Abstract
Human prefrontal cortex (PFC) is associated with broad individual variabilities in functions linked to personality, social behaviors, and cognitive functions. The phenotype variabilities associated with brain functions can be caused by genetic or epigenetic factors. The interactions between these factors in human subjects is, as of yet, poorly understood. The heterogeneity of cerebral tissue, consisting of neuronal and nonneuronal cells, complicates the comparative analysis of gene activities in brain specimens. To approach the underlying neurogenomic determinants, we performed a deep analysis of open chromatin–associated histone methylation in PFC neurons sorted from multiple human individuals in conjunction with whole-genome and transcriptome sequencing. Integrative analyses produced novel unannotated neuronal genes and revealed individual-specific chromatin “blueprints” of neurons that, in part, relate to genetic background. Surprisingly, we observed gender-dependent epigenetic signals, implying that gender may contribute to the chromatin variabilities in neurons. Finally, we found epigenetic, allele-specific activation of the testis-specific gene nucleoporin 210 like (NUP210L) in brain in some individuals, which we link to a genetic variant occurring in <3% of the human population. Recently, the NUP210L locus has been associated with intelligence and mathematics ability. Our findings highlight the significance of epigenetic-genetic footprinting for exploring neurologic function in a subject-specific manner.—Gusev, F. E., Reshetov, D. A., Mitchell, A. C., Andreeva, T. V., Dincer, A., Grigorenko, A. P., Fedonin, G., Halene, T., Aliseychik, M., Goltsov, A. Y., Solovyev, V., Brizgalov, L., Filippova, E., Weng, Z., Akbarian, S., Rogaev, E. I. Epigenetic-genetic chromatin footprinting identifies novel and subject-specific genes active in prefrontal cortex neurons.
Keywords: ChIP-seq, H3K4me3, brain, histone
Exploration of the epigenetic landscape in human tissues provides a tissue-specific genomic map of gene activities. Significant efforts have been expended by several consortia [e.g., Encyclopedia of DNA Elements (ENCODE) (1), RoadMap Epigenomics (2), and Psych Encyclopedia of DNA Elements (PsychENCODE) (3)] to generate comprehensive data on the regulatory structure of the human genome. However, the role of individual variabilities caused by genetic and nongenetic factors on the epigenetic chromatin profiles in specialized cells has yet to be elucidated. A landmark study by Chen et al. (4) suggested that the genetic effect on gene expression can be higher than the epigenetic effect (after adjusting for the genetic component) in human monocytes. The variations in gene activities can be predicted from chromatin modification features (5). It is thus conceivable that genetic variations across a genome can contribute to chromatin variations in specific loci harboring protein-coding and noncoding genes, affecting the expression patterns of these genes. Therefore, we hypothesized that behavioral phenotypes, including risk for disorders, can be defined by an interplay between genetic and epigenetic regulatory components producing an individual diversity of gene activities in brain cells and, specifically, in cortical neurons (6–8).
Apart from the genetic component, variations in epigenetic architecture of differentiated cells are thought to reflect the developmental history of the tissue as well as environmental influences. Although epigenome-wide association studies have reported specific epigenetic markers for some phenotypes (9), the role of individual epigenetic variabilities on brain functions is poorly known.
In this regard, the study of neuronal populations and, specifically, of the human prefrontal cortex (PFC) is of particular interest. The PFC is among the most rapidly evolved and disproportionally enlarged brain regions responsible for cognition, social behaviors, and abstract thinking (10–12). The PFC also plays a key role in cognitive and psychiatric diseases, such as schizophrenia and autism.
Nucleosome core histones are decorated with post-translational epigenetic modifications that define chromatin structure and function. For example, trimethylation of histone H3 at lysine 4 (H3K4me3) of nucleosomes is linked to the RNA polymerase II transcriptional initiation complex and specifically marks transcription start sites (TSSs) and gene promoters in open (or active) chromatin states (13, 14). We suggest that by mapping thousands of loci marked by H3K4me3 across the genome in many individuals, we can identify TSS for known genes as well as yet unknown human genes. Combining epigenomic profiling of H3K4me3 in mature neurons with whole-genome sequencing (WGS) and transcriptome sequencing, we sought to highlight subject-specific epigenetic-genetic chromatin footprints for genes active in neurons. These data, along with large genome-wide association studies (GWASs) (15–22), provide a roadmap for future studies aimed at the dissection of interindividual variability in cognitive performance and vulnerability to disease.
MATERIALS AND METHODS
H3K4me3-tagged chromatin profiling of PFC neurons
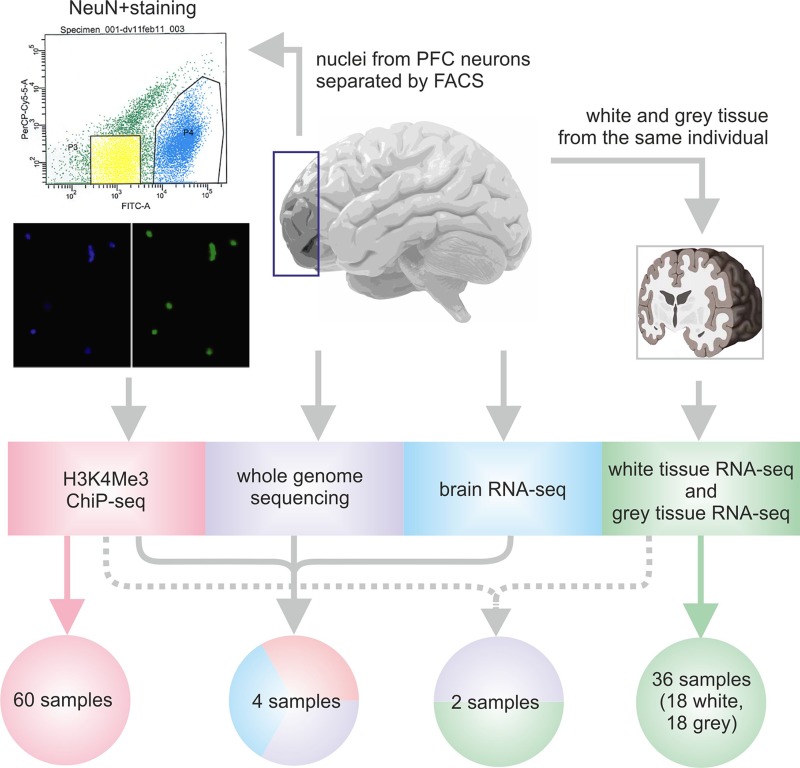
Whole-genome data for chromatin immunoprecipitation followed by sequencing (ChIP-seq) were generated with H3K4me3-specific antibodies using dorsolateral PFC neurons extracted from post mortem brain samples in a cohort of unrelated patients with autism and schizophrenia as well as control individuals with no psychiatric illnesses (15, 16, 20, 22, 23 and Supplemental Text S1). Because the human PFC glia-neuron ratio in different areas of II/III layers is 1.55–2.19 (24), we avoided cell composition differences as a potential confound by separating neuronal and nonneuronal nuclei by fluorescence-activated cell sorting against the neuronal nucleus marker, as previously described by Jiang et al. (25) (Fig. 1). ChIP-seq for neuronal nuclei was performed as previously described (20, 23) and in Supplemental Text S1. Our study included detailed analysis of neuronal H3K4me3 profiles of 56 subjects after rigorous quality control: 27 control individuals (aged 0–81 yr; mean age 30.6 yr; 8 females), 13 subjects diagnosed with autism spectrum disorders (aged 2–29 yr; mean age 14.5 yr; 2 females), and 16 subjects diagnosed with schizophrenia (aged 21–79 yr; mean age 39.9 yr; 4 females). Nonneuronal cells from 3 control subjects were used for H3K4me3 profiling for quality control. The detailed description of samples is available in Supplemental Table S1.
Figure 1.
Experimental design of the study. ChIP-seq analysis was performed for open chromatin extracted from neuronal nuclei of post mortem PFC of multiple human subjects. Sample subsets from unaffected individuals and patients with psychiatric disorders were also used for WGS and RNA-seq analysis of total PFC tissue or white and gray matter of PFC. The arrows indicate the individual samples used in each analysis. FACS, fluorescence-activated cell sorting; FITC, fluorescein isothiocyanate; NeuN, neuronal nuclei.
Matching WGS and transcriptome-sequencing data
In addition to ChIP-seq, whole-genome and brain transcriptome profiling was performed for 4 subjects (Fig. 1). Genomic DNA (2 µg) was extracted from frozen brain tissue, sheared using Covaris S2 (Thermo Fisher Scientific, Waltham, MA, USA), and used for WGS library preparation according to the Paired-End DNA Sample Prep Kit (Illumina, San Diego, CA, USA); this was followed by 101-bp paired-end sequencing on the Illumina HiSeq 2000 platform. Furthermore, ∼100 mg of brain tissue per sample was used for extracting RNA fractions >200 nt using the Ambion MirVana Protein and RNA Isolation System Kit (Thermo Fisher Scientific), followed by treatment with RNAse-Free DNase I (Illumina) and the Ribo-Zero rRNA Removal Kit (Illumina). RNA (∼50 ng) was used to prepare indexed libraries with the ScriptSeq v.2 RNA Sequencing (RNA-Seq) Library Kit (Illumina). Libraries were then purified with Agencourt AMPure XP beads (Beckman Coulter, Brea, CA, USA) and sequenced on the HiSeq 2000.
Brain transcriptome profiling
PFC specimens (70–100 mg; cytoarchitectonic Brodmann area BA10 and surrounding subdivisions) separated for cortical gray and subcortical white matter from 20 individuals (Supplemental Table S1) were used for total RNA extraction. The RNeasy Lipid Tissue Mini Kit (74804; Qiagen, Hilden, Germany) was used for total RNA extraction, which was followed by treatment with DNAse I, purification, and dilution to 20 ng/µl. Libraries for RNA-seq were prepared according to the NuGen Ovation RNA-Seq v.2 (NuGen, San Carlos, CA, USA) protocol and run on the paired-end 50-bp module of the Illumina HiSeq 2000 (Eurofins Scientific, Brussels, Belgium). Additionally, we used previously published RNA-seq profiles of superior temporal gyrus for 9 individuals with schizophrenia and 9 control individuals (26) (Supplemental Text S1). Altogether, this data set consisted of 58 brain transcriptome profiles.
Assays for expression and regulation analysis of nucleoporin 210 like gene
Primers for RT-PCR experiments and Sanger sequencing are described in Supplemental Text S1. For gel-shift analysis, double-stranded oligonucleotides were synthesized (Supplemental Text S1), and then EMSA was performed as previously described by Bryzgalov et al. (27). For competition experiments, cold double-stranded oligonucleotides were added to the reaction mix at 0.1 pM and incubated on ice for 20 min before the addition of labeled probes (Supplemental Text S1).
Bioinformatic analysis
For ChIP-seq analysis, we mapped reads to the human reference genome Genome Reference Consortium Human Build (GRCh) 37 with Bowtie2 (28) using default parameters and then identified enriched loci with Model-Based Analysis of ChIP-Seq (MACS) v.1.4.3 (29) at P values <1×10−10; the signal was normalized to the number of reads that map to known gene promoters (4 kb with the center in a TSS). We defined individual peaks as those with P values <1×10−20 in a single individual and P values >1×10−10 in all others.
Comparative analysis of mean H3K4me3 tag occupancy in male and female individuals was performed with an enhanced negative binomial test implemented in R package EdgeR (30). For comparative analysis, we generated a list of loci that are enriched in at least 1 neuronal sample and quantified H3K4me3 read occupancy in each region per individual by normalizing read count to a total number of reads mapped to known gene promoters.
WGS reads were mapped to the human reference genome GRCh37 with Burrows-Wheeler Aligner (31), and Genome Analysis Toolkit (32) was used for single nucleotide polymorphism (SNP)/indel calling; copy number variants (CNVs) were first identified with control-FREE Copy number caller, FREEC, (33) and CNVnator (34) (q0 > 0.5), and then the intersection was used for analysis. We estimated the effect of SNP rs114697636 on transcription factor binding using PrEdicting Regulatory Functional Effect by Approximate P-value Estimation (PERFECTOS-APE) (35) using HOmo sapiens COmprehensive MOdel COllection (HOCOMOCO) v11 (36).
RNA-seq reads were mapped to the reference genome with TopHat2 (37), and expression was quantified using Cufflinks (38). Gene models for the identification of novel genes were also assembled with Cufflinks, and ab initio predictions were generated using FGENESH (39). Protein-coding potential was estimated with the Coding Potential Calculator (40). To generate a list of novel genes, we used the cuffcompare utility against Ensembl v.87 (41) and the University of California–Santa Cruz (42) Known Genes table for hg19 assembly (accessed April 4, 2018) and selected transcripts classified as i (a transfrag falling entirely within a reference intron), p (possible polymerase run-on fragment within 2 kbp of a reference transcript), or u (unknown, intergenic transcript). Additionally, we lifted the gene models to the GRCh38 reference genome with CrossMap (42) and executed a similar procedure against Ensembl v.91 annotation.
Approval
All procedures were approved by the Institutional Review Board of the University of Massachusetts Medical School (Worcester, MA, USA) and the Ethical Committee of Vavilov Institute of General Genetics Russian Academy of Sciences (Moscow, Russia).
RESULTS
Comparative analysis of open chromatin loci in human brain neurons across the genome
We analyzed H3K4me3 ChIP-seq data of neural nuclei extracted from human PFC for a cohort of human subjects consisting of patients with schizophrenia and autism and control individuals with no history of psychiatric illness (age range 0–89 yr). Quality control was performed for sequencing depth, gender match, and activity of neuron and glia-specific genes (Supplemental Fig. S1 and Supplemental Text S1). We used 56 neuronal-specific H3K4me3 profiles to carefully estimate the individual diversity of active chromatin in PFC neurons (Fig. 1 and Supplemental Table S1). Overall, we identified 29,547 neuronal H3K4me3 peaks, with 17,499 ± 1418 peaks per sample and only 12,523 (42%) peaks called across all 56 samples (Supplemental Table S2). We also found that 638 of the neuronal peaks were robustly identified in single individuals only (Supplemental Fig. S2), indicating that 2% of the H3K4me3-neuronal peaks are rare in the human population or are even individual specific (Supplemental Table S3). However, most of such peaks are found in infants (<1 yr old) and preadolescent children (<10 yr old), which concurs with reports of significant H3K4me3-tagged chromatin remodeling in immature PFCs (12). Thus, when we considered subjects 18 yr and older, the mean number of individual-specific peaks was limited to 2 per adult individual, with a total of 65 peaks.
Next, we quantified H3K4me3 activity at each of the 29,547 neuronal peaks per individual [after normalizing read counts for sequencing depth (Supplemental Text S1)] and performed pairwise and groupwise analyses. First, we used a >2-fold (up or down) difference as a filter and found that, on average, 5874 ± 1633 genomic loci showed substantial differences between any 2 individuals. Furthermore, we found 837 individual-specific up-peaks and 354 down-peaks across all subjects in this cohort in exactly 1 subject but not in the remaining 55 subjects (when taking into account only adult subjects, there were 237 up-peaks and 210 down-peaks; Supplemental Table S4). On average, this corresponds to 22 (9 for adults) individual-specific up-peaks and 12 (11 for adults) down-peaks per individual sample. Taken together, these findings strongly indicate that the large majority of neuronal H3K4me3 peaks in PFC neurons is shared among the adult population. Consistent with this observation, individual neuronal H3K4me3 profiles showed a high similarity (0.77 < Pearson correlation coefficient < 0.99) with each other but were significantly different from nonneuronal samples (Supplemental Fig. S3). There was no global genome-wide shift of H3K4me3 profiles regarding gender, diagnosis (schizophrenia and autism), or age among subjects older than 2 yr. These findings further affirm that neuronal H3K4me3 profiles are largely similar in PFCs of different individuals, with each subject defined by an individual H3K4me3 footprint.
Gender-specific variations in H3K4me3 profiles of human cortical neurons
The incidence of certain neuropsychiatric diseases and brain tumors is different between men and women. However, the biologic causes for this gender-associated bias in disease frequency remain unknown. During primary screening of the whole cohort, we observed apparent gender-associated Y and X chromosome epichromatin marks (Supplemental Fig. S4 and Supplemental Table S5). After excluding the Y chromosome and including only adult individuals (29 male and 10 female samples older than 18 yr), we searched for specific loci that may exhibit differential levels of methylated histones (H3K4me3) in PFC neurons between men and women and found gender-linked differences in 132 H3K4me3-marked sites. In addition to 60 loci on the X chromosome that were only found or strongly up-regulated in females, we uncovered 2 loci (2 phosphatidylinositol specific phospholipase C X domain containing 1 promoters and a protocadherin 11 X-linked promoter) on the X chromosome that were up-regulated in males and 69 loci on autosomes predominantly up- or down-regulated between the genders.
On the X chromosome, 63 loci exhibited significant shifts in H3k4me3 levels. These loci include sites of female-specific H3K4me3 peaks that were completely absent in males [e.g., X inactive specific transcript promoter, 3 clusters of tandem repeats (44), and 7 loci located in pseudoautosomal regions (45, 46)]. The haloacid dehalogenase-like hydrolase domain-containing protein 1A gene partially escapes X chromosome inactivation (47), which is in agreement with the shift in H3K4me3 occupancy (P = 5.46×10−11; male-to-female fold change = 0.66; Supplemental Fig. S4C). The following most significant hit was brain neuronal RNA 100701, BNRNA_109701, (P = 5.41×10−9; male-to-female fold change = 0.63; Supplemental Fig. S4C), a noncoding RNA (ncRNA) gene discovered in this study and present also in deep sequencing transcriptome (48). Altogether, there were 14 protein-coding and 3 ncRNA genes with significant differences in H3K4me3 occupancy on the X chromosome.
For autosomes, 28 out of the 69 peaks were proximate to known gene promoters. These include the NADPH oxidase 5 gene and sperm equatorial segment protein 1 gene promoters (P = 3×10−5; male-to-female fold change = 1.7) and the POU class 5 homeobox 1 (POU5F1) promoter (P = 6.80×10−5; male-to-female fold change = 1.84) that were found up-regulated in males (Supplemental Fig. S4A, B). Interestingly, the POU5F1 gene on chromosome 6 encodes octamer-binding transcription factor 4 (OCT4), a dedifferentiating protein and an immunohistochemical marker for CNS germinomas (49, 50). The FKBP prolyl isomerase 5 gene (P = 1.13×10−5; male-to-female fold change = 0.62), a regulator of the stress hormone system (51), and the brain-testis–specific glucuronidase, beta pseudogene 12 (P = 1.38×10−7; male-to-female fold change = 0.42) were the most differentially H3K4me3-marked protein-coding genes across all autosomes.
Gender-specific gene expression in the brain has been mainly reported for genes located on X and Y chromosomes (52). Our data on epichromatin signatures in PFC neurons combined with a recent study on RNA transcripts in total brain tissue (53) demonstrate the gender-dependent activity of certain genes located on autosomal chromosomes. The differential epigenetic modifications of genes in brain cells may underlie the disproportional incidence of brain pathologies between females and males.
Searching for novel genes marked by H3K4me3 peaks
H3K4me3 is commonly found at TSSs and gene promoters poised for activation. In our data set, only 70% of 29,547 ChIP-seq peaks were proximate within a 2 kb region to any gene promoter present in Ensembl v.87 (41) (Fig. 2). We theorized that the remaining 30% either correspond to yet undescribed genes or harbor unannotated TSS and cis-regulatory elements. Notably, this subgroup of unannotated-TSS H3K4me3 peaks was significantly enriched for individual-specific peaks (hypergeometric test P < 0.01; Supplemental Table S6). To identify cryptic TSSs, we integrated H3K4me3 (N = 56 subjects) with the 40 RNA-seq data sets (N = 20 subjects, both gray and white matter separately for each subject) generated in this study. The RNA was extracted from PFC samples of nonpsychotic control and disease subjects. Another RNA-seq data set (N = 18 subjects) from the superior temporal gyrus of normal subjects and individuals with schizophrenia (26) was also included in the analysis.
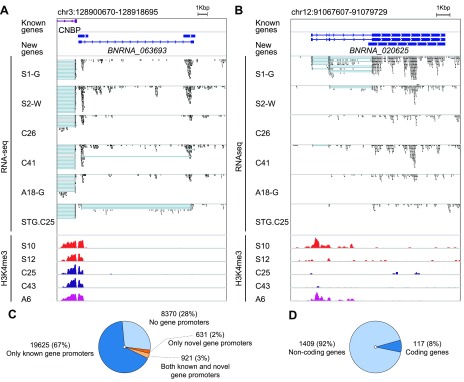
Figure 2.
Integration of H3K4me3 and RNA-seq data identified novel protein-coding and noncoding neuronal genes active in the human brain. A) Novel unannotated ncRNA gene with TSS mapped to the H3K4me3-marked region also harboring a promoter and TSS for the CCHC-type zinc finger nucleic acid binding protein gene. B) A novel ncRNA multiexonic gene with widely variable neuronal H3K4me3 abundance and expression in neurons from different individuals. C) Relative proportion of H3K4me3-marked promoter regions for previously annotated genes. D) Proportion of novel genes with notable protein-coding potential. Sample prefix indicates disease status; suffix indicates tissue for RNA-seq samples. H3K4me3 peaks are color-coded (red, schizophrenia; purple, autism; blue, control). A, autism; C, control; G, cortical gray matter; S, schizophrenia; W, cortical white matter; STG, superior temporal gyrus. For RNA-seq, blue lines show the connection of exons in spliced transcript reads.
Altogether, we called 1526 novel, previously unannotated genes that encode transcripts in close proximity to any of the 29,547 H3K4me3 peaks. At least 8370 H3K4me3 peaks have no closely located transcripts. Whether they mark cryptic promoters of yet unannotated genes or are unrelated to active genes remains to be clarified. The newly predicted genes (Supplemental File S1) were not present in widely used databases, such as Ensembl and the University of California–Santa Cruz genome browser, and have not been annotated in the reference genome. Up to 42% (or 637 of 1526) of novel genes were proximate to 631 H3K4me3 peaks but with no annotated genes nearby. Interestingly, another 58% of the novel genes started from 921 regions marked by H3K4me peaks that directly overlapped with TSSs for already annotated gene transcripts. In many cases, the novel and annotated genes were transcribed from opposite strands (Fig. 2A). Therefore, these H3K4me3-marked regions represent bidirectional promoters in cortical neurons. Of these putative genes, 161 possessed at least 1 multiexon transcript, whereas 105 exhibited multiple spliced transcripts and active chromatin H3K4me3 states in all or only a subset of individuals (Fig. 2). We also predicted that at least 117 (8%) of novel genes have notable protein-coding potential (Fig. 2D and Supplemental Table S7).
During manuscript preparation, we constantly checked the new versions of gene annotation databases. Some genes obtained through our analyses were found in these most recently updated databases, supporting the viability of this experimental setup. Among these genes, we discovered and characterized in detail a gene on chromosome 19, which we had originally named brain neuronal paraneoplastic protein [BNPP; now present in the Ensembl database as paraneoplastic Ma (PNMA) family member 8C (PNMA8C)], that is distantly related to the PNMA gene family, originally identified as onco-protein antigens (Supplemental Fig. S5A–I). BNPP expression was higher in gray than in white matter and BNPP exhibited low but detectable expression levels in the ovary and testis (Supplemental Fig. S5B, D). BNPP was also robustly expressed and epigenetically regulated in all of our samples, measured by both H3K4me3 ChIP-seq and RNA-seq (Supplemental Fig. S5A, B). Furthermore, BNPP was expressed in the brain and testis, a type of tissue-specific expression common for some genes of the PNMA family (Supplemental Fig. S5D). Phylogenetic history provided additional evidence for the protein-coding potential of BNPP, as it originates from an insertion of a gypsy element after the split of placental and marsupial mammals 160 million years ago (Supplemental Fig. S5C, E). We found that the BNPP amino acid sequence is highly conserved with stabilizing selection (synonymous/nonsynonymous mutations = 0.0–0.3), including recent evolution in primates, in line with the active H3K4me3 state for this gene in PFC neurons of chimpanzee and macaque brains (Supplemental Fig. S5F–H). Interestingly, no novel nonsynonymous mutations became fixed in the human lineage after splitting with other higher primates. However, we found that the Neanderthals and Denisovans share a novel G154R substitution in a conservative position (Supplemental Fig. S5B), which is consistent with the notion that these archaic hominines had a common ancestor after splitting with the Homo sapiens lineage (54).
Another example of a novel protein-coding gene is brain neuronal Lbh, BN-LBH, [now present in the Ensembl database as limb, bud, and heart (LBH) domain containing 2 (LBHD2)], which has a predicted 106 aa sequence homologous to the protein domain of a family of transcriptional regulators named Lbh (short for limb, bud, and heart) [PFAM (55) search; 65–67% identity, P = 1×10−23 to 5×10−23 (Supplemental Fig. S6)]. Lbh proteins act as essential regulators of the heart in embryonic development and MAPK signaling (56). Unlike BNPP, H3K4me3 peaks in the brain neuronal Lbh locus of the PFC were not consistently present among all 56 individuals included in ChIP-seq, demonstrating the individual-based variety in neuronal activity of this novel gene.
Rare H3K4me3 peaks were enriched for ncRNA genes and unannotated gene regions (Supplemental Table S6). Even considering the newly discovered genes, at least 8370 H3K4me3 peaks remained with no matches to any genes. However, RNA-seq based predictions may be incomplete due to the low spatial-temporal expression of transcripts. To overcome these limitations, we applied the FGENESH algorithm (39), which uses only the reference genome sequence. We were able to predict putative protein-coding genes located in proximity of 901 H3K4me3 peaks (Supplemental File S1). However, no protein-coding gene predictions were found for 7469 peaks; of these, 4375 bore promoter sequence signatures, including 3246 with the classic TATA-box sequence. For the remaining 3094 peaks (10% of total 29,547), we identified neither predicted genes nor promoter-like structures (Supplemental Table S8).
Genetic variation affects the chromatin architecture of gene promoters in cortical neurons
To directly elucidate the genetic and epigenetic interplay in gene regulation, brain PFC specimens from 4 individuals were subjected to transcriptome (RNA-seq) and WGS in addition to H3K4me3 profiling.
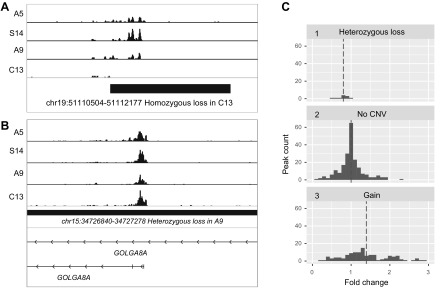
As expected, the H3K4me3 peak was abolished for carriers of homozygous CNVs (Fig. 3A). Nonetheless, the anticipated 2-fold reduction in H3K4me3 peak size for heterozygous CNV loss was not evident, which could be explained, in part, by compensatory epigenetic effects for the open-chromatin loci. There were clear examples for heterozygous CNV loss affecting peak size [e.g., the golgin A8 family member A gene (Fig. 3B)]. Globally, there was an anticipated increase in H3K4me3 levels for CNV-gain regions (median fold change = 1.4; P = 7×10−16; Mann-Whitney-Wilcoxon test) and a decrease in H3K4me3 levels for heterozygous CNV-loss regions (median fold change = 0.8; P = 0.003; Mann-Whitney-Wilcoxon test; Fig. 3C). In line with this, we found a relatively weak but statistically significant correlation between H3K4me3 peak size change and copy number (Pearsons correlation coefficient = 0.33; P = 2×10−11).
Figure 3.
Correlation of CNV and epigenetic differences among individual human neurogenomes. A) As expected, the peak within a homozygous deletion is completely lost. B) Example of heterozygous CNV loss in 2 regions of chromosome 15. The CNV loss of 1 allele results in a diminished H3K4me3 peak at the TSS position of golgin A8 family member A inside the CNV region. C) To quantify the effect of CNV on peak size for each locus within the CNV, we estimated the mean peak size for individuals with no individual CNVs and computed the fold change for subjects’ individual CNVs. 1) Heterozygous deletions do not reduce peak size by 2-fold, yet the median fold change was 0.8; 2) when 2 subjects had no CNVs in the peak, the fold change was computed to the mean and almost symmetrical spread on the histogram, corresponding to peak size variation, which is not caused by CNVs; 3) in loci with duplications, the median fold change was 1.4. Peaks with fold change >3.0 are omitted to save space.
Next, we tested whether H3K4me3 signals can be detected in an allele-specific manner using the ChIP-seq data for PFC neurons. Imprinted genes are exclusively expressed from a single allele of paternal or maternal origin. Using WGS data obtained in this study from 4 individuals, we revealed a set of single nucleotide variants (SNVs) in heterozygous state in the promoter regions of several imprinted genes. By verifying these SNVs in the ChIP-seq reads available for these individuals, we demonstrated that H3K4me3 signals for the imprinted genes correspond to only 1 of 2 alleles (Supplemental Fig. S7).
Next, we tested whether SNPs/indels occurring in the H3K4me3 peak region are associated with alterations in H3K4me3 peak size. To this end, we selected 22,502 H3K4me3 peaks with any local genetic polymorphisms within the genomic region occupied by the peaks (mean peak size = 1785 bp) using WGS and ChIP-seq data from the same 4 subjects. Next, we selected 7580 H3K4me3 peaks with significant individual-specific differences (>1.5-fold) in 1 subject compared with the remaining 3 samples. We found that 1573 of 7580 (21%) of individual-specific peaks have an individual genetic variant in H3K4me3-marked regions in the same individual. Such overlap, however, did not reach statistical significance for any type of SNV (hypergeometric test P = 1.0; Supplemental Table S9) in this small subset of subjects. Larger sample sizes are required to further elucidate the direct interplay between genetic variants and epigenetic state in TSS/promoter regions and distantly located positions.
After combining ChIP-seq and RNA-seq data from these 4 samples, we found a statistically significant medium Spearman correlation between H3K4me3 peak size in gene promoters and gene expression (rS = 0.49; P < 1×10−10; Supplemental Table S10). However, this was a nonlinear relationship based on the Pearson correlation coefficient. This may be explained by posttranscriptional regulation of expression or a mismatch between ChIP-seq (neuronal cells) and RNA-seq (unselected brain cells) of tissue sources.
In summary, interindividual epigenetic chromatin variability is not only modulated by environmental interactions (57) but also by genetic variations. Among these, CNVs have the most pronounced effect on a global scale, but SNPs and small indels within promoter regions cannot alone explain the observed variance in histone H3K4me3 signals in neurons in vivo on a global scale. However, the SNP-driven chromatin remodeling in neuronal promoters can clearly be observed for some genes. Through one example, we elucidated such epigenetic-genetic interactions in gene promoters in more detail.
Epigenetic-genetic activation of a testis-specific gene in human brain cells
We addressed the question of whether individual-specific epigenetic promoter activation (for a gene that is commonly silent in cortical neurons) results in subject-specific gene expression. To this end, we searched for strong locus-specific H3K4me3 signals in brain cells that are found in some human subjects and are completely absent in others by scanning our cohort of unrelated adults for rare H3K4me3 signals that are present in no more than 10% of individuals. As noted above, the mean number of “induced” individual peaks found in a single subject was 2 per individual. The total number of rare peaks found in a subset of no more than 10% of the cohort was 79 (P < 1×10−20 in a sample with a signal and >1×10−10 in all others).
One representative example was nucleoporin 210 like (NUP210L), a gene associated with strong neuronal H3K4me3 signals in 5 individuals and no signals exhibited in the other 51 subjects of different ages (Supplemental Fig. S8A). Interestingly, 3 of the 5 individuals were patients with schizophrenia (S14) and autism (A5, A11). BodyMap (Gene Expression Omnibus accession E-MTAB-513) and ChIP-seq data (58) inspection showed that NUP210L has an open-chromatin state with RNA expression in the testis but not in any other tissues (Supplemental Fig. S4D). We hypothesized that a genetic regulatory mutation may contribute to the activation of testis-specific NUP210L in nontestis tissue in some individuals.
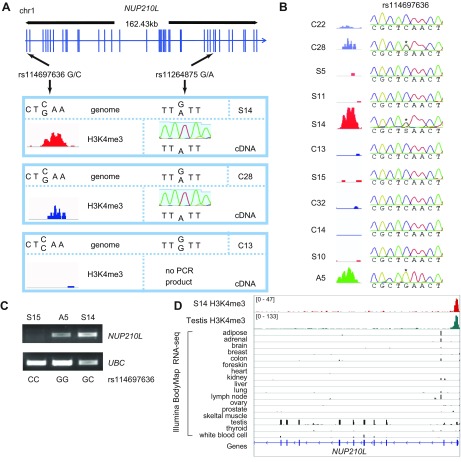
By analyzing the WGS data obtained in this study, we found that subjects S14 and A5 shared allele G for the rs114697636 SNP located 28 bp upstream of the NUP210L start codon. S14 was heterozygous (GC) and A5 was homozygous (GG) for the rare G allele [minor allele frequency = 3% in the European population according to 1000 Genomes phase 3 data (59)] (Supplemental Fig. S8B, H). Analysis of genomes of individuals lacking the NUP210L H3K4me3 peak (C13 and A9) indicated that they have the reference CC genotype (Supplemental Fig. S8A, B). This SNP was verified by Sanger sequencing of the set of individuals included in H3K4me3 analysis. Only carriers of the G variant exhibited an H3K4me3 peak overlapping the promoter and 5ʹ region of NUP210L in PFC neurons (Fig. 4A, B). For one G allele carrier (C28), we were able to obtain ChIP-seq data for H3K4me3-binding sites in both neuronal and nonneuronal PFC cells and observed the NUP210L promoter-associated H3K4me3 peak in both cell types (Supplemental Fig. S8C). Thus, we hypothesized that the presence of the G allele leads to induction of an open-chromatin state in this locus and activation of NUP210L in brain cells. Subsequently, we searched for NUP210L transcripts in brain samples and found significant levels of these transcripts in the brain cells of G allele carriers but not CC carriers (Fig. 4C and Supplemental Fig. S8D, E).
Figure 4.
Single nucleotide substitutions in the promoter are associated with induction of open chromatin and neuronal transcription activation of the testis-specific gene NUP21OL. A) Linkage of the rs114697636 SNP G allele, located near a start codon, and the rs11264875 SNP A allele, located at an exonic region of NUP210L. Monoallelic expression is demonstrated by direct Sanger sequencing of brain transcript cDNA from heterozygous carriers. B) Presence of the G allele at rs114697636 was strongly associated with an emerging H3K4me3 peak at the NUP210L promoter in all carriers. Asterisks denote the presence of the G allele in heterozygous (C28 and S14) and homozygous (A5) individuals. C) RT-PCR analysis of NUP210L relative to ubiquitin C (UBC) gene shows that NUP210L expression occurs in carriers of the rs114697636-G allele variant only (see also Supplemental Fig. S8). D) In general, NUP210L was characterized by testis-specific transcription and an active chromatin state of the promoter. ChIP-seq of testis (68) and RNA-seq of a panel of tissues from the Illumina BodyMap project are shown. A, autism; C, control; S, schizophrenia.
Next, we provided direct evidence that allele-specific cis-regulation contributes to the activation of NUP210L in the brain. Because the rs114697636 SNP is located at the proximal 5ʹ region of the NUP210L gene, it would be difficult to directly test the transcriptional allelic imbalance for this polymorphism. Therefore, we searched for other SNPs in NUP210L and found the exonic rs11264875 SNP. Using 1000 Genomes data, we found that the rs114697636-G variant is linked in cis-position to the exonic rs11264875-A allelic variant (Fig. 4A). We analyzed the RNA transcripts from PFCs by sequencing RT-PCR products of 2 individuals (S14 and C28) who were heterozygous for these SNPs. Both S14 and C28 expressed the A allele only of the rs11264875 SNP only, confirming our hypothesis of monoallelic expression in heterozygous carriers (Fig. 4A).
Given that the SNP is located in the 5ʹ region of NUP210L, this region may be important for interaction with transcriptional factors that are either activators or repressors. Indeed, gel-shift analysis of oligonucleotide sequences overlapping the rs114697636 SNP in this region demonstrated a band-shift after incubation with cell line nuclear extracts (Supplemental Fig. S8G). Finally, we demonstrated that the rs114697636-G substitution alters the evolutionary conserved site in this putative regulatory region of NUP210L, which we predicted to change the binding affinity of multiple transcription factors, with the most robust emerging binding site for nuclear receptor subfamily 2 group C member 1, also known as testicular receptor 2 (Supplemental Fig. S8F, I). Interestingly, the DNA methylation state of NUP210L has recently been linked to psychologic development disorders (60), and the common SNPs in this region are associated with mathematical abilities (61) and intelligence (62). Thus, the consequences of abnormal NUP210L activation found in the brain of some individuals warrants further investigation.
DISCUSSION
In the present study, we performed high-resolution mapping of the open-chromatin mark H3K4me3, which was found primarily associated with active gene promoters, in a panel of 56 individuals (normal and with psychiatric pathologies), in conjunction with whole-genome and transcriptome sequencing (Fig. 1).
Our data suggest that although the histone methylation landscapes of prefrontal neurons are strikingly similar across adults, their subject-specific epigenomic blueprints of neurons reveal distinctive patterns between individuals. The extracted neuronal populations could be a mixture of neuronal subtypes with a predominant population of excitatory over inhibitory neurons. Thus, a separate analysis of neuronal classes and single-nucleus epigenetic profiling may reveal even more individual neuron-type–specific variabilities.
Gender affects the epigenetic profile in several loci encompassing genes associated with diseases disproportionally presented in males and females. Interestingly, the up-regulated H3K4me3 signal in male neurons was found for the POU5F1 gene. This gene encodes octamer-binding transcription factor 4, which is a marker for brain germinomas (49), a disease known for its male predominance (gender ratio 14:1) and high prevalence in people younger than 20 yr of age (90%), when tumor develops in the pineal region (43). However, these correlations should be taken cautiously given the different brain region location and cell origin of germinomas.
In this study, we revealed at least 9001 loci marked by H3K4me3 in neurons, which cannot be annotated to promoters of known genes. By mapping transcripts to the novel H3K4me3-marked loci, we predicted 1526 novel genes (mainly ncRNA genes). The origins of the remaining 8370 H3K4me3 loci with no transcripts annotated previously or in our study is unknown. The transcripts for these open-chromatin loci can be presumably found by utilizing a larger cohort of deeply sequenced transcriptomes from multiple human subjects. In part, these loci may also be sites of open chromatin unrelated to TSS of promoter regions.
Integrative analyses of WGS and ChIP-seq data demonstrated a correlation between the accumulation of individual genetic CNVs and open-chromatin signals in neuronal genomes. The significant contribution of genetic variations to chromatin variability in cells, including cells cultured in vitro, was recently reported (62–65). It is conceivable that somatic cells and tissues from human subjects, such as PFC neurons in this study, were exposed to diverse biologic conditions in different individuals and would therefore bear more nongenetically induced variability of chromatin signals than cells transformed and cultured in uniform-media conditions.
In this study, we focused on analyzing genetic variations mapped within the genomic regions occupied by H3K4me3 peaks. The role of rare genetic variant SNPs on H3K4me3 marks was exemplified at the 5ʹ region of the NUP210L gene. However, the effect of distantly located genetic variations on the open-chromatin regions of promoters (e.g., enhancers and insulators) can also be anticipated (65, 66).
Correlation analysis of ChIP-seq and RNA-seq data revealed a modest (but statistically significant) correspondence between the two experiments. Such correlative analyses may, in part, be confounded by a mismatch in tissues used in the experiments (sorted neurons for ChIP-seq and unsorted brain tissue cells for RNA-seq). At the same time, state-of-the-art machine-learning algorithms for prediction of gene expression using multiple epigenetic marks similarly demonstrate a significant but incomplete predictive power (16), presumably due to distal epigenetic effects and posttranscriptional gene regulation.
Furthermore, we provided strong evidence that a significant number of both novel and previously annotated genes exhibit active chromatin states in the PFC of certain individuals only. The origin of rare or private H3K4me3 peaks found in some adult individuals has yet to be clarified. These loci are enriched for ncRNAs (Supplemental Table S6). A small subset of these loci overlapped with promoters commonly active in the testis, suggesting their reactivation in the brain. Surprisingly, we did find evidence that, in some subjects, induction of open chromatin occurs at gene promoter regions that are normally silenced in PFC cells. For example, we found epigenomic neuronal activation of the testis-specific gene NUP210L in the brain tissue of several human subjects. We linked this activation to a single genetic mutation in the close vicinity of the NUP210L start codon. NUP210L gene is poorly studied. We explored the largest GWAS data known to date and found no association between the NUP210L locus and major psychiatric illnesses. No association was found with schizophrenia (17), bipolar disorder (18), major depressive disorder (67), or autism spectrum disorders (19), even when we applied the relaxed P value threshold (P < 1×10−6). However, genome-wide screening of DNA methylation at birth revealed differences for NUP210L locus in carriers of 22q11.2 syndrome with psychologic development disorders compared with the disorder-free 22q11.2 carriers (60). Moreover, by exploring recently reported GWAS data (61) of 279,930 subjects, we found that the genomic region harboring NUP210L is among the loci showing an association with intelligence. A large GWAS study involving one million people also revealed a highly significant association of intronic SNP in NUP210L region with self-reported mathematical abilities (P 2.21×10−25) and highest math class taken (P 5.82×10−10) (62). Thus, our finding that the SNP-dependent activity of this testes-specific gene can be detected in neurons of some humans (<3%) is not only interesting but also warrants further exploration of the link between the SNP, epigenetic activation of the gene in brain, and cognitive abilities.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Dr. Andree Lessard (Maryland Psychiatric Research Center, Catonsville, MD, USA), Dr. William E. Bunney Jr. (University of California–Irvine, Irvine, CA, USA), and Dr. Edward G. Jones (deceased; University of California–Davis, Davis, CA, USA) for providing post mortem brain tissue. The authors also thank Dr. Hennady Shulha (University of Massachusetts Medical School, Worcester, MA, USA) for helpful discussions on chromatin immunoprecipitation followed by sequencing (ChIP-seq) analysis, Dr. Iris Cheung (University of Massachusetts Medical School) for help with performing ChIP-seq assays, and the University of Massachusetts Flow Cytometry and Deep Sequencing Cores, including Dr. R. Konz (Flow Cytometry Core Facility, University of Massachusetts Medical School) and Dr. E. Kittler (Deep Sequencing Core Lab, University of Massachusetts Medical School), for technical support. This work was supported in part by the U.S. National Institutes of Health (NIH)/National Institute on Aging (NIA; Grant R01 AG054712 to E.I.R.), the Government of the Russian Federation (Grant 14. B25.31.0033, sequencing analysis, and gel-shift assay), the Russian Scientific Foundation (RSF; Grant 14-44-00077, bioinformatic analysis of ChIP-seq data), the Russian Foundation for Basic Research (RFBR; Grant 18-29-13051), and the Brain and Behavior Research Foundation, Autism Speaks, and the NIH/National Institute of Mental Health (NIMH; Grant R01 MH106056 to S.A., A.C.M., and A.D.). The authors declare no conflicts of interest.
Glossary
- BNPP
brain neuronal paraneoplastic protein
- BNRNA
brain neuronal RNA
- ChIP-seq
chromatin immunoprecipitation followed by sequencing
- CNV
copy number variant
- GRCh
Genome Reference Consortium Human Build
- GWAS
genome-wide association study
- H3K4me3
trimethylation of histone H3 at lysine 4
- Lbh
limb, bud, and heart
- ncRNA
noncoding RNA
- NUP210L
nucleoporin 210 like
- PFC
prefrontal cortex
- PNMA
paraneoplastic Ma
- POU5F1
POU class 5 homeobox 1
- RNA-seq
RNA sequencing
- SNV
single nucleotide variant
- SNP
single nucleotide polymorphism
- TSS
transcription start site
- WGS
whole-genome sequencing
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTION
E. I. Rogaev designed the genetic and epigenetic experiments and analysis; S. Akbarian supervised nucleus sorting and ChIP-seq data set generation; T. V. Andreeva and A. P. Grigorenko performed whole-genome sequencing; D. A. Reshetov, A. P. Grigorenko, A. Y. Goltsov, and E. Filippova performed genotyping analysis; A. C. Mitchell and A. Dincer performed ChIP-seq; F. E. Gusev, D. A. Reshetov, G. Fedonin, M. Aliseychik, and Z. Weng obtained and analyzed primary ChIP-seq data; F. E. Gusev performed bioinformatic analysis of genome sequences and open-chromatin loci; V. Solovyev performed bioinformatic prediction of promoters and genes; L. Brizgalov performed gel-shift assays; and T. Halene provided and analyzed clinical data of some brain specimens.
REFERENCES
- 1.The ENCODE Project Consortium (2012) An integrated encyclopedia of DNA elements in the human genome. Nature 489, 57–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roadmap Epigenomics Consortium , Kundaje A., Meuleman W., Ernst J., Bilenky M., Yen A., Heravi-Moussavi A., Kheradpour P., Zhang Z., Wang J., Ziller M. J., Amin V., Whitaker J. W., Schultz M. D., Ward L. D., Sarkar A., Quon G., Sandstrom R. S., Eaton M. L., Wu Y.-C., Pfenning A. R., Wang X., Claussnitzer M., Liu Y., Coarfa C., Harris R. A., Shoresh N., Epstein C. B., Gjoneska E., Leung D., Xie W., Hawkins R. D., Lister R., Hong C., Gascard P., Mungall A. J., Moore R., Chuah E., Tam A., Canfield T. K., Hansen R. S., Kaul R., Sabo P. J., Bansal M. S., Carles A., Dixon J. R., Farh K.-H., Feizi S., Karlic R., Kim A.-R., Kulkarni A., Li D., Lowdon R., Elliott G., Mercer T. R., Neph S. J., Onuchic V., Polak P., Rajagopal N., Ray P., Sallari R. C., Siebenthall K. T., Sinnott-Armstrong N. A., Stevens M., Thurman R. E., Wu J., Zhang B., Zhou X., Beaudet A. E., Boyer L. A., De Jager P. L., Farnham P. J., Fisher S. J., Haussler D., Jones S. J. M., Li W., Marra M. A., McManus M. T., Sunyaev S., Thomson J. A., Tlsty T. D., Tsai L.-H., Wang W., Waterland R. A., Zhang M. Q., Chadwick L. H., Bernstein B. E., Costello J. F., Ecker J. R., Hirst M., Meissner A., Milosavljevic A., Ren B., Stamatoyannopoulos J. A., Wang T., Kellis M. (2015) Integrative analysis of 111 reference human epigenomes. Nature 518, 317–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akbarian S., Liu C., Knowles J. A., Vaccarino F. M., Farnham P. J., Crawford G. E., Jaffe A. E., Pinto D., Dracheva S., Geschwind D. H., Mill J., Nairn A. C., Abyzov A., Pochareddy S., Prabhakar S., Weissman S., Sullivan P. F., State M. W., Weng Z., Peters M. A., White K. P., Gerstein M. B., Amiri A., Armoskus C., Ashley-Koch A. E., Bae T., Beckel-Mitchener A., Berman B. P., Coetzee G. A., Coppola G., Francoeur N., Fromer M., Gao R., Grennan K., Herstein J., Kavanagh D. H., Ivanov N. A., Jiang Y., Kitchen R. R., Kozlenkov A., Kundakovic M., Li M., Li Z., Liu S., Mangravite L. M., Mattei E., Markenscoff-Papadimitriou E., Navarro F. C. P., North N., Omberg L., Panchision D., Parikshak N., Poschmann J., Price A. J., Purcaro M., Reddy T. E., Roussos P., Schreiner S., Scuderi S., Sebra R., Shibata M., Shieh A. W., Skarica M., Sun W., Swarup V., Thomas A., Tsuji J., van Bakel H., Wang D., Wang Y., Wang K., Werling D. M., Willsey A. J., Witt H., Won H., Wong C. C. Y., Wray G. A., Wu E. Y., Xu X., Yao L., Senthil G., Lehner T., Sklar P., Sestan N. (2015) The PsychENCODE project. Nature Neuroscience 18, 1707–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen L., Ge B., Casale F. P., Vasquez L., Kwan T., Garrido-Martín D., Watt S., Yan Y., Kundu K., Ecker S., Datta A., Richardson D., Burden F., Mead D., Mann A. L., Fernandez J. M., Rowlston S., Wilder S. P., Farrow S., Shao X., Lambourne J. J., Redensek A., Albers C. A., Amstislavskiy V., Ashford S., Berentsen K., Bomba L., Bourque G., Bujold D., Busche S., Caron M., Chen S.-H., Cheung W., Delaneau O., Dermitzakis E. T., Elding H., Colgiu I., Bagger F. O., Flicek P., Habibi E., Iotchkova V., Janssen-Megens E., Kim B., Lehrach H., Lowy E., Mandoli A., Matarese F., Maurano M. T., Morris J. A., Pancaldi V., Pourfarzad F., Rehnstrom K., Rendon A., Risch T., Sharifi N., Simon M.-M., Sultan M., Valencia A., Walter K., Wang S.-Y., Frontini M., Antonarakis S. E., Clarke L., Yaspo M.-L., Beck S., Guigo R., Rico D., Martens J. H. A., Ouwehand W. H., Kuijpers T. W., Paul D. S., Stunnenberg H. G., Stegle O., Downes K., Pastinen T., Soranzo N. (2016) Genetic drivers of epigenetic and transcriptional variation in human immune cells. Cell 167, 1398–1414.e24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karlić R., Chung H.-R., Lasserre J., Vlahoviček K., Vingron M. (2010) Histone modification levels are predictive for gene expression. Proc. Natl. Acad. Sci. USA 107, 2926–2931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Houston I., Peter C. J., Mitchell A., Straubhaar J., Rogaev E., Akbarian S. (2013) Epigenetics in the human brain. Neuropsychopharmacology 38, 183–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rogaev E. I. (2012) Genomics of behavioral diseases. Front. Genet. 3, 45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dincer A., Gavin D. P., Xu K., Zhang B., Dudley J. T., Schadt E. E., Akbarian S. (2015) Deciphering H3K4me3 broad domains associated with gene-regulatory networks and conserved epigenomic landscapes in the human brain. Transl. Psychiatry 5, e679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rakyan V. K., Down T. A., Balding D. J., Beck S. (2011) Epigenome-wide association studies for common human diseases. Nat. Rev. Genet. 12, 529–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Somel M., Rohlfs R., Liu X. (2014) Transcriptomic insights into human brain evolution: acceleration, neutrality, heterochrony. Curr. Opin. Genet. Dev. 29, 110–119 [DOI] [PubMed] [Google Scholar]
- 11.Buckner R. L., Krienen F. M. (2013) The evolution of distributed association networks in the human brain. Trends Cogn. Sci. 17, 648–665 [DOI] [PubMed] [Google Scholar]
- 12.Fu X., Giavalisco P., Liu X., Catchpole G., Fu N., Ning Z.-B., Guo S., Yan Z., Somel M., Pääbo S., Zeng R., Willmitzer L., Khaitovich P. (2011) Rapid metabolic evolution in human prefrontal cortex. Proc. Natl. Acad. Sci. USA 108, 6181–6186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kouzarides T. (2007) Chromatin modifications and their function. Cell 128, 693–705 [DOI] [PubMed] [Google Scholar]
- 14.Shen E., Shulha H., Weng Z., Akbarian S. (2014) Regulation of histone H3K4 methylation in brain development and disease. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369, pii: 20130514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shulha H. P., Cheung I., Guo Y., Akbarian S., Weng Z. (2013) Coordinated cell type-specific epigenetic remodeling in prefrontal cortex begins before birth and continues into early adulthood. PLoS Genet. 9, e1003433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh R., Lanchantin J., Robins G., Qi Y. (2016) DeepChrome: deep-learning for predicting gene expression from histone modifications. Bioinformatics 32, i639–i648 [DOI] [PubMed] [Google Scholar]
- 17.Pardiñas A. F., Holmans P., Pocklington A. J., Escott-Price V., Ripke S., Carrera N., Legge S. E., Bishop S., Cameron D., Hamshere M. L., Han J., Hubbard L., Lynham A., Mantripragada K., Rees E., MacCabe J. H., McCarroll S. A., Baune B. T., Breen G., Byrne E. M., Dannlowski U., Eley T. C., Hayward C., Martin N. G., McIntosh A. M., Plomin R., Porteous D. J., Wray N. R., Caballero A., Geschwind D. H., Huckins L. M., Ruderfer D. M., Santiago E., Sklar P., Stahl E. A., Won H., Agerbo E., Als T. D., Andreassen O. A., Bækvad-Hansen M., Mortensen P. B., Pedersen C. B., Børglum A. D., Bybjerg-Grauholm J., Djurovic S., Durmishi N., Pedersen M. G., Golimbet V., Grove J., Hougaard D. M., Mattheisen M., Molden E., Mors O., Nordentoft M., Pejovic-Milovancevic M., Sigurdsson E., Silagadze T., Hansen C. S., Stefansson K., Stefansson H., Steinberg S., Tosato S., Werge T., Collier D. A., Rujescu D., Kirov G., Owen M. J., O’Donovan M. C., Walters J. T. R.; GERAD1 Consortium ; CRESTAR Consortium (2018) Common schizophrenia alleles are enriched in mutation-intolerant genes and in regions under strong background selection. Nat. Genet. 50, 381–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stahl E., Breen G., Forstner A., McQuillin A., Ripke S., Cichon S., Scott L., Ophoff R., Andreassen O. A., Kelsoe J., Sklar P.; Bipolar Disorder Working Group of the Psychiatric Genomics Consortium (2018) Genomewide association study identifies 30 loci associated with bipolar disorder. bioRxiv doi: 10.1101/173062 [Google Scholar]
- 19.Grove J., Ripke S., Als T. D., Mattheisen M., Walters R., Won H., Pallesen J., Agerbo E., Andreassen O. A., Anney R., Belliveau R., Bettella F., Buxbaum J. D., Bybjerg-Grauholm J., Bækved-Hansen M., Cerrato F., Chambert K., Christensen J. H., Churchhouse C., Dellenvall K., Demontis D., Rubeis S. D., Devlin B., Djurovic S., Dumont A., Goldstein J., Hansen C. S., Hauberg M. E., Hollegaard M. V., Hope S., Howrigan D. P., Huang H., Hultman C., Klei L., Maller J., Martin J., Martin A. R., Moran J., Nyegaard M., Nærland T., Palmer D. S., Palotie A., Pedersen C. B., Pedersen M. G., Poterba T., Poulsen J. B., Pourcain B. S., Qvist P., Rehnström K., Reichenberg A., Reichert J., Robinson E., Roeder K., Roussos P., Saemundsen E., Sandin S., Satterstrom F. K., Smith G. D., Stefansson H., Stefansson K., Steinberg S., Stevens C., Sullivan P. F., Turley P., Walters G. B., Xu X., Geschwind D., Nordentoft M., Hougaard D. M., Werge T., Mors O., Mortensen P. B., Neale B. M., Daly M. J., Børglum A.D. (2017) Identification of common risk variants for autism spectrum disorder. Nat Genet. 51, 431–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shulha H. P., Crisci J. L., Reshetov D., Tushir J. S., Cheung I., Bharadwaj R., Chou H.-J., Houston I. B., Peter C. J., Mitchell A. C., Yao W.-D., Myers R. H., Chen J.-F., Preuss T. M., Rogaev E. I., Jensen J. D., Weng Z., Akbarian S. (2012) Human-specific histone methylation signatures at transcription start sites in prefrontal neurons. PLoS Biol. 10, e1001427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roussos P., Mitchell A. C., Voloudakis G., Fullard J. F., Pothula V. M., Tsang J., Stahl E. A., Georgakopoulos A., Ruderfer D. M., Charney A., Okada Y., Siminovitch K. A., Worthington J., Padyukov L., Klareskog L., Gregersen P. K., Plenge R. M., Raychaudhuri S., Fromer M., Purcell S. M., Brennand K. J., Robakis N. K., Schadt E. E., Akbarian S., Sklar P. (2014) A role for noncoding variation in schizophrenia. Cell Rep. 9, 1417–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell A. C., Javidfar B., Pothula V., Ibi D., Shen E. Y., Peter C. J., Bicks L. K., Fehr T., Jiang Y., Brennand K. J., Neve R. L., Gonzalez-Maeso J., Akbarian S. (2018) MEF2C transcription factor is associated with the genetic and epigenetic risk architecture of schizophrenia and improves cognition in mice. Mol. Psychiatry 23, 123–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shulha H. P., Cheung I., Whittle C., Wang J., Virgil D., Lin C. L., Guo Y., Lessard A., Akbarian S., Weng Z. (2012) Epigenetic signatures of autism: trimethylated H3K4 landscapes in prefrontal neurons. Arch. Gen. Psychiatry 69, 314–324 [DOI] [PubMed] [Google Scholar]
- 24.Sherwood C. C., Stimpson C. D., Raghanti M. A., Wildman D. E., Uddin M., Grossman L. I., Goodman M., Redmond J. C., Bonar C. J., Erwin J. M., Hof P. R. (2006) Evolution of increased glia-neuron ratios in the human frontal cortex. Proc. Natl. Acad. Sci. USA 103, 13606–13611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang Y., Matevossian A., Huang H.-S., Straubhaar J., Akbarian S. (2008) Isolation of neuronal chromatin from brain tissue. BMC Neurosci. 9, 42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu J. Q., Wang X., Beveridge N. J., Tooney P. A., Scott R. J., Carr V. J., Cairns M. J. (2012) Transcriptome sequencing revealed significant alteration of cortical promoter usage and splicing in schizophrenia. PLoS One 7, e36351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bryzgalov L. O., Antontseva E. V., Matveeva M. Y., Shilov A. G., Kashina E. V., Mordvinov V. A., Merkulova T. I. (2013) Detection of regulatory SNPs in human genome using ChIP-seq ENCODE data. PLoS One 8, e78833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langmead B., Salzberg S. L. (2012) Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y., Liu T., Meyer C. A., Eeckhoute J., Johnson D. S., Bernstein B. E., Nusbaum C., Myers R. M., Brown M., Li W., Liu X. S. (2008) Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robinson M. D., McCarthy D. J., Smyth G. K. (2010) edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H., Durbin R. (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DePristo M. A., Banks E., Poplin R., Garimella K. V., Maguire J. R., Hartl C., Philippakis A. A., del Angel G., Rivas M. A., Hanna M., McKenna A., Fennell T. J., Kernytsky A. M., Sivachenko A. Y., Cibulskis K., Gabriel S. B., Altshuler D., Daly M. J. (2011) A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat. Genet. 43, 491–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boeva V., Popova T., Bleakley K., Chiche P., Cappo J., Schleiermacher G., Janoueix-Lerosey I., Delattre O., Barillot E. (2012) Control-FREEC: a tool for assessing copy number and allelic content using next-generation sequencing data. Bioinformatics 28, 423–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abyzov A., Urban A. E., Snyder M., Gerstein M. (2011) CNVnator: an approach to discover, genotype, and characterize typical and atypical CNVs from family and population genome sequencing. Genome Res. 21, 974–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vorontsov I. E., Kulakovskiy I. V., Khimulya G., Nikolaeva D. D., Makeev V. J. (2015) PERFECTOS-APE–predicting regulatory functional effect of SNPs by approximate P-value estimation. In Proceedings of the International Conference on Bioinformatics Models, Methods and Algorithms (BIOSTEC 2015) 1, 102–108 [Google Scholar]
- 36.Kulakovskiy I. V., Vorontsov I. E., Yevshin I. S., Sharipov R. N., Fedorova A. D., Rumynskiy E. I., Medvedeva Y. A., Magana-Mora A., Bajic V. B., Papatsenko D. A., Kolpakov F. A., Makeev V. J. (2018) HOCOMOCO: towards a complete collection of transcription factor binding models for human and mouse via large-scale ChIP-Seq analysis. Nucleic Acids Research 46, D252–D259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim D., Pertea G., Trapnell C., Pimentel H., Kelley R., Salzberg S. L. (2013) TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 14, R36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trapnell C., Roberts A., Goff L., Pertea G., Kim D., Kelley D. R., Pimentel H., Salzberg S. L., Rinn J. L., Pachter L. (2012) Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 7, 562–578; erratum: 9, 2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salamov A. A., Solovyev V. V. (2000) Ab initio gene finding in Drosophila genomic DNA. Genome Res. 10, 516–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kong L., Zhang Y., Ye Z.-Q., Liu X.-Q., Zhao S.-Q., Wei L., Gao G. (2007) CPC: assess the protein-coding potential of transcripts using sequence features and support vector machine. Nucleic Acids Res. 35(Suppl2), W345–W349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flicek P., Amode M. R., Barrell D., Beal K., Billis K., Brent S., Carvalho-Silva D., Clapham P., Coates G., Fitzgerald S., Gil L., Girón C. G., Gordon L., Hourlier T., Hunt S., Johnson N., Juettemann T., Kähäri A. K., Keenan S., Kulesha E., Martin F. J., Maurel T., McLaren W. M., Murphy D. N., Nag R., Overduin B., Pignatelli M., Pritchard B., Pritchard E., Riat H. S., Ruffier M., Sheppard D., Taylor K., Thormann A., Trevanion S. J., Vullo A., Wilder S. P., Wilson M., Zadissa A., Aken B. L., Birney E., Cunningham F., Harrow J., Herrero J., Hubbard T. J. P., Kinsella R., Muffato M., Parker A., Spudich G., Yates A., Zerbino D. R., Searle S. M. J. (2014) Ensembl 2014. Nucleic Acids Res. 42, D749–D755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenbloom K. R., Armstrong J., Barber G. P., Casper J., Clawson H., Diekhans M., Dreszer T. R., Fujita P. A., Guruvadoo L., Haeussler M., Harte R. A., Heitner S., Hickey G., Hinrichs A. S., Hubley R., Karolchik D., Learned K., Lee B. T., Li C. H., Miga K. H., Nguyen N., Paten B., Raney B. J., Smit A. F. A., Speir M. L., Zweig A. S., Haussler D., Kuhn R. M., Kent W. J. (2015) The UCSC Genome Browser database: 2015 update. Nucleic Acids Res. 43, D670–D681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao H., Sun Z., Wang J., Huang H., Kocher J.-P., Wang L. (2014) CrossMap: a versatile tool for coordinate conversion between genome assemblies. Bioinformatics 30, 1006–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horakova A. H., Moseley S. C., McLaughlin C. R., Tremblay D. C., Chadwick B. P. (2012) The macrosatellite DXZ4 mediates CTCF-dependent long-range intrachromosomal interactions on the human inactive X chromosome. Hum. Mol. Genet. 21, 4367–4377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Freije D., Helms C., Watson M. S., Donis-Keller H. (1992) Identification of a second pseudoautosomal region near the Xq and Yq telomeres. Science 258, 1784–1787 [DOI] [PubMed] [Google Scholar]
- 46.Gianfrancesco F., Esposito T., Montanini L., Ciccodicola A., Mumm S., Mazzarella R., Rao E., Giglio S., Rappold G., Forabosco A. (1998) A novel pseudoautosomal gene encoding a putative GTP-binding protein resides in the vicinity of the Xp/Yp telomere. Hum. Mol. Genet. 7, 407–414 [DOI] [PubMed] [Google Scholar]
- 47.Preumont A., Rzem R., Vertommen D., Van Schaftingen E. (2010) HDHD1, which is often deleted in X-linked ichthyosis, encodes a pseudouridine-5′-phosphatase. Biochem. J. 431, 237–244 [DOI] [PubMed] [Google Scholar]
- 48.Hu H. Y., He L., Khaitovich P. (2014) Deep sequencing reveals a novel class of bidirectional promoters associated with neuronal genes. BMC Genomics 15, 457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hattab E. M., Tu P.-H., Wilson J. D., Cheng L. (2005) OCT4 immunohistochemistry is superior to placental alkaline phosphatase (PLAP) in the diagnosis of central nervous system germinoma. Am. J. Surg. Pathol. 29, 368–371 [DOI] [PubMed] [Google Scholar]
- 50.Cuccia V., Galarza M. (2006) Pure pineal germinomas: analysis of gender incidence. Acta Neurochir. (Wien) 148, 865–871, discussion 871 [DOI] [PubMed] [Google Scholar]
- 51.Klengel T., Mehta D., Anacker C., Rex-Haffner M., Pruessner J. C., Pariante C. M., Pace T. W. W., Mercer K. B., Mayberg H. S., Bradley B., Nemeroff C. B., Holsboer F., Heim C. M., Ressler K. J., Rein T., Binder E. B. (2013) Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat. Neurosci. 16, 33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vawter M. P., Evans S., Choudary P., Tomita H., Meador-Woodruff J., Molnar M., Li J., Lopez J. F., Myers R., Cox D., Watson S. J., Akil H., Jones E. G., Bunney W. E. (2004) Gender-specific gene expression in post-mortem human brain: localization to sex chromosomes. Neuropsychopharmacology 29, 373–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trabzuni D., Ramasamy A., Imran S., Walker R., Smith C., Weale M. E., Hardy J., Ryten M.; North American Brain Expression Consortium (2013) Widespread sex differences in gene expression and splicing in the adult human brain. Nat. Commun. 4, 2771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prüfer K., Racimo F., Patterson N., Jay F., Sankararaman S., Sawyer S., Heinze A., Renaud G., Sudmant P. H., de Filippo C., Li H., Mallick S., Dannemann M., Fu Q., Kircher M., Kuhlwilm M., Lachmann M., Meyer M., Ongyerth M., Siebauer M., Theunert C., Tandon A., Moorjani P., Pickrell J., Mullikin J. C., Vohr S. H., Green R. E., Hellmann I., Johnson P. L., Blanche H., Cann H., Kitzman J. O., Shendure J., Eichler E. E., Lein E. S., Bakken T. E., Golovanova L. V., Doronichev V. B., Shunkov M. V., Derevianko A. P., Viola B., Slatkin M., Reich D., Kelso J., Pääbo S. (2014) The complete genome sequence of a Neanderthal from the Altai Mountains. Nature 505, 43–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Finn R. D., Coggill P., Eberhardt R. Y., Eddy S. R., Mistry J., Mitchell A. L., Potter S. C., Punta M., Qureshi M., Sangrador-Vegas A., Salazar G. A., Tate J., Bateman A. (2016) The Pfam protein families database: towards a more sustainable future. Nucleic Acids Res. 44, D279–D285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Briegel K. J., Joyner A. L. (2001) Identification and characterization of Lbh, a novel conserved nuclear protein expressed during early limb and heart development. Dev. Biol. 233, 291–304 [DOI] [PubMed] [Google Scholar]
- 57.Feil R., Fraga M. F. (2012) Epigenetics and the environment: emerging patterns and implications. Nat. Rev. Genet. 13, 97–109 [DOI] [PubMed] [Google Scholar]
- 58.Pratto F., Brick K., Khil P., Smagulova F., Petukhova G. V., Camerini-Otero R. D. (2014) DNA recombination. Recombination initiation maps of individual human genomes. Science 346, 1256442; erratum: 362, eaav6294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Auton A., Brooks L. D., Durbin R. M., Garrison E. P., Kang H. M., Korbel J. O., Marchini J. L., McCarthy S., McVean G. A., Abecasis G. R.; 1000 Genomes Project Consortium (2015) A global reference for human genetic variation. Nature 526, 68–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Starnawska A., Hansen C. S., Sparsø T., Mazin W., Olsen L., Bertalan M., Buil A., Bybjerg-Grauholm J., Bækvad-Hansen M., Hougaard D. M., Mortensen P. B., Pedersen C. B., Nyegaard M., Werge T., Weinsheimer S. (2017) Differential DNA methylation at birth associated with mental disorder in individuals with 22q11.2 deletion syndrome. Transl. Psychiatry 7, e1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee J. J., Wedow R., Okbay A., Kong E., Maghzian O., Zacher M., Nguyen-Viet T. A., Bowers P., Sidorenko J., Karlsson Linnér R., Fontana M. A., Kundu T., Lee C., Li H., Li R., Royer R., Timshel P. N., Walters R. K., Willoughby E. A., Yengo L., Alver M., Bao Y., Clark D. W., Day F. R., Furlotte N. A., Joshi P. K., Kemper K. E., Kleinman A., Langenberg C., Mägi R., Trampush J. W., Verma S. S., Wu Y., Lam M., Zhao J. H., Zheng Z., Boardman J. D., Campbell H., Freese J., Harris K. M., Hayward C., Herd P., Kumari M., Lencz T., Luan J., Malhotra A. K., Metspalu A., Milani L., Ong K. K., Perry J. R. B., Porteous D. J., Ritchie M. D., Smart M. C., Smith B. H., Tung J. Y., Wareham N. J., Wilson J. F., Beauchamp J. P., Conley D. C., Esko T., Lehrer S. F., Magnusson P. K. E., Oskarsson S., Pers T. H., Robinson M. R., Thom K., Watson C., Chabris C. F., Meyer M. N., Laibson D. I., Yang J., Johannesson M., Koellinger P. D., Turley P., Visscher P. M., Benjamin D. J., Cesarini D., 23andMe Research TeamCOGENT (Cognitive Genomics Consortium)Social Science Genetic Association Consortium (2018) Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat. Genet. 50, 1112–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Savage J. E., Jansen P. R., Stringer S., Watanabe K., Bryois J., de Leeuw C. A., Nagel M., Awasthi S., Barr P. B., Coleman J. R. I., Grasby K. L., Hammerschlag A. R., Kaminski J. A., Karlsson R., Krapohl E., Lam M., Nygaard M., Reynolds C. A., Trampush J. W., Young H., Zabaneh D., Hägg S., Hansell N. K., Karlsson I. K., Linnarsson S., Montgomery G. W., Muñoz-Manchado A. B., Quinlan E. B., Schumann G., Skene N. G., Webb B. T., White T., Arking D. E., Avramopoulos D., Bilder R. M., Bitsios P., Burdick K. E., Cannon T. D., Chiba-Falek O., Christoforou A., Cirulli E. T., Congdon E., Corvin A., Davies G., Deary I. J., DeRosse P., Dickinson D., Djurovic S., Donohoe G., Conley E. D., Eriksson J. G., Espeseth T., Freimer N. A., Giakoumaki S., Giegling I., Gill M., Glahn D. C., Hariri A. R., Hatzimanolis A., Keller M. C., Knowles E., Koltai D., Konte B., Lahti J., Le Hellard S., Lencz T., Liewald D. C., London E., Lundervold A. J., Malhotra A. K., Melle I., Morris D., Need A. C., Ollier W., Palotie A., Payton A., Pendleton N., Poldrack R. A., Räikkönen K., Reinvang I., Roussos P., Rujescu D., Sabb F. W., Scult M. A., Smeland O. B., Smyrnis N., Starr J. M., Steen V. M., Stefanis N. C., Straub R. E., Sundet K., Tiemeier H., Voineskos A. N., Weinberger D. R., Widen E., Yu J., Abecasis G., Andreassen O. A., Breen G., Christiansen L., Debrabant B., Dick D. M., Heinz A., Hjerling-Leffler J., Ikram M. A., Kendler K. S., Martin N. G., Medland S. E., Pedersen N. L., Plomin R., Polderman T. J. C., Ripke S., van der Sluis S., Sullivan P. F., Vrieze S. I., Wright M. J., Posthuma D. (2018) Genome-wide association meta-analysis in 269,867 individuals identifies new genetic and functional links to intelligence. Nat. Genet. 50, 912–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McVicker G., van de Geijn B., Degner J. F., Cain C. E., Banovich N. E., Raj A., Lewellen N., Myrthil M., Gilad Y., Pritchard J. K. (2013) Identification of genetic variants that affect histone modifications in human cells. Science 342, 747–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kilpinen H., Waszak S. M., Gschwind A. R., Raghav S. K., Witwicki R. M., Orioli A., Migliavacca E., Wiederkehr M., Gutierrez-Arcelus M., Panousis N. I., Yurovsky A., Lappalainen T., Romano-Palumbo L., Planchon A., Bielser D., Bryois J., Padioleau I., Udin G., Thurnheer S., Hacker D., Core L. J., Lis J. T., Hernandez N., Reymond A., Deplancke B., Dermitzakis E. T. (2013) Coordinated effects of sequence variation on DNA binding, chromatin structure, and transcription. Science 342, 744–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Waszak S. M., Delaneau O., Gschwind A. R., Kilpinen H., Raghav S. K., Witwicki R. M., Orioli A., Wiederkehr M., Panousis N. I., Yurovsky A., Romano-Palumbo L., Planchon A., Bielser D., Padioleau I., Udin G., Thurnheer S., Hacker D., Hernandez N., Reymond A., Deplancke B., Dermitzakis E. T. (2015) Population variation and genetic control of modular chromatin architecture in humans. Cell 162, 1039–1050 [DOI] [PubMed] [Google Scholar]
- 66.Grubert F., Zaugg J. B., Kasowski M., Ursu O., Spacek D. V., Martin A. R., Greenside P., Srivas R., Phanstiel D. H., Pekowska A., Heidari N., Euskirchen G., Huber W., Pritchard J. K., Bustamante C. D., Steinmetz L. M., Kundaje A., Snyder M. (2015) Genetic control of chromatin states in humans involves local and distal chromosomal interactions. Cell 162, 1051–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wray N. R., Ripke S., Mattheisen M., Trzaskowski M., Byrne E. M., Abdellaoui A., Adams M. J., Agerbo E., Air T. M., Andlauer T. M. F., Bacanu S.-A., Bækvad-Hansen M., Beekman A. F. T., Bigdeli T. B., Binder E. B., Blackwood D. R. H., Bryois J., Buttenschøn H. N., Bybjerg-Grauholm J., Cai N., Castelao E., Christensen J. H., Clarke T.-K., Coleman J. I. R., Colodro-Conde L., Couvy-Duchesne B., Craddock N., Crawford G. E., Crowley C. A., Dashti H. S., Davies G., Deary I. J., Degenhardt F., Derks E. M., Direk N., Dolan C. V., Dunn E. C., Eley T. C., Eriksson N., Escott-Price V., Kiadeh F. H. F., Finucane H. K., Forstner A. J., Frank J., Gaspar H. A., Gill M., Giusti-Rodríguez P., Goes F. S., Gordon S. D., Grove J., Hall L. S., Hannon E., Hansen C. S., Hansen T. F., Herms S., Hickie I. B., Hoffmann P., Homuth G., Horn C., Hottenga J.-J., Hougaard D. M., Hu M., Hyde C. L., Ising M., Jansen R., Jin F., Jorgenson E., Knowles J. A., Kohane I. S., Kraft J., Kretzschmar W. W., Krogh J., Kutalik Z., Lane J. M., Li Y., Li Y., Lind P. A., Liu X., Lu L., MacIntyre D. J., MacKinnon D. F., Maier R. M., Maier W., Marchini J., Mbarek H., McGrath P., McGuffin P., Medland S. E., Mehta D., Middeldorp C. M., Mihailov E., Milaneschi Y., Milani L., Mill J., Mondimore F. M., Montgomery G. W., Mostafavi S., Mullins N., Nauck M., Ng B., Nivard M. G., Nyholt D. R., O’Reilly P. F., Oskarsson H., Owen M. J., Painter J. N., Pedersen C. B., Pedersen M. G., Peterson R. E., Pettersson E., Peyrot W. J., Pistis G., Posthuma D., Purcell S. M., Quiroz J. A., Qvist P., Rice J. P., Riley B. P., Rivera M., Saeed Mirza S., Saxena R., Schoevers R., Schulte E. C., Shen L., Shi J., Shyn S. I., Sigurdsson E., Sinnamon G. B. C., Smit J. H., Smith D. J., Stefansson H., Steinberg S., Stockmeier C. A., Streit F., Strohmaier J., Tansey K. E., Teismann H., Teumer A., Thompson W., Thomson P. A., Thorgeirsson T. E., Tian C., Traylor M., Treutlein J., Trubetskoy V., Uitterlinden A. G., Umbricht D., Van der Auwera S., van Hemert A. M., Viktorin A., Visscher P. M., Wang Y., Webb B. T., Weinsheimer S. M., Wellmann J., Willemsen G., Witt S. H., Wu Y., Xi H. S., Yang J., Zhang F., Arolt V., Baune B. T., Berger K., Boomsma D. I., Cichon S., Dannlowski U., de Geus E. C. J., DePaulo J. R., Domenici E., Domschke K., Esko T., Grabe H. J., Hamilton S. P., Hayward C., Heath A. C., Hinds D. A., Kendler K. S., Kloiber S., Lewis G., Li Q. S., Lucae S., Madden P. F. A., Magnusson P. K., Martin N. G., McIntosh A. M., Metspalu A., Mors O., Mortensen P. B., Müller-Myhsok B., Nordentoft M., Nöthen M. M., O’Donovan M. C., Paciga S. A., Pedersen N. L., Penninx B. W. J. H., Perlis R. H., Porteous D. J., Potash J. B., Preisig M., Rietschel M., Schaefer C., Schulze T. G., Smoller J. W., Stefansson K., Tiemeier H., Uher R., Völzke H., Weissman M. M., Werge T., Winslow A. R., Lewis C. M., Levinson D. F., Breen G., Børglum A. D., Sullivan P. F.; 23andMe ; Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium (2018) Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat. Genet. 50, 668–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pratto F., Brick K., Khil P., Smagulova F., Petukhova G. V., Camerini-Otero R. D. (2014) DNA recombination. Recombination initiation maps of individual human genomes. Science 346, 1256442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.