values were used to generate a weighted adjacency matrix to construct the gene expression network.
RESULTS
FOXA2 protein is located specifically in glands of the human endometrium
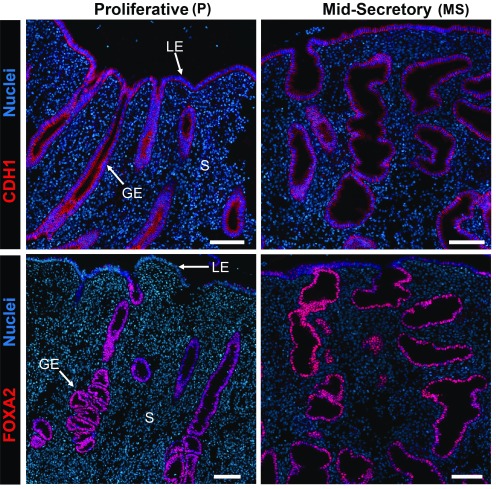
Biopsy samples of P- and MS-phase human endometrium were evaluated for FOXA2 by immunofluorescence analysis (Fig. 1). Immunoreactive FOXA2 was observed predominantly in the nucleus of GE cells as well as some luminal epithelium cells (particularly in the MS phase) but not in the stroma or other cells of the functionalis endometrium. The hypertrophy of glands in the MS-phase endometrium is distinctive of secretory transformation by progesterone.
Figure 1.
FOXA2 and cadherin 1 (CDH1), an epithelial marker protein, in the human endometrium. Endometrial biopsies (n = 4/phase) were analyzed by immunofluorescence and counterstained with DAPI to visualize nuclei. GE, glandular epithelium; LE, luminal epithelium; S, stroma. Scale bars, 100 μm.
Identification of FOXA2 binding sites in human endometrium
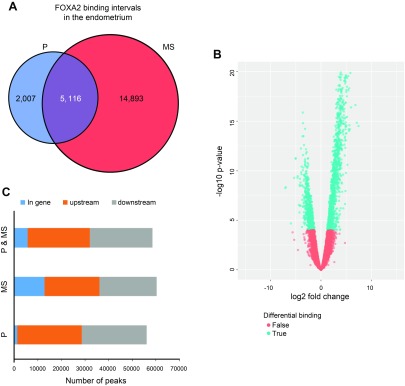
To identify direct targets of FOXA2, in vivo FOXA2 binding sites in P- and MS-phase endometrium was determined by ChIP-Seq analysis. A total of 22,016 FOXA2 binding intervals were identified in both samples of P and MS endometrium (Fig. 2A, B). A more significant number of FOXA2 binding intervals were identified in the MS (20,009) as compared with P endometrium (7123), with 5116 intervals in common. Representative University of California–Santa Cruz genome browser custom tracks for FOXA2 binding intervals near the transcription start site (TSS) of the E2F-associated phosphoprotein, kinesin family member 9, leucyl-cystinyl aminopeptidase, and protein-L-isoaspartate (D-aspartate) O-methyltransferase domain containing 1 (PCMTD1) genes is provided in Supplemental Fig. S1. Overall, more FOXA2 binding intervals were located in the upstream or downstream regions of genes than within genes in both P- and MS-phase endometrium samples (Fig. 2C). Furthermore, there were more FOXA2 binding intervals located within genes in the MS than the P phase and in the upstream regions of genes in the P than the MS phase.
Figure 2.
ChIP-Seq analysis of FOXA2 binding in the P- or MS-phase endometrium. A) Venn diagram of nonredundant FOXA2 intervals in the P- and MS-phase endometrium. B) Volcano plot illustrating the pattern of differential binding of FOXA2 binding locations in the endometrium during the P and MS phases. The locations with log-fold change >0 are associated with MS phase, and those with log fold change <0 are associated with the P phase. Thus, more FOXA2-bound genes are found in the MS than in the P phase. C) Distribution of genome-wide FOXA2 binding intervals relative to TSSs of nearby genes.
HOMER analysis was conducted to identify and compare TFBS motifs that were enriched in FOXA2 binding intervals (30). As expected, the FOXA2 motif was highly enriched in FOXA2 binding intervals in both P and MS endometrium samples (Table 1). HOMER analysis revealed differential enrichment of TFBS motifs in FOXA2 binding intervals from P as compared with MS endometrium. In the P endometrium, the top 15 enriched motifs included sex-determining region Y-box 17 (SOX17) and estrogen receptor 1 (ESR1) (Supplemental Table S1). The top 15 motifs enriched in the MS endometrium included fos-like antigen 2 (FOSL2) and CCAAT enhancer binding protein alpha (CEBPA) (Supplemental Table S2), whereas motifs common to both P and MS endometrium included ELK1 and Yin Yang 1 (Supplemental Table S3).
TABLE 1.
HOMER motif analysis of FOXA2 binding intervals in P- and MS-phase endometrium
| Rank | Best-match motif |
Target (%) |
Background (%) |
|---|---|---|---|
| P and MS endometrium | |||
| 1 | FOXA2 | 11.6 | 2.4 |
| 2 | NAC025 | 53.2 | 36.6 |
| 3 | JUNB | 8.3 | 2.5 |
| 4 | MAC1 | 59.6 | 48.2 |
| 5 | NFY | 7.2 | 2.8 |
| 6 | MBNL1 | 27.64 | 18.7 |
| 7 | CEBPA | 4.0 | 1.1 |
| 8 | MYB3R1 | 43.2 | 33.0 |
| 9 | ETV2 | 24.9 | 16.8 |
| 10 | RBP1 | 23.8 | 16.0 |
| Only P endometrium | |||
| 1 | FOXA2 | 29.3 | 5.5 |
| 2 | SOX17 | 30.0 | 13.8 |
| 3 | ERE | 5.8 | 0.5 |
| 4 | MYOG | 16.4 | 8.1 |
| 5 | MOD | 1.7 | 0.1 |
| 6 | ZNF467 | 34.2 | 24.3 |
| 7 | MAC1 | 44.0 | 33.4 |
| 8 | DOF42 | 16.5 | 9.7 |
| 9 | RBM6 | 4.5 | 1.5 |
| 10 | HOXA6 | 0.6 | 0.0 |
| Only MS endometrium | |||
| 1 | FOSL2 | 9.1 | 2.2 |
| 2 | FOXA2 | 8.8 | 2.5 |
| 3 | HAL9 | 40.6 | 26.8 |
| 4 | CEBPA | 5.1 | 1.1 |
| 5 | EIP74EF | 47.3 | 34.4 |
| 6 | YLR278C | 41.6 | 30.7 |
| 7 | CCAAT-box | 7.3 | 3.0 |
| 8 | RBM4.3 | 26.0 | 18.3 |
| 9 | MSN2 | 16.7 | 10.6 |
| 10 | CRE | 12.1 | 7.1 |
Association of FOXA2 binding with differential expression of genes between P and MS phases
The transcriptome of endometrial biopsies from P- and MS-phase endometrium was determined by RNA-Seq (NCBI GEO: GSE119209). Consistent with other RNA-Seq studies of human endometrium (33, 34), substantial alterations in gene expression were observed between the P- and MS-phase endometrium (Supplemental Fig. S2). Overall, there were 8641 DEGs with about an equal number of genes increased (4401) and decreased (4240) between the 2 phases. Overall, genes with increased expression in the MS endometrium were enriched in biologic pathways involved in neutrophil degranulation, signaling by interleukins, and innate immune response. In contrast, genes with increased expression in the P endometrium were enriched in pathways involved in cell cycle, DNA replication, and chromosome maintenance. As expected from FOXA2 protein localization in P and MS endometrium (Fig. 1), FOXA2 expression was not different between the P and MS endometrium samples. Of note, the other FOXA family members (FOXA1 and FOXA3) were not expressed [<1 fragment per kilobase of transcript per million mapped reads (FPKM)] in the P and MS endometrium.
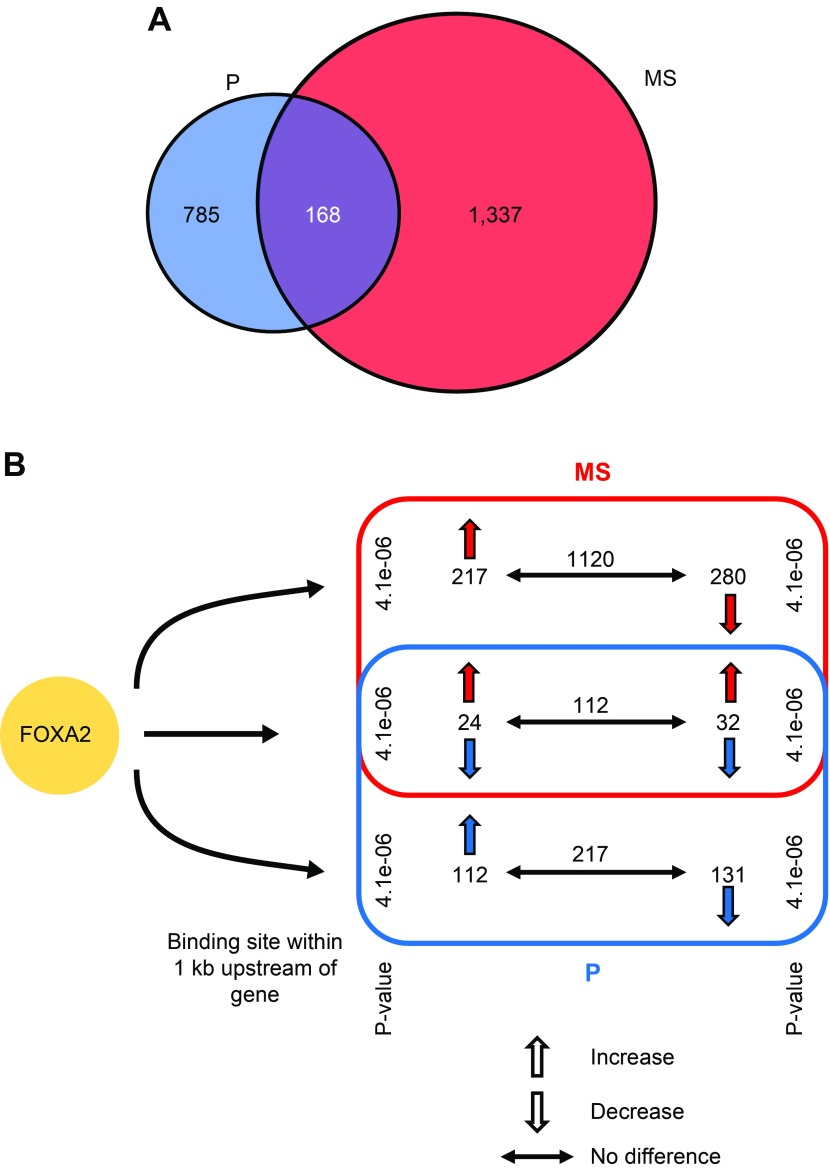
Integration of RNA-Seq and ChIP-Seq data found that 2290 expressed genes in the P and MS endometrium contained a FOXA2 binding interval near or within them (Fig. 3A). Of those 2290 genes, 785 were specific to the P phase, 1337 were specific to the MS phase, and 168 were common to both. The 785 P phase–specific genes were enriched in many biologic processes, including cell motility, cell migration, and regulation of actin filament–based processes. The 1337 MS phase–specific genes were enriched for different biologic processes, including chromosome segregation, cell cycle, and cytoskeletal organization. Expression of the 2290 genes either increased, decreased, or did not change between the P and MS phase (Fig. 3B). RNA-Seq analysis found that 153 DEGs (59 increased, 94 decreased) exhibited greater than a 3-fold change in expression level between the P and MS phase, and all of those DEGs contained an upstream FOXA2 binding interval within 1 kb from their predicted TSS. Biologic processes associated with the 94 genes decreased in the MS phase included cell cycle and mitosis, whereas the 59 genes increased in the MS phase included negative regulation of gene expression.
Figure 3.
Impact of FOXA2 binding upstream of genes on their expression in the P- and MS-phase endometrium. A) Genes that are expressed in the endometrium with a FOXA2 binding interval located within 1 kb of their predicted TSS based on integration of ChIP-Seq and RNA-Seq analysis. B) Differential expression of genes with FOXA2 binding intervals located within 1 kb upstream of the TSS. The P values show significance of overrepresentation of FOXA2 binding sites to the DEGs between the 2 phases.
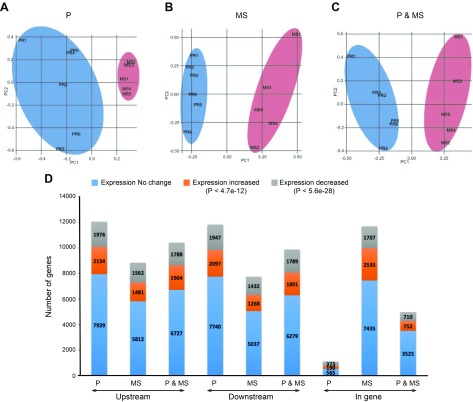
Principal component analysis was then used to analyze the influence of FOXA2 binding intervals on gene expression variation in P and MS endometrium (Fig. 4). The presence of FOXA2 binding intervals in genes within a specific phase (P or MS) displayed higher variance in expression than in the other phase (Fig. 4A, B). In contrast, there was no change in expression of genes that contained FOXA2 binding intervals in both P and MS endometrium (Fig. 4C). The proportion of genes displaying an increased or decreased level of expression varied depending upon the location of the FOXA2 binding interval relative to the genes (Fig. 4D). A regression analysis was performed to determine whether FOXA2 binding in 1 phase compared with the other (or both the phases) influences changes in gene expression between the 2 phases. This analysis revealed that genes associated with FOXA2 binding intervals specifically in the P endometrium displayed reduced variation in expression. Based on the regression coefficient, a unit increase of FOXA2 binding in the P phase was predicted to reduce the number of DEGs by 9.1% (P = 3.3e−08). In contrast, genes bound by FOXA2 specifically in the MS endometrium (sites were not found in P endometrium) displayed a higher variation of expression. A unit increase of FOXA2 binding in the MS phase was predicted to increase the number of DEGs by 7.1% (P = 3.3e−08). However, if FOXA2 binds genes in both P and MS endometrium, there was no effect (P = 0.18) on gene expression variation and, hence, the number of DEGs.
Figure 4.
Influence of FOXA2 on gene expression variation between P- and MS-phase endometrium. A–C) Gene expression variation between P and MS endometrium analyzed by principal component (PC) analysis. D) Genes displaying an increased or decreased level of or no change in expression varied depending upon location of the FOXA2 binding interval relative to the position of the gene.
FOXA2 and uterine receptivity
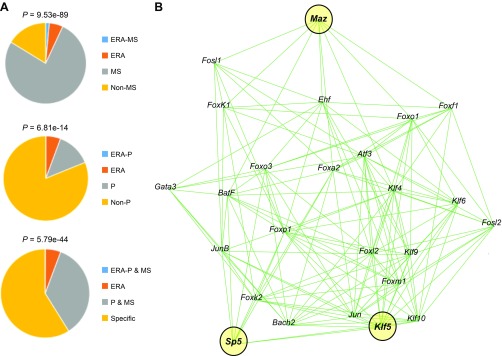
The endometrial receptivity array (ERA) is a collection of 238 genes associated with uterine receptivity for blastocyst implantation in women (35). Hypergeometric tests were performed based on the number of genes expressed in the ERA, phase, and both in ERA and phase using RNA-Seq data. As expected, significant enrichment of ERA genes was found in the MS phase (Fig. 5A). Of the 238 ERA genes, 62 of them were found to contain FOXA2 binding intervals unique to the MS phase (Supplemental Table S4). HOMER motif enrichment analysis of the FOXA2 binding intervals in those 62 genes identified 46 different TFBS motifs. RNA-Seq data indicated that 28 of those 46 transcription factors are expressed in the endometrium, with 14 displaying increased expression in the MS endometrium (Table 2). MI analysis was conducted to discern potential crosstalk among the 28 transcription factors (Fig. 5B). Centrality analysis of that network predicted 3 top key players [Kruppel like factor (KLF) 5, MYC-associated zinc finger protein (purine-binding transcription factor) (MAZ), trans-acting transcription factor 5 (SP5); centrality score = 7.99] that likely have a fundamental role in endometrial receptivity for blastocyst implantation.
Figure 5.
Analysis of FOXA2 binding intervals in ERA genes. A) Venn diagrams comparing unique or common nonredundant genes with an associated FOXA2 binding interval in the proliferative (P)- and mid-secretory (MS)-phase endometrium based on RNA-Seq and overlap with ERA genes. Hypergeometric P values of overrepresentation of ERA genes with genes expressed in the specific phase or common to both the phases are shown. B) MI and centrality analysis of 28 transcription factors expressed in MS endometrium that have binding motifs in FOXA2 binding intervals associated with ERA genes determined by HOMER motif analysis of ChIP-Seq data. The top key players are highlighted in yellow.
TABLE 2.
Expression of transcription factors in P and MS endometrium corresponding to enriched motifs in FOXA2 binding intervals from the ERA
| Gene | Ensembl identifier | Log FCa | FDR P | P endometrium (FPKM)b | MS endometrium (FPKM)b |
|---|---|---|---|---|---|
| ATF3 | ENSG00000162772 | 1.24 | 0.01 | 5.87 | 13.67 |
| BACH2 | ENSG00000112182 | 0.72 | 0.07 | 3.83 | 6.69 |
| BATF | ENSG00000156127 | 1.66 | 0.01 | 0.51 | 1.42 |
| EHF | ENSG00000135373 | −0.37 | 0.30 | 65.21 | 48.58 |
| FOSL1 | ENSG00000175592 | 0.75 | 0.08 | 0.93 | 1.61 |
| FOSL2 | ENSG00000075426 | 2.13 | 0.00 | 84.84 | 363.01 |
| FOXA2 | ENSG00000125798 | −0.26 | 0.35 | 26.09 | 21.64 |
| FOXA3 | ENSG00000170608 | 0.89 | 0.28 | 0.23 | 0.44 |
| FOXF1 | ENSG00000103241 | −0.25 | 0.53 | 1.58 | 1.33 |
| FOXK1 | ENSG00000164916 | −0.23 | 0.34 | 35.71 | 29.53 |
| FOXK2 | ENSG00000141568 | 0.18 | 0.31 | 47.1 | 53.54 |
| FOXL2 | ENSG00000183770 | −1.91 | 0.01 | 73.95 | 19.56 |
| FOXM1 | ENSG00000111206 | −2.77 | 0.01 | 48.01 | 7.77 |
| FOXO1 | ENSG00000150907 | 2.85 | 0.01 | 52.18 | 382.49 |
| FOXO3 | ENSG00000118689 | −0.5 | 0.01 | 69.45 | 48.44 |
| FOXP1 | ENSG00000114861 | 1.14 | 0.01 | 28.56 | 64.6 |
| GATA3 | ENSG00000107485 | 1.72 | 0.01 | 1.11 | 3.78 |
| HOXA9 | ENSG00000078399 | −0.65 | 0.01 | 34.8 | 21.58 |
| JUN | ENSG00000177606 | 1.09 | 0.01 | 53.94 | 115.33 |
| JUNB | ENSG00000171223 | 1.78 | 0.01 | 8.63 | 25.69 |
| KLF10 | ENSG00000155090 | −0.97 | 0.01 | 96.23 | 49.62 |
| KLF4 | ENSG00000136826 | 1.46 | 0.01 | 15.33 | 42.01 |
| KLF5 | ENSG00000102554 | 2.28 | 0.01 | 18.75 | 93.77 |
| KLF6 | ENSG00000067082 | 3.15 | 0.01 | 49.56 | 438.92 |
| KLF9 | ENSG00000119138 | 1.67 | 0.01 | 25.11 | 79.84 |
| MAZ | ENSG00000103495 | −0.43 | 0.10 | 19.38 | 14.55 |
| MITF | ENSG00000187098 | 1.43 | 0.01 | 58.56 | 154.12 |
| SP5 | ENSG00000204335 | 1.1 | 0.03 | 2.71 | 6.70 |
Log-fold change based on RNA-Seq analysis. Log FC, log-fold change.
Mean expression of gene based on RNA-Seq analysis.
FOXA2 regulated ligands and stromal cell decidualization
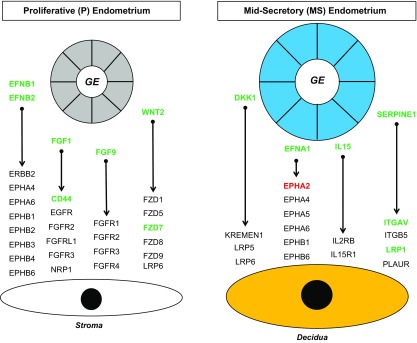
Recent evidence in mice and humans strongly supports the idea that secretions and products of the GE impact stromal cell decidualization in the endometrium (1, 2, 36, 37). First, the FANTOM5 database (31) of ligands and receptors was used to determine ligands whose encoded genes were associated with FOXA2 binding intervals based on P- and MS-phase endometrium ChIP-Seq data. Those ligands likely emanate from the glands given that FOXA2 is expressed only in the GE and not the stroma (Fig. 1). Second, receptors for those ligands were determined using transcriptome data from human ESCs before and after decidualization in vitro (NCBI GEO: GSE112362) (32). This analysis predicted 140 ligand-receptor interactions in the P-phase endometrium and 540 ligand-receptor interactions in the MS-phase endometrium. Of note, 46 ligand-receptor pairs have ligands that are also differentially expressed between the P- and MS-phase endometrium and corresponding receptors that are differentially expressed in human ESCs before and after decidualization (Fig. 6).
Figure 6.
Predicted ligand-receptor interactions between the GE and stroma in the endometrium. Selected ligands that are encoded by genes associated with FOXA2 binding intervals from ChIP-Seq analysis are presented for proliferative (P) and mid-secretory (MS) endometrium. Corresponding receptors are presented based on RNA-Seq analysis of human ESCs before (stroma) and after in vitro decidualization. Genes shown in green have higher expression levels than those in black, and those in red have lower expression than those in black, based on RNA-Seq analysis.
DISCUSSION
FOXA2 is termed a pioneer transcription factor because it can displace linker histone [H1], allowing for the decompaction of heterochromatin in an ATP-independent manner and facilitating binding of other transcription factors to gene enhancers (17, 38). FOXA proteins also have an N-terminal transactivation domain that recruits coregulators or other transcription factors, which in turn can facilitate gene expression. The present study found differential enrichment of TFBSs in FOXA2 binding intervals between the P- and MS-phase endometrium. Thus, FOXA2 is hypothesized to enable access of other transcription factors to coregulate gene expression in the endometrial GE of the human uterus. Indeed, dynamic changes in FOXA2 and associated transcription factors in relation to gene expression programs occurs in the developing embryo (39) as well as in the liver (40). Furthermore, FOXA1 and FOXA2 act in concert with other nuclear receptors, including ESR1 and glucocorticoid receptor (41, 42). Of note, FOXA2 is a tumor suppressor gene that is frequently mutated during the development of adenocarcinoma (22, 23) and down-regulated in endometriosis (43). Future studies should explore how FOXA2 regulates chromatin accessibility and impacts gene regulation in concert with steroid hormone receptors and other transcription factors in the GE of normal and diseased human endometrium.
The P phase of the endometrial cycle occurs after menstruation and is characterized by the growth of the functionalis endometrium and an increase in circulating levels of estrogen from the ovary (7). Growth of the functionalis endometrium involves proliferation of the luminal epithelium, GE, and stroma from stem cells in the underlying stratum basalis adjacent to the myometrium (44). The cyclical regeneration of glands in the functionalis endometrium may be similar to GE morphogenesis that occurs in the neonatal and prepubertal uterus (45, 46). Conditional deletion of Foxa2 in the neonatal mouse immediately after birth completely inhibited GE differentiation in the neonatal mouse (4), establishing that Foxa2 is a key regulator of GE differentiation and growth before puberty. A Foxa2 ChIP-Seq study of the postnatal d–12 mouse uterus found that GE-expressed genes with Foxa2 binding intervals were enriched for cell cycle, cell junction, focal adhesion, and WNT signaling (18). Of note, canonical WNT signaling is critical for uterine gland morphogenesis in the neonatal mouse uterus (47, 48). In the present study, FOXA2 binding intervals unique to P-phase endometrium contained SOX17, ESR1, ELK1, and nuclear receptor subfamily 4 group A member 2 TFBS motifs; all of those transcription factors are expressed in the GE of the human uterus, and their expression decreases in the MS phase based on RNA-Seq analysis. Of note, Sox17–conditional knockout mice lack uterine glands, indicating a possible reciprocal interaction between Sox17 and Foxa2 (49). Although glands initially develop in neonatal Esr1-null mice, Esr1 is required for maintenance of differentiated GE before puberty and GE function in the adult mouse uterus (46). There is no available information on the mechanistic role of ELK1 and nuclear receptor subfamily 4 group A member 2 in endometrial function. The present study identified many genes with FOXA2 binding intervals in the P-phase endometrium that encode secreted ligands, such as ephrin B1, fibroblast growth factor (FGF) 1, FGF9, and WNT2, with corresponding receptors on undecidualized ESCs (Fig. 6). Other studies have implicated WNTs and FGFs in endometrial regeneration (50, 51) and found that FGF9 stimulates ESC growth (52). Collective evidence supports the idea that FOXA2 in the P-phase endometrium cooperates with or enables the action of specific transcription factors that regulate genes involved in cell motility, cell migration, and other biologic processes essential for endometrial growth and regeneration.
The secretory phase of the menstrual cycle begins after ovulation and involves differentiation of the stratum functionalis because of rising levels of ovarian progesterone after ovulation (7). Progesterone-dependent changes in the endometrium include the secretory transformation of the glands as well as the onset of stromal cell decidualization. Between 7 and 10 d after ovulation, the endometrium becomes receptive to blastocyst implantation, termed the “window of implantation” (53, 54). Although microarray and RNA-Seq analysis have investigated gene expression changes between the P and MS phase, little is known about the molecular mechanisms mediating gene expression changes and which genes and their biologic functions are essential for acquisition of uterine receptivity for blastocyst implantation. The present study found that FOXA2 binding intervals in the MS-phase endometrium were associated with biologic pathways, including cell cycle, cytoskeletal organization, and regulation of gene expression. A number of TFBS motifs in FOXA2 binding intervals were unique to the MS-phase endometrium, including FOSL2, CEBPA, and CCCTC binding factor (CTCF) motifs. RNA-Seq data found no change in expression of CEBPA, a modest decrease in CTCF (−1.3 fold), and an increase in FOSL2 (4.4-fold) between the P- and MS-phase endometrium. All of those transcription factors (except CEBPA) are known to be expressed in the glands of the human endometrium. CTCF can function as a transcriptional activator, a repressor, or an insulator and regulates cell polarity of the GE (55). FOSL2 is a component of the activator protein 1 transcription factor complex and regulates cell proliferation, differentiation, and transformation, but its role in uterine glands is not known. Similarly, CEBPA function in endometrial glands is not known. These results support the idea that FOXA2 regulates genes in the MS-phase endometrium in concert with other transcription factors that influence biologic processes important for uterine receptivity.
The ERA test consists of a customized microarray based on the transcriptomic signature of human receptive endometrium, specifically when the endometrium is receptive to blastocyst adhesion and attachment (35, 53). Thus, the ERA test is employed to determine the optimal timing of the window of implantation for embryo transfer in a clinical setting. In the present study, about one-quarter of the 238 ERA genes were bound by FOXA2, suggesting that FOXA2 may have an essential role in the regulation of genes involved in the coordination the window of implantation. Establishment of pregnancy requires not only uterine receptivity and blastocyst implantation but also stromal cell decidualization (12, 54). The differentiation of ESCs into specialized decidual cells controls endometrial receptivity, embryo selection, embryo implantation, and placentation (12, 54). In women, altered or failed endometrial decidualization is an essential contributor to later pregnancy complications, such as preeclampsia and pregnancy loss (13), and may be involved in fetal growth restriction and preterm labor (56–58). Recent evidence supports the idea that GE-derived factors regulate stromal cell decidualization and placental growth in mice (2, 59) and humans (8–10, 60). In the present study, we identified many potential FOXA2-regulated genes in the MS phase that encode secreted ligands with receptors on decidualized stromal cells. Both Dickkopf 1 and IL-15 are expressed in the glands of the MS-phase endometrium (61, 62), but their effects on stromal cell decidualization have not been reported, although IL-11 enhances human ESC decidualization in vitro (36). The present study provides a solid foundation to begin exploring crosstalk between the GE and stroma of the human endometrium.
In summary, this study presents the first comprehensive analysis of the FOXA2 cistrome in the endometrium of the human uterus, thereby providing an important resource to understand FOXA2 biology and function. The results strongly support the idea that FOXA2 acts in concert with other transcription factors and nuclear receptors in a cycle phase–dependent manner to regulate genes and biologic pathways governing uterine gland differentiation and function that influence key events in pregnancy establishment, including blastocyst implantation, endometrial receptivity, and stromal cell decidualization as well as disease (endometrial cancer and endometriosis) in women.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This work was supported by the U.S. National Institutes of Health (NIH) Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant 1 R01 HD096266 (to T.E.S.). The authors declare no conflicts of interest.
Glossary
- CEBPA
CCAAT enhancer binding protein α
- ChIP
chromatin immunoprecipitation
- ChIP-Seq
ChIP sequencing
- CTCF
CCCTC binding factor
- DEG
differentially expressed gene
- ERA
endometrial receptivity array
- ESC
endometrial stromal cell
- ESR1
estrogen receptor 1
- FDR
false discovery rate
- FGF
fibroblast growth factor
- FPKM
fragment per kilobase of transcript per million mapped reads
- FOX
forkhead box
- GE
glandular epithelium
- GEO
Gene Expression Omnibus
- HOMER
Hypergeometric Optimization of Motif Enrichment
- KLF
Kruppel-like factor
- MI
mutual information
- MS
midsecretory
- NCBI
National Center for Biotechnology Information
- P
proliferative
- RNA-Seq
RNA sequencing
- RRID
Research Resource Identifier
- SOX17
sex-determining region Y-box 17
- TFBS
transcription factor binding site
- TSS
transcription start site
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
S. L. Young, F. J. DeMayo, and T. E. Spencer designed the research; A. M. Kelleher, S. K. Behura, and G. W. Burns performed the research and analyzed the data; and A. M. Kelleher, S. K. Behura, and T. E. Spencer wrote the manuscript.
REFERENCES
- 1.Kelleher A. M., Peng W., Pru J. K., Pru C. A., DeMayo F. J., Spencer T. E. (2017) Forkhead box a2 (FOXA2) is essential for uterine function and fertility. Proc. Natl. Acad. Sci. USA 114, E1018–E1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelleher A. M., Milano-Foster J., Behura S. K., Spencer T. E. (2018) Uterine glands coordinate on-time embryo implantation and impact endometrial decidualization for pregnancy success. Nat. Commun. 9, 2435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Filant J., Spencer T. E. (2013) Endometrial glands are essential for blastocyst implantation and decidualization in the mouse uterus. Biol. Reprod. 88, 93 [DOI] [PubMed] [Google Scholar]
- 4.Jeong J. W., Kwak I., Lee K. Y., Kim T. H., Large M. J., Stewart C. L., Kaestner K. H., Lydon J. P., DeMayo F. J. (2010) Foxa2 is essential for mouse endometrial gland development and fertility. Biol. Reprod. 83, 396–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooke P. S., Ekman G. C., Kaur J., Davila J., Bagchi I. C., Clark S. G., Dziuk P. J., Hayashi K., Bartol F. F. (2012) Brief exposure to progesterone during a critical neonatal window prevents uterine gland formation in mice. Biol. Reprod. 86, 63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelleher A. M., Burns G. W., Behura S., Wu G., Spencer T. E. (2016) Uterine glands impact uterine receptivity, luminal fluid homeostasis and blastocyst implantation. Sci. Rep. 6, 38078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noyes R. W. (1973) Normal phases of the endometrium. In The Uterus (Norris H. J., Hertig A. T., Abell M. R., eds.), pp. 110–135, Williams & Wilkins, Baltimore, MD, USA [Google Scholar]
- 8.Burton G. J., Scioscia M., Rademacher T. W. (2011) Endometrial secretions: creating a stimulatory microenvironment within the human early placenta and implications for the aetiopathogenesis of preeclampsia. J. Reprod. Immunol. 89, 118–125 [DOI] [PubMed] [Google Scholar]
- 9.Kelleher A. M., DeMayo F. J., Spencer T. E. (2019) Uterine glands: developmental biology and functional roles in pregnancy. Endocr. Rev. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spencer T. E. (2014) Biological roles of uterine glands in pregnancy. Semin. Reprod. Med. 32, 346–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das S. K. (2009) Cell cycle regulatory control for uterine stromal cell decidualization in implantation. Reproduction 137, 889–899 [DOI] [PubMed] [Google Scholar]
- 12.Gellersen B., Brosens J. J. (2014) Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr. Rev. 35, 851–905 [DOI] [PubMed] [Google Scholar]
- 13.Garrido-Gomez T., Dominguez F., Quiñonero A., Diaz-Gimeno P., Kapidzic M., Gormley M., Ona K., Padilla-Iserte P., McMaster M., Genbacev O., Perales A., Fisher S. J., Simón C. (2017) Defective decidualization during and after severe preeclampsia reveals a possible maternal contribution to the etiology. Proc. Natl. Acad. Sci. USA 114, E8468–E8477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conrad K. P., Rabaglino M. B., Post Uiterweer E. D. (2017) Emerging role for dysregulated decidualization in the genesis of preeclampsia. Placenta 60, 119–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedman J. R., Kaestner K. H. (2006) The Foxa family of transcription factors in development and metabolism. Cell. Mol. Life Sci. 63, 2317–2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaestner K. H. (2010) The FoxA factors in organogenesis and differentiation. Curr. Opin. Genet. Dev. 20, 527–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Golson M. L., Kaestner K. H. (2016) Fox transcription factors: from development to disease. Development 143, 4558–4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Filant J., Lydon J. P., Spencer T. E. (2014) Integrated chromatin immunoprecipitation sequencing and microarray analysis identifies FOXA2 target genes in the glands of the mouse uterus. FASEB J. 28, 230–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filant J., Spencer T. E. (2013) Cell-specific transcriptional profiling reveals candidate mechanisms regulating development and function of uterine epithelia in mice. Biol. Reprod. 89, 86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stewart C. L., Kaspar P., Brunet L. J., Bhatt H., Gadi I., Köntgen F., Abbondanzo S. J. (1992) Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature 359, 76–79 [DOI] [PubMed] [Google Scholar]
- 21.Villacorte M., Suzuki K., Hirasawa A., Ohkawa Y., Suyama M., Maruyama T., Aoki D., Ogino Y., Miyagawa S., Terabayashi T., Tomooka Y., Nakagata N., Yamada G. (2013) β-Catenin signaling regulates Foxa2 expression during endometrial hyperplasia formation. Oncogene 32, 3477–3482 [DOI] [PubMed] [Google Scholar]
- 22.Le Gallo M., Rudd M. L., Urick M. E., Hansen N. F., Merino M. J., Mutch D. G., Goodfellow P. J., Mullikin J. C., Bell D. W.; National Institutes of Health Intramural Sequencing Center Comparative Sequencing Program (2018) The FOXA2 transcription factor is frequently somatically mutated in uterine carcinosarcomas and carcinomas. Cancer 124, 65–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith B., Neff R., Cohn D. E., Backes F. J., Suarez A. A., Mutch D. G., Rush C. M., Walker C. J., Goodfellow P. J. (2016) The mutational spectrum of FOXA2 in endometrioid endometrial cancer points to a tumor suppressor role. Gynecol. Oncol. 143, 398–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hawkins S. M., Creighton C. J., Han D. Y., Zariff A., Anderson M. L., Gunaratne P. H., Matzuk M. M. (2011) Functional microRNA involved in endometriosis. Mol. Endocrinol. 25, 821–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noyes R. W., Hertig A. T., Rock J. (1950) Dating the endometrial biopsy. Fertil. Steril. 1, 3–25 [DOI] [PubMed] [Google Scholar]
- 26.Kim D., Langmead B., Salzberg S. L. (2015) HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12, 357–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liao Y., Smyth G. K., Shi W. (2014) featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 [DOI] [PubMed] [Google Scholar]
- 28.Zhou X., Lindsay H., Robinson M. D. (2014) Robustly detecting differential expression in RNA sequencing data using observation weights. Nucleic Acids Res. 42, e91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J., Bardes E. E., Aronow B. J., Jegga A. G. (2009) ToppGene Suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Res. 37, W305–W311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heinz S., Benner C., Spann N., Bertolino E., Lin Y. C., Laslo P., Cheng J. X., Murre C., Singh H., Glass C. K. (2010) Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramilowski J. A., Goldberg T., Harshbarger J., Kloppmann E., Lizio M., Satagopam V. P., Itoh M., Kawaji H., Carninci P., Rost B., Forrest A. R. (2015) A draft network of ligand-receptor-mediated multicellular signalling in human. Nat. Commun. 6, 7866; erratum: 7, 10706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szwarc M. M., Hai L., Gibbons W. E., Peavey M. C., White L. D., Mo Q., Lonard D. M., Kommagani R., Lanz R. B., DeMayo F. J., Lydon J. P. (2018) Human endometrial stromal cell decidualization requires transcriptional reprogramming by PLZF. Biol. Reprod. 98, 15–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sigurgeirsson B., Åmark H., Jemt A., Ujvari D., Westgren M., Lundeberg J., Gidlöf S. (2017) Comprehensive RNA sequencing of healthy human endometrium at two time points of the menstrual cycle. Biol. Reprod. 96, 24–33 [DOI] [PubMed] [Google Scholar]
- 34.Hu S., Yao G., Wang Y., Xu H., Ji X., He Y., Zhu Q., Chen Z., Sun Y. (2014) Transcriptomic changes during the pre-receptive to receptive transition in human endometrium detected by RNA-Seq. J. Clin. Endocrinol. Metab. 99, E2744–E2753 [DOI] [PubMed] [Google Scholar]
- 35.Díaz-Gimeno P., Horcajadas J. A., Martínez-Conejero J. A., Esteban F. J., Alamá P., Pellicer A., Simón C. (2011) A genomic diagnostic tool for human endometrial receptivity based on the transcriptomic signature. Fertil. Steril. 95, 50–60, 60.e1-15 [DOI] [PubMed] [Google Scholar]
- 36.Dimitriadis E., Robb L., Salamonsen L. A. (2002) Interleukin 11 advances progesterone-induced decidualization of human endometrial stromal cells. Mol. Hum. Reprod. 8, 636–643 [DOI] [PubMed] [Google Scholar]
- 37.Shuya L. L., Menkhorst E. M., Yap J., Li P., Lane N., Dimitriadis E. (2011) Leukemia inhibitory factor enhances endometrial stromal cell decidualization in humans and mice. PLoS One 6, e25288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zaret K. S., Carroll J. S. (2011) Pioneer transcription factors: establishing competence for gene expression. Genes Dev. 25, 2227–2241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Godini R., Fallahi H. (2019) Dynamics changes in the transcription factors during early human embryonic development. J. Cell. Physiol. 234, 6489–6502 [DOI] [PubMed] [Google Scholar]
- 40.Iwafuchi-Doi M., Donahue G., Kakumanu A., Watts J. A., Mahony S., Pugh B. F., Lee D., Kaestner K. H., Zaret K. S. (2016) The pioneer transcription factor FoxA maintains an accessible nucleosome configuration at enhancers for tissue-specific gene activation. Mol. Cell 62, 79–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whirledge S., Kisanga E. P., Taylor R. N., Cidlowski J. A. (2017) Pioneer factors FOXA1 and FOXA2 assist selective glucocorticoid receptor signaling in human endometrial cells. Endocrinology 158, 4076–4092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carroll J. S., Liu X. S., Brodsky A. S., Li W., Meyer C. A., Szary A. J., Eeckhoute J., Shao W., Hestermann E. V., Geistlinger T. R., Fox E. A., Silver P. A., Brown M. (2005) Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell 122, 33–43 [DOI] [PubMed] [Google Scholar]
- 43.Lin A., Yin J., Cheng C., Yang Z., Yang H. (2018) Decreased expression of FOXA2 promotes eutopic endometrial cell proliferation and migration in patients with endometriosis. Reprod. Biomed. Online 36, 181–187 [DOI] [PubMed] [Google Scholar]
- 44.Gargett C. E., Nguyen H. P., Ye L. (2012) Endometrial regeneration and endometrial stem/progenitor cells. Rev. Endocr. Metab. Disord. 13, 235–251 [DOI] [PubMed] [Google Scholar]
- 45.Cooke P. S., Spencer T. E., Bartol F. F., Hayashi K. (2013) Uterine glands: development, function and experimental model systems. Mol. Hum. Reprod. 19, 547–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nanjappa M. K., Medrano T. I., March A. G., Cooke P. S. (2015) Neonatal uterine and vaginal cell proliferation and adenogenesis are independent of estrogen receptor 1 (ESR1) in the mouse. Biol. Reprod. 92, 78 [DOI] [PubMed] [Google Scholar]
- 47.Jeong J. W., Lee H. S., Franco H. L., Broaddus R. R., Taketo M. M., Tsai S. Y., Lydon J. P., DeMayo F. J. (2009) beta-catenin mediates glandular formation and dysregulation of beta-catenin induces hyperplasia formation in the murine uterus. Oncogene 28, 31–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Farah O., Biechele S., Rossant J., Dufort D. (2018) Regulation of porcupine-dependent Wnt signaling is essential for uterine development and function. Reproduction 155, 93–102 [DOI] [PubMed] [Google Scholar]
- 49.Guimarães-Young A., Neff T., Dupuy A. J., Goodheart M. J. (2016) Conditional deletion of Sox17 reveals complex effects on uterine adenogenesis and function. Dev. Biol. 414, 219–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tulac S., Nayak N. R., Kao L. C., Van Waes M., Huang J., Lobo S., Germeyer A., Lessey B. A., Taylor R. N., Suchanek E., Giudice L. C. (2003) Identification, characterization, and regulation of the canonical Wnt signaling pathway in human endometrium. J. Clin. Endocrinol. Metab. 88, 3860–3866 [DOI] [PubMed] [Google Scholar]
- 51.Nallasamy S., Li Q., Bagchi M. K., Bagchi I. C. (2012) Msx homeobox genes critically regulate embryo implantation by controlling paracrine signaling between uterine stroma and epithelium. PLoS Genet. 8, e1002500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsai S. J., Wu M. H., Chen H. M., Chuang P. C., Wing L. Y. (2002) Fibroblast growth factor-9 is an endometrial stromal growth factor. Endocrinology 143, 2715–2721 [DOI] [PubMed] [Google Scholar]
- 53.Díaz-Gimeno P., Ruiz-Alonso M., Blesa D., Bosch N., Martínez-Conejero J. A., Alamá P., Garrido N., Pellicer A., Simón C. (2013) The accuracy and reproducibility of the endometrial receptivity array is superior to histology as a diagnostic method for endometrial receptivity. Fertil. Steril. 99, 508–517 [DOI] [PubMed] [Google Scholar]
- 54.Carson D. D., Bagchi I., Dey S. K., Enders A. C., Fazleabas A. T., Lessey B. A., Yoshinaga K. (2000) Embryo implantation. Dev. Biol. 223, 217–237 [DOI] [PubMed] [Google Scholar]
- 55.Marshall A. D., Bailey C. G., Champ K., Vellozzi M., O’Young P., Metierre C., Feng Y., Thoeng A., Richards A. M., Schmitz U., Biro M., Jayasinghe R., Ding L., Anderson L., Mardis E. R., Rasko J. E. J. (2017) CTCF genetic alterations in endometrial carcinoma are pro-tumorigenic. Oncogene 36, 4100–4110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Teklenburg G., Salker M., Heijnen C., Macklon N. S., Brosens J. J. (2010) The molecular basis of recurrent pregnancy loss: impaired natural embryo selection. Mol. Hum. Reprod. 16, 886–895 [DOI] [PubMed] [Google Scholar]
- 57.Ewington L. J., Tewary S., Brosens J. J. (2019) New insights into the mechanisms underlying recurrent pregnancy loss. J. Obstet. Gynaecol. Res. 45, 258–265 [DOI] [PubMed] [Google Scholar]
- 58.Brosens I., Benagiano G., Brosens J. J. (2015) The potential perinatal origin of placentation disorders in the young primigravida. Am. J. Obstet. Gynecol. 212, 580–585 [DOI] [PubMed] [Google Scholar]
- 59.Behura S. K., Kelleher A. M., Spencer T. E. (2019) Evidence for functional interactions between the placenta and brain in pregnant mice. FASEB J. 33, 4261–4272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moser G., Windsperger K., Pollheimer J., de Sousa Lopes S. C., Huppertz B. (2018) Human trophoblast invasion: new and unexpected routes and functions. Histochem. Cell Biol. 150, 361–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Macdonald L. J., Sales K. J., Grant V., Brown P., Jabbour H. N., Catalano R. D. (2011) Prokineticin 1 induces Dickkopf 1 expression and regulates cell proliferation and decidualization in the human endometrium. Mol. Hum. Reprod. 17, 626–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mariee N., Li T. C., Laird S. M. (2012) Expression of leukaemia inhibitory factor and interleukin 15 in endometrium of women with recurrent implantation failure after IVF; correlation with the number of endometrial natural killer cells. Hum. Reprod. 27, 1946–1954 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.