Abstract
BACKGROUND
Neuropeptides are regulators of critical life processes in insects and, due to their high specificity, represent potential targets in the development of greener insecticidal agents. Fundamental to this drive is understanding neuroendocrine pathways that control key physiological processes in pest insects and the screening of potential analogues. The current study investigated neuropeptide binding sites of kinin and CAPA (CAPA‐1) in the aphids Myzus persicae and Macrosiphum rosae and the effect of biostable analogues on aphid fitness under conditions of desiccation, starvation and thermal (cold) stress.
RESULTS
M. persicae and M. rosae displayed identical patterns of neuropeptide receptor mapping along the gut, with the gut musculature representing the main target for kinin and CAPA‐1 action. While kinin receptor binding was observed in the brain and VNC of M. persicae, this was not observed in M. rosae. Furthermore, no CAPA‐1 receptor binding was observed in the brain and VNC of either species. CAP2b/PK analogues (with CAPA receptor cross‐activity) were most effective in reducing aphid fitness under conditions of desiccation and starvation stress, particularly analogues 1895 (2Abf‐Suc‐FGPRLa) and 2129 (2Abf‐Suc‐ATPRIa), which expedited aphid mortality. All analogues, with the exception of 2139‐Ac, were efficient at reducing aphid survival under cold stress, although were equivalent in the strength of their effect.
CONCLUSION
In demonstrating the effects of analogues belonging to the CAP2b neuropeptide family and key analogue structures that reduce aphid fitness under stress conditions, this research will feed into the development of second generation analogues and ultimately the development of neuropeptidomimetic‐based insecticidal agents. © 2019 The Authors. Pest Management Science published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Keywords: aphicide, cold tolerance, desiccation, ligand‐binding, G‐protein coupled receptors receptor‐mapping, pest control
1. INTRODUCTION
With a global dependence on broad‐spectrum insecticides, the damaging effects of which are well documented,1, 2 there is increasing need for the development of greener, target‐specific insecticides. The development and employment of neuropeptide synthetic analogues offers a promising avenue in the drive for greener and target‐specific insecticidal agents. Within the insects, neuropeptides are regulatory peptides with functional roles in growth and development, behavior and reproduction, metabolism and homeostasis, and muscle movement.3 Due to their high specificity, neuropeptides and their cognate receptors (G‐protein coupled receptors, GPCRs) may be developed as an insecticidal target system4, 5 to selectively reduce the fitness of target pest insects, while minimizing detrimental environmental impacts.
The two neuropeptide families selected for study include the insect kinins and CAP2b/CAPA peptides. Insect kinins are multifunctional neuropeptides which share a conserved C‐terminal pentapeptide motif Phe‐X1‐X2‐Trp‐Gly‐NH2, where X1 can be His, Asn, Ser or Tyr, and X2 can be Ser, Pro or Ala.6 The insect kinins have been identified in most insects, with the exception of Coleoptera,7 and have diverse roles in the stimulation of muscle,8 fluid secretion in renal tubules,9, 10 digestive enzyme release,11 inhibition of larval weight gain12 and the desiccation and starvation stress response.13, 14 The second family, the CAPA peptides, were first identified from the moth Manduca sexta (CAP2b)15 and have since been identified in many insect families.16 Although function varies depending on insect species, life stage, and lifestyle, CAPA peptides play a key role in myomodulation and osmoregulation16 and have more recently been linked to desiccation and cold tolerance in Drosophila species.17, 18
The CAPA peptides belong to the PRXamide superfamily which can be further subdivided into three major classes: CAPA peptides, pyrokinins (PK) and ecdysis triggering hormone (ETH).19 The pyrokinins are further subdivided into diapause hormone (DH) and pheromone biosynthesis activating neuropeptides (PBAN) and by their C‐terminal motifs WFGPRLamide and FXPRLamide, respectively.20 The GPCRs of this ligand group form a homologous cluster, suggesting co‐evolution of ancestrally related ligand‐receptor partners. As a result, some cross activity by analogues of the ligand sub‐groups with respective, recombinant receptors has been observed.21, 22 For this reason, certain PK analogues that have previously demonstrated cross activity on recombinant CAPA receptors of Tribolium castaneum have been included in this study. In particular, analogue 1895 (Table 1) has exhibited agonist activity, and analogues 1896 and 1902 (Table 1) antagonistic activity on T. castaneum TcCAPAr.22 Furthermore, PRXamide analogues with the addition of hydrophobic moieties at the N‐terminus have been shown to display greater biostability in‐vivo,23 as featured in 1895, 1896 and 1902. Subsequently, 2089, 2123, 2125 and 2129 (Table 1) were designed and synthesized as second generation analogues of 1895, 1896 and 1902 to be evaluated in the current study.
Table 1.
The structure of biostable CAP2b, pyrokinin (with CAPA receptor cross activity) and kinin analogues used in aphid stress tolerance assays. Modifications are shown in bold
| Code | Structure |
|---|---|
| CAP2b/PK | |
| 1895 | 2Abf‐Suc‐FGPRLa |
| 1896 | 2Abf‐Suc‐FTPRIa |
| 1902 | 2Abf‐Suc‐FKPRLa |
| 2089 | 2Abf‐Suc‐FTPRVa |
| 2123 | 2Abf‐Suc‐FT[Hyp]RVa |
| 2125 | 2Abf‐Suc‐FT[Oic]RVa |
| 2129 | 2Abf‐Suc‐ATPRIa |
| Kinin | |
| 1728 | [Aib]FF[Aib]WGa |
| 2139 | FF[Aib]WGa |
| 2139‐AC | Ac‐FF[Aib]WGa |
Insect kinins are prone to rapid degradation by peptidases within the insect hemolymph and bound to tissues. The angiotensin converting enzyme (ACE) from the housefly is capable of cleaving insect kinin at the primary hydrolysis site, and neprilysin (NEP), at both the primary and secondary hydrolysis sites,24, 25, 26, 27, 28 thus representing biostability issues. In order to overcome this issue, incorporation of a single α‐amino isobutyric acid (Aib) into the third position of the insect kinin active core protects the primary hydrolysis site from tissue‐bound peptidase.26, 27, 28, 29, 30 Incorporation of a second Aib residue adjacent to the secondary peptidase hydrolysis site further enhances biostability.26 Insect kinin analogues that incorporate sterically‐bulky Aib residues, adjacent to both primary and secondary peptidase hydrolysis sites were previously evaluated on two recombinant receptor assays from the southern cattle tick, Rhipicephalus (Boophilus) microplus,31, 32 and the dengue vector, the mosquito Aedes aegypti.33 These results demonstrated that biostable Aib analogues of the insect kinins can retain potent activity on these two receptors.30
Recent studies have documented the potent effects of biostable neuropeptide analogues on pest insects.30, 34, 35 By using two aphid pest species, kinin36 and CAPA37 receptor sites were first mapped in aphid tissue. The potential effects of kinin and CAP2b/PK analogues on aphid stress tolerance and fitness (desiccation, starvation, cold) were subsequently screened, representing the first study into the role of these neuropeptide families in aphid stress tolerance. Aphids (Hemiptera: Aphididae) are one of the most significant groups of agricultural pests38 and are vectors in the transmission of approximately 50% of all insect transmitted plant viruses.39 The primary study species for the current study, the peach potato aphid Myzus persicae, is the most economically important aphid crop pest worldwide,40 with a global distribution and host range encompassing more than 400 species in 40 different plant families.41 The secondary study species, the rose aphid Macrosiphum rosae, was selected to represent a major pest of horticulture. M. rosae is an important pest of cultivated species of Rosa and is a vector in the transmission of 12 plant viruses including the strawberry mild yellow edge virus.41 The results of this study will inform design and development of novel, specific insecticidal agents.
2. MATERIAL AND METHODS
2.1. Aphid rearing
Stock cultures of anholocyclic M. persicae were established using aphids supplied by the Smagghe laboratory, Ghent University, Belgium. Cultures were reared under a 12:12 h LD photocycle at 22 °C on Chinese cabbage (Brassica rapa var. Wong Bok) contained within a BugDorm fine mesh cage (44545F) (45 cm × 45 cm × 45 cm). A fresh supply of Chinese cabbage of approximately 4 weeks from sowing was supplied to the cages on a once‐weekly basis to maintain the aphid cultures.
M. rosae was selected as a secondary aphid species and a sub‐set of experiments was performed on the species to determine the overlap in response between aphid species of different genera. Stock cultures of anholocyclic M. rosae were set up from individual aphids originally collected on Rosa species within the grounds of the University of Glasgow, Scotland, UK. A stock culture was set up within the laboratory and maintained on supermarket‐bought miniature rose plants and under identical conditions to M. persicae. Prior to use, the leaves of the rose plants were thoroughly washed using 70% ethanol followed by distilled water to ensure the removal of any potential chemical residues present on the plant.
2.2. Neuropeptide synthesis
Native and fluorescently labelled neuropeptides CAPA‐1 (CAPA‐1‐F) and kinin (Kinin‐F) were synthesized by Cambridge Peptides (Birmingham, UK) as previously detailed7 and based on the CAPA and kinin structures of Drosophila melanogaster. In brief, native kinin was synthesized and coupled to Alexfluor488 resulting in fluorescent kinin (Alexa‐488‐C5‐maleimide‐CNSVVLGKKQRFHSWGamide). The same rationale was used for the production of CAPA‐1 (GANMGLYAFPRVamide) and labelled CAPA‐1‐F with the addition of TMR‐C5‐Maleimide Bodipy dye (TMR‐C5‐ maleimide‐CGANMGLYAFPRVamide).
The synthesis of PK analogues (with CAPA receptor cross‐activity) 1895 and 1902,22, 23 CAP2b analogue 1896,22 and insect kinin analogues 1728 and 213929, 30 have been previously described. CAP2b analogues 2089, 2123, 2125, and 2129;23 as well as insect kinin analogue 2139‐Ac29 were synthesized and cleaved according to procedures that have been previously described. The analogues were purified on a Waters Delta‐Pak C18 reverse‐phase column (8 × 100 mm, 15 µm particle size, 100 Å pore size) with a Waters 510 HPLC system with detection at 214 nm at ambient temperature. Solvent A = 0.1% aqueous trifluoroacetic acid (TFA); Solvent B = 80% aqueous acetonitrile containing 0.1% TFA. Initial conditions were 10% B followed by a linear increase to 90% B over 40 min.; flow rate, 2 mL min−1. Delta‐Pak C18 retention times: 2089, 12.0 min; 2123, 9.0 min; 2139‐Ac, 5.9 min; 2125, 12.5 min; 2129, 7.5 min. The analogues were further purified on a Waters Protein Pak I 125 column (7.8 × 300 mm). Conditions: isocratic using 80% acetonitrile containing 0.1% TFA; flow rate, 2 mL min−1. Waters Protein Pak retention times: 2089, 6.0 min; 2123, 5.5 min; 2139‐Ac, 5.9 min; 2125, 5.5 min; 2129, 6.0 min. Amino acid analysis was carried out under previously reported conditions (Nachman et al., 2004) to quantify the analogues and to confirm identity: 2089: F[1.0], P[1.0], R[1.0], T[1.0], V[1.0]; 2123: F[1.0], R[0.9], T[0.9], V[0.9]; 2139‐Ac: F[2.0], G[0.9]; 2125: F[1.0], R[0.8], T[0.7], V[0.8]; 2129: A[1.0], I[0.9], P[0.9], R[0.9], T[0.9]. The identity of the analogues was also confirmed by MALDI‐MS on a Kratos Kompact Probe MALDI‐MS instrument (Shimadzu, Columbia, Maryland). The following molecular ions (MH+) were observed: 2089, 961.0 (calc.961.8); 2123, 976.2 (calc.976.1); 2139‐Ac, 704.7 (calc.704.5, [MNa+]); 2125, 1014.1 (calc.1014.0); 2129, 898.8 (calc.898.8). The structures of the biostable analogues are displayed in Table 1.
2.3. Receptor mapping assay using fluorescently labelled neuropeptides
Aphids were cold anesthetized and the tissue of interest dissected out in a 1:1 solution of Schneider's insect medium and optimized saline.7, 42 The dissected tissue was mounted on a poly‐L‐lysine‐covered 35 mm glass bottom dish containing 1:1 saline. Nuclei were stained via incubation in DAPI (1 µg mL−1) for 1 min and then washed with the optimized saline solution. A baseline image was taken to determine the level of autofluorescence and adjust exposure settings accordingly. All images were recorded on an inverted confocal microscope (Zeiss LSM 510 Meta). A labelled neuropeptide (10−7 m) was subsequently added to the tissue and the tissue incubated for 1 min before washing with the optimized saline solution. The sample tissue was immediately imaged. The concentration of 10−7 m was chosen for labelled neuropeptides because it represents the minimal concentration required to produce a saturated receptor response, thereby optimizing the conditions for optical detection of ligand‐receptor complexes.7 Following imagining, unlabeled neuropeptide (10−5 m) was added to the sample and a time‐lapse experiment set up to determine if the unlabeled neuropeptide outcompeted the labelled neuropeptide, thus affirming detection of the ligand‐receptor complexes. Images were collected every 30 s for a duration of 20–30 m. All imaging was repeated on a minimum of three specimens to ensure consistency and further re‐affirm conclusions. All images were exported as JPEG files and subsequently viewed in FIJI and Microsoft Illustrator. When specific binding was observed in muscle tissue, this was supported by the addition of rhodamine phalloidin; a high‐affinity F‐actin probe conjugated to tetramethylrhodamine (TRITC) that specifically binds to muscle.
2.4. Neuropeptide treatment via microinjection
Neuropeptides were administered to test aphids via microinjection to allow for rapid mass screening of neuropeptide analogue efficacy. For this, native neuropeptides were diluted in double distilled water (DDH20) to a concentration of 1 × 10−5 m. Neuropeptide analogues were diluted in DDH20 to the following concentrations: kinin analogues 1728 (2.5 × 10−5 m), 2139 (3.5 × 10−5 m), 2139‐Ac (3.5 × 10−5 m); CAP2b/PK analogues 1895 (3.5 × 10−5 m), 1896 (3.5 × 10−5 m), 1902 (3.5 × 10−5 m), 2089 (3.9 × 10−5 m), 2123 (1.0 × 10−5 m), 2125 (1.0 × 10−5 m), 2129 (2.0 × 10−5 m). Once at the desired concentration, neuropeptide solutions were administered to test aphids at an injection volume of 9 nL based on total hemolymph volume, to produce an approximate 1:20 dilution of injection volume to hemolymph. Injections were performed using a pulled glass needle and a Nanoject II Auto‐Nanoliter Injector (Drummond Scientific Company, Broomall, Pennsylvania). A vehicle control was set up for each treatment / day of experiments to account for variation in needle pulling. For this, control aphids were injected with 9 nL of DDH20 and subsequently exposed to the same experiments as aphids receiving the neuropeptide treatment. Neuropeptide treated and vehicle control aphids were subsequently used in the stress bioassays detailed below.
2.5. Desiccation / starvation tolerance bioassay
Anholocyclic adults of mixed age of either M. persicae or M. rosae were selected from the stock cultures and treated with a native neuropeptide or neuropeptide analogue via microinjection using the method detailed above. Treated and vehicle control aphids were allowed to recover on excised leaves of the host plant for 1 h before being placed in an empty ventilated microcage (L = 4 cm, Ø = 9.5 cm) at densities of 10 per cage. In total, 30–40 aphids were treated for each neuropeptide treatment group and a further 30–40 for the associated vehicle control group (i.e. 3–4 biological replicates of 10). From the point of placement in the microcage (taken as 0 h), aphid survival was checked every hour during daylight hours and approximately every 4 h during night‐time hours until the final aphid died. Survival data were subsequently analyzed using a Log‐rank (Mantel‐Cox) test in GraphPad Prism version 7.0. LTime50 (the time taken to kill 50% of the test population) values were calculated via Probit Analysis in Minitab 17 (Minitab Inc., State College, Pennsylvania). Neuropeptide analogues which significantly impacted aphid survival under desiccation / starvation stress were further tested under non‐stress conditions. For this, aphids were treated with the neuropeptide analogue as detailed above and maintained on excised leaves of the host plant for a duration of 7 days. Survival data at day 7 were arcsine square root transformed and analyzed using analysis of variance (ANOVA) and Tukey's multiple range test in MINITAB version 17.
2.6. Cold tolerance bioassay
2.6.1. Calculation of discriminating temperatures
M. persicae and M. rosae displayed identical results in desiccation / starvation stress assays. For this reason, and given its global pest status, only M. persicae was taken forward in cold stress assays. Survival curves were first established to determine a species‐specific discriminating temperature for subsequent neuropeptide testing. Aphids were selected at the pre‐reproductive adult stage for cold tolerance bioassays since aphid cold tolerance is known to significantly vary throughout an aphid's life cycle.43, 44 Temperature ranges were selected to encompass 0–100% mortality. Anholocyclic pre‐reproductive adults (approximately 9 days old at 22 °C) of M. persicae were exposed to a range of low temperatures (−14 °C to −7 °C at 1 °C intervals) using a direct plunge method.45, 46 For each temperature treatment, 30 adults were placed within plastic 0.5 mL Eppendorf tubes at densities of ten adults per tube, which, in turn, were placed within a glass boiling tube held within an alcohol bath (Haake G50 and PC200; Thermo Scientific, Germany) pre‐set to the desired temperature. Pieces of cotton wool were used to stopper the boiling tubes to limit air circulation and to ensure a more stable internal temperature within the tubes. Adults were held at the desired exposure temperature for 1 h. Following exposure, aphids were allowed to recover at the culture temperature in microcages containing excised leaves of the host plant and survival was assessed after 48 h. The procedure was repeated for each exposure temperature.
Survival data were analyzed using Probit analysis in MINITAB, version 17 (Minitab Inc., State College, Pennsylvania) and the LT30 (the lethal temperature resulting in 30% mortality of a test population) was elucidated. The LT30 was chosen to act as a discriminating temperature for subsequent neuropeptide testing since it enabled detection of directional effects of subsequent neuropeptide treatment, but primarily in the direction of interest i.e. which neuropeptides significantly increased mortality in the species of interest.
2.6.2. Peptide analogue treatment and testing at the discriminating temperature
Pre‐reproductive anholocyclic adult aphids of M. persicae were treated with neuropeptide analogues using the microinjection method detailed above. Following microinjection treatment, individuals were returned to microcages containing excised leaves of the host plant at densities of approximately 20–30 per microcage and allowed to recover for 24 h at the culture temperature. Following the 24 h recovery period, adults were placed within plastic 0.5 mL Eppendorf tubes at densities of ten adults per tube to a total of 30 for each species × neuropeptide treatment group (i.e. three biological replicates of 10 per replicate). Eppendorf tubes were then placed within glass boiling tubes held within the alcohol bath pre‐set to the desired discriminating temperature. Pieces of cotton wool were used to stopper the boiling tubes to limit air circulation and to ensure a more stable internal temperature within the tubes. Adults were held at the desired exposure temperature for 1 h. Following exposure, adults were allowed to recover at the culture temperature in microcages containing excised leaves of the host plant and survival was assessed after 48 h. The procedure was repeated for each species × peptide analogue treatment group.
Statistical analyses were performed using R Software (R Development Core Team, 2013). A generalized linear model (GLM) with binomial family was fitted to survival data with analogue ‘Treatment’ (peptide analogue), treatment ‘Type’ (test vs. control), and analogue treatment × treatment type interaction as factors.
3. RESULTS
3.1. Receptor mapping assay using fluorescently labelled neuropeptides
A fluorescent ligand‐receptor binding assay was used to map specificity of binding of Kinin and CAPA‐1 within M. persicae and M. rosae. Fluorophore‐labelled kinin (kinin‐F) and CAPA‐1 (CAPA‐1‐F) revealed the neuropeptides to bind to circular muscles along the aphid gut (Fig. 1). Kinin‐F binding to longitudinal muscles in the hindgut region of the gut was further suggested (data not shown). Both the kinin‐F and CAPA‐1‐F signals were displaced by excess unlabeled peptide in the ligand competition assay, thus confirming specificity of binding. Additional labelling with rhodamine phalloidin acted to confirm the gut muscle as the site of binding (Fig. 1(C)). Interestingly, specific kinin‐F and CAPA‐1‐F binding of the gut musculature was not evident under low magnification (x10) (Fig. 2). The presence of smaller cells, running the length of the gut, were detected as a site of kinin‐F binding (Fig. 1(B) and 2 indicated by white arrows), although were not a site of CAPA‐1‐F binding (Fig. 1(A)). In addition, CAPA‐1‐F specific binding was detected in a region of the aphid midgut (stomach) closest to the foregut (Fig. 3) and may represent enterocytes, although further testing is required to confirm this.
Figure 1.
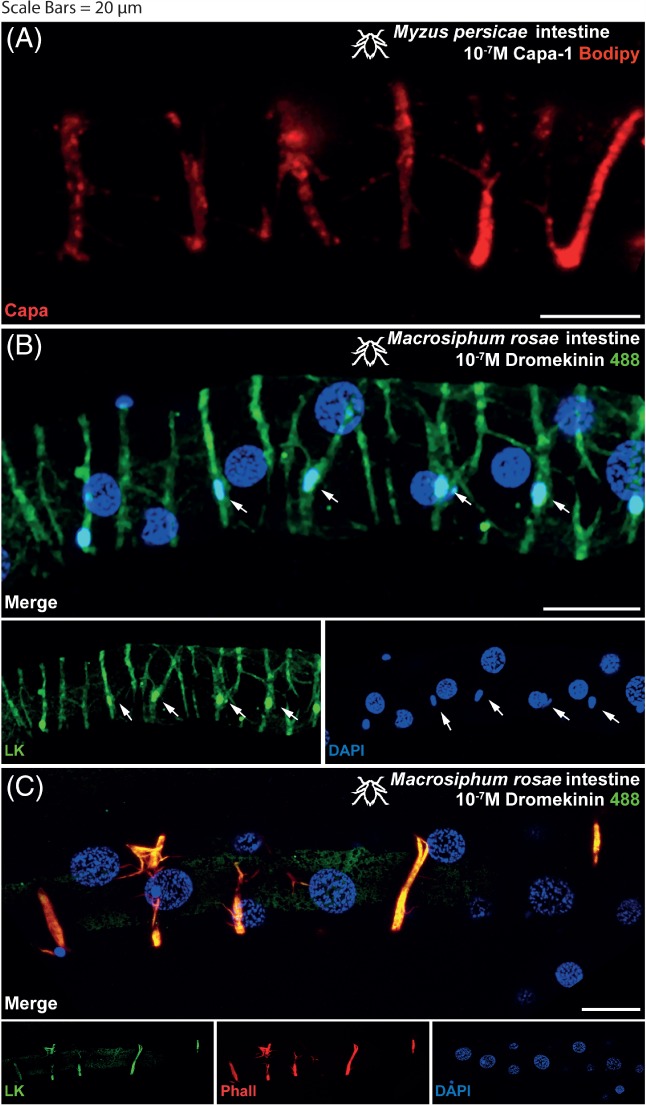
Aphid intestine (distal midgut and proximal hindgut) stained with 10−7 m CAPA‐1‐F labelled with TMR C5‐Maleimide (A) shows receptor localization in the gut muscles (Myzus persicae shown). Excess unlabeled CAPA‐1 (10−5 m) displaces fluorescent signal in a ligand competition assay (not shown), thus confirming the specificity of binding. Aphid intestine stained with 10−7 m kinin labelled with alexafluor488 (B) shows receptor localization in the gut muscles (M. rosae shown). Excess unlabeled kinin (10−5 m) displaces fluorescent signal in a ligand competition assay (not shown), thus confirming the specificity of binding. Staining by kinin‐F was also present in a population of basal cells, characterized by smaller nuclei, as indicated by the white arrows. DAPI was used for nuclear staining (blue). Staining with rhodamine phalloidin labelled with tetramethylrhodamine (TRITC) reaffirms the gut musculature as the site of receptor binding (C). (A and B): Kinin‐F, green; CAPA‐1‐F, red; DAPI, blue. (C) Kinin‐F, green; rhodamine phalloidin, red; DAPI, blue. Scale bars = 20 µm.
Figure 2.
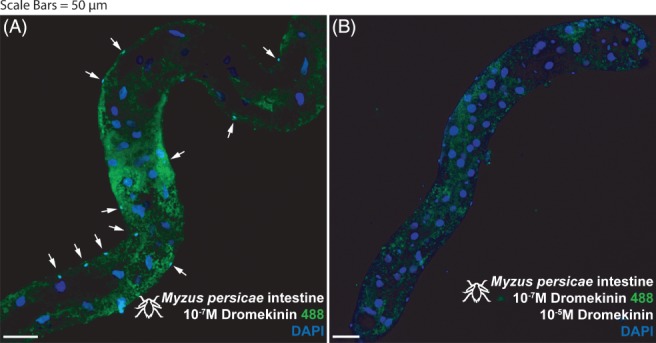
Myzus persicae intestine (distal midgut and proximal hindgut) stained with 10−7 m kinin labelled with alexafluor488 (A) and then out‐competed with 10−5 m unlabeled kinin (B). (A) Staining apparent in a population of basal cells, characterized by overtly smaller nuclei (arrows). (B) Staining abrogated in basal (small nuclei) cells (realized by DAPI staining, arrows) during out‐competition with unlabeled 10−5 m kinin. Kinin‐F, green; DAPI, blue. Scale bars = 50 µm.
Figure 3.
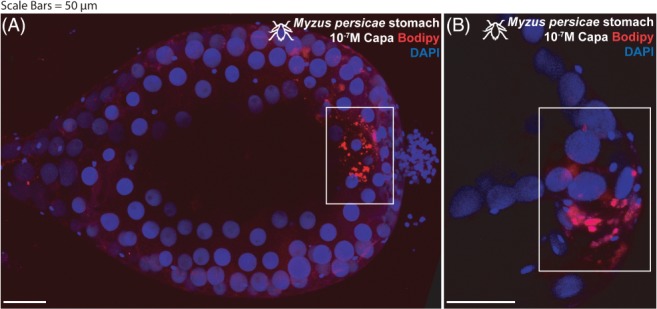
Myzus persicae stomach (midgut) stained with 10−7 m CAPA‐1‐F labeled with TMR C5‐Maleimide. (A) Staining apparent at junctional area between the fore‐ and midgut (white box). (B) Higher magnification detail of staining associated with this junctional area. Staining is abrogated when outcompeted with unlabeled 10−5 m CAPA‐1 (not shown). CAPA‐1‐F, red; DAPI, blue. Scale bars = 50 µm.
Receptor mapping of the M. persicae brain and ventral nerve cord (VNC) revealed kinin‐F staining apparent in a bilateral symmetrical ‘ladder’ of neuronal clusters (two to three neurons) and a set of baso‐lateral neurons in the suboesophageal ganglion (Fig. 4(A)). Staining was also apparent in symmetrical pairs of neurons/neuronal clusters in the ventro‐ to dorso‐lateral protocerebrum. Little to no kinin‐F staining was observed in the VNC with the exception of a set of cells in the most distal tip of the abdominal ganglion (Fig. 4(B)). In contrast, no specific staining with kinin‐F was observed in the brain or VNC of M. rosae. Labelling with CAPA‐1‐F revealed no sites of receptor binding in either the brain (Fig. 4(C)) or the VNC of both species (Fig. 4(D)).
Figure 4.
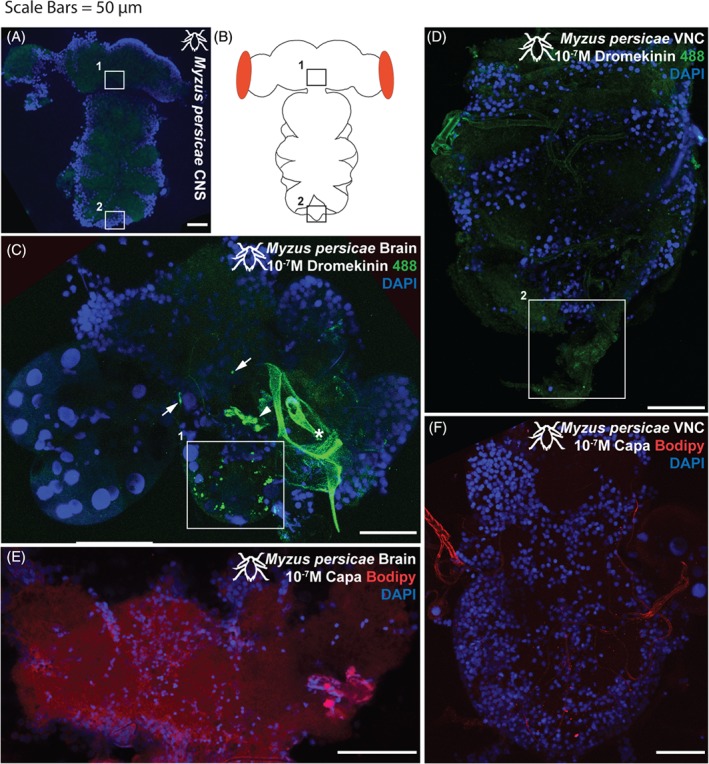
(A) Unstained Myzus persicae CNS, demonstrating baseline autofluorescent levels (488 nm excitation range; green). (B) cartoon schematic. (C) Myzus persicae brain and (D) VNC incubated with 10−7 m kinin‐F labelled with alexafluor488. (C) Staining apparent in a bilateral ‘ladder’ of neurons and a set of more baso‐lateral neurons in the suboesophageal ganglion (1white box). Position of 1white box indicated by 1boxes in (A) and (B). Staining also apparent in symmetrical pairs of neurons/neuronal clusters in the ventro‐ to dorso‐lateral protocerebrum (arrows). Some neurons obscured by cuticular material associated with the aphid feeding stylus (asterisk). (D) Little to no kinin‐F staining apparent in the VNC, although a faint set of cells in the most distal tip of the abdominal ganglion (2white box) are consistently observed. Position of 2white box indicated by 2boxes in (A) and (B). (E) Myzus persicae brain and (F) VNC incubated with 10−7 m CAPA‐1‐F labelled with TMR C5‐Maleimide. No apparent staining with CAPA‐1‐F in either brain or VNC. Kinin‐F, green; CAPA‐1‐F, red; DAPI, blue. Scale bars = 50 µm.
3.2. Desiccation stress
The CAP2b analogues 1895 and 2129 significantly increased desiccation / starvation mortality in both species (Table 2, Fig. 5). Here, treatment with 1895 acted to reduce the LTime50 by 3.5 and 9.6 h in M. persicae and M. rosae respectively, and median survival by 4.0 and 10.5 h respectively (Table 2). Treatment with 2129 acted to reduce the LTime50 by 7.1 and 11.6 h in M. persicae and M. rosae respectively, and median survival by 9.8 and 12.8 h respectively (Table 2). Neither 1895 nor 2129 impacted survival under non‐stress conditions (ANOVA DF = 2, F = 0.00, P = 0.999) with 1 out of 32 aphids treated with 1895, 1 out of 31 aphids treated with 2129, and 1 out of 29 aphids from the control group dying within 7 days post treatment. None of the kinin analogues significantly affected desiccation/starvation mortality in either species (Table 2).
Table 2.
The effect of neuropeptide analogue treatment via microinjection on the desiccation and starvation tolerance of M. persicae and M. rosae. Neuropeptide analogues were administered to a final concentration of ×10−5 m. Survival is shown as both a median survival (h) ± IQR and an LTime50 (h). Values in bold significantly increased desiccation / starvation mortality in relation to a vehicle control group. Example survival curves are displayed in Fig. 5
| LTime50 (h) | Median ± IQR survival (h) | ||||||
|---|---|---|---|---|---|---|---|
| Documented effect | Control | test | Control | test | χ2 | P | |
| Myzus persicae | |||||||
| 1728 | No effect | 18.3 | 25.0 | 22.0 ± 16.5 | 26.0 ± 23.5 | 2.260 | 0.133 |
| 2139 | No effect | 18.3 | 22.4 | 22.0 ± 16.5 | 25.0 ± 21.0 | 1.341 | 0.247 |
| 2139‐AC | No effect | 19.4 | 16.7 | 22.5 ± 19.8 | 20.0 ± 14.0 | 2.498 | 0.114 |
| 1895 | Increases mortality | 15.9 | 12.4 | 17.8 ± 12.0 | 13.8 ± 11.8 | 3.948 | 0.047 |
| 1896 | No effect | 22.2 | 24.8 | 24.0 ± 09.0 | 25.0 ± 10.5 | 1.939 | 0.164 |
| 1902 | No effect | 22.2 | 21.8 | 24.0 ± 09.0 | 23.5 ± 07.0 | 0.030 | 0.863 |
| 2089 | No effect | 10.9 | 13.0 | 11.0 ± 12.0 | 16.5 ± 10.3 | 2.197 | 0.138 |
| 2123 | No effect | 24.3 | 21.1 | 25.0 ± 13.5 | 21.0 ± 13.5 | 2.092 | 0.148 |
| 2125 | No effect | 10.8 | 13.0 | 11.0 ± 12.0 | 11.0 ± 12.0 | 1.309 | 0.253 |
| 2129 | Increases mortality | 15.9 | 08.8 | 17.8 ± 12.0 | 08.0 ± 17.0 | 10.200 | 0.001 |
| Macrosiphum rosae | |||||||
| 1728 | No effect | 31.7 | 31.6 | 29.5 ± 22.0 | 24.0 ± 28.5 | 0.431 | 0.512 |
| 2139 | No effect | 31.7 | 29.8 | 29.5 ± 22.0 | 27.0 ± 26.0 | 0.176 | 0.675 |
| 2139‐AC | No effect | 44.0 | 40.1 | 39.0 ± 37.0 | 35.0 ± 32.0 | 0.210 | 0.647 |
| 1895 | Increases mortality | 18.7 | 09.1 | 17.5 ± 19.5 | 07.0 ± 12.5 | 14.060 | <0.0001 |
| 1896 | No effect | 28.9 | 24.7 | 27.0 ± 17.3 | 23.0 ± 18.5 | 0.065 | 0.799 |
| 1902 | No effect | 28.9 | 26.4 | 27.0 ± 17.3 | 25.0 ± 10.0 | 0.224 | 0.636 |
| 2089 | No effect | 23.2 | 16.4 | 23.0 ± 17.0 | 17.0 ± 21.3 | 2.002 | 0.157 |
| 2123 | No effect | 21.6 | 19.1 | 24.0 ± 11.0 | 16.0 ± 18.0 | 1.806 | 0.179 |
| 2125 | No effect | 21.6 | 20.1 | 24.0 ± 9.25 | 20.5 ± 18.0 | 0.215 | 0.643 |
| 2129 | Increases mortality | 24.3 | 12.7 | 23.8 ± 20.5 | 11.0 ± 13.0 | 14.320 | <0.0001 |
Figure 5.
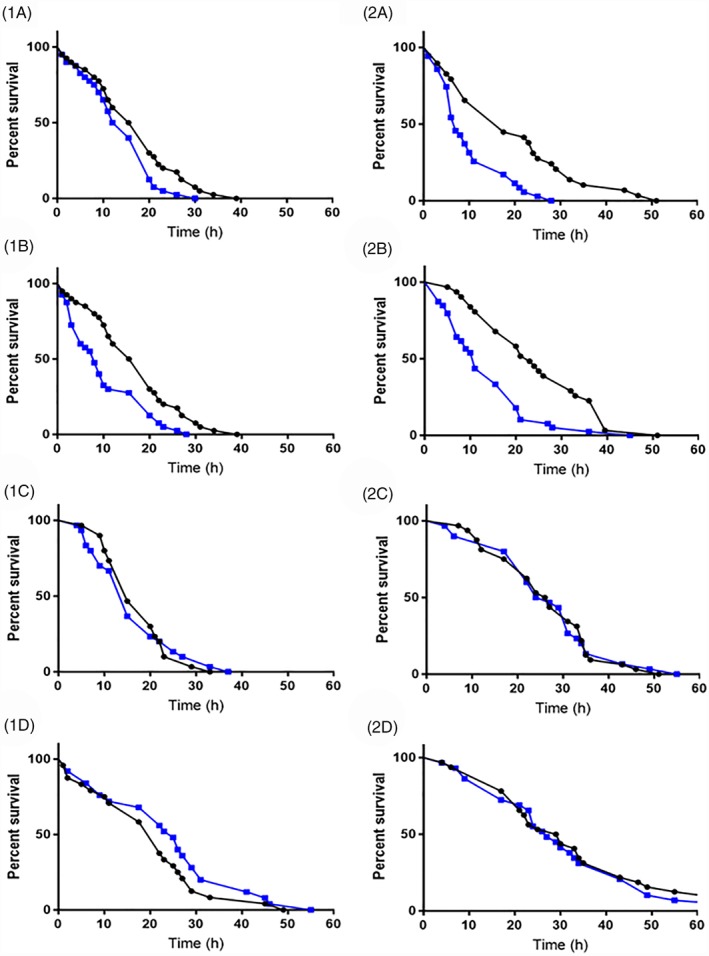
Effect of CAP2b and kinin analogue treatment on the survival of Myzus persicae (1) and M. rosae (2) under conditions of desiccation and starvation stress. Control aphids are indicated by the black line and analogue‐treated aphids by the blue line. CAP2b analogues 1895 (a) and 2129 (b) were administered to a final concentration of ×10−5 m via microinjection and acted to significantly increase mortality relative to the control. CAP2b analogue 2125 (c) and kinin analogue 2139 (d) are presented to illustrate non‐significant survival curves.
3.3. Cold stress
A survival curve was calculated for M. persicae (Fig. 6) and the LT30 (discriminating temperature) calculated as −9.7 °C. There was a significant effect of ‘Type’ (control vs. treatment) on the cold stress survival of M. persicae following cold shock at the discriminating temperature of −9.7 °C (GLM DF = 1, χ2 = 5.9844, P = 0.014), indicating that all analogues are efficient at increasing the mortality of test aphids under conditions of cold stress. However, there was no effect of the factor ‘Treatment’ (peptide analogue) on aphid cold stress survival (GLM DF = 9, χ2 = 7.8355, P = 0.551), indicating that all analogues appear equivalent in their effect, with no analogue having a stronger effect than another. Interestingly, treatment with analogue 2139‐AC implies a reverse effect on aphid survival (Fig. 7), acting to increase survival relative to the control. However, further examination restricted to this case against its control proved non‐significant (GLM DF = 1, χ2 = 7.8355, P = 0.3771). It must be concluded that all studied analogues increase the mortality of aphids under conditions of cold stress, with the exception of 2139‐Ac, although no individual peptide is significantly more powerful in its effect.
Figure 6.
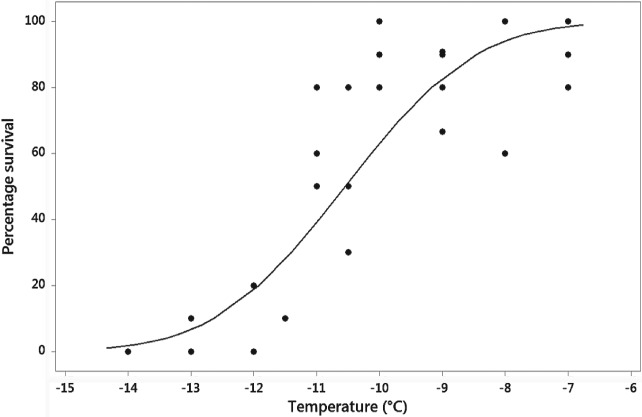
Survival curve calculated via Probit analysis for Myzus persicae pre‐reproductive adults following a 1 h exposure at the desired temperature. Raw data values are indicated by black circles.
Figure 7.
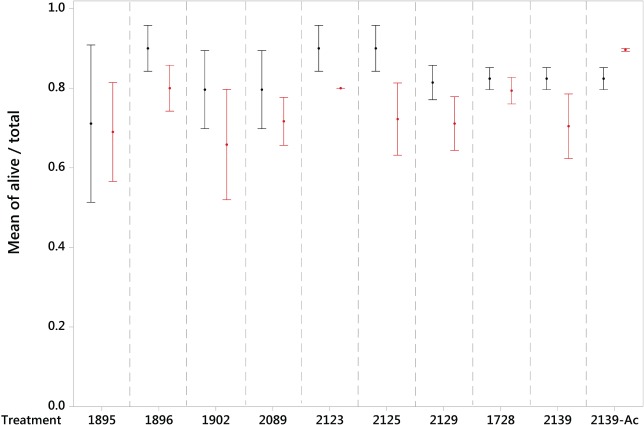
Mean ± standard error proportion survival of M. persicae when treated with biostable peptide analogues (CAP2b/PK: 1895, 1896, 1902, 2089, 2123, 2125, 2129; kinin: 1728, 2139, 2139‐Ac) via microinjection and subjected to a discriminating temperature for a 1 h exposure. Control groups are shown in black and peptide treatment groups in red.
4. DISCUSSION
Neuropeptides are regulators of critical life processes in insects and, due to their high specificity, hold great potential in the drive for target‐specific and environmentally friendly insecticidal agents.5 In pursuit of the development of neuropeptidomimetic‐based insecticides, the current study mapped kinin (kinin‐F) and CAPA (CAPA‐1‐F) neuropeptide binding sites within M. persicae and M. rosae to determine the location of neuropeptide receptors and thus the targets of neuropeptide action. A total of 10 kinin and CAP2b/PK (active on the CAPA receptor) biostable analogues were subsequently assayed for target‐insect‐specificity and an ability to reduce aphid pest fitness under a range of environmental stressors.
Receptor mapping employing fluorescently labelled kinin revealed the gut musculature as a main target for kinin activity in both M. persicae and M. rosae, as previously shown for M. persicae.7 Additional areas in the brain and VNC were also indicated in M. persicae. In the pea aphid, Acyrthosiphon pisum, it is thought that kinin regulates gut motility, digestive enzyme release, fluid cycling and nutrient transport across the gut.34 Indeed, kinin analogues have shown great potential in the laboratory for their aphicidal properties, acting as antifeedant agents during artificial diet trials on the pea aphid (A. pisum).34 Interestingly, while kinin analogues, including the present analogue 1728, have displayed prior antifeedant potential, none of the kinin analogues in the current study acted to reduce aphid fitness under desiccation and starvation stress conditions.
As with receptor mapping of kinin activity, receptor mapping with fluorescently labelled CAPA revealed the muscles of the aphid midgut as the target for neuropeptide binding, with no CAPA receptor binding detected in the aphid brain or VNC. However, in contrast to the kinin analogues, CAP2b/PK analogues displayed greater promise in stress tolerance assays, with analogues 1985 (2Abf‐Suc‐FGPRLa) and 2129 (2Abf‐Suc‐ATPRIa) acting to expedite aphid (M. persicae and M. rosae) mortality under conditions of desiccation and starvation stress. Furthermore, all tested analogues (kinin and CAP2b/PK), with the exception of 2139‐Ac, enhanced M. persicae mortality under cold stress conditions, although were all considered equivalent in the strength of their effect.
Neuropeptides of the CAPA family have roles in the stimulation of fluid secretion in Malpighian (renal) tubules47 and, more recently, have been linked to desiccation and cold tolerance in Drosophila.17 Unlike most insects, aphids lack Malpighian tubules;48 organs with vital roles in osmoregulation, detoxification and immunity.49, 50 Due to this secondary loss of Malpighian tubules in the aphids, key osmoregulatory roles have been reassigned to other organs, particularly the aphid gut.50 Receptor mapping assays performed in the current study offer support to this, highlighting the presence of CAPA receptors along the aphid gut and implicating the gut as a primary target for CAPA neuropeptide action. The role of CAPA neuropeptides in osmoregulation further offers explanation for the relative effectiveness of the CAP2b analogues tested in expediting aphid mortality under conditions of desiccation stress.
5. CONCLUSION
Kinin and CAPA (CAPA‐1) binding was confirmed in the aphids M. persicae and M. rosae, with the musculature of the gut the primary target of neuropeptide action. In‐vivo assays revealed CAP2b/PK analogues to be the most effective in expediting aphid mortality under conditions of desiccation and starvation stress. In highlighting the PRXamide neuropeptide family more generally, and the structures of promising CAP2b/PK analogues more specifically, this research will feed into the development of second and third generation analogues and ultimately drive forward the development of neuropeptidomimetic‐based insecticidal agents. Current testing of new generation analogues is focusing on mode of application to elucidate the best method of delivery to apply neuropeptide‐based insecticides in the field.
ACKNOWLEDGEMENTS
This work was funded by the European Union's Horizon 2020 Research and Innovation program under grant agreement No. 634361 (SD/JATD) (nEUROSTRESSPEP). The authors are grateful to the Smagghe laboratory, Ghent University, for providing M. persicae. Thanks also to Angela Douglas, Cornell University, for discussion on aphid internal morphology.
REFERENCES
- 1. Pimentel D, Acquay H, Biltonen M, Rice P, Silva M, Nelson J et al., Environmental and economic costs of pesticide use. Bioscience 42:750–760 (1992). [Google Scholar]
- 2. Wilson C and Tisdell C, Why farmers continue to use pesticides despite environmental, health and sustainability costs. Ecol Econ 39:449–462 (2001). [Google Scholar]
- 3. Altstein M and Nässel DR, Neuropeptide signaling in insects. Adv Exp Med Biol 692:155–165 (2010). [DOI] [PubMed] [Google Scholar]
- 4. Nachman RJ and Pietrantonio PV, Interaction of mimetic analogs of insect kinin neuropeptides with arthropod receptors. Adv Exp Med Biol 692:27–48 (2010). [DOI] [PubMed] [Google Scholar]
- 5. Audsley N and Down RE, G protein coupled receptors as targets for next generation pesticides. Insect Biochem Molec 67:27–37 (2015). [DOI] [PubMed] [Google Scholar]
- 6. Holman GM, Nachman RJ and Coast GM, Isolation, characterization and biological activity of a diuretic myokinin neuropeptide from the housefly, Musca domestica . Peptides 20:1–10 (1999). [DOI] [PubMed] [Google Scholar]
- 7. Halberg KA, Terhzaz S, Cabrero P, Davies SA and Dow JAT, Tracing the evolutionary origins of insect renal function. Nat Commun 6:6800 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schoofs L, Vanden Broeck J and De Loof A, The myotropic peptides of Locusta migratoria: structures, distribution, functions and receptors. Insect Biochem Mol Biol 23:859–881 (1993). [DOI] [PubMed] [Google Scholar]
- 9. Coast GM, Holman GM and Nachman RJ, The diuretic activity of a series of cephalomyotropic neuropeptides, the achetakinins, on isolated Malpighian tubules of the house cricket Acheta domesticus . J Insect Physiol 36:481–488 (1990). [Google Scholar]
- 10. Dow JA, Insights into the Malpighian tubule from functional genomics. J Exp Biol 212:435–445 (2009). [DOI] [PubMed] [Google Scholar]
- 11. Harshini S, Nachman RJ and Sreekumar S, Inhibition of digestive enzyme release by neuropeptides in larvae of Opisina arenosella (Lepidoptera: Cryptophasidae). Comp Biochem Physiol B132:353–358 (2002). [DOI] [PubMed] [Google Scholar]
- 12. Nachman RJ, Coast GM, Douat C, Fehrentz JA, Kaczmarek K, Zabrocki J et al., A C‐terminal aldehyde insect kinin analog enhances inhibition of weight gain and induces significant mortality in Helicoverpa zea larvae. Peptides 24:1615–1621 (2003). [DOI] [PubMed] [Google Scholar]
- 13. Cannell E, Dornan AJ, Halberg KA, Terhzaza S, Dow JAT and Davies SA, The corticotropin‐releasing factor‐like diuretic hormone 44 (DH44) and kinin neuropeptides modulate desiccation and starvation tolerance in Drosophila melanogaster . Peptides 80:96–107 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zandawala M, Marley R, Davies SA and Nässel DR, Characterization of a set of abdominal neuroendocrine cells that regulate stress physiology using colocalized diuretic peptides in drosophila. Cell Mol Life Sci 75:1099–1115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huesmann GR, Cheung CC, Loi PK, Lee TD, Swiderek KM and Tublitz NJ, Amino acid sequence of CAP2b, an insect cardioacceleratory peptide from the tobacco hawkmoth Manduca sexta . FEBS Lett 371:311–314 (1995). [DOI] [PubMed] [Google Scholar]
- 16. Davies SA, Cabrero P, Povsic M, Johnston NR, Terhzaz S and Dow JAT, Signaling by drosophila capa neuropeptides. Gen Comp Endocr 188:60–66 (2013). [DOI] [PubMed] [Google Scholar]
- 17. Terhzaz S, Teets NM, Cabrero P, Henderson L, Ritchie MG, Nachman RJ et al., Insect capa neuropeptides impact desiccation and cold tolerance. Proc Natl Acad Sci USA 112:2882–2887 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Terhzaz S, Alford L, Yeoh JGC, Marley R, Dornan AT, Dow JAT et al., Renal neuroendocrine control of desiccation and cold tolerance by Drosophila suzukii . Pest Manag Sci 74:800–810 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Predel R and Wegener C, Biology of the CAPA peptides in insects. Cell Mol Life Sci 63:2477–2490 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Predel R and Nachman RJ, The FXPRLamide (Pyrokinin/PBAN) peptide family, in Handbook of Biologically Active Peptides, ed. by Kastin AJ. Elsevier, New York, pp. 207–212 (2006). [Google Scholar]
- 21. Jiang H, Wei Z, Nachman RJ, Adams ME and Park Y, Functional phylogenetics reveals contributions of pleiotropic peptide action to ligand‐receptor coevolution. Sci Rep 4:6800 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jiang H, Wei Z, Nachman RJ, Kaczmarek K, Zabrocki J and Park Y, Functional characterization of five different PRXamide receptors of the red flour beetle Tribolium castaneum with peptidomimetics and identification of agonists and antagonists. Peptides 68:246–252 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang Q, Nachman RJ, Kaczmarek K, Zabrocki J and Denlinger DL, Disruption of insect diapause using agonists and an antagonist of diapause hormone. Proc Natl Acad Sci U S A 108:16922–16926 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cornell MJ, Williams TA, Lamango NS, Coates D, Corvol P, Soubier F et al., Cloning and expression of an evolutionary conserved single‐domain angiotensin converting enzyme from Drosophila melanogaster . J Biol Chem 270:13613–13619 (1995). [DOI] [PubMed] [Google Scholar]
- 25. Lamango NS, Nachman RJ, Hayes TK, Strey A and Isaac RE, Hydrolysis of insect neuropeptides by an angiotensin converting enzyme from the housefly, M. domestica . Peptides 18:47–52 (1997). [DOI] [PubMed] [Google Scholar]
- 26. Nachman RJ, Strey A, Isaac E, Pryor N, Lopez JD, Deng JG et al., Enhanced in vivo activity of peptidase‐resistant analogs of the insect kinin neuropeptide family. Peptides 23:735–745 (2002). [DOI] [PubMed] [Google Scholar]
- 27. Nachman RJ, Isaac RE, Coast GM, Roberts VA, Lange A, Orchard I et al., Active conformation and mimetic analog development for the Pyrokinin/PBAN and Myosuppressin insect neuropeptide families, in Phytochemicals for Pest Control, ACS Symposium Series 658, ed. by Hedin PA, Hollingworth RM, Masler EP, Miyamoto J. and Thompson DG. ACS, Washington, DC, pp. 277–291 (1997). [Google Scholar]
- 28. Nachman RJ, Isaac RE, Coast GM and Holman GM, Aib‐containing analogues of the insect kinin neuropeptide family demonstrate resistance to an insect angiotensin‐converting enzyme and potent diuretic activity. Peptides 18:53–57 (1997). [DOI] [PubMed] [Google Scholar]
- 29. Taneja‐Bageshwar S, Strey A, Zubrzak P, Pietrantonio PV and Nachman RJ, Comparative structure–activity analysis of insect kinin core analogs on recombinant kinin receptors from southern cattle tick Boophilus microplus (Acari: Ixodidae) and mosquito Aedes aegypti (Diptera: Culicidae). Arch Insect Biochem Physiol 62:128–140 (2006). [DOI] [PubMed] [Google Scholar]
- 30. Taneja‐Bageshwar S, Strey A, Isaac ER, Coat GM, Zubrzak P, Pietrantonio PV et al., Biostable agonists that match or exceed activity of native insect kinins on recombinant arthropod GPCRs. Gen Comp Endocr 162:122–128 (2009). [DOI] [PubMed] [Google Scholar]
- 31. Holmes SP, He H, Chen AC, lvie GW and Pietrantonio PV, Cloning and transcriptional expression of a leucokinin‐like peptide receptor from the southern cattle tick Boophilus microplus (Acari: Ixodidae). Insect Mol Biol 9:457–465 (2000). [DOI] [PubMed] [Google Scholar]
- 32. Holmes SP, Barhoumi R, Nachman RJ and Pietrantonio PV, Functional analysis of a G protein‐coupled receptor from the southern cattle tick Boophilus microplus (Acari: Ixodidae) identifies it as the first arthropod myokinin receptor. Insect Mol Biol 12:27–38 (2003). [DOI] [PubMed] [Google Scholar]
- 33. Pietrantonio PV, Jagge C, Taneja‐Bageshwar S, Nachman RJ and Barhoumi R, The mosquito Aedes aegypti (L.) leucokinin receptor is a multiligand receptor for the three Aedes kinins . Insect Mol Biol 14:55–67 (2005). [DOI] [PubMed] [Google Scholar]
- 34. Smagghe G, Mahdian K, Zubrzak P and Nachman RJ, Antifeedant activity and high mortality in the pea aphid Acyrthosiphon pisum (Hemiptera: Aphidae) induced by biostable insect kinin analog. Peptides 31:498–505 (2010). [DOI] [PubMed] [Google Scholar]
- 35. Zhang C, Qu Y, Wu X, Song D, Ling Y and Yang X, Design, synthesis and aphicidal activity of N‐terminal modified insect kinin analogs. Peptides 68:233–238 (2015). [DOI] [PubMed] [Google Scholar]
- 36. Radford JC, Davies SA and Dow JA, Systematic G‐protein‐coupled receptor analysis in Drosophila melanogaster identifies a leucokinin receptor with novel roles. J Biol Chem 277:38810–38817 (2002). [DOI] [PubMed] [Google Scholar]
- 37. Terhzaz S, Cabrero P, Robben JH, Radford JC, Hudson BD, Milligan G et al., Mechanism and function of Drosophila capa GPCR: a desiccation stress‐responsive receptor with functional homology to human neuromedinU receptor. PLoS One 7:e29897 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Christie AE, In silico analyses of peptide paracrines/hormones in Aphidoidea. Gen Comp Endocr 159:67–79 (2008). [DOI] [PubMed] [Google Scholar]
- 39. Nault LR, Arthropod transmission of plant viruses: a new synthesis. Ann Entomol Soc Am 90:521–541 (1997). [Google Scholar]
- 40. van Emden HF and Harrington R, Aphids as Crop Pests. CABI Publishing, Wallingford: (2007). [Google Scholar]
- 41. Blackman RL and Eastop VF, Aphids on the World's Crops: An Identification Guide. John Wiley & Sons Ltd, Chichester: (2000). [Google Scholar]
- 42. Dow JAT, Maddrell SHP, Gortz A, Skaer NJV, Brogan S and Kaiser K, The Malpighian tubules of Drosophila melanogaster ‐ a novel phenotype for studies of fluid secretion and its control. J Exp Biol 197:421–428 (1994). [DOI] [PubMed] [Google Scholar]
- 43. Clough MS, Bale JS and Harrington R, Differential cold hardiness in adults and nymphs of the peach‐potato aphid Myzus‐Persicae . Ann Appl Biol 116:1–9 (1990). [Google Scholar]
- 44. Powell SJ and Bale JS, Intergenerational acclimation in aphid overwintering. Ecol Entomol 33:95–100 (2008). [Google Scholar]
- 45. Sinclair BJ and Chown SL, Rapid cold‐hardening in a Karoo beetle, Afrinus sp. Physiol Entomol 31:98–101 (2006). [Google Scholar]
- 46. Terblanche JS, Clusella‐Trullas S, Deere JA and Chown SL, Thermal tolerance in a south‐east African population of the tsetse fly Glossina pallidipes (Diptera, Glossinidae): implications for forecasting climate change impacts. J Insect Physiol 54:114–127 (2008). [DOI] [PubMed] [Google Scholar]
- 47. Davies SA, Huesmann GR, Maddrell SH, O'Donnell MJ, Skaer NJ, Dow JAT et al., CAP2b, a cardioacceleratory peptide, is present in Drosophila and stimulates tubule fluid secretion via cGMP. Am J Physiol 269:R1321–R1326 (1995). [DOI] [PubMed] [Google Scholar]
- 48. Douglas AE, Nutritional physiology of aphids. Adv Insect Physiol 31:73–140 (2003). [Google Scholar]
- 49. Beyenbach KW, Skaer H and Dow JA, The developmental, molecular, and transport biology of Malpighian tubules. Annu Rev Entomol 55:351–374 (2010). [DOI] [PubMed] [Google Scholar]
- 50. Jing X, White TA, Yang X and Douglas AE, The molecular correlates of organ loss: the case of insect Malpighian tubules. Biol Lett 11:20150154 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]


