Abstract
Background:
Patellofemoral pain is common in the young and active populations. Nonoperative management is limited and focuses on physical therapy. Hyaluronic acid (HA) is an injectable device that has been used for the treatment of knee osteoarthritis.
Hypothesis:
A single injection of HA would reduce pain and improve function in patients with patellofemoral pain who had previously failed conservative management.
Study Design:
Randomized controlled trial; Level of evidence, 2.
Methods:
A total of 86 patients with patellofemoral pain (65 females, 21 males; mean ± SD age, 27.0 ± 7.7 years; height, 168.6 ± 8.9 cm; weight, 74.6 ± 17.0 kg; body mass index, 26.2 ± 5.2 kg/m2) enrolled in this study after failing conservative management. Patients were randomly allocated to either 6 mL of HA or a sham injection. All patients were prescribed an additional home exercise program, including lower extremity strengthening and flexibility exercises, and were evaluated at 1, 3, and 6 months. Outcome assessments included patellofemoral pain assessment with a visual analog scale during a single-legged squat, KOOS (Knee injury and Osteoarthritis Outcome Score), Kujala score, Tegner activity rating, and normalized isometric knee extension strength. Group assignment was revealed after the 6-month assessment, and crossover treatment was offered to patients in the sham group who were still symptomatic. Linear mixed models were used to compare outcomes between groups and across time.
Results:
A total of 45 patients were randomized to HA injection and 41 to sham, with 6 patients lost to follow-up (93% follow-up rate). Patients in both groups experienced a significant reduction in visual analog pain ratings and significant improvements in all domains of the KOOS and in Kujala scores at 6 months when compared with baseline measurement (P < .05); however, there was no significant difference between groups. There were no differences observed over time or between groups for normalized knee extension strength or Tegner activity rating (P > .05).
Conclusion:
HA injection had no clinically meaningful effect on pain or functional outcomes in patients diagnosed with patellofemoral pain. Improvements were observed for both groups in patient-reported pain and function, with no change in quadriceps strength or activity rating.
Registration:
NCT01771952 (ClinicalTrials.gov identifier).
Keywords: knee, anterior knee pain, patellofemoral pain, hyaluronic acid, clinical trial
Patellofemoral pain is common in young and active populations25 and presents a challenge to treating health care providers owing to persistence of symptoms over time.17 Patients with anterior knee pain often exhibit muscle weakness, arthrogenic muscle inhibition of the quadriceps, reduced physical activity levels, altered gait mechanics, and poor patient-reported outcomes, including quality of life.9,10,12,14,19 Surgical management options are limited in this patient population; therefore, the focus for treatments is on conservative management such as physical therapy.
Among the more commonly used therapies for treating nonosteoarthritic patellofemoral pain are rest, exercise, physical therapy, and oral analgesics; however, evidence is limited to support the long-term efficacy of these interventions.6 Physical therapy interventions that focus on hip and thigh muscle strengthening and pain control treatments have shown the most promise to alleviate symptoms and improve outcomes in patients with patellofemoral joint pain.9 When conservative therapies fail, there are few nonsurgical options for patients with persistent anterior knee pain,22 thereby making this diagnosis particularly challenging for both the treating clinician and patient. Corticosteroid injections may provide an alternative to operative care in patients with persistent pain. However, the use of such injections is usually limited owing to conflicting outcome studies, short-term efficacy, and concerns for steroid-related cartilage damage,29 which is concerning for younger patients.
Hyaluronic acid (HA) is an injectable device that has been used for the treatment of tibiofemoral28 and patellofemoral joint5 osteoarthritis. HA injections have been proposed to provide pain relief, stimulate existing synovial fibroblasts to produce native synovial fluid, and provide cushioning and lubrication to joint surfaces.18 HA has also been found to affect joint nociceptive response,18 thereby interfering with reflex muscle inhibition in the quadriceps. Therefore, for patients with rehabilitation-resistant, nonosteoarthritic, chronic patellofemoral joint pain, we hypothesized that HA may provide a suitable nonoperative therapy to reduce pain and improve muscle strength and function, thereby promoting long-term joint health and muscle function.
There are currently no clinical trials studying patient-reported or strength outcomes in patients with patellofemoral pain treated with HA. Therefore, the purpose of this study was to determine the clinical efficacy of a single-dose HA injection for the treatment of chronic patellofemoral pain. The primary aim of this study was to compare anterior knee pain rating during single-legged squat, patient-reported outcomes, and quadriceps function in patients treated with a single dose of HA versus those receiving a sham injection. We compared patient-reported outcomes and quadriceps muscle function between groups at 1, 3, and 6 months following injection. We hypothesized that a single injection with HA would reduce pain, improve patient-reported function, and improve muscle strength and activation in patients who had previously failed conservative management when compared with a sham injection treatment.
Methods
This was a prospective, randomized, double-blind, sham-controlled, parallel-group clinical trial to compare outcomes for 6 months after a single injection of HA in the knees of patients diagnosed with patellofemoral pain. All participants read and signed an informed consent agreement that was approved by our university’s institutional review board for health sciences research. This was an investigator-initiated clinical trial with an investigational device exemption approved by the US Food and Drug Administration for off-label use of HA in the absence of arthritis and for the patellofemoral joint.
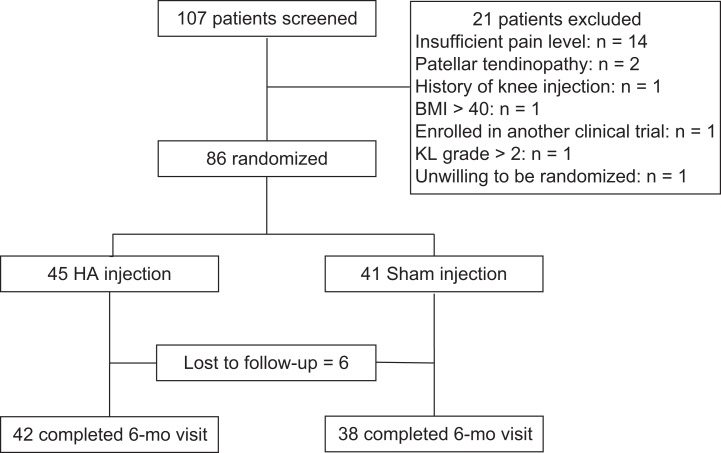
A total of 107 patients were screened for enrollment. Of these, 21 patients did not meet all inclusion and exclusion criteria and were excluded; therefore, 86 patients were enrolled and randomized (65 females, 21 males; mean ± SD age, 27.0 ± 7.7 years; height, 168.6 ± 8.9 cm; weight, 74.6 ± 17.0 kg; body mass index, 26.2 ± 5.2) (Table 1). A flowchart of patient selection is shown in Figure 1. Patients qualified if they were 15 to 45 years old, with a history and clinical diagnosis of anterior knee pain for longer than 3 months, pain and crepitus with patellar grind, 4 or greater pain ratings (out of 10), and a minimum of 4 weeks of failed physical therapy. Patients were excluded if they had any of the following: joint effusion, patellar maltracking or instability, patellar tendinitis, any evidence of tibiofemoral or patellofemoral joint space narrowing or osteoarthritis (defined as greater than grade II Kellgren-Lawrence rating) confirmed on radiographs at the time of enrollment, any indications for arthroscopy (eg, meniscus tear or instability), prior steroid injection within 6 months, any prior use of viscosupplementation, allergy to avian products, body mass index >40, prior knee surgery, evidence of hip injury, inflammatory arthritis, or other comorbid or known psychiatric conditions. Patients refrained from any treatments outside the clinical trial, including pain medication other than acetaminophen for the duration of the study.
TABLE 1.
Patient Demographics by Group Membershipa
| HA Injection | Sham Injection | Combined | P | |
|---|---|---|---|---|
| Age, y | 26.0 ± 7.0 | 28.1 ± 8.4 | 27.0 ± 7.7 | .24 |
| Height, cm | 168.9 ± 9.2 | 168.3 ± 8.6 | 168.6 ± 8.9 | .66 |
| Mass, kg | 75.7 ± 16.6 | 73.5 ± 17.6 | 74.6 ± 17.0 | .59 |
| BMI | 26.4 ± 5.3 | 25.8 ± 5.1 | 26.1 ± 5.2 | .62 |
| Injection side, right:left:both, n | 17:24:4 | 21:14:6 | 38:38:10 | .19 |
aValues are expressed as mean ± SD unless otherwise indicated. BMI, body mass index; HA, hyaluronic acid.
Figure 1.
CONSORT (Consolidated Standards of Reporting Trials) flowchart. BMI, body mass index; HA, hyaluronic acid; KL, Kellgren-Lawrence.
After screening, eligible patients completed baseline assessments for all outcome measures. The outcome assessments included patellofemoral pain assessment per a visual analog scale (VAS) during a single-legged squat, composite Knee injury and Osteoarthritis Outcome Score (KOOS; sum of all subdomain scores), Kujala patellofemoral pain questionnaire, and Tegner activity rating. Body mass–normalized isometric maximal voluntary knee extension contraction torque and quadriceps central activation ratio were measured with the knee flexed to 60° in a multimode dynamometer (System 3; Biodex Inc) and according to previously published methods.16
A sealed envelope containing group assignment from a random number generator was opened just prior to injection. Patients were randomly assigned (in 1:1 ratio) to receive either an injection of 6 mL of HA (Synvisc-One; Sanofi-Aventis Inc) or a sham injection (needle stick). To maintain blinding, patients lay supine on a treatment table with a screen that shielded their view of the knee injection. Under a sterile technique, a 21-gauge needle was carefully inserted into the intra-articular space via a superolateral approach. Based on the randomization, either 6 mL of HA was injected, or the needle was left in place and removed for a similar length of time to simulate injection.
Each patient was given instructions to perform home stretching and strengthening exercises 4 times per week for the first month postinjection (Table 2). Patients returned at 1, 3, and 6 months for repeat outcomes assessment with a blinded observer using the same procedures as in the baseline assessment. Group assignment was revealed to the patients after the 6-month outcome assessment, and a crossover treatment (single injection of 6 mL of HA with the same technique described earlier) was offered to patients receiving the sham injection who were still symptomatic. In patients who had bilateral qualifying knee pain, we treated both knees with the same randomized treatment as before.
TABLE 2.
Home Exercise Programa
| Exercise | Description |
|---|---|
| Quadriceps sets | 10-s hold × 20 repetitions |
| Straight-legged raises (hip flexion) | 1- to 2-s hold × 20 repetitions |
| Side-lying hip abduction | 1- to 2-s hold × 20 repetitions |
| Seated isometric hamstring contractions | 10 s × 10 repetitions |
| Standing calf raises | 1- to 2-s hold × 20 repetitions |
| Prone bent knee hip adduction | 10-s hold × 20 repetitions |
| Static stretching (calf, hamstring, quadriceps) | 20- to 25-s hold × 3 repetitions |
aPatients were instructed to complete 4 times per week for the first 4 weeks postinjection.
Sample size was calculated with published data reporting changes in VAS pain scores in patients with patellofemoral pain after an exercise intervention.1 Given the variability in VAS scores, we determined that 100 patients (50 per group) would be sufficient to detect a 1.0-point difference in VAS scores between treatment groups at the 6-month time point. This estimate assumes 80% power and an alpha level of .05. This sample size accounts for 10% attrition rate. We performed an interim power analysis based on variability in pain scores from the first 76 patients (34 sham, 42 HA injection) who had completed the study. We decided to stop enrollment early (N = 86) owing to low statistical power (ie, futility) and to proceed with full statistical analyses.
Statistical Analyses
Linear mixed analysis of covariance (ANCOVA) models—for nonequally spaced repeated measures—were constructed to compare the distributions of the primary and secondary outcome variables between the 2 clinical trial arms (ie, HA injected and sham). All ANCOVAs were conducted under the guidelines of the intent-to-treat principle.
ANCOVA Model Specification
For each ANCOVA, the data of the response variable represented the 1-, 3-, and 6-month postbaseline changes in the outcome variable, and the independent variables identified the clinical trial arm of randomization (HA injected or sham) and the postbaseline assessment time (1, 3, or 6 months). The preintervention measurements of the outcome variable (eg, VAS score) served as the ANCOVA covariate so that all of the between-arm comparisons could be adjusted to reflect a between–clinical arm comparison between 2 groups of participants who started the intervention period at a common baseline outcome value.
Hypothesis Testing
With regard to hypothesis testing, a traditional hierarchical hypothesis testing approach was utilized. Type III F tests were first conducted to test for “clinical arm” and “postbaseline assessment time” main effects and “clinical arm” by “postbaseline assessment time” interaction. A P ≤ .05 null hypothesis decision rule was established a priori as the null hypothesis rejection criterion for all ANCOVA type III F tests. Linear contrasts of the ANCOVA least square means were constructed to derive the 2-sample t tests for comparing the mean 1-, 3-, and 6-month postbaseline changes in the outcome variable between the 2 clinical arms. A Bonferroni-corrected P ≤ .05 null hypothesis decision rule was established a priori as the null hypothesis rejection criterion for the 1-, 3-, and 6-month between-arm comparisons of the postbaseline mean change in the outcome variable.
Results
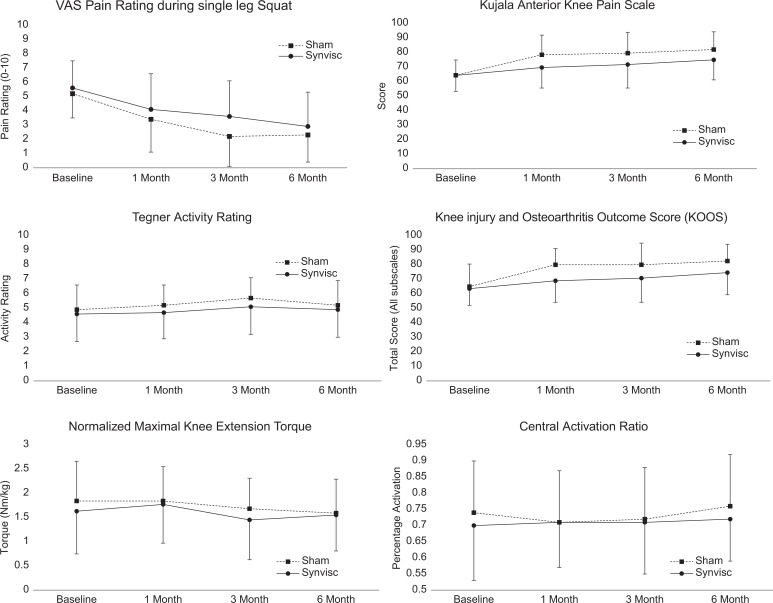
Between March 2010 and April 2016, a total of 86 patients provided consent and were enrolled, including 45 patients randomized to HA injection (34 females, 11 males) and 41 to sham (31 females, 10 males). Six patients were lost to follow-up (3 HA injection, 3 sham injection; 93% follow-up rate) (Figure 1). There were no significant group differences in patient characteristics (Table 1). For our primary outcome measure of VAS pain, there was a main effect for time (P = .005) but no group interaction (P = .451). There were no significant group × time interactions for any of the other analyzed outcome measures (Figure 2). Specifically, there were no significant between-intervention differences (HA injected vs sham) in the 1-, 3-, or 6-month changes in outcome scores, patient-reported function, muscle strength, or activation from at baseline.
Figure 2.
Between-group comparisons of anterior knee pain during single-legged squat, Kujala score, Tegner activity rating, Knee injury and Osteoarthritis Outcome Score, normalized maximal voluntary knee extension torque, and central activation ratio, measured at baseline and at 1, 3, and 6 months postrandomization. Values are presented as mean ± SD. VAS, visual analog scale.
Discussion
This is the first study to compare patient-reported outcomes, pain, muscle strength, and activation in patients with patellofemoral pain, without a radiographic diagnosis of patellofemoral osteoarthritis, who were treated with a single dose of HA. The study provides evidence that HA does not affect pain, patient-reported outcomes, or muscle function beyond the effect of sham intervention and home exercises in a 6-month follow-up period for patients with patellofemoral pain. Prior clinical trials3,4,15 studying outcomes following HA injections for chronic osteoarthritic knee pain have had conflicting results, suggesting that HA has a varying effect in patient populations. In the current study, patients were free from osteoarthritis diagnosis and had failed to improve after 1 month of conservative treatment. The time effect for the primary outcome variable suggests that VAS pain scores measured during a single-legged squat were reduced, on average across groups, over the 6-month treatment period. The magnitude of pain reduction experienced in patients enrolled in the current study may be clinically relevant; however, factors other than HA injection likely played a role in any observed changes in pain rating.
Nonsurgical management of anterior knee pain is an area for extensive research given the recurrent and persistent nature of the complaint. In a prior review,9 short-term pain reductions in patients with anterior knee pain were observed across multiple studies of clinical hip and/or quadriceps muscle strengthening. Outcomes in patients with patellofemoral pain tended to deteriorate with mid- and long-term follow-up, suggesting that patellofemoral pain is recurrent and may require longer-term interventions to maintain muscle strength and function.20,24 These findings agree with those reported in the current study, where the sharpest reduction in VAS pain was observed in the first month, when patients were actively engaged in home muscle activation exercises.
HA injections have been studied extensively in patients with osteoarthritis. Serum and synovial biomarker changes in patients with arthroscopically diagnosed chondromalacia patella are similar to those with early-stage cartilage changes associated with osteoarthritis.27 Pain reduction has been reported in patients with patellofemoral osteoarthritis after a 3-injection series of HA.7 HA was also reported to be effective in reducing pain in young, active patients with patellofemoral or tibiofemoral chondropathies; however, that study was not a controlled trial.26 The current study reports reduced pain levels over time in all patients but no differences between treatment groups. Our study was controlled by a sham injection—that is, a simple needle stick under sterile conditions. Prior studies comparing intra-articular injection efficacy were controlled by a saline placebo injection. Saline placebo injections have been proven to effectively reduce knee pain23; therefore, we selected a sham control to avoid confounding because of a potential placebo effect.
The home exercise program used in the current study was not progressive, so it is not surprising that we did not observe changes in knee extension strength or central activation over time. Strength and central activation ratio have been found to be reliable over time in patients with patellofemoral pain21; therefore, we are confident in this outcome measure. The patients in the current study were enrolled after a failed course of conservative management, so the exercise program was designed to determine whether the addition of an intra-articular injection of HA could supplement the potential benefits of exercise. A similar exercise program was utilized in a small clinical trial13 for patients with muscle weakness following knee ligament reconstructions. That study combined both supervised and home-based exercises and was found that exercise increased knee extension strength. Future studies may evaluate different forms of therapy in conjunction with intra-articular therapies in patients with chronic patellofemoral pain.
Limitations
The current study population was majority female. This is expected since anterior knee pain affects females more than males. The study enrollment was stopped prematurely on the basis of an interim power analysis demonstrating that several hundred patients (272 per clinical arm) would be needed to achieve an acceptable level of statistical power (ie, 0.80 or greater) for the VAS postbaseline change between clinical arms. Therefore, we decided to stop enrollment and perform statistical analyses. The results of this study suggest that there is no clinically meaningful effect of intra-articular injections on pain or muscle-related outcomes in patients with patellofemoral pain. Because we did stop early and thus had fewer patients, there is a possibility of type II error. Another limitation is the possibility that 1 month of conservative management was not enough time to be considered a failed attempt at treatment, although prior studies have found large-magnitude effects in reducing patellofemoral pain after a monthlong or less rehabilitation intervention.8,11 Challenges with blinding may have also confounded patient reports, as patients may have been able to tell the difference between a sham and HA injection. We purposefully chose not to control with a saline injection given the potential effect that has been reported.2 Finally, during patient screening, we used radiographs, not magnetic resonance imaging, to rule out knee osteoarthritis.
Conclusion
We observed improvements in both groups for patient-reported pain and function, with no change in quadriceps strength or activity rating. We observed no differences between groups, meaning that HA injection had no effect on pain or functional outcomes in patients with patellofemoral pain. Therefore, the use of an HA injection within this population should be utilized with caution.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: This study was funded by an investigator-initiated grant from Sanofi-Aventis. D.R.D. has received consulting fees from DePuy Synthes and receives royalties from Smith & Nephew. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from the University of Virginia (HSR No. 14386).
References
- 1. Bakhtiary AH, Fatemi E. Open versus closed kinetic chain exercises for patellar chondromalacia. Br J Sports Med. 2008;42(2):99–102. [DOI] [PubMed] [Google Scholar]
- 2. Bar-Or D, Rael LT, Brody EN. Use of saline as a placebo in intra-articular injections in osteoarthritis: potential contributions to nociceptive pain relief. Open Rheumatol J. 2017;11:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;2:CD005321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berenbaum F, Grifka J, Cazzaniga S, et al. A randomised, double-blind, controlled trial comparing two intra-articular hyaluronic acid preparations differing by their molecular weight in symptomatic knee osteoarthritis. Ann Rheum Dis. 2012;71(9):1454–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clarke S, Lock V, Duddy J, Sharif M, Newman JH, Kirwan JR. Intra-articular hylan G-F 20 (Synvisc) in the management of patellofemoral osteoarthritis of the knee (POAK). Knee. 2005;12(1):57–62. [DOI] [PubMed] [Google Scholar]
- 6. Crossley K, Bennell K, Green S, McConnell J. A systematic review of physical interventions for patellofemoral pain syndrome. Clin J Sport Med. 2001;11(2):103–110. [DOI] [PubMed] [Google Scholar]
- 7. Cubukcu D, Ardic F, Karabulut N, Topuz O. Hylan G-F 20 efficacy on articular cartilage quality in patients with knee osteoarthritis: clinical and MRI assessment. Clin Rheumatol. 2005;24(4):336–341. [DOI] [PubMed] [Google Scholar]
- 8. Ferber R, Kendall KD, Farr L. Changes in knee biomechanics after a hip-abductor strengthening protocol for runners with patellofemoral pain syndrome. J Athl Train. 2011;46(2):142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Frye JL, Ramey LN, Hart JM. The effects of exercise on decreasing pain and increasing function in patients with patellofemoral pain syndrome: a systematic review. Sports Health. 2012;4(3):205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Glaviano NR, Baellow A, Saliba S. Physical activity levels in individuals with and without patellofemoral pain. Phys Ther Sport. 2017;27:12–16. [DOI] [PubMed] [Google Scholar]
- 11. Glaviano NR, Marshall AN, Mangum LC, et al. Impairment-based rehabilitation with patterned electrical neuromuscular stimulation and lower extremity function in individuals with patellofemoral pain: a preliminary study. J Athl Train. 2019;54(3):255–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Glaviano NR, Saliba S. Association of altered frontal plane kinematics and physical activity levels in females with patellofemoral pain. Gait Posture. 2018;65:86–88. [DOI] [PubMed] [Google Scholar]
- 13. Hart JM, Kuenze CM, Diduch DR, Ingersoll CD. Quadriceps muscle function after rehabilitation with cryotherapy in patients with anterior cruciate ligament reconstruction. J Athl Train. 2014;49(6):733–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hart JM, Pietrosimone B, Hertel J, Ingersoll CD. Quadriceps activation following knee injuries: a systematic review. J Athl Train. 2010;45(1):87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jorgensen A, Stengaard-Pedersen K, Simonsen O, et al. Intra-articular hyaluronan is without clinical effect in knee osteoarthritis: a multicentre, randomised, placebo-controlled, double-blind study of 337 patients followed for 1 year. Ann Rheum Dis. 2010;69(6):1097–1102. [DOI] [PubMed] [Google Scholar]
- 16. Kuenze CM, Blemker SS, Hart JM. Quadriceps function relates to muscle size following ACL reconstruction. J Orthop Res. 2016;34(9):1656–1662. [DOI] [PubMed] [Google Scholar]
- 17. Lankhorst NE, van Middelkoop M, Crossley KM, et al. Factors that predict a poor outcome 5-8 years after the diagnosis of patellofemoral pain: a multicentre observational analysis. Br J Sports Med. 2016;50(14):881–886. [DOI] [PubMed] [Google Scholar]
- 18. Moreland LW. Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: mechanisms of action. Arthritis Res Ther. 2003;5(2):54–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Neal BS, Lack SD, Lankhorst NE, Raye A, Morrissey D, van Middelkoop M. Risk factors for patellofemoral pain: a systematic review and meta-analysis. Br J Sports Med. 2019;53(5):270–281. [DOI] [PubMed] [Google Scholar]
- 20. Nimon G, Murray D, Sandow M, Goodfellow J. Natural history of anterior knee pain: a 14- to 20-year follow-up of nonoperative management. J Pediatr Orthop. 1998;18(1):118–122. [PubMed] [Google Scholar]
- 21. Norte GE, Frye JL, Hart JM. Reliability of the superimposed-burst technique in patients with patellofemoral pain: a technical report. J Athl Train. 2015;50(11):1207–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Outerbridge RE, Dunlop JA. The problem of chondromalacia patellae. Clin Orthop Relat Res. 1975;110:177–196. [DOI] [PubMed] [Google Scholar]
- 23. Rosseland LA, Helgesen KG, Breivik H, Stubhaug A. Moderate-to-severe pain after knee arthroscopy is relieved by intraarticular saline: a randomized controlled trial. Anesth Analg. 2004;98(6):1546–1551. [DOI] [PubMed] [Google Scholar]
- 24. Sandow MJ, Goodfellow JW. The natural history of anterior knee pain in adolescents. J Bone Joint Surg Br. 1985;67(1):36–38. [DOI] [PubMed] [Google Scholar]
- 25. Smith BE, Selfe J, Thacker D, et al. Incidence and prevalence of patellofemoral pain: a systematic review and meta-analysis. PLoS One. 2018;13(1):e0190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tamburrino P, Castellacci E. Intra-articular injections of HYADD4-G in male professional soccer players with traumatic or degenerative knee chondropathy: a pilot, prospective study. J Sports Med Phys Fitness. 2016;56(12):1534–1539. [PubMed] [Google Scholar]
- 27. Vaatainen U, Lohmander LS, Thonar E, et al. Markers of cartilage and synovial metabolism in joint fluid and serum of patients with chondromalacia of the patella. Osteoarthritis Cartilage. 1998;6(2):115–124. [DOI] [PubMed] [Google Scholar]
- 28. Wen DY. Intra-articular hyaluronic acid injections for knee osteoarthritis. Am Fam Physician. 2000;62(3):565–570. [PubMed] [Google Scholar]
- 29. Wernecke C, Braun HJ, Dragoo JL. The effect of intra-articular corticosteroids on articular cartilage: a systematic review. Orthop J Sports Med. 2015;3(5):2325967115581163. [DOI] [PMC free article] [PubMed] [Google Scholar]