Abstract
Objective
Real‐world clinical effectiveness of liraglutide 3.0 mg, in combination with diet and exercise, was investigated 4 and 6 months post initiation. Changes in absolute and percent body weight were examined from baseline.
Methods
A cohort of liraglutide 3.0 mg initiators in 2015 and 2016 was identified from six Canadian weight‐management clinics. Post initiation values at 4 and 6 months were compared with baseline values using a paired t test.
Results
The full cohort consisted of 311 participants, with 210 in the ≥ 4‐month persistence group and 167 in the ≥ 6‐month persistence group. Average baseline BMI was 40.7 kg/m2, and weight was 114.8 kg. There was a significant change in body weight 6 and 4 months after initiation of treatment in persistent subjects (≥ 6‐month: −8.0 kg, P < 0.001; ≥ 4‐month: −7.0 kg, P < 0.001) and All Subjects, regardless of persistence (−7.3 kg; P < 0.001). Percentage change in body weight from baseline was −7.1% in the ≥ 6‐month group and −6.3% in the ≥ 4‐month group, and All Subjects lost 6.5% body weight. Of participants in the ≥ 6‐month group, 64.10% and 34.5% lost ≥ 5% and > 10% body weight, respectively.
Conclusions
In a real‐world setting, liraglutide 3.0 mg, when combined with diet and exercise, was associated with clinically meaningful weight loss.
Introduction
Global rates of overweight and obesity are increasing at an alarming rate, with projections estimating the number of afflicted individuals to reach 1.35 billion and 573 million, respectively, by 2030 1. In 2014, roughly 14.2 million (54%) adult Canadians self‐reported as having overweight or obesity 2, with 28.1% of Canadians classified as having obesity in 2015 3.
Several prominent associations, including the Canadian Medical Association, recognize obesity as a chronic relapsing primary disease characterized by excessive accumulation and storage of fat in the body 4, 5, 6, 7. Obesity is associated with many serious comorbidities, including hypertension, type 2 diabetes (T2D), cardiovascular disease, osteoarthritis, and certain types of cancer 8, 9, 10. The continued rise in the prevalence of obesity makes prevention and treatment public health priorities because it has been shown that an increase in BMI is correlated with a reduction of life expectancy and with an increase of obesity‐related comorbidities 11, 12, 13.
Although it has been shown that a weight reduction of 5% to 10% can provide clinically relevant improvements in obesity‐related comorbidities and quality of life 14, 15, 16, 17, 18, 19, average weight loss achieved from lifestyle intervention is 3%, which does not lead to substantial improvements of clinical indicators 20. For some individuals, pharmacotherapy and bariatric surgery are recommended for long‐term weight management 7, 21, 22, 23 and may serve as effective complements to traditional approaches. In fact, pharmacological intervention, as an adjunct to lifestyle intervention, is recommended to assist in reducing obesity‐related symptoms for appropriate adults with overweight or obesity who are not attaining or are unable to maintain clinically important weight loss with diet and exercise therapy 21.
Liraglutide, an acylated glucagon‐like peptide 1 (GLP‐1) receptor agonist with 97% homology to human GLP‐1, is used for glycemic control in T2D but is also a physiological regulator of appetite 24, 25, 26. In 2015, liraglutide was approved for use in Canada at a daily dose of 3.0 mg for weight management, as an adjunct to a reduced‐calorie diet and increased physical activity 27.
The clinical efficacy of liraglutide 3.0 mg has been established in randomized controlled clinical trials. Although these clinical trials offer a wealth of valuable information about safety and efficacy, the data derived from the highly controlled and specific population in the trials might not translate to a real‐world setting 28. Patients in the real world are subject to an uncontrolled environment and often experience a wider range of comorbidities and variable adherence to treatment. Despite several trial‐extension studies, the real‐world clinical effectiveness of liraglutide 3.0 mg has yet to be investigated. Using real‐world data more than 2 years post launch, this study investigates the clinical effectiveness of liraglutide 3.0 mg treatment, in combination with diet and exercise, among a real‐world sample of patients from a medically supervised interdisciplinary obesity‐management program.
Methods
Data source
This study used a database of deidentified electronic medical records (EMRs) from the Wharton Medical Clinic (WMC), a network of six secondary‐care weight‐ and diabetes‐management clinics funded by the Ontario Health Insurance Plan and based in Southern Ontario, Canada. Further information about WMC and the specific treatments patients engage in can be found in online Supporting Information (Clinical Setting). The database contained demographic, diagnosis, prescription, and laboratory information (extracted directly from the EMR). Prior to study initiation and without study‐sponsor involvement, fields such as ethnicity, adherence to nonpharmaceutical interventions for weight management (e.g., physical activity, diet), adherence to medication, smoking history, and failure of previous weight‐management interventions were standard coded by WMC (from the EMR). As of August 2008, patients at WMC indicated whether they were willing to consent to the use of their electronic medical data for research purposes and were informed that their participation or lack of participation would not alter treatment. Patients who opted out of, or had not expressly given consent to, their data being used for research by WMC were not included in this study. To maintain the anonymity of patients, the database was deidentified by Privacy Analytics (Ottawa, Canada). Further details on the deidentification process are available in online Supporting Information. To ensure that the deidentification process did not compromise data integrity, the entire original and final data sets were compared by WMC for 20 participants, and key demographic data were compared for all participants in the final analysis data set. All data sharing was conducted in a remote, secure manner.
Study design and ethics
A retrospective, observational, pre‐post study design was applied to investigate weight 4 and 6 months after initiation of liraglutide 3.0 mg compared with weight at initiation of treatment. Data were available for the period of September 15, 2014, to April 30, 2017. To allow for a 12‐month look‐back period to collect baseline characteristics and for a 6‐month follow‐up, the selection period for treatment initiation was from September 15, 2015, to September 30, 2016. The index date was defined as the date a participant initiated liraglutide 3.0 mg treatment. Estimates of the initiation date were made from assumptions based on back calculations described in Table 1. The study design and protocol were reviewed and approved by the Center for IRB Intelligence.
Table 1.
Index date calculation
| Reported dose, mg | Assumed initiation date |
|---|---|
| 0.0‐0.6 | Initiated on date of visit to WMC |
| 0.7‐1.2 | Initiated 1 week prior to visit to WMC |
| 1.3‐1.8 | Initiated 2 weeks prior to visit to WMC |
| 1.9‐2.4 | Initiated 3 weeks prior to visit to WMC |
| 2.5‐3.0 | Initiated 4 weeks prior to visit to WMC |
Participants
Participants were included in the study if they fulfilled all of the following criteria: had at least one prescription for liraglutide 3.0 mg at the discretion of the physician (further details in online Supporting Information [WMC Weight Management Protocol]) and initiated treatment during the selection period (September 15, 2015, to September 30, 2016), were ≥ 18 years of age at the index date, had at least one reported baseline weight measurement within 3 months prior to the index date, had at least one visit to WMC within 6 months after the index date, and (prior to index date) had BMI ≥ 30 kg/m2 or BMI ≥ 27 kg/m2 with at least one weight‐related comorbidity (e.g., hypertension, T2D, dyslipidemia).
Participants were excluded if they fulfilled any of the following criteria: had previously taken liraglutide 3.0 mg; had previously taken or were currently taking GLP‐1 receptor agonists liraglutide 1.2 or 1.8 mg, exenatide, exenatide extended release, or dulaglutide; and had ever had bariatric surgery. Participants meeting all of the inclusion criteria and none of the exclusion criteria were included in the All Subjects cohort. Two additional cohorts were defined as participants known to be persistent on liraglutide 3.0 mg for at least 4 months (≥ 4‐month) and those known to be persistent on liraglutide 3.0 mg for at least 6 months (≥ 6‐month). Participants in the ≥ 4‐month cohort were assessed at 4 months, aligning to the monograph recommendation of 4 weeks of titration as well as with the monograph review point that recommends treatment cessation in participants in whom at least 5% of initial body weight is not lost following 12 weeks of adherence to 3.0 mg daily 26.
Variables and outcomes
Baseline demographics and adherence to weight‐management interventions (physical activity and caloric recommendations) were collected from the visit prior to the index date; baseline comorbid conditions were defined as evidence of the condition in the 12 months prior to index, and baseline physiology data were those closest to the index date in the 12 months prior, with the exception of weight, which had to be within the 3 months prior to index. Definitions of the baseline variables can be found in Supporting Information Table S1. The primary outcome was weight 6 months after the index date. There were several secondary outcomes. Percentage loss of body weight was defined as the difference in weight at follow‐up compared with the weight recorded at baseline, i.e., ([body weight at follow‐up − body weight at baseline] ÷ body weight at baseline) × 100. Participants were flagged if they lost at least 5%, as well as more than 10%, of their body weight. Weight 4 months after liraglutide 3.0 mg initiation, as well as glycated hemoglobin A1c (HbA1c), systolic blood pressure (SBP), and diastolic blood pressure (DBP) 6 months after liraglutide 3.0 mg initiation, was also reported. The value closest to the follow‐up date, within 30 days, was used for all outcomes.
Statistical analysis
Baseline demographics and clinical data were reported for all subjects as n (percentage) and mean (SD) or median (interquartile range), as appropriate. Body weight, HbA1c, SBP, and DBP at 6 months post index were compared with their respective baseline values using paired t test analyses for subjects in the ≥ 6‐month cohort. As sensitivity analyses, weight, HbA1c, SBP, and DBP analyses were repeated for the All Subjects cohort at 6 months, and weight analyses were repeated for subjects in the ≥ 4‐month cohort, comparing baseline and postindex values at 4 months. After accounting for baseline weight, the impact of baseline variables (age [in years], sex [reference: male], ethnicity [reference: white], prediabetes [reference: no], and diabetes [reference: no]) was examined on body weight at 6 months using multivariate linear regression. The mean (SD) percentage weight loss, as well as the number and percentage of subjects with at least a 5% loss in body weight and greater than a 10% loss in body weight, was reported for subjects in all three cohorts. The impact of the same variables on at least a 5% loss in body weight and greater than a 10% loss in body weight was examined using logistic regression. Missing data were not imputed but were reported as a proportion for all analyses. All analyses were conducted by IQVIA (Montreal, Canada) using SAS version 9.3 (SAS Institute, Inc., Cary, North Carolina).
Results
Cohort and baseline characteristics
Of 849 consented WMC patients included in the database during the study selection period, 355 patients had at least one prescription for, and had initiated treatment with, liraglutide 3.0 mg. The All Subjects cohort comprised 311 subjects who met all of the inclusion criteria and none of the exclusion criteria (Figure 1). One patient reported use of an additional weight‐loss medication (orlistat) alongside liraglutide 3.0 mg; however, this patient did not meet the inclusion criteria for either the ≥ 4‐ or the ≥ 6‐month cohort. Of those subjects, 210 were persistent on liraglutide 3.0 mg for at least 4 months (42 were lost to follow‐up, and 59 actively discontinued liraglutide 3.0 mg), and 167 were persistent for at least 6 months (58 were lost to follow‐up, and 86 actively discontinued liraglutide 3.0 mg). The data set was 83.0% female. For All Subjects, the average age was 49.7 years. At baseline, the average BMI was 40.68 kg/m2, and the average weight was 114.8 kg. Among All Subjects, 74.9% had normoglycemia, 19.9% had prediabetes, and 5.1% had diabetes; 33.1% had evidence of hypertension, and 61.1% had evidence of dyslipidemia. Average baseline values for HbA1c, SBP, and DPB were 5.8%, 127.2 mmHg, and 77.2 mmHg, respectively (Table 2).
Figure 1.
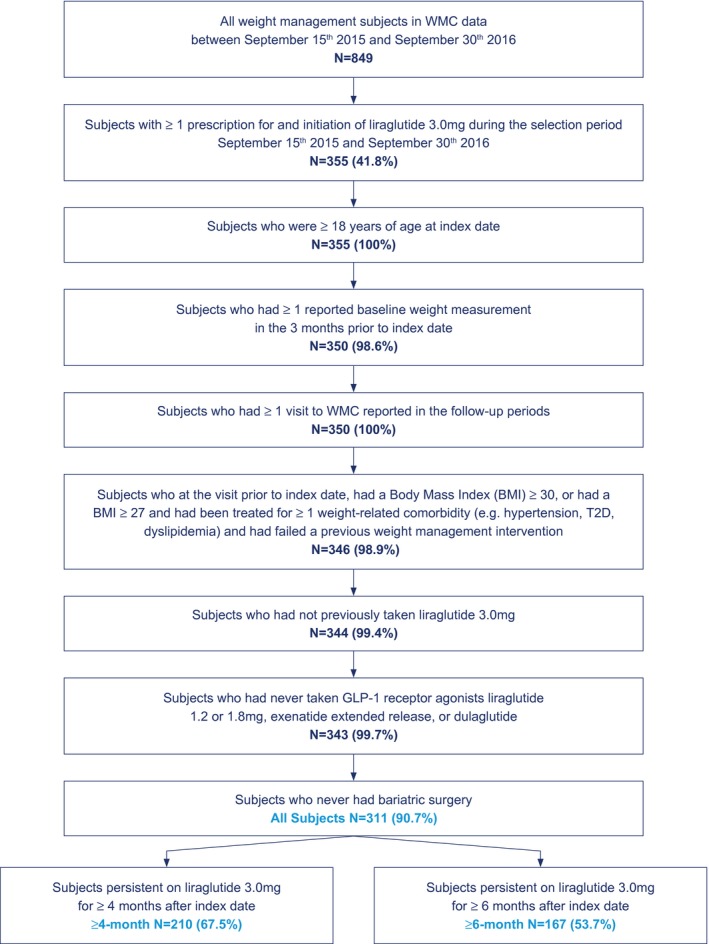
Flowchart of study population. [Colour figure can be viewed at wileyonlinelibrary.com]
Table 2.
Baseline characteristics
| All Subjects, N = 311 | |
|---|---|
| Age | |
| Mean (SD) | 49.7 (11.6) |
| Median (IQR) | 50.0 (42.0‐58.0) |
| Sex, n (%) | |
| Male | 53 (17.0) |
| Female | 258 (83.0) |
| Ethnicity, n (%) | |
| Missing | 22 (7.1) |
| White | 241 (77.5) |
| Aboriginal | 2‐5 (0.6‐1.6) |
| African American | 2‐5 (0.6‐1.6) |
| African heritage | 2‐5 (0.6‐1.6) |
| East Asian | 2‐5 (0.6‐1.6) |
| South Asian | 10 (3.2) |
| West Indian black | 8 (2.6) |
| Other | 17 (5.5) |
| Index year, n (%) | |
| 2015 | 16 (5.1) |
| 2016 | 295 (94.9) |
| BMI | |
| Mean (SD) | 40.7 (7.1) |
| Median (IQR) | 39.9 (35.1‐44.9) |
| BMI categories, n (%) | |
| Overweight | 2‐5 (0.3‐1.3) |
| Class 1 obesity | 70 (22.5) |
| Class 2 obesity | 83 (26.7) |
| Class 3 obesity | 155 (49.8) |
| Weight | |
| Mean (SD) | 114.8 (26.3) |
| Median (IQR) | 111.1 (95.3‐129.7) |
| HbA1c | |
| Missing, n (%) | 143 (46.0) |
| Mean (SD) | 5.8 (0.9) |
| Median (IQR) | 5.7 (5.4‐6.1) |
| SBP | |
| Mean (SD) | 127.2 (11.2) |
| Median (IQR) | 126.0 (120.0‐135.0) |
| DBP | |
| Mean (SD) | 77.2 (7.2) |
| Median (IQR) | 78.0 (72.0‐82.0) |
| Diabetes, n (%) | |
| None | 233 (74.9) |
| Prediabetes | 62 (19.9) |
| T2D | 16 (5.1) |
| Hypertension, n (%) | |
| No | 208 (66.9) |
| Yes | 103 (33.1) |
| Dyslipidemia, n (%) | |
| No | 121 (38.9) |
| Yes | 190 (61.1) |
| Smoking status, n (%) | |
| Missing | 2‐5 (0.1‐1.1) |
| Nonsmoker | 169 (54.3) |
| Current smoker | 25 (8.0) |
| Ex‐smoker | 116 (37.3) |
| Adherence to exercise program, n (%) | |
| Missing | 87 (28.0) |
| No physical activity | 41 (13.2) |
| Some physical activity | 78 (25.1) |
| Meeting or exceeding physical activity recommendations | 105 (33.8) |
| Adherence to diet, n (%) | |
| Missing | 178 (57.2) |
| Exceeding caloric prescription every day | 16 (5.1) |
| Meeting caloric prescription sometimes | 40 (12.9) |
| Always meeting caloric recommendations | 77 (24.8) |
DBP, diastolic blood pressure; HbA1c, glycated hemoglobin A1c; IQR, interquartile range; SBP, systolic blood pressure; T2D, type 2 diabetes.
Differences in absolute weight
On average, there was an 8.0‐kg decrease in body weight (P < 0.001) between baseline and 6 months for the 145 subjects in the ≥ 6‐month cohort who were persistent for at least 6 months and had a reported 6‐month weight value. Significant weight loss was also observed in subjects persistent on treatment for ≥ 4 months (−7.0 kg; P < 0.001) and in All Subjects, regardless of persistence (−7.3 kg; P < 0.001) (Table 3 and Figure 2A).
Table 3.
Unadjusted outcomes
| Outcome (cohort) | Cohort N | N a | n (%) | Baseline, mean (SD) | Follow‐up, mean (SD) | Difference, mean (SD) | P value |
|---|---|---|---|---|---|---|---|
| Weight at 6‐months (≥ 6‐month), kg | 167 | 145 | N/A | 117.6 (31.0) | 109.6 (31.0) | −8.00 (6.12) | <0.001 |
| Weight at 6 months (All Subjects), kg | 311 | 203 | N/A | 115.5 (28.4) | 108.2 (28.2) | −7.28 (6.20) | <0.001 |
| Weight at 4 months (≥ 4‐month), kg | 210 | 187 | N/A | 115.9 (28.8) | 108.9 (28.5) | −7.00 (4.53) | <0.001 |
| % Weight loss at 6 mo (≥ 6‐month), % | 167 | 145 | N/A | N/A | N/A | −7.1 (5.4) | N/A |
| % Weight loss at 6 mo (All Subjects), % | 311 | 203 | N/A | N/A | N/A | −6.5 (5.5) | N/A |
| % Weight loss at 4 mo (≥ 4‐month), % | 210 | 187 | N/A | N/A | N/A | −6.3 (4.1) | N/A |
| Loss of ≥ 5 % body weight at 6 mo (≥ 6‐month) | 167 | 145 | 93 (64.1) | N/A | N/A | N/A | N/A |
| Loss of ≥ 5 % body weight at 6 mo (All Subjects) | 311 | 203 | 119 (58.6) | N/A | N/A | N/A | N/A |
| Loss of ≥ 5 % body weight at 4 mo (≥ 4‐month) | 210 | 187 | 118 (63.1) | N/A | N/A | N/A | N/A |
| Loss of > 10% body weight at 6 mo (≥ 6‐month) | 167 | 145 | 50 (34.5) | N/A | N/A | N/A | N/A |
| Loss of > 10% body weight at 6 mo (All Subjects) | 311 | 203 | 60 (29.6) | N/A | N/A | N/A | N/A |
| Loss of > 10% body weight at 4 mo (≥ 4‐month) | 210 | 187 | 34 (18.2) | N/A | N/A | N/A | N/A |
| HbA1c at 6 mo (≥ 6‐month), % | 167 | 30 | N/A | 5.7 (0.5) | 5.3 (0.4) | −0.35 (0.28) | <0.001 |
| HbA1c at 6 mo (All Subjects), % | 311 | 39 | N/A | 5.6 (0.5) | 5.3 (0.4) | −0.30 (0.31) | <0.001 |
| SBP at 6 mo (≥ 6‐month), mmHg | 167 | 136 | N/A | 127.8 (10.5) | 124.8 (11.7) | −2.98 (10.67) | <0.01 |
| SBP at 6 mo (All Subjects), mmHg | 311 | 185 | N/A | 127.6 (10.6) | 125.4 (12.2) | −2.23 (11.20) | <0.01 |
| DBP at 6 mo (≥ 6‐month), mmHg | 167 | 136 | N/A | 77.5 (7.6) | 77.5 (8.4) | 0.10 (8.57) | 0.897 |
| DBP at 6 mo (All Subjects), mmHg | 311 | 187 | N/A | 77.2 (7.7) | 77.7 (8.5) | 0.51 (8.91) | 0.437 |
These are patients for whom baseline and postindex values were available, rendering them eligible for each measure.
N values may differ from overall cohort numbers because of a difference in the number of participants with baseline and postindex values.
DBP, diastolic blood pressure; HbA1c, glycated hemoglobin A1c; N/A, not applicable; SBP, systolic blood pressure.
Figure 2.
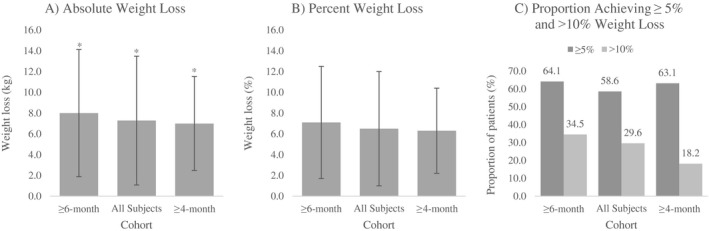
(A) Mean absolute weight loss, (B) mean percent weight loss, and (C) proportion achieving ≥ 5% or > 10% weight loss for ≥ 6‐month (n = 145), All Subjects (n = 203), and ≥ 4‐month (n = 187) persistence cohorts. Error bars represent ± SD. *Significant change in weight (P< 0.05).
Differences in percent body weight
Participants in the ≥ 6‐month cohort lost a mean 7.1% body weight at 6 months, with 93 (64.1%) and 50 (34.5%) participants losing ≥ 5% and > 10% body weight, respectively. Percentage change in body weight in the ≥ 4‐month group was −6.3%, with 118 (63.1%) and 34 (18.2%) subjects losing ≥ 5 % and > 10% body weight, respectively. All Subjects lost 6.5% body weight, with 119 (58.6%) and 60 (29.6%) subjects losing ≥ 5% and > 10% body weight, respectively (Table 3 and Figure 2B‐2C).
Adjusted weight analyses
In the ≥ 6‐month cohort, only baseline weight was a predictor of weight at 6 months when multivariate linear regression was applied (Supporting Information Table S2a‐S2c). When investigating the factors influencing percent body weight using logistic regression, age was the sole predictor of attaining ≥ 5% weight loss at 6 months (odds ratio [95% CI]: 1.040 [1.005‐1.077]; P < 0.05). None of the variables studied was associated with attaining > 10% weight loss after 6 months of treatment with liraglutide 3.0 mg.
Differences in cardiometabolic values
Differences in cardiometabolic values were observed across the cohorts. For the ≥ 6‐month treatment group, there was a statistically significant decrease of 0.4% in HbA1c levels (P < 0.001) based on a small sample of 30 subjects with available HbA1c values (Table 3). On average, 6‐month SBP significantly decreased by 3.0 mmHg (P < 0.01), whereas DBP did not change (mean difference: 0.10 mmHg; P = 0.90) for the 167 subjects in the ≥ 6‐monthscohort with blood pressure results. Similar results were observed in the All Subjects cohort after 6 months, regardless of persistence. Among All Subjects, there was a 0.3% decrease in HbA1c levels (P < 0.001; SD: 0.3), with a 2.2‐mmHg decrease in SBP (P < 0.01; SD: 11.2) and no change in DBP (mean difference: 0.5 mmHg; P = 0.4; SD: 8.9), between baseline and 6 months for 187 participants with relevant blood pressure values.
Discussion
To our knowledge, this is the first study to evaluate the real‐world clinical effectiveness of liraglutide 3.0 mg in a general population with overweight or obesity. We found that in a real‐world setting, treatment with liraglutide 3.0 mg, in addition to recommended diet and exercise, was associated with a significant loss of 7.0 to 8.0 kg and with a 6.5% to 7.1% decrease in body weight 4 and 6 months post initiation. This study thus confirms that the effectiveness of liraglutide 3.0 mg, as an adjunct to diet and exercise, demonstrated in randomized clinical trials is also evident in a real‐world clinical setting, which may not always be evident given variability in the general population and in adherence to treatment 28.
These real‐world study results reflect those reported in the randomized clinical trial investigating liraglutide 3.0 mg compared with a placebo, in adjunct to diet and exercise, in individuals with obesity and prediabetes in which after 56 weeks of treatment, participants lost 8.4 kg (8.4%) of body weight 29. Moreover, a ≥ 5% clinically meaningful weight loss was observed in 58.6% to 64.1% of subjects from the three real‐world cohorts studied, with 18.2% to 34.5% of subjects achieving a loss of 10% body weight, whereas the randomized clinical trial reported 63.2% achieving a ≥ 5% loss in body weight and 33.1% losing 10% body weight 30. Despite the absence of a comparator group in this real‐world study, the categorical weight‐loss results were well within the Food and Drug Administration guidelines used to establish the efficacy of weight management, which require the proportion of active‐product group subjects losing ≥ 5% of baseline body weight to be at least 35%, as well as approximately twice the proportion of the placebo group, and require the difference between groups to be statistically significant 6.
Baseline characteristics of the populations in the current real‐world study and in the randomized clinical trial investigating liraglutide 3.0 mg in individuals with obesity and prediabetes were largely similar and predominantly comprised middle‐aged white women 29. Primary differences were in obesity class because 49.8% of the real‐world study subjects had class 3 obesity (≥ 40 kg/m2), compared with only 33.3% in the randomized clinical trial 29. Furthermore, a smaller proportion of patients in our real‐world study had prediabetes (19.9% vs. 61.4% in the randomized clinical trial) and hypertension (33.1% vs. 34.2%) but not dyslipidemia (61.1% vs. 29.6%). Accordingly, the comparable weight loss observed in this real‐world setting should be seen in the context of a study population with generally higher weight but fewer cases of prediabetes compared with that of the randomized clinical trial.
Variables influencing weight loss were investigated, and after accounting for baseline weight, logistic regression analyses suggested that older subjects had increased odds of attaining ≥ 5% weight loss at 6 months. Several studies, including a previous study performed by WMC, have reported greater weight‐loss success in older patients 30, 31, 32, with low attrition and greater persistence believed to be the contributing factors of this success. However, other studies have reported no influence attributable to age 33, 34, 35.
Changes in cardiometabolic markers were also investigated. Although these cardiometabolic changes often require sufficient time for detection, our study showed statistically significant improvements in HbA1c and SBP, after 6 months of treatment, for All Subjects and for those persistent on treatment for at least 6 months (≥ 6‐month). These positive findings are in keeping with the results of the randomized controlled clinical trial 29. Given the population of patients at risk for prediabetes and diabetes in our study, the HbA1c improvements observed can be considered clinically relevant. Changes in both SBP and DBP in our study were subtler than those reported in the randomized clinical trial, and although SBP was still statistically significant, it is worth noting that our study population was less hypertensive.
Further real‐world evidence is required to better understand the longer‐term effectiveness of treatment with liraglutide 3.0 mg for weight management in a real‐world setting. Future analysis is planned once sufficient follow‐up data are available.
This real‐world clinical effectiveness study used a longitudinal database of deidentified EMR data, making it possible to analyze continued subject care. Data were of high quality and representative of the specific target population of this pharmacotherapeutic intervention. WMC is government funded and thus provides a reasonable weight‐management approach that is generalizable to the public. After applying the selection criteria, this real‐world database produced a data set that was capable of powering the study, which is often a challenge in real‐world studies 28. Moreover, this collaboration permitted the validation of EMR data and the collection of free‐text fields coded in the EMR without breaching subject confidentiality.
Several important limitations need to be considered when evaluating the study findings. Given the observational pre‐post nature of this study, it was not possible to compare subjects receiving liraglutide 3.0 mg with contemporary controls.
It is also important to note that WMC is a referral‐based clinic; thus, study participants may represent a population more motivated to lose weight than the general eligible population. Moreover, this population is representative of subjects who would choose to initiate pharmaceuticals for weight management because subjects who refused treatment with liraglutide 3.0 mg, despite being prescribed it, were not included in the study. On a related note, patients did not always initiate liraglutide 3.0 mg when prescribed; thus, not all dates of liraglutide 3.0 mg initiation are exact. Estimates of the initiation date were made from assumptions based on back calculations using the dose reported at the appointment in which initiation was reported. Similarly, not all dates of liraglutide 3.0 mg discontinuation are exact because some participants may have stopped taking the medication and reported it to the physician at a later time or some subjects may have been lost to follow‐up but continued to take the medication. Furthermore, some participants may have been lost to follow‐up after treatment initiation, with no further information on their subsequent management and clinical outcomes.
Given the real‐world nature of the data used in this study, participants who did not have a value recorded within 30 days of the specified time point, i.e., 4 months or 6 months, were not included in the analysis of that specific outcome. The All Subjects cohort included any subject with the required measurements to calculate the specified outcome, i.e., a baseline value, and a value ± 30 days within the time point being analyzed. As such, it is possible that a subject could be excluded from any 4‐month analyses and included in subsequent 6‐month analyses.
Finally, it is important to note that an EMR is not a perfect database. The impact of coding errors or missing information was mitigated during the data‐cleaning phase of the study, with outliers and biologically unrealistic observations removed from the study. However, given the challenges of completing rapid and sufficiently powered drug‐safety and drug‐effectiveness studies, computerized databases such as EMR have become preferred and cost‐effective data sources 28.
Conclusion
This study demonstrates the clinical effectiveness of liraglutide 3.0 mg in a real‐world setting 4 and 6 months post initiation. Treatment, combined with a reduced‐calorie diet and increased physical activity, was associated with a clinically significant decrease in absolute and percent body weight and with improvement of cardiometabolic markers.
Supporting information
Acknowledgments
The authors wish to acknowledge Tahir Feroz (Novo Nordisk Canada Inc., Mississauga, Canada) and Jason Goodfield (IQVIA, Mississauga, Canada) for their contributions to the study design and planning, Nicole Bhoop and Elham Kamran (WMC, Burlington, Canada) for their assistance with data collection and data verification, and Drew Neish (IQVIA, Montreal, Canada) for his work in data analyses.
Funding agencies: This study was sponsored by Novo Nordisk A/S.
Disclosure: SW is the owner and director of Wharton Medical Clinic (WMC) and is an internal medicine specialist with privileges at Toronto East General Hospital and Hamilton Health Sciences. He has previously received funding in the forms of grants for research from the Canadian Institutes of Health Research and Mitacs. He has also received funding from Novo Nordisk, Eli Lilly and Company, Janssen Pharmaceutica, and AstraZeneca for advisory work. SV is an employee of WMC. RAGC was employed as a research coordinator for WMC. JM and GSP are employees of IQVIA Canada, responsible for study management, analysis, and dissemination. AL and AP are employees of Novo Nordisk Canada Inc. EN and CLH are employees of Novo Nordisk A/S.
Author contributions: SW was responsible for the conception and design of the study, for acquisition and interpretation of the data, and for review of the manuscript. SV was responsible for the acquisition of the data. RAGC was responsible for the conception and design of the study, for acquisition and interpretation of the data, and for review of the manuscript. AL was responsible for the interpretation of the data and for review of the manuscript. AP, EN, and CLH were responsible for the conception and design of the study, for interpretation of the data, and for review of the manuscript. JM was responsible for the conception and design of the study, for interpretation of the data, and for the development and review of the manuscript. GSP was responsible for the conception and design of the study, for the analysis and interpretation of the data, and for the development and review of the manuscript.
References
- 1. Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 2008;32:1431‐1437. [DOI] [PubMed] [Google Scholar]
- 2. Statistics Canada . Table 13-10-0096-20. Body mass index, overweight or obese, self‐reported, adult age Groups (18 Years and Older). https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310009620. Updated April 5, 2019. [Google Scholar]
- 3. Statistics Canada . Table 13-10-0323-01 Adult body mass index - Health Canada classification, inactive. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310032301. Updated April 5, 2019. [Google Scholar]
- 4. Canadian Medical Association (CMA) . Obesity as a chronic medical disease. Policy resolution. https://policybase.cma.ca/en. Published October 3, 2015. [Google Scholar]
- 5. Mechanick JI, Garber AJ, Handelsman Y, Garvey WT. American Association of Clinical Endocrinologists’ position statement on obesity and obesity medicine. Endocr Pract 2012;18:642‐648. [DOI] [PubMed] [Google Scholar]
- 6. US Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Research . Guidance for Industry Developing Products for Weight Management. Rockville, MD: Food and Drug Administration; 2007. [Google Scholar]
- 7. Jastreboff AM, Kotz CM, Kahan S, Kelly AS, Heymsfield SB. Obesity as a disease: The Obesity Society 2018 position statement. Obesity (Silver Spring) 2019;27:7‐9. [DOI] [PubMed] [Google Scholar]
- 8. Public Health Agency of Canada; Canadian Institute for Health Information . Obesity in Canada: A Joint Report From the Public Health Agency of Canada and the Canadian Institute for Health Information. Ottawa, Canada: Public Health Agency of Canada, The Canadian Institute for Health Information; 2011. [Google Scholar]
- 9. Tjepkema M. Adult obesity. Health Rep 2006;17:9‐25. [PubMed] [Google Scholar]
- 10. Fujioka K, Sparre T, Sun LY, Krogsgaard S, Kushner RF. Usability of the novel liraglutide 3.0 mg pen injector among overweight or obese adult patients with or without prior injection experience. J Diabetes Sci Technol 2015;10:164‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Malnick SD, Knobler H. The medical complications of obesity. QJM 2006;99:565‐579. [DOI] [PubMed] [Google Scholar]
- 12. Nagai M, Kuriyama S, Kakizaki M, et al. Impact of obesity, overweight and underweight on life expectancy and lifetime medical expenditures: the Ohsaki Cohort Study. BMJ Open 2012;2:e000940. doi: 10.1136/bmjopen-2012-000940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Whitlock G, Lewington S, Sherliker P, et al; Prospective Studies Collaboration . Body‐mass index and cause‐specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 2009;373:1083‐1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Knowler WC, Barrett‐Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li G, Zhang P, Wang J, et al. Cardiovascular mortality, all‐cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23‐year follow‐up study. Lancet Diabetes Endocrinol 2014;2:474‐480. [DOI] [PubMed] [Google Scholar]
- 16. Dattilo AM, Kris‐Etherton PM. Effects of weight reduction on blood lipids and lipoproteins: a meta‐analysis. Am J Clin Nutr 1992;56:320‐328. [DOI] [PubMed] [Google Scholar]
- 17. Wing RR, Lang W, Wadden TA, et al; Look AHEAD Research Group . Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 2011;34:1481‐1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Warkentin LM, Das D, Majumdar SR, Johnson JA, Padwal RS. The effect of weight loss on health‐related quality of life: systematic review and meta‐analysis of randomized trials. Obes Rev 2014;15:169‐182. [DOI] [PubMed] [Google Scholar]
- 19. Wright F, Boyle S, Baxter K, et al. Understanding the relationship between weight loss, emotional well‐being and health‐related quality of life in patients attending a specialist obesity weight management service. J Health Psychol 2013;18:574‐586. [DOI] [PubMed] [Google Scholar]
- 20. Anderson JW, Konz EC, Frederich RC, Wood CL. Long‐term weight‐loss maintenance: a meta‐analysis of US studies. Am J Clin Nutr 2001;74:579‐584. [DOI] [PubMed] [Google Scholar]
- 21. Lau DC, Douketis JD, Morrison KM, Hramiak IM, Sharma AM, Ur E; Obesity Canada Clinical Practice Guidelines Expert Panel . 2006 Canadian clinical practice guidelines on the management and prevention of obesity in adults and children [summary]. CMAJ 2007;176:S1‐S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Apovian CM, Aronne LJ, Bessesen DH, et al. Endocrine Society . Pharmacological management of obesity: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2015;100:342‐362. [DOI] [PubMed] [Google Scholar]
- 23. Jensen MD, Ryan DH, Donato KA, Guidelines (2013) for managing overweight and obesity in adults. Obesity (Silver Spring) 2014;22(S2):S1‐S410. [Google Scholar]
- 24. Knudsen LB. Liraglutide: the therapeutic promise from animal models. Int J Clin Pract Suppl 2010;167:4‐11. [DOI] [PubMed] [Google Scholar]
- 25. van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WH. Effects of the once‐daily GLP‐1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non‐diabetic adults. Int J Obes (Lond) 2014;38:784‐793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Novo Nordisk Canada Inc. Product Monograph: SAXENDA®: Liraglutide 6 mg/mL: Solution for Injection in a Pre‐filled Pen Human Glucagon Like Peptide‐1 (GLP‐1) Weight Management. Mississauga, Canada: Novo Nordisk Canada Inc; 2017. https://www.novonordisk.ca/content/dam/Canada/AFFILIATE/www-novonordisk-ca/OurProducts/PDF/Saxenda_PM_English.pdf [Google Scholar]
- 27.Novo Nordisk. Effective obesity management: it’s more than reducing numbers on the scale [press release]. http://www.multivu.com/players/English/7610251-novo-nordisk-saxenda/. Published August 27, 2015.
- 28. Lin KJ, Schneeweiss S. Considerations for the analysis of longitudinal electronic health records linked to claims data to study the effectiveness and safety of drugs. Clin Pharmacol Ther 2016;100:147‐159. [DOI] [PubMed] [Google Scholar]
- 29. Pi‐Sunyer X, Astrup A, Fujioka K, et al; SCALE Obesity and Prediabetes NN8022‐1839 Study Group . A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med 2015;373:11‐22. [DOI] [PubMed] [Google Scholar]
- 30. Jiandani D, Wharton S, Rotondi MA, Ardern CI, Kuk JL. Predictors of early attrition and successful weight loss in patients attending an obesity management program. BMC Obes 2016;3:14. doi: 10.1186/s40608-016-0098-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Honas JJ, Early JL, Frederickson DD, O’Brien MS. Predictors of attrition in a large clinic‐based weight‐loss program. Obes Res 2003;11:888‐894. [DOI] [PubMed] [Google Scholar]
- 32. Gill RS, Karmali S, Hadi G, Al‐Adra DP, Shi X, Birch DW. Predictors of attrition in a multidisciplinary adult weight management clinic. Can J Surg 2012;55:239‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huisman S, Maes S, De Gucht VJ, Chatrou M, Haak HR. Low goal ownership predicts drop‐out from a weight intervention study in overweight patients with type 2 diabetes. Int J Behav Med 2010;17:176‐181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grossi E, Dalle Grave R, Mannucci E, et al. Complexity of attrition in the treatment of obesity: clues from a structured telephone interview. Int J Obes (Lond) 2006;30:1132‐1137. [DOI] [PubMed] [Google Scholar]
- 35. Greenberg I, Stampfer MJ, Schwarzfuchs D, Shai I; DIRECT Group . Adherence and success in long‐term weight loss diets: the dietary intervention randomized controlled trial (DIRECT). J Am Coll Nutr 2009;28:159‐168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


