Abstract
Background
In October 2014, the US Food and Drug Administration released a draft guidance for the development of drugs for the acute treatment of migraine. This guidance offered the option of replacing the previously required 4 co‐primary endpoints: pain freedom, freedom from nausea, freedom from photophobia, and freedom from phonophobia, all at 2 hours posttreatment, with 2 co‐primary endpoints: pain freedom and freedom from most bothersome symptom (MBS) other than pain, both at 2 hours posttreatment. At the time the new draft guidance was released, no large clinical trials had been undertaken with these 2 co‐primary endpoints, posing a challenge in determining the sample size that might be required to achieve statistical significance. As a number of trials have now been completed, we conducted a review of the observed placebo responses, drug effect sizes, and sample sizes to better inform the design of future trials.
Methods
We searched PubMed, Embase, Web of Science, and the Cochrane library for primary publications of phase 3 randomized, placebo‐controlled, double‐blind acute migraine treatment trials that used pain freedom and MBS freedom as primary or planned secondary endpoints. For each endpoint, placebo response rates were determined and used to generate estimates of sample size, assuming differences between placebo and active treatment groups of 10%, 15%, and 20%. Sample size calculations were based on 80% power using a 2‐group continuity corrected chi‐square test with a 5% 2‐sided significance level.
Results
We identified abstracts or full‐length papers describing results of 8 clinical trials employing the new co‐primary endpoints. The mean placebo response rate for 2‐hour pain freedom was 16.75% (range 11.8‐21.3%) and treatment effect (difference in response rates between active and placebo groups) ranged from 5.0% to 27.2%. For 2‐hour MBS freedom, the mean placebo response rate was 32.8% (range 25.2‐48.1%), and the range of treatment effect was 8.9% to 25.4%. Based on a placebo response rate of 17% for pain freedom, the sample sizes that would have been required to achieve statistical significance were n = 269, n = 128, and n = 77, for treatment effect sizes of 10%, 15%, and 20%, respectively. For MBS, assuming a placebo response rate of 33%, the corresponding required sample sizes would have been n = 389, n = 181, and n = 105.
Conclusions
The observed range of placebo response and treatment effect sizes suggests that use of the newly recommended 2 co‐primary endpoints could reduce the sample sizes required to achieve significance compared with past trials using 4 primary endpoints (in which mean and median group sizes for recent trials were 375 and 362, respectively). However, the initial trials using the newly recommended co‐primary endpoints tended to treat more participants than would have been minimally required. We anticipate that with the growing body of information regarding the use of these new endpoints, samples sizes may be more aligned with treatment efficacy, enabling faster and more cost‐effective trials for acute migraine treatment.
Keywords: migraine, headache, most bothersome symptom, trial endpoints
Introduction
Estimating or calculating sample size is a crucial early step in planning a clinical trial. Inappropriate sample size can lead to overpowering a trial and showing a statistical difference that may be of no clinical relevance, or underpowering the trial and inappropriately failing to reject the null hypothesis. In placebo‐controlled trials where the primary outcome measure is a binary endpoint, it is essential to have a reliable estimate of the effect size, which is determined by the efficacy of the intervention and the rate of placebo response. For novel endpoints, there may limited or no literature on rates of placebo response to aid in the determination of sample size.
Such was the case in 2014 when the US Food and Drug Administration (FDA) released draft guidance for the development of drugs for the acute treatment of migraine. Prior to this guidance, clinical trials were required to reach statistical significance when compared to placebo on 4 co‐primary endpoints: pain freedom (or relief), freedom from nausea, freedom from photophobia, and freedom from phonophobia, all at 2 hours posttreatment. While this approach is still considered acceptable, a new and preferable approach was offered that “aims to better align the study outcome with the symptom(s) of primary importance to patients.” This guidance, which subsequently became final in February 2018,1 suggests that trials be designed with 2 co‐primary outcome measures, freedom from pain, and freedom from most bothersome symptom (MBS) other than pain, both assessed at 2 hours postadministration of study drug.
To determine MBS, subjects may choose from nausea/vomiting, photophobia, or phonophobia. Selection of MBS may take place either at a baseline visit or at the time of treatment. In the former case, MBS is based on recall and is fixed for the course of the trial. Conversely, election immediately prior to dosing ensures that the selected MBS specifically reflects the treated migraine attack but may not reflect the participant’s historical MBS. Both approaches have merits and have been discussed elsewhere.2
In recent trials using 4 co‐primary endpoints, the mean and median group sizes were 375 and 362, respectively,3, 4, 5 but prior to the new FDA guidance, no large clinical trials had been conducted using 2 co‐primary endpoints. Investigators and sponsors conducting trials subsequent to the guidance were thus faced with a challenge in estimating sample size for appropriate power. Several trials with the new endpoints have been concluded, and the results should inform the future refinement of trial design. Here we review the literature to November 2018 regarding clinical trials that used MBS as an endpoint to assist in estimating appropriate sample size for use in future treatment of acute migraine trial designs.
Methods
We searched titles and abstracts in PubMed, Embase, Web of Science, and the Cochrane library for conference abstracts and publications describing results from clinical trials using the newly recommended endpoints for acute migraine treatment.1 The search terms used were (“most bothersome symptom” OR “most bothersome migraine‐associated symptom” OR “MBS”) AND “acute” AND “migraine.”
We included primary publications (conference abstracts or full‐length articles) of phase 3 randomized, placebo‐controlled, double‐blind trials that used pain freedom and MBS freedom, both at 2 hours posttreatment, as primary or planned secondary endpoints. Trials were required to be interventional and had a design in which a single migraine attack that had progressed to moderate or severe was treated. No language or time restrictions were used.
After removal of duplicate or redundant articles and abstracts, 2 independent authors extracted pertinent information regarding trial design and treatment effects. In certain cases, when a conference abstract did not provide complete information regarding trial design, information from Clinicaltrials.gov and other publicly available sources was used to ascertain this information.
For each endpoint, the average and the range of placebo response rates were determined and used to generate estimates of sample size assuming differences between placebo and active treatment groups of 10%, 15%, and 20%. Sample size calculations were based on 80% power using a 2‐group continuity corrected chi‐square test with a 5% 2‐sided significance level. Analyses were performed using Statistical Analysis System (SAS) software version 9.4 (SAS Institute, Cary, NC, USA).
Results
Database searches occurred on November 11 and 12, 2018. Thirty‐one records were identified. Among these, 14 were either duplicates or were abstracts presenting the same results at multiple different conferences. Of the remaining 17 records, 9 were excluded for the following reasons: Phase 2 studies (n = 2), noninterventional trial (n = 2), open‐label study (n = 2), post hoc analysis (n = 1), subanalysis (n = 1), and presented aggregate results (n = 1).
Characteristics of the trials described in the remaining 8 studies are presented in Table 1. Trials differed in size and design, with several trials investigating multiple drug doses. Notably, in trials investigating ubrogepant and sumatriptan injection, MBS was identified at the time of treatment,6, 7, 8 whereas in the trial investigating ADAM zolmitriptan, patients prespecified their usual MBS on the first day of the run‐in period and were required to have that symptom at the time of the treated migraine.2 For the remaining trials, insufficient information has been published thus far to ascertain when MBS was specified.
Table 1.
Study Design for Included Trials
| Study Clinicaltrials.gov Identifier | Active Intervention | Primary Efficacy Endpoint(s) | Secondary Efficacy Endpoint(s) | Number Randomized, Number in mITT | Number Randomized/Number Treatment Groups |
|---|---|---|---|---|---|
|
ACHIEVE I8
NCT02828020 |
Ubrogepant 50 mg† |
|
1672, 1327 | 557 | |
|
ACHIEVE II7
NCT02867709 |
Ubrogepant 50 mg§ |
|
1686, 1355 | 562 | |
|
RESTOR6
NCT02569853 |
DFN‐11 (Sumatriptan injection 3 mg) |
|
|
268, NR | 134 |
|
SPARTAN10
NCT02605174 |
Lasmitidan 200 mg¶ |
|
3005, 2156 | 751 | |
|
SAMURAI11
NCT02439320 |
Lasmitidan 200 mg‡ |
|
2232‡,12 NR | 743 | |
|
Study 30113
NCT03235479 |
Rimegepant 75 mg |
|
1162, 1084 | 581 | |
|
Study 30214
NCT03237845 |
Rimegepant 75 mg |
|
1186, 1072 | 593 | |
| ZOTRIP15 | ADAM zolmitriptan 3.8 mg‡ |
|
|
365, 360 | 91 |
Additional dose was 100 mg.
Photophobia, phonophobia, nausea.
Additional dose was 25 mg.
Additional doses were 100 mg, 50 mg.
Additional dose was 100 mg.
Enrolled.
Additional doses were 1 mg, 1.9 mg.
MBS = most bothersome symptom; mITT = modified intent to treat population; NR = not reported.
All of the studies achieved a statistically significant treatment effect for both 2‐hour pain freedom and 2‐hour MBS freedom. However, for sumatriptan injection, MBS freedom (which was a secondary endpoint) was only significant in a post hoc analysis that employed observed cases rather than Last Observation Carried Forward imputation.6
The mean placebo response rate for 2‐hour pain freedom was 16.75% (range 11.8‐21.3%) (Table 2). For active treatment groups, the mean response rate was 30.41% (range 19.2‐51%). Correspondingly, treatment effect (difference in response rates between active and placebo groups) ranged from 5.0% to 27.2%.
Table 2.
Treatment Effects on Freedom From Pain and From Most Bothersome Other Symptom at 2 Hours Postdose
|
Active Intervention Trial |
2‐Hour Pain Freedom | 2‐Hour MBS Freedom | |||||
|---|---|---|---|---|---|---|---|
| Placebo (%) | Active Treatment (%) | Difference Between Groups (%) | Placebo (%) | Active Treatment (%) | Difference Between Groups (%) | ||
|
Ubrogepant 50 mg ACHIEVE I8 |
11.8 | 19.2 | 7.4 | 27.8 | 38.6 | 10.8 | |
|
Ubrogepant 50 mg ACHIEVE II7 |
14.3 | 21.8 | 7.5 | 27.4 | 38.9 | 11.5 | |
|
DFN‐11 RESTOR6 |
30.8 | 51.0 | 21.0 | 48.1 | 64.1 | 16.0 | |
|
Lasmitidan 200 mg SPARTAN10 |
21.3 | 38.8 | 17.5 | 33.5 | 48.7 | 15.2 | |
|
Lasmitidan 200 mg SAMURAI11 |
15.3 | 32.2 | 16.9 | 29.5 | 40.7 | 11.2 | |
|
Rimegepant 75 mg Study 30113 |
14.2 | 19.2 | 5.0 | 27.7 | 36.6 | 8.9 | |
|
Rimegepant 75 mg Study 30214 |
12.0 | 19.6 | 7.6 | 25.2 | 37.6 | 12.4 | |
|
ADAM zolmitriptan 3.8 mg ZOTRIP15 |
14.3 | 41.5 | 27.2 | 42.9 | 68.3 | 25.4 | |
MBS = Most bothersome symptom.
For 2‐hour MBS freedom, the mean placebo response rate was 32.8% (range 25.2‐48.1%). The mean response rate for active treatment groups was 46.7% (range 36.6‐68.3%), yielding a range of treatment effects of 8.9% to 25.4%.
Based on an average placebo response rate of 17% for 2‐hour pain freedom and 33% for 2‐hour MBS freedom, we calculated the group sizes that would be required to achieve a statistically significant result in a clinical trial if response rates were 10%, 15%, or 20% (approximating the observed ranges in the currently published studies). The results are shown in Figure 1. Sample size calculations were based on 80% power.
Figure 1.
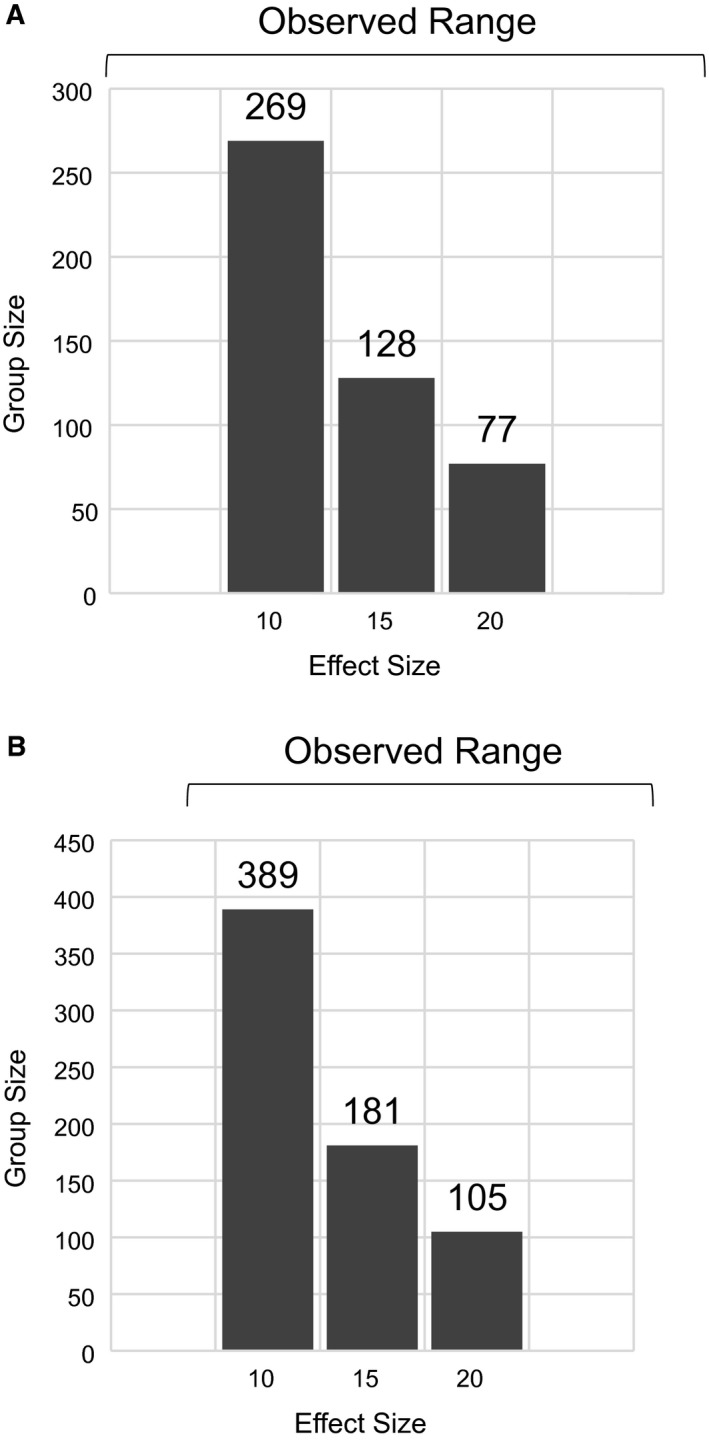
Calculated group size required to achieve a statistically significant result based on different effect sizes. (A) Group sizes required to achieve significance for 2‐hour pain freedom. (B) Group sizes required to achieve a significance for 2‐hour MBS freedom. Calculations are powered at 80%. Bars at the top represent the range of observed effect sizes in currently reported studies.
Discussion and Conclusions
Symptoms of migraine attacks are highly variable among and within patients, and often include nausea, photophobia, and/or phonophobia in addition to headache pain.16 The newly recommended co‐primary endpoints (2‐hour pain freedom and 2‐hour MBS‐freedom) for clinical trials of acute migraine treatments are intended to better address the symptoms considered most important to patients. One additional potential advantage to the use of the new endpoints is that sample sizes might be reduced, leading to faster and more cost‐effective clinical trials.2 With the emerging data on placebo responses and effect sizes observed in trials using the newly revised endpoints, we can now begin to determine whether this is the case.
It is well recognized that placebo responses can be substantial in migraine trials and are variable, depending on numerous factors, including patient expectation, route of administration, and patient demographics.17, 18 It is therefore not surprising to observe quite a bit of heterogeneity among the trials identified in this review, with the highest placebo responses occurring in trials employing parenteral routes of administration.
The drug effect size (difference between active treatment group and placebo group) was also quite variable in the identified studies, ranging from 5% to 27% for 2‐hour pain freedom, and from 9% to 25% for 2‐hour MBS freedom. Based on this range of values and the observed placebo responses, we calculated the minimum group size that would have been required to establish statistical significance for each of the co‐endpoints.
It is important to note that our calculations did not take into consideration any correlation between the 2 co‐primary endpoints. Correlation between 2‐hour MBS freedom and 2‐hour pain freedom is unknown and may be inconstant. Notably however, in the recently completed ZOTRIP trial, only 1 patient (<1%) achieved pain freedom without achieving MBS freedom.2
Our calculations suggest that the initial trials using the newly recommended co‐primary endpoints tended to treat more patients than would have been minimally required. Surprisingly, group sizes in the currently reviewed trials (with the exception of the RESTOR and ZOTRIP trials) also tended to substantially exceed those in 4 of the most recent trials (NCT00434083, NCT00433732, NCT00623636, NCT00330850) completed using 4 co‐primary endpoints in similar patient populations. The mean and median group sizes in those trials were 375 and 362, respectively.3, 4, 5
This observation likely suggests an initial conservative approach reflecting uncertainty regarding placebo response and effect size. It also may reflect differences in trial design, such as the time at which MBS was selected (ie, prospectively or at the time of treatment). As many of the details of these trials have yet to be fully published, it is difficult to ascertain what considerations led to the determination of sample size. We anticipate that with a growing body of information regarding the use of these new endpoints, samples sizes may be more aligned with treatment efficacy, enabling faster and more cost‐effective trials for acute migraine treatment.
Key Conclusions
Eight clinical trials in the acute treatment of migraine have now been completed that employed the 2 new co‐primary endpoints (pain freedom and freedom from most bothersome symptom other than pain, both at 2 hours posttreatment) recommended in 2018 by the US Food and Drug Administration.
In addition to providing the potential to better align study outcomes with symptoms of primary importance to patients, the use of these endpoints may allow the enrollment of fewer trial participants than have been used in the past, when 4 co‐primary outcome measures were required. Placebo response rates and drug effect sizes in the 8 trials using these new endpoints support this notion.
However, these initial trials had enrollment of more patients than would have been minimally required. We anticipate that with the growing body of information regarding the use of these new endpoints, sample sizes may be more aligned with treatment efficacy, enabling faster and more cost‐effective trials for acute migraine treatment.
Acknowledgments
Statistical analysis was performed by Jean M. Engels and funded by Zosano Pharma. Medical writing support was provided by Pam Foreman, PhD, and funded by Zosano Pharma.
Conflict of Interest: DJK and PCS are employees of Zosano Pharma. NAH serves on speaker’s bureaus for Amgen, Eli Lilly, and Electrocore, and serves on advisory boards for Amgen, Eli Lilly, Alder, and Zosano Pharma.
Funding: Support for this publication was provided by Zosano Pharma.
References
- 1. Migraine: Developing Drugs for Acute Treatment Guidance for Industry. Available at https://www.fda.gov/downloads/drugs/guidances/ucm419465.pdf. [Google Scholar]
- 2. Dodick DW, Tepper SJ, Friedman DI, Gelfand AA, Kellerman DJ, Schmidt PC. Use of most bothersome symptom as a coprimary endpoint in migraine clinical trials: A post‐hoc analysis of the pivotal ZOTRIP randomized, controlled trial. Headache. 2018;58:986‐992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lipton RB, Grosberg B, Singer RP, et al. Efficacy and tolerability of a new powdered formulation of diclofenac potassium for oral solution for the acute treatment of migraine: Results from the International Migraine Pain Assessment Clinical Trial (IMPACT). Cephalalgia. 2010;30:1336‐1345. [DOI] [PubMed] [Google Scholar]
- 4. Aurora SK, Silberstein SD, Kori SH, et al. MAP0004, orally inhaled DHE: A randomized, controlled study in the acute treatment of migraine. Headache. 2011;51:507‐517. [DOI] [PubMed] [Google Scholar]
- 5. Brandes JL, Kudrow D, Stark SR, et al. Sumatriptan‐naproxen for acute treatment of migraine: A randomized trial. JAMA. 2007;297:1443‐1454. [DOI] [PubMed] [Google Scholar]
- 6. Landy S, Munjal S, Brand‐Schieber E, Rapoport AM. Efficacy and safety of DFN‐11 (sumatriptan injection, 3 mg) in adults with episodic migraine: A multicenter, randomized, double‐blind, placebo‐controlled study. J Head Pain. 2018;19:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lipton RB, Dodick DW, Ailani J, et al. Efficacy, safety, and tolerability of ubrogepant for the acute treatment of migraine: Results from a single attack phase iii study, ACHIEVE II. Headache. 2018;58:1315‐1316. [Google Scholar]
- 8. Trugman J, Finnegan M, Lipton R, et al. Efficacy, safety, and tolerability of ubrogepant for the acute treatment of migraine: Results from a single‐attack phase II study, ACHIEVE I. Neurology. 2018;90:e2186. [Google Scholar]
- 9.Available at https://clinicaltrials.gov/. Accessed November 18, 2018.
- 10. Wietecha LA, Kuca B, Case MG, Selzler KJ, Aurora S. Phase 3 study (Spartan) of lasmiditan compared to placebo for acute treatment of migraine. Cephalalgia. 2018;38:57‐58. [Google Scholar]
- 11. Wietecha LA, Kuca B, Asafu‐Adjei J, Aurora SK. Phase 3 studies (Samurai, Spartan) of lasmiditan compared to placebo for acute treatment of migraine. Neurology. 2018;90(Suppl. 15):S50.008. [Google Scholar]
- 12. CoLucid Pharmaceuticals Announces Achievement of Both Primary and Key Secondary Endpoints in the SAMURAI Phase 3 Pivotal Trial of Lasmiditan in Migraine [press release]. Cambridge, MA: GLOBE NEWSWIRE; 2016. [Google Scholar]
- 13. Lipton RB, Conway CM, Stock EG, et al. Efficacy, safety, and tolerability of rimegepant 75 mg, an oral CGRP receptor antagonist, for the acute treatment of migraine: Results from a double‐blind, randomized, placebo‐controlled trial, study 301. Headache. 2018;58:1336‐1337. [Google Scholar]
- 14. Lipton RB, Coric V, Stock EG, et al. Efficacy, safety, and tolerability of rimegepant 75 mg, an oral CGRP receptor antagonist, for the acute treatment of migraine: Results from a double‐blind, randomized, placebo‐controlled trial, study 302. Headache. 2018;58:1289. [Google Scholar]
- 15. Spierings EL, Brandes JL, Kudrow DB, et al. Randomized, double‐blind, placebo‐controlled, parallel‐group, multi‐center study of the safety and efficacy of ADAM zolmitriptan for the acute treatment of migraine. Cephalalgia. 2018;38:215‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Viana M, Sances G, Ghiotto N, et al. Variability of the characteristics of a migraine attack within patients. Cephalalgia. 2016;36:825‐830. [DOI] [PubMed] [Google Scholar]
- 17. Macedo A, Farre M, Banos JE. A meta‐analysis of the placebo response in acute migraine and how this response may be influenced by some of the characteristics of clinical trials. Eur J Clin Pharmacol. 2006;62:161‐172. [DOI] [PubMed] [Google Scholar]
- 18. Diener HC. Placebo effects in treating migraine and other headaches. Curr Opin Invest Drugs. 2010;11:735‐739. [PubMed] [Google Scholar]


