Abstract
A considerable number of drugs were withdrawn from the world market in the last decades due to safety reasons. A retrospective review of withdrawals is important in determining the adequacy of regulations regarding the safety and efficacy of drugs. The scope of the present study was to focus on cardiovascular adverse reactions of 61 withdrawn medicinal products, as well as 40 additional drugs withdrawn due to non-cardiovascular toxicity, while being cardiovascular agents themselves. A detailed web-based data search was held to draw a list of withdrawn pharmaceutical products from the pharmaceutical market by regulatory authorities between 1950 and 2017 due to safety reasons. A total of 464 medicinal products were withdrawn from the pharmaceutical markets between 1950 and 2017 due to safety reasons. Hepatotoxicity was the most commonly reported adverse drug reaction (ADR) that led to withdrawal, followed by immune-related reactions, neurotoxicity, and cardiovascular toxicity. The underlying mechanisms leading to cardiovascular toxicity should be investigated in depth to avoid the use of risky drugs for long periods, especially in consideration of the fact that some cardiovascular drugs persisted in the market for many decades. Furthermore, improved reporting of suspected adverse reactions and stricter regulations will lead to quicker detection of ADRs, thus emphasizing the importance of this public health problem and highlighting the need for improved “early warning systems” to manage the risks of high-risk drugs.
Keywords: Cardiovascular toxicity, drug safety, drug withdrawal
A newly designed drug passes through a long and tough route before reaching the market, and throughout its way, the safety, efficacy, and quality are the most compellable issues. Although the drugs are tested very elaborately for their safety in addition to their efficacy during preclinical and clinical trials, some concerns, such as difficulties in extrapolating drug data from animal to man, sample size that is required to emphasize to assess clinical benefit, limited duration in relatively healthy adults, and detection of rare adverse drug reactions (ADRs) and drug interactions, remain as problems to overcome. During preclinical and clinical studies, as well as post-marketing surveillance, the drug must satisfy all safety and efficacy concerns to launch and remain in the pharmaceutical market. These concerns are addressed by the system of pharmacovigilance, the partners of which are health authorities, hospitals, academia, healthcare professionals, pharmaceutical industry, and patients. A meta-analysis published by Lazarou et al. [1] reported that ADRs are responsible for >100,000 deaths and 2 million hospital admissions, making them between the fourth and sixth most common causes of death. This prevalence of ADR-related problems also revealed the requirement for national pharmacovigilance systems in the last decades. Although the pharmacovigilance data collected in developed countries initially appeared appropriate for extrapolation to other countries, the role of many confounding factors, primarily cultural and genetic predisposition, has proven the fallacy of this practice.
Therefore, the World Health Organization (WHO) recommends every country to establish their own pharmacovigilance systems and become full members of the WHO Programme for International Drug Monitoring. As of September 2017, this program has 127 participating member countries, and further 29 countries are associate members whose national pharmacovigilance centers are under establishment [2].
Despite the establishment of the Turkish regulatory system in 1985 and membership in the WHO Programme for International Drug Monitoring since 1987, effective oversight and monitoring commenced in 2005 with the establishment of the Turkish Pharmacovigilance Centre (TUFAM), which became responsible for detecting post-marketing drug safety problems [3].
In the present study, particular emphasis was placed on cardiovascular ADRs due to many concerns; because of being among the potentially life-threatening conditions and, due to their wide prevalence, being the fourth reason for withdrawal (n=61), after hepatotoxicity (n=81), immune-related reactions (n=79), and neurotoxicity (n=76). Moreover, the treatment options for negative cardiovascular effects are either lifelong or cured with major surgery, which both impart a severe medical, psychological, and economic burden on the patient, as well as additional costs on the healthcare system [4, 5].
The objective of the present study was to evaluate drugs withdrawn due to their cardiovascular risks, as well as cardiovascular drugs that were removed from the pharmaceutical market due to adverse effects either related or unrelated to their own pharmacological action worldwide between the period of 1950 and 2017.
METHODS
In the present study, detailed web-based data search and concurrent systematic reviews that summarize all removed drugs worldwide have been conducted [4, 6, 7]. Taking the above statement into account, our review updates and evaluates the data on drugs related to the cardiovascular system. The reputable sources given below compile a list of withdrawn cardiovascular drugs and drugs withdrawn for their cardiovascular concerns:
WHO’s Drug Information (volumes 1–30)
The WHO’s Pharmaceutical Newsletters (1997–2017)
The UK Medicine and Healthcare Products Regulatory Agency website
The UK Medicine and Healthcare Products Regulatory Agency, Drug Safety Monthly Updates (January 2015–May 2017)
The US Food and Drug Administration (FDA) website
PubMed
Records of Turkish Medicines and Medical Devices Agency
European Medicines Agency (EMA) website.
Veterinary medicines and drugs removed due to commercial reasons by pharmaceutical companies were not included in our data.
RESULTS
A total of 464 drugs were withdrawn from 88 different pharmacological classes by 2017 according to our recent research. Of the 464 withdrawn drugs, 53 had cardiovascular indications (Table 1). There were 11 different pharmacological classes, namely antihypertensives, vasodilators, antilipidemics, antianginals, antiarrhythmics, antithrombotics, anticholesterol agents, cardiac stimulants, thrombolytics, vasopressors, and prostaglandins as shown in Figure 1.
TABLE 1.
Cardiovascular drugs removed from the market due to adverse drug reactions between 1950 and 2017
| Medicinal product | Class | Therapeutic indication | Reason for withdrawal | Total years in the market |
|---|---|---|---|---|
| Adenosine phosphate | Antiarrhythmic | Cardiac arrhythmia | Cardiovascular | 43 |
| Aliskiren | Antihypertensive | Hypertension | Angioedema | 4 |
| Amoproxan | Antianginal | Angina | Sensory systems, skin | 1 |
| Beclobrate | Antilipidemic | Hyperlipidemia | Hepatotoxicity | 5 |
| Benzarone | Thrombolytic | Varicose veins | Liver | 28 |
| Bepridil | Antiarrhythmic | Cardiac arrhythmia | Cardiovascular | 23 |
| Buflomedil | Vasodilator | Peripheral arterial occlusive disease | Neurotoxicity, cardiotoxicity | 36 |
| Cadralazine | Antihypertensive | Hypertension | Immunologic | 3 |
| Cerivastatin | Antilipidemic | Hyperlipidemia | Renal, musculoskeletal | 4 |
| Cinepazide | Vasodilator | Cerebrovascular disease | Hematologic | 14 |
| Clofibrate | Antilipidemic | Hyperlipidemia | Accelerated deaths | 11 |
| Cyclandelate | Vasodilator | Raynaud’s disease | Not effective for use | 9 |
| Diethyl-aminoethoxyexestrol | Antianginal | Angina pectoris | Liver | 6 |
| Dilevalol | Antihypertensive | Hypertension | Liver | 1 |
| Dinoprostone* | Prostaglandin | Induction of labor | Fetal distress, uterine hypertonia | 19 |
| Dofetilide | Antiarrhythmic | Cardiac arrhythmia | Cardiovascular | 5 |
| Drotrecogin alfa (activated) | Antithrombotic | Sepsis | Insufficient evidence, bleeding risk | 10 |
| Encainide | Antiarrhythmic | Cardiac arrhythmia | Cardiovascular | 6 |
| Erythrityl tetranitrate | Antihypertensive | Angina pectoris | Insufficient evidence, skin | 43 |
| Flosequinan | Vasodilator | Congestive heart failure | Death | 1 |
| Gallopamil | Antiarrhythmic | Cardiac arrhythmia | Not specified, cardiovascular | 18 |
| Gemfibrozil | Anticholesterol | Dyslipidemia | Negative benefit-to-harm balance | 5 |
| Guanethidine | Antihypertensive | Hypertension | Sensory systems | 13 |
| Hexestrol bis | Vasodilator | Hypertension | Hepatotoxicity | 17 |
| (β-diethylaminoethyl ether) | ||||
| Hydrochlorothiazide + sotalol | Antihypertensive | Hypertension | Cardiovascular, drug interactions | 16 |
| Indoramin | Vasodilator | Benign prostatic hyperplasia, hypertension | Cardiovascular | 30 |
| Isoprenaline | Cardiac stimulant | Bradycardia and heart block, asthma | Cardiovascular | 43 |
| Laropiprant/nicotinic acid | Antilipidemic | Facial flushing | Higher frequency of non-fetal but serious side effects | 0 |
| Levarterenol | Vasopressor | Nonhemorrhagic shock | Nervous, Cardiovascular | 69 |
| Lysine amidotrizoate** | Radiography | Vascular diagnosis | Cardiovascular, hematologic, immunologic, urinary tract (safer alternatives) | 20 |
| Metipranolol | Antihypertensive | Hypertension | Sensory systems (uveitis) | 5 |
| Mibefradil | Antihypertensive | Hypertension | Drug interactions, musculoskeletal | 1 |
| Molsidomine | Antianginal | Angina pectoris | Tumorigenicity | 13 |
| Moxisylyte (thymoxamine uroalpha) | Antianginal | Benign prostatic hyperplasia | Liver | 4 |
| Muzolimine | Antihypertensive | Hypertension | Nervous system | 4 |
| Naftidrofuryl | Vasodilator | Intermittent | Cardiovascular, | 18 |
| oxalate (IV) | claudication | immunologic, liver, urinary tract | ||
| Nifedipine (10 mg) | Antihypertensive | Hypertension | Cardiovascular | 21 |
| Pargyline | Antihypertensive | Hypertension | Interaction with tyramine | 16 |
| Pentosan polysulfate Sodium | Antithrombotic | Cystitis, osteoarthritis | Hematologic, thrombocytopenia | 29 |
| Perhexiline maleate | Antianginal | Angina pectoris | Hypoglycemia, liver, musculoskeletal, nervous system | 11 |
| Phentolamine mesylate | Antihypertensive | Erectile dysfunction | Carcinogenicity | 2 |
| Polidexide | Antilipidemic | Hyperlipidemia | Oculomucocutaneous syndrome | 3 |
| Potassium canrenoate | Antihypertensive | Hypertension, ascites | Carcinogenic | 18 |
| Potassium nitrate | Antihypertensive | Hypertension | Tumorigenicity | 80 |
| Practolol | Antihypertensive | Hypertension | Gastrointestinal, sensory systems, skin | 11 |
| Prenylamine | Antianginal | Angina pectoris | Cardiovascular: multifactorial ventricular tachycardia | 29 |
| Probucol*** | Antioxidant | Hyperlipidemia | Cardiovascular, Torsade de pointes | 9 |
| Pronethalol | Antihypertensive | Angina pectoris | Tumorigenicity | 2 |
| Sitaxentan sodium | Antihypertensive | Pulmonary arterial hypertension | Hepatotoxicity | 4 |
| Suloctidil | Vasodilator | Intermittent claudication | Liver, hepatotoxicity | 10 |
| Tienilic acid (ticrynafen) | Antihypertensive | Hypertension, kidney stones | Liver, urinary tract | 4 |
| Tocainide | Antiarrhythmic | Cardiac arrhythmia | Hematologic, agranulocytosis, aplastic anemia | 5 |
| Triparanol | Antilipidemic | Hyperlipidemia | Sensory systems, skin | 3 |
Dinoprostone is a prostaglandin that has a direct mechanism of action as a vasodilator;
Lysine amidotrizoate is a radiographic agent but it is used for vascular diagnosis;
Probucol is an antioxidant that acts as an anti-hyperlipidemic drug.
FIGURE 1.
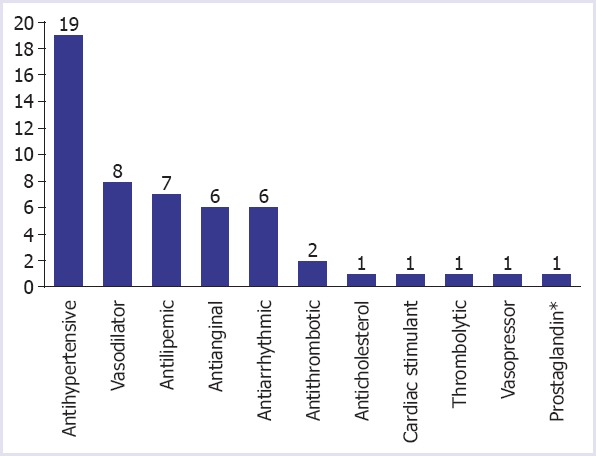
Cardiovascular classes of withdrawn drugs.
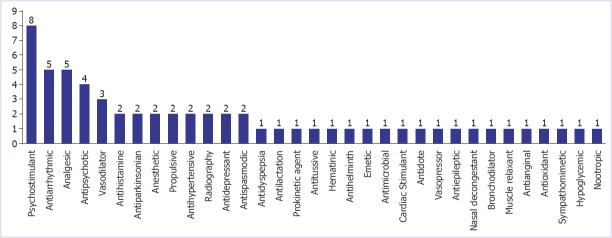
In addition, a total of 61 medicinal products from 33 different pharmacological classes were subject to withdrawal due to cardiovascular adverse effects. Those most likely to cause cardiovascular toxicity were psychostimulants (n=8), analgesics (n=5), antiarrhythmics (n=5), antipsychotics (n=4), and vasodilators (n=3), with the remaining 36 drugs distributed among 28 classes as shown in Figure 2. There were 13 drugs that were both cardiovascular agents and were withdrawn due to cardiovascular toxicity as shown in Table 2.
FIGURE 2.
Pharmacological classes of drugs withdrawn in response to their cardiovascular toxicity.
TABLE 2.
Cardiovascular drugs withdrawn due to their adverse effects on the cardiovascular system
| Medicinal product | Class | Reason for withdrawal |
|---|---|---|
| Prenylamine | Antianginal | Cardiovascular: multifactorial ventricular tachycardia |
| Adenosine phosphate | Antiarrhythmic | Cardiovascular |
| Bepridil | Antiarrhythmic | Cardiovascular |
| Dofetilide | Antiarrhythmic | Cardiovascular |
| Encainide | Antiarrhythmic | Cardiovascular |
| Gallopamil | Antiarrhythmic | Not specified, cardiovascular |
| Hydrochlorothiazide+sotalol | Antihypertensive | Cardiovascular, drug interactions |
| Nifedipine (10mg) | Antihypertensive | Cardiovascular |
| Isoprenaline | Cardiac stimulant | Cardiovascular |
| Buflomedil | Vasodilator | Neurotoxicity, cardiotoxicity |
| Indoramin | Vasodilator | Cardiovascular |
| Naftidrofuryl oxalate (IV) | Vasodilator | Cardiovascular, immunologic, liver, urinary tract |
| Levarterenol | Vasopressor | Nervous, cardiovascular |
DISCUSSION
The benefits provided by a medication might come with associated risks to health and well-being. Benefits must outweigh risks by a significant margin in all cases. The ideal medication should combine a high benefit–risk ratio, optimal treatment with the least number of medications, and affordable cost of treatment.
It is noteworthy that some of the withdrawn drugs remained in the market for long periods. For instance, potassium nitrate despite its tumorigenic effects remained as an antihypertensive agent in the market for 80 years.
In contrast, synthetic Coumarin (anticoagulant) and Laropiprant/nicotinic acid (antilipidemic) were both withdrawn within a year of their launch, which is a testament to the improvements in the ADR reporting mechanisms and the awareness and acceptance of the pharmacovigilance systems worldwide. In Turkey, withdrawals were done at the same time as the EMA or FDA withdrawals, except sibutramine, which was withdrawn in Turkey due to the efforts of the TUFAM a month ahead of the EMA and 8 months ahead of the FDA [8].
At least 200 ADR reports are expected to be submitted per million individuals annually according to the WHO [9]. ADR reports to the FDA between 2006 and 2014 have shown a 2.7-fold increase in the United States [10]. These high rates of ADRs and the withdrawal of products even that had appeared as “good drugs” in the market for many years cause loss of confidence to both drugs and the health authorities [6]. In the present study, most drugs were conventional drugs. On the other hand, the number of biotechnological drugs is increasing as both original products and biosimilar products due to the expiration of biopharmaceutical patents. The number of ADRs on VigiBase Data has exceeded 12 million by 2015 [7, 11, 12], thus warranting increased awareness of pharmacovigilance.
Similarly, the EudraVigilance system, established by the EMA, systematically works to encourage reporting of potential ADRs in Europe. In response to systematic works, the number of submitted reports showed an increasing trend, and the number of reports submitted to the EudraVigilance system exceeded 1 million in 2015 [13].
National centers, such as TUFAM, also play a significant role in increasing public awareness of drug safety. The legal time limit of submitting traced ADR reports to the TUFAM is restricted to 15 days. The annual number of ADR report submissions to the TUFAM follows an increasing trend between 2006 and 2013, with the number of reports increasing by 8.2 times during this interval. However, the elevation of the number of ADR reports (2600 reports in 2013) is not high enough to reach the point expected from a population of 75 million according to a study [14]. Therefore, extensive collaboration between all partners of the pharmacovigilance system is necessary to ensure that a sufficient level of ADR reporting is achieved after marketing [3].
There are multiple intrinsic and extrinsic factors that can determine the patient’s response to any pharmaceutical agent. A meta-analysis by Hakkarainen et al. [15] suggested that 45%–52% of ADRs are preventable. One significant intrinsic factor is the genetic make-up of the patient. The emerging field of pharmacogenomics can greatly facilitate the tailoring of a patient-specific dose regimen and aid in the reduction of ADRs occurring as the genetic make-up of each person is unique. He et al. [16] showed a correlation between a functional polymorphism in the CYP3A4 gene and an increased risk of coronary heart disease in the study population.
The main target of both pharmacogenomics and pharmacovigilance is to understand the heterogeneity and the structure of the drug efficacy and safety distribution signals among the population. Although these two disciplines are synergistic conceptually and practically, these disciplines do not have a sensible convergence up to now [17].
The current implementation of pre-licensing Phase III trials involves the participation of 500–10,000 individuals, followed by the full licensing of the drug for the market. Post-marketing studies, which succeed the launch of the drug, are optional under the current regime. In light of the pharmacovigilance concept, it has become necessary that drugs undergoing Phase III trials with at least 3000 individuals should only obtain a conditional approval, followed by compulsory post-marketing studies involving at least 30,000 individuals by taking ADR reports into consideration for newly discovered drug molecules, only after which the full license should be issued. Building on these considerations, health problems caused by ADRs can be highly mitigated [18, 19].
CONCLUSION
In light of the above information, the existing drug safety procedures should be widely reconsidered. In conclusion, collaboration between the participants of the pharmacovigilance system is necessary to develop and maintain an effective system for detecting post-marketing safety risks to protect public health.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
Financial Disclosure: The authors declare that they have received no financial support.
Authorship contributions: Concept – SSar.; Design – KK; Supervision – SS; SSar.; Materials – KK; FBA; Data collection and/or processing – KK; SS; FBA; Analysis and/or interpretation – KK, SSar.; Literature search – KK; SS; FBA; SSar.; Writing – KK; SS; FBA; SSar.; Critical review – SS; SSar.
REFERENCES
- 1.Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients:a meta-analysis of prospective studies. JAMA. 1998;279:1200–5. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- 2.UMC - Members. [Accessed May 27, 2017]. Available at: https://www.who-umc.org/global-pharmacovigilance/members/
- 3.Uppsala:the Uppsala Monitoring Center - Uppsala Reports October 2005. 1st ed. [Accessed May 2, 2019]. p. 5. Available at: https://www.who-umc.org .
- 4.Onakpoya IJ, Heneghan CJ, Aronson JK. Post-marketing withdrawal of 462 medicinal products because of adverse drug reactions:a systematic review of the world literature. BMC Med. 2016;14:10. doi: 10.1186/s12916-016-0553-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sultana J, Cutroneo P, Trifirò G. Clinical and economic burden of adverse drug reactions. J Pharmacol Pharmacother. 2013;4:S73–7. doi: 10.4103/0976-500X.120957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giacomini KM, Krauss RM, Roden DM, Eichelbaum M, Hayden MR, Nakamura Y. When good drugs go bad. Nature. 2007;446:975–7. doi: 10.1038/446975a. [DOI] [PubMed] [Google Scholar]
- 7.Singh JA, Wells GA, Christensen R, Tanjong Ghogomu E, Maxwell L, Macdonald JK, et al. Adverse effects of biologics:a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev. 2011 doi: 10.1002/14651858.CD008794.pub2. CD008794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.TC Sağlık Bakanlığı. Pepper Time Kapsül (Biber hapı) hakkında, Sağlık Bakanlığıİlaçve Eczacılık Genel Müdürlüğü(05.08.2010 ve B.10.0.İEG.0.11.00.01-330.06 sayılıyazı) [Google Scholar]
- 9.WHO. Safety Monitoring of Medicinal Products:Guidelines for Setting Up and Running a Pharmacovigilance Centre:5. Special Issues In Reporting:5.3 Under-reporting. 2000. [Accessed May 2, 2019]. Available at: http://apps.who.int/medicinedocs/en/d/Jh2934e/6.3.html .
- 10.FDA. Reports Received and Reports Entered into FAERS by Year. 2015. [Accessed May 2, 2019]. Available at: https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm070434.htm .
- 11.Prinz JC. Biologics. New drugs, new adverse reactions. [Article in German] Hautarzt. 2010;61:668–75. doi: 10.1007/s00105-010-1941-8. [DOI] [PubMed] [Google Scholar]
- 12.Scherer K, Spoerl D, Bircher AJ. Adverse drug reactions to biologics. J Dtsch Dermatol Ges. 2010;8:411–26. doi: 10.1111/j.1610-0387.2010.07339.x. [DOI] [PubMed] [Google Scholar]
- 13.European Medicines Agency. 2015 Annual Report on EudraVigilance for the European Parliament, the Council and the Commission. [Accessed May 2, 2019]. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Report/2016/03/WC500203705.pdf .
- 14.Ozcan G, Aykac E, Kasap Y, Nemutlu N, Sen E, Aydinkarahaliloglu N. Adverse Drug Reaction Reporting Pattern in Turkey:Analysis of the National Database in the Context of the First Pharmacovigilance Legislation. Drugs Real World Outcomes. 2016;3:33–43. doi: 10.1007/s40801-015-0054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hakkarainen KM, Hedna K, Petzold M, Hägg S. Percentage of patients with preventable adverse drug reactions and preventability of adverse drug reactions--a meta-analysis. PLoS One. 2012;7:e33236. doi: 10.1371/journal.pone.0033236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He BX, Shi L, Qiu J, Tao L, Li R, Yang L, et al. A functional polymorphism in the CYP3A4 gene is associated with increased risk of coronary heart disease in the Chinese Han population. Basic Clin Pharmacol Toxicol. 2011;108:208–13. doi: 10.1111/j.1742-7843.2010.00657.x. [DOI] [PubMed] [Google Scholar]
- 17.Phillips KA, Veenstra DL, Oren E, Lee JK, Sadee W. Potential role of pharmacogenomics in reducing adverse drug reactions:a systematic review. JAMA. 2001;286:2270–9. doi: 10.1001/jama.286.18.2270. [DOI] [PubMed] [Google Scholar]
- 18.Strom BL. How the US drug safety system should be changed. JAMA. 2006;295:2072–5. doi: 10.1001/jama.295.17.2072. [DOI] [PubMed] [Google Scholar]
- 19.Downing NS, Shah ND, Aminawung JA, Pease AM, Zeitoun JD, Krumholz HM, et al. Postmarket Safety Events Among Novel Therapeutics Approved by the US Food and Drug Administration Between 2001 and 2010. JAMA. 2017;317:1854–63. doi: 10.1001/jama.2017.5150. [DOI] [PMC free article] [PubMed] [Google Scholar]