Abstract
Background & Aims
Hepatocellular adenomas (HCA) are rare, hormone‐driven, benign liver tumours. HCA >50 mm are associated with haemorrhage and malignant transformation. Guidelines recommend cessation of oral contraceptive pills (OCP) for size reduction; however, it is currently unknown how HCA respond to cessation of OCP. We sought to investigate the effect of OCP cessation on HCA size.
Methods
A retrospective cohort study was performed including HCA patients who stopped OCP intake within 6 months of imaging between 2005 and 2018. Biometrics and hormonal medication use were evaluated with self‐designed questionnaires. Response of the largest HCA was evaluated according to Response Evaluation Criteria in Solid Tumours (RECISTv1.1). Cox regression was performed for analysis of factors influencing HCA regression.
Results
Seventy‐eight HCA patients were included, diagnosed at a median (interquartile range) age of 32 (26‐41) years. Follow‐up was 1.6 (0.4‐2.9) years. HCA size at diagnosis ranged 10‐167 mm. After a median time of 1.3 (0.6‐2.6) years after OCP cessation, 37.2% of HCA showed ≥30% regression, 5.1% complete regression, 56.4% stability and 1.3% progression. No HCA‐induced complications were observed during follow‐up. Cox regression analysis demonstrated a significant association of HCA size with rate of regression; 50 ≤ HCA < 100 mm (HR 2.4, 95% CI 1.1‐5.3; P < 0.05), HCA ≥ 100 mm (HR 8.3, 95% CI 3.3‐21.6; P < 0.001).
Conclusions
Ninety‐eight per cent of HCA remained stable or regressed after OCP cessation. A longer wait‐and‐see period was associated with a larger proportion of regressing HCA, without HCA‐related complications during follow‐up.
Keywords: hepatocellular adenoma, oral contraceptive pill, regression, treatment algorithm
Abbreviations
- b‐HCA
b‐Catenin activated Hepatocellular Adenoma
- BMI
Body Mass Index
- CT
Computed Tomography
- GSD
Glycogen Storage Disease
- HCA
Hepatocellular Adenoma
- HCC
Hepatocellular Carcinoma
- H‐HCA
Hepatocyte Nuclear Factor 1a inactivated Hepatocellular Adenoma
- HNF1A‐MODY
Hepatocyte Nuclear Factor 1a Maturity Onset Diabetes of the Young
- I‐HCA
Inflammatory Hepatocellular Adenoma
- MRI
Magnetic Resonance Imaging
- OCP
Oral Contraceptive Pill
- RECIST
Response Evaluation Criteria in Solid Tumours
Key points.
Liver adenomas are benign liver tumours probably associated with long‐term oral contraceptive pill use. Large liver adenomas are at risk for bleeding and development of cancer. Although it is already known they could shrink after cessation of oral contraceptive use, it is unclear what their exact response is. We observed that 98% of liver adenomas remained stable or decreased in size after oral contraceptive pill cessation, with no complications – information which could render liver surgery not necessary anymore in a majority of patients.
1. INTRODUCTION
Hepatocellular adenomas (HCA) are rare, hormone‐driven benign liver tumours. They mainly develop in young women in their reproductive age. While HCA have an incidence rate around one per million per year in the general population, long‐term (>2 years) users of oral contraceptive pills (OCP) have 30‐ to 40‐fold increased risk of developing HCA.1, 2, 3 Main complications are (potentially lethal) haemorrhage (15‐20%) and malignant transformation to hepatocellular carcinoma (HCC) (5%).4, 5, 6 HCA of ≥50 mm size are especially at risk for these complications.4, 5, 7, 8, 9
Hepatocellular adenomas can be classified into subtypes. Inflammatory HCA (I‐HCA) comprise 40% to 55% and are most common in patients with obesity and/or metabolic syndrome. They are associated with prolonged oestrogen exposure.6 Hepatocyte nuclear factor 1a inactivated HCA (H‐HCA) comprise 30% to 40% of HCA and bleed only in rare cases. They are seen in patients with significantly less oestrogen exposure than I‐HCA, suggesting a higher oestrogen sensitivity or alternative pathophysiology.6 Less common are the b‐catenin activated adenomas (b‐HCA). b‐HCA have an increased tendency to transform into HCC and are mostly seen in men. Half of b‐HCA are hybrid b‐catenin/I‐HCA.6 b‐HCA are diagnosed through immunohistochemical analysis of glutamine synthetase. Finally, there are unclassified HCA and sonic hedgehog activated HCA, each accounting for 5% to 10% of HCA.
Sex hormones or androgens stimulate both de novo formation as well as growth of HCA.2, 10 Hence, women using OCP and those using anabolic steroids are particularly at risk of HCA development. Extra gonadal oestrogen is most notably formed by adipose tissue which accounts for 10% to 50% of total oestrogen production.11 Oestrogen production has been proven to increase with obesity.12 Vital for a non‐invasive management of HCA is that regression can be induced by reduction of circulating oestrogen levels, which occurs naturally after the onset of the menopause, or after substantial weight loss.13
Hepatocellular adenomas are also known to form in patients with metabolic disorders such as glycogen storage disease (GSD) and hepatocyte nuclear factor 1A maturity onset diabetes of the young (HNF1A‐MODY). The incidence, aetiology, behaviour and treatment of these HCA differ from androgen‐induced HCA.
The clinical guideline for treatment of benign liver tumours was published by the European Association for the Study of the Liver (EASL) in 2016.14 It advices an initial conservative and oestrogen level reducing treatment through life style changes in females diagnosed with HCA larger than 50 mm. This reduction is mainly accomplished by weight loss and cessation of all hormonal medication.15, 16 OCP containing progesterone only are excluded, as this hormone has no role in HCA (patho)physiology. If during follow‐up HCA increase significantly in size (>20%), or if after 6 months of wait‐and‐see policy HCA remain equal to or larger than 50 mm, resection is advised.
It is well established that OCP are the main risk factor for HCA formation and growth, and that HCA show regression after OCP cessation. However, detailed information on exact OCP use in HCA patients, and HCA behaviour after OCP cessation is still lacking. The age of commencement, total duration and age of cessation of OCP have not yet been observed to influence rate of regression, although research is lacking. Secondly, the current EASL guideline does not take HCA diameter at baseline into account – a 6 month wait‐and‐see period is advised for all HCA ≥ 50 mm. This period could prove to be frankly too short for regression of large HCA to sub 50 mm size. The aim of this study was to evaluate the response of HCA after OCP cessation, and to evaluate any factors associated with this response.
2. MATERIALS AND METHODS
2.1. Study design and population
All female HCA patients treated at the University Medical Center Groningen (UMCG) between 2005 and 2018 were included. After obtaining informed consent, patients were subjected to a self‐designed questionnaire regarding biometrical information, comorbidities and exact intake of all hormonal medication such as OCP. Analysis was performed on female HCA patients with a history of oestrogen containing OCP intake and consecutive imaging available. Baseline imaging was defined as imaging within 6 months prior to or after OCP cessation. The minimum amount of follow‐up was at least one scan obtained by either magnetic resonance imaging (MRI) or computed tomography (CT), 6 months after OCP cessation or 6 months after baseline imaging if the HCA was imaged after cessation. Patients were excluded if: OCP intake was stopped after an HCA induced or post biopsy haemorrhage (as tumour diameter could not be observed accurately thereafter), had HCA in concordance with GSD or HNF1A‐MODY, or if they did not comply with the local opt‐out research registry. The study protocol was approved by the UMCG ethics committee (UMCG research registry 201700324—METc 2017/270).
Hepatocellular adenomas response was evaluated in accordance with the Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1.17 These criteria recommend that lesion response should only be evaluated using CT or MRI acquired imaging. Lesions should not be smaller than 10 mm, and should be measured in their longest diameter. As advised, lesions were measured in the transversal plane on post contrast series. Imaging from CT was only used when MRI was not available. Response criteria defined by RECIST are: ‘complete response’ defined by disappearance of all lesions, ‘partial response’ defined by at least 30% regression, ‘progressive disease’ defined by at least 20% increase of diameter and ‘stable disease’ defined by neither sufficient growth for being classified as ‘progressive disease’ nor regression for ‘partial response’.
2.2. Endpoints
Primary endpoint was HCA response to cessation of OCP as defined by the RECISTv1.1 criteria. Secondary outcomes were HCA‐related complications, frequency, indications and outcomes of invasive treatment, and independent predictors of the rate of HCA regression.
2.3. Data collection and definitions
Relevant information was obtained from electronic patient files and a self‐designed questionnaire. Obesity was defined by body mass index (BMI) above 30 kg/m2 and was measured at baseline. One target lesion per patient was followed, defined by the single largest HCA at baseline imaging. These were classified into HCA < 50 mm, 50 ≤ HCA < 100 mm and HCA ≥ 100 mm.
2.4. Data analysis
Continuous variables were described using the median with interquartile range, whereas nominal and ordinal variables were described using totals, frequencies and percentages. Normality was tested using the Shapiro‐Wilk test. The Student t test or Mann‐Whitney U test was used to investigate differences between groups for continuous variables and the chi‐square or Fisher exact test for categorical variables. For comparison of three groups, either ANOVA or the Kruskal‐Wallis test was used. Spearman's rank‐order was used for analysis of correlation for non‐parametric values. A P ≤ 0.05 was considered statistically significant. Cox proportional hazard model was used to determine factors that are independently associated with >30% regression in HCA diameter. Patients were categorized into < or ≥ median values. Categories were made (1) on less than and (2) equal to or larger than the median. Variables with a P < 0.10 at univariate analysis were included in the multivariate analysis. The statistical analyses were performed using IBM SPSS statistics version 23.0 (SPSS Inc, Chicago, IL). Figures were generated with GraphPad Prism version 5.0 (GraphPad Software, La Jolla, CA).
3. RESULTS
3.1. Study population and baseline characteristics
In total, 267 HCA patients were treated between 2005 and 2018. Twenty‐one patients with concurring GSD, six HNF1A‐MODY patients and two male patients diagnosed with HCA were excluded. Of the remaining 238 patients, another 116 were excluded as their OCP use outmatched the inclusion criteria. Thirty‐one patients in whom an intervention was performed before the effect of the life style advices could be observed, and 13 patients in whom no observation of OCP cessation was possible, were also excluded, thereby leaving 78 patients available for analysis (Figure 1).
Figure 1.
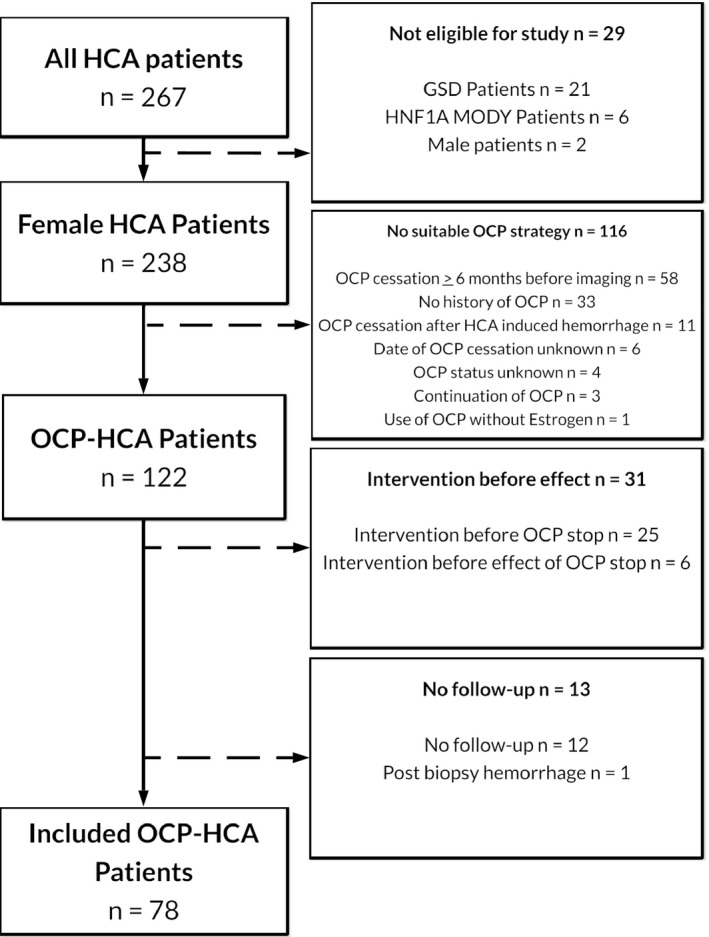
Flowchart of included patients. Abbreviations: HCA, hepatocellular adenoma; GSD, glycogen storage disease; HNF1A‐MODY, hepatocyte nuclear factor 1a inactivated maturity onset diabetes of the young; OCP, oral contraceptive pill.
Hepatocellular adenomas were diagnosed at a median age of 32 (27‐41) years. About a third of patients had obesity and 11.5% had a BMI ≥ 40 kg/m2. HCA size at diagnosis ranged from 10 mm to 167 mm, and had a median size of 49 mm. Patients started the intake of OCP at a median age of 15 (14‐17) years and stopped after 15 (10‐24) years of intake. Two‐thirds of patients took an oestrogen/progesterone combination preparation containing less than 50 µg oestrogen, 3.8% took a preparation with 50 µg, and oestrogen dosage was unknown in 21 patients (26.9%). Obese patients were more often diagnosed with larger HCA, as the median size of the HCA was 37 (27.5‐79.5) mm in patients with a BMI ≤30 kg/m2 compared to 57.5 (45.0‐94.3) mm of patients with BMI ≥30 kg/m2 (P = 0.01). Age at diagnosis, however, was similar (34.0 vs 32.5 years; P = 0.74). A Spearman's rank‐order correlation was performed to analyse the correlation of the age of commencement and total duration of OCP intake to HCA size at diagnosis. Earlier commencement was not associated with larger HCA (r s(11) = 0.085, P = 0.53), and neither was total duration of OCP intake (rs(11) = 0.050, P = 0.71) (Table 1).
Table 1.
Baseline characteristics
| HCA size at diagnosis | <50 mm, n = 39 | ≥50‐<100 mm, n = 26 | ≥100 mm, n = 13 | P‐value |
|---|---|---|---|---|
| Female | 39 (100%) | 26 (100%) | 15 (100%) | — |
| Age at diagnosis, y | 31.0 (26.0‐40.0) | 36.5 (29‐44) | 31.0 (24.5‐41.0) | 0.21a |
| Body mass index, kg/m2 | 27.0 (22.9‐33.4) | 32.0 (25.7‐35.3) | 30.6 (28.1‐34.3) | 0.08a |
| Oral contraceptive use | ||||
| Age at start, y | 15.0 (14.0‐16.0) | 16.0 (14.0‐18.0) | 15.0 (14.0‐17.0) | 0.62b |
| Age at cessation, y | 31.0 (27.0‐40.0) | 33.0 (29.0‐41.0) | 29.5 (25.0‐39.3) | 0.47a |
| Duration of intake, y | 13.0 (7.0‐27.0) | 17.0 (11.5‐23.8) | 17.0 (10.5‐23.0) | 0.85a |
Values are given in median (interquartile range) or n (%).
Kruskal‐Wallis test.
ANOVA.
3.2. HCA response to OCP cessation
At the end of follow‐up, four HCA demonstrated a complete response (5.1%), 29 HCA (37.2%) showed a partial response, 44 HCA (56.4%) remained stable, and one HCA (1.3%) showed progression (Figure 2). The HCA that demonstrated growth progressed from 10 to 16 mm during 7 months, however, no further follow‐up was available. All of the four HCA with a complete response were smaller than 50 mm at diagnosis. Of the remaining 35 HCA <50 mm, eight demonstrated a partial response (20.5%), one was progressive (2.6%) and 26 (66.7%) remained stable. None of the HCA <50 mm progressed to a ≥ 50 mm diameter. Thirty‐nine HCA were larger than 50 mm at diagnosis, median size at diagnosis 86 (60‐110) mm and final diameter 55 (41‐81) mm after 1 (0.4‐2.9) year. In this subgroup, stable disease was seen in 18 HCA (46.2%), and 21 HCA (53.8%) demonstrated a partial response. Fourteen patients (35.9%) of the latter group regressed to <50 mm size from a median diameter of 65.5 (56.5‐95.5) mm after a median follow‐up of 1.3 (0.9‐3.3) year. Analysis of response per HCA subtype was not possible due to insufficient patient numbers for HCA subtypes other than I‐HCA (Table 2).
Figure 2.
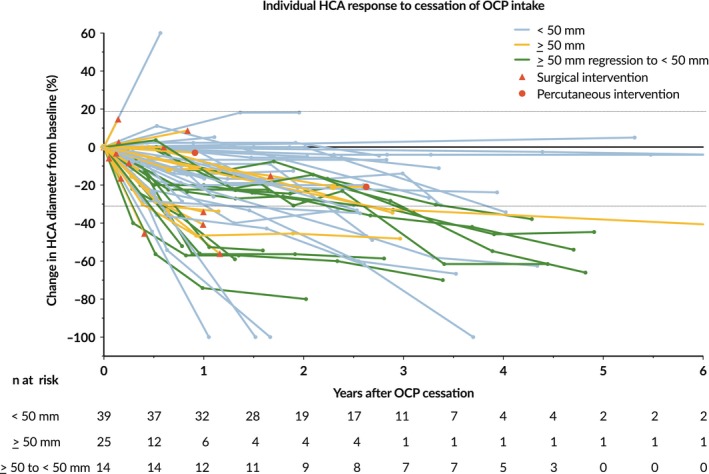
Spider plot of the relative change of largest HCA diameter from baseline over time for all evaluable patients (n = 78), defined as those with baseline tumour assessments and at least one postbaseline assessment. Lines are colour coded based on overall response. Horizontal dashed lines represent Response Evaluation Criteria In Solid Tumours version 1.1 guideline for partial response (≥30% decrease in target lesion) and progressive disease (≥20% increase in target lesion). Abbreviations: HCA, hepatocellular adenoma; OCP, oral contraceptive pill. Legend: Blue, HCA diameter <50 mm; Orange, HCA diameter >50 mm; Green, HCA diameter >50 mm at baseline, regression to <50 mm size; Red triangle, surgical intervention; Red circle, percutaneous intervention
Table 2.
HCA response and management after cessation of OCP
|
Included patients with HCA
n = 78 |
|
|---|---|
| T0: HCA diagnosis | |
| HCA diameter at diagnosis, mm | 49.0 (30.8‐86.0) |
| Interval between OCP cessation ‐ T0, months | 0 (−0.8 to 1.0) |
| No. of observed HCA | 78 |
| First follow‐up (T1) | |
| HCA diameter at first follow‐up, mm | 50 (27.0‐65.0) |
| Interval between T0 ‐ T1, months | 5.4 (4.1‐6.4) |
| No. of observed HCA | 59 |
| Second follow‐up (T2) | |
| HCA diameter at second follow‐up, mm | 41.0 (24.0‐61.0) |
| Interval between T0 ‐ T2, months | 11.6 (9.9‐13.2) |
| No. of observed HCA | 42 |
| Total follow‐up time, y | 1.1 (0.5‐2.6) |
| HCA subtype | |
| H‐HCA | 2 (2.6%) |
| I‐HCA | 23 (29.5%) |
| β‐HCA | — |
| β‐IHCA | 2 (2.3%) |
| U‐HCA | 2 (2.3%) |
| No histopathology or subtype analysis available | 49 (62.8%) |
| Management | |
| Conservative | 60 (76.9%) |
| Intervention | 18 (23.1%) |
HCA, hepatocellular adenoma; OCP, oral contraceptive pill. Values are given in median (interquartile range) or n (%). For HCA subtype explanation, see introduction.
3.3. HCA‐related complications
No HCA‐related complications occurred during follow‐up. Although two b‐HCA were observed, at risk for malignant transformation, there were no cases of actual malignant degeneration to HCC during the follow‐up period.
3.4. Interventions
Eighteen patients underwent invasive treatment for HCA, at a median of 8.5 (5.8‐14.1) months after diagnosis (Table 3). The majority of cases was treated through either segment resection (n = 10) or (extended) hemihepatectomy (n = 5). Open thermal ablation of an additional lesion was performed in three patients with segment resections. Three cases were treated through minimally invasive percutaneous treatment – radiofrequency or microwave ablation or transarterial embolization. Almost two‐thirds of ≥50 mm HCA (25 of 39) remained larger than 50 mm in size and thus were potential candidates for surgery. Nearly a quarter of patients in whom HCA were resected, the indication for surgery was the inability to exclude (well‐differentiated) HCC at either MRI or histopathologic analysis. All of these HCA demonstrated washout on contrast‐enhanced MRI. Percutaneous biopsy was performed in two patients, which had a suspicion for malignant characteristics on immunohistochemical analysis. No HCC were found and only one case had a beta catenin mutation (Table 4). There were two major complications and one minor complication (urine tract infection) postoperatively. One patient developed an abdominal incisional hernia after an open Couinaud segment 2, 3, and 6 resection requiring reoperation for mesh repair. Another patient experienced biliary leakage after a left hemihepatectomy requiring a reoperation with a roux‐en‐Y hepaticojejunostomy.
Table 3.
Patients with invasive treatment
|
Included patients with HCA n = 18 |
|
|---|---|
| Age at treatment, y | 32 (32.0‐39.5) |
| HCA and OCP characteristics | |
| HCA diameter at baseline, mm | 93.5 (71.5‐120.8) |
| HCA diameter before intervention, mm | 80.5 (61.0‐102.3) |
| Interval between cessation of OCP and intervention, months | 11.0 (4.8‐14.3) |
| Treatment type | |
| Resection | 15 (83.3%) |
| Percutaneous thermal ablation | 2 (11.1%) |
| Transarterial embolization | 1 (5.6%) |
| Indication | |
| HCA size ≥50mm after lifestyle advices | 9 (50.0%) |
| Unable to rule out malignancy | 4 (22.2%) |
| Patient’s own wish | 3 (16.7%) |
| HCA‐induced symptoms | 1 (5.6%) |
| Wish to become pregnant | 1 (5.6%) |
HCA, hepatocellular adenoma; OCP, oral contraceptive pill. Values are given in median (interquartile range) or n (%).
Table 4.
Patients with suspected malignancy
| Case 1 | |
| Age at diagnosis, y | 32 |
| HCA diameter at diagnosis, mm | 112 |
| HCA diameter at last follow‐up, mm | 100 |
| Total follow‐up duration, months | 12 |
| MRI, type and findings | MRI gadoxetic acid: wash out on venous phase |
| Percutaneous histopathology | I‐HCA: no b‐Cat activation, though some malignant characteristics |
| Postoperative histopathology | b‐IHCA |
| Type of intervention | Segment 2, 3 resection |
| Case 2 | |
| Age at diagnosis, y | 32 |
| HCA diameter at diagnosis, mm | 75 |
| HCA diameter at last follow‐up, mm | 86 |
| Total follow‐up duration, months | 3 |
| MRI, type and findings | MRI gadoxetic acid: atypical HCA, wash out on venous phase |
| Percutaneous histopathology | N/A |
| Postoperative histopathology | HCA, no subtype |
| Type of intervention | Segment 2, 3 and caudal part of 6 resection |
| Case 3 | |
| Age at diagnosis, y | 48 |
| HCA diameter at diagnosis, mm | 73 |
| HCA diameter at last follow‐up, mm | 61 |
| Total follow‐up duration, months | 2 |
| MRI, type and findings | MRI gadoxetic acid: wash out on venous phase |
| Percutaneous histopathology | N/A |
| Postoperative histopathology | I‐HCA |
| Type of intervention | Right hemihepatectomy |
| Case 4 | |
| Age at diagnosis, y | 49 |
| HCA diameter at diagnosis, mm | 167 |
| HCA diameter at last follow‐up, mm | 110 |
| Total follow‐up duration, months | 12 |
| MRI, type and findings | MRI gadoteridol: washout on portal venous phase |
| Percutaneous histopathology | b‐IHCA |
| Postoperative histopathology | I‐HCA: GS neg., b‐Cat expression on membrane |
| Type of intervention | Right hemihepatectomy |
HCA, Hepatocellular Adenoma; b‐Cat, b‐Catenin; GS, glutamine synthetase. For HCA subtype explanation, see introduction.
3.5. Factors associated with HCA regression
Rate of HCA regression was analyzed (Figure 3A). HCA size at baseline was significantly associate with rate of regression (Figure 3B). Duration of intake was not associated with rate of regression though (Figure 3C). Increased BMI was associated with larger HCA diameter at diagnosis. Analysis using a Cox proportional hazard model, however, did not demonstrate a relation with the rate of HCA regression (Figure 3D). Duration of OCP intake was not related to the rate of HCA regression. HCA diameter at baseline was significantly related to the rate of HCA regression. Next, a Cox proportional hazard model was performed for univariate analysis of factors influencing regression including the following variables: BMI, total duration of OCP use, age of OCP commencement, age of HCA diagnosis and largest HCA diameter categorized into <50 mm, 50 ≤ HCA<100 mm and ≥100 mm (Table 5). Only HCA diameter was associated with rate of regression. Compared to HCA smaller than 50 mm, both HCA categorized 50 to 100 mm and larger than 100 mm were significantly more likely to regress 30% or more. They demonstrated a hazard ratio with 95% confidence interval of 2.37 (1.1‐5.3) and 8.39 (3.3‐21.6) respectively. No multivariate analysis was performed as none of the other variables demonstrated a univariate P < 0.10.
Figure 3.
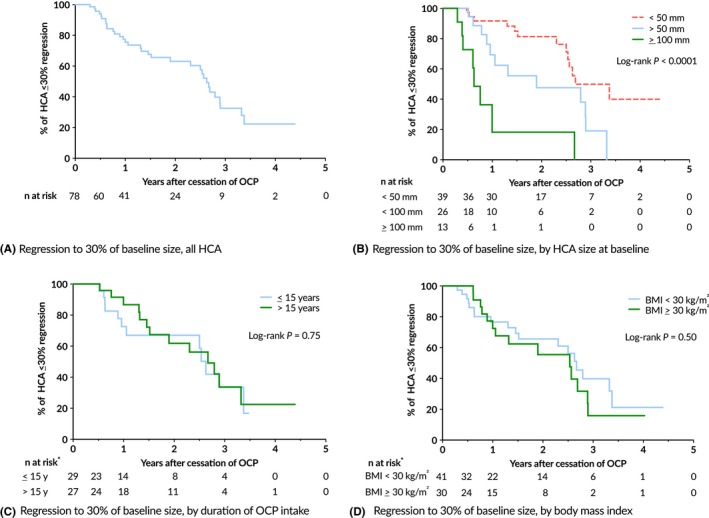
(A‐D) Kaplan‐Meier curves for the percentage of HCA showing 30% or more regression. (A) All HCA, (B) subdivided by initial HCA diameter, (C) duration of OCP use (*22 missing cases) and (D) BMI (*7 missing cases). HCA, hepatocellular adenoma; OCP, oral contraceptive pill; BMI, body mass index
Table 5.
Univariate analysis of HCA regression to ≥30% of baseline size by the Cox proportional hazards model
| Variable | Univariate analysis | |||
|---|---|---|---|---|
| Hazard ratio | 95% confidence interval | P‐value | ||
| Weight, kg/m2 | <30 vs ≥30 | 1.273 | 0.630‐2.574 | 0.50 |
| Start of OCP use | <15 vs ≥15 | 1.102 | 0.491‐2.475 | 0.81 |
| Duration of OCP use | <15 vs ≥15 | 0.880 | 0.401‐1.935 | 0.75 |
| Age of HCA diagnosis | <32 vs ≥32 | 0.868 | 0.435‐1.733 | 0.69 |
| Largest HCA diameter | <50 vs ≥50 to <100 | 2.368 | 1.057‐5.304 | 0.04 |
| <50 vs ≥100 | 8.394 | 3.260‐21.612 | <0.001 | |
HCA, hepatocellular adenoma; OCP, oral contraceptive pill. Bold values indicate statistical significance.
4. DISCUSSION
To our knowledge, this is the first study measuring the radiological HCA response after cessation of OCP using the RECIST criteria. We observed at least 30% regression in almost 40% of HCA patients, and half of all HCA remained stable after cessation of OCP intake. Only one HCA experienced growth during follow‐up imaging. Apart from being effective, this non‐invasive therapy was safe as there were no HCA‐related complications during the follow‐up period. Finally, we demonstrated that HCA diameter was significantly associated with the rate of HCA regression.
Till date, there has been only one report describing HCA regression rate and the timing of HCA resection. Klompenhouwer et al stated that 15% of HCA in their cohort showed regression to 50 mm or smaller after 6 months. This increased to 25% of patients after 1 year.18 Also, they observed that larger HCA often require more than 6 months to regress to a diameter <50 mm.18 Their sample size allowed them to exclude a correlation between HCA subtype and response. We were not able to reproduce this in our dataset because of a smaller sample size. Although the authors stated that patients were advised to halt OCP intake and lose weight, it is unclear when OCP intake was exactly stopped in their cohort. This could lead to an underestimation of the effect of OCP cessation and makes estimation of regression time inaccurate.
Apart from including few other HCA subtypes than I‐HCA, our study has potential weaknesses. We cannot rule out any residual bias, which is inherent to the retrospective design of our study. We were unable to perform extensive subanalyses in the group with regression to <50 mm in size because of our sample size (n = 49 at baseline). Our study focused on the relative change of HCA diameter. In the clinical setting, however, absolute HCA size (≥50 mm) remains crucial for treatment. Hence, we are not able to recommend any specific prolongation of the current wait‐and‐see period. Also, measurement errors could have been made during analyses of HCA diameter. OCP cessation date was obtained through either patient files or patient reporting – both may be subject to recall bias. Finally, we were not able to take a possible change in BMI through time into account as it turned out to be rarely reported more than once (at baseline) in the electronic patient files.
It is important to note that not all HCA responded to a reduction of circulating oestrogen levels and that oestrogen sensitivity may possibly vary across subtypes. It has previously been hypothesized that the oestrogen (and general androgen) sensitivity of HCA is because of an increased expression of all androgen receptors, and oestrogen receptors in specific. Unfortunately, studies which identified, characterized and quantified these receptors, only analysed small patient series (<20 cases). In addition, none of the authors performed any subtype analysis as these have been identified since 2008.19, 20 This might have resulted in unwittingly staining a mix of HCA subtypes. Future research will determine the definite role of oestrogen receptors in the HCA (subtype) response.
We observed that HCA diameter at presentation was larger in obese patients. While one could argue this could be explained through a reduction of auto‐sensation of any liver masses in obese patients compared to lean patients, age of diagnosis did not differ between the two groups. A possible mechanism is the additional growth stimulation through oestrogen synthesis by the excessive adipose tissue. It is currently unclear to what extent obesity contributes to HCA formation and growth, and weight loss to HCA regression. Till date, there has only been one report on weight loss‐induced HCA regression and consists of three cases.16 Future studies will need to be performed for more definite answers.
We found large HCA to regress at a relatively faster rate. This could be an indication for a stronger metabolic activity and dependency on oestrogen (induced stimulants). Clinically, this observation is of importance as current guidelines do not take baseline HCA size into account for selection of patients suited for a wait‐and‐see period. We confirmed the observation of Klompenhouwer et al: large HCA show significant regression, even at a faster rate than their smaller counterparts, and are able to reduce themselves to <50 mm size – but only when provided sufficient time. Extending the wait‐and‐see period, which was a safe strategy in our cohort, may potentially prevent surgical interventions in some patients. Although complication rates are low, the two major surgical complications we observed underscore the potential risk associated with surgery.
In conclusion, we found that the 98% of HCA remain either stable or show regression after OCP cessation. Large HCA showed faster regression than small HCA, but this required a longer time than the currently advised 6‐month period. No HCA‐induced complications were observed during follow‐up. A conservative approach could lead to HCA regression below 50 mm and thereby potentially prevent unnecessary hepatic surgery in a majority of patients.
CONFLICTS OF INTEREST
The authors do not have any disclosures to report.
Haring MPD, Gouw ASH, de Haas RJ, Cuperus FJC, de Jong KP, de Meijer VE. The effect of oral contraceptive pill cessation on hepatocellular adenoma diameter: a retrospective cohort study. Liver Int. 2019;39:905–913. 10.1111/liv.14074
Handling Editor: Janus Ong
REFERENCES
- 1. Rooks JB, Ory HW, Ishak KG, et al. Epidemiology of hepatocellular adenoma. JAMA. 1979;242:644. [PubMed] [Google Scholar]
- 2. Edmondson HA, Henderson B, Benton B. Liver‐cell adenomas associated with use of oral contraceptives. N Engl J Med. 1976;294:470‐472. [DOI] [PubMed] [Google Scholar]
- 3. Nault JC, Bioulac‐Sage P, Zucman‐Rossi J. Hepatocellular benign tumors‐from molecular classification to personalized clinical care. Gastroenterology. 2013;144:888‐902. [DOI] [PubMed] [Google Scholar]
- 4. Dokmak S, Paradis V, Vilgrain V, et al. A single‐center surgical experience of 122 patients with single and multiple hepatocellular adenomas. Gastroenterology. 2009;137:1698‐1705. [DOI] [PubMed] [Google Scholar]
- 5. Van Aalten SM, De Man RA, Ijzermans J, Terkivatan T. Systematic review of haemorrhage and rupture of hepatocellular adenomas. Br J Surg. 2012;99:911‐916. [DOI] [PubMed] [Google Scholar]
- 6. Nault J‐C, Couchy G, Balabaud C, et al. Molecular classification of hepatocellular adenoma associates with risk factors, bleeding, and malignant transformation. Gastroenterology. 2017;152:880‐894. [DOI] [PubMed] [Google Scholar]
- 7. Bieze M, Phoa S, Verheij J, Van Lienden KP, Van Gulik TM. Risk factors for bleeding in hepatocellular adenoma. Br J Surg. 2014;101:847‐855. [DOI] [PubMed] [Google Scholar]
- 8. Bossen L, Grønbaek H, Lykke Eriksen P, Jepsen P. Men with biopsy‐confirmed hepatocellular adenoma have a high risk of progression to hepatocellular carcinoma: A nationwide population‐based study. Liver Int. 2017. [DOI] [PubMed] [Google Scholar]
- 9. Stoot J, Coelen R, De Jong MC, et al. Malignant transformation of hepatocellular adenomas into hepatocellular carcinomas: a systematic review including more than 1600 adenoma cases. HPB (Oxford). 2010;12:509‐522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grangé JD, Guéchot J, Legendre C, Giboudeau J, Darnis F, Poupon R. Liver adenoma and focal nodular hyperplasia in a man with high endogenous sex steroids. Gastroenterology. 1987;93:1409‐1413. [DOI] [PubMed] [Google Scholar]
- 11. Siiteri P, MacDonald P. Role of extraglandular estrogen in human endocrinology In Greep R, Astwood E, eds. Handbook of physiology: endocrinology. Washington, DC: American Physiological Society;1973:615. [Google Scholar]
- 12. Edman CD, MacDonald PC. Effect of obesity on conversion of plasma androstenedione to estrone in ovulatory and anovulatory young women. Am J Obstet Gynecol. 1978;130:456‐461. [DOI] [PubMed] [Google Scholar]
- 13. Klompenhouwer AJ, Sprengers D, Willemssen F, Gaspersz MP, Ijzermans J, De Man RA. Evidence of good prognosis of hepatocellular adenoma in post‐menopausal women. J Hepatol. 2016;65:1163‐1170. [DOI] [PubMed] [Google Scholar]
- 14. Colombo M, Forner A, Ijzermans J, et al. EASL Clinical Practice Guidelines on the management of benign liver tumours. J Hepatol. 2016;65:386‐398. [DOI] [PubMed] [Google Scholar]
- 15. Buhler H, Pirovino M, Akobiantz A, et al. Regression of liver cell adenoma. a follow‐up study of three consecutive patients after discontinuation of oral contraceptive use. Gastroenterology. 1982;82:775‐782. [PubMed] [Google Scholar]
- 16. Dokmak S, Belghiti J. Will weight loss become a future treatment of hepatocellular adenoma in obese patients? Liver Int. 2015;35:2228‐2232. [DOI] [PubMed] [Google Scholar]
- 17. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228‐247. [DOI] [PubMed] [Google Scholar]
- 18. Klompenhouwer AJ, Bröker M, Thomeer M, Gaspersz MP, de Man RA, IJzermans J. Retrospective study on timing of resection of hepatocellular adenoma. Br J Surg. 2017;104:1695–1703. [DOI] [PubMed] [Google Scholar]
- 19. Cohen C, Lawson D, Derose PB. Sex and androgenic steroid receptor expression in hepatic adenomas. Hum Pathol. 1998;29:1428‐1432. [DOI] [PubMed] [Google Scholar]
- 20. Porter LE, Elm MS, Van Thiel DH, Eagon PK. Hepatic estrogen receptor in human liver disease. Gastroenterology. 1987;92:735‐745. [DOI] [PubMed] [Google Scholar]
- 21. Masood S, West AB, Barwick KW. Expression of steroid hormone receptors in benign hepatic tumors: An immunocytochemical study. Arch Pathol Lab Med. 1992;116:1355‐1359. [PubMed] [Google Scholar]
- 22. Torbenson M, Lee J‐H, Choti M, et al. Hepatic adenomas: analysis of sex steroid receptor status and the Wnt signaling pathway. Mod Pathol. 2002;15:189‐196. [DOI] [PubMed] [Google Scholar]
- 23. Rebouissou S, Bioulac‐Sage P, Zucman‐Rossi J. Molecular pathogenesis of focal nodular hyperplasia and hepatocellular adenoma. J Hepatol. 2008;48:163‐170. [DOI] [PubMed] [Google Scholar]


