Abstract
Aromatase inhibitors are the preferred treatment for certain women with estrogen receptor (ER)-positive breast cancer, but evidence suggests that women with obesity experience aromatase inhibitor resistance at higher rates. To compare how stromal cells derived from women who are lean or obese influence response to the aromatase inhibitor (anastrazole), we incorporated patient-derived stroma in a previously characterized MCF7-derived in vitro duct model. Coculture with adipose stromal cells enabled the metabolism of testosterone (T) to E2, which induced estrogen response element activity, epithelial proliferation, and hyperplasia in MCF7 cells. The effects of T were inhibited by the ER antagonist tamoxifen and aromatase inhibitor anastrazole and were increased by the aromatase inducer dexamethasone. Primary mammary adipose stromal cells derived from women with obesity displayed increased aromatase mRNA compared with lean controls. MCF7-derived ducts cocultured with obese stromal cells exhibited higher maximal aromatization-induced ER transactivation and reduced anastrazole sensitivity, a difference not seen in 2-dimensional coculture. Finally, tamoxifen was more effective than anastrazole at reducing aromatization-induced ER transactivation and proliferation. These findings suggest that patient-specific responses to hormone therapies can be modeled and studied organotypically in vitro and add to evidence advocating obesity as a parameter to consider when identifying treatments for patients with ER-positive breast cancer.—Morgan, M. M., Arendt, L. M., Alarid, E. T., Beebe, D. J., Johnson, B. P. Mammary adipose stromal cells derived from obese women reduce sensitivity to the aromatase inhibitor anastrazole in an organotypic breast model.
Keywords: obesity, estrogen, cancer, hormone therapy
Approximately two thirds of all breast cancer cases are estrogen receptor (ER) α positive. ER is thought to regulate the progression of ER-positive breast cancer by controlling the growth and death of breast cancer cells through estrogen-regulated signaling (1). In postmenopausal women, treatment of ER-positive breast cancer typically involves directly targeting ER-mediated signaling with ER antagonists such as tamoxifen, or indirectly limiting local estrogen by suppressing the conversion of testosterone (T) to E2 with the use of aromatase inhibitors such as anastrazole (2). Although both tamoxifen and aromatase inhibitors are effective treatments, clinical studies indicate that aromatase inhibitors are more effective at reducing recurrence and mortality rates than tamoxifen (3–7).
Unfortunately, the patient response rates using aromatase inhibitors range from 20 to 50%, and understanding the underlying causes of treatment resistance is a persistent challenge (8). In particular, obesity is a risk factor for aromatase inhibitor resistance. Clinical trials have found that in comparison to patients who are lean, patients with obesity with breast cancer who are treated with aromatase inhibitors are at higher risk of recurrence and may be less responsive to treatment (9–11). There is some evidence that obese postmenopausal women may benefit from other therapies such as tamoxifen (9, 12), although these findings are controversial and warrant further investigation (13, 14). Understanding the mechanisms that predispose obese individuals to aromatase inhibitor resistance may increase our ability to predict which patients are poor candidates for aromatase inhibitors as well as pinpoint alternative therapeutic strategies.
A major challenge with studying aromatase inhibitor resistance is a lack of suitable model systems. Studies examining aromatase inhibitor resistance in obese vs. lean individuals typically rely on mouse models where deciphering mechanisms can be challenging due to difficulties with pinpointing specific cell:cell or chemical:cell interactions (15, 16). Additionally, extrapolating data between species is particularly difficult due to the differences between the physiology of the mouse and human mammary gland. For instance, ER expression differs in the mammary gland of the mouse and human; ER is expressed in the stroma of the mouse mammary gland but not in the stroma of the human mammary gland (17). Unlike humans, mice lack the promoters that regulate aromatase expression in peripheral tissues, such as the breast (18, 19). Although using an in vitro model mitigates these issues, traditional in vitro breast cancer cell models neglect to include stromal cells. Stromal cells are essential for studying aromatase inhibitor resistance because breast stromal cells are primarily responsible for producing aromatase (20, 21). Researchers have started to incorporate mammary stroma into in vitro breast cancer platforms, which have revealed striking differences in how obese and lean stromal cells influence the behavior of breast cancer cells (22) and have confirmed that mammary stromal cells can induce ER-driven responses by metabolizing T to estrogen (23). However, these studies cultured cells in platforms that did not include an extracellular matrix or tissue geometry, both of which have been shown to be important to recapitulating in vivo responses (24). These studies also did not compare resistance to aromatase inhibitors in patients who are obese or lean, and, consequently, the increased risk of aromatase inhibitor resistance in obese individuals remains largely understudied. Altogether, an in vitro breast model that incorporates aromatase signaling would be useful for deciphering mechanisms of aromatase inhibitor resistance.
To this end, we used a previously characterized organotypic mammary duct model to investigate how the mammary stromal cells of women who are lean or obese differentially influence response to the aromatase inhibitor, anastrazole, in vitro. We chose to use the organotypic model because it enables the study of stromal:epithelial interactions in a more physiologically relevant environment than a traditional 2-dimensional (2D) model (25). Importantly, we found that the organotypic coculture system was able to segregate differences in anastrazole sensitivity between patients who are lean or obese, whereas a 2D coculture model could not. Our results suggest that 1) the mammary stroma regulates resistance to aromatase inhibitors, 2) patient-specific responses to anastrazole can be modeled and studied in vitro, and 3) that obesity may be a useful parameter to consider when choosing hormone therapies for patients with breast cancer.
MATERIALS AND METHODS
Primary human tissue isolation
All breast tissue procurement for these experiments was performed in compliance with the laws and institutional guidelines as approved by the Institutional Review Board committee from the University of Wisconsin–Madison. Disease-free, deidentified breast tissues were obtained from female patients undergoing elective reduction mammoplasty with informed consent through the Translational Science BioCore BioBank at the Carbone Cancer Center at the University of Wisconsin–Madison. This research study was approved by the Institutional Review Board as not human subject research, with a limited patient data set including patient age, date of surgery, and body mass index (BMI). All mammary stromal cells used in the manuscript were isolated from the stromal vascular fraction of breast tissue of premenopausal patients undergoing reduction mammoplasty, as previously described in Chamberlin et al. (26). Briefly, after collection breast tissue was incubated for 8 h with 1.5 mg/ml collagenase I (MilliporeSigma, Burlington, MA, USA) diluted in DMEM:F12 medium (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 5% calf serum (Thermo Fisher Scientific). After digestion, the tissue was incubated for 10 min at room temperature then the lipid-rich portion was discarded. The stromal fraction was incubated with red blood cell lysis buffer (ACK Lysing Buffer; Lonza, Basel, Switzerland) then plated in DMEM supplemented with 10% fetal bovine serum (FBS; Thermo Fisher Scientific) and 1% antibiotic/antimycotic solution. For experiments that evaluated the effect of cell density on anastrazole resistance, we used mammary stromal cells derived from patients with obesity that were at a late passage (P > 7). Unless otherwise indicated, when comparing patients who are lean or obese, we used mammary stromal cells at an early passage (P ≤ 3). Supplemental Table S1 lists the BMI and age of the 12 patients (6 lean and 6 obese) used in the obese vs. lean comparison studies. All patients in the study who reported their ethnicity were non-Hispanic white; 1 patient did not include race.
Cell culture
Adipose-derived mesenchymal stem cells (AdMSCs) were purchased from American Type Culture Collection (ATCC; Manassas, VA, USA). The Michigan Cancer Foundation-7 (MCF7) cells were previously transfected with an estrogen response element (ERE)-luciferase reporter to detect ER activation using luminescent activity (27). All cell types were maintained in DMEM (Thermo Fisher Scientific) supplemented with 10% FBS and 1% penicillin-streptomycin (Thermo Fisher Scientific). Forty-eight hours before experiment seeding, culture flasks were washed with PBS then replenished with estrogen-free medium, which contains phenol-red–free DMEM (Thermo Fisher Scientific) with 10% charcoal-stripped FBS, 2 mM glutamine, and 1% penicillin-streptomycin. All cultures were maintained in an incubator at 37°C and 5% CO2.
Generation of MCF7-derived ducts
MCF7-derived ducts were generated as previously described in Morgan et al. (25). Briefly, the 2-layered microfluidic devices were constructed from polydimethylsiloxane (PDMS) using the Sylgard 184 Silicone Elastomer Kit (Dow Corning, Midland, MI, USA) and standard photolithography techniques. After PDMS devices were treated with 2% poly (ethyleneimine) (MilliporeSigma) and 0.4% glutaraldehyde (MilliporeSigma) for 10 and 30 min, respectively, devices were washed 3 times with water. Each device was loaded with 6.5 µl of a 4.5 mg/ml neutralized rat-tail collagen I solution (Corning, Corning, NY, USA) that contained medium or stromal cells in medium. Four thousand AdMSCs per device were used for all experiments validating metabolism of T to E2, except for experiments evaluating aromatase induction via dexamethasone (DEX); DEX experiments used 2000 AdMSCs per device. Experiments that evaluated different AdMSC densities used ∼2000, 8000, and 16,000 AdMSCs per device for the low, medium, and high concentrations, respectively. Experiments comparing lean to obese cultures used ∼4000 stromal cells per device. After loading, collagen was polymerized at room temperature for 10 min and then transferred to the incubator for 1 h. Afterward, the PDMS rod was removed and the resulting luminal structure was filled with 1.5 µl of MCF7s at 50,000/µl. Cultures were flipped every 20 min for 1 h, then excess cells were aspirated from the large port and medium were replenished through the small port. An evenly seeded confluent lumen contains ∼4000 MCF7 cells. All organotypic cultures were seeded in estrogen-free medium supplemented with serum-free fibroblast growth supplement (ScienCell, Carlsbad, CA, USA). Experiments were dosed with E2, T, anastrazole, DEX, 5α-dihydrotestosterone (DHT), or 4-hydroxytamoxifen (OHT). All chemicals were dissolved in ethanol and were bought from MilliporeSigma [except for DHT, which was bought from Cerilliant Corporation (Round Rock, TX, USA)]. Drugs were dosed in organotypic culture medium containing 2 mM of VivoGlo luciferin (Promega, Madison, WI, USA), which cleaves luciferase to produce a luminescent signal linear to ER transactivation. Cultures were exposed to test chemicals the day after seeding, and dosing medium were replenished daily.
2D coculture
We used a coculture approach similar to a previous study (28). Mammary stromal cells were seeded in a 384-well plate at 2500 cells/well and allowed to adhere for 3 h. In total, 2500 MCF7s were then seeded on top of the stromal cells. Similar to the organotypic system, we used a 1:1 ratio of adipose stromal cell:MCF7 cell and dosed the cultures the day after seeding. Additionally, the same types of medium were used for seeding and dosing as in the organotypic system.
ER transactivation assay
The MCF7s used in the model were previously transfected with a reporter placing a luciferase gene downstream of the ERE located in the vitellogenin gene; therefore, the luciferase produced by the cells is linear to ER transactivation (27). Luminescence produced in organotypic cultures was measured with Chemidoc imager (BioRad, Hercules, CA, USA), whereas luminescence in well plates was measured by a Pherastar plate reader (BMG Labtech, Cary, NC, USA).
RNA isolation and quantitative real-time PCR
To lyse cells grown on 96-well plates, medium was removed from each well and replaced with the lysis binding buffer of the Dynabead mRNA Direct Purification Kit (Thermo Fisher Scientific). To lyse cells from organotypic cultures, the top layer of the PDMS device was removed and the collagen/cell mixture was transferred to a tube containing lysis buffer. Collagen was then pulverized with a 23-gauge needle (Thermo Fisher Scientific). RNA was isolated from the lysates using the Dynabead mRNA Direct Purification Kit according to the manufacturer’s protocol and was reverse transcribed using the High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). PCR was conducted using the Light Cycler 480 Probes Master Mix and Taqman primers to trefoil factor 1 (TFF1) (Hs00907239_m1), progesterone receptor (PGR) (Hs01556702_m1), or cytochrome P450 family 19 subfamily A member 1 (CYP19A1) (Hs00903411_m1) and normalized to hypoxanthine phosphoribosyltransferase 1 (HPRT) (Hs02800695_m1) and 60S acidic ribosomal protein P0 (RPLP0) (Hs99999902_m1). We used HPRT and RPLP0 as housekeeping genes because they have been used previously as housekeeping genes for breast cancer studies (29, 30) and are lowly (HPRT) and highly (RPLP0) expressed housekeeping genes. Relative gene expression was determined using the ΔΔCt method.
Evaluation of cell density
Cell density was quantified as previously described in Morgan et al. (25). Cells were fixed with 4% paraformaldehyde (Alfa Aesar, Haverhill, MA, USA) for 15 min then washed twice with PBS (25). To examine cell density, cells were stained with Hoescht (Thermo Fisher Scientific) and Texas red phalloidin (Thermo Fisher Scientific) to visualize nuclei and F-actin, respectively. The semiautomated Image J (National Institutes of Health, Bethesda, MD, USA)–based program, JEXperiment (https://github.com/davidenunes/jexperiment), was used for image quantification (31), where regions of interests of equal size were drawn over the ducts of each image, and a rolling ball background subtraction was used. The number of nuclei was then identified.
Cross-sectioning
After 5 d in culture, lumens were fixed and stained for nuclei and F-actin and then embedded in agarose and cross-sectioned with a Compresstome (Precisionary Instruments, Greenville, NC, USA) as previously described in Morgan et al. (25). To quantify ductal thickness, the number of nuclei was counted at 6 evenly spaced points along each duct.
Statistics
Excel (Microsoft, Redmond, WA, USA) and GraphPad Prism (GraphPad Software, La Jolla, CA, USA) were used to conduct statistical tests. Nonlinear regression was used to calculate dose response curves and half-maximal inhibitory concentration (IC50) concentrations, which is the concentration that induces, or represses, a response by 50%. Specifically, Prism models log (agonist) vs. response (3 parameters) and log (inhibitor) vs. response (3 parameters) were used. Error bars in all graphs represent se, and significance is defined as P < 0.05. A Student’s t test was used to evaluate significance in experiments comparing 2 conditions. Experiments evaluating more than 2 conditions used a 1-way ANOVA to determine significance. A 2-way ANOVA followed by a multiple comparisons test was used when evaluating the effect of adipose stromal cells on responses to T.
RESULTS
T induces ER-driven responses when AdMSCs are in the matrix surrounding MCF7-derived ducts
In the mammary gland, adipose stromal cells—a mixture of adipose stem cells, fibroblasts, and other stromal cell types (32)—produce the enzyme aromatase that metabolizes T and androstenedione to E2 and estrone, respectively (20, 21). To mimic the conversion of androgens to estrogens that occurs in breast tissue in vivo, we incorporated a commercially available source of primary human adipose stromal cells, AdMSCs, into the collagen matrix that surrounds a ductal structure lined with MCF7 breast cancer cells.
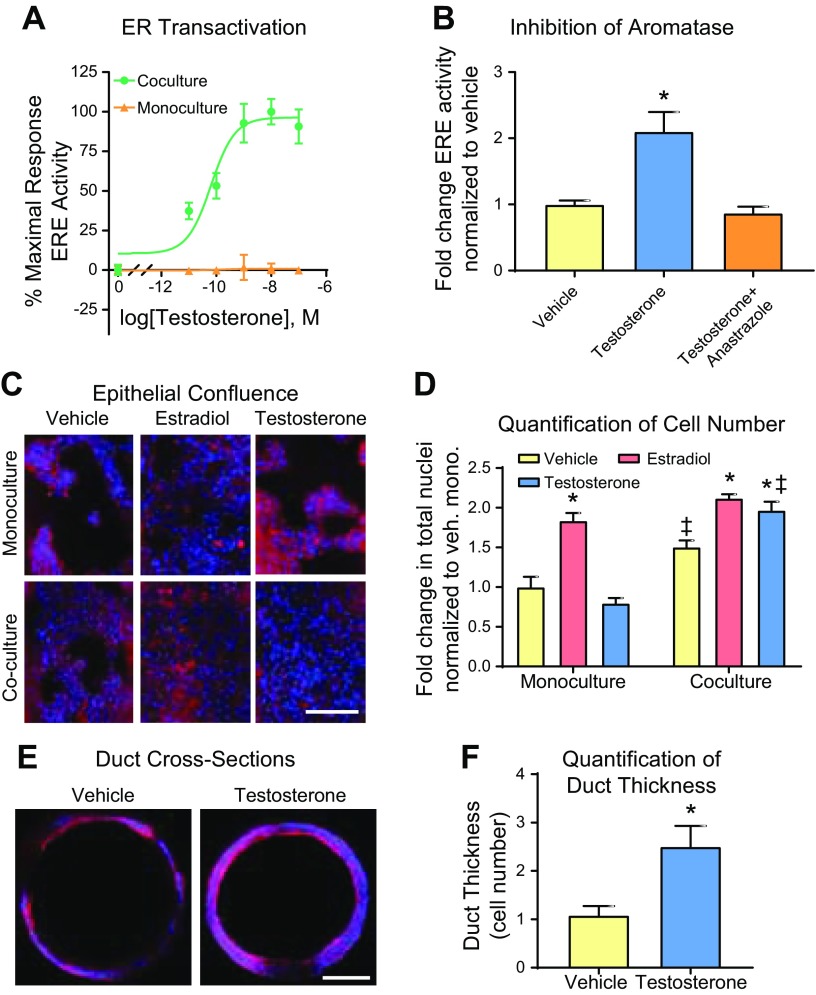
To validate the conversion of T to E2, MCF7-derived ducts cultured alone or with AdMSCs in the matrix were evaluated for ER transactivation after a 48-h exposure to 5 concentrations of T; we chose to evaluate T rather than androstenedione because a previous in vitro study demonstrated that breast adipose fibroblasts could metabolize T to E2, which induced ER-driven responses in MCF7 cells (28). A dose-dependent relationship was observed between T exposure and ER transactivation in the coculture but not in the monoculture (Fig. 1A).
Figure 1.
T induces ER-driven responses in MCF7-derived ducts cocultured with AdMSCs. A) ER transactivation was evaluated in MCF7-derived ducts grown alone or with AdMSCs after a 48-h exposure to 5 doses of T. B) MCF7-derived ducts cocultured with AdMSCs were exposed to a vehicle control (0.1% ethanol), T (100 nM), or T and anastrazole (1 µM) for 24 h then evaluated for ER transactivation. C) After a 5-d exposure to a vehicle control, E2 (100 nM), or T, MCF7-derived ducts cultured alone or in coculture with AdMSCs were fixed then stained for nuclei (blue) and F-actin (red). D) Cell number was quantified by counting the number of nuclei within each duct. E) After a 5-d exposure to a vehicle control or T, MCF7-derived ducts cocultured with AdMSCs were fixed, stained for nuclei and F-actin and cross-sectioned to evaluate hyperplasia. F) Hyperplasia was quantified by counting the number of cells lining the duct at 6 evenly spaced points along each duct. *P < 0.05 vs. vehicle, ‡P < 0.05 vs. respective monoculture.
To confirm that the T-induced ER transactivation was due to estrogen production via aromatase, we evaluated how ER transactivation was affected by T when cotreated with an aromatase inhibitor or inducer. First, we exposed MCF7-derived ducts cocultured with AdMSCs to a vehicle control, 100 nM T, or 100 nM T and 1 μM of the aromatase inhibitor anastrazole. As expected, although T increased ER transactivation 2-fold, cotreatment with anastrazole inhibited this effect (Fig. 1B). Next, MCF7-derived ducts cocultured with AdMSCs were exposed to a vehicle control, 100 nM T, or 100 nM T and 1 μM of the aromatase inducer DEX. ER transactivation was significantly higher when the cultures were exposed to DEX and T (4.6-fold relative to vehicle) compared with exposure to T alone (2.5-fold relative to vehicle) (Supplemental Fig. S1A). Next, MCF7-derived ducts cocultured with AdMSCs were exposed to 5 doses (0.1–1000 nM) of DEX, alone or in combination with T. Although DEX had no effect on ER transactivation in the absence of T, when exposed in the presence of T, DEX increased ER transactivation in a dose-dependent manner (Supplemental Fig. S1B).
To evaluate if T induces proliferation, cell number was evaluated after MCF7-derived ducts cultured alone or with AdMSCs were exposed to a vehicle control, 100 nM of E2, or 100 nM of T for 5 d. Although E2 significantly increased cell numbers in both culture conditions, T treatment showed no effect in the monoculture. In contrast, exposure to T led to a significant increase in cell number in the coculture (Fig. 1C, D), which we suspect was due to the conversion of T to estrogen. The vehicle and T-treated cocultures were cross-sectioned to evaluate the presence of hyperplasia, a defining feature of preinvasive breast lesions (Fig. 1E). Quantification revealed a single cell layer in the vehicle-treated coculture, whereas the T-treated coculture had ∼2.5 cell layers per lumen (Fig. 1F).
Previous studies have reported that androgens can influence the growth of breast cancer cells by acting on the androgen receptor (AR) (23, 33). To examine if some of the effects of T are mediated through AR, we exposed MCF7-derived ducts cocultured with AdMSCs to a vehicle control, T, or DHT. DHT is a potent androgen but is not a substrate for aromatase. ER transactivation was evaluated after 48 h (Supplemental Fig. S2A) and lumen confluency after 5 d (Supplemental Fig. S2B). Consistent with our previous findings, T increased ER transactivation and increased lumen confluency. However, no detectable response was observed after DHT treatment.
Breast cancer cells cultured with a higher concentration of AdMSCs exhibit decreased sensitivity to anastrazole
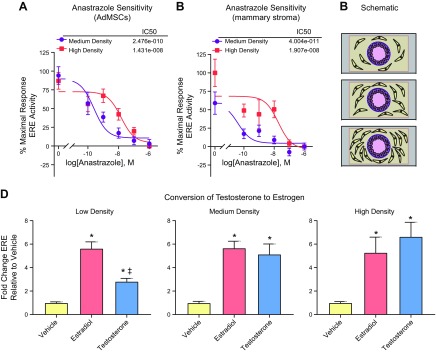
We hypothesized that increased adipose volume and, consequently, increased number of adipose stromal cells, would reduce anastrazole sensitivity in breast cancer cells due to enhanced levels of aromatase. We measured ER transactivation when MCF7-derived ducts were cocultured with a medium (2:1 AdMSC:MCF7) or high concentration (4:1 AdMSC:MCF7) of AdMSCs and exposed to 10 nM T in the presence of 5 concentrations of anastrazole. MCF7-derived ducts cocultured with a high concentration of AdMSCs exhibited an IC50 of 1.4e−8 M compared with an IC50 2.5e−10 M for the medium concentration coculture (Fig. 2A). The IC50 is a measure of drug potency because it describes the concentration needed to inhibit a response by 50%. The dose response experiment was repeated using human adipose stromal cells derived from the stromal vascular fraction of reduction mammoplasty breast tissues. Similar to our findings with the AdMSCs, an increased concentration of human mammary stromal cells was associated with a decreased sensitivity to anastrazole, as indicated by a significantly increased IC50 (IC50 of high concentration cultures was 1.9e−8 M compared with 4e−11 M of medium concentration cultures) (Fig. 2B).
Figure 2.
Anastrazole sensitivity and T metabolism are dependent on the concentration of AdMSCs. A) MCF7-derived ducts cultured with a medium and high concentration of AdMSCs in the presence of 10 nM T were exposed to 5 concentrations of anastrazole for 48 h then evaluated for ER transactivation. B) MCF7-derived ducts cultured with a medium and high concentration of late passage primary human mammary adipose stromal cells derived from women with obesity in the presence of 10 nM T were exposed to 5 concentrations of anastrazole for 48 h then evaluated for ER transactivation. C) Schematic showing MCF7-derived ducts cultured with a low, medium, or high concentration of AdMSCs; the ratio of cancer cells to AdMSCs for the low, medium, and high concentration conditions are 1:2, 2:1, and 4:1, respectively. D) MCF7-derived ducts cultured with different concentrations of AdMSCs were exposed to a vehicle control, E2, or T for 48 h then evaluated for ER transactivation. *P < 0.05 vs. vehicle, ‡P < 0.05 vs. E2.
To confirm that T to estrogen metabolism is dependent on the number of adipose stromal cells, ER transactivation was evaluated for MCF7-derived ducts cocultured with a low (0.5:1 AdMSC:MCF7), medium (2:1 AdMSC:MCF7), or high concentration of AdMSCs (4:1 AdMSC:MCF7) (Fig. 2C), when exposed to a vehicle control, 100 nM of T, or 100 nM of E2 (Fig. 2D). T-induced ER transactivation was significantly lower when MCF7s were cocultured with a low concentration of AdMSCs, compared to when cocultured with a medium or high concentration of AdMSCs. There was no difference between the T-induced ER transactivation in the medium and high concentration of AdMSCs, and E2-induced ER transactivation was the same across the 3 culture conditions.
Breast cancer cells cultured with obese-derived stromal cells exhibit decreased sensitivity to anastrazole compared to when cultured with lean-derived stromal cells
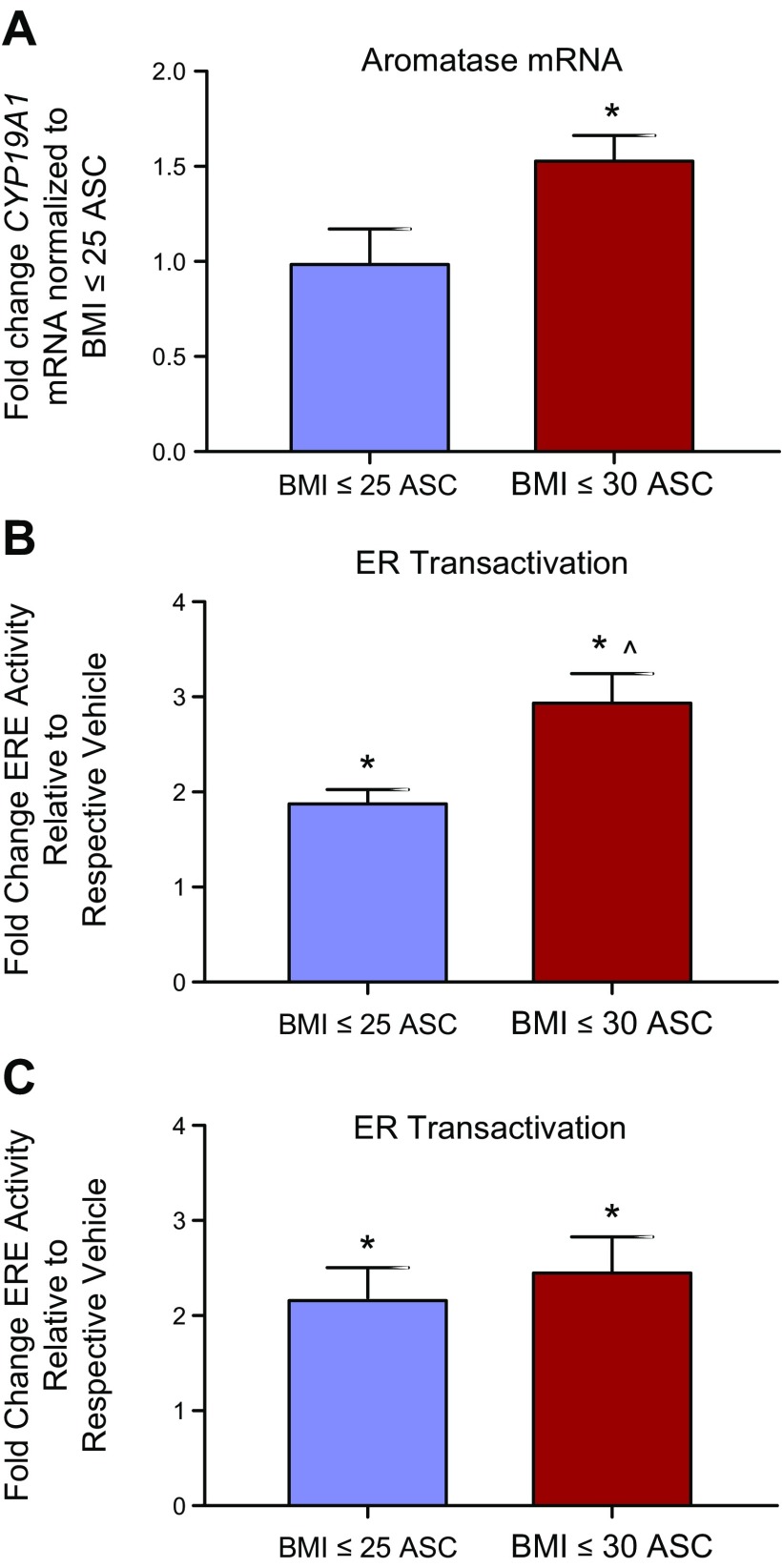
Accumulating evidence suggests that women with obesity are less sensitive to anastrazole than women who are lean (9–11). To test this in vitro, we acquired mammary adipose stromal cells derived from the reduction mammoplasties of women who are lean (BMI ≤ 25) or obese (BMI ≥ 30). Obese stromal cells and lean stromal cells were cultured in a 2D monoculture for 48 h and then evaluated for CYP19A1, the gene encoding aromatase. Quantitative real-time PCR revealed that obese stromal cells had 1.5-fold higher levels of CYP19A1 mRNA than lean stromal cells (Fig. 3A). To compare the conversion of T to E2, MCF7-derived ducts cocultured with lean or obese stromal cells at a low passage number (P ≤ 3) were exposed to a vehicle control or T for 48 h. Evaluation of ER transactivation revealed that obese cocultures converted more T to estrogen, as T-induced ER transactivation was 3-fold higher than the vehicle in obese cocultures, compared to 2-fold in the lean cocultures (Fig. 3B). Because studies have found that the molecular profiles of primary cells can change over time in culture (34, 35), aromatization-induced ER transactivation was compared again when the lean and obese stromal cells were at a late passage number (P > 7). Although obese cocultures exhibited slightly increased ER transactivation compared with lean cocultures, the difference was not significant (Fig. 3C).
Figure 3.
Mammary adipose stromal cells (ASCs) derived from women with obesity exhibit increased aromatase expression and aromatization-induced ER transactivation. A) The expression of CYP19A1 mRNA was evaluated in the mammary stroma of lean female donors and the mammary stroma of obese female donors. B, C) MCF7-derived ducts were exposed to a vehicle control or T when cocultured with mammary stromal cells of patients who are lean or obese at an early passage (B) (P ≤ 3) and a late passage (C) (P > 7), then evaluated for ER transactivation. T-treated cultures were normalized to the vehicle-treated cultures of each respective patient. *P < 0.05 vs. respective vehicle, ^P < 0.05 vs. lean cultures.
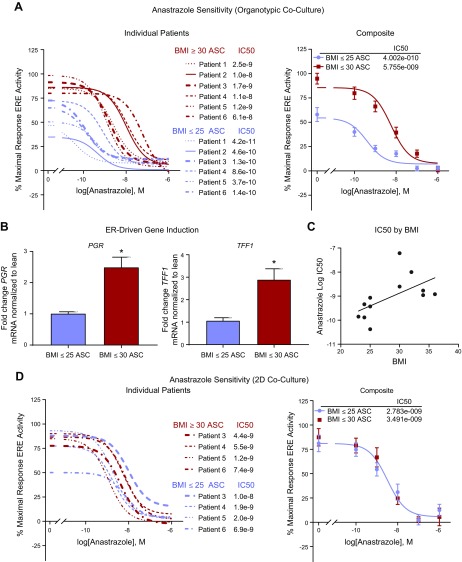
To assess response to anastrazole, we evaluated ER transactivation after MCF7-derived ducts cultured with mammary stromal cells derived from women who are either lean or obese were exposed to 5 concentrations of anastrazole. All cultures were seeded with the same concentration of stromal cells (1:1 adipose stromal cell:MCF7; passage ≤3) and supplemented with 10 nM of T. ER transactivation was compared for 6 patients with obesity and 6 patients who were lean (Fig. 4A). Evaluation of dose response curves revealed a dramatically increased IC50 (P < 0.01) and increased maximal ER transactivation (P < 0.0001) in the obese cultures compared with the lean cultures. The vehicle-treated organotypic cultures (e.g., cultures treated with 10 nM T but no anastrazole) were evaluated at d 4 for ER-driven genes TFF1 and PGR, and there was an ∼2.9-fold and 2.5-fold higher expression of each gene, respectively, in the obese cultures (Fig. 4B). Anastrazole IC50 was graphed against BMI, which revealed a weak positive trend (r2 = 0.34; P < 0.05) (Fig. 4C). For 8 of the 12 patient samples (4 lean and 4 obese), sensitivity to anastrazole was also compared when MCF7 cells were cocultured with mammary stromal cells on a 2D well plate. The 2D coculture experiment used the same ratio of stromal cells to MCF7 cells (1:1) and was seeded, dosed, and evaluated at the same time points as the organotypic cultures. In contrast to the organotypic platform, the obese and lean dose response curves did not segregate or differ in IC50 (Fig. 4D).
Figure 4.
MCF7-derived ducts cocultured with adipose stromal cells (ASCs) derived from obese individuals are more resistant to anastrazole treatment. A) MCF7-derived ducts cocultured with lean or obese mammary stromal cells in the presence of 10 nM T were exposed to 5 concentrations of anastrazole for 48 h then evaluated for ER transactivation. Right panel shows individual patients and left panel shows patients merged into a single curve. B) The vehicle-treated (e.g., no anastrazole but exposed to 10 nM of T) cultures of D were lysed and evaluated for ER-driven genes TFF1 and PGR. C) Patient BMI was graphed against their correlating anastrazole IC50. D) MCF7 cells cocultured with lean or obese mammary stromal cells in a 2D well plate in the presence of 10 nM T were exposed to 5 concentrations of anastrazole for 48 h, then evaluated for ER transactivation. Right panel shows individual patients and left panel shows patients merged into a single curve. *P < 0.05 vs. BMI < 25 ASC.
Tamoxifen is more effective than anastrazole at reducing ER transactivation and proliferation
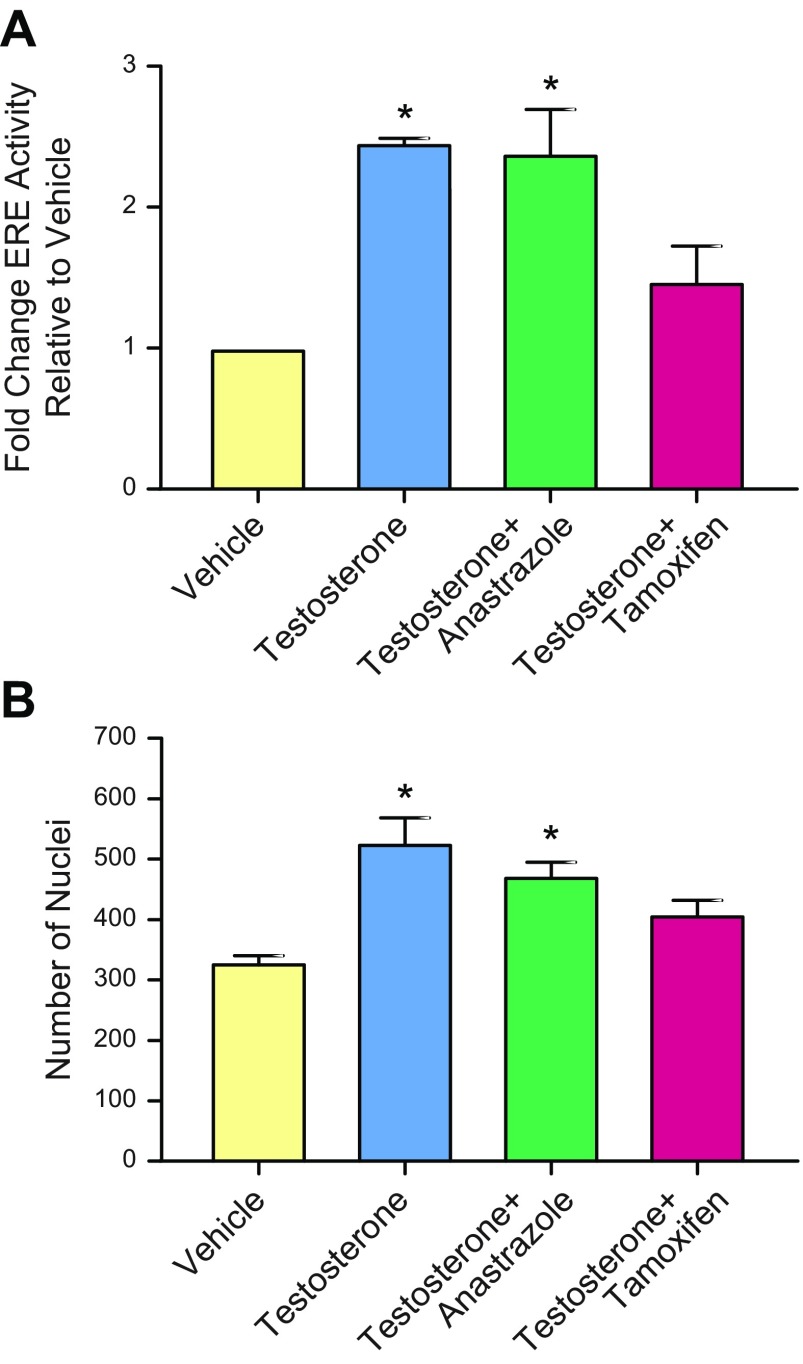
Although anastrazole has been found to be as effective or more effective than tamoxifen, there is evidence that tamoxifen may be more effective in women with obesity (9, 12). To examine this hypothesis in vitro, MCF7-derived ducts cocultured with stromal cells derived from women with obesity were exposed to a vehicle control, 10 nM T, 10 nM T in combination with 1 nM anastrazole, or 10 nM T in combination with 1 nM of OHT (the active metabolite of tamoxifen). Evaluation of ER transactivation after 48 h revealed that tamoxifen prevented T-induced ER transactivation, whereas anastrazole did not (Fig. 5A). Similarly, quantification of cell number after a 5-d exposure showed a similar number of cells in the vehicle and tamoxifen treated cultures, whereas the T- and anastrazole-treated cultures were significantly increased relative to the vehicle (Fig. 5B).
Figure 5.
Tamoxifen is more effective than anastrazole at reducing T-induced ER transactivation and proliferation. MCF7-derived ducts cultured with obese stromal cells were exposed to a vehicle control, 10 nM T, 10 nM T with 1 nM anastrazole, or 10 nM T with 1 nM of the active metabolite of tamoxifen, OHT. A) After 48 h, ER transactivation was evaluated. B) After 5 d, cultures were fixed and evaluated for nuclei. *P < 0.05 vs. vehicle.
DISCUSSION
The effectiveness of aromatase inhibitors may vary depending on adiposity, although the mechanisms are not fully understood. Using an organotypic mammary model, we found that adipose stromal cells converted T to estrogen via aromatase and induced ER-driven responses proliferation and hyperplasia in breast cancer cells, and that T metabolism and anastrozole resistance were dependent on the concentration of adipose stromal cells. We also examined how sensitivity to the aromatase inhibitor anastrazole differs in women who are lean or obese. We provided in vitro evidence that anastrazole is less effective in obese individuals compared with lean individuals. Importantly, when MCF7 cells were cultured with adipose stromal cells in a conventional 2D coculture system, we did not detect differences in anastrazole sensitivity of patients who were obese or lean. These data support the use of organotypic models for future in vitro breast cancer studies and introduces an in vitro system that can be used to study the mechanisms of aromatase inhibitor resistance. Together, these data suggest that patient-specific responses to hormone therapies can be modeled in vitro and that tamoxifen may be a more effective treatment for women with obesity.
Although T is an AR ligand and AR has been shown previously to influence the growth of breast cancer cells (23, 33), our data suggest that the effects induced by T were not induced by AR and instead occurred through ER. If some of the effects of T were mediated through AR, we would expect that T should have induced responses in the MCF7 monocultures; however, we did not observe significant responses. Breast epithelial cells produce little aromatase except for in very advanced breast cancers (36, 37), suggesting that T was not metabolized to estrogen when MCF7s are grown alone. When cocultured with AdMSCs, T exposure induced ER transactivation and increased proliferation in MCF7 cells. This is in agreement with a previous study that cocultured adipose stromal cells with ER-positive breast cancer cells in 2D, which revealed that adipose stromal cells metabolize T to estrogen and induce proliferation in breast cancer cells (23). We also found that induction of aromatase via DEX increased ER transactivation, and inhibition of aromatase via anastrazole prevented ER transactivation, suggesting that the effects observed were related to the metabolism of T to E2 via aromatase. When estrogen signaling was antagonized via tamoxifen, T had no effect on ER transactivation or proliferation. In addition, exposure to the nonaromatizable androgen DHT had no effect on ER transactivation or proliferation. Together, these results suggest that T was metabolized to estrogen via aromatase and exerted effects through ER. Our data also validate that AdMSCs can metabolize T to E2 and are thus a commercially available cell line that can be used for aromatase studies.
Previous studies have speculated that increased adipose volume and, consequently, increased number of adipose stromal cells, in the breast tissue of obese individuals contributes to aromatase inhibitor resistance (5). Our finding that the conversion of T to E2 and anastrazole sensitivity are dependent on the number of AdMSCs supports the idea that the number of adipose stromal cells can influence aromatase concentration. These findings also suggest that the ratio of adipose stromal cells to cancer cells should be optimized when conducting aromatase experiments because too few or too many adipose stromal cells may complicate detection of aromatase inhibitors or inducers, respectively. However, clinical studies have found that aromatase expression and activity increases with BMI (38, 39), and in vitro and in vivo studies have reported increased CYP19A1 mRNA and activity in obese stromal cells compared with lean stromal cells (16, 22). In concordance with these studies, we found that stromal cells derived from obese individuals exhibited increased aromatase mRNA compared with stromal cells derived from lean individuals. We have expanded upon these studies to demonstrate that the mammary stromal cells of women who are lean or obese differently affect aromatase-driven responses in breast cancer cells in vitro. Specifically, we showed that obese stromal cells conferred higher rates of aromatization-induced ER transactivation and decreased sensitivity to the aromatase inhibitor anastrazole, as indicated by an increased IC50. We suspect that the increased aromatase expression is likely responsible for the increased aromatization-induced maximal ER transactivation in the obese cocultures, as well as the decreased sensitivity to anastrazole; we hypothesize that the heightened aromatase expression in obese cultures increased the conversion of T to E2. This hypothesis is supported by clinical data that found that aromatase inhibitors are less effective at reducing serum estrogen levels in women with obesity than in women who are lean (40, 41). Together, these findings support the hypothesis that aromatase inhibitor resistance is mediated by changes in the mammary stroma and introduce an in vitro method that can be used to study the mechanisms responsible for aromatase inhibitor resistance.
Our finding that the increased aromatization-induced ER transactivation observed in the obese compared with lean cultures is diminished at later passages is consistent with studies that have reported changes in the molecular and functional profiles of primary cells that are cultured for several passages (34, 35, 42). These data underscore the challenges with using primary cells and highlights the importance of using freshly isolated cells.
A major obstacle to studying the mechanisms of drug resistance is that traditional in vitro models poorly recapitulate in vivo biology. Several authors have argued that increasing the physiologic relevance of in vitro platforms may improve the ability to predict drug responses (1, 43, 44). In support of this, we found that a 2D coculture system did not segregate the anastrazole responses of patients who are lean or obese as did our organotypic culture platform. These findings are important because they suggest that some aspect of the organotypic culture is needed to recapitulate in vivo responses to aromatase inhibitors. Several variables that differ between the platforms, including material (PDMS vs. polystyrene), culture volume (5 μl vs. 40 μl), matrix proteins (collagen vs. plastic), confluency (confluent vs. not confluent), and structure (lumen vs. flat surface) have been previously shown to influence the behavior of stromal cells and breast cancer cells as well as paracrine signaling and drug sensitivity. For instance, microfluidic systems are thought to be more sensitive at detecting stromal:epithelial interactions because the higher surface area to volume ratio inherent in microfluidics increases the concentration of secreted factors (45). Previous studies from our lab and others have reported striking differences in cell phenotype and behavior when cells are cultured in conventional 2D platforms, compared to when cultured in 3-dimensional matrices and organotypic models (46–49). Therefore, we suspect that the organotypic coculture platform modulated the function of the stromal and epithelial cells, which enabled the model to recapitulate the differences in anastrazole resistance in patients who are lean or obese. Additional studies are needed to clarify the mechanism(s) responsible for the different anastrazole responses observed in the 2D and organotypic system.
One limitation of the study was that due to sample availability, these experiments were conducted using normal cells from premenopausal women. To better understand the interactions between obesity and aromatase inhibitor resistance in breast cancer, future studies could integrate the stroma of postmenopausal patients with breast cancer. The proposed study should also include individuals of varying ethnicities because in this study all patients who reported their ethnicity were non-Hispanic white. We suspect that a similar trend will be observed because a previous study that included women of various ethnicities found that BMI correlated with increased aromatase expression in both pre- and postmenopausal women (39).
Multiple clinical trials have reported a reduced efficacy of anastrazole in patients with breast cancer who are obese compared with patients with breast cancer who are lean, suggesting that higher doses or alternative therapies may be beneficial for women who are overweight (10). However, body weight is not considered when choosing therapies for patients with ER-positive breast cancer (12). In contrast to aromatase inhibitors, clinical evidence suggests that other breast cancer therapies such as tamoxifen (50) are not influenced by BMI. Our data support this hypothesis because we found that tamoxifen was more effective than anastrazole at reducing aromatization-induced ER transactivation and proliferation. Although our preliminary results are promising, a prospective clinical study is needed to verify if the MCF7-derived coculture model predicts responses to hormone therapies.
Altogether, this study adds to evidence that suggests obese individuals may be less responsive to anastrazole compared with patients who are lean. These findings support the notion that body weight may be a useful parameter when choosing therapies for patients with ER-positive breast cancer (9, 11) and suggest that patient-specific responses to hormone therapies can be modeled and studied in vitro.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Linda Schuler for guidance, expertise, and positivity. The authors acknowledge Megan Livingston and Jordan Ciciliano for support. This work was supported by the University of Wisconsin Carbone Cancer Center Support Grant P30 CA014520, Environmental Protection Agency (EPA) Science to Achieve Results (STAR) Grant 83573701, U.S. National Institutes of Health (NIH), National Cancer Institute (NCI) Grants R01 CA186134 (to D.J.B.) and T32 CA157322, and National Institute of Environmental Health Science (NIEHS) Grant K99ES028744 to B.P.J., NIEHS Grant T32 ES007015-39 to M.M.M., and Susan G. Komen Grant CCR15332611 to L.M.A. Three of the authors have competing financial interests. D.J.B. holds equity in Bellbrook Labs, Tasso, Turba, Stacks to the Future, Salus Discovery, Lynx Biosciences, and Onexio Biosystems. B.P.J. holds equity in Onexio Biosystems. M.M.M. is an employee at Salus Discovery and has received compensation.
Glossary
- 2D
2-dimensional
- ASC
adipose stromal cell
- AdMSC
adipose-derived mesenchymal stem cell
- AR
androgen receptor
- BMI
body mass index
- CYP19A1
cytochrome P450 family 19 subfamily A member 1
- DEX
dexamethasone
- DHT
5α-dihydrotestosterone
- ER
estrogen receptor
- ERE
estrogen response element
- FBS
fetal bovine serum
- HPRT
hypoxanthine phosphoribosyltransferase 1
- IC50
half maximal inhibitory concentration
- OHT
4-hydroxytamoxifen
- MCF7
Michigan Cancer Foundation-7
- PDMS
polydimethylsiloxane
- PGR, progesterone receptor; RPLP0
60S acidic ribosomal protein P0
- T
testosterone
- TFF1
trefoil factor 1
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
E. T. Alarid, D. J. Beebe, and B. P. Johnson oversaw the research; M. M. Morgan and B. P. Johnson planned the experiments and M. M. Morgan performed the experiments; L. M. Arendt collected and processed the patient samples and provided experimental input; M. M. Morgan drafted the manuscript; and B. P. Johnson revised the manuscript.
REFERENCES
- 1.Morgan M. M., Johnson B. P., Livingston M. K., Schuler L. A., Alarid E. T., Sung K. E., Beebe D. J. (2016) Personalized in vitro cancer models to predict therapeutic response: challenges and a framework for improvement. Pharmacol. Ther. 165, 79–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osborne C. K., Schiff R. (2011) Mechanisms of endocrine resistance in breast cancer. Annu. Rev. Med. 62, 233–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rugo H. S., Rumble R. B., Macrae E., Barton D. L., Connolly H. K., Dickler M. N., Fallowfield L., Fowble B., Ingle J. N., Jahanzeb M., Johnston S. R., Korde L. A., Khatcheressian J. L., Mehta R. S., Muss H. B., Burstein H. J. (2016) Endocrine therapy for hormone receptor-positive metastatic breast cancer: American society of clinical oncology guideline. J. Clin. Oncol. 34, 3069–3103 [DOI] [PubMed] [Google Scholar]
- 4.Howell A., Cuzick J., Baum M., Buzdar A., Dowsett M., Forbes J. F., Hoctin-Boes G., Houghton J., Locker G. Y., Tobias J. S.; ATAC Trialists’ Group (2005) Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet 365, 60–62 [DOI] [PubMed] [Google Scholar]
- 5.Bulun S. E., Chen D., Moy I., Brooks D. C., Zhao H. (2012) Aromatase, breast cancer and obesity: a complex interaction. Trends Endocrinol. Metab. 23, 83–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milla-Santos A., Milla L., Portella J., Rallo L., Pons M., Rodes E., Casanovas J., Puig-Gali M. (2003) Anastrozole versus tamoxifen as first-line therapy in postmenopausal patients with hormone-dependent advanced breast cancer: a prospective, randomized, phase III study. Am. J. Clin. Oncol. 26, 317–322 [DOI] [PubMed] [Google Scholar]
- 7.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) (2015) Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 386, 1341–1352 [DOI] [PubMed] [Google Scholar]
- 8.Chen S., Masri S., Wang X., Phung S., Yuan Y. C., Wu X. (2006) What do we know about the mechanisms of aromatase inhibitor resistance? J. Steroid Biochem. Mol. Biol. 102, 232–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfeiler G., Königsberg R., Fesl C., Mlineritsch B., Stoeger H., Singer C. F., Pöstlberger S., Steger G. G., Seifert M., Dubsky P., Taucher S., Samonigg H., Bjelic-Radisic V., Greil R., Marth C., Gnant M. (2011) Impact of body mass index on the efficacy of endocrine therapy in premenopausal patients with breast cancer: an analysis of the prospective ABCSG-12 trial. J. Clin. Oncol. 29, 2653–2659 [DOI] [PubMed] [Google Scholar]
- 10.Sestak I., Distler W., Forbes J. F., Dowsett M., Howell A., Cuzick J. (2010) Effect of body mass index on recurrences in tamoxifen and anastrozole treated women: an exploratory analysis from the ATAC trial. J. Clin. Oncol. 28, 3411–3415 [DOI] [PubMed] [Google Scholar]
- 11.Gnant M., Pfeiler G., Stöger H., Mlineritsch B., Fitzal F., Balic M., Kwasny W., Seifert M., Stierer M., Dubsky P., Greil R., Steger G., Samonigg H., Fesl C., Jakesz R. (2013) The predictive impact of body mass index on the efficacy of extended adjuvant endocrine treatment with anastrozole in postmenopausal patients with breast cancer: an analysis of the randomised ABCSG-6a trial. Br. J. Cancer 109, 589–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolters R., Schwentner L., Regierer A., Wischnewsky M., Kreienberg R., Wöckel A. (2012) Endocrine therapy in obese patients with primary breast cancer: another piece of evidence in an unfinished puzzle. Breast Cancer Res. Treat. 131, 925–931 [DOI] [PubMed] [Google Scholar]
- 13.Ioannides S. J., Barlow P. L., Elwood J. M., Porter D. (2014) Effect of obesity on aromatase inhibitor efficacy in postmenopausal, hormone receptor-positive breast cancer: a systematic review. Breast Cancer Res. Treat. 147, 237–248 [DOI] [PubMed] [Google Scholar]
- 14.Goodwin P. J. (2013) Obesity and endocrine therapy: host factors and breast cancer outcome. Breast 22 (Suppl 2), S44–S47 [DOI] [PubMed] [Google Scholar]
- 15.Giles E. D., Jindal S., Wellberg E. A., Schedin T., Anderson S. M., Thor A. D., Edwards D. P., MacLean P. S., Schedin P. (2018) Metformin inhibits stromal aromatase expression and tumor progression in a rodent model of postmenopausal breast cancer. Breast Cancer Res. 20, 50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Subbaramaiah K., Howe L. R., Bhardwaj P., Du B., Gravaghi C., Yantiss R. K., Zhou X. K., Blaho V. A., Hla T., Yang P., Kopelovich L., Hudis C. A., Dannenberg A. J. (2011) Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev. Res. (Phila.) 4, 329–346 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Dabydeen S. A., Furth P. A. (2014) Genetically engineered ERα-positive breast cancer mouse models. Endocr. Relat. Cancer 21, R195–R208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao H., Pearson E. K., Brooks D. C., Coon J. S., V, Chen D., Demura M., Zhang M., Clevenger C. V., Xu X., Veenstra T. D., Chatterton R. T., DeMayo F. J., Bulun S. E. (2012) A humanized pattern of aromatase expression is associated with mammary hyperplasia in mice. Endocrinology 153, 2701–2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao H., Zhou L., Shangguan A. J., Bulun S. E. (2016) Aromatase expression and regulation in breast and endometrial cancer. J. Mol. Endocrinol. 57, R19–R33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simpson E. R. (2003) Sources of estrogen and their importance. J. Steroid Biochem. Mol. Biol. 86, 225–230 [DOI] [PubMed] [Google Scholar]
- 21.Yaghjyan L., Colditz G. A. (2011) Estrogens in the breast tissue: a systematic review. Cancer Causes Control 22, 529–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strong A. L., Ohlstein J. F., Biagas B. A., Rhodes L. V., Pei D. T., Tucker H. A., Llamas C., Bowles A. C., Dutreil M. F., Zhang S., Gimble J. M., Burow M. E., Bunnell B. A. (2015) Leptin produced by obese adipose stromal/stem cells enhances proliferation and metastasis of estrogen receptor positive breast cancers. Breast Cancer Res. 17, 112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chottanapund S., Van Duursen M. B., Navasumrit P., Hunsonti P., Timtavorn S., Ruchirawat M., Van den Berg M. (2013) Effect of androgens on different breast cancer cells co-cultured with or without breast adipose fibroblasts. J. Steroid Biochem. Mol. Biol. 138, 54–62 [DOI] [PubMed] [Google Scholar]
- 24.Furuta S., Bissell M. J. (2016) Pathways involved in formation of mammary organoid architecture have keys to understanding drug resistance and to discovery of druggable targets. Cold Spring Harb. Symp. Quant. Biol. 81, 207–217 [DOI] [PubMed] [Google Scholar]
- 25.Morgan M. M., Livingston M. K., Warrick J. W., Stanek E. M., Alarid E. T., Beebe D. J., Johnson B. P. (2018) Mammary fibroblasts reduce apoptosis and speed estrogen-induced hyperplasia in an organotypic MCF7-derived duct model. Sci. Rep. 8, 7139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chamberlin T., D’Amato J. V., Arendt L. M. (2017) Obesity reversibly depletes the basal cell population and enhances mammary epithelial cell estrogen receptor alpha expression and progenitor activity. Breast Cancer Res. , 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Demirpence E., Duchesne M. J., Badia E., Gagne D., Pons M. (1993) MVLN cells: a bioluminescent MCE-7-derived cell line to study the modulation of estrogenic activity. J. Steroid Biochem. Mol. Biol. 46, 355–364 [DOI] [PubMed] [Google Scholar]
- 28.Heneweer M., Muusse M., Dingemans M., de Jong P. C., van den Berg M., Sanderson J. T. (2005) Co-culture of primary human mammary fibroblasts and MCF-7 cells as an in vitro breast cancer model. Toxicol. Sci. 83, 257–263 [DOI] [PubMed] [Google Scholar]
- 29.Oh T. G., Bailey P., Dray E., Smith A. G., Goode J., Eriksson N., Funder J. W., Fuller P. J., Simpson E. R., Tilley W. D., Leedman P. J., Clarke C. L., Grimmond S., Dowhan D. H., Muscat G. E. (2014) PRMT2 and RORγ expression are associated with breast cancer survival outcomes. Mol. Endocrinol. 28, 1166–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Kok J. B., Roelofs R. W., Giesendorf B. A., Pennings J. L., Waas E. T., Feuth T., Swinkels D. W., Span P. N. (2005) Normalization of gene expression measurements in tumor tissues: comparison of 13 endogenous control genes. Lab. Invest. 85, 154–159 [DOI] [PubMed] [Google Scholar]
- 31.Warrick J. W., Timm A., Swick A., Yin J. (2016) Tools for single-cell kinetic analysis of virus-host interactions. PLoS One 11, e0145081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bourin P., Bunnell B. A., Casteilla L., Dominici M., Katz A. J., March K. L., Redl H., Rubin J. P., Yoshimura K., Gimble J. M. (2013) Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 15, 641–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ortmann J., Prifti S., Bohlmann M. K., Rehberger-Schneider S., Strowitzki T., Rabe T. (2002) Testosterone and 5 alpha-dihydrotestosterone inhibit in vitro growth of human breast cancer cell lines. Gynecol. Endocrinol. 16, 113–120 [PubMed] [Google Scholar]
- 34.Zaitseva M., Vollenhoven B. J., Rogers P. A. (2006) In vitro culture significantly alters gene expression profiles and reduces differences between myometrial and fibroid smooth muscle cells. Mol. Hum. Reprod. 12, 187–207 [DOI] [PubMed] [Google Scholar]
- 35.Baer P. C. (2014) Adipose-derived mesenchymal stromal/stem cells: an update on their phenotype in vivo and in vitro. World J. Stem Cells 6, 256–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sasano H., Miki Y., Nagasaki S., Suzuki T. (2009) In situ estrogen production and its regulation in human breast carcinoma: from endocrinology to intracrinology. Pathol. Int. 59, 777–789 [DOI] [PubMed] [Google Scholar]
- 37.Santner S. J., Pauley R. J., Tait L., Kaseta J., Santen R. J. (1997) Aromatase activity and expression in breast cancer and benign breast tissue stromal cells. J. Clin. Endocrinol. Metab. 82, 200–208 [DOI] [PubMed] [Google Scholar]
- 38.Morris P. G., Hudis C. A., Giri D., Morrow M., Falcone D. J., Zhou X. K., Du B., Brogi E., Crawford C. B., Kopelovich L., Subbaramaiah K., Dannenberg A. J. (2011) Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev. Res. (Phila.) 4, 1021–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown K. A., Iyengar N. M., Zhou X. K., Gucalp A., Subbaramaiah K., Wang H., Giri D. D., Morrow M., Falcone D. J., Wendel N. K., Winston L. A., Pollak M., Dierickx A., Hudis C. A., Dannenberg A. J. (2017) Menopause is a determinant of breast aromatase expression and its associations with BMI, inflammation, and systemic markers. J. Clin. Endocrinol. Metab. 102, 1692–1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pfeiler G., Königsberg R., Hadji P., Fitzal F., Maroske M., Dressel-Ban G., Zellinger J., Exner R., Seifert M., Singer C., Gnant M., Dubsky P. (2013) Impact of body mass index on estradiol depletion by aromatase inhibitors in postmenopausal women with early breast cancer. Br. J. Cancer 109, 1522–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Folkerd E. J., Dixon J. M., Renshaw L., A’Hern R. P., Dowsett M. (2012) Suppression of plasma estrogen levels by letrozole and anastrozole is related to body mass index in patients with breast cancer. J. Clin. Oncol. 30, 2977–2980 [DOI] [PubMed] [Google Scholar]
- 42.Hopkinson B. M., Klitgaard M. C., Petersen O. W., Villadsen R., Rønnov-Jessen L., Kim J. (2017) Establishment of a normal-derived estrogen receptor-positive cell line comparable to the prevailing human breast cancer subtype. Oncotarget 8, 10580–10593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vidi P. A., Bissell M. J., Lelièvre S. A. (2013) Three-dimensional culture of human breast epithelial cells: the how and the why. Methods Mol. Biol. 945, 193–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shamir E. R., Ewald A. J. (2014) Three-dimensional organotypic culture: experimental models of mammalian biology and disease. Nat. Rev. Mol. Cell Biol. 15, 647–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu H., Alexander C. M., Beebe D. J. (2007) Understanding microchannel culture: parameters involved in soluble factor signaling. Lab Chip 7, 726–730 [DOI] [PubMed] [Google Scholar]
- 46.Vantangoli M. M., Madnick S. J., Huse S. M., Weston P., Boekelheide K. (2015) MCF-7 human breast cancer cells form differentiated microtissues in scaffold-free hydrogels. PLoS One 10, e0135426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sung K. E., Su X., Berthier E., Pehlke C., Friedl A., Beebe D. J. (2013) Understanding the impact of 2D and 3D fibroblast cultures on in vitro breast cancer models. PLoS One 8, e76373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang F., Weaver V. M., Petersen O. W., Larabell C. A., Dedhar S., Briand P., Lupu R., Bissell M. J. (1998) Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc. Natl. Acad. Sci. USA 95, 14821–14826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lelièvre S. A., Weaver V. M., Nickerson J. A., Larabell C. A., Bhaumik A., Petersen O. W., Bissell M. J. (1998) Tissue phenotype depends on reciprocal interactions between the extracellular matrix and the structural organization of the nucleus. Proc. Natl. Acad. Sci. USA 95, 14711–14716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dignam J. J., Wieand K., Johnson K. A., Fisher B., Xu L., Mamounas E. P. (2003) Obesity, tamoxifen use, and outcomes in women with estrogen receptor-positive early-stage breast cancer. J. Natl. Cancer Inst. 95, 1467–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.