Abstract
Background
Despite increasing awareness of the disease, rates of undiagnosed psoriatic arthritis (PsA) are high in patients with psoriasis (PsO). The validated Psoriasis Epidemiology Screening Tool (PEST) is a five‐item questionnaire developed to help identify PsA at an early stage.
Objectives
To assess the risk of possible undiagnosed PsA among patients with PsO and characterize patients based on PEST scores.
Methods
This study included all patients enrolled in the Corrona PsO Registry with data on all five PEST questions. Demographics, clinical characteristics and patient‐reported outcomes were compared in Corrona PsO Registry patients with PEST scores ≥3 and <3 using t‐tests for continuous variables and chi‐squared tests for categorical variables; scores ≥3 may indicate PsA.
Results
Of 1516 patients with PsO, 904 did not have dermatologist‐reported PsA; 112 of these 904 patients (12.4%) scored ≥3 and were significantly older, female, less likely to be working, and had higher BMI than patients with scores <3. They also had significantly longer PsO duration, were more likely to have nail PsO and had worse health status, pain, fatigue, Dermatology Life Quality Index and activity impairment.
Conclusions
Improved PsA screening is needed in patients with PsO because the validated PEST identified over one‐tenth of registry patients who were not noted to have PsA as having scores ≥3, who could have had undiagnosed PsA. Appropriate, earlier care is important because these patients were more likely to have nail PsO, worse health‐related quality of life and worse activity impairment.
Introduction
Psoriasis (PsO), a chronic inflammatory disease that affects the skin, has an estimated global prevalence of 2–4%.1, 2 Psoriatic arthritis (PsA) is one of the conditions most frequently associated with PsO; up to 30% of patients with PsO – approximately 0.3–1.0% of the global population – may have a concurrent diagnosis of PsA.3, 4, 5, 6, 7 The presence or absence of PsA plays a major role in determining which therapy should be used in patients with PsO.8 In most patients with PsO who develop PsA, arthritis usually occurs within 10 years following the first manifestation of their skin disease.9 Scalp and flexural skin involvement, nail lesions, certain HLA alleles and increased levels of acute‐phase proteins and matrix metalloproteinase 3 in the sera have been implicated as risk factors for the development of PsA in patients with PsO.10, 11, 12 Enthesitis and dactylitis are characteristic features of PsA and are indicative of erosive forms of the disease and worse prognostic outcomes.13, 14
However, undiagnosed PsA is common in patients with PsO. A meta‐analysis revealed that between 10.1% and 15.5% of patients with PsO may have undiagnosed PsA,15 while other observational studies have shown that even larger proportions of patients with PsO may have undiagnosed PsA.4, 16 For example, of 949 patients with PsO evaluated at 34 dermatology centres across seven countries in North America and Europe, 285 (30%) had PsA, 117 (41%) of whom were not previously diagnosed.4 Undiagnosed PsA, or even a delay in diagnosis of PsA by 6 months, may lead to physical disability and peripheral joint erosion.17 Patients with PsA must see a rheumatologist for a definitive diagnosis; therefore, patients in consultation with a general practitioner or a dermatologist for their PsO and joint pain may not receive a timely diagnosis of PsA.18 The Classification Criteria for Psoriatic Arthritis was developed for use by rheumatologists to classify inflammatory musculoskeletal disease, using rheumatologist assessment, diagnostic measures for inflammatory articular disease and patient‐reported symptoms.19 However, because most patients with PsO are under the care of a dermatologist or general practitioner, it is necessary to have a simple and sensitive tool that can be used by these providers to identify patients who may have early‐stage PsA and prompt a timely referral to a rheumatologist for PsA evaluation.
The currently available screening tools for PsA have been validated in various clinical settings; these tools include the Early Arthritis for Psoriatic Patients (EARP),20 German PsO Arthritis Diagnostic questionnaire,21 PsO Assessment Questionnaire (PAQ),22 Psoriatic Arthritis Screening and Evaluation (PASE),23 PsO and Arthritis Screening Questionnaire (PASQ),24 Psoriasis Epidemiology Screening Tool (PEST),18 Toronto Psoriatic Arthritis Screening (ToPAS)25 and ToPAS 2.26 For this analysis, the validated PEST questionnaire was selected because it is an effective tool that can be used in clinical practice, is easy to use (just five questions) and is available in the Corrona PsO Registry. This questionnaire can be effectively used in non‐rheumatology practices to detect possibly undiagnosed PsA and identify patients who may benefit from consultation with a rheumatologist.18
In this analysis of the US‐based Corrona PsO Registry, we aimed to determine the proportion of patients with possibly undiagnosed PsA based on PEST scores (≥3) and compared demographic and clinical characteristics of patients with a PEST score ≥3 with those of patients with a PEST score <3.
Methods
Data source
The Corrona PsO Registry is a large, independent, prospective, observational cohort of patients with PsO, launched in April 2015. Patients in this analysis were recruited from 114 private and academic practice sites across 34 states in the United States, with 263 participating dermatologists. As of 10 May 2018, the Corrona PsO Registry had enrolled 4864 patients, with data on 11 562 patient visits and 3890.8 patient‐years of follow‐up observation time. The mean time of patient follow‐up was 1.36 years (median, 1.28 years).
All participating investigators were required to obtain full institutional review board (IRB) approval for conducting research involving human subjects. Sponsor approval and continuing review were obtained through a central IRB (IntegReview, Corrona‐PSO‐500). For academic investigative sites that did not receive a waiver to use the central IRB, full board approval was obtained from the respective governing IRBs and documentation of approval was submitted to the Sponsor prior to initiating any study procedures. All registry subjects were required to provide written informed consent prior to participating.
Study population
Study inclusion criteria were the same as those used for enrolment in the Corrona PsO Registry: ≥18 years old, diagnosed with PsO by a dermatologist, and initiated or switched to a systemic (biologic or non‐biologic) PsO treatment on the enrolment date or within 12 months preceding the enrolment date. This descriptive study included all patients with PsO and non‐missing data on all five questions of the PEST questionnaire (Table 1) at time of enrolment in the Corrona PsO Registry, between April 2015 and June 2016. The PEST consists of five simple yes/no questions. Each ‘yes’ answer has a value of 1 point, and a score of ≥3 indicates risk of having PsA and that a rheumatology referral may be needed.
Table 1.
Psoriasis Epidemiology Screening Tool (PEST)
| Question | Yes | No |
|---|---|---|
| Have you ever had a swollen joint (joints)? | □ | □ |
| Has a doctor ever told you that you have arthritis? | □ | □ |
| Do your fingernails or toenails have holes or pits? | □ | □ |
| Have you had pain in your heel? | □ | □ |
| Have you had a finger or toe that was completely swollen or painful for no reason? | □ | □ |
Study outcomes
Data on patient demographics, treatment history, clinical characteristics, patient‐reported outcome measures and work productivity were collected using questionnaires from patients and their treating dermatologists at the enrolment visit. Demographics included age, sex, race/ethnicity, bodyweight, body mass index (BMI), physician‐reported history of comorbidities, work status, family history of PsO and smoking status. Treatment history included prior and current use of biologic and non‐biologic systemic therapies. Clinical characteristics evaluated included PsO morphology, affected body surface area (BSA; 0–100%), Investigator Global Assessment (IGA; 0–4) and PsO Area and Severity Index (PASI; 0–72), which measures disease severity. Patient‐reported outcome measures included patient‐reported pain and fatigue visual analog scale (VAS; 0–100), the Dermatology Life Quality Index (DLQI; 0–30) and EuroQol VAS (EQ VAS; 0–100). Work productivity was measured by the Work Productivity and Activity Impairment (WPAI) questionnaire.
Data analysis
Among patients without a diagnosis of PsA at enrolment, a descriptive summary of patient demographics, treatment history, clinical characteristics and patient‐reported outcome measures (including quality of life measures and work productivity) was provided. Continuous variables were summarized by the number of observations, the mean and the SD, or the median and interquartile range; categorical variables were summarized using frequency counts and percentages. Statistical comparisons between PEST groups (PEST score <3 and PEST score ≥3) were made using two‐sample t‐tests for continuous variables and chi‐squared or Fisher's exact tests for categorical variables.
Results
As of June 2016, 1516 of 1529 patients (99.1%) in the Corrona PsO Registry had non‐missing data on all five PEST questions. A total of 612 patients (40.4%) had dermatologist‐reported PsA at enrolment. Of the remaining 904 patients (59.6%) without dermatologist‐reported PsA, 112 (12.4%) had a PEST score ≥3 (Fig. 1). Of the 112 patients without dermatologist‐reported PsA who had a PEST score ≥3, patients most commonly answered ‘yes’ to ‘Have you ever had a swollen joint (or joints)?’ (89%) and ‘Has a doctor ever told you that you have arthritis?’ (86%), followed by ‘Do your finger nails have holes or pits?’ (63%), ‘Have you ever had pain in your heel?’ (62%) and ‘Have you had a finger or toe that was completely swollen and painful for no apparent reason?’ (52%).
Figure 1.
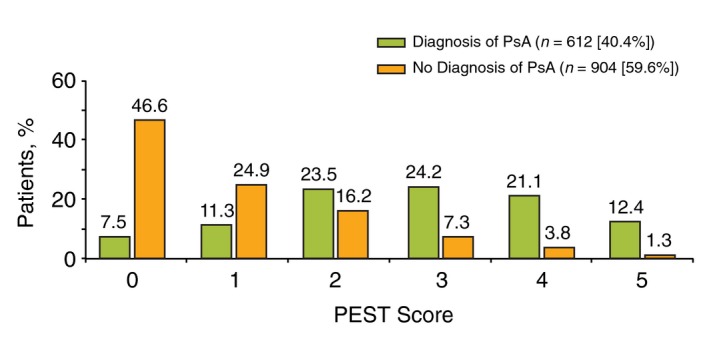
Distribution of Psoriasis Epidemiology Screening Tool (PEST) scores among patients with and without a diagnosis of psoriatic arthritis (PsA) in the Corrona Psoriasis Registry.
Demographics and treatment history
As shown in Table 2, patients with a PEST score ≥3 were significantly older than patients with a PEST score <3 [mean (SD), 52.9 (14.4) vs. 49.2 (15.2) years, respectively; P = 0.016]. They were also more likely to be female (55.4% vs. 42.8%; P = 0.012) and have a higher BMI [mean (SD), 32.2 (8.0) vs. 29.5 (6.9) kg/m2; P = 0.001], and were less likely to have a full‐ or part‐time job (53.2% vs. 70.9%; P < 0.001). Patients with a PEST score ≥3 were also more likely have certain comorbidities (cardiovascular disease, serious infection, depression/anxiety, hypertension and hyperlipidaemia) and a family history of PsO than patients with a PEST score <3 (all P < 0.05). No differences in use of treatments for PsO and smoking status were observed between groups (Table 2).
Table 2.
Demographic, clinical and treatment characteristics of patients with PsO and no diagnosis of PsA, stratified by PEST score
| Characteristic | PEST ≥3 n = 112 | PEST <3 n = 792 | P value |
|---|---|---|---|
| Age, mean (SD), years | 52.9 (14.4) | 49.2 (15.2) | 0.016 |
| Female, n (%) | 62 (55.4) | 339 (42.8) | 0.012 |
| White, n (%) | 96 (85.7) | 593 (74.9) | 0.012 |
| Hispanic, n (%) | 7 (6.5) | 75 (9.6) | 0.289 |
| Bodyweight, mean (SD), kg | 94.1 (25.3) | 86.4 (22.6) | 0.052 |
| BMI, mean (SD), kg/m2 | 32.2 (8.0) | 29.5 (6.9) | 0.001 |
| BMI (in kg/m 2 ) classifications, n (%) | n = 111 | n = 787 | |
| Normal/underweight (<25.0) | 22 (19.8) | 218 (27.7) | <0.001 |
| Overweight (25.0 to <30.0) | 26 (23.4) | 268 (34.1) | |
| Obese (≥30.0) | 63 (56.8) | 301 (38.2) | |
| Work status, n (%) | n = 111 | n = 787 | |
| Full/part time | 59 (53.2) | 561 (70.9) | <0.001 |
| Retired | 29 (26.1) | 121 (15.3) | |
| Disabled | 18 (16.2) | 30 (3.8) | |
| Other | 5 (4.5) | 79 (10.0) | |
| History of comorbidities, n (%) | n = 112 | n = 792 | |
| Cardiovascular disease† | 9 (8.0) | 21 (2.7) | 0.003 |
| Cancer‡ | 10 (8.9) | 64 (8.1) | 0.759 |
| Serious infection§ | 9 (8.0) | 26 (3.3) | 0.015 |
| Diabetes | 16 (14.3) | 85 (10.7) | 0.266 |
| Depression/anxiety¶ | 28 (25.0) | 113 (14.3) | 0.003 |
| Hypertension | 50 (44.6) | 260 (32.9) | 0.014 |
| Hyperlipidaemia | 39 (34.8) | 190 (24.0) | 0.014 |
| Psoriasis duration, mean (SD) years | 17.3 (14.8) | 14.6 (13.2) | <0.001 |
| Prior medication use, median (IQR) | |||
| Biologics†† | 1 (0–2) | 0 (0–1) | 0.186 |
| Non‐biologic systemic therapy‡‡ | 0 (0–1) | 0 (0–1) | 0.034 |
| Current medication use, n (%) | n = 112 | n = 792 | |
| Biologic monotherapy | 66 (58.9) | 490 (61.9) | 0.569 |
| Biologic combination therapy | 9 (8.0) | 48 (6.1) | 0.421 |
| Non‐biologic systemic use | 37 (33.0) | 254 (32.1) | 0.838 |
| Current smoking status, n (%) | n = 112 | n = 786 | |
| Non‐smoker§§ | 87 (77.7) | 660 (84.0) | 0.096 |
| Current | 25 (22.3) | 126 (16.0) | |
| Family history of psoriasis, n (%) | 14 (12.6) | 36 (4.6) | <0.001 |
†Combined histories of myocardial infarction, acute coronary syndrome, congestive heart failure and peripheral artery disease. ‡Includes non‐melanoma of the skin. §Infections that led to hospitalization or intravenous antibiotics, including joint/bursa, cellulitis, sinusitis, Candida infections, diverticulitis, sepsis, pneumonia, bronchitis, gastroenteritis, urinary tract infection, tuberculosis or others as specified by a physician. ¶Physician‐reported depression from the adverse event portion of enrolment form. ††Prior biologic use included adalimumab, alefacept, certolizumab, efalizumab, etanercept, golimumab, infliximab, ixekizumab, secukinumab, ustekinumab and other investigative biologics. ‡‡Prior non‐biologic use included acitretin, apremilast, cyclosporine, hydroxyurea, methotrexate, mycophenolate mofetil, sulfasalazine, 6‐thioguanine, tofacitinib and other non‐biologic therapies. §§Non‐smokers include never and former smokers.
BMI, body mass index; IQR, interquartile range; PEST, Psoriatic Arthritis Screening Tool; PsA, psoriatic arthritis; PsO, psoriasis.
Clinical characteristics
Patients with a PEST score ≥3 had a longer duration of PsO [mean (SD), 17.3 (14.8) vs. 14.6 (13.2) years; P < 0.001] than patients with a PEST score <3 (Table 2). Additionally, patients with a PEST score ≥3 were more likely to exhibit nail PsO (21.4% vs. 10.9%; P = 0.001; Fig. 2). However, the groups did not differ significantly in terms of other PsO morphology subgroups or PsO disease severity, as measured by categorical IGA score, per cent of affected BSA, and mean PASI score (Fig. 3).
Figure 2.
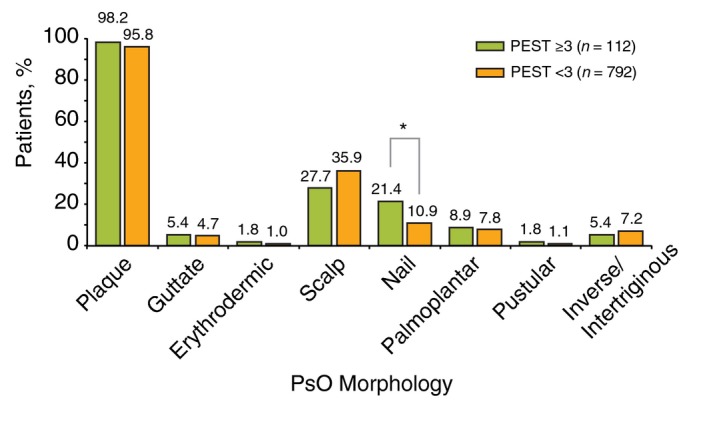
Psoriasis (PsO) morphology in patients stratified by Psoriasis Epidemiology Screening Tool (PEST) score; *P < 0.05.
Figure 3.
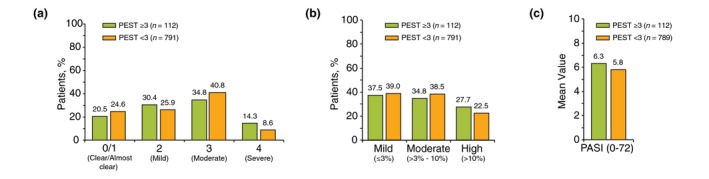
Disease severity among patients with Psoriasis Epidemiology Screening Tool (PEST) scores ≥3 and <3 as measured by (a) categorical Investigator's Global Assessment (IGA) score, (b) per cent of affected body surface area (BSA) and (c) mean Psoriatic Arthritis Severity Index (PASI) score.
Patient‐reported outcome measures
Among the 904 patients without dermatologist‐reported PsA, those with a PEST score ≥3 had significantly worse mean (SD) pain [28.3 (30.6) vs. 21.3 (28.7)] and fatigue [41.7 (27.8) vs. 25.0 (26.8)] than patients with a PEST score < 3 (P = 0.015 and <0.001, respectively; Table 3). Patients with a PEST score ≥3 also demonstrated significantly worse mean (SD) health status [EQ VAS, 67.0 (22.9) vs. 76.4 (21.2); P = 0.002] and DLQI score [8.1 (6.5) vs. 6.2 (5.9); P = 0.002]. Higher proportions of patients with a PEST score ≥3 had DLQI scores of 11–20 or 21–30 (‘very large’ and ‘extremely large’ effects on quality of life, respectively) than patients with a PEST score <3. In the WPAI domains, patients with a PEST score ≥3 had significantly worse mean (SD) activity impairment than those with a PEST score <3 [24.0% (28.6%) vs. 15.0% (23.6%); P < 0.001]; the other WPAI domains (work time missed, impairment while working and overall work impairment) were also worse in patients with a PEST score ≥3, but the differences were not statistically significant.
Table 3.
Patient‐reported outcome measures for patients with PsO and no diagnosis of PsA, stratified by PEST score
| Characteristic | PEST ≥3 n = 112 | PEST <3 n = 792 | P value |
|---|---|---|---|
| Patient pain (VAS 0–100), mean (SD) | 28.3 (30.6) | 21.3 (28.7) | 0.015 |
| Patient‐reported fatigue (VAS 0–100), mean (SD) | 41.7 (27.8) | 25.0 (26.8) | <0.001 |
| EQ VAS (0–100), mean (SD) | 67.0 (22.9) | 76.4 (21.2) | <0.001 |
| DLQI (0–30), mean (SD) | 8.1 (6.5) | 6.2 (5.9) | 0.002 |
| DLQI (‘effect on life’), n (%) | n = 112 | n = 792 | |
| None (0–1) | 21 (18.8) | 196 (24.7) | 0.035 |
| Small (2–5) | 27 (24.1) | 258 (32.6) | |
| Moderate (6–10) | 27 (24.1) | 160 (20.2) | |
| Very large (11–20) | 33 (29.5) | 145 (18.3) | |
| Extremely large (21–30) | 4 (3.6) | 33 (4.2) | |
| WPAI summary scores | |||
| Currently employed, n (%) | 60 (53.6) | 561 (71.0) | |
| WPAI domains, mean (SD) [n] | |||
| % Work time missed | 2.9 (7.9) [n = 57] | 2.6 (10.7) [n = 505] | 0.795 |
| % Impairment while working | 13.3 (21.9) [n = 57] | 10.2 (18.7) [n = 502] | 0.238 |
| % Overall work impairment | 14.8 (23.5) [n = 57] | 11.5 (20.2) [n = 502] | 0.251 |
| % Activity impairment | 24.0 (28.6) [n = 111] | 15.0 (23.6) [n = 787] | <0.001 |
DLQI, Dermatology Life Quality Index; EQ VAS, EuroQol visual analogue scale; PEST, Psoriasis Epidemiology Screening Tool; PsA, psoriatic arthritis; PsO, psoriasis; VAS, visual analog scale; WPAI, Work Productivity and Activity Impairment.
Discussion
Among the 904 patients without dermatologist‐reported PsA in the US‐based Corrona PsO Registry as of June 2016, 112 (12.4%) had a PEST score ≥3 at enrolment, indicating a need for further evaluation for a possible diagnosis of PsA. Patients with PEST score ≥3 were more likely to have nail disease, a longer duration of PsO, a higher BMI, and worse pain, fatigue and health‐related quality of life. Given current opinion that nail disease and obesity are among the strongest predictors for development of PsA,27 these results also suggest that further evaluation for a possible diagnosis of PsA is needed for patients with a PEST score ≥3.
Results of screening questionnaires administered by dermatologists, such as PEST, may allow for timely rheumatologist referral and lead to earlier diagnosis of PsA in patients with PsO. Because joint erosions have been documented in 27% of patients within 10 months of PsA onset and in 47% of patients within 2 years, early screening and diagnosis of PsA may result in earlier therapeutic intervention28; observational studies have shown improved patient outcomes in patients with PsA who are treated soon after a diagnosis of PsA.17, 29, 30 In addition, improved outcomes after early detection and treatment may be long‐term, as shown in a study of the Swedish Early PsA Register, in which a shorter duration of PsA symptoms and lower health assessment questionnaire scores independently predicted achievement of minimal disease activity at the 5‐year follow‐up.31 Furthermore, the long‐term burden of PsA eventually increases the mean cost of health care, particularly among those with critical loss of physical function.32 Therefore, the loss of productivity and the availability of effective treatment also warrant earlier screening and detection of PsA.33
Some of the screening tests developed for PsA present unique features that may be advantageous in clinical settings. For instance, the PASE can also be used to monitor a patient's response to therapy,23 while the PEST, PASQ and ToPAS questionnaires include visual aids so that patients can quickly and easily identify areas of pain, stiffness or swelling.18, 24, 25 The PEST showed high specificity and sensitivity during its development,18 with similar results in one real‐world study of patients with PsO evaluated by a dermatologist.34 Lower specificity of PEST was observed in another real‐world study, but this may have been related to study design and patient population, and the results were still comparable to those with other screening tools.35 The PEST demonstrated superior performance compared with PAQ, with a sensitivity of 0.92 and a specificity of 0.78.18 Two ‘head‐to‐head’ evaluations of three screening tools (comparing PASQ, PEST and ToPAS and PEST, EARP and PASE, respectively) in detecting PsA concluded that the PEST had the most favourable balance between sensitivity and specificity to screen for PsA.25, 36 Additional head‐to‐head comparisons with other available tools should be performed to determine the optimal tool to identify patient populations at risk for developing PsA, thus leading to earlier accurate diagnosis and treatment of PsA in clinical practice.36 The use of screening tools can be beneficial in the detection of PsA, and comprehensive efforts to validate them in multiple clinical settings must continue, along with collection of critical feedback from patients and clinicians.
As with any observational study, there are possibilities of other unmeasured confounding variables. Care received by patients enrolled in the Corrona registry may not be representative of the frequency or type received by the general population of patients with PsO. All diagnoses of PsA were reported by dermatologists, and confirmation by a rheumatologist is not reported. Some patients may have had musculoskeletal symptoms that resulted in evaluation for PsA by their dermatologists and a subsequent diagnosis of another non‐PsA musculoskeletal disorder (e.g. osteoarthritis or fibromyalgia). Additionally, all patients initiated or switched to a systemic biologic or non‐biologic for treatment of PsO within 12 months of enrolment, which may have selected for patients with unstable or more active disease. Some of these agents are indicated for both PsO and PsA, and as such, select subclinical PsA symptoms may already have been treated, thus affecting the patients’ responses to the PEST and potentially reducing their scores. Furthermore, no corrections for potential confounders in the multivariate analyses were performed. Further research is needed to characterize patients by individual PEST score and to assess outcomes over time.
In conclusion, using the validated PEST, over one‐tenth of patients with PsO enrolled in the US‐based Corrona PsO Registry were identified as having PEST scores of ≥3, raising the possibility that many of these patients could have undiagnosed PsA and highlighting a need for improved screening for PsA in dermatology settings. Appropriate and earlier care of these patients with possible undiagnosed PsA is important because they are more likely to have nail PsO, higher activity impairment and worse health‐related quality of life.
Acknowledgements
Support for third‐party writing assistance for this manuscript, furnished by Kheng Bekdache, PhD, and Nicola Gillespie, DVM, of Health Interactions, Inc, was provided by Novartis Pharmaceuticals Corporation, East Hanover, NJ.
Conflicts of interest
P. J. Mease has received research grants from AbbVie, Amgen, BMS, Celgene, Eli Lilly and Company, Novartis, Pfizer, SUN Bioscience, and UCB; consulting fees from AbbVie, Amgen, BMS, Celgene, Corrona, Novartis, Eli Lilly and Company, Janssen, Merck, Pfizer, SUN, and UCB; and speakers’ bureau fees from AbbVie, Amgen, BMS, Celgene, Genentech, Janssen, Pfizer, and UCB. J. B. Palmer and P. Hur are employees of Novartis. B. E. Strober is a consultant for AbbVie, Amgen, Almirall, AstraZeneca, Boehringer Ingelheim, Celgene, Cutanea‐Maruho, Dermira, Eli Lilly and Company, Janssen, Leo Pharma, Medac, Novartis, Ortho Dermatologics, Pfizer, Sun Pharma, and UCB; an investigator for AbbVie, Amgen, Boehringer Ingelheim, Celgene, GlaxoSmithKline, Novartis, Eli Lilly and Company, Janssen, Merck, and Sun Pharma (payments to the University of Connecticut); and a scientific director for the Corrona Psoriasis Registry; he receives grant support through the University of Connecticut Fellowship Program from AbbVie and Janssen. M. Lebwohl is an employee of Icahn School of Medicine at Mount Sinai, which receives research funds from AbbVie, Boehringer Ingelheim, Celgene, Eli Lilly and Company, Incyte, Janssen/Johnson & Johnson, Leo Pharma, Medimmune/AstraZeneca, Novartis, Pfizer, Sciderm, UCB, Valeant, and Vidac; he has received consulting fees for Allergan plc, Aqua, Arcutis, Boehringer Ingelheim, Leo Pharma, Menlo, and Promius. C. Karki was an employee of Corrona at the time of this study and writing of this manuscript. G. W. Reed is an employee and stockholder of Corrona. C. J. Etzel is an employee and stockholder of Corrona, and is a member of an advisory board for Merck. J. D. Greenberg is an employee and shareholder of Corrona; and has received consulting fees from Eli Lilly and Company, Genentech, Janssen, Novartis, and Pfizer. P. S. Helliwell has received consulting fees from Amgen, BMS, Eli Lilly and Company, Janssen, Pfizer, and UCB; and speaking fees from AbbVie, Eli Lilly and Company, Janssen, Novartis, Pfizer, and UCB.
Funding source
Corrona has been supported through contracted subscriptions in the last 2 years by AbbVie, Amgen, Boehringer Ingelheim, Bristol‐Myers Squibb, Crescendo, Eli Lilly and Company, Genentech, Gilead, GSK, Horizon Pharma USA, Janssen, Momenta Pharmaceuticals, Novartis, Pfizer Inc, Roche, Merck, UCB, and Valeant. The study design and conduct were the result of a collaborative effort between Corrona and Novartis, and financial support for the study was provided by Novartis. Novartis participated in the interpretation of data and review and approval of the manuscript.
References
- 1. Parisi R, Symmons DP, Griffiths CE et al Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol 2013; 133: 377–385. [DOI] [PubMed] [Google Scholar]
- 2. Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol 2017; 31: 205–212. [DOI] [PubMed] [Google Scholar]
- 3. Gelfand JM, Gladman DD, Mease PJ et al Epidemiology of psoriatic arthritis in the population of the United States. J Am Acad Dermatol 2005; 53: 573. [DOI] [PubMed] [Google Scholar]
- 4. Mease PJ, Gladman DD, Papp KA et al Prevalence of rheumatologist‐diagnosed psoriatic arthritis in patients with psoriasis in European/North American dermatology clinics. J Am Acad Dermatol 2013; 69: 729–735. [DOI] [PubMed] [Google Scholar]
- 5. Gladman DD, Antoni C, Mease P et al Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis 2005; 64: 14–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gladman DD. Clinical features and diagnostic considerations in psoriatic arthritis. Rheum Dis Clin North Am 2015; 41: 569–579. [DOI] [PubMed] [Google Scholar]
- 7. Huynh D, Kavanaugh A. Psoriatic arthritis: current therapy and future approaches. Rheumatology (Oxford) 2015; 54: 20–28. [DOI] [PubMed] [Google Scholar]
- 8. Kaushik SB, Lebwohl MG. CME part I psoriasis: which therapy for which patient psoriasis comorbidities and preferred systemic agents. J Am Acad Dermatol 2019; 80: 27–40. [DOI] [PubMed] [Google Scholar]
- 9. Greb JE, Goldminz AM, Elder JT et al Psoriasis. Nat Rev Dis Primers 2016; 2: 16082. [DOI] [PubMed] [Google Scholar]
- 10. Haroon M, Kirby B, FitzGerald O. High prevalence of psoriatic arthritis in patients with severe psoriasis with suboptimal performance of screening questionnaires. Ann Rheum Dis 2013; 72: 736–740. [DOI] [PubMed] [Google Scholar]
- 11. Chandran V, Cook RJ, Edwin J et al Soluble biomarkers differentiate patients with psoriatic arthritis from those with psoriasis without arthritis. Rheumatology (Oxford) 2010; 49: 1399–1405. [DOI] [PubMed] [Google Scholar]
- 12. Eder L, Chandran V, Gladman DD. What have we learned about genetic susceptibility in psoriasis and psoriatic arthritis? Curr Opin Rheumatol 2015; 27: 91–98. [DOI] [PubMed] [Google Scholar]
- 13. Kaeley GS, Eder L, Aydin SZ et al Dactylitis: a hallmark of psoriatic arthritis. Semin Arthritis Rheum 2018; 48: 263–273. [DOI] [PubMed] [Google Scholar]
- 14. Kaeley GS, Eder L, Aydin SZ et al Enthesitis: a hallmark of psoriatic arthritis. Semin Arthritis Rheum 2018; 48: 35–43. [DOI] [PubMed] [Google Scholar]
- 15. Villani AP, Rouzaud M, Sevrain M et al Prevalence of undiagnosed psoriatic arthritis among psoriasis patients: systematic review and meta‐analysis. J Am Acad Dermatol 2015; 73: 242–248. [DOI] [PubMed] [Google Scholar]
- 16. Reich K, Kruger K, Mossner R, Augustin M. Epidemiology and clinical pattern of psoriatic arthritis in Germany: a prospective interdisciplinary epidemiological study of 1511 patients with plaque‐type psoriasis. Br J Dermatol 2009; 160: 1040–1047. [DOI] [PubMed] [Google Scholar]
- 17. Haroon M, Gallagher P, FitzGerald O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis 2015; 74: 1045–1050. [DOI] [PubMed] [Google Scholar]
- 18. Ibrahim GH, Buch MH, Lawson C et al Evaluation of an existing screening tool for psoriatic arthritis in people with psoriasis and the development of a new instrument: the Psoriasis Epidemiology Screening Tool (PEST) questionnaire. Clin Exp Rheumatol 2009; 27: 469–474. [PubMed] [Google Scholar]
- 19. Raychaudhuri SP, Wilken R, Sukhov AC et al Management of psoriatic arthritis: early diagnosis, monitoring of disease severity and cutting edge therapies. J Autoimmun 2017; 76: 21–37. [DOI] [PubMed] [Google Scholar]
- 20. Tinazzi I, Adami S, Zanolin EM et al The early psoriatic arthritis screening questionnaire: a simple and fast method for the identification of arthritis in patients with psoriasis. Rheumatology (Oxford) 2012; 51: 2058–2063. [DOI] [PubMed] [Google Scholar]
- 21. Harle P, Letschert K, Wittig B, Mrowietz U. Sensitivity of the GEPARD patient questionnaire to identify psoriatic arthritis in patients with psoriasis in daily practice: the GEPARD‐Life Study. Dermatology 2016; 232: 597–605. [DOI] [PubMed] [Google Scholar]
- 22. Alenius GM, Stenberg B, Stenlund H et al Inflammatory joint manifestations are prevalent in psoriasis: prevalence study of joint and axial involvement in psoriatic patients, and evaluation of a psoriatic and arthritic questionnaire. J Rheumatol 2002; 29: 2577–2582. [PubMed] [Google Scholar]
- 23. Husni ME, Meyer KH, Cohen DS et al The PASE questionnaire: pilot‐testing a psoriatic arthritis screening and evaluation tool. J Am Acad Dermatol 2007; 57: 581–587. [DOI] [PubMed] [Google Scholar]
- 24. Khraishi M, Chouela E, Bejar M et al High prevalence of psoriatic arthritis in a cohort of patients with psoriasis seen in a dermatology practice. J Cutan Med Surg 2012; 16: 122–127. [DOI] [PubMed] [Google Scholar]
- 25. Gladman DD, Schentag CT, Tom BD et al Development and initial validation of a screening questionnaire for psoriatic arthritis: the Toronto Psoriatic Arthritis Screen (ToPAS). Ann Rheum Dis 2009; 68: 497–501. [DOI] [PubMed] [Google Scholar]
- 26. Tom BD, Chandran V, Farewell VT et al Validation of the Toronto Psoriatic Arthritis Screen version 2 (ToPAS 2). J Rheumatol 2015; 42: 841–846. [DOI] [PubMed] [Google Scholar]
- 27. McHugh NJ. Verna Wright lecture: psoriatic arthritis: the need for early intervention. J Rheumatol Suppl 2015; 93: 10–13. [DOI] [PubMed] [Google Scholar]
- 28. Kane D, Stafford L, Bresnihan B, FitzGerald O. A prospective, clinical and radiological study of early psoriatic arthritis: an early synovitis clinic experience. Rheumatology (Oxford) 2003; 42: 1460–1468. [DOI] [PubMed] [Google Scholar]
- 29. Gladman DD, Thavaneswaran A, Chandran V, Cook RJ. Do patients with psoriatic arthritis who present early fare better than those presenting later in the disease? Ann Rheum Dis 2011; 70: 2152–2154. [DOI] [PubMed] [Google Scholar]
- 30. Tillett W, Jadon D, Shaddick G et al Smoking and delay to diagnosis are associated with poorer functional outcome in psoriatic arthritis. Ann Rheum Dis 2013; 72: 1358–1361. [DOI] [PubMed] [Google Scholar]
- 31. Theander E, Husmark T, Alenius GM et al Early psoriatic arthritis: short symptom duration, male gender and preserved physical functioning at presentation predict favourable outcome at 5‐year follow‐up. Results from the Swedish Early Psoriatic Arthritis Register (SwePsA). Ann Rheum Dis 2014; 73: 407–413. [DOI] [PubMed] [Google Scholar]
- 32. Poole CD, Lebmeier M, Ara R et al Estimation of health care costs as a function of disease severity in people with psoriatic arthritis in the UK. Rheumatology (Oxford) 2010; 49: 1949–1956. [DOI] [PubMed] [Google Scholar]
- 33. Gladman DD. Recent advances in understanding and managing psoriatic arthritis. F1000Res 2016; 5: 2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mease PJ, Gladman DD, Helliwell P et al Comparative performance of psoriatic arthritis screening tools in patients with psoriasis in European/North American dermatology clinics. J Am Acad Dermatol 2014; 71: 649–655. [DOI] [PubMed] [Google Scholar]
- 35. Coates LC, Aslam T, Al Balushi F et al Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol 2013; 168: 802–807. [DOI] [PubMed] [Google Scholar]
- 36. Karreman MC, Weel AEAM, van der Ven M et al Performance of screening tools for psoriatic arthritis: a cross‐sectional study in primary care. Rheumatology (Oxford) 2017; 56: 597–602. [DOI] [PubMed] [Google Scholar]


