Key Points
Question
Does estradiol mitigate the negative association of visceral fat with structural brain networks and cognitive health?
Findings
In this cross-sectional study of a German population-based cohort of 974 adults, higher estradiol levels were associated with increased structural brain network covariance and a reduction in the negative association of visceral fat with network covariance, but only for women. In women, higher estradiol levels were associated with better structural network covariance and cognitive performance during midlife.
Meaning
Assessing visceral adipose tissue and hormone profiles, particularly in women during midlife, may be essential for promoting a healthy brain aging trajectory.
This cross-sectional study of a German population-based cohort assesses whether visceral adipose fat moderates the association between age and brain network structure and whether estradiol moderates the association between visceral adipose fat and brain network structure.
Abstract
Importance
Changes in estradiol during aging are associated with increased dementia risk. It remains unclear how estradiol supports cognitive health and whether risk factors, such as midlife obesity, are exacerbated by estrogen loss.
Objectives
To assess whether visceral adipose tissue (VAT) moderates the association between age and brain network structure and to investigate whether estradiol moderates the association between VAT and brain network structure.
Design, Setting, and Participants
Cross-sectional study of data from 974 cognitively healthy adults in Germany who participated in the Health Study of the Leipzig Research Centre for Civilization Diseases, a previously described population-based cohort study. Two moderation analyses were performed, including VAT as the moderator variable between age and brain network structure and estradiol as the moderator variable between VAT and brain network structure. The study was conducted from August 1, 2011, to November 23, 2014. Analyses were conducted from August 2017 to September 2018.
Exposures
Serum estradiol levels from fasting blood and visceral adipose tissue volume from T1-weighted magnetic resonance imaging (MRI).
Main Outcomes and Measures
Brain network covariance (individual loading on structural network derived from T1-weighted MRI) and memory performance (composite score from the Consortium to Establish a Registry for Alzheimer Disease [CERAD] verbal episodic memory test on learning [score range, 0-30], recall [score range, 0-10], and recognition [score range, 0-20]).
Results
Final analyses included data from 473 women (mean [SD] age, 50.10 [15.63] years) and 501 men (mean [SD] age, 51.24 [15.67] years). Visceral adipose tissue was associated with an exacerbation of the negative association of aging with network covariance for women (interaction term β = −0.02; 95% bias-corrected bootstrap CI, −0.03 to −0.01; P = .001) and men (interaction term β = −0.02; 95% bias-corrected bootstrap CI, −0.03 to −0.01; P < .001). Estradiol level was associated with a reduction in the negative association of VAT with network covariance in women (interaction term β = 0.63; 95% bias-corrected bootstrap CI, 0.14-1.12; P = .01), with no significant association in men. In the female midlife subgroup (age range, 35-55 years, when menopause transition occurs), low estradiol levels were associated with lower memory network covariance (Cohen d = 0.61; t80 = 2.76; P = .007) and worse memory performance (Cohen d = 0.63; t76 = 2.76; P = .007).
Conclusions and Relevance
This study reports a novel association between VAT, estradiol, and structural brain networks as a potential mechanism underlying cognitive decline in women. These findings appear to highlight the need for sex-specific strategies, including VAT and hormonal screening during midlife, to support healthy cognitive aging.
Introduction
Neuroimaging evidence suggests an association between age-related brain atrophy, cognitive decline, and obesity.1 Emerging data linking obesity to increased cognitive impairment in old age are concerning given that approximately 39% of the world’s adult population are overweight and 13% are obese.2 Visceral adipose tissue (VAT) is a known risk factor for vascular and metabolic diseases,3,4,5 but the results of some studies6,7 suggest that elevated VAT may also impair cognitive function. Behaviorally, increased VAT is associated with reduced verbal memory, attenuated attention, and lower executive function. Expanded VAT is further linked to brain atrophy, such as decreased hippocampal volume, cortical thickness, and total brain volume.6,8 Consequently, researchers are starting to view visceral obesity in midlife as a risk factor for dementia and depression in later life9,10 independent of type 2 diabetes and cardiovascular comorbidities, with the findings of other studies11,12 suggesting an even stronger association in women.
Expanded VAT is associated with systemic inflammatory biomarkers, proinflammatory cytokines,13,14,15 and reduced levels of adipocyte-specific proteins with anti-inflammatory properties, for which substantial sex differences are reported.13,16 Visceral adipose tissue is also associated with dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis and may contribute to cortisol regeneration17 and vulnerability to stress-induced cortisol reactivity in women.18 Moreover, if VAT compromises brain structural integrity owing to its association with systemic inflammation and HPA dysregulation, estradiol may have a protective role due to its anti-inflammatory properties and ability to strengthen HPA robustness.19 This is in line with menopause transition risk models for neuropsychiatric disorders, which suggest that ovarian hormone fluctuations induce alterations in stress response pathways.20 Further support for the moderating role of estradiol stems from the finding that estradiol replacement reduces metabolic syndrome symptoms in estradiol-depleted women.21,22,23 Estradiol also has vasodilatory, antiapoptotic, and antioxidative effects, which could help preserve myelin architecture.24,25,26 Although VAT and estradiol appear to have opposing roles in association with healthy brain aging, it remains unclear how they interactively alter brain network structure. This has serious implications for female cognitive decline in later life because women are potentially more sensitive to the effects of VAT on cognition.7 Moreover, dementia in women is likely influenced by midlife obesity,27 a time also characterized by rapid, unstable decreases in estradiol.28 To investigate these associations in adults, we require integration of brain, abdominal, hormonal, and cognitive data.
We addressed these issues in a novel way by integrating measures of brain network structure, VAT, sex hormones, and cognitive function in a comprehensive data set of 974 participants (473 women) aged 19 to 79 years. We first characterized a structural brain network that shows accelerated degeneration with aging and, when compromised, has been associated with poor memory performance and vulnerability to unhealthy aging and disease.29,30 After validating that this network correlated with memory performance, we explored how associations between VAT, estradiol levels, and structural brain network covariance differ between men and women. To our knowledge, this is the first study to examine how these factors interact to shed light on biological mechanisms underlying cognitive decline. In a secondary analysis, we investigated how estradiol levels are associated with network covariance and memory performance specifically in midlife women (age range, 35-55 years) because this is a crucial transition point when midlife obesity is a recognized risk factor for dementia27 and estradiol levels are known to fluctuate during the menopause transition.28 Based on the research summarized above linking brain network structure, VAT, estradiol levels, and cognitive function, we hypothesized (1) that VAT would be associated with an amplification of the negative association of age with brain network structure and cognitive health and (2) that estradiol would be associated with a reduction in the negative association of VAT with structural network covariance and cognitive performance in women.
Methods
Participants
Data from 1159 participants were taken from a German population-based cohort study, the Health Study of the Leipzig Research Centre for Civilization Diseases (LIFE). Participants provided written informed consent after all procedures were explained. The protocol and informed consent forms were approved by the research ethics board of the University of Leipzig. Study design and assessment information were described previously31 (eAppendix 1 in the Supplement). Excluded from analysis were 183 individuals owing to medication intake altering the central nervous system, immunosuppressive medication, previous stroke or other lesions, current diagnosed cancer or cancer treatment during the previous year, head tumors, epilepsy, multiple sclerosis, or Parkinson disease. One individual was excluded because of failure of the macro for fat tissue segmentation and another individual owing to missing anthropometric data needed for VAT normalization. In total, 974 participants were included in the final analyses, all of whom were confirmed to not have dementia during a neuropsychological assessment, including the Mini-Mental State Examination, by a trained study physician. Dates of the original cohort study were August 1, 2011, to November 23, 2014. Analyses were conducted from August 2017 to September 2018. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.32
Brain and Abdomen Imaging
Magnetic resonance imaging (MRI) data were acquired on a 3-T imaging system (Magnetom Verio; Siemens). It was equipped with a 32-channel head array coil and body coil for the transmit-receive coil of abdominal scans.
Abdominal Data Acquisition and Analysis
Magnetic resonance imaging was performed using an axial T1-weighted fast spin-echo technique with the following variables: echo time of 18 milliseconds per repetition time of 520 milliseconds, echo train length of 7; slice thickness of 5 mm, 5 mm between slices; scanning matrix of 320 × 306 pixels (no partial Fourier); and field of view of 500 mm ×375 mm, final voxel size of 1.6 mm ×1.6 mm ×5.0 mm, water saturation. Nine slices under and 10 slices above the umbilicus, diaphragm excluded, were segmented. The abdominal fat tissue segmentation was graphically evaluated using a macro to distinguish visceral or subcutaneous fat semiautomatically in ImageJ (https://imagej.nih.gov/ij/download/) by 4 raters (M.H., M.R., U.S., and a nonauthor) for accuracy. The results were inspected visually (slice by slice, identifying misclassified fat and nonfat voxels) and corrected for minor voxel misclassifications manually in 897 participants (eAppendix 2 in the Supplement).
Brain Data Acquisition and Analysis
We collected a 3-dimensional magnetization-prepared rapid acquisition with gradient echo (MPRAGE) sequence using the Alzheimer Disease Neuroimaging Initiative (ADNI) standard protocol with the following variables: inversion time of 900 milliseconds, repetition time of 2300 milliseconds, echo time of 2.98 milliseconds, flip angle of 9°, band width of 240 Hz per pixel, image matrix of 256 × 240 pixels, 176 partitions, field of view of 256 × 240 × 176 mm3, sagittal orientation, 1 average. Voxel size was 1 × 1 × 1 mm3, with no interpolation.33,34 We processed structural T1-weighted data with FSL-VBM (FSL, version 5.0.9), an optimized voxel-based morphometry protocol using FMRIB Software Library (FSL) tools. All structural images were brain extracted, gray matter segmented, and registered to the Montreal Neurological Institute (MNI) 152 standard space using nonlinear registration. A symmetric study-specific gray matter template was built from images of the study population. All native gray matter images were nonlinearly registered to this study-specific template and “modulated” to correct for local expansion or contraction due to the nonlinear component of the spatial transformation. Modulated images were smoothed with an isotropic gaussian kernel with a sigma of 4 mm (approximately 9.4-mm full width at half maximum). Before FSL-VBM processing, volumes were masked by the full brain–segmented volume output from FreeSurfer (FreeSurfer, version 5.3.0) to exclude nonbrain compartments. Brain structural information was derived from vertexwise cortical thickness, and surface area was calculated in FreeSurfer by an automated surface reconstruction scheme. We inspected all surface reconstructions for misplaced boundaries in FreeView (implemented in FreeSurfer) and manually corrected 142 cases. Cortical thickness and surface area maps were sampled from participant space to the common FsAverage template (163 842 vertices) and smoothed with a surface full width at half maximum of 10 mm. Linked independent component analysis was then applied to measures of gray matter volume, cortical thickness, and pial area (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FLICA), decomposing the data into 70 independent components. For each component, we calculated an individual course per participant, further indicated as network covariance,35,36 which reflects individual loading on the brain network.
Memory Testing
We used the 10-word verbal episodic memory test from the neuropsychological test battery of the Consortium to Establish a Registry for Alzheimer Disease (CERAD),37 from which we calculated a composite score per participant according to previous studies30,38,39,40 (eAppendix 3 in the Supplement). The composite score was from the CERAD verbal episodic memory test on learning (score range, 0-30), recall (score range, 0-10), and recognition (score range, 0-20). To assess performance independent of educational achievement, all memory performance scores used are unstandardized education residuals.
Hormone Measurement
Serum estradiol levels from fasting blood were assessed in a subsample of 390 participants (181 women). Estradiol level was measured by electrochemiluminescence immunoassay (ECLIA) (Cobas; Roche), with a sensitivity of 5.01 pg/mL and representative interassay coefficients of variation of 4.4% to 9.9% for the range of 85 to 110 pg/mL and 2.7% to 5.6% for the range of 501 to 555 pg/mL (to convert estradiol level to picomoles per liter, multiply by 3.671).
Statistical Analysis
Statistical analyses were performed using SPSS Statistics 24 (IBM) and R 3.3.2 (R Foundation). Visceral adipose tissue values were height standardized and log-transformed. Estradiol levels were log-transformed. We performed a linear regression with memory network covariance as the independent variable and memory performance as the dependent variable, controlling for age. To investigate VAT accumulation rates, we generated regression models for women and men separately using age as the independent variable and VAT as the dependent variable for each 1-year age bin. To assess best fit, we compared R2 values of linear, quadratic, and polynomial fit of third degree. To examine sex interactions in the association between VAT and memory network covariance, we performed a multiple linear regression with VAT as the independent variable and network covariance as the dependent variable. To assess the association between estradiol level and this network independent of age, we performed a linear regression with estradiol level as the independent variable and network covariance as the dependent variable using unstandardized age residuals of network covariance.
Moderation analyses were conducted separately per sex with the PROCESS macro (SAS Institute), a modeling program using an ordinary least squares–based path analytical framework to test for direct and indirect associations.41 We tested regression pathways in a moderation model (first model) (PROCESS, version 3.0) (eFigure 1 in the Supplement). Variables were mean centered before analyses. A 95% bias-corrected bootstrap CI (BBCI), excluding zero and based on 10 000 bootstrap samples, was considered to be a robust result.41 In the first model, we assessed significance and stability of the interaction of VAT and age in association with network covariance by defining age as the independent variable, network covariance as the outcome variable, and VAT as the moderator variable. In the second model, we defined VAT as the independent variable, memory network covariance as the outcome variable, and estradiol as the moderator variable.
Finally, we assessed a subgroup of 82 women in midlife (age range, 35-55 years, when menopause transition occurs). In these women, we performed an estradiol-level median split and compared the mean differences in memory network covariance and memory performance using independent-samples t tests. All testing was 2 sided, and P < .05 was considered statistically significant.
Results
In total, 974 participants were included in analyses (Table). The female sample (n = 473) had a mean (SD) age of 50.10 (15.63) years (age range, 20-78 years), and the male sample (n = 501) had a mean (SD) age of 51.24 (15.67) years (age range, 19-79) years.
Table. Study Population Demographic Characteristics.
| Variable | All | Women | Men | P Value |
|---|---|---|---|---|
| Age, y | ||||
| No. of participants | 974 | 473 | 501 | .26 |
| Mean (SD) | 50.69 (15.65) | 50.10 (15.63) | 51.24 (15.67) | |
| BMI | ||||
| No. of participants | 974 | 473 | 501 | <.001 |
| Mean (SD) | 26.47 (4.62) | 25.87 (5.13) | 27.04 (4.00) | |
| VAT, cm3 | ||||
| No. of participants | 974 | 473 | 501 | <.001 |
| Mean (SD) | 2188.40 (1462.27) | 1569.93 (1059.40) | 2772.30 (1548.57) | |
| Estradiol, pg/mL | ||||
| No. of participants | 390 | 181 | 209 | <.001 |
| Mean (SD) | 40.76 (73.68) | 61.84 (103.89) | 22.50 (9.61) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); VAT, visceral adipose fat tissue.
SI conversion factor: To convert estradiol level to picomoles per liter, multiply by 3.671.
Structural Brain Network Covariance and Memory Performance
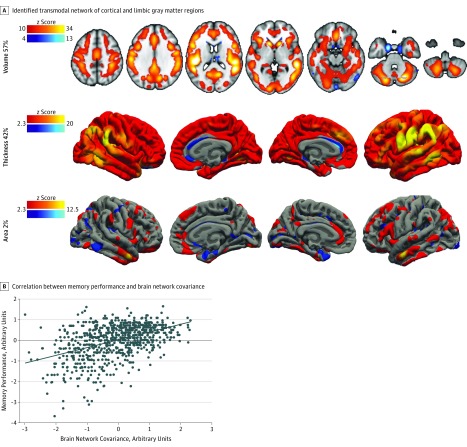
We replicated a structural network linked to memory performance and cognitive decline29 (Figure 1A, eAppendix 4 in the Supplement for network reproducibility analyses, and eFigure 2 in the Supplement for other networks). The network explains 17% of total variance in the imaging data, revealing a transmodal network of cortical and limbic gray matter regions. The network is composed of 57% gray matter volume (explaining 20% of total gray matter volume variance), 42% cortical thickness (explaining 29% of total cortical thickness variance), and 2% pial area (explaining 1% of total pial area variance). Higher individual structural covariance was associated with better memory performance (adjusted R2 = 0.21; β = 0.46; P < .001) (Figure 1B). Data indicate an expected ceiling effect for memory performance in a healthy population.42
Figure 1. Brain Network Covariance and Memory Performance.
Color bars indicate z-scored positive (red/yellow) or negative (blue/light-blue) covariations within the network.
Sex Differences in VAT Volume Across Age
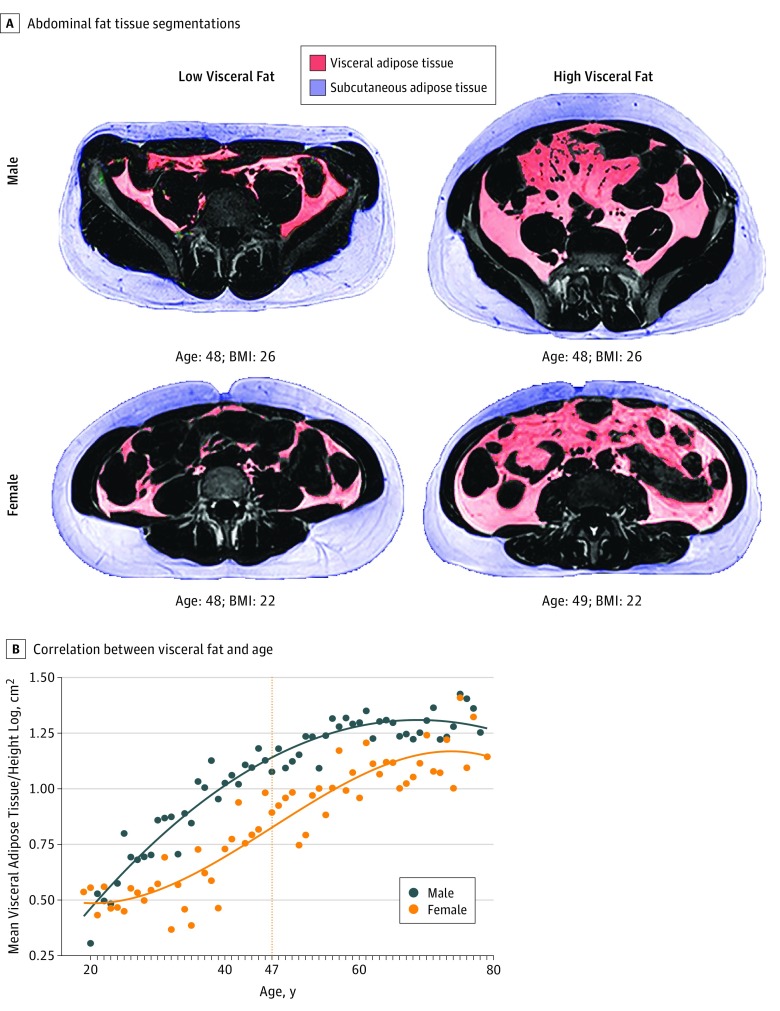
We obtained VAT volume information by segmenting visceral from subcutaneous fat in abdominal MRI (Figure 2A). Men and women displayed different trends in VAT accumulation. For men, a quadratic model revealed the best fit (adjusted R2 = 0.93; P < .001) compared with the linear model (F1,58 = 101.56; P < .001) (Figure 2B). The quadratic function showed a concave progression with a steady slope decrease. For women, a polynomial model of third degree showed the best fit (adjusted R2 = 0.85; P < .001) compared with the linear model (F1,59 = 4.25; P = .02) and quadratic model (F2,58 = 7.74; P = .007). The cubic curve progression began convex and became concave at the inflection point of 47 years. Therefore, men in this sample had the highest VAT to age ratio at an earlier age, and this decreased with age; in contrast, women had the highest VAT to age ratio during midlife.
Figure 2. Sex Differences in the Association Between Visceral Adipose Tissue and Age.
A, Representative visceral adipose tissue segmentations in 2 women and 2 men. Participants of the same sex, age, and body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) can have vastly different fat distribution profiles. B, Nonlinear correlation between visceral adipose tissue and age in women and men. For women, the dashed line shows the inflection point.
Sex Differences in VAT and Memory Network Association
We observed an interaction of VAT and sex on memory network covariance (F3 = 163.37; adjusted R2 = 0.33; P = .02): men showed a stronger negative association between VAT and network covariance (adjusted R2 = 0.33; β = −0.57; P < .001) than women (adjusted R2 = 0.29; β = −0.54; P < .001) (Figure 3A). We conducted a moderation analysis in both sexes (Figure 3B) and found that overall models for men (F3,497 = 474.43; R2 = 0.74; P < .001) and women (F3,469 = 322.31; R2 = 0.67; P < .001) were significant. The interaction of VAT and age was significant for women (interaction term β = −0.02; t469 = 4.07; 95% BBCI, −0.03 to −0.01; P = .001) and men (interaction term β = −0.02; t497 = −4.83; 95% BBCI, −0.03 to −0.01; P < .001), suggesting that VAT is a moderator of the association between age and network covariance in both sexes and is associated with an exacerbation of the negative association of aging with memory network covariance.
Figure 3. Visceral Adipose Tissue (VAT) and Structural Network Covariance.
A, The plot shows simple slopes of VAT and structural network covariance for each sex, which differ significantly. The VAT values were height standardized and log-transformed. B, Moderation analysis of interaction of VAT and age on network covariance in both sexes.
Sex Hormones and Memory Network Covariance
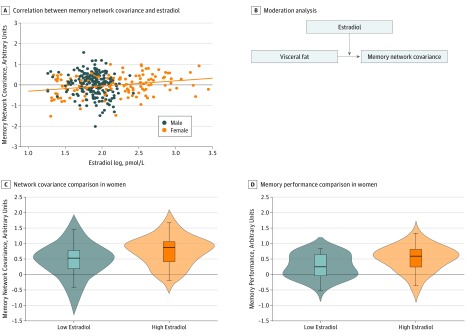
Linear regression analysis revealed a significant positive association between estradiol levels and memory network covariance in women after adjustment for age (adjusted R2 = 0.07; P = .002) (Figure 4A). In the next set of moderation analyses (Figure 4B), we again found that overall models were significant for both men (F3,205 = 30.13; R2 = 0.31; P < .001) and women (F3,177 = 19.60; R2 = 0.25; P < .001). However, the interaction of VAT and estradiol level was significant only for women (interaction term β = 0.63; 95% BBCI, 0.14-1.12; t177 = 2.52; P = .01), suggesting that estradiol is a moderator of the association between VAT and network covariance in women and is associated with a mitigation of the negative association of VAT with memory network covariance.
Figure 4. Estradiol and Structural Network Covariance in Women Only.
A, The plot shows a simple slope of estradiol and structural network covariance. B, Moderation analysis of interaction of visceral adipose tissue and estradiol on network covariance. C and D, The inner box plot shows the median (centers) and interquartile range (borders), with whiskers extending 1.5 times the interquartile range. The width of the shaded area shows the proportion of data located there. To convert estradiol level to picograms per milliliter, divide by 3.671.
Finally, in the female subgroup (age range, 35-55 years), the low estradiol and high estradiol groups did not differ by age or VAT. However, low estradiol level was associated with lower memory network covariance (Cohen d = 0.61; t80 = 2.76; P = .007) (Figure 4C) and worse memory performance (Cohen d = 0.63; t76 = 2.76; P = .007) (Figure 4D).
Discussion
To our knowledge, this is the first large, population-based study to investigate associations between structural patterns of brain aging, VAT as a metabolic risk factor for structural brain atrophy, and estradiol levels in adults of a broad age range. Our primary finding is that, while VAT was associated with increased risk for compromised brain network structure and cognitive impairment in both men and women, estradiol level was associated with reducing the negative consequences of VAT in women. Specifically, men had the highest VAT to age ratio at an earlier age; in women during midlife, VAT was associated with accelerated cognitive aging, and estradiol may protect the female brain against these structural patterns of atrophy, particularly during midlife. Our results have important clinical implications for developing sex-specific strategies to support healthy cognitive aging.
To effectively preserve cognitive abilities throughout life, it is imperative to consider sex-specific risk trajectories and identify biological mechanisms that may degrade or protect relevant brain network integrity. Integrating MRI-based VAT in large cross-sectional neuroimaging studies on cognitive function represents a novel approach to identify such a mechanism. We specifically included VAT as the main adiposity measure given the well-established role of VAT in conferring metabolic and inflammatory risk.3,4,5,13 Assessing VAT volume by MRI rather than with more conventional and easily accessible anthropometric proxies, such as body mass index or waist to hip ratio, is even more critical given the findings indicating VAT as a unique risk factor for neurodegenerative processes.10,43,44,45 Our analysis revealed substantial sex differences not only in VAT accumulation but also in the association between VAT and brain health: while both sexes showed a negative association of VAT with memory network covariance, this association was stronger in men.
When studying a brain network implicated in cognitive decline, it is difficult to control for changes accompanying natural aging. To disentangle the associations of biological aging from the association of VAT with memory network covariance, we tested VAT as a moderator variable in the association between age and the memory network. Our analysis revealed a significant interaction of VAT and age on network covariance, suggesting that VAT is associated with accelerated brain aging. Our findings thus substantially extend prior research showing an association of VAT with brain tissue damage independent of age associations.46 While those authors applied age correction in their analysis, the study was conducted in elderly individuals (mean age, 65 years); therefore, the possibility of age as a confounder for the sample could not be excluded.
Although multiple mechanisms may have contributed to the observed sex differences in the association between VAT and network covariance herein, differences in ovarian hormone states across the life span, particularly estrogen fluctuations, have been shown to influence cognitive aging processes.47,48 Thus, we investigated the role of estradiol and identified a positive association between estradiol and network covariance in women after age correction. Although the effect size appears small, small associations can suggest strong support for a given phenomenon, particularly if they have substantial cumulative consequences.49 Our findings indicate that ovarian aging goes beyond biological age-related brain changes and adds an additional layer to this process. This is important because of the prominent sexual dimorphism in neuropsychiatric disorder rates. A detailed understanding of these processes may provide critical insight into how to address the higher rates of depression50 and dementia51,52 in women. A subsequent moderation analysis revealed a significant interaction of VAT and estradiol on network covariance in women, suggesting that estradiol is a significant moderator of the VAT-brain association in women. This association could be accounted for by several mechanisms. First, estradiol has anti-inflammatory properties, and systemic inflammation has been shown to occur in response to estradiol depletion in women (eg, after ovariectomy,53 natural menopause,54 or treatment with the antiestrogenic drug tamoxifen55). Second, VAT increases HPA axis dysregulation, which could harm the brain, and estradiol strengthens robustness of HPA activity. Third, estradiol has vasodilatory, antiapoptotic, and antioxidative effects that could have a neuroprotective role and help preserve myelin architecture.24,25,26 The present study cannot identify what the mechanism is behind how VAT damages structural networks or how estradiol provides a potential protective association. However, our study results argue for longitudinal studies to assess changes in ovarian hormones, VAT accumulation, cognitive performance, and structural brain networks in women during perimenopause to provide insight into a transition period that may serve as the tipping point into neurodegenerative disease. Moreover, given growing evidence that fluctuations in ovarian hormones and their derived neurosteroids induce HPA axis dysregulation, thereby driving vulnerability to psychosocial stress and neuropsychiatric disease,20 a future study could benefit from including markers of HPA axis function in conjunction with psychosocial stress evaluation.
Finally, we investigated the association of estradiol with cognitive health in women during midlife (age range, 35-55 years) because this is when we observed an inflection point in the curve for VAT ratios in women in our cross-sectional sample. It is also the age range when women typically experience rapid estradiol fluctuations and significant estradiol depletion during perimenopause.28 We found less healthy patterns of memory network covariance and weaker memory performance in women with lower estradiol levels compared with women with higher estradiol levels during the perimenopausal age range. Because these women were VAT- and age-matched, our findings suggest that associations of ovarian aging with body and brain may extend beyond changes in body composition and brain network structure typically observed during biological aging.56,57 This could explain why VAT had less of a negative association with network covariance in women than in men.
Limitations
It is important to acknowledge the methodological limitations of data-driven neuroimaging analysis, particularly regarding the interpretation of structural brain covariance changes in terms of cellular mechanisms.29,58 Specifically, structural MRI is limited to probing information on a macroscopic scale, and extraction of morphological features of interest from MRI remains imperfect, thus making it difficult to disentangle the contributing cellular mechanisms. Despite these constraints, experts29,58 still agree that this methodological approach represents a powerful tool to obtain unique insights into human brain organization. By combining this approach with cutting-edge abdominal adiposity imaging and assessment of sex hormone levels—and ultimately relating these multimodal measures to memory performance in a large sample—we provide a biologically relevant view of the consequences of VAT on brain network structure and cognitive abilities. Furthermore, not all measures herein (estradiol levels and educational achievement) were available for all enrolled participants owing to technical reasons; therefore, some analyses reported could only be conducted in subgroups. We also acknowledge that the CERAD verbal episodic memory test shows ceiling associations in young healthy participants, but the observation that the identified brain network in our sample is associated with memory performance and cognitive decline has also been demonstrated with other cognitive tests.29 In addition, brain aging is a multifaceted process; while we applied strict screening criteria to exclude neurodegenerative or cerebrovascular disease and controlled for age and educational achievement, we cannot exclude that other variables could contribute to this process. Inherent to the cross-sectional design, no causal association can be inferred because all reported associations are correlational; therefore, future studies applying within-subject modeling are needed.
Conclusions
Given the dramatic increase in human life expectancy over the past century, age-related cognitive decline is rapidly becoming one of the biggest health challenges we face.59,60,61 Herein, we identified sex-specific risk trajectories of brain and cognitive aging. We provide evidence for a detrimental association of VAT with structural brain networks important for memory and a potential protective association of estradiol with cognitive health in women through maintaining gray matter network integrity. These data underscore the need to consider adipose tissue and hormonal profiles during primary care visits in midlife to support healthy brain aging and maintain cognitive abilities in later life. Our findings highlight the perimenopausal transition as a window of opportunity to prevent accelerated brain aging and neurodegenerative disease development in women.
eAppendix 1. Participant Recruitment
eAppendix 2. Abdomen: Data Acquisition and Analysis
eAppendix 3. Memory Testing
eAppendix 4. Reproducibility of Structural Network From Linked-Independent Component Analysis
eFigure 1. Conceptual and Statistical Diagram for Moderation Analyses
eFigure 2. Spatial Maps of Brain Networks
eReferences.
References
- 1.Raji CA, Ho AJ, Parikshak NN, et al. . Brain structure and obesity. Hum Brain Mapp. 2010;31(3):-. doi: 10.1002/hbm.20870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization Obesity and overweight. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Published February 16, 2018. Accessed September 20, 2018.
- 3.Mathieu P, Pibarot P, Larose E, Poirier P, Marette A, Després JP. Visceral obesity and the heart. Int J Biochem Cell Biol. 2008;40(5):821-836. doi: 10.1016/j.biocel.2007.12.001 [DOI] [PubMed] [Google Scholar]
- 4.Britton KA, Massaro JM, Murabito JM, Kreger BE, Hoffmann U, Fox CS. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. J Am Coll Cardiol. 2013;62(10):921-925. doi: 10.1016/j.jacc.2013.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carr DB, Utzschneider KM, Hull RL, et al. . Intra-abdominal fat is a major determinant of the National Cholesterol Education Program Adult Treatment Panel III criteria for the metabolic syndrome. Diabetes. 2004;53(8):2087-2094. doi: 10.2337/diabetes.53.8.2087 [DOI] [PubMed] [Google Scholar]
- 6.Isaac V, Sim S, Zheng H, Zagorodnov V, Tai ES, Chee M. Adverse associations between visceral adiposity, brain structure, and cognitive performance in healthy elderly. Front Aging Neurosci. 2011;3:12. doi: 10.3389/fnagi.2011.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartz DH, Leonard G, Perron M, et al. . Visceral fat is associated with lower executive functioning in adolescents. Int J Obes (Lond). 2013;37(10):1336-1343. doi: 10.1038/ijo.2013.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Debette S, Beiser A, Hoffmann U, et al. . Visceral fat is associated with lower brain volume in healthy middle-aged adults. Ann Neurol. 2010;68(2):136-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008;71(14):1057-1064. doi: 10.1212/01.wnl.0000306313.89165.ef [DOI] [PubMed] [Google Scholar]
- 10.Cereda E, Sansone V, Meola G, Malavazos AE. Increased visceral adipose tissue rather than BMI as a risk factor for dementia. Age Ageing. 2007;36(5):488-491. doi: 10.1093/ageing/afm096 [DOI] [PubMed] [Google Scholar]
- 11.Everson-Rose SA, Lewis TT, Karavolos K, Dugan SA, Wesley D, Powell LH. Depressive symptoms and increased visceral fat in middle-aged women. 2009;71(4):410-416. doi: 10.1097/PSY.0b013e3181a20c9c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murabito JM, Massaro JM, Clifford B, Hoffmann U, Fox CS. Depressive symptoms are associated with visceral adiposity in a community-based sample of middle-aged women and men. Obesity (Silver Spring). 2013;21(8):1713-1719. doi: 10.1002/oby.20130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pou KM, Massaro JM, Hoffmann U, et al. . Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116(11):1234-1241. doi: 10.1161/CIRCULATIONAHA.107.710509 [DOI] [PubMed] [Google Scholar]
- 14.Vohl MC, Sladek R, Robitaille J, et al. . A survey of genes differentially expressed in subcutaneous and visceral adipose tissue in men. Obes Res. 2004;12(8):1217-1222. doi: 10.1038/oby.2004.153 [DOI] [PubMed] [Google Scholar]
- 15.Choi J, Joseph L, Pilote L. Obesity and C-reactive protein in various populations: a systematic review and meta-analysis. Obes Rev. 2013;14(3):232-244. doi: 10.1111/obr.12003 [DOI] [PubMed] [Google Scholar]
- 16.Bidulescu A, Liu J, Hickson DA, et al. . Gender differences in the association of visceral and subcutaneous adiposity with adiponectin in African Americans: the Jackson Heart Study. BMC Cardiovasc Disord. 2013;13(1):9. doi: 10.1186/1471-2261-13-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrew R, Westerbacka J, Wahren J, Yki-Järvinen H, Walker BR. The contribution of visceral adipose tissue to splanchnic cortisol production in healthy humans. Diabetes. 2005;54(5):1364-1370. doi: 10.2337/diabetes.54.5.1364 [DOI] [PubMed] [Google Scholar]
- 18.Epel ES, McEwen B, Seeman T, et al. . Stress and body shape: stress-induced cortisol secretion is consistently greater among women with central fat. Psychosom Med. 2000;62(5):623-632. doi: 10.1097/00006842-200009000-00005 [DOI] [PubMed] [Google Scholar]
- 19.Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28(5):521-574. doi: 10.1210/er.2007-0001 [DOI] [PubMed] [Google Scholar]
- 20.Gordon JL, Girdler SS, Meltzer-Brody SE, et al. . Ovarian hormone fluctuation, neurosteroids, and HPA axis dysregulation in perimenopausal depression: a novel heuristic model. Am J Psychiatry. 2015;172(3):227-236. doi: 10.1176/appi.ajp.2014.14070918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tchernof A, Calles-Escandon J, Sites CK, Poehlman ET. Menopause, central body fatness, and insulin resistance: effects of hormone-replacement therapy. Coron Artery Dis. 1998;9(8):503-511. doi: 10.1097/00019501-199809080-00006 [DOI] [PubMed] [Google Scholar]
- 22.Salpeter SR, Cheng J, Thabane L, Buckley NS, Salpeter EE. Bayesian meta-analysis of hormone therapy and mortality in younger postmenopausal women. Am J Med. 2009;122(11):1016-1022.e1. doi: 10.1016/j.amjmed.2009.05.021 [DOI] [PubMed] [Google Scholar]
- 23.Shea KL, Gavin KM, Melanson EL, et al. . Body composition and bone mineral density after ovarian hormone suppression with or without estradiol treatment. Menopause. 2015;22(10):1045-1052. doi: 10.1097/GME.0000000000000430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linford N, Wade C, Dorsa D. The rapid effects of estrogen are implicated in estrogen-mediated neuroprotection. J Neurocytol. 2000;29(5-6):367-374. doi: 10.1023/A:1007113323582 [DOI] [PubMed] [Google Scholar]
- 25.Perillo B, Sasso A, Abbondanza C, Palumbo G. 17β-Estradiol inhibits apoptosis in MCF-7 cells, inducing bcl-2 expression via two estrogen-responsive elements present in the coding sequence. Mol Cell Biol. 2000;20(8):2890-2901. doi: 10.1128/MCB.20.8.2890-2901.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Urata Y, Ihara Y, Murata H, et al. . 17β-Estradiol protects against oxidative stress–induced cell death through the glutathione/glutaredoxin-dependent redox regulation of Akt in myocardiac H9c2 cells. J Biol Chem. 2006;281(19):13092-13102. doi: 10.1074/jbc.M601984200 [DOI] [PubMed] [Google Scholar]
- 27.Azad NA, Al Bugami M, Loy-English I. Gender differences in dementia risk factors. Gend Med. 2007;4(2):120-129. doi: 10.1016/S1550-8579(07)80026-X [DOI] [PubMed] [Google Scholar]
- 28.Brinton RD, Yao J, Yin F, Mack WJ, Cadenas E. Perimenopause as a neurological transition state. Nat Rev Endocrinol. 2015;11(7):393-405. doi: 10.1038/nrendo.2015.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Douaud G, Groves AR, Tamnes CK, et al. . A common brain network links development, aging, and vulnerability to disease. Proc Natl Acad Sci U S A. 2014;111(49):17648-17653. doi: 10.1073/pnas.1410378111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kharabian Masouleh S, Arélin K, Horstmann A, et al. . Higher body mass index in older adults is associated with lower gray matter volume: implications for memory performance. Neurobiol Aging. 2016;40:1-10. doi: 10.1016/j.neurobiolaging.2015.12.020 [DOI] [PubMed] [Google Scholar]
- 31.Loeffler M, Engel C, Ahnert P, et al. . The LIFE-Adult-Study: objectives and design of a population-based cohort study with 10,000 deeply phenotyped adults in Germany. BMC Public Health. 2015;15(1):691. doi: 10.1186/s12889-015-1983-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies [published correction appears in Ann Intern Med. 2008;148(2):168]. Ann Intern Med. 2007;147(8):573-577. doi: 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 33.Jack CR Jr, Bernstein MA, Fox NC, et al. . The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27(4):685-691. doi: 10.1002/jmri.21049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jack CR Jr, Bernstein MA, Borowski BJ, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Update on the magnetic resonance imaging core of the Alzheimer’s Disease Neuroimaging Initiative. Alzheimers Dement. 2010;6(3):212-220. doi: 10.1016/j.jalz.2010.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Groves AR, Beckmann CF, Smith SM, Woolrich MW. Linked independent component analysis for multimodal data fusion. Neuroimage. 2011;54(3):2198-2217. doi: 10.1016/j.neuroimage.2010.09.073 [DOI] [PubMed] [Google Scholar]
- 36.Groves AR, Smith SM, Fjell AM, et al. . Benefits of multi-modal fusion analysis on a large-scale dataset: life-span patterns of inter-subject variability in cortical morphometry and white matter microstructure. Neuroimage. 2012;63(1):365-380. doi: 10.1016/j.neuroimage.2012.06.038 [DOI] [PubMed] [Google Scholar]
- 37.Morris JC, Heyman A, Mohs RC, et al. . The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD), I: clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39(9):1159-1165. doi: 10.1212/WNL.39.9.1159 [DOI] [PubMed] [Google Scholar]
- 38.van de Rest O, Geleijnse JM, Kok FJ, et al. . Effect of fish oil on cognitive performance in older subjects: a randomized, controlled trial. Neurology. 2008;71(6):430-438. doi: 10.1212/01.wnl.0000324268.45138.86 [DOI] [PubMed] [Google Scholar]
- 39.Witte AV, Kerti L, Hermannstädter HM, et al. . Long-chain omega-3 fatty acids improve brain function and structure in older adults. Cereb Cortex. 2014;24(11):3059-3068. doi: 10.1093/cercor/bht163 [DOI] [PubMed] [Google Scholar]
- 40.Zhang R, Beyer F, Lampe L, et al. . White matter microstructural variability mediates the relation between obesity and cognition in healthy adults. Neuroimage. 2018;172:239-249. doi: 10.1016/j.neuroimage.2018.01.028 [DOI] [PubMed] [Google Scholar]
- 41.Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. 2nd ed New York, NY: Guilford Publications; 2017. [Google Scholar]
- 42.Luck T, Pabst A, Rodriguez FS, et al. . Age-, sex-, and education-specific norms for an extended CERAD Neuropsychological Assessment Battery: results from the population-based LIFE-Adult-Study. Neuropsychology. 2018;32(4):461-475. doi: 10.1037/neu0000440 [DOI] [PubMed] [Google Scholar]
- 43.Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: the Rotterdam Study. Neurology. 1999;53(9):1937-1942. doi: 10.1212/WNL.53.9.1937 [DOI] [PubMed] [Google Scholar]
- 44.Solomon A, Kivipelto M, Wolozin B, Zhou J, Whitmer RA. Midlife serum cholesterol and increased risk of Alzheimer’s and vascular dementia three decades later. Dement Geriatr Cogn Disord. 2009;28(1):75-80. doi: 10.1159/000231980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kivipelto M, Helkala EL, Laakso MP, et al. . Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. BMJ. 2001;322(7300):1447-1451. doi: 10.1136/bmj.322.7300.1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Widya RL, Kroft LJ, Altmann-Schneider I, et al. ; Leiden Longevity Study Group . Visceral adipose tissue is associated with microstructural brain tissue damage. Obesity (Silver Spring). 2015;23(5):1092-1096. doi: 10.1002/oby.21048 [DOI] [PubMed] [Google Scholar]
- 47.Lamberts SW, van den Beld AW, van der Lely AJ. The endocrinology of aging. Science. 1997;278(5337):419-424. doi: 10.1126/science.278.5337.419 [DOI] [PubMed] [Google Scholar]
- 48.McCarthy MM. Estradiol and the developing brain. Physiol Rev. 2008;88(1):91-124. doi: 10.1152/physrev.00010.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matz SC, Gladstone JJ, Stillwell D. In a world of big data, small effects can still matter: a reply to Boyce, Daly, Hounkpatin, and Wood (2017). Psychol Sci. 2017;28(4):547-550. doi: 10.1177/0956797617697445 [DOI] [PubMed] [Google Scholar]
- 50.Bromet E, Andrade LH, Hwang I, et al. . Cross-national epidemiology of DSM-IV major depressive episode. BMC Med. 2011;9(1):90. doi: 10.1186/1741-7015-9-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prince M, Knapp M, Guerchet M, et al. . Dementia UK: Second Edition: Overview. London, England: Alzheimer’s Society; September 2014. [Google Scholar]
- 52.Ruitenberg A, Ott A, van Swieten JC, Hofman A, Breteler MM. Incidence of dementia: does gender make a difference? Neurobiol Aging. 2001;22(4):575-580. doi: 10.1016/S0197-4580(01)00231-7 [DOI] [PubMed] [Google Scholar]
- 53.Abu-Taha M, Rius C, Hermenegildo C, et al. . Menopause and ovariectomy cause a low grade of systemic inflammation that may be prevented by chronic treatment with low doses of estrogen or losartan. J Immunol. 2009;183(2):1393-1402. doi: 10.4049/jimmunol.0803157 [DOI] [PubMed] [Google Scholar]
- 54.Cioffi M, Esposito K, Vietri MT, et al. . Cytokine pattern in postmenopause. Maturitas. 2002;41(3):187-192. doi: 10.1016/S0378-5122(01)00286-9 [DOI] [PubMed] [Google Scholar]
- 55.Nissen MJ, Shapiro A, Swenson KK. Changes in weight and body composition in women receiving chemotherapy for breast cancer. Clin Breast Cancer. 2011;11(1):52-60. doi: 10.3816/CBC.2011.n.009 [DOI] [PubMed] [Google Scholar]
- 56.Raz N, Lindenberger U, Rodrigue KM, et al. . Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15(11):1676-1689. doi: 10.1093/cercor/bhi044 [DOI] [PubMed] [Google Scholar]
- 57.Fjell AM, Walhovd KB. Structural brain changes in aging: courses, causes and cognitive consequences. Rev Neurosci. 2010;21(3):187-221. doi: 10.1515/REVNEURO.2010.21.3.187 [DOI] [PubMed] [Google Scholar]
- 58.Alexander-Bloch A, Giedd JN, Bullmore E. Imaging structural co-variance between human brain regions. Nat Rev Neurosci. 2013;14(5):322-336. doi: 10.1038/nrn3465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xie J, Matthews FE, Jagger C, Bond J, Brayne C. The oldest old in England and Wales: a descriptive analysis based on the MRC Cognitive Function and Ageing Study. Age Ageing. 2008;37(4):396-402. doi: 10.1093/ageing/afn061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brayne C. The elephant in the room: healthy brains in later life, epidemiology and public health. Nat Rev Neurosci. 2007;8(3):233-239. doi: 10.1038/nrn2091 [DOI] [PubMed] [Google Scholar]
- 61.Oeppen J, Vaupel JW. Demography: broken limits to life expectancy. Science. 2002;296(5570):1029-1031. doi: 10.1126/science.1069675 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Participant Recruitment
eAppendix 2. Abdomen: Data Acquisition and Analysis
eAppendix 3. Memory Testing
eAppendix 4. Reproducibility of Structural Network From Linked-Independent Component Analysis
eFigure 1. Conceptual and Statistical Diagram for Moderation Analyses
eFigure 2. Spatial Maps of Brain Networks
eReferences.