Abstract
Despite continuous exposure to environmental pathogens, injured mucosa within the oral cavity heals faster and almost scar free compared with skin. Saliva is thought to be one of the main contributing factors. Saliva may possibly also stimulate skin wound healing. If so, it would provide a novel therapy for treating skin wounds, for example, burns. This study aims to investigate the therapeutic wound healing potential of human saliva in vitro. Human saliva from healthy volunteers was filter sterilized before use. Two different in vitro wound models were investigated: (a) open wounds represented by 2D skin and gingiva cultures were used to assess fibroblast and keratinocyte migration and proliferation and (b) blister wounds represented by introducing freeze blisters into organotypic reconstructed human skin and gingiva. Re‐epithelialization and differentiation (keratin K10, K13, K17 expression) under the blister and inflammatory wound healing mediator secretion was assessed. Saliva‐stimulated migration of skin and oral mucosa fibroblasts and keratinocytes, but only fibroblast proliferation. Topical saliva application to the blister wound on reconstructed skin did not stimulate re‐epithelization because the blister wound contained a dense impenetrable dead epidermal layer. Saliva did promote an innate inflammatory response (increased CCL20, IL‐6, and CXCL‐8 secretion) when applied topically to the flanking viable areas of both wounded reconstructed human skin and oral mucosa without altering the skin specific keratin differentiation profile. Our results show that human saliva can stimulate oral and skin wound closure and an inflammatory response. Saliva is therefore a potential novel therapeutic for treating open skin wounds.
Keywords: cell migration, inflammatory response, oral mucosa, proliferation, reconstructed human skin, saliva, therapy, wound healing
1. INTRODUCTION
Due to advancements in patient care, survival chances have increased significantly for severe burns patients. Now the major issue has become the prevention of infection and improving the quality of the final scar. Both of these are directly related to the rate of wound closure, the longer the wound remains open the greater the risk of adverse healing. The prevalence of post‐burn pathological fibrosis (hypertrophic scar) is very common (77%; Gangemi et al., 2008). The quality of life of burns patients with hypertrophic scars can be severely affected due to chronic itching, loss of joint mobility, contractures, and disfigurements, which lead to accompanying psychological problems (Bayat, McGrouther, & Ferguson, 2003). Also, patient care is extremely expensive due to the repeated surgical interventions needed to release scar contracture (Bayat et al., 2003). Hypertrophic scars occur most often after full thickness wounding, where no viable dermis is left (Deitch, Wheelahan, Rose, Clothier, & Cotter, 1983). Although there are various treatment strategies (including meshed autografts and skin substitutes), it is generally accepted that current strategies are still far from optimal. Excessive granulation tissue forms within the meshed area of autografts resulting in hypertrophic scar formation (Finnerty et al., 2016). Initial results with skin substitutes look promising (Gardien et al., 2016), but such advanced therapy medicinal products are extremely expensive to produce and require complicated logistics to receive patient (autologous) biopsies to the cleanroom facility and to transfer the living skin substitute back to the specialized hospital (Hartmann‐Fritsch, Marino, & Reichmann, 2016). Therefore, there is an urgent need to develop easy to use, inexpensive therapies which will enhance wound closure, as these in turn are expected to reduce the risk of wound infection and granulation tissue formation, and in doing so improve the final scar quality.
Wound healing involves four overlapping phases (hemostasis, inflammation, proliferation, and tissue remodelling; Gurtner, Werner, Barrandon, & Longaker, 2008; Martin, 1997; Martin & Leibovich, 2005; van den Broek, Limandjaja, Niessen, & Gibbs, 2014). Hemostasis occurs directly after injury and results in vasoconstriction and activation of platelets, which secrete many soluble wound healing factors to activate the coagulation pathway leading to the deposition of a fibrin clot. At the onset of trauma, inflammatory cytokines are released for the recruitment of different cell types. Monocytes and macrophages infiltrate the wounded area to combat infection and remove the damaged tissue. Upon wounding, it is most essential that the skin barrier function is restored as quickly as possible. Re‐epithelialization of a wound involves keratinocyte proliferation, migration, and differentiation in order to restore the breached epithelial barrier (Hakkinen, Uitto, & Larjava, 2000). In the underlying connective tissue, fibroblasts proliferate and migrate into the wound bed and deposit new extracellular matrix, which remodels into scar tissue. The challenge for scientists and clinicians is to develop therapies to guide the wound healing process in order to achieve optimal repair and ultimately regeneration (full restoration of structure and function) of the damaged tissue.
Notably, oral wounds heal much faster than skin wounds and with relatively much less scar formation (Hakkinen et al., 2000; Oudhoff et al., 2008; Oudhoff, Kroeze, et al., 2009; Oudhoff, van den Keijbus, et al., 2009; Wong et al., 2009). A major difference between skin and oral mucosa which regulates the tissue wound healing response is the presence of saliva in the oral cavity (Brand, Ligtenberg, & Veerman, 2014; Dawes et al., 2015). In addition to its lubricating function, saliva contains a vast cocktail of proteins (>1,000 proteins) which function in synergy so that saliva is mitogenic, enhances cell migration, and also acts in an anti‐microbial manner resulting in a healthy oral microflora (Denny et al., 2008; Mohanty et al., 2017). For example, defensins, LL37, histatins, and mucins protect against the formation of a pathogenic microbiome; whereas cytokines (Boink et al., 2017), chemokines, and growth factors directly enhance wound closure (Behm, Babilas, Landthaler, & Schreml, 2012). Saliva also stimulates the innate immune system, and therefore, the inflammatory phase of wound healing by stimulating fibroblasts to produce, for example, IL‐6 and CXCL8 (Cvikl, Lussi, Moritz, Sculean, & Gruber, 2015). By regulating the wound healing response and microbiota, saliva is thought to contribute to the almost scar‐free healing in the oral cavity. The wound healing properties of saliva was already acknowledged by the ancient Greeks 2,000 years ago when they applied snake saliva to open wounds to enhance cutaneous wound healing. Also, rats, which were allowed to lick burn wounds, showed enhanced wound healing compared with controls (Hakkinen et al., 2000). Notably, a primary human instinct is to directly apply saliva to a wound (e.g., on a finger) by licking the injured skin if possible. Taken together, saliva may contain components that are beneficial to skin wound healing as well as oral wound healing.
Oral wound healing is guided by both the intrinsic properties of the cells within the oral mucosa and interactions with saliva (Boink et al., 2016; Glim, van Egmond, Niessen, Everts, & Beelen, 2013). This suggests that the synergistically functioning multitude of saliva‐derived molecules may potentially be used as a potent novel therapeutic for enhancing skin wound closure. However, before such a saliva therapy can be investigated in human clinical studies, pre‐clinical experiments must first be performed. Animal studies are unsuitable for such a preclinical investigation of the therapeutic properties of human saliva because its consistency and many of the corresponding cellular receptors are specific for primates (de Sousa‐Pereira et al., 2013). The aim of this study was therefore to investigate the therapeutic wound healing potential of whole human saliva in vitro.
Human saliva was collected from healthy volunteers and filter sterilized before applying to cell cultures. Two types of in vitro wound models were investigated: (a) open wound represented by 2D culture models (Monsuur et al., 2016) and (b) a blistering wound represented by introducing freeze blisters into organotypic 3D reconstructed human skin (RHS) and gingiva (RHG; air‐exposed, differentiated epithelium on a fibroblast populated hydrogel; Breetveld, Richters, Rustemeyer, Scheper, & Gibbs, 2006; Kosten, Buskermolen, Spiekstra, de Gruijl, & Gibbs, 2015).
2. MATERIALS AND METHODS
2.1. Isolation and culture of human skin and gingiva keratinocytes and fibroblasts
Human skin was obtained after informed consent from healthy donors who underwent an abdominal dermolipectomy. Human gingiva was obtained as surgical waste after dental implant surgery. Both skin and gingiva were used in an anonymous fashion in accordance with the “Human Tissue and Medical Research: Code of conduct for responsible use” as formulated by the Federation of Dutch Medical Scientific Societies (www.federa.org).
Skin and gingiva keratinocytes were isolated and cultured as described previously (Waaijman et al., 2010). In short, epithelial sheets were separated from the dermis (skin) or lamina propria (gingiva) by an overnight incubation in dispase II (Sigma‐Aldrich, St. Louis, MO, USA) at 4°C. The epithelial sheet was then trypsinized and the primary keratinocytes were cultured at 37°C and 7.5% CO2 in KC‐medium consisting of Dulbecco's Modified Eagle Medium (DMEM; Lonza, Basel, Switzerland)/Ham's F‐12 (Gibco, Grand Island, USA) (3:1), 1% Ultroser G (BioSepra S.A. Cergy‐Saint‐Christophe, France), 1% penicillin–streptomycin (Gibco), 1 μM hydrocortisone (Sigma‐Aldrich), 1 μM isoproterenol (Sigma‐Aldrich), and 0.1 μM insulin (Sigma‐Aldrich) and supplemented with 1 ng/ml keratinocyte growth factor (KGF; Sigma‐Aldrich) for skin keratinocytes or 1 ng/ml epidermal growth factor (EGF; Sigma‐Aldrich) for gingival keratinocytes. The keratinocytes were used at Passage 1. This culture medium enables keratinocytes to differentiate as well as proliferate. A separate skin donor was used for each individual experiment described below, skin donors were not pooled.
Skin and gingival fibroblasts were isolated from the lamina propria (after removal of the epithelium) by incubation in collagenase (Gibco) for 3 hr. After passing the suspension through a 40 μm cell strainer, to remove extracellular matrix, the fibroblasts where cultured at 37°C, 5% CO2 in fibroblast medium containing DMEM, 1% Ultroser G, and 1% penicillin–streptomycin. Fibroblasts were used at Passage 1 or 2. A separate skin donor was used for each individual experiment described below, skin donors were not pooled.
2.2. Saliva collection and dilution
Unstimulated saliva was collected from self‐reported healthy volunteers in the morning between 8:00 and 12:00 a.m. Participants were instructed not to consume food or drink beverages other than water for at least 1 hr prior to the collection of saliva and to wash their mouth thoroughly with water before the collection of saliva. Saliva was collected for 10 min by dribbling into sterile 50 ml tube (Greiner Bio‐One, Alphen aan de Rijn, the Netherlands). Fresh saliva was collected and immediately used for the experiments. No protease or phosphatase inhibitors were added to the saliva. Saliva from a total of eight volunteers was collected; ranging from pH 6.2 to 8.3. This saliva from two to five volunteers was pooled, depending on the required volume necessary for each experiment, and filter sterilized through a 0.22 μm filter (Merck, Darmstadt, Germany) to remove pathogens. Pooled saliva resulted in batches ranging from pH 6.5 to 7.6. For the fibroblast and keratinocyte monolayer experiments (see below), the pooled saliva was diluted in PBS (as PBS is most compatible with cell culture and would be easy to implement in a future clinical study) to concentrations of 0%, 20%, 40%, and 100% saliva and further diluted in the culture medium (1:1) to final concentrations of 0%, 10%, 20%, and 50% saliva, so that all conditions contained the same percentage of culture medium (50%) and only the ratio of saliva to PBS differed between conditions. In the blister wound healing experiments, the saliva was added topically and undiluted (100%) to the RHS and RHG, because RHS and RHG have a fully differentiated, keratinized, and stratified epithelial layer, which provides a barrier to topically applied substances and therefore higher concentrations are required.
2.3. Fibroblast wound healing scratch assay
To investigate the ability of saliva to stimulate skin and gingiva fibroblast wound closure, a wound healing scratch assay was performed as previously described (Monsuur et al., 2016). The fibroblasts were cultured in a 48‐well plate (Corning, Tewksbury, MA, USA) in fibroblast medium (see above). When cultures reached 100% confluency, a scratch was made across the middle of the well with a 1 ml pipet tip. After washing with PBS to remove any loose fibroblasts, fibroblasts were further cultured in DMEM with 0.1% BSA (Sigma‐Aldrich), supplemented with different concentrations of pooled saliva (see above) or 1 ng/ml EGF (positive control). At day 0 and 2 after introduction of the scratch, phase contrast micrographs were made. The surface area of the scratch was measured using an image processing algorithm previously described in detail (Monsuur et al., 2016; Topman, Sharabani‐Yosef, & Gefen, 2012). Reduction of the scratch surface area relative to the 0% saliva control (50% PBS and 50% culture medium) was calculated for each experiment. Three independent experiments were performed; each experiment had an intra‐experiment duplicate.
2.4. Keratinocyte epithelialization model
In order to assess epithelial outgrowth from a 1 cm diameter confluent circle, 5 × 105 skin or gingiva keratinocytes in 100 μl KC‐medium (see above) were seeded into a metal ring (1 cm diameter) on collagen IV‐coated 10 cm culture dishes (Corning). After 4 hr of incubation, to enable keratinocyte attachment, the metal ring was removed and cells were further cultured in medium containing DMEM/Ham's F‐12 (3:1), 0.2% Ultroser G, 1% penicillin–streptomycin, 1 μM hydrocortisone, 1 μM isoproterenol, 0.1 μM insulin, with 1 ng/ml KGF for skin keratinocytes, and 1 ng/ml EGF for gingiva keratinocytes. This culture medium contains a high calcium concentration (1.6 mM) and therefore enables keratinocyte differentiation with limited stratification as well as proliferation (P. E. M. Gibbs & Lawrence, 1993; S. Gibbs, Backendorf, & Ponec, 1996). The culture medium was further supplemented with different concentrations of pooled saliva (see above). After 1 week, during which time the culture medium with saliva was refreshed at Day 3, the culture medium was exchanged for 2 ml of 2 mg/ml Thiazolyl Blue Tetrazolium Bromide (MTT; Sigma‐Aldrich) in PBS. After 1 hr of incubation at 37°C in the MTT solution, during which metabolic active keratinocytes stained blue/purple, photographs were made to determine the surface area of the expanding epithelium relative to 0% saliva. Three independent experiments were performed; each experiment had an intra‐experiment duplicate.
2.5. Keratinocyte and fibroblast proliferation
Skin and gingiva fibroblasts or keratinocytes were seeded into a 48‐well plate at 5 × 103 cells per well. Keratinocytes were cultured in collagen IV coated plates in KC‐medium supplemented with saliva (as described above). Fibroblasts were cultured in DMEM supplemented with 0.5% UltraserG and 1% penicillin–streptomycin supplemented with saliva as described above. After four days of culture, cells were harvested and DNA content per well was determined using a DNA quantification kit (CyQuant, Thermo Fisher Scientific, Waltham, USA) according to manufacturer's instructions. For quantification of cell number a serial dilution of a known amount of fibroblasts or keratinocytes was used. Three individual experiments were performed; each experiment had an intra‐experiment duplicate and results are expressed relative to the unexposed cultures.
2.6. Reconstructed human skin and gingiva wound model
RHS and RHG (reconstructed epithelium on fibroblast‐populated hydrogel) were constructed exactly as previously described (Kosten et al., 2015). Keratinocytes and fibroblasts were donor matched from a single donor within each independent experiment. In short, gingiva fibroblasts (1 × 105 cells/ml) were mixed with a 4 mg/ml collagen solution and pipetted into trans‐well inserts (2.4 cm diameter, 0.4 μm pore size [Corning]). After overnight incubation in fibroblast medium, keratinocytes (5 × 105 cells/hydrogel) were seeded on top. RHS and RHG were cultured, submerged in KC‐medium for 3 days and then further cultured for 10 days at the air liquid interface in medium containing DMEM/Ham's F12 (3:1), supplemented with 0.2% Ultroser G, 1% penicillin–streptomycin, 2 μM hydrocortisone, 0.1 μM insulin, 1 μM isoproterenol, 10 μM carnitine (Sigma‐Aldrich), 10 mM l‐serine (Sigma‐Aldrich), 0.4 mmol l‐ascorbic acid (Sigma‐Aldrich), 2 ng/ml KGF (RHS), or 2 ng/ml EGF (RHG). Culture medium was refreshed every 3 days.
After 10 days of air exposed culture, full thickness freeze blister wounds were introduced as previously described (Breetveld et al., 2006; Buskermolen et al., 2016). In short, a spatula cooled in liquid nitrogen was pressed lightly against the RHS and RHG surface for 5 s to freeze a narrow line (2‐cm long and 2‐mm wide) across the models (2‐cm diameter). This resulted in death of fibroblasts within the gel and detachment (blister forming) of the dead epithelium from the gel in the vicinity of the freeze wound only. Parallel cultures were used to examine the effect of saliva on RHS and RHG without wounds. New culture medium (without KGF or EGF) was added underneath the wounded and unwounded RHS and RHG. The models were subsequently topically exposed to 100 μl of undiluted pooled saliva or PBS (control) so that the entire culture surface was covered. After 24 hr, culture supernatant was collected and stored at −20°C for further analysis of inflammatory cytokine secretion by ELISA. At 24 hr and 3 days after the introduction of the wound, RHS and RHG were harvested for standard paraffin embedment and histological analysis in order to measure re‐epithelialization of the wound area as previously described (Buskermolen et al., 2016). Three independent experiments were performed; each experiment had an intra‐experiment duplicate.
2.7. Histology and immunohistochemistry
RHS and RHG were fixed in 4% paraformaldehyde and processed for paraffin embedment. After rehydration, tissue sections (5 μm) were stained with haematoxylin and eosin (H&E) for histological examination or processed for immunohistochemistry (IHC). For IHC, after antigen retrieval, sections were incubated for 1 hr with mouse antibodies against K10 (Santa Cruz Biotechnology), K13, or K17 (Monosan), as previously described (Kosten et al., 2015; Vriens et al., 2008). Hereafter, the sections were washed in PBS and incubated for 30 min with Brightvision (Immunologic). Next, the sections were incubated with AEC substrate for 10 min followed by haematoxylin staining. The microscopic slides were visualized with a Nikon Eclipse 80i. Quantification of re‐epithelialization on H&E sections were done with NIS‐Elements software (Nikon Instruments Europe B.V.).
2.8. ELISA for cytokine production
The amount of IL‐6 (R&D systems, Minneapolis, USA), CXCL8 (Sanquin, Amsterdam, the Netherlands), and CCL20 (R&D systems) in the culture supernatant was quantified by ELISA according to the manufacturers specifications as previously described (Spiekstra et al., 2005).
2.9. Statistics
Statistical analysis was performed with the aid of GraphPad Prism, version 6 (GraphPad Software, Inc.). Data were analyzed with the Friedman test followed by Dunn's multiple comparison test, except the re‐epithelialization of RHS, which was analyzed with a two‐way ANOVA, followed by Fisher's LSD test to analyze keratinocyte outgrowth. Differences were considered significant when p < .05. Data are represented as mean ± standard error of mean (SEM); * p < .05; ** p < .01; and *** p < .001.
3. RESULTS
3.1. Saliva stimulates fibroblast but not keratinocyte proliferation
In order to determine whether saliva could increase fibroblast and keratinocyte proliferation, conventional submerged cultures were exposed to increasing concentrations of saliva (Figure 1). For fibroblasts, 5% saliva resulted in the highest increase in proliferation (gingiva: 1.9 ± 0.2 fold; skin: 1.8 ± 0.3 fold compared with unstimulated fibroblasts). In contrast, saliva did not stimulate keratinocyte proliferation, and indeed 50% saliva appeared to have a cytotoxic effect on both skin and gingiva keratinocytes as observed by the dose‐dependent decrease in proliferation. Overall, saliva had a stimulating effect on gingiva and skin fibroblast but not keratinocyte proliferation.
Figure 1.
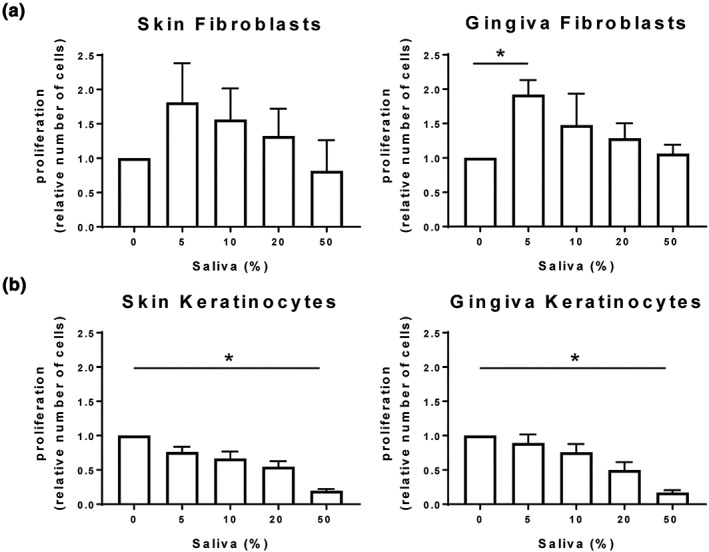
Saliva stimulates fibroblast (a) but not keratinocyte (b) proliferation. Proliferation, as determined by DNA quantification after exposure to saliva for 4 days is shown. Three independent experiments each with an intra‐experiment triplicate; mean ± SEM relative to unstimulated controls; * p < .05; Friedman test followed by Dunn's multiple comparisons test
3.2. Saliva stimulates wound closure in fibroblast scratch assay
Using the scratch wound closure assay, it was found that gingiva fibroblasts had a significantly greater intrinsic capacity to migrate into the wound area than skin fibroblasts (0.1 ± 0.01 mm2 versus 0.07 ± 0.01 mm2, respectively, in 48 hr; Figure 2) in agreement with previous results shown by (Boink et al., 2016; Oudhoff, Kroeze, et al., 2009). Supplementation of the culture medium with saliva resulted in an increase in wound closure in both skin and gingiva fibroblast cultures. Stimulation with 20% saliva resulted in the greatest increase in scratch closure relative to the controls: 2.5 ± 0.4 fold increase for both skin and gingiva fibroblasts. For comparison, stimulation with the potent mitogen EGF (2 ng/ml) only increased wound closure by 1.5 ± 0.2 fold for skin fibroblasts and 1.8 ± 0.2 fold for gingiva fibroblasts (data not shown).
Figure 2.
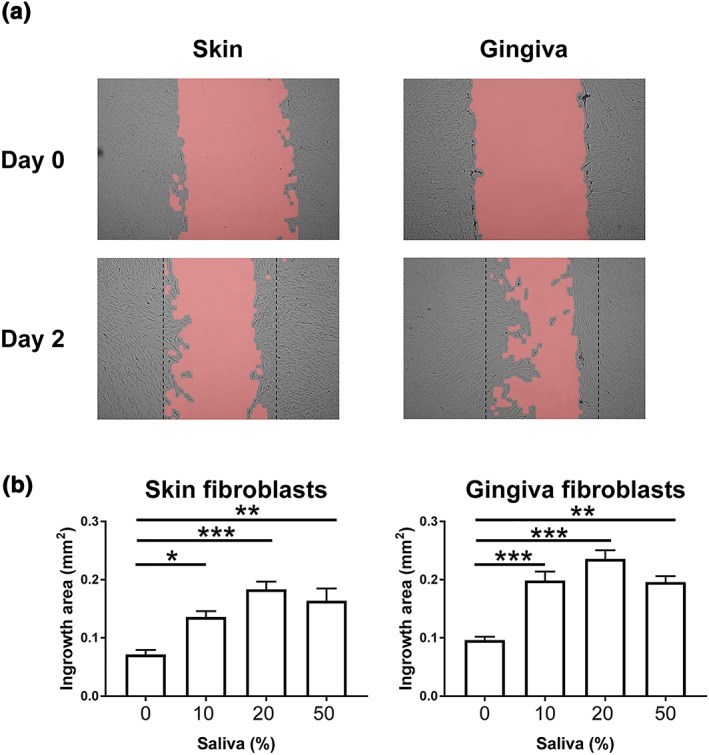
Saliva stimulates fibroblast migration in a wound healing scratch assay. (a) Representative photographs of Day 0 (top) and Day 2 (bottom) showing intrinsic scratch wound closure of skin fibroblasts (left) and gingiva fibroblasts (right). Dotted line represents initial scratch border on Day 0. (b) Quantification of scratch area closure (ingrowth area) after exposure to saliva. Data represents mean ± SEM of three independent experiments, each with an intra‐experiment duplicate; * p < .05; ** p < .01; *** p < .001; Friedman test followed by Dunn's multiple comparisons test [Colour figure can be viewed at wileyonlinelibrary.com]
3.3. Saliva stimulates keratinocyte epithelialization
The epithelial expansion from a confluent circle of proliferating and differentiating keratinocytes derived from skin or gingiva (submerged culture conditions) was used to mimic epithelialization over an open wound area (Figure 3a). Gingiva keratinocytes had a greater capacity to grow out from the circle than skin keratinocytes (3.5 ± 1.1 cm2 versus 1.4 ± 0.02 cm2, respectively). Supplementation of culture medium with saliva resulted in a further expansion reaching a maximum for skin keratinocytes with 50% saliva (1.5 ± 0.1 fold) and for gingiva keratinocytes with 20% saliva (1.8 ± 0.2 fold). Under the experimental conditions used in this study, 50% saliva inhibited the basal outgrowth of gingiva keratinocytes from the seeded area, possibly due to cytotoxicity (Figure 3a,b). Keratinocytes from both skin and gingiva at the outgrowth edge showed a different morphology when stimulated with saliva compared with unstimulated keratinocytes (Figure 3c). Under conditions where maximum outgrowth was obtained (skin: 50%; gingiva: 20%), the typical intact epithelial sheet observed in control cultures at the migrating front appeared less compact with intercellular spaces becoming visible, particularly in the case of the gingiva epithelium. This is in line with the finding that saliva stimulates keratinocyte migration but not proliferation (compare Figure 1 and 2).
Figure 3.
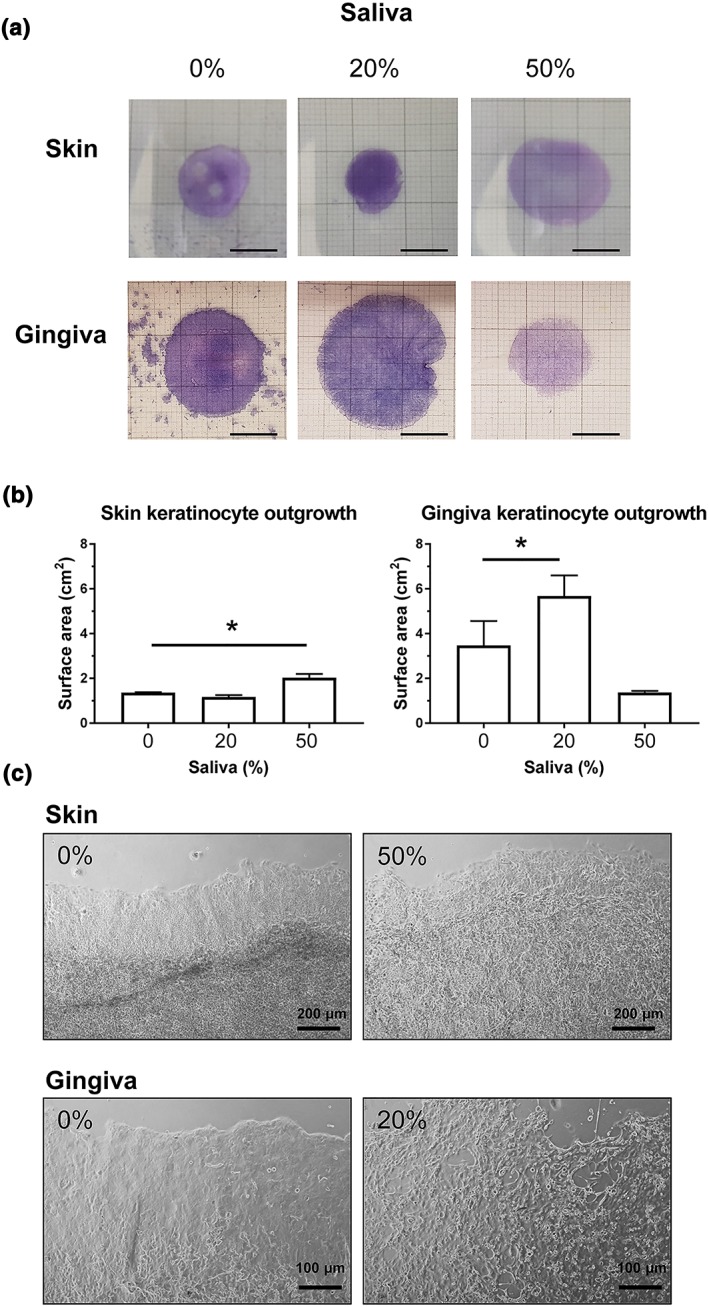
Saliva stimulates keratinocyte epithelium expansion. (a) Representative macroscopic photographs showing skin keratinocyte (top) and gingiva keratinocyte (bottom) outgrowth from a 1‐cm diameter seeding circle over a cell culture plate upon exposure to saliva. Keratinocytes are grown in traditional submerged culture conditions. Bar = 1 cm. (b) Quantification of epithelial surface area after exposure to saliva. Data represents mean ± SEM of three independent experiments, each with an intra‐experiment duplicate; * p < .05. (c) Representative phase contrast images picturing skin (top) and gingiva (bottom) keratinocytes at the outgrowth margin under influence of 0%, 20%, or 50% saliva [Colour figure can be viewed at wileyonlinelibrary.com]
3.4. Saliva does not stimulate skin blister closure
Organotypic RHS and RHG (each batch made from a separate skin or gingiva donor) were used to investigate healing from beneath a blister (Figure 4). Both RHS and RHG consist of a stratified, differentiated epithelium on a fibroblast populated collagen hydrogel. The stratum corneum of RHS contains no cell nuclei, which is typical for ortho‐keratinized (skin) epithelium; whereas RHG clearly has nuclei still visible in the most differentiated upper cells, which is typical for para‐keratinized (oral mucosa) epithelium (Figure 4a). Upon freezing, the wounded epithelium detaches from the hydrogel mimicking blister formation in vivo (Breetveld et al., 2006) (Figure 4b). Three days after introduction of the freeze wound, re‐epithelialization is visible underneath the blister of dead epithelium for both the RHS and RHG. Topical application of saliva to the surface of RHS did not stimulate the RHS wound closure above that observed for unexposed RHS (Figure 4c). For RHG, which exhibits a different type of terminal differentiation (para‐keratinized and less cornified) to ortho‐keratinized skin, it was not possible to measure the ingrowth of the epithelial sheet because it was not possible to accurately identify the wound margin, which is needed for such a quantification. In all experiments, the RHG wound area was totally closed (re‐epithelialized and fibroblasts had repopulated the hydrogel) within 72 hr after wounding (Figure 4b).
Figure 4.
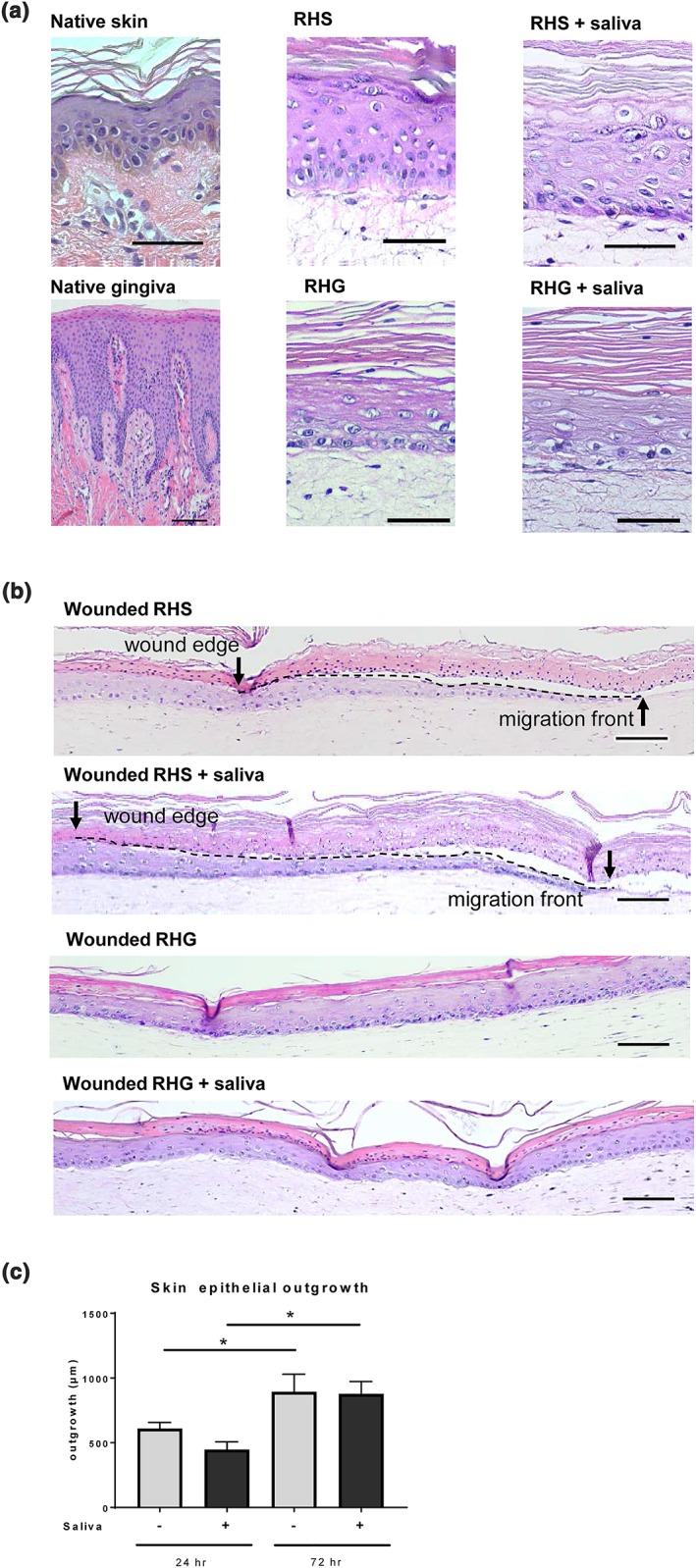
Blister wound healing model. (a) Representative haematoxylin and eosin staining is shown for native skin and native gingiva (left); reconstructed human skin (RHS; orthokeratinized epithelium) and reconstructed human gingiva (RHG; parakeratinized epitheliu; middle); RHS and RHG topically exposed to 100% saliva (right). (b) Freeze blisters were introduced into RHS and RHG. Re‐epithelialization is shown 72 hr after wounding with and without saliva treatment. RHS tissue sections showing original wound edge (black arrow), interface between dead epithelial layer and ingrowing epithelial layer (dotted line) and migrating front (arrow head). RHG tissue sections show that 72 hr post wounding, the fibroblasts have already migrated into the wound bed and the wound is completely closed, making it impossible to adequately define the exact wound margin. Scale bars represent 100 μm. (c) Quantification of re‐epithelialization after topical exposure to 0% (PBS) and 100% saliva for 72 hr, starting at time of introduction of freeze wound. * p < .05; ** p < .01; *** p < .001; two‐way ANOVA followed by Fisher's LSD test [Colour figure can be viewed at wileyonlinelibrary.com]
3.5. Saliva stimulates secretion of inflammatory cytokines in blister wounded RHS and RHG
When unwounded RHS and RHG were topically exposed to saliva for 24 hr, the secretion of IL‐6 and CXCL8 was increased from each of the three donor batches (Figure 5). The secretion of these cytokines was also increased during the 24 hr after creating the freeze blister wound in the absence of saliva. Particularly in the case of CXCL8, a combination of a freeze wound and the exposure to saliva had an additional effect, causing a higher cytokine release than the separate treatments. CCL20 secretion was not increased by topical saliva application but did show an increase in two of the three batches of RHS and RHG after creating the freeze blister wound. For RHG, these same two donors showed a further increase in CCL20 secretion when saliva was applied to the wounded RHG.
Figure 5.
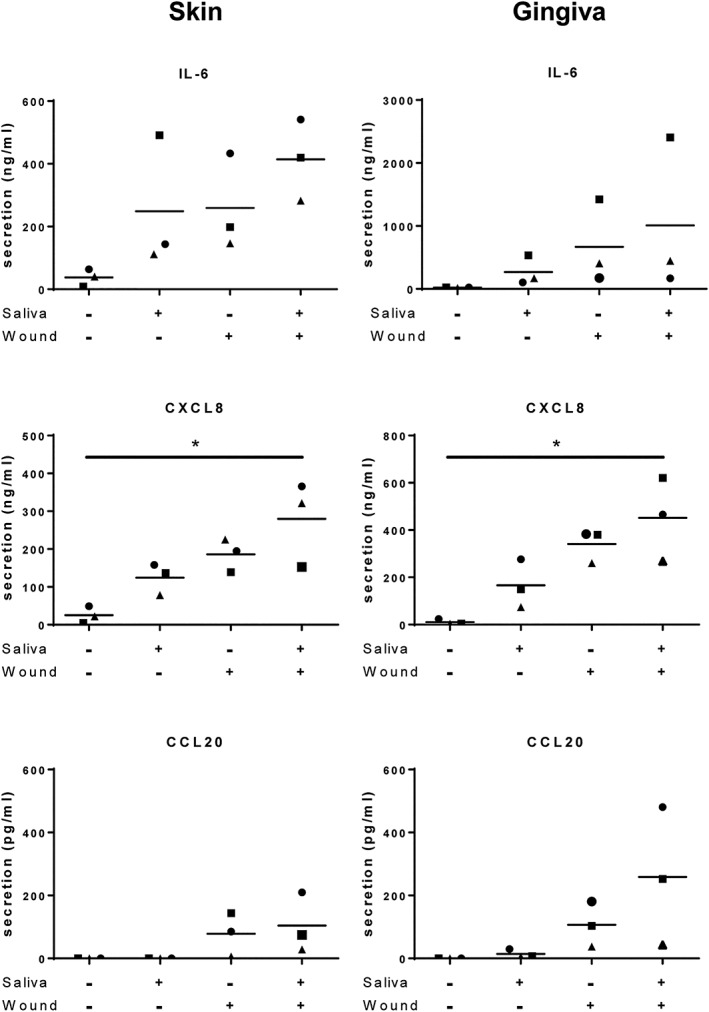
Cytokine IL‐6, CXCL8, and CCL20 secretion in culture supernatants of RHS (left) and RHG (right) was determined by ELISA 24 hr after introduction of the wound and/or topical application of 0% (PBS) or 100% saliva * p < .05; Friedman test followed by Dunn's multiple comparisons test. Data represents mean ± SEM of three independent experiments each with an intra‐experiment duplicate. Each experiment represents a batch of RHS or RHG constructed from a single skin donor; three different skin and gingiva donors were used in total
3.6. Saliva does not influence skin or gingiva epithelial differentiation
Because skin and gingiva epithelium have different barrier functions adapted to an air versus moist environment, they are histologically very distinct from each other (Figure 4 and 6). Skin has a stratum corneum, suprabasal K10 expression and no expression of K13 or K17. In contrast, gingiva has no stratum corneum and nuclei were still visible in the terminally differentiated keratinocytes being sloughed from the upper surface. K10 and K17 are expressed only in the upper differentiated cell layers and K13 has intermittent expression. Therefore, it is most important to ensure that a potential skin saliva therapy will not result in an epithelium more representative of gingiva than skin. After 3 days of topical exposure to saliva, the characteristic intrinsic properties of the epithelium of RHS and RHG were maintained (Figure 4 and 6). A typical compact basal layer of keratinocytes, a spinous layer, and a stratum granulosum and stratum corneum could still be observed in the epidermis of unwounded RHS. The epithelium of RHG lacked a clearly defined stratum granulosum and stratum corneum, and nuclei were still visible in the differentiated keratinocytes being sloughed from the surface. Furthermore, the expression of keratinocyte markers K10, K13, and K17 in RHS and RHG still correlated closely to that in native skin and gingiva (S. Gibbs & Ponec, 2000) and were not influenced by the topical application of saliva in the unwounded area of the cultures. Re‐epithelializing RHS did show increased K17 expression in line with its increased expression in healing wounds (McGowan & Coulombe, 1998; Figure 6). Re‐epithelializing RHG showed K10, K13, and K17 expression similar to native gingiva. Both saliva‐exposed RHS and RHG showed the same expression of keratins as their control, non‐saliva exposed counterparts (data not shown). Taken together, these results show that saliva will not influence the differentiation phenotype typical of skin epidermis.
Figure 6.
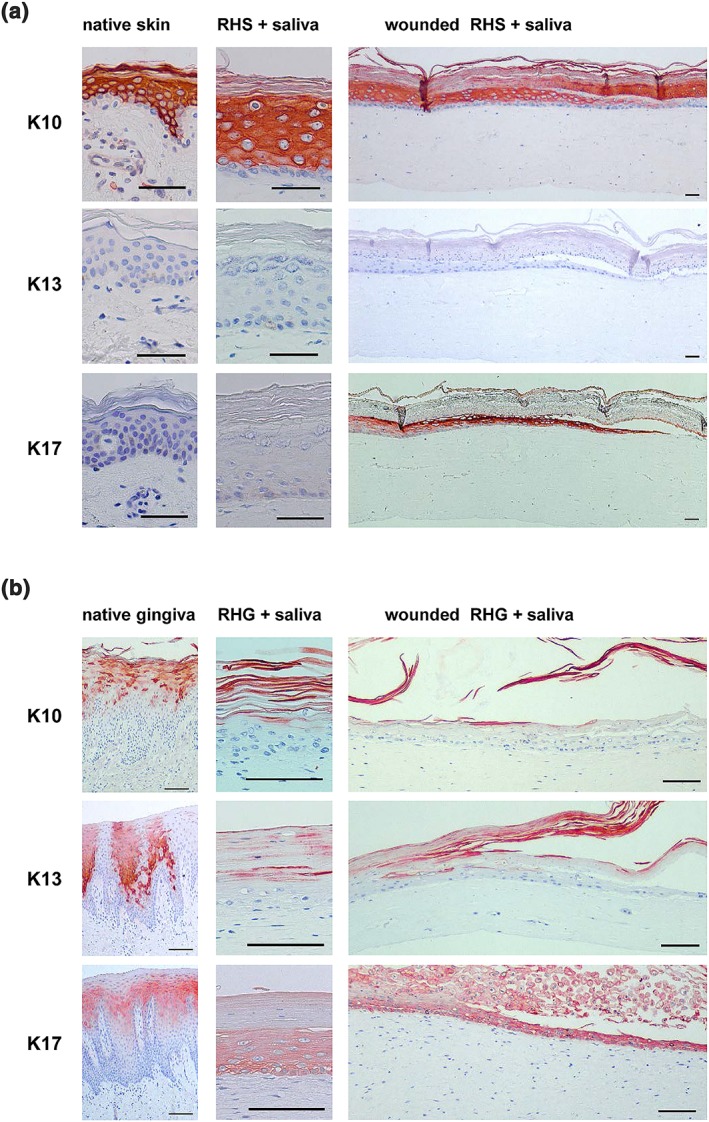
Immunohistochemical staining with K10, K13, and K17 of (a) skin and (b) gingiva. Left: Healthy native skin or gingiva biopsy; middle: Unwounded area of RHS or RHG, which was exposed topically to 100% saliva for 72 hr; right: Epithelial migrating front of wounded RHS or RHG exposed topically to saliva for 72 hr. Scale bars represent 100 μm [Colour figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
Our results show that human saliva has the potential to stimulate skin wound closure and an inflammatory response, whereas not influencing normal epidermal differentiation. These findings may have significant clinical implications as a patient own (autologous, non‐cell‐based) saliva therapy would provide an extremely easy to implement and low cost means to enhance wound closure. This is in contrast to drugs or cell‐based products (advanced therapy medicinal products), which involve extensive development, safety, and efficacy testing, thus making them expensive and/or difficult to implement in a regular clinical setting. A saliva therapy could also be combined with standard surgical (autograft) and wound care procedures. In our study, we choose to investigate whole saliva as it is easy to collect without the aid of any complicated collection devices (Henskens et al., 1996). Previously, a positive effect of human parotid saliva was found on the migratory properties of human buccal mucosa keratinocytes (squamous cell carcinoma cell line TR146; Oudhoff et al., 2008). However, collection of parotid saliva requires suction devices being attached to the oral gland which would involve an unnecessary extra burden to the patient involved and require handling expertise by the clinical staff.
Saliva contains more than a 1,000 active peptides, which function in a complementary and synergistic fashion (Amerongen & Veerman, 2002; Ashcroft et al., 2000; Ghosh, Gupta, Jiang, & Weinberg, 2011; Oudhoff, Kroeze, et al., 2009; Prodan et al., 2015; Veerman, Oudhoff, & Brand, 2011). For example, the salivary protein histatin 1, which is only present in higher primates, has shown to have a positive influence on wound closure. Synthesized and HPLC purified histatin‐1 peptide was found to promote endothelial cell adhesion, migration, and angiogenesis in vivo as well as in vitro (Torres et al., 2017). Previously, we investigated the effect of histatin 1 on skin and gingiva fibroblast migration (Boink et al., 2016). It was found that histatin 1 stimulated migration slightly and to a lesser extent than EGF (10 ng/ml). The effect of whole saliva on human skin or non‐cancerous human oral cells has not been researched before. In this study, we show for the first time the wound healing potential of whole human saliva in healthy human wound healing models.
The models used in our study represented either an open wound or a blister wound. The scratch wound healing assay is representative of cells migrating into an open wound area. Previously, we showed that gingiva fibroblasts have a greater intrinsic capacity to migrate in this assay than skin fibroblasts (Boink et al., 2016; Oudhoff, van den Keijbus, et al., 2009). In this study, we show that migration reached a maximum in cultures supplemented with 20% saliva and decreased when 50% saliva was used. The reason for this is currently unknown, but it needs to be considered that saliva contains many peptides, for example, LL37, which have also been shown to be cytotoxic to cultured cells at high concentrations (>10 μM; Boink et al., 2017) and that in vivo saliva rarely has direct contact with fibroblasts because it first needs to penetrate granulation tissue forming in a wound bed or the epithelium in intact gingiva. Using a model to investigate epithelial expansion, we were indeed able to show that 50% saliva was not cytotoxic but resulted in maximum migration of differentiating skin keratinocytes growing out from a confluent circle over a cell culture surface. By using a 3‐(4,5‐di methyl thiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT) dye, we were able to clearly visualize the metabolically active migrating keratinocytes. As with gingiva fibroblasts, gingiva keratinocytes migrated faster than skin keratinocytes. Of note, saliva stimulated fibroblast proliferation but not keratinocyte proliferation. This later was also noticeable in the microscopic analysis of the edges of the epithelial sheets where under conditions with maximum outgrowth (50% and 20% for skin and gingiva respectively), the migrating front appeared less compact than in the control cultures. This finding was surprising considering the large number of molecules present in saliva, including salivary leptins, CCL5 (RANTES), IL‐1α, and KGF, which are described as mitogens (Dawes et al., 2015; Groschl et al., 2005; Kroeze et al., 2012). However, it must also be considered that cytotoxic proteins such as LL37, as mentioned above, are also present in saliva, indicating that a delicate balance exists between mitogens and antimicrobial proteins (Boink et al., 2017). It has been shown that a prolonged acidic wound environment can prevent wound closure and re‐epithelialization (Kruse et al., 2017). Even though pH values of saliva can differ between donors, pooled saliva results in batches within the pH range (6.5 to 8.5) that have been shown to be well‐tolerated for keratinocyte proliferation (Sharpe, Harris, Jubin, Bainbridge, & Jordan, 2009). For the majority of the experiments, the pooled saliva was added directly to the buffered cell culture medium. The pH of the medium remained at approximately 7.5 as observed by phenol red indicator in DMEM. Therefore, we can conclude that no cytotoxicity occurred due to extreme pH values and that the pH was not the reason for the decrease in keratinocyte proliferation. For the experiments with the blistering wound where undiluted saliva was applied topically to RHS and RHG, no cytotoxicity was observed as assessed by haematoxylin eosin staining of tissue sections. Our findings are in line with our previous study, investigating extensive cold injury in which we also found that keratinocytes when rapidly re‐epithelializing a dermal matrix migrate but do not proliferate in the outermost migrating front (Breetveld et al., 2006).
In order to mimic re‐epithelialization under a healing blister, freeze blisters were introduced into organotypic RHS (Buskermolen et al., 2016). Saliva, when topically applied to the blister wound, did not result in increased epithelialization. Because saliva did increase skin keratinocyte migration and fibroblast proliferation and migration in the open wound models discussed above, these results indicate that saliva cannot penetrate the dense layer of dead epidermis with stratum corneum in the blister model. Therefore, if a saliva therapy is anticipated for healing skin wounds in a future phase 1 clinical study, blisters should first be removed and the saliva only applied to the open wound. Due to technical limitations (wound width: 2.56 ± 0.77 mm) it was not feasible to remove the blisters in this in vitro model. For this reason, the epithelial outgrowth model was designed in order to mimic an open wound. Importantly, our results show that topical saliva application to RHS did not influence epidermal differentiation and that the typical expression profiles of skin keratinocyte differentiation markers K10, K13, and K17 (Vriens et al., 2008) were not altered. The increased expression of K17 at the wound edges of RHS is in line with wound healing where K17 is induced within hours (together with K6 and K16) upon acute injury (McGowan & Coulombe, 1998). Even though it was not possible to define accurately the exact wound margins of RHG due to technical issues with the histological sections, it was possible to determine keratin expression in the region. After topical exposure to saliva, RHG maintained the keratin expression, which is typical to that of native gingiva and is different to that of skin.
Topical application of saliva to RHS and RHG or introduction of a blister wound stimulated the secretion of inflammatory cytokines involved in wound healing (IL‐6, CXCL8 and to a lesser extent CCL20). This effect became even more pronounced when saliva was applied topically to cultures with a blister wound. Previously, we have shown that histatin 1 and LL37 (both salivary peptides) stimulate inflammatory cytokine release from keratinocytes, the latter via an IL‐1α‐dependent pathway (Boink et al., 2017). Since IL‐1α is pre‐stored in the stratum corneum and is also readily synthesized by keratinocytes, it is most probable that saliva stimulates pro‐inflammatory cytokine release from keratinocytes, which in turn stimulates an inflammatory cytokine cascade via the fibroblasts (Boink et al., 2017; Dawes et al., 2015; Spiekstra, Breetveld, Rustemeyer, Scheper, & Gibbs, 2007). Such an effect would be even more pronounced in the presence of a wound.
As with all in vitro studies, the limitations of the models used in this study should be acknowledged. The models were designed to investigate skin and gingiva keratinocyte proliferation and migration under defined conditions and to mimic wound closure. The models do not take into account the inflammatory status of a wound bed, infiltrating cells, and vascular effects. Neither do they take into account the microbiome, which would be expected to strongly influence re‐epithelialization (Shang et al., 2018). The experiments in this study represent multiple saliva donors (pooled saliva) and multiple skin or gingiva donors. Our results suggest that donor variation is apparent (in particular regarding the inflammatory response). Therefore, before progressing to a phase 1 clinical study, it would be necessary to further investigate the potential impact of the skin and saliva donor variation in vitro. In particular, the influence of the trauma (e.g., burn) on saliva composition and the age of the saliva donor needs to be investigated. In addition, burns are known to influence the immune status of the patient and wound closure occurs slower in the elderly. However, we do show here the potential of human saliva as a therapeutic for skin wound healing. Whether saliva may also have the ability to reduce scar formation or stimulate chronic wound closure still has to be investigated.
CONFLICT OF INTEREST
The authors have declared that there is no conflict of interest.
ACKNOWLEDGEMENTS
This work has been financed in part by the Dutch Burns Foundation (Nederlandse Brandwonden Stichting) grant 16.107 and we would like to thank dentist Henri JJ Uijlenbroek for supplying the fresh healthy gingiva biopsies.
Rodrigues Neves C, Buskermolen J, Roffel S, et al. Human saliva stimulates skin and oral wound healing in vitro. J Tissue Eng Regen Med. 2019;13:1079–1092. 10.1002/term.2865
REFERENCES
- Amerongen, A. V. , & Veerman, E. C. (2002). Saliva–The defender of the oral cavity. Oral Diseases, 8(1), 12–22. 10.1034/j.1601-0825.2002.1o816.x [DOI] [PubMed] [Google Scholar]
- Ashcroft, G. S. , Lei, K. J. , Jin, W. W. , Longenecker, G. , Kulkarni, A. B. , Greenwell‐Wild, T. , … Wahl, S. M. (2000). Secretory leukocyte protease inhibitor mediates non‐redundant functions necessary for normal wound healing. Nature Medicine, 6(10), 1147–1153. 10.1038/80489 [DOI] [PubMed] [Google Scholar]
- Bayat, A. , McGrouther, D. A. , & Ferguson, M. W. (2003). Skin scarring. BMJ, 326(7380), 88–92. 10.1136/bmj.326.7380.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behm, B. , Babilas, P. , Landthaler, M. , & Schreml, S. (2012). Cytokines, chemokines and growth factors in wound healing. Journal of the European Academy of Dermatology and Venereology, 26(7), 812–820. 10.1111/j.1468-3083.2011.04415.x [DOI] [PubMed] [Google Scholar]
- Boink, M. A. , Roffel, S. , Nazmi, K. , Bolscher, J. G. M. , Veerman, E. C. I. , & Gibbs, S. (2017). Saliva‐derived host defense peptides histatin1 and LL‐37 increase secretion of antimicrobial skin and oral mucosa chemokine CCL20 in an IL‐1 α ‐independent manner. Journal of Immunology Research, 2017, 3078194 10.1155/2017/3078194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boink, M. A. , van den Broek, L. J. , Roffel, S. , Nazmi, K. , Bolscher, J. G. , Gefen, A. , … Gibbs, S. (2016). Different wound healing properties of dermis, adipose, and gingiva mesenchymal stromal cells. Wound Repair and Regeneration, 24(1), 100–109. 10.1111/wrr.12380 [DOI] [PubMed] [Google Scholar]
- Brand, H. S. , Ligtenberg, A. J. , & Veerman, E. C. (2014). Saliva and wound healing. Monographs in Oral Science, 24, 52–60. 10.1159/000358784 [DOI] [PubMed] [Google Scholar]
- Breetveld, M. , Richters, C. D. , Rustemeyer, T. , Scheper, R. J. , & Gibbs, S. (2006). Comparison of wound closure after burn and cold injury in human skin equivalents. The Journal of Investigative Dermatology, 126(8), 1918–1921. 10.1038/sj.jid.5700330 [DOI] [PubMed] [Google Scholar]
- Buskermolen, J. K. , Reijnders, C. M. , Spiekstra, S. W. , Steinberg, T. , Kleverlaan, C. J. , Feilzer, A. J. , … Gibbs, S. (2016). Development of a full‐thickness human gingiva equivalent constructed from immortalized keratinocytes and fibroblasts. Tissue Engineering. Part C, Methods, 22(8), 781–791. 10.1089/ten.TEC.2016.0066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvikl, B. , Lussi, A. , Moritz, A. , Sculean, A. , & Gruber, R. (2015). Sterile‐filtered saliva is a strong inducer of IL‐6 and IL‐8 in oral fibroblasts. Clinical Oral Investigations, 19(2), 385–399. 10.1007/s00784-014-1232-3 [DOI] [PubMed] [Google Scholar]
- Dawes, C. , Pedersen, A. M. , Villa, A. , Ekstrom, J. , Proctor, G. B. , Vissink, A. , … Wolff, A. (2015). The functions of human saliva: A review sponsored by the World Workshop on Oral Medicine VI. Archives of Oral Biology, 60(6), 863–874. 10.1016/j.archoralbio.2015.03.004 [DOI] [PubMed] [Google Scholar]
- de Sousa‐Pereira, P. , Amado, F. , Abrantes, J. , Ferreira, R. , Esteves, P. J. , & Vitorino, R. (2013). An evolutionary perspective of mammal salivary peptide families: Cystatins, histatins, statherin and PRPs. Archives of Oral Biology, 58(5), 451–458. 10.1016/j.archoralbio.2012.12.011 [DOI] [PubMed] [Google Scholar]
- Deitch, E. A. , Wheelahan, T. M. , Rose, M. P. , Clothier, J. , & Cotter, J. (1983). Hypertrophic burn scars: Analysis of variables. The Journal of Trauma, 23(10), 895–898. 10.1097/00005373-198310000-00009 [DOI] [PubMed] [Google Scholar]
- Denny, P. , Hagen, F. K. , Hardt, M. , Liao, L. , Yan, W. , Arellanno, M. , … Fisher, S. J. (2008). The proteomes of human parotid and submandibular/sublingual gland salivas collected as the ductal secretions. Journal of Proteome Research, 7(5), 1994–2006. 10.1021/pr700764j [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnerty, C. C. , Jeschke, M. G. , Branski, L. K. , Barret, J. P. , Dziewulski, P. , & Herndon, D. N. (2016). Hypertrophic scarring: the greatest unmet challenge after burn injury. Lancet, 388(10052), 1427–1436. 10.1016/S0140-6736(16)31406-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangemi, E. N. , Gregori, D. , Berchialla, P. , Zingarelli, E. , Cairo, M. , Bollero, D. , … Stella, M. (2008). Epidemiology and risk factors for pathologic scarring after burn wounds. Archives of Facial Plastic Surgery, 10(2), 93–102. 10.1001/archfaci.10.2.93 [DOI] [PubMed] [Google Scholar]
- Gardien, K. L. , Marck, R. E. , Bloemen, M. C. , Waaijman, T. , Gibbs, S. , Ulrich, M. M. , … Dutch Outback Study, G. (2016). Outcome of burns treated with autologous cultured proliferating epidermal cells: A prospective randomized multicenter intrapatient comparative trial. Cell Transplantation, 25(3), 437–448. 10.3727/096368915X689569 [DOI] [PubMed] [Google Scholar]
- Ghosh, S. K. , Gupta, S. , Jiang, B. , & Weinberg, A. (2011). Fusobacterium nucleatum and human beta‐defensins modulate the release of antimicrobial chemokine CCL20/macrophage inflammatory protein 3alpha. Infection and Immunity, 79(11), 4578–4587. 10.1128/IAI.05586-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs, P. E. M. , & Lawrence, C. W. (1993). U‐U and T‐T cyclobutane dimers have different mutational properties. Nucleic Acids Research, 21(17), 4059–4065. 10.1093/nar/21.17.4059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs, S. , Backendorf, C. , & Ponec, M. (1996). Regulation of keratinocyte proliferation and differentiation by all‐trans‐retinoic acid, 9‐cis‐retinoic acid and 1,25‐dihydroxy vitamin D‐3. Archives of Dermatological Research, 288(12), 729–738. 10.1007/Bf02505289 [DOI] [PubMed] [Google Scholar]
- Gibbs, S. , & Ponec, M. (2000). Intrinsic regulation of differentiation markers in human epidermis, hard palate and buccal mucosa. Archives of Oral Biology, 45(2), 149–158. 10.1016/S0003-9969(99)00116-8 [DOI] [PubMed] [Google Scholar]
- Glim, J. E. , van Egmond, M. , Niessen, F. B. , Everts, V. , & Beelen, R. H. J. (2013). Detrimental dermal wound healing: What can we learn from the oral mucosa? Wound Repair and Regeneration, 21(5), 648–660. 10.1111/wrr.12072 [DOI] [PubMed] [Google Scholar]
- Groschl, M. , Topf, H. G. , Kratzsch, J. , Dotsch, J. , Rascher, W. , & Rauh, M. (2005). Salivary leptin induces increased expression of growth factors in oral keratinocytes. Journal of Molecular Endocrinology, 34(2), 353–366. 10.1677/jme.1.01658 [DOI] [PubMed] [Google Scholar]
- Gurtner, G. C. , Werner, S. , Barrandon, Y. , & Longaker, M. T. (2008). Wound repair and regeneration. Nature, 453(7193), 314–321. 10.1038/nature07039 [DOI] [PubMed] [Google Scholar]
- Hakkinen, L. , Uitto, V. J. , & Larjava, H. (2000). Cell biology of gingival wound healing. Periodontology 2000, 24, 127–152. 10.1034/j.1600-0757.2000.024001127.x [DOI] [PubMed] [Google Scholar]
- Hartmann‐Fritsch, F. , Marino, D. , & Reichmann, E. (2016). About ATMPs, SOPs and GMP: The hurdles to produce novel skin grafts for clinical use. Transfusion Medicine and Hemotherapy, 43(5), 344–352. 10.1159/000447645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henskens, Y. M. C. , vandenKeijbus, P. A. M. , Veerman, E. C. I. , VanderWeijden, G. A. , Timmerman, M. F. , Snoek, C. M. , … Amerongen, A. V. N. (1996). Protein composition of whole and parotid saliva in healthy and periodontitis subjects–Determination of cystatins, albumin, amylase and IgA. Journal of Periodontal Research, 31(1), 57–65. 10.1111/j.1600-0765.1996.tb00464.x [DOI] [PubMed] [Google Scholar]
- Kosten, I. J. , Buskermolen, J. K. , Spiekstra, S. W. , de Gruijl, T. D. , & Gibbs, S. (2015). Gingiva equivalents secrete negligible amounts of key chemokines involved in langerhans cell migration compared to skin equivalents. Journal of Immunology Research, 2015, 627125 10.1155/2015/627125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeze, K. L. , Vink, L. , de Boer, E. M. , Scheper, R. J. , van Montfrans, C. , & Gibbs, S. (2012). Simple wound exudate collection method identifies bioactive cytokines and chemokines in (arterio) venous ulcers. Wound Repair and Regeneration, 20(3), 294–303. 10.1111/j.1524-475X.2012.00789.x [DOI] [PubMed] [Google Scholar]
- Kruse, C. R. , Singh, M. , Targosinski, S. , Sinha, I. , Sorensen, J. A. , Eriksson, E. , & Nuutila, K. (2017). The effect of pH on cell viability, cell migration, cell proliferation, wound closure, and wound reepithelialization: In vitro and in vivo study. Wound Repair and Regeneration, 25(2), 260–269. 10.1111/wrr.12526 [DOI] [PubMed] [Google Scholar]
- Martin, P. (1997). Wound healing–Aiming for perfect skin regeneration. Science, 276(5309), 75–81. 10.1126/science.276.5309.75 [DOI] [PubMed] [Google Scholar]
- Martin, P. , & Leibovich, S. J. (2005). Inflammatory cells during wound repair: The good, the bad and the ugly. Trends in Cell Biology, 15(11), 599–607. 10.1016/j.tcb.2005.09.002 [DOI] [PubMed] [Google Scholar]
- McGowan, K. M. , & Coulombe, P. A. (1998). Onset of keratin 17 expression coincides with the definition of major epithelial lineages during skin development. The Journal of Cell Biology, 143(2), 469–486. 10.1083/jcb.143.2.469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty, T. , Alberius, P. , Schmidtchen, A. , Reiss, K. , Schroder, J. M. , & Sorensen, O. E. (2017). Saliva induces expression of antimicrobial peptides and promotes intracellular killing of bacteria in keratinocytes by epidermal growth factor receptor transactivation. The British Journal of Dermatology, 176(2), 403–412. 10.1111/bjd.14883 [DOI] [PubMed] [Google Scholar]
- Monsuur, H. N. , Boink, M. A. , Weijers, E. M. , Roffel, S. , Breetveld, M. , Gefen, A. , … Gibbs, S. (2016). Methods to study differences in cell mobility during skin wound healing in vitro. Journal of Biomechanics, 49(8), 1381–1387. 10.1016/j.jbiomech.2016.01.040 [DOI] [PubMed] [Google Scholar]
- Oudhoff, M. J. , Bolscher, J. G. , Nazmi, K. , Kalay, H. , van 't Hof, W. , Amerongen, A. V. , & Veerman, E. C. (2008). Histatins are the major wound‐closure stimulating factors in human saliva as identified in a cell culture assay. The FASEB Journal, 22(11), 3805–3812. 10.1096/fj.08-112003 [DOI] [PubMed] [Google Scholar]
- Oudhoff, M. J. , Kroeze, K. L. , Nazmi, K. , van den Keijbus, P. A. , van 't Hof, W. , Fernandez‐Borja, M. , … Veerman, E. C. (2009). Structure‐activity analysis of histatin, a potent wound healing peptide from human saliva: Cyclization of histatin potentiates molar activity 1,000‐fold. The FASEB Journal, 23(11), 3928–3935. 10.1096/fj.09-137588 [DOI] [PubMed] [Google Scholar]
- Oudhoff, M. J. , van den Keijbus, P. A. , Kroeze, K. L. , Nazmi, K. , Gibbs, S. , Bolscher, J. G. , & Veerman, E. C. (2009). Histatins enhance wound closure with oral and non‐oral cells. Journal of Dental Research, 88(9), 846–850. 10.1177/0022034509342951 [DOI] [PubMed] [Google Scholar]
- Prodan, A. , Brand, H. S. , Ligtenberg, A. J. , Imangaliyev, S. , Tsivtsivadze, E. , van der Weijden, F. , … Veerman, E. C. (2015). Interindividual variation, correlations, and sex‐related differences in the salivary biochemistry of young healthy adults. European Journal of Oral Sciences, 123(3), 149–157. 10.1111/eos.12182 [DOI] [PubMed] [Google Scholar]
- Shang, L. , Deng, D. , Buskermolen, J. K. , Janus, M. M. , Krom, B. P. , Roffel, S. , … Gibbs, S. (2018). Multi‐species oral biofilm promotes reconstructed human gingiva epithelial barrier function. Scientific Reports, 8(1), 16061 10.1038/s41598-018-34390-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe, J. R. , Harris, K. L. , Jubin, K. , Bainbridge, N. J. , & Jordan, N. R. (2009). The effect of pH in modulating skin cell behaviour. The British Journal of Dermatology, 161(3), 671–673. 10.1111/j.1365-2133.2009.09168.x [DOI] [PubMed] [Google Scholar]
- Spiekstra, S. W. , Breetveld, M. , Rustemeyer, T. , Scheper, R. J. , & Gibbs, S. (2007). Wound‐healing factors secreted by epidermal keratinocytes and dermal fibroblasts in skin substitutes. Wound Repair and Regeneration, 15(5), 708–717. 10.1111/j.1524-475X.2007.00280.x [DOI] [PubMed] [Google Scholar]
- Spiekstra, S. W. , Toebak, M. J. , Sampat‐Sardjoepersad, S. , van Beek, P. J. , Boorsma, D. M. , Stoof, T. J. , … Gibbs, S. (2005). Induction of cytokine (interleukin‐1alpha and tumor necrosis factor‐alpha) and chemokine (CCL20, CCL27, and CXCL8) alarm signals after allergen and irritant exposure. Experimental Dermatology, 14(2), 109–116. 10.1111/j.0906-6705.2005.00226.x [DOI] [PubMed] [Google Scholar]
- Topman, G. , Sharabani‐Yosef, O. , & Gefen, A. (2012). A standardized objective method for continuously measuring the kinematics of cultures covering a mechanically damaged site. Medical Engineering & Physics, 34(2), 225–232. 10.1016/j.medengphy.2011.07.014 [DOI] [PubMed] [Google Scholar]
- Torres, P. , Diaz, J. , Arce, M. , Silva, P. , Mendoza, P. , Lois, P. , … Torres, V. A. (2017). The salivary peptide histatin‐1 promotes endothelial cell adhesion, migration, and angiogenesis. The FASEB Journal, 31(11), 4946–4958. 10.1096/fj.201700085R [DOI] [PubMed] [Google Scholar]
- van den Broek, L. J. , Limandjaja, G. C. , Niessen, F. B. , & Gibbs, S. (2014). Human hypertrophic and keloid scar models: Principles, limitations and future challenges from a tissue engineering perspective. Experimental Dermatology, 23(6), 382–386. 10.1111/exd.12419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerman, E. C. , Oudhoff, M. J. , & Brand, H. S. (2011). Saliva and wound healing. Nederlands Tijdschrift voor Tandheelkunde, 118(5), 253–256. 10.5177/ntvt.2011.05.10268 [DOI] [PubMed] [Google Scholar]
- Vriens, A. P. , Waaijman, T. , van den Hoogenband, H. M. , de Boer, E. M. , Scheper, R. J. , & Gibbs, S. (2008). Comparison of autologous full‐thickness gingiva and skin substitutes for wound healing. Cell Transplantation, 17(10–11), 1199–1209. 10.3727/096368908787236521 [DOI] [PubMed] [Google Scholar]
- Waaijman, T. , Breetveld, M. , Ulrich, M. , Middelkoop, E. , Scheper, R. J. , & Gibbs, S. (2010). Use of a collagen/elastin matrix as transport carrier system to transfer proliferating epidermal cells to human dermis in vitro. Wound Repair and Regeneration, 18(6), A98–A98. [DOI] [PubMed] [Google Scholar]
- Wong, J. W. , Gallant‐Behm, C. , Wiebe, C. , Mak, K. , Hart, D. A. , Larjava, H. , & Hakkinen, L. (2009). Wound healing in oral mucosa results in reduced scar formation as compared with skin: Evidence from the red Duroc pig model and humans. Wound Repair and Regeneration, 17(5), 717–729. 10.1111/j.1524-475X.2009.00531.x [DOI] [PubMed] [Google Scholar]


