Abstract
Damage-induced long noncoding RNA (DINO) is a long noncoding RNA that directly interacts with p53 and thereby enhances p53 stability and activity in response to various cellular stresses. Here, we demonstrate that nuclear receptor subfamily 2 group E member 3 (NR2E3) plays a crucial role in maintaining active DINO epigenetic status for its proper induction and subsequent p53 activation. In acetaminophen (APAP)- or carbon tetrachloride–induced acute liver injuries, NR2E3 knockout (KO) mice exhibited far more severe liver injuries due to impaired DINO induction and p53 activation. Mechanistically, NR2E3 loss both in vivo and in vitro induced epigenetic DINO repression accompanied by reduced DINO chromatin accessibility. Furthermore, compared with the efficient reversal by a typical antidote N-acetylcysteine (NAC) treatment of APAP-induced liver injury in wild-type mice, the liver injury of NR2E3 KO mice was not effectively reversed, indicating that an intact NR2E3-DINO-p53–signaling axis is essential for NAC-mediated recovery against APAP-induced hepatotoxicity. These findings establish that NR2E3 is a critical component in p53 activation and a novel susceptibility factor to drug- or toxicant-induced acute liver injuries.—Khanal, T., Leung, Y.-K., Jiang, W., Timchenko, N., Ho, S.-M., Kim, K. NR2E3 is a key component in p53 activation by regulating a long noncoding RNA DINO in acute liver injuries.
Keywords: NR2E3, p53, DINO, long noncoding RNA
The p53 protein is a key transcription factor that directs a cell’s fate in terms of cell cycle arrest or apoptosis in response to various cellular stresses (1). The abundance or stability of p53 finely tunes the magnitude of p53 signaling cascades (2, 3). Recently, damage-induced long noncoding RNA (DINO) was identified as a long noncoding RNA induced by and interacting with p53. The DINO-p53 (RNA protein) complex enhances p53 stability and amplifies its signaling (4). However, the underlying mechanism that regulates DINO remains unknown.
Nuclear receptor subfamily 2 group E member 3 (NR2E3) is an orphan nuclear receptor that plays a crucial role in retinal development (5, 6). Previously, we reported that NR2E3 is essential for maintaining the basal expression of estrogen receptor α (ER) and is a significantly good prognostic marker for relapse-free survival in patients with breast cancer (7). Loss of NR2E3 induced the recruitment of lysine-specific histone demethylase 1A (LSD1) and thereby epigenetically repressed ER expression (8). We further demonstrated that NR2E3 formed active transcriptional complex with specificity protein 1 (Sp1) transcription factor and maintained the basal expression of aryl hydrocarbon receptor (AHR) by preventing LSD1-mediated epigenetic reprogramming. We also showed that NR2E3 loss is also correlated with the development of human liver diseases and cancer (9).
Acute liver failures are usually caused by viral infection, drug, or toxicant exposure (10). Among these, acetaminophen (APAP)-induced hepatotoxicity is the leading cause of acute liver failure in the US (11). APAP is a widely used analgesic and antipyretic that is safe and effective at therapeutic doses. However, APAP overdose facilitates the excessive formation of a reactive metabolite, N-acetyl-p-benzoquinone imine, via CYP2E1-dependent metabolic activation (12). N-acetyl-p-benzoquinone imine production depletes glutathione (GSH), increases mitochondrial oxidative stress, and eventually leads to liver necrosis (13). Recently, it was reported that p53 plays a protective role in APAP-induced liver injury by suppressing persistent activation of JNK (14), a key player in APAP-induced mitochondrial oxidative damage (15, 16). In clinical settings, N-acetylcysteine (NAC) has been used as an effective antidote for treating APAP-induced hepatotoxicity (17, 18). Nonetheless, the molecular mechanism or factor that determines the susceptibility to APAP-induced hepatotoxicity, particularly at the epigenetic level, remains largely unknown.
Here, we present the novel molecular link between the NR2E3 and p53 signaling pathways. Without altering basal p53 levels, NR2E3 ablation at both in vivo and in vitro induces epigenetic DINO repression, resulting in inefficient DINO induction and p53 activation. This disruption of NR2E3-DINO–p53 signaling axis leads to more severe liver injuries and ineffective reversal by antioxidant NAC treatment. Collectively, our findings show that NR2E3 is critical in DINO-mediated p53 signaling pathways and is a susceptibility factor that determines drug- or toxicant-induced acute liver injury responses.
MATERIALS AND METHODS
Cell line, reagents, and chemicals
HepG2 cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) and were maintained at 37°C in the presence of 5% CO2 in MEMα medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin solution (MilliporeSigma, Burlington, MA, USA). The cells (2.5 × 104/well) were seeded, and the fetal bovine serum content of the medium was reduced to 5%. Alanine aminotransferase (ALT) Colorimetric/Fluorometric assay diagnostic kits were purchased from BioVision (Milpitas, CA, USA); carbon tetrachloride (ccl4) was purchased from MilliporeSigma; the Glutathione Assay Kit and APAP were purchased from Cayman Chemicals (Ann Arbor, MI, USA).
Small interfering RNAs (siRNAs) targeting Sp1 (siRNA identification: SASI_Hs_00363664 and SASI_Hs01_00070994) (19) and negative control siRNAs were purchased from MilliporeSigma. The validated small interfering RNAs targeting DINO from the previous study were used (4). Small hairpin RNAs targeting NR2E3 were previously described (6, 7).
Generation of Nr2e3−/− knockout mice
A whole body NR2E3 knockout (KO) mouse was generated using the clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (CAS9) gene editing system in the transgenic core of the Cincinnati Children Hospital Medical Center. Briefly, we used a total of 4 single guide RNAs (sgRNAs) to create Nr2e3−/− KO mice (Supplemental Table S1). The sgRNAs were selected according to the location and the on- and off-target scores from the web tool CRISPOR (http://www.crispor.tefor.net) (20). The selected target sequences were sg143 and sg144, which target the exon 1 location, and sg145 and sg146, which target the exon 2 location of the NR2E3 gene, respectively. The sgRNAs were synthesized in vitro using the MEGAshortscript T7 kit (Thermo Fisher Scientific, Waltham, MA, USA) and purified using the MEGAclear Kit (Thermo Fisher Scientific), as previously described in Yang et al. (21). sgRNAs (25 ng/μl of each) were mixed with 200 ng/μl Cas9 protein (Thermo Fisher Scientific) and incubated at 37°C for 15 min to form a RNP complex. We then injected the mix into the cytoplasm of one-cell-stage embryos of the C57BL/6 genetic background using a piezo-driven microinjection technique, as previously described (21, 22). Injected embryos were immediately transferred into the oviducal ampulla of pseudopregnant CD1 females. The founder mice and their progeny carrying 519 bp deletion derived from Nr2e3 gene region between exon1 and exon 2 were identified by the PCR with the primer set (Supplemental Table S2) as bands shifted down compared with that of wild-type (WT) mice. To confirm the deletion, the lower bands were purified and further confirmed by DNA sequencing. In general, off-target mutations by CAS9/CRISPR gene editing in mice are rare (23). However, using the CRISPOR program, we analyzed potential off-target effects of the 4 sgRNAs. Based on the analysis results, we listed the top 10 off-target sequences in each sgRNA (Supplemental Tables S3 and S4). Of note, the off-target score (off-target score range for MIT specificity score was 0–100 and for CFP specificity score 0–1) for each predicted off-target sequence was either significantly low or located in an intron, consistently indicating that off-target effects were minimal. Human DINO expression plasmid was kindly provided by Dr. Howard Chang from Stanford University (Stanford, CA, USA).
Animal experiments
All the animal experiments were performed in compliance with the guidelines established by the Animal Care Committee of University of Cincinnati. The animals were acclimated to temperature- and humidity-controlled rooms with a 12-h light/dark cycle for 1 wk prior to use. The mice had access to laboratory chow and tap water ad libitum. C57BL/6J WT mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). C57BL/6J WT and age-matched Nr2e3−/− KO male mice (6–8 wk old, 20–25 g) were allocated for treatment with saline, APAP dissolved in saline, NAC dissolved in water, or ccl4 dissolved in olive oil. Before any treatment, mice were not fed up to 12 h. Mice were treated with APAP (300 mg/kg) intraperitoneally, and 90 min after APAP treatment, mice were injected with NAC (500 mg/kg) dissolved in saline. Tissue samples and blood were collected at 0, 6, 12, or 24 h after APAP or APAP plus NAC treatment. Ccl4 (0.7 ml/kg) was injected intraperitoneally into mice, which were then anesthetized after 24 h. Liver tissues were frozen immediately in liquid nitrogen, and samples were collected for further analyses.
Chromatin immunoprecipitation assay
A chromatin immunoprecipitation (ChIP) assay was performed using the ChiP-IT Express Chromatin Immunoprecipitation Kit or the Re-ChIP-IT Kit (Active Motif, Carlsbad, CA, USA), according to the manufacturer’s protocol. Approximately 150–300 mg of liver tissue was minced, homogenized, and fixed with 1% formaldehyde for in vivo ChIP or Re-ChIP assays. The sequences of all the ChIP primer sets are listed in Supplemental Table S5. All the ChIP-grade antibodies used and incubation conditions employed are listed in Supplemental Table S6. All the ChIP-PCR reactions were carried out using a 7300HT Real-Time PCR system or a QuantStudio 3 Real-Time PCR system with a 96-well block module (Thermo Fisher Scientific). The cycling conditions were 56°C for 30 min and 95°C for 10 min, followed by 50 cycles of 95°C for 25 s and 60°C for 60 s.
Chromatin accessibility assay (chromatin repositioning)
Mouse Dino or human DINO gene promoter (DINO) chromatin accessibility was determined by nuclease (Nse)-dependent chromatin degradation coupled to quantitative PCR using an Epiquik Chromatin Accessibility Assay Kit (EpiGentek, Farmingdale, NY, USA), according to the manufacturer’s protocol. Briefly, chromatins were isolated from tissues or cells and then treated with or without an Nse mix. DNA was then isolated and amplified using gene-specific primers for mouse Dino or human DINO gene promoter region (Supplemental Table S7). The fold enrichment was calculated by the ratio of amplification efficiency of the Nse-treated DNA sample over that of the control sample not treated with Nse (no Nse): [fold enrichment = 2 (Nse Ct − no Nse Ct) × 100%]. Changes in chromatin accessibility or repositioning were determined by the degree of Ct shift between the digested and undigested samples. DNA in heterochromatin was resistant to the Nse treatment, resulting in insignificant Ct shifts between digested and undigested (control) samples, whereas DNA in euchromatin was accessible to Nse, resulting in a large Ct shift. All the employed chromatin accessibility primer sequences are listed in Supplemental Table S7. Additional details about all other experiments are provided in the Supplemental Data.
RNA-ChIP assay
To detect the level of interaction between DINO and p53, we performed RNA-ChIP assay as previously established in Zhao et al. (24). Briefly, cells were fixed with formaldehyde and sheared by sonication. After DNase treatment, the fixed chromatins were immunoprecipitated with IgG or p53 antibody overnight with addition of RNAse inhibitor followed by stringent washing of protein A/G bead pellets. Next, cross-linking was reversed and RNA was extracted with Trizol. The purified RNAs were analyzed by quantitative real-time PCR to determine the levels of interaction between RNA and protein of interest such as p53.
Serum biochemistry
An ALT assay was used to assess liver injury levels. The ALT activity was measured using ALT Activity Colorimetric Assay Kit (BioVision), following the manufacturer’s protocol. Briefly, samples were centrifuged at 1000 g for 10 min within 1 h after collection. The serum was stored in the −80°C freezer prior to analysis. ALT activity in the serum was evaluated by OD 570 nm.
Determination of GSH levels
Reduced and oxidized forms of GSH (GSH and GSSG, respectively) were measured using a GSH/GSSG Ratio Detection Assay Kit (Cayman Chemicals), according to the manufacturer’s protocol. A total of 50 mg of liver tissue was rinsed in PBS prior to dissection and homogenized in cold buffer (i.e., 50 mM MES or phosphate, pH 6–7, containing 1 mM EDTA) (ratio of weight to volume, 1:20) while on ice. Tissue homogenates were centrifuged at 10,000 g for 15 min at 4°C. Supernatant was divided into aliquots and stored at −80°C until use. A total of 50 µl of homogenate was used, in duplicate, for detection of GSH at OD 412 nm.
Real-time quantitative PCR
Total RNA was extracted from liver tissues or cells, and the cDNAs were synthesized using a high-capacity cDNA Reverse Transcription Kit (Applied Biosystems) to perform real-time quantitative PCR. The obtained results were normalized to glyceraldehyde-3-phosphate dehydrogenase or β-actin control. All the primer set sequences for detecting specific mouse or human genes by quantitative real-time PCR are listed in Supplemental Table S8. We used previously reported human DINO and mouse DINO gene primer sequences (4).
TUNEL assay
For detection of apoptotic cells, an In Situ Cell Death Detection Kit AP was purchased from Roche (Indianapolis, IN, USA). This kit was based on the TUNEL assay. After the sections were deparaffinized in xylene and rehydrated through a series of graded ethanol solutions, the slides were placed in 0.1 M Citrate buffer, with a pH of 6 for 5 min, and 350 W microwave irradiation was applied for 5 min. Sections were permeabilized with 0.1% triton × 100 for 2 min at room temperature. Finally, the TUNEL reaction (enzyme solution plus label solution) mixture was added, and the slides were incubated for 60 min at 37°C in a humidified atmosphere in the dark. The color was developed using NBT/BCIP and visualized under a microscope. The apoptotic index of each field (region of interest) was calculated as the percent of TUNEL-positive cells.
Histologic analysis
Paraffin-embedded liver sections were sectioned, deparaffinized in xylene, and rehydrated through a series of graded ethanol solutions. The extent of APAP- or ccl4-induced hepatic injury was determined by assessing the morphologic changes in liver sections stained with hematoxylin and eosin (H&E), followed by examination under a light microscope for histologic analysis.
Caspase-3/7 assay
This assay was carried out according to the manufacturer’s protocol. Cells were treated with etoposide and after 16 h this assay was performed.
Western blotting
Total proteins extracted from either cells or minced liver tissues (50–80 μg/lane) were resolved by SDS-PAGE and transferred onto PVDF membranes. After being blocked with Tris-buffered saline with Tween-20 or phosphate-buffered saline with Tween-20 buffer containing 5% nonfat milk, the membranes were incubated with appropriate primary antibody. Then, proteins of interest were detected with either anti-rabbit or anti-mouse horseradish peroxidase–conjugated secondary antibodies (1: 3000 dilutions; Cell Signaling Technology, Danvers, MA, USA) and visualized with an ECL detection system using a C-DiGit Blot Scanner from Li-Cor Biosciences (Lincoln, NE, USA). The antibodies and incubation conditions used for Western blotting are described (Supplemental Table S9). All the immunoblotting experiments were carried out at least 2 or 3 times.
Immunohistochemical staining
After liver sections were deparaffinized, the sections were immersed in 0.01 mM sodium citrate (pH 6.0) and heated in a microwave oven (100°C) for antigenic retrieval. The deparaffinized sections were incubated with peroxidase-blocking reagent (BioGenex Laboratories, Fremont, CA, USA) to block endogenous peroxidase activity and then incubated with nonspecific staining blocking reagent (Vector Laboratories, Burlingame, CA, USA). The sections were incubated with primary antibodies at 4°C overnight. Anti-nitrotyrosine antibody (ab7048; Abcam, Cambridge, MA, USA), anti-NR2E3 antibody (14246-1; Proteintech, Rosemont, IL, USA), and anti-p53 antibody (10442-1; Proteintech) were employed as primary antibodies. The sections were subsequently incubated with peroxidase-conjugated secondary antibodies (Vector Laboratories) and 3,3-diaminobenzine-tetrachloride (Vector Laboratories), according to the manufacturer’s instructions. The sections were counterstained with hematoxylin and observed under a microscope.
Statistical analysis
The results are representative of at least 2–3 independent experiments, with 3 replicates per experiment. Statistical difference was determined by the unpaired Student’s t test with a 2-tailed distribution. Data are presented as the means ± sd. For statistical difference between multiple groups, 1-way ANOVA with Bonferroni correction was performed using Prism 6.0 software (GraphPad, La Jolla, CA, USA). Statistical significance was set at a value of P < 0.05.
RESULTS
CRISPR/CAS9-Mediated NR2E3 gene ablation in vivo
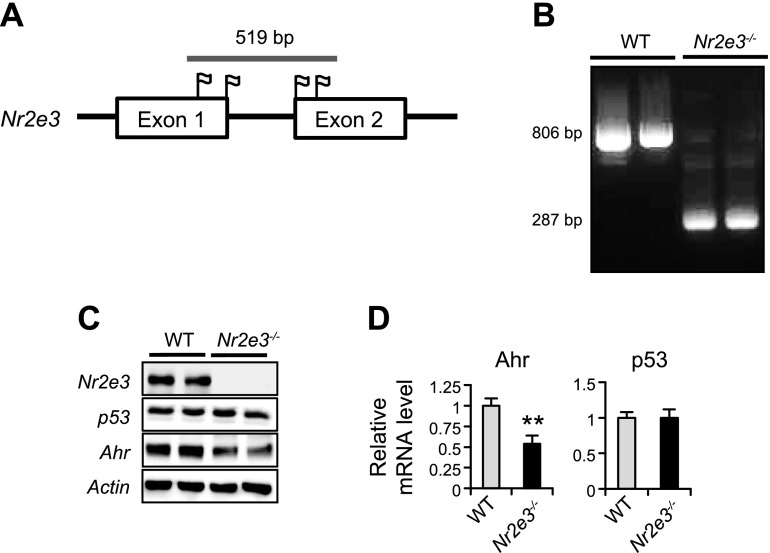
To generate a whole body Nr2e3 (Nr2e3−/−) KO mouse with a C57BL/6 genetic background, 4 separate sgRNAs targeted to the 2 sites on exon 1 and the other 2 sites of exon 2 of NR2E3 were selected; the red tag indicates protospacer adjacent motif sites targeted by sgRNAs (Fig. 1A). The deleted region and surrounding exon sequences are indicated in Supplemental Fig. S1, and the sgRNA sequences are shown in Supplemental Table S1. Following injection and screening, we identified the founder mouse containing biallelic deletions in Nr2e3 exon1 and exon 2. The 519 bp deletion was confirmed by PCR, with the primer set flanking the target region (Fig. 1B and Supplemental Table S2). Liver tissue lysate from the founder mice showed the absence of Nr2e3 expression by immunoblotting. Consistent to the previous results (9), Nr2e3 ablation in vivo decreased Ahr protein and mRNA expressions with no alteration in the basal p53 levels (Fig. 1C, D). In addition, similar to the previous results (9), immunohistochemical Nr2e3 staining analysis confirmed that Nr2e3 protein expressions were detected in the hepatocytes of WT mice but not in Nr2e3−/− KO mice (Supplemental Fig. S2). No distinctive morphologic differences between WT and Nr2e3−/− KO mice were observed.
Figure 1.
Generation of NR2E3 KO mouse using the CRISPR/CAS9 gene editing system. A) Four selected sgRNA sites (flag) in exon 1 and exon 2 are shown. B) CRISPR/CAS9-mediated deletion of 519 bp from mouse NR2E3 exon 1 and exon 2 regions was confirmed by the size difference between 806 bp fragment amplification from WT vs. 287 bp bands from NR2E3−/− KO founder mice after PCR amplification of the target region. C, D) NR2E3 ablation in vivo was confirmed by immunoblotting. Consistently, reduced protein and mRNA levels of previously identified NR2E3 downstream target gene Ahr were detected but not p53 (WT: n = 4, Nr2e3−/− KO: n = 4). Values are represented as means ± sd. **Significant repression; P < 0.05.
Nr2e3−/− KO mice exhibited severe APAP-induced liver damage
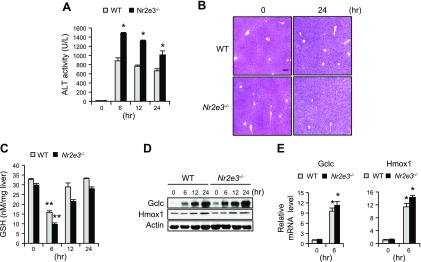
Our previous report showed that NR2E3 loss is clinically associated with development of liver diseases and cancer (9), the end points of many liver injuries. Thus, we first examined the role of NR2E3 by employing a typical liver toxicant APAP. Both WT and Nr2e3−/− KO mice mice were treated with APAP, and then plasma and livers were collected at 0-, 6-, 12-, and 24-h time points. The APAP-treated Nr2e3−/− KO mice exhibited far more severe liver injury levels, confirmed by higher ALT activity, than those of WT mice at all time points (Fig. 2A). Consistently, the APAP-treated livers of Nr2e3−/− KO mice exhibited clear evidence of increased necrosis in H&E-stained histology sections (Fig. 2B). These results indicate that Nr2e3 loss exacerbated APAP-induced hepatotoxicity.
Figure 2.
Increased APAP-induced liver damage without altering GSH replenishment process in the absence of NR2E3 in vivo. A) Mice were treated with APAP (300 mg/kg), and serum ALT levels were measured at 0-, 6-, 12-, and 24-h time points (only male WT and Nr2e3−/− KO mice were employed; n = 4–5 per each time point). B) Liver damage was evaluated by H&E staining of liver sections derived from APAP-treated WT and Nr2e3−/− KO mice (0 and 24 h). C) GSH levels were reduced at 6 h and then similarly recovered at 24 h in the livers of WT and Nr2e3−/− KO mice after APAP treatment. D, E) Effects of APAP treatment on protein and mRNA induction levels of glutamate-cysteine ligase catalytic subunit and heme oxygenase 1 were shown in the livers of WT and Nr2e3−/− KO mice. Scale bar, 100 μm. Values are represented as means ± sd. *Significant induction or **repression; P < 0.05.
Given that GSH replenishment plays a protective role against APAP-induced hepatotoxicity (25), we examined whether the GSH replenishment process might be compromised in the livers of the APAP-treated Nr2e3−/− KO mice. However, there is no difference observed in the GSH replenishment process between WT and Nr2e3−/− KO mice after APAP treatment (Fig. 2C). Consistently, no significant differences were detected in the induction of γ-glutamylcysteine synthetase, a rate-limiting enzyme for GSH replenishment, or of heme oxygenase 1, which plays a protective role against APAP-induced liver injuries at both the protein and mRNA levels (Fig. 2D, E). Both genes are typically induced in APAP-induced liver damage responses and are well-established downstream target genes of Nrf2 transcription factor (26–28). However, we did not observe any effects of Nr2e3 loss on the basal mRNA and protein levels of Nrf2 and Cyp2e1 (Supplemental Fig. S3A, B), which are key players in APAP metabolic activation as well as in the GSH replenishment process (12, 26, 27). Taken together, these results suggest that Nr2e3 ablation in vivo increased the APAP-induced liver damage levels without affecting the GSH replenishment process.
APAP-treated Nr2e3−/− KO mice exhibited significantly reduced apoptosis
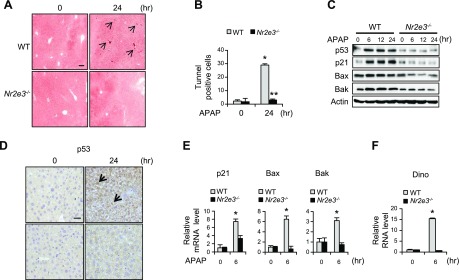
Next, to investigate the underlying mechanism responsible for the increased liver damage in APAP-treated Nr2e3−/− KO mice, we examined whether Nr2e3 loss dysregulates apoptosis. Intriguingly, the result from TUNEL staining showed that there were significantly fewer apoptotic cell deaths in the livers of APAP-treated Nr2e3−/− KO mice at 24 h, indicating that NR2E3 is required for mediating apoptosis in APAP-induced liver injury responses (Fig. 3A). The markedly reduced number of TUNEL-positive cells in the livers of APAP-treated Nr2e3−/− KO mice was subsequently confirmed (Fig. 3B).
Figure 3.
Effects of NR2E3 ablation in vivo on APAP-induced p53 activation and apoptosis. A) Representative TUNEL-stained liver sections from WT and Nr2e3−/− KO mice are shown. TUNEL-stained cells are indicated by black arrow. Scale bar, 100 μm. B) The markedly reduced number of TUNEL-positive cells per region of interest (>10 regions) in Nr2e3−/− KO mice treated with APAP are shown. C) The protein level changes of p53 and its downstream target genes, including p21, Bax, and Bak1, in WT and Nr2e3−/− KO mice treated with APAP are shown. D) Immunostaining confirmed increased p53 protein levels and p53 nucleus localization (thick black arrow) in the liver section of WT 24 h after APAP treatment but not in the Nr2e3−/− KO mice. Scale bar, 20 μm. E) Major p53 downstream target gene mRNA level changes are shown (WT vs. Nr2e3−/− KO; n = 4–5 per each time point). F) No induction of DINO in the livers of Nr2e3−/− KO mice treated with APAP. Values are represented as means ± sd. *Significant induction or **repression; P < 0.05.
Because p53 is a master regulator of cellular injury responses in relation to apoptosis, we examined whether Nr2e3 loss dysregulated APAP-induced p53 activation. Of note, compromised induction of p53 and its major downstream target genes, including p21, Bax, and Bak1, was detected by immunoblotting (Fig. 3C). The increased p53 protein levels and its localization in the nucleus in the APAP-treated livers of WT mice (0 and 24 h) were further confirmed by immunostaining, whereas their increases were not observed in the liver tissues of APAP-treated Nr2e3−/− KO mice (Fig. 3D). Consistently, reduced inductions of p53 downstream target gene mRNAs were detected (Fig. 3E and Supplemental Fig. S5A). It was previously reported that p53 functional loss sustained JNK phosphorylation, an active JNK form, and thereby promoted APAP-induced hepatotoxicity (14). In fact, previous reports indicated that persistent JNK activation disrupted mitochondrial bioenergenetics and increased mitochondrial oxidative stress that promotes peroxynitrite formation, leading to increased necrotic cell death (29, 30). Thus, we investigated whether Nr2e3 ablation in vivo increased JNK activation and the formation of nitrotyrosine, a biomarker for peroxynitrite formation. Correspondingly, we observed increased JNK phosphorylation and nitrotyrosine staining in the livers of APAP-treated Nr2e3−/− KO mice (Supplemental Fig. S4A, B). Taken together, these results consistently suggested that Nr2e3 ablation in vivo disrupted p53 activation and its downstream events, resulting in sustained JNK activation and increased APAP-induced mitochondrial oxidative stress.
Intriguingly, we observed that the basal p53 protein levels were unchanged between WT and Nr2e3−/− KO mice (Supplemental Fig. S3B); however, p53 was not stabilized in APAP-treated NR2E3 KO mice (Fig. 3C). These results suggest that NR2E3 likely regulates p53 protein stability. Recently, a long noncoding RNA DINO was identified as a novel player that increases p53 stability and amplifies p53 signaling via direct interaction with p53 (4). Therefore, we examined whether the DINO gene was properly induced in the livers of APAP-treated Nr2e3−/− KO mice. Intriguingly, DINO induction was significantly compromised in the Nr2e3−/− KO mice (Fig. 3F), demonstrating that NR2E3 loss dysregulated the induction.
Effects of NR2E3 loss on ccl4-induced acute liver injury
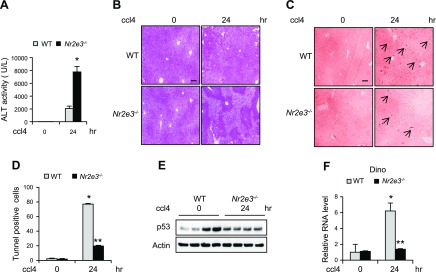
To examine whether NR2E3 also plays a role in other toxicant-induced acute liver injury, we treated mice with ccl4. Unlike APAP-induced hepatotoxicity (where JNK inhibition is highly protective), in ccl4-induced acute hepatotoxicity, JNK inhibition was not effective (15). However, p53 still plays a role by mediating apoptosis in ccl4-induced hepatotoxicity (31, 32). Increased acute liver injury levels accompanied by enhanced ALT activity and necrosis by H&E staining were detected in ccl4-treated livers of Nr2e3−/− KO mice (Fig. 4A, B). The significant reduction in apoptosis was detected in the ccl4-treated Nr2e3−/− KO mice (Fig. 4C, D). Consistently, the compromised DINO induction and p53 activation confirmed the important role of NR2E3 in the p53 activation also in ccl4-induced acute liver injury responses (Fig. 4E, F). Consistently, the impaired induction of p53 downstream target genes, including p21, Bax, Bak1, and mdm2, was detected by quantitative real-time PCR analysis (Supplemental Fig. S5B).
Figure 4.
Effects of NR2E3 ablation in vivo on ccl4-induced p53 activation and apoptotic cell death. A) Serum ALT levels at 24 h after ccl4 treatment (0.7 ml/kg, i.p.) (n = 4–5). B) H&E staining images of representative liver sections from ccl4-treated or untreated WT and Nr2e3−/− KO mice. C) TUNEL staining of representative liver sections (0 and 24 h). D) The significantly decreased number of TUNEL-positive cells in Nr2e3−/− KO mice treated with ccl4 is shown. E) Impaired p53 activation was confirmed by immunoblotting in the livers of ccl4-treated Nr2e3−/− KO mice. F) Significantly reduced DINO induction was observed in the livers of ccl4-treated NR2E3−/− KO mice. Scale bars, 100 μm. Black arrows indicate TUNEL-positive cells. Values are represented as means ± sd. *Significant induction or **repression; P < 0.05.
NR2E3 loss induced repressive DINO epigenetic status with reduced chromatin accessibility
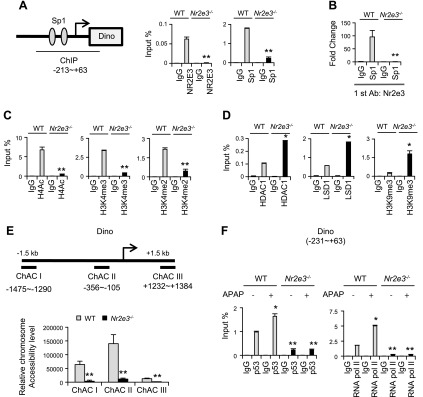
Our previous reports showed that NR2E3 played a role in maintaining the active epigenetic status of ER and AHR gene promoters, the depletion of which resulted in epigenetic repression (8, 9). Therefore, we investigated whether NR2E3 loss could also dysregulate the epigenetic status of DINO. It was previously reported that NR2E3 formed an active complex with Sp1 transcription factor to sustain active AHR epigenetic status and its expression (9). Interestingly, the result from an online promoter analysis showed that there are 2 consensus Sp1 binding sites in the proximal DINO gene promoter region (33) (Fig. 5A. left). We performed a ChIP assay with the multiple primer sets covering the distal region (ChIP I:−1808 ∼ −1521 and ChIP III: +1377 ∼ +1559) and the proximal DINO gene promoter regions (ChIP II: −213 ∼ +63), which contains 2 consensus Sp1 binding sequences. We detected significant Nr2e3 bindings to the proximal region, not the distal regions (ChIP I and III) using liver lysate from WT mice. However, no NR2E 3 binding was detected when we used the liver lysate from Nr2e3−/− KO mice (Supplemental Fig. S6A). Consistently, we detected both NR2E3 and Sp1 binding to the region (Fig. 5A). A Re-ChIP assay that pulled down the liver ChIP lysate with NR2E3 antibody first and then subsequently with Sp1 antibody further corroborated the presence of NR2E3–Sp1 protein complex in this promoter region, but it was absent in the Nr2e3−/− KO lysate (Fig. 5B). Together, these results clearly show that NR2E3 formed a complex with Sp1 in the DINO gene proximal promoter region. Next, we determined whether NR2E3 loss resulted in the repressive epigenetic status of the DINO gene promoter, similar to what we previously observed (8, 9). The results from the ChIP assays showed that NR2E3 loss significantly decreased the active histone marks of H4Ac, H3K4me2, and H3K4me3 (Fig. 5C) (34, 35), suggesting that this loss induced repressive DINO chromatin status. This repression was further confirmed by the enhanced binding of histone deacetylase 1 and LSD1, a histone demethylase that can induce gene silencing by demethylating H3K4me2 (36), accompanied by increased binding of H3K9me3, an inactive histone mark (Fig. 5D) (37). Taken together, the results indicate that NR2E3 loss, disrupting NR2E3–Sp1 protein complex, induced epigenetic DINO repression, possibly via multiple histone modification status changes.
Figure 5.
Effects of NR2E3 ablation in vivo on DINO epigenetic status and chromatin accessibility. A, right) Schematic diagram for mouse DINO gene proximal promoter region containing two consensus Sp1 binding sequences (oval shape). A, left) Effect of NR2E3 loss on the binding status of NR2E3 and Sp1 on the Dino gene promoter proximal region (−213 to +63), as determined by ChIP-PCR assay. B) Re-ChIP analysis of NR2E3–Sp1 complex formation on the DINO proximal promoter region in the presence or absence of NR2E3 in vivo. C) Active histone modification status, including H4Ac, H3K4me2, and H3K4me3. D) The binding status of LSD1, histone deacetylase 1, and repressive histone mark H3K9me3 in the DINO gene promoter proximal region. E) Effects of NR2E3 loss on the chromatin accessibility of DINO gene promoter region. ChACI, II and III are the primer sets used for measuring chromatin accessibility of three different regions of DINO gene promoter. F) The recruitment of p53 and RNA pol II to DINO gene proximal promoter region after APAP treatment (0 and 6 h) in the livers of APAP-treated WT and Nr2e3−/− KO mice. Values are represented as means ± sd. *Significant induction or **repression; P < 0.05.
The decreases of active histone marks, including H4Ac, H3k4me2, and H3K4me3, or increased repressive H3K9me3 mark were often associated with compact chromatin accompanied by reduced chromatin accessibility via chromatin repositioning (38–41), which is associated with epigenetic repression. Therefore, we sought to determine whether NR2E3 loss that disrupts the NR2E3–Sp1 complex formation alters DINO chromatin accessibility. We carried out a chromatin accessibility assay with 3 different primer sets that cover upstream (−1475 ∼ −1290), proximal (−356 ∼ −105), and downstream (+1232 ∼ +1384) of the DINO gene promoter region (Fig. 5E, upper). Each region exhibited relatively different levels of chromatin accessibility. Corresponding to the results from the ChIP assays, the chromatin accessibility of the DINO gene was significantly reduced in all of these regions, indicating that NR2E3 or NR2E3–Sp1 complex plays a crucial role in maintaining accessible DINO chromatin status (Fig. 5E, bottom). To further determine whether the reduced chromatin accessibility interfered with active transcriptional complex recruitment and formation, we performed a ChIP assay using APAP-treated and -untreated liver ChIP lysate (0 and 6 h). Efficient recruitments of p53 and RNA pol II to the DINO gene promoter region were observed in APAP-treated WT liver ChIP lysate; however, no recruitments were observed in Nr2e3−/− KO mice (Fig. 5F). In addition, the Re-ChIP assay results using WT and Nr2e3−/− KO liver ChIP lysate with or without APAP treatment (0 and 6 h) clearly demonstrated that NR2E3 is essential for recruiting p53 and for the formation of an active transcriptional complex with Sp1 during APAP-induced DINO induction (Supplemental Fig. S6B, C). Consistently, compromised p53 and RNA pol II recruitments to the promoter regions of p53 downstream target genes such as Bax and Bak1 gene promoters were observed (Supplemental Fig. S7A, B). Bak1 was previously reported as a p53 target gene, which was regulated by both p53 and Sp1 (42, 43). The decreased Bax and Bak1 induction were likely due to the failure of the DINO–p53 complex formation necessary for p53-mediated transcriptional activation.
Effects of NR2E3 loss on DINO-mediated p53 activation in human liver cancer cells
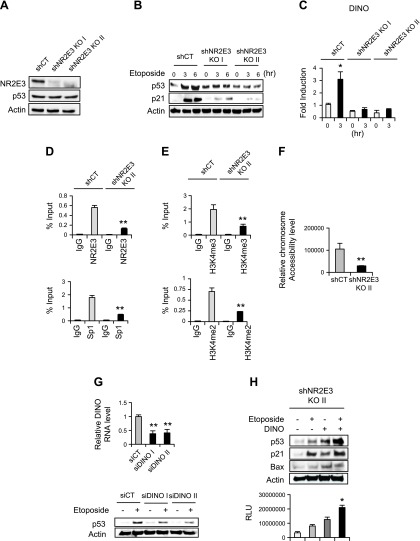
By using p53-positive HepG2 liver cancer cells, we determined whether NR2E3 depletion in HepG2 similarly dysregulated DINO induction and p53 activation. NR2E3 depletion in HepG2 cells did not alter basal p53 protein and mRNA levels (Fig. 6A and Supplemental Fig. S8). However, when NR2E3-depleted HepG2 cells were treated with etoposide, a chemotherapeutic agent that activates p53, no substantial induction of p53 and p21 (major p53 downstream target genes) was observed (Fig. 6B). Likewise, DINO induction was markedly compromised in the NR2E3-depleted cells (Fig. 6C). Taken together, results showed that NR2E3 is a critical component in DINO induction and p53 activation at the cellular level.
Figure 6.
Effects of NR2E3 loss on DINO-mediated p53 activation in vitro. A) NR2E3 was depleted in HepG2 cells using small hairpin (sh) RNAs targeting NR2E3 (shNR2E3 I and II) with scrambled control (shCT). B) Control (shCT) and NR2E3-depleted (shNR2E3 I and II) HepG2 cells were treated with etoposide, a chemotherapeutic agent that activates p53 (25 μM; 0, 3, and 6 h). No significant induction of p53 and p21, p53 downstream target genes, was detected in NR2E3-depleted HepG2 cells. C) DINO was not effectively induced in NR2E3-depleted HepG2 cells. D, E) Compared to control hepG2 cells (shCT), NR2E3 depletion in HepG2 cells (shNR2E3 KO II) disrupted NR2E3 and Sp1 binding to human DINO gene promoter region (−692 ∼ −497) and reduced active histone marks such as H3K4me2 and H3K4me3. F) NR2E3 depletion in HepG2 cells consistently reduced the chromatin accessibility of the DINO gene promoter region (−653 ∼ 496). G, top) The depletion of DINO using siDINOs I and II in HepG2 cells reduced p53 activation. siCT was used as a negative control. G, bottom) Cells transfected with either siDINOs or siCT were treated with etoposide and then p53 levels were determined by immunoblotting. H, top) Overexpression of DINO in NR2E3-depleted HepG2 cells restored p53 activation and subsequently increased p21 and Bax expressions. H, bottom) The DINO restoration consistently enhanced caspase3/7 activities relevant to the levels of apoptosis. The caspase3/7 activities were measured 16 h after etoposide treatment, as determined by relative luciferase activity unit (RLU). Values are represented as means ± sd. Values are represented as means ± sd. *Significant induction or **repression; P < 0.05.
Next, by utilizing control (shCT) and NR2E3-depleted (shNR2E3 KO II) HepG2 cell lysate, we performed a ChIP assay to determine whether NR2E3 depletion induced similar repressive epigenetic status of human DINO gene, with a ChIP primer set covering the proximal region containing consensus Sp1 site (−692 ∼ −497). NR2E3 depletion in HepG2 cells resulted in decreased Sp1 binding accompanied by decreased active histone marks, including H3K4em2 and H3K4me3 (Fig. 6D, E). Furthermore, to access the effects of NR2E3 depletion on DINO chromatin accessibility, we performed a chromatin accessibility assay, with the primer set covering the similar region (−653 ∼ −496). Consistently, inaccessible and compact DINO chromatin status was detected in NR2E3-depleted cells (Fig. 6F). These results together indicate the role of NR2E3 in maintaining active and accessible DINO epigenetic status at the cellular level, whereas its loss induced epigenetic DINO repression with reduced chromatin accessibility.
In order to determine the role of DINO in p53 activation, by using siRNAs targeting DINO (siDINO) previously validated (siDINOs I and II) and scrambled control siRNA (siCT), we specifically depleted DINO in HepG2 cells (Fig. 6G, top) and then treated with etoposide. The result from p53 immunoblotting showed that DINO depletion inhibited the p53 activation (Fig. 6G, bottom). On the contrary, overexpression of DINO in NR2E3-depleted HepG2 cells increased p53 stability and activation and also expressions of downstream target genes, including p21 and Bax (Fig. 6H, top). Despite that the DINO levels in cells overexpressing DINO were much higher comparing to control (shCT) or NR2E3-depleted (shNR2E3 II) cells (Supplemental Fig. S9), the result clearly demonstrated the functionality of DINO that can restore p53 stability and activation. Correspondingly, the DINO overexpression increased caspase-3/7 activities involving apoptosis, demonstrating that DINO is indispensable for p53-mediated apoptosis (Fig. 6H, bottom). Next, to confirm the direct interaction between DINO and p53 under HepG2 liver cancer cell context, an RNA-ChIP assay was carried out (24). We detected significantly enhanced physical interaction between DINO and p53 in the control cells treated with etoposide, not in NR2E3-depleted cells (Supplemental Fig. S10A). Furthermore, the role of DINO that stabilizes p53 proteins even in the absence of etoposide treatment was accessed. Cells transfected with empty vector or DINO were treated with cycloheximide. This cycloheximide chase assay showed that enforced DINO expression increased p53 and its downstream target p21 proteins levels, not in the control cells transfected with empty vector (Supplemental Fig. S10B). These results are in line with the mechanistic role of DINO that stabilizes p53 from the previous report (4). Taken together, the results showed that DINO is essential for p53 activation and p53-mediated apoptosis under liver cancer cell context.
We previously reported that NR2E3 interacted with Sp1 and formed active transcriptional complex for AHR expression (9). Human DINO gene promoter also contained consensus Sp1 binding sequence (−692 ∼ −497) and NR2E3 depletion markedly reduced Sp1 binding (Fig. 5D). Thus, to determine the role of Sp1 in DINO-mediated p53 activation, we depleted Sp1 in HepG2 cells using siRNAs targeting Sp1 (Supplemental Fig. S11A). The Sp1 depletion resulted in decreased DINO induction in HepG2 cells, confirming the necessary role of Sp1 for DINO induction and p53 activation (Supplemental Fig. S11B).
No efficient reversal of APAP-induced liver damage by NAC treatment
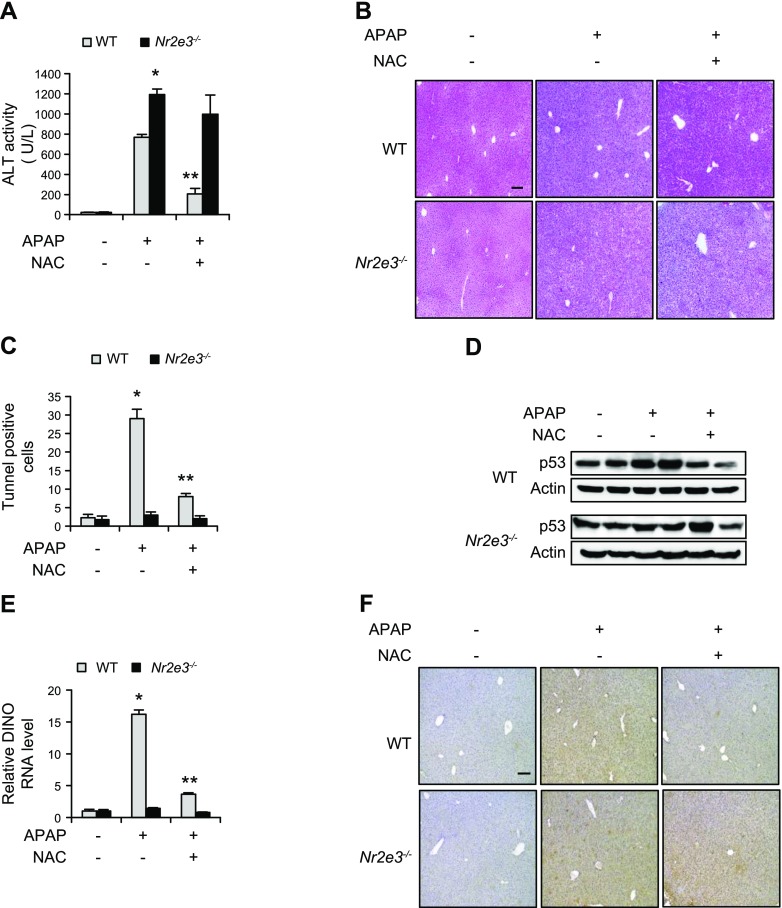
In patients with APAP-induced liver failure, NAC has been used as a major antidote for decades (17, 18). NAC treatment reduces APAP-hepatotoxicity by replenishing antioxidant GSH and forming adducts with APAP-derived toxic metabolites (44). To examine whether NAC treatment effectively reduced APAP-induced hepatotoxicity in Nr2e3−/− KO mice, mice were treated with APAP (300 mg/kg), with NAC (500 mg/kg) treatment following 1.5 h later (45). After 24 h, plasma and livers from the mice were collected and plasma ALT levels were measured. We detected significantly lower ALT activity in the NAC-treated WT mice but not in the Nr2e3−/− KO mice (Fig. 7A), indicating that NAC treatment was effective in decreasing APAP-induced hepatotoxicity in WT mice but not in Nr2e3−/− KO mice. Similarly, the H&E-stained histology sections from Nr2e3−/− KO mice treated with APAP and followed by NAC treatment showed almost no reversal of necrosis (Fig. 7B). Next, we determined the effects of NAC treatment on APAP-induced apoptotic cell death. A marked decrease in the number of TUNEL-positive apoptotic cells in APAP plus NAC-treated liver sections of WT mice was observed, whereas no significant increase of TUNEL-positive apoptotic cells by APAP or APAP plus NAC treatment was detected in the liver sections of APAP-treated Nr2e3−/− KO mice (Fig. 7C and Supplemental Fig. S12). Consistently, we observed that NAC treatment significantly reduced DINO induction and p53 activation in the livers of APAP-treated WT mice. However, there was no significant DINO induction or p53 activation in APAP-treated or APAP plus NAC-treated livers of Nr2e3−/− KO mice (Fig. 7D, E). Furthermore, NAC treatment markedly reduced the staining levels of nitrotyrosine, a marker for mitochondrial oxidative stress, in APAP-treated livers of WT mice but not in the APAP-treated livers of Nr2e3−/− KO mice (Fig. 7F). These results were consistent with previous reports showing that p53 inactivation sustained JNK activation (Supplemental Fig. S4), of which activation led to enhanced mitochondrial oxidative stress (14), and that NAC was typically much less efficient antioxidant to reverse the mitochondrial oxidative stress than the antioxidant specifically targeting mitochondrial oxidative stress (46). Collectively, these data suggested that NR2E3 is essential for NAC-mediated protection against APAP-induced hepatotoxicity likely by regulating DINO-mediated p53 signaling pathway activation and its downstream effects.
Figure 7.
Ineffective NAC-mediated protection against APAP-treated hepatotoxicity. A) Mice were treated with APAP (300 mg/kg) followed by NAC (500 mg/kg) 90 min later. Blood and liver tissues were collected at 24h after APAP treatment. ALT activities were measured in WT and Nr2e3−/− KO mice treated with APAP ± NAC. B) Representative H&E-stained liver sections. C) Quantification of TUNEL-positive cells. D) Immunoblotting analysis for detection of p53 level changes using liver lysate from WT and Nr2e3−/− KO mice treated with APAP ± NAC. E) Effects of NAC treatment on APAP-induced DINO induction. F) Nitrotyrosine staining intensity in the liver sections of WT mice and Nr2e3−/− KO mice treated first with APAP and then 90 min later with NAC (top panels). All tissues were collected 24 h after APAP treatment. No effective reversal of nitrotyrosine staining intensity by NAC treatment in the livers of Nr2e3−/− KO mice was seen (bottom panels). Scale bars, 100 μm. *Significant induction or **repression; P < 0.05.
DISCUSSION
NR2E3 has been primarily characterized as a major player in retinal development and diseases (5, 6). Previously, we demonstrated that NR2E3 is a critical epigenetic player that positively regulates both ER and AHR expressions and serves as a significantly good prognostic marker in breast and liver cancers (7–9). NR2E3 loss disrupts active transcription complex and induces the epigenetic repression of ER and AHR. However, the detailed mechanistic role of NR2E3 in epigenetic regulation remains largely elusive. To investigate the role of NR2E3 in vivo, we generated a complete whole body Nr2e3−/− KO mouse using CRISPR/CAS9 gene editing technology (20, 21). In our previous study, we employed Nr2e3Rd7/Rd7 (retinal degeneration 7, Rd7) mice that contain a mutation in the NR2E3 gene; however, these mice still expressed a minimal level of NR2E3 as hypomorphs (9). Consistent with the previous report, reduced expressions of AHR were observed in the livers of Nr2e3−/− KO mice. However, NR2E3 ablation itself in vivo did not cause any liver damage (Fig. 2A, B) or alter the expression of genes, including Cyp2e1, Nrf2, and p53, which are critical in liver injury responses (Fig. 1C, D and Supplemental Fig. S3A, B).
The present study has newly established that NR2E3 is a critical component in p53 activation. NR2E3 played a role in maintaining active and accessible DINO epigenetic status for proper DINO induction. NR2E3 formed an active complex with Sp1 and maintained active and accessible DINO epigenetic status (Figs. 5B–F and 6D–F). In the presence of NR2E3, DINO was properly induced (Figs. 3F and 6C), stabilizing p53 and amplifying p53 signaling cascades in response to APAP-induced liver injury (Figs. 3C, 4E and 6B). In contrast, NR2E3 ablation in vivo or in vitro induced the repressive and inaccessible epigenetic DINO status, accompanied by repressive histone modification status (Figs. 5D–F and 6D–F), and thereby DINO induction and p53 activation were significantly compromised in response to APAP- or etoposide-induced cell damage responses (Fig. 3C, F). The p53 proteins were not properly stabilized, and the p53 inactivation led to necrosis rather than apoptosis (Figs. 2B and 3B). Similarly, the deactivation of DINO–p53 signaling pathways was observed in Nr2e3−/− KO mice treated with ccl4 (Fig. 4). We present our model for the NR2E3–DINO–p53 signaling axis in Fig. 8. Intriguingly, NR2E3 loss specifically induced repressive epigenetic DINO status without altering the basal p53 levels (Supplemental Fig. S3) or Nrf2-mediated gene expressions involving GSH replenishment (Fig. 2C–E).
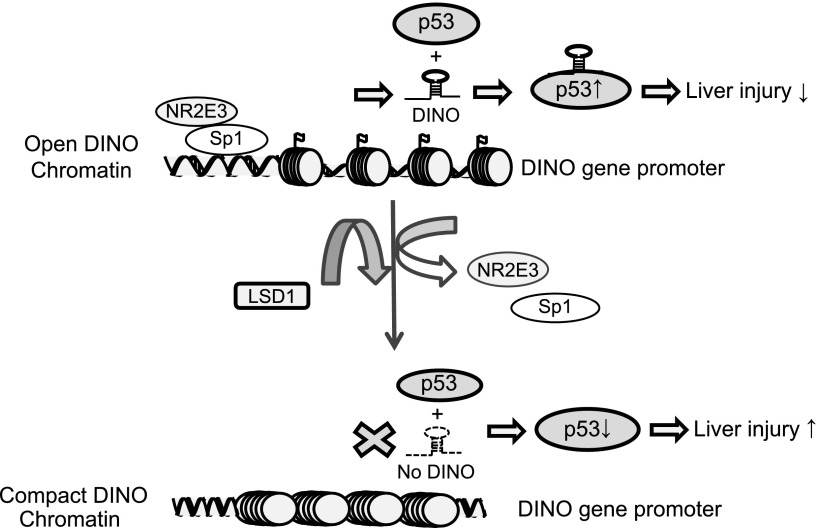
Figure 8.
A model proposed for the role of the NR2E3–Sp1 complex in maintaining active DINO epigenetic status and chromatin accessibility essential for p53 activation and downstream events. NR2E3 that forms active complex with Sp1 transcription factor maintains active DINO chromatin status, whereas NR2E3 loss induced repressive DINO chromatin. The proper induction of DINO in turn stabilizes p53 through RNA–protein interaction, amplifying p53 signaling pathways.
It was previously reported that NR2E3 forms a complex with p53 and enhances p53 activity via p53 acetylation (47). However, this study primarily focused on the effects of NR2E3 overexpression with regard to enhancing p53 activity at the cellular level. The effects of NR2E3 functional loss on p53 function and activity were not fully investigated at both in vivo and in vitro levels. This study demonstrated the role of NR2E3 in coordinating the regional chromatin structure and epigenetic status, involving p53 activation, both in vitro and in vivo. NR2E3 played a critical role in maintaining open accessible DINO chromatin, whereas NR2E3 loss induced compact DINO chromatin (Figs. 5E and 6F). These results suggested that NR2E3 status is a critical factor that directs the levels of p53 activity by regulating DINO. Thus, NR2E3 status is likely an important indicator for p53 activity. These findings also strengthen the importance of NR2E3 status possibly in other drug- or toxicant-induced liver injury responses. In APAP- or ccl4-induced acute liver injuries, NR2E3 ablation in vivo caused far more severe liver damage, at least in part, due to the inactivation of DINO–p53 signaling pathways (Figs. 3 and 4). NR2E3 is required for protecting cells or tissues against toxicant- or drug-induced damage responses, reinforcing that NR2E3 is a crucial susceptibility factor in drug- or toxicant-induced liver damage responses, in part, by regulating DINO–p53 signaling pathways, and it is also likely independent of the DINO-p53 signaling pathways. This possibility will be further investigated in different experimental settings in the future. In addition, by depleting DINO or Sp1 and DINO restoration, we showed that DINO or Sp1 play a key role in the p53 activation and apoptosis in human liver cancer cell context (Fig. 6G, H, and Supplemental Fig. S11). Intriguingly, unlike the effective reversal in APAP-treated WT mice, NAC treatment did not efficiently reverse APAP-induced acute liver damage in Nr2e3−/− KO mice (Fig. 7A, B). These findings highlight the key role of NR2E3 signaling axis in NAC-mediated protection against APAP-induced hepatotoxicity. An intact NR2E3–DINO–p53 signaling axis is likely required for inhibiting persistent JNK activation (14) and in NAC-mediated reduction of mitochondrial oxidative stress (44) (Fig. 7F). Taken together, NR2E3 is a novel susceptibility factor to and a determining factor for NAC-mediated protection against APAP-induced hepatotoxicity.
In summary, this study identifies NR2E3 as a novel epigenetic player that control p53 signaling pathways by regulating DINO epigenetic status and induction, which is essential for p53 stability and activity. Furthermore, the role of NR2E3 in regulating DINO-mediated p53 activation suggests its broader role in other human diseases and cancers in which p53 plays a role. Our previous reports showed that NR2E3 is a significantly good prognostic indicator in breast and liver cancers (7, 9), and that decreased NR2E3 expressions were also correlated with other liver diseases (9). Taken together, these results suggest that Nr2e3−/− KO mice may be tumor prone, similar to p53 hypomorphs, as previously reported in Hemann et al. (48). It is likely that the NR2E3–DINO–p53 signaling axis or other NR2E3-regulated signaling pathways that are unknown contribute to the development of liver cancer or chronic liver diseases such as cirrhosis. Future studies are necessary to understand the role of NR2E3 in the development of liver cancer and diseases.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank for Prof. Howard Chang (Department of Genetics, School of Medicine, Stanford University) for kindly providing the damage-induced long noncoding RNA plasmid. This work was supported by U.S. National Institutes of Health/National Institute of Environmental Health Sciences (NIEHS) Grant P30-ES006096 from the Center for Environmental Genetics at the University of Cincinnati. The authors declare no conflicts of interest.
Glossary
- AHR
aryl hydrocarbon receptor
- ALT
alanine aminotransferase
- APAP
acetaminophen
- Bak1
BCL2 antagonist/killer 1
- Bax
Bcl-2-associated X protein
- BCIP
5-bromo-4-chloro-3-indolyl phosphate
- CAS9
CRISPR-associated protein 9
- ccl4
carbon tetrachloride
- ChIP
chromatin immunoprecipitation
- CRISPR
clustered regularly interspaced short palindromic repeats
- DINO
damage-induced long noncoding RNA
- ER
estrogen receptor α
- GSH
glutathione
- H3K4me2
histone 3 lysine 4 dimethylation
- H3K4me3
histone 3 lysine 4 trimethylation
- H4Ac
histone 4 acetylation
- H&E
hematoxylin and eosin
- KO
knockout
- LSD1
lysine-specific histone demethylase 1A
- NAC
N-acetylcysteine
- NBT
p-nitroblue tetrazolium chloride
- Nrf2
nuclear factor (erythroid-derived 2)-like 2
- NR2E3
nuclear receptor subfamily 2 group E member 3
- Nse
nuclease
- Rd7
retinal degeneration 7
- sgRNA
single guide RNA
- OD
optical density
- p21
cyclin-dependent kinase inhibitor 1
- shCT
small hair RNA scrambled control
- shNR2E3
small haripin RNA targeting NR2E3
- siCT
scrambled control siRNA
- siDINO
siRNA targeting DINO
- siRNA
small interfering RNA
- Sp1
specificity protein 1
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
K. Kim conceived of and supervised the study; T. Khanal, Y.-K. Leung, and K. Kim designed and performed all the experiments; W. Jiang performed immunohistochemical analysis; N. Timchenko and S.-M. Ho contributed to the design, analysis, and interpretation of the data; K. Kim wrote the manuscript; and all authors approved the final version of this manuscript.
REFERENCES
- 1.Reinhardt H. C., Schumacher B. (2012) The p53 network: cellular and systemic DNA damage responses in aging and cancer. Trends Genet. 28, 128–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loewer A., Batchelor E., Gaglia G., Lahav G. (2010) Basal dynamics of p53 reveal transcriptionally attenuated pulses in cycling cells. Cell 142, 89–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Purvis J. E., Karhohs K. W., Mock C., Batchelor E., Loewer A., Lahav G. (2012) p53 dynamics control cell fate. Science 336, 1440–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmitt A. M., Garcia J. T., Hung T., Flynn R. A., Shen Y., Qu K., Payumo A. Y., Peres-da-Silva A., Broz D. K., Baum R., Guo S., Chen J. K., Attardi L. D., Chang H. Y. (2016) An inducible long noncoding RNA amplifies DNA damage signaling. Nat. Genet. 48, 1370–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haider N. B., Jacobson S. G., Cideciyan A. V., Swiderski R., Streb L. M., Searby C., Beck G., Hockey R., Hanna D. B., Gorman S., Duhl D., Carmi R., Bennett J., Weleber R. G., Fishman G. A., Wright A. F., Stone E. M., Sheffield V. C. (2000) Mutation of a nuclear receptor gene, NR2E3, causes enhanced S cone syndrome, a disorder of retinal cell fate. Nat. Genet. 24, 127–131 [DOI] [PubMed] [Google Scholar]
- 6.Milam A. H., Rose L., Cideciyan A. V., Barakat M. R., Tang W. X., Gupta N., Aleman T. S., Wright A. F., Stone E. M., Sheffield V. C., Jacobson S. G. (2002) The nuclear receptor NR2E3 plays a role in human retinal photoreceptor differentiation and degeneration. Proc. Natl. Acad. Sci. USA 99, 473–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park Y. Y., Kim K., Kim S. B., Hennessy B. T., Kim S. M., Park E. S., Lim J. Y., Li J., Lu Y., Gonzalez-Angulo A. M., Jeong W., Mills G. B., Safe S., Lee J. S. (2012) Reconstruction of nuclear receptor network reveals that NR2E3 is a novel upstream regulator of ESR1 in breast cancer. EMBO Mol. Med. 4, 52–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khanal T., Kim D., Johnson A., Choubey D., Kim K. (2015) Deregulation of NR2E3, an orphan nuclear receptor, by benzo(a)pyrene-induced oxidative stress is associated with histone modification status change of the estrogen receptor gene promoter. Toxicol. Lett. 237, 228–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khanal T., Choi K., Leung Y. K., Wang J., Kim D., Janakiram V., Cho S. G., Puga A., Ho S. M., Kim K. (2017) Loss of NR2E3 represses AHR by LSD1 reprogramming, is associated with poor prognosis in liver cancer. Sci. Rep. 7, 10662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee W. M., Squires R. H., Jr., Nyberg S. L., Doo E., Hoofnagle J. H. (2008) Acute liver failure: summary of a workshop. Hepatology 47, 1401–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larson A. M., Polson J., Fontana R. J., Davern T. J., Lalani E., Hynan L. S., Reisch J. S., Schiødt F. V., Ostapowicz G., Shakil A. O., Lee W. M.; Acute Liver Failure Study Group (2005) Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology 42, 1364–1372 [DOI] [PubMed] [Google Scholar]
- 12.Lee S. S., Buters J. T., Pineau T., Fernandez-Salguero P., Gonzalez F. J. (1996) Role of CYP2E1 in the hepatotoxicity of acetaminophen. J. Biol. Chem. 271, 12063–12067 [DOI] [PubMed] [Google Scholar]
- 13.Jaeschke H., Bajt M. L. (2006) Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol. Sci. 89, 31–41 [DOI] [PubMed] [Google Scholar]
- 14.Huo Y., Yin S., Yan M., Win S., Aung Than T., Aghajan M., Hu H., Kaplowitz N. (2017) Protective role of p53 in acetaminophen hepatotoxicity. Free Radic. Biol. Med. 106, 111–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gunawan B. K., Liu Z. X., Han D., Hanawa N., Gaarde W. A., Kaplowitz N. (2006) c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology 131, 165–178 [DOI] [PubMed] [Google Scholar]
- 16.Hanawa N., Shinohara M., Saberi B., Gaarde W. A., Han D., Kaplowitz N. (2008) Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J. Biol. Chem. 283, 13565–13577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prescott L. F., Park J., Ballantyne A., Adriaenssens P., Proudfoot A. T. (1977) Treatment of paracetamol (acetaminophen) poisoning with N-acetylcysteine. Lancet 2, 432–434 [DOI] [PubMed] [Google Scholar]
- 18.Smilkstein M. J., Knapp G. L., Kulig K. W., Rumack B. H. (1988) Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985). N. Engl. J. Med. 319, 1557–1562 [DOI] [PubMed] [Google Scholar]
- 19.Yang W. S., Chadalapaka G., Cho S. G., Lee S. O., Jin U. H., Jutooru I., Choi K., Leung Y. K., Ho S. M., Safe S., Kim K. (2014) The transcriptional repressor ZBTB4 regulates EZH2 through a MicroRNA-ZBTB4-specificity protein signaling axis. Neoplasia 16, 1059–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haeussler M., Schönig K., Eckert H., Eschstruth A., Mianné J., Renaud J. B., Schneider-Maunoury S., Shkumatava A., Teboul L., Kent J., Joly J. S., Concordet J. P. (2016) Evaluation of off-target and on-target scoring algorithms and integration into the guide RNA selection tool CRISPOR. Genome Biol. 17, 148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang H., Wang H., Jaenisch R. (2014) Generating genetically modified mice using CRISPR/Cas-mediated genome engineering. Nat. Protoc. 9, 1956–1968 [DOI] [PubMed] [Google Scholar]
- 22.Yuan C. L., Hu Y. C. (2017) A transgenic core facility’s experience in genome editing revolution. Adv. Exp. Med. Biol. 1016, 75–90 [DOI] [PubMed] [Google Scholar]
- 23.Iyer V., Shen B., Zhang W., Hodgkins A., Keane T., Huang X., Skarnes W. C. (2015) Off-target mutations are rare in Cas9-modified mice. Nat. Methods 12, 479 [DOI] [PubMed] [Google Scholar]
- 24.Zhao J., Ohsumi T. K., Kung J. T., Ogawa Y., Grau D. J., Sarma K., Song J. J., Kingston R. E., Borowsky M., Lee J. T. (2010) Genome-wide identification of polycomb-associated RNAs by RIP-seq. Mol. Cell 40, 939–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corcoran G. B., Wong B. K. (1986) Role of glutathione in prevention of acetaminophen-induced hepatotoxicity by N-acetyl-L-cysteine in vivo: studies with N-acetyl-D-cysteine in mice. J. Pharmacol. Exp. Ther. 238, 54–61 [PubMed] [Google Scholar]
- 26.Chan K., Han X. D., Kan Y. W. (2001) An important function of Nrf2 in combating oxidative stress: detoxification of acetaminophen. Proc. Natl. Acad. Sci. USA 98, 4611–4616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Enomoto A., Itoh K., Nagayoshi E., Haruta J., Kimura T., O’Connor T., Harada T., Yamamoto M. (2001) High sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol. Sci. 59, 169–177 [DOI] [PubMed] [Google Scholar]
- 28.Farombi E. O., Surh Y. J. (2006) Heme oxygenase-1 as a potential therapeutic target for hepatoprotection. J. Biochem. Mol. Biol. 39, 479–491 [DOI] [PubMed] [Google Scholar]
- 29.Saito C., Lemasters J. J., Jaeschke H. (2010) c-Jun N-terminal kinase modulates oxidant stress and peroxynitrite formation independent of inducible nitric oxide synthase in acetaminophen hepatotoxicity. Toxicol. Appl. Pharmacol. 246, 8–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.LoGuidice A., Boelsterli U. A. (2011) Acetaminophen overdose-induced liver injury in mice is mediated by peroxynitrite independently of the cyclophilin D-regulated permeability transition. Hepatology 54, 969–978 [DOI] [PubMed] [Google Scholar]
- 31.Guo X. L., Liang B., Wang X. W., Fan F. G., Jin J., Lan R., Yang J. H., Wang X. C., Jin L., Cao Q. (2013) Glycyrrhizic acid attenuates CCl4-induced hepatocyte apoptosis in rats via a p53-mediated pathway. World J. Gastroenterol. 19, 3781–3791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elgawish R. A. R., Rahman H. G. A., Abdelrazek H. M. A. (2015) Green tea extract attenuates CCl4-induced hepatic injury in male hamsters via inhibition of lipid peroxidation and p53-mediated apoptosis. Toxicol. Rep. 2, 1149–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farré D., Roset R., Huerta M., Adsuara J. E., Roselló L., Albà M. M., Messeguer X. (2003) Identification of patterns in biological sequences at the ALGGEN server: PROMO and MALGEN. Nucleic Acids Res. 31, 3651–3653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hebbes T. R., Thorne A. W., Crane-Robinson C. (1988) A direct link between core histone acetylation and transcriptionally active chromatin. EMBO J. 7, 1395–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santos-Rosa H., Schneider R., Bannister A. J., Sherriff J., Bernstein B. E., Emre N. C., Schreiber S. L., Mellor J., Kouzarides T. (2002) Active genes are tri-methylated at K4 of histone H3. Nature 419, 407–411 [DOI] [PubMed] [Google Scholar]
- 36.Shi Y., Lan F., Matson C., Mulligan P., Whetstine J. R., Cole P. A., Casero R. A., Shi Y. (2004) Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 119, 941–953 [DOI] [PubMed] [Google Scholar]
- 37.Maison C., Bailly D., Peters A. H., Quivy J. P., Roche D., Taddei A., Lachner M., Jenuwein T., Almouzni G. (2002) Higher-order structure in pericentric heterochromatin involves a distinct pattern of histone modification and an RNA component. Nat. Genet. 30, 329–334 [DOI] [PubMed] [Google Scholar]
- 38.Lee D. Y., Hayes J. J., Pruss D., Wolffe A. P. (1993) A positive role for histone acetylation in transcription factor access to nucleosomal DNA. Cell 72, 73–84 [DOI] [PubMed] [Google Scholar]
- 39.Lan F., Collins R. E., De Cegli R., Alpatov R., Horton J. R., Shi X., Gozani O., Cheng X., Shi Y. (2007) Recognition of unmethylated histone H3 lysine 4 links BHC80 to LSD1-mediated gene repression. Nature 448, 718–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akimzhanov A. M., Yang X. O., Dong C. (2007) Chromatin remodeling of interleukin-17 (IL-17)-IL-17F cytokine gene locus during inflammatory helper T cell differentiation. J. Biol. Chem. 282, 5969–5972 [DOI] [PubMed] [Google Scholar]
- 41.Bannister A. J., Zegerman P., Partridge J. F., Miska E. A., Thomas J. O., Allshire R. C., Kouzarides T. (2001) Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature 410, 120–124 [DOI] [PubMed] [Google Scholar]
- 42.Graupner V., Alexander E., Overkamp T., Rothfuss O., De Laurenzi V., Gillissen B. F., Daniel P. T., Schulze-Osthoff K., Essmann F. (2011) Differential regulation of the proapoptotic multidomain protein Bak by p53 and p73 at the promoter level. Cell Death Differ. 18, 1130–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deng M., Chen P., Liu F., Fu S., Tang H., Fu Y., Xiong Z., Hui S., Ji W., Zhang X., Zhang L., Gong L., Hu X., Hu W., Sun S., Liu J., Xiao L., Liu W. B., Xiao Y. M., Liu S. J., Liu Y., Li D. W. (2012) The p53-Bak apoptotic signaling axis plays an essential role in regulating differentiation of the ocular lens. Curr. Mol. Med. 12, 901–916 [DOI] [PubMed] [Google Scholar]
- 44.Zafarullah M., Li W. Q., Sylvester J., Ahmad M. (2003) Molecular mechanisms of N-acetylcysteine actions. Cell. Mol. Life Sci. 60, 6–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miners J. O., Drew R., Birkett D. J. (1984) Mechanism of action of paracetamol protective agents in mice in vivo. Biochem. Pharmacol. 33, 2995–3000 [DOI] [PubMed] [Google Scholar]
- 46.Du K., Farhood A., Jaeschke H. (2017) Mitochondria-targeted antioxidant Mito-Tempo protects against acetaminophen hepatotoxicity. Arch. Toxicol. 91, 761–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wen Z., Pyeon D., Wang Y., Lambert P., Xu W., Ahlquist P. (2012) Orphan nuclear receptor PNR/NR2E3 stimulates p53 functions by enhancing p53 acetylation. Mol. Cell. Biol. 32, 26–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hemann M. T., Fridman J. S., Zilfou J. T., Hernando E., Paddison P. J., Cordon-Cardo C., Hannon G. J., Lowe S. W. (2003) An epi-allelic series of p53 hypomorphs created by stable RNAi produces distinct tumor phenotypes in vivo. Nat. Genet. 33, 396–400 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.