Abstract
Objectives
To evaluate whether the systemic inflammation score (SIS) could predict postoperative outcomes for patients undergoing video-assisted thoracoscopic surgery (VATS) lobectomy for early-stage non-small-cell lung cancer (NSCLC).
Methods
This retrospective study was conducted on the prospectively maintained database in our institution between January 2016 and December 2017. Preoperative SIS comprising serum albumin (sALB) and lymphocyte-to-monocyte ratio (LMR) was graded into 0, 1 and 2, and then utilized to distinguish patients at high surgical risks. Multivariable logistic-regression analysis was conducted to determine independent risk factors for postoperative outcomes.
Results
There were 1,025 patients with TNM-stage I-II NSCLC included, with an overall morbidity rate of 31.1% and mortality rate of 0.3%. We applied the sALB at 40 g/L and the median LMR of our series at 4.42 as dichotomized cutoffs for modified SIS scoring criteria. Both minor and major morbidity rates in patients with SIS=2 were significantly higher than those in patients with SIS=0 and with SIS=1 (P<0.001). No difference was found in overall morbidity rate between patients with SIS=1 and with SIS=0 (P=0.20). No significant difference was found in the mortality rate between these 3 groups. Patients with SIS=2 had the highest probability to experience most of individual complications. Finally, multivariable logistic-regression analysis suggested that preoperative SIS=2 could independently predict the morbidity risks following VATS lobectomy (OR=1.73; 95% CI=1.11–2.71; P=0.016).
Conclusions
The SIS scoring system can be employed as a simplified, effective and routinely operated risk stratification tool in patients undergoing VATS lobectomy.
Keywords: systemic inflammation score, video-assisted thoracoscopic surgery, non-small-cell lung cancer, prediction
Introduction
Rationale
Non-small-cell lung cancer (NSCLC) is the leading cause of malignancy-related deaths worldwide.1 Radical surgery is generally regarded as the optimal therapeutic option for early-stage NSCLCs. Since the 1990s, single-lobectomy via video-assisted thoracoscopic surgery (VATS) has been popularized in the modern surgical modality, offering more advantages than traditional thoracotomy in terms of cosmetic wounds, operative stress control, preservation of pulmonary function and prolonged survival time.2–4 However, despite advances in surgical techniques, anesthetic techniques and perioperative care, the overall morbidity rate still reaches at 26.2–36.3% after VATS lobectomy.3–5 A better understanding of possible predisposing factors will be extremely crucial to assist thoracic surgeons to prevent morbidity risks.
Current evidence recognizes that a systemic inflammatory response, which is featured by changes in peripheral hematological indicators and alterations in levels of inflammation-linked proteins, frequently accompany with cancer patients, especially in advanced cases, and plays a vital role in carcinomatous pathogenesis and progression.6–8 Systemic inflammation indicated by a range of circulating hematological and biochemical markers, such as the elevated neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and lymphocyte-to-monocyte ratio (LMR), and the decreased hemoglobin and serum albumin (sALB) levels, has been reported to predict unfavorable cancer prognosis.6–8 These biomarkers can provide readily available and objective information to help oncologists to evaluate patient outcomes since they are easily obtained with a low healthcare cost in routine clinical practice.7
Recently, a novel scoring system comprising the ordinal variables of LMR and sALB, termed systemic inflammation score (SIS), was firstly developed among clear-cell renal cell carcinoma (ccRCC) patients, showing a great efficacy for prognostic prediction. Preoperative SIS was then reported to serve as a potent prognostic factor for patients undergoing gastric, colorectal and hepatic surgery.7–10 However, until recently, there has been no study addressing on the clinical significance of SIS for either short-term or long-term outcomes after lung cancer surgery.
Objectives
The purpose of our study was to elucidate the predictive roles of preoperative SIS for morbidity and mortality risks following VATS lobectomy for early-stage NSCLC.
Materials and methods
Ethics approval and consent to participate
This present study was approved by the regional ethics committee of Sichuan University West China Hospital (ID: 2016–255). We declared that all relevant procedures were in compliance with the Helsinki Declaration. All enrolled patients signed the written informed consent forms, including the use of participant medical characteristics and files for research in this manuscript.
Study design and protocol
This single-center retrospective study was conducted on the prospectively maintained dataset in our institution. We wrote it in compliance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.11
Patient selection
Settings
We retrospectively reviewed clinical data of consecutive patients undergoing VATS lobectomy for early-stage NSCLCs at our unit between January 2016 and December 2017. Patient characteristics could be extracted from our medical records.
Inclusion and exclusion criteria
On the basis of existing studies reporting the SIS, we established the following eligibility criteria to determine the appropriateness of patients included:7–10
The target diseases were primary TNM-stage I-II NSCLCs. Patients with any concomitant or previous malignancy were not included;
Only standardized single-lobectomy with systematic mediastinal lymph node dissection operated by a completely VATS procedure would be included. Any extended or sleeve resection was not considered;
Patients who received neoadjuvant or adjuvant chemotherapy were not considered, in order to avoid potential confounding influence from perioperative anti-cancer therapy, which might complicate the actual significance of the SIS;
Patients who experienced major intraoperative events, resulting in unexpected conversion to thoracotomy, were excluded due to their confounding influence on postoperative outcomes when evaluating the significance of the SIS;
The laboratorial indexes must be obtained within 5 days before surgery. Patients with loss of accurate laboratorial records were not included;
Patients must finish the entire clinical pathways according to our institutional policies during the hospitalization;
Outcome data, measures and definitions
Patient characteristics
Baseline information included the age, gender, body mass index (BMI), FEV1, FEV1 to FVC ratio (FEV1/FVC) and smoking history.
Preoperative comorbidities included the respiratory comorbidities, cardio-cerebrovascular comorbidities, diabetes mellitus, renal insufficiency and steroid use. Respiratory comorbidity was defined as the existence of one or more respiratory diseases, including the chronic obstructive pulmonary disease, emphysema, lung bullae, asthma, tuberculosis, pneumonia (including the bacterial/viral/fungal respiratory tract infections, obstructive pneumonia and aspiration pneumonia), bronchiectasis, lung abscess and interstitial lung diseases (including the idiopathic interstitial pneumonia, idiopathic pulmonary fibrosis, eosinophilic pneumonia and Langerhans cell histiocytosis). Cardio-cerebrovascular comorbidity was comprised of the hypertension, coronary heart diseases, peripheral arterial diseases, stroke, aortic aneurysm and chronic heart failure.
Laboratorial markers
The amounts of neutrophils, lymphocytes, monocytes and platelets, and the levels of hemoglobin and sALB were gathered from preoperative blood sampling to extrapolate the NLR, PLR and LMR.
Establishment and modification of SIS
The SIS was formulated by dichotomous variables of sALB and LMR. The lower range of normal measurement at 40 g/L was applied to dichotomize the sALB level according to its clinical meaning to define the “hypoalbuminemia”. As for the LMR dichotomization, the original SIS, which was first proposed based on the survival data of 441 ccRCC patients, utilized a median value at 4.44 as the cutoff since no international consensus had been recommended on the reference values for LMR. Accordingly, in this study, we chose the median LMR value of our NSCLC cohort and sALB at 40 g/L as the dichotomized cutoffs to modify the original SIS scoring criteria.
Therefore, the modified SIS system was as follows: patients with both sALB ≥40 g/L and LMR ≥ the median value of our cohort were assigned a score of 0; patients with either sALB <40 g/L or LMR < the median value of our cohort were assigned a score of 1; patients with both sALB <40 g/L and LMR < the median value of our cohort were assigned a score of 2.
Operative notes
Estimated intraoperative parameters included the tumor location, dense pleural adhesion, pulmonary fissure completeness, estimated intraoperative blood loss (EIBL) and operation time.
Pathological factors
The following three pathological variables were assessed: histological subtypes, tumor invasion (T-stage) and lymph node metastasis (LNM), all of which were defined according to the Union for International Cancer Control Seventh Edition.
Outcomes of interest
Our outcome of interest was any Clavien-Dindo grade ≥II complication developed within 30 days after surgery, which was judged in compliance with the Society of Thoracic Surgeons and the European Society of Thoracic Surgeons joint definitions.12,13 In-hospital mortality was defined as any death during the hospitalization.
All Clavien-Dindo grade II complications only requiring pharmacological intervention were categorized as the minor morbidity, including the prolonged air leak (PAL) (>5 days) requiring suction drainage only, pneumonia (fever >38°C, purulent sputum, abnormal findings on radiography) requiring antibacterial drugs, mild-atelectasis, subcutaneous emphysema requiring suction drainage only, mild-pneumothorax, pleural effusion requiring suction drainage only, hemoptysis, sinus irregularity and atrial arrhythmia requiring medical care, gastrointestinal discomforts and wound infection requiring antibacterial drugs.
We categorized all Clavien-Dindo grade ≥III complications requiring endoscopy, surgical intervention or life support as the major morbidity, including the PAL requiring pleurodesis or re-operation, severe-atelectasis requiring endoscopic intervention, severe subcutaneous emphysema and pneumothorax requiring re-operation, pulmonary artery embolism, acute respiratory distress syndrome (ARDS), chylothorax, bronchial fistula, ventricular arrhythmia, myocardial infarction and urinary tract retention requiring catheterization.
Grouping criteria
In our series, we adopted the same grouping criteria that had been validated in previous studies estimating the prognostic significance of the SIS.7–10 We divided the patients into 3 groups based on their preoperative SIS for initial risk stratification. Then, we compared the baseline characteristics and postoperative outcomes between patients who were stratified into 3 degrees of systemic inflammation indicated by the SIS of 0, 1 and 2. With respect to potential predictors for postoperative morbidity, we further explored the demographic differences in clinicopathological variables between patients with and without any complication in the univariable analysis.
Surgical procedure and perioperative care
Our VATS lobectomy was operated through a 3-portal access, using a modified “hilum-first-fissure-last” thoracoscopic technique known as “single-direction lobectomy”.3 Mechanical staplers were implemented in all patients to divide the incomplete inter-lobar fissures and close the bronchial stumps.
All of our patients were routinely managed in compliance with a standardized clinical pathway, including the pulmonary physiotherapy, antibiotic prophylaxis, respiratory drug intervention, breath training, venous thromboembolism prophylaxis, and surgical pain control, which had been described in our previous studies.3,14 One chest tube was placed on the suction device (−10–20 cm H2O) at the end of operation, and then either removed or converted to water seal according to our institutional policies after chest radiography done on postoperative day 1. Chest tube removal would be allowed with a 24 hr pleural drainage <200 mL and air leak cessation detected from the chest drainage system.
Statistical analysis
We employed the Pearson’s chi-squared test (when 0% of data cells have expected count <5) with Yates-correction (when 25% of data cells have expected count <5) or Fisher’s exact test (when 50% of data cells have expected count <5) to compare the categorical variables (number with percentage), and the Mann–Whitney U-test to compare the continuous variables [median with 25th–75th percentile-interval].
A receiver operating characteristic (ROC) analysis was performed to estimate the discriminative power of preoperative SIS for predicting morbidity risks. Area under the curve (AUC) with its 95% confidence interval (CI) was then calculated.
Finally, as Sato et al9 previously reported, a preoperative SIS of 2 and other clinicopathological variables with univariable P<0.20 would be included in the multivariable binary logistic-regression model, which utilized the Hosmer–Lemeshow test for goodness-of-fit and the C-statistic for discrimination, to determine independent risk factors for postoperative complications. OR with its 95% CI was then obtained.
We used the IBM SPSS 22.0 software (IBM SPSS Statistics, Version 22.0; IBM Corp., Armonk, NY, USA) to accomplish above statistical analyses. Statistical significance was indicated by P<0.05.
Results
Basic information and outcomes
Patient characteristics
During the study period, there were 1,025 patients undergoing VATS lobectomy for primary early-stage NSCLCs included. Patient characteristics are presented in Table 1. Our cohort consists of 530 male (ratio =51.7%) and 495 female (ratio =48.3%) patients, with a median age of 62 years (25th–75th percentile-interval =57–67 years) and median BMI of 23.3 kg/m2 (25th–75th percentile-interval = 21.1–25.2 kg/m2), and 380 patients were active smokers (ratio =37.1%). There were 665 patients suffered from one or more underlying comorbidities (ratio =64.9%). Lung adenocarcinoma was diagnosed in 822 patients (ratio =80.2%), followed by squamous cell carcinoma in 181 patients (ratio =17.7%) and other subtypes in 22 patients (ratio =2.1%). LNM was found in 59 patients postoperatively by the pathological criteria (ratio =5.8%).
Table 1.
Patient characteristicsa
| Characteristicsb | Total (N=1,025) | SIS=0 (N=470) | SIS=1 (N=413) | P1-value | SIS=2 (N=142) | P2-value |
|---|---|---|---|---|---|---|
| Median age (years) | 62 (57–67) | 61 (55–66) | 63 (56–69) | 0.004 | 66 (60–71) | <0.001 |
| Gender | ||||||
| Male | 530 (51.7%) | 170 (36.2%) | 256 (62.0%) | <0.001 | 104 (73.2%) | <0.001 |
| Female | 495 (48.3%) | 300 (63.8%) | 157 (38.0%) | 38 (26.8%) | ||
| Median body mass index (kg/m2) | 23.3 (21.1–25.2) | 23.2 (21.1–25.1) | 23.4 (21.2–25.4) | 0.090 | 22.9 (20.5–26.3) | 0.92 |
| Median FEV1% | 83.8 (73.8–92.7) | 87.3 (80.3–96.5) | 80.8 (70.3–89.8) | <0.001 | 74.7 (64.9–86.8) | <0.001 |
| Median FEV1/FVC (%) | 79.0 (74.2–83.5) | 81.3 (76.4–84.7) | 77.9 (73.0–82.9) | <0.001 | 74.9 (66.8–79.4) | <0.001 |
| Median neutrophil-to-lymphocyte ratio | 1.9 (1.4–2.5) | 1.5 (1.2–1.9) | 2.3 (1.8–3.1) | <0.001 | 2.4 (1.7–3.1) | <0.001 |
| Median platelet-to-lymphocyte ratio | 91.5 (72.4–120.0) | 82.7 (64.9–102.6) | 101.6 (78.0–133.8) | <0.001 | 105.7 (81.6–162.7) | <0.001 |
| Median hemoglobin (g/dL) | 13.6 (12.8–14.6) | 13.5 (12.9–14.5) | 13.8 (12.9–14.8) | 0.11 | 13.2 (12.1–14.2) | <0.001 |
| Smoking history | 380 (37.1%) | 99 (21.1%) | 198 (47.9%) | <0.001 | 83 (58.5%) | <0.001 |
| Respiratory comorbidity | 400 (39.0%) | 146 (31.1%) | 183 (44.3%) | <0.001 | 71 (50.0%) | <0.001 |
| Cardio-cerebrovascular comorbidity | 382 (37.3%) | 134 (28.5%) | 181 (43.8%) | <0.001 | 67 (47.2%) | <0.001 |
| Diabetes mellitus | 112 (10.9%) | 36 (7.7%) | 59 (14.3%) | 0.002 | 17 (12.0%) | 0.11 |
| Renal insufficiency | 89 (8.7%) | 35 (7.4%) | 38 (9.2%) | 0.35 | 16 (11.3%) | 0.15 |
| Steroid use | 31 (3.0%) | 11 (2.3%) | 14 (3.4%) | 0.35 | 6 (4.2%) | 0.23 |
| Tumor location | ||||||
| Right upper lobe | 334 (32.6%) | 164 (34.9%) | 136 (32.9%) | 0.44 | 34 (23.9%) | 0.25 |
| Left upper lobe | 261 (25.5%) | 127 (27.0%) | 99 (24.0%) | 35 (24.6%) | ||
| Right lower lobe | 193 (18.8%) | 81 (17.2%) | 78 (18.9%) | 34 (23.9%) | ||
| Left lower lobe | 149 (14.5%) | 56 (11.9%) | 65 (15.7%) | 28 (19.7%) | ||
| Right middle lobe | 88 (8.6%) | 42 (8.9%) | 35 (8.5%) | 11 (7.7%) | ||
| Dense pleural adhesion | ||||||
| Absent | 935 (91.2%) | 429 (91.3%) | 374 (90.6%) | 0.71 | 132 (93.0%) | 0.53 |
| Present | 90 (8.8%) | 41 (8.7%) | 39 (9.4%) | 10 (7.0%) | ||
| Pulmonary fissure completeness | ||||||
| Complete | 596 (58.1%) | 285 (60.6%) | 242 (58.6%) | 0.54 | 69 (48.6%) | 0.011 |
| Incomplete | 429 (41.9%) | 185 (39.4%) | 171 (41.4%) | 73 (51.4%) | ||
| Median estimated intraoperative blood loss (mL) | 50 (20–50) | 30 (20–50) | 50 (20–60) | 0.053 | 50 (30–60) | <0.001 |
| Median Operation time (mins) | 100 (80–130) | 100 (75–130) | 105 (80–130) | 0.016 | 120 (90–150) | <0.001 |
| Histological subtypes | ||||||
| Adenocarcinoma | 822 (80.2%) | 386 (82.1%) | 339 (82.1%) | 0.99 | 97 (68.3%) | <0.001 |
| Non-adenocarcinoma | 203 (19.8%) | 84 (17.9%) | 74 (17.9%) | 45 (31.7%) | ||
| Tumor invasion (T-stage) | ||||||
| T1 | 627 (61.2%) | 322 (68.5%) | 237 (57.4%) | 0.001 | 68 (47.9%) | <0.001 |
| T2-3 | 398 (38.8%) | 148 (31.5%) | 176 (42.6%) | 74 (52.1%) | ||
| Lymph node metastasis (N-stage) | ||||||
| N0 | 966 (94.2%) | 453 (96.4%) | 389 (94.2%) | 0.12 | 124 (87.3%) | 0.001 |
| N1-2 | 59 (5.8%) | 17 (3.6%) | 24 (5.8%) | 18 (12.7%) | ||
Notes: aP1-value indicates demographic differences between patients with SIS=1 and with SIS=0. P2-value indicates demographic differences between patients with SIS=2 and with SIS=0. bThe continuous data were presented as medians with 25th–75th percentile-intervals.
Abbreviations: FEV1/FVC, FEV1 to FVC ratio; SIS, Systemic inflammation score.
SIS evaluation
The median sALB and LMR of our cohort were 43.1 g/L (25th–75th percentile-interval =40.7–45.4 g/L) and 4.42 (25th–75th percentile-interval =3.30–5.48), respectively. Therefore, the sALB at 40 g/L and the median LMR at 4.42 were adopted to formulate our modified SIS. Accordingly, there were 470 patients got a SIS=0 (ratio =45.9%), 413 patients got a SIS=1 (ratio =40.3%) and 142 patients got a SIS=2 (ratio =13.9%), respectively.
Outcomes
There were 319 patients experienced Clavien-Dindo grade ≥II complications postoperatively, with an overall morbidity rate of 31.1%. The minor morbidity and major morbidity rate of the entire cohort was 27.7% (n=284) and 14.8% (n=152), respectively. There were 3 deaths during the hospitalization, with an in-hospital mortality rate of 0.3%. One patient died of sudden ventricular fibrillation, and another 2 patients dead of ARDS and pulmonary artery embolism. The incidences of individual complications are presented in Table 2.
Table 2.
Postoperative outcomesa
| Characteristics | Total (N=1,025) | SIS=0 (N=470) | SIS=1 (N=413) | P1-value | SIS=2 (N=142) | P2-value |
|---|---|---|---|---|---|---|
| Overall morbidity | ||||||
| Any morbidity | 319 (31.1%) | 124 (26.4%) | 125 (30.3%) | 0.20 | 70 (49.3%) | <0.001 |
| Minor morbidity (Clavien-Dindo Grade II) | 284 (27.7%) | 111 (23.6%) | 109 (26.4%) | 0.34 | 64 (45.1%) | <0.001 |
| Major morbidity (Clavien-Dindo Grade ≥III) | 152 (14.8%) | 54 (11.5%) | 62 (15.0%) | 0.12 | 36 (25.4%) | <0.001 |
| In-hospital mortality | 3 (0.3%) | 1 (0.2%) | 2 (0.5%) | 0.60 | 0 (0.0%) | 1.0 |
| Individual complications | ||||||
| Prolonged air leak (>5 days) | 152 (14.8%) | 47 (10.0%) | 65 (15.7%) | 0.011 | 40 (28.2%) | <0.001 |
| Pneumonia | 118 (11.5%) | 36 (7.7%) | 48 (11.6%) | 0.045 | 34 (23.9%) | <0.001 |
| Atelectasis | 95 (9.3%) | 45 (9.6%) | 35 (8.5%) | 0.57 | 15 (10.6%) | 0.73 |
| Subcutaneous emphysema | 73 (7.1%) | 26 (5.5%) | 28 (6.8%) | 0.44 | 19 (13.4%) | 0.002 |
| Pneumothorax | 49 (4.8%) | 26 (5.5%) | 17 (4.1%) | 0.33 | 6 (4.2%) | 0.54 |
| Pleural effusion requiring chest tube drainage | 30 (2.9%) | 9 (1.9%) | 5 (1.2%) | 0.40 | 16 (11.3%) | <0.001 |
| Hemoptysis requiring medical care | 29 (2.8%) | 11 (2.3%) | 8 (1.9%) | 0.68 | 10 (7.0%) | 0.015 |
| Sinus irregularity requiring medical care | 17 (1.7%) | 9 (1.9%) | 4 (1.0%) | 0.24 | 4 (2.8%) | 0.75 |
| Chylothorax | 15 (1.5%) | 2 (0.4%) | 9 (2.2%) | 0.019 | 4 (2.8%) | 0.028 |
| Atrial arrhythmia | 11 (1.1%) | 3 (0.6%) | 6 (1.5%) | 0.32 | 2 (1.4%) | 0.33 |
| Ventricular arrhythmia | 3 (0.3%) | 1 (0.2%) | 0 (0.0%) | 1.0 | 2 (1.4%) | 0.14 |
| Pulmonary artery embolism | 8 (0.8%) | 6 (1.3%) | 2 (0.5%) | 0.30 | 0 (0.0%) | 0.35 |
| Acute respiratory distress syndrome | 4 (0.4%) | 0 (0.0%) | 4 (1.0%) | 0.047 | 0 (0.0%) | — |
| Hemothorax | 2 (0.2%) | 0 (0.0%) | 2 (0.5%) | 0.22 | 0 (0.0%) | — |
| Bronchial fistula | 1 (0.1%) | 0 (0.0%) | 1 (0.2%) | 0.47 | 0 (0.0%) | — |
| Wound infection | 4 (0.4%) | 2 (0.4%) | 2 (0.5%) | 1.0 | 0 (0.0%) | 1.0 |
| Gastrointestinal discomforts requiring medical care | 13 (1.3%) | 5 (1.1%) | 8 (1.9%) | 0.28 | 0 (0.0%) | 0.60 |
| Urinary retention | 7 (0.7%) | 3 (0.6%) | 2 (0.5%) | 1.0 | 2 (1.4%) | 0.33 |
Notes: aP1-value indicates demographic differences between patients with SIS=1 and with SIS=0. P2-value indicates demographic differences between patients with SIS=2 and with SIS=0.
Abbreviation: SIS, systemic inflammation score.
Preoperative SIS and patient characteristics
Table 1 shows the association between preoperative SIS and baseline characteristics. We found the significantly increased age, NLR, PLR and operation time but the decreased FEV1% and FEV1/FVC with each 1 point increase of SIS. When compared to patients with SIS=0, both patients with SIS=1 and with SIS=2 had significantly higher ratios of males, smoking history, respiratory and cardio-cerebrovascular comorbidities and T2-3-stage tumors. In addition, patients with SIS=2 had a larger volume of EIBL and higher ratios of poorly developed fissure, LNM and non-adenocarcinoma subtypes. No difference was found in the other clinicopathological variables between these 3 groups.
Preoperative SIS and surgical outcomes
Morbidity and mortality
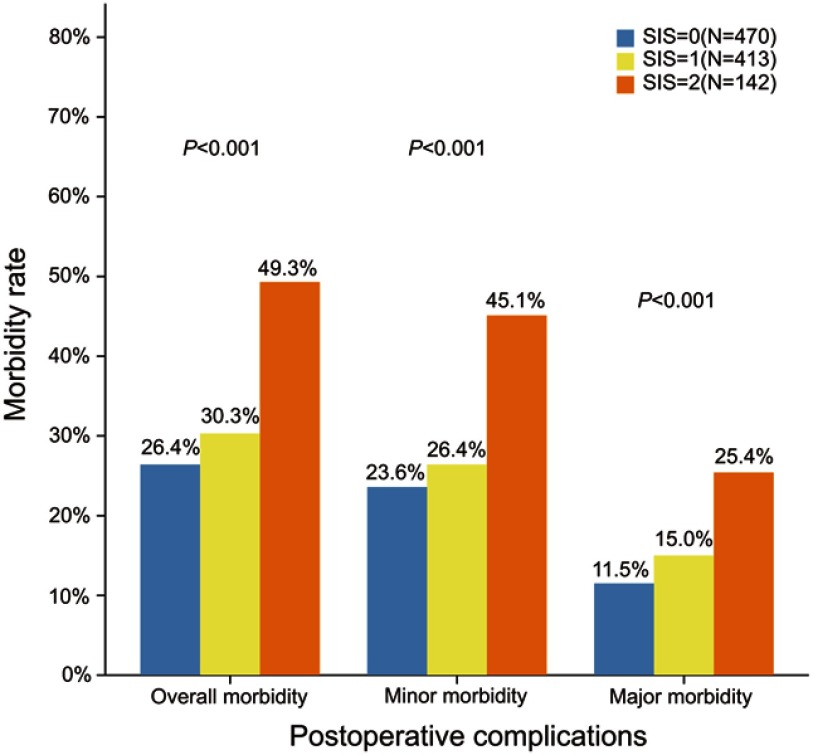
The overall morbidity rate in patients with SIS=0, SIS=1 and SIS=2 was 26.4% (n=124), 30.3% (n=125) and 49.3% (n=70), respectively (Figure 1; Table 2). The overall morbidity rate in patients with SIS=2 was significantly higher than that in patients with SIS ranged 0–1 (P<0.001). No difference was found in overall morbidity rate between patients with SIS=1 and with SIS=0 (P=0.20). Of the 3 dead cases, one got an SIS=0 and another two got an SIS=1. No difference was found in the mortality rate between these 3 groups.
Figure 1.
Overall, minor and major morbidity rates between 3 SIS groups.
Abbreviation: SIS, systemic inflammation score.
The data for minor and major morbidities between 3 SIS groups are shown in Table 2. Both minor morbidity and major morbidity rates in patients with SIS=2 were significantly higher than those in patients with SIS ranged 0–1 (P<0.001). However, no difference was observed in either minor morbidity (P=0.34) or major morbidity (P=0.12) between patients with SIS=0 and with SIS=1.
Individual complications
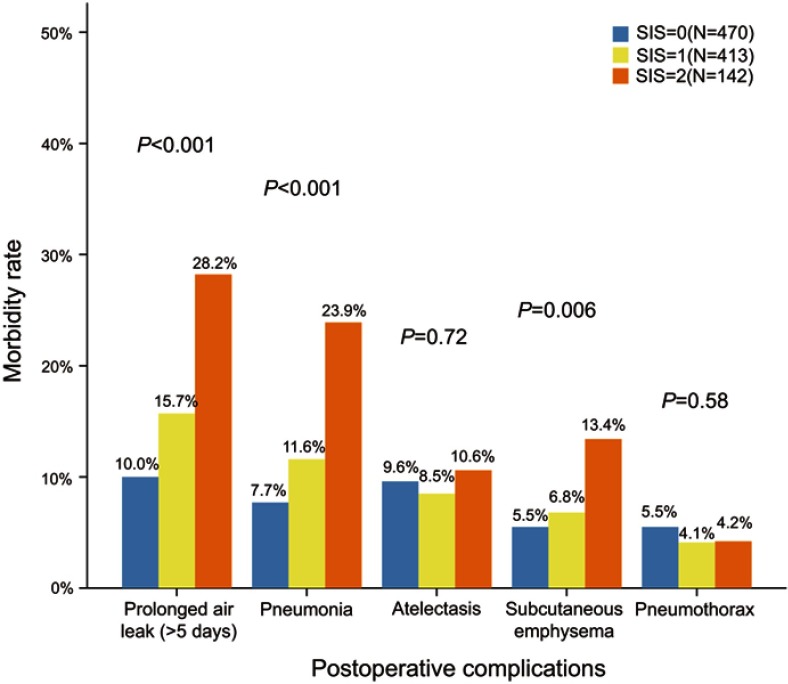
Demographic data for individual complications are summarized in Table 2. Among five most frequent complications, there was a significant step-wise increase in the incidences of PAL and pneumonia in proportion to the SIS, as shown in Figure 2. Patients with SIS=2 had the highest probability to experience most of individual complications.
Figure 2.
Incidences of five most frequent complications between 3 SIS groups.
Abbreviation: SIS, systemic inflammation score.
Compared to patients with SIS=0, patients with SIS=2 had significantly higher incidences of PAL (28.2 vs 10.0%; P<0.001), pneumonia (23.9 vs 7.7%; P<0.001), subcutaneous emphysema (13.4 vs 5.5%; P=0.002), pleural effusion (11.3 vs 1.9%; P<0.001), hemoptysis (7.0 vs 2.3%; P=0.015) and chylothorax (2.8 vs 0.4%; P=0.028). In addition, patients with SIS=1 had significantly higher incidences of PAL (15.7 vs 10.0%; P=0.011), pneumonia (11.6 vs 7.7%; P=0.045), chylothorax (2.2 vs 0.4%; P=0.019) and ARDS (1.0 vs 0.0%; P=0.047) than those of patients with SIS=0. No significant difference was observed in the other individual complications between these 3 groups.
ROC analysis of the SIS for morbidity prediction
The ROC analysis of our modified SIS showed an AUC of 0.58 (95% CI=0.54–0.62; P<0.001) for predicting overall morbidity. Moreover, the Chang’s original SIS got an approximately equal AUC of 0.57 (95% CI=0.54–0.61; P<0.001). With regard to the prediction of overall morbidity, sALB at 40 g/L had 84% sensitivity and 26% specificity, LMR at 4.42 had 54% sensitivity and 58% specificity, and LMR at 4.44 had 53% sensitivity and 58% specificity. Finally, SIS=2 was found to be the threshold value at which the sensitivity (22.0%) plus specificity (90.0%) were maximal.
Univariable analysis of predictors for postoperative complications
The morbidity group patients had significantly higher age (P<0.001), NLR (P=0.007), PLR (P=0.014), EIBL (P<0.001) and operation time (P<0.001) but lower FEV1% (P<0.001) and FEV1/FVC (P<0.001) than those of non-morbidity group patients. Compared to patients without any complication, patients who experienced complications had significantly higher ratios of males (P<0.001), SIS=2 (P<0.001), smoking history (P<0.001), respiratory comorbidity (P<0.001), cardio-cerebrovascular comorbidity (P=0.002), incomplete pulmonary fissure (P<0.001), dense pleural adhesion (P=0.017), LNM (P<0.001) and T2-3-stage tumors (P=0.001). No significant difference was found in the other clinicopathological variables between patients with and without any morbidity (Table 3).
Table 3.
Univariable analysis of predictors for overall morbidity
| Characteristicsa | Total (N=1,025) | Without morbidity (N=706) | With morbidity | P-valueb | ||
|---|---|---|---|---|---|---|
| Overall (N=319) | Minor morbidity (N=284) | Major morbidity (N=152) | ||||
| Median age (years) | 62 (57–67) | 62 (55–67) | 63 (58–69) | 63 (59–69) | 63 (57–69) | <0.001 |
| Gender | ||||||
| Male | 530 (51.7%) | 330 (46.7%) | 200 (62.7%) | 177 (62.3%) | 100 (65.8%) | <0.001 |
| Female | 495 (48.3%) | 376 (53.3%) | 119 (37.3%) | 107 (37.7%) | 52 (34.2%) | |
| Median body mass index (kg/m2) | 23.3 (21.1–25.2) | 23.3 (21.4–25.1) | 22.7 (20.4–25.6) | 22.8 (20.5–25.8) | 22.3 (19.7–25.6) | 0.11 |
| Median FEV1% | 83.8 (73.8–92.7) | 85.5 (76.5–94.7) | 79.5 (67.5–89.8) | 79.1 (65.8–90.0) | 79.0 (65.6–90.2) | <0.001 |
| Median FEV1/FVC (%) | 79.0 (74.2–83.5) | 79.4 (75.4–84.1) | 77.9 (70.9–82.3) | 77.3 (70.7–82.4) | 77.3 (68.9–82.5) | <0.001 |
| Median neutrophil-to-lymphocyte ratio | 1.9 (1.4–2.5) | 1.8 (1.4–2.5) | 2.0 (1.6–2.5) | 1.9 (1.6–2.4) | 2.0 (1.6–2.7) | 0.007 |
| Median platelet-to-lymphocyte ratio | 91.5 (72.4–120.0) | 90.4 (70.2–117.0) | 95.9 (77.4–126.7) | 94.8 (77.2–125.5) | 102.9 (86.6–134.4) | 0.014 |
| Median Hemoglobin (g/dL) | 13.6 (12.8–14.6) | 13.5 (12.8–14.5) | 13.7 (12.9–14.8) | 13.7 (12.9–14.7) | 13.6 (12.8–14.8) | 0.091 |
| Systemic inflammation score | ||||||
| 0 | 470 (45.9%) | 346 (49.0%) | 124 (38.9%) | 111 (39.1%) | 54 (35.5%) | <0.001 |
| 1 | 413 (40.3%) | 288 (40.8%) | 125 (39.2%) | 109 (38.4%) | 62 (40.8%) | |
| 2 | 142 (13.9%) | 72 (10.2%) | 70 (21.9%) | 64 (22.5%) | 36 (23.7%) | |
| Smoking history | 380 (37.1%) | 235 (33.3%) | 145 (45.5%) | 130 (45.8%) | 79 (52.0%) | <0.001 |
| Respiratory comorbidity | 400 (39.0%) | 219 (31.0%) | 181 (56.7%) | 156 (54.9%) | 101 (66.4%) | <0.001 |
| Cardio-cerebrovascular comorbidity | 382 (37.3%) | 241 (34.1%) | 141 (44.2%) | 124 (43.7%) | 74 (48.7%) | 0.002 |
| Diabetes mellitus | 112 (10.9%) | 78 (11.0%) | 34 (10.7%) | 28 (9.9%) | 16 (10.5%) | 0.85 |
| Renal insufficiency | 89 (8.7%) | 56 (7.9%) | 33 (10.3%) | 30 (10.6%) | 19 (12.5%) | 0.21 |
| Steroid use | 31 (3.0%) | 23 (3.3%) | 8 (2.5%) | 8 (2.8%) | 7 (4.6%) | 0.52 |
| Tumor location | ||||||
| Right upper lobe | 334 (32.6%) | 217 (30.7%) | 117 (36.7%) | 107 (37.7%) | 48 (31.6%) | 0.060 |
| Left upper lobe | 261 (25.5%) | 189 (26.8%) | 72 (22.6%) | 66 (23.2%) | 39 (25.7%) | |
| Right lower lobe | 193 (18.8%) | 124 (17.6%) | 69 (21.6%) | 59 (20.8%) | 35 (23.0%) | |
| Left lower lobe | 149 (14.5%) | 113 (16.0%) | 36 (11.3%) | 33 (11.6%) | 15 (9.9%) | |
| Right middle lobe | 88 (8.6%) | 63 (8.9%) | 25 (7.8%) | 19 (6.7%) | 15 (9.9%) | |
| Dense pleural adhesion | ||||||
| Absent | 935 (91.2%) | 654 (92.6%) | 281 (88.1%) | 250 (88.0%) | 129 (84.9%) | 0.017 |
| Present | 90 (8.8%) | 52 (7.4%) | 38 (11.9%) | 34 (12.0%) | 23 (15.1%) | |
| Pulmonary fissure completeness | ||||||
| Complete | 596 (58.1%) | 433 (62.4%) | 145 (45.5%) | 127 (44.7%) | 56 (36.8%) | <0.001 |
| Incomplete | 429 (41.9%) | 255 (36.1%) | 174 (54.5%) | 157 (55.3%) | 96 (63.2%) | |
| Median estimated intraoperative blood loss (mL) | 50 (20–50) | 30 (20–50) | 50 (30–100) | 50 (30–100) | 50 (30–100) | <0.001 |
| Median operation time (mins) | 100 (80–130) | 95 (75–120) | 120 (100–160) | 120 (100–170) | 130 (110–175) | <0.001 |
| Histological subtypes | ||||||
| Adenocarcinoma | 822 (80.2%) | 559 (79.2%) | 263 (82.4%) | 234 (82.4%) | 116 (76.3%) | 0.22 |
| Non-adenocarcinoma | 203 (19.8%) | 147 (20.8%) | 56 (17.6%) | 50 (17.6%) | 36 (23.7%) | |
| Tumor invasion (T-stage) | ||||||
| T1 | 627 (61.2%) | 455 (64.4%) | 172 (53.9%) | 155 (54.6%) | 79 (52.0%) | 0.001 |
| T2-3 | 398 (38.8%) | 251 (35.6%) | 147 (46.1%) | 129 (45.4%) | 73 (48.0%) | |
| Lymph node metastasis (N-stage) | ||||||
| N0 | 966 (94.2%) | 678 (96.0%) | 288 (90.3%) | 256 (90.1%) | 135 (88.8%) | <0.001 |
| N1-2 | 59 (5.8%) | 28 (4.0%) | 31 (9.7%) | 28 (9.9%) | 17 (11.2%) | |
Notes: aThe continuous data were presented as medians with 25th–75th percentile intervals. bP-value indicates demographic differences between patients with and without any Clavien-Dindo grade ≥II complication. FEV1/FVC, FEV1 to FVC ratio.
Multivariable analysis of predictors for postoperative complications
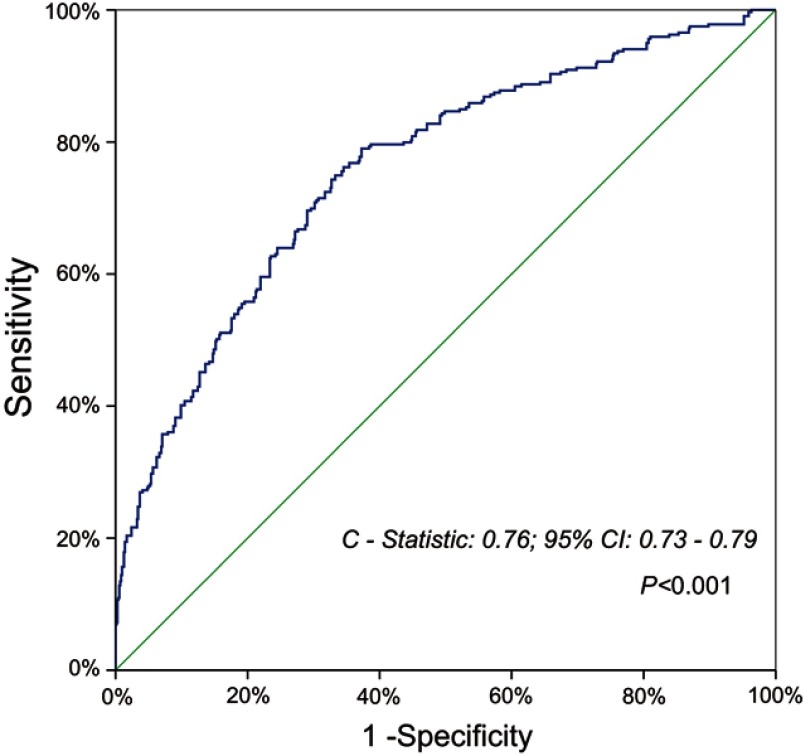
Multivariable logistic-regression analysis was conducted on clinicopathological parameters with univariable P<0.20 regarding to postoperative morbidity, as shown in Table 4. The multivariable logistic-regression model with Hosmer–Lemeshow-P=0.40 and C-statistic=0.76 (95% CI=0.73–0.79; P<0.001) demonstrated that preoperative respiratory comorbidity (OR=2.13; 95% CI=1.56–2.91; P<0.001), SIS=2 (OR=1.73; 95% CI=1.11–2.71; P=0.016), incomplete pulmonary fissure (OR=1.69; 95% CI=1.25–2.29; P=0.001) and prolonged operation time (OR=1.01; 95% CI=1.01–1.02; P<0.001) could independently predict overall morbidity after VATS lobectomy (Table 4; Figure 3).
Table 4.
Multivariable analysis of predictors for overall morbidity
| Estimated factors | OR | 95% CI | P-value |
|---|---|---|---|
| Age (per 1-year increase) | 1.004 | 0.98–1.02 | 0.72 |
| Gender (male vs female) | 1.24 | 0.79–1.93 | 0.35 |
| Body mass index (kg/m2) (per unit decrease) | 1.04 | 0.991–1.10 | 0.11 |
| FEV1% (per unit decrease) | 1.01 | 1.00–1.03 | 0.054 |
| FEV1/FVC (%) (per unit decrease) | 1.004 | 0.98–1.03 | 0.75 |
| Systemic inflammation score (2 vs 0–1) | 1.73 | 1.11–2.71 | 0.016 |
| Neutrophil-to-lymphocyte ratio (per unit increase) | 0.99 | 0.89–1.10 | 0.79 |
| Platelet-to-lymphocyte ratio (per unit increase) | 1.002 | 0.998–1.006 | 0.38 |
| Hemoglobin (g/dL) (per unit decrease) | 1.007 | 0.997–1.02 | 0.17 |
| Smoking history | 1.49 | 0.97–2.31 | 0.071 |
| Respiratory comorbidity | 2.13 | 1.56–2.91 | <0.001 |
| Cardio-cerebrovascular comorbidity | 1.18 | 0.85–1.64 | 0.33 |
| Right upper lobectomy | 1.32 | 0.96–1.81 | 0.087 |
| Pulmonary fissure completeness (incomplete vs complete) | 1.69 | 1.25–2.29 | 0.001 |
| Dense pleural adhesion (present vs absent) | 1.33 | 0.78–2.25 | 0.30 |
| Operation time (mins) (per 10 mins increase) | 1.01 | 1.01–1.02 | <0.001 |
| Estimated intraoperative blood loss (mL) (per 10 mL increase) | 1.002 | 1.00–1.004 | 0.083 |
| Tumor invasion (T2-3vs.T1) | 1.06 | 0.78–1.46 | 0.70 |
| Lymph node metastasis | 1.46 | 0.78–2.71 | 0.24 |
Note: FEV1/FVC, FEV1 to FVC ratio.
Figure 3.
C-statistic revealing discriminative power of multivariable logistic-regression model for the prediction of overall morbidity.
In addition, we also established two effective multivariable logistic-regression models to demonstrate independent risk factors for minor morbidity and major morbidity, respectively (see Tables S1–S2). Accordingly, we found that the SIS=2 could be predictive of both minor morbidity (OR=1.79; 95% CI=1.15–2.80; P=0.011) and major morbidity (OR=1.87; 95% CI=1.09–3.20; P=0.023) after adjusting the confounding influence from other clinicopathological factors.
Table S1.
Multivariable analysis of predictors for minor morbidity (Clavien-Dindo Grade II complications)a
| Estimated factors | Odds ratio | 95% CI | P-value |
|---|---|---|---|
| Age (year) (per 1-year increase) | 1.006 | 0.98–1.03 | 0.59 |
| Gender (male vs female) | 1.25 | 0.81–1.93 | 0.32 |
| FEV1% (per unit decrease) | 1.01 | 0.999–1.03 | 0.064 |
| FEV1/FVC (%) (per unit decrease) | 1.005 | 0.98–1.03 | 0.68 |
| Systemic inflammation score (2 vs 0-1) | 1.79 | 1.15–2.80 | 0.011 |
| Neutrophil-to-lymphocyte ratio (per unit increase) | 0.98 | 0.88–1.10 | 0.72 |
| Platelet-to-lymphocyte ratio (per unit increase) | 1.001 | 0.997–1.006 | 0.51 |
| Smoking history | 1.40 | 0.89–2.22 | 0.14 |
| Respiratory comorbidity | 1.99 | 1.44–2.76 | <0.001 |
| Cardio-cerebrovascular comorbidity | 1.08 | 0.77–1.52 | 0.66 |
| Right upper lobectomy | 1.46 | 1.05–2.01 | 0.024 |
| Pulmonary fissure completeness (incomplete vs complete) | 1.66 | 1.21–2.28 | 0.002 |
| Dense pleural adhesion (present vs absent) | 1.31 | 0.76–2.27 | 0.33 |
| Operation time (mins) (per 10 mins increase) | 1.01 | 1.009–1.02 | <0.001 |
| Estimated intraoperative blood loss (mL) (per 10 mL increase) | 1.003 | 1.000–1.005 | 0.018 |
| Tumor invasion (T2-3 vs T1) | 1.004 | 0.72–1.39 | 0.98 |
| Lymph node metastasis | 1.51 | 0.80–2.84 | 0.20 |
Notes: aThe multivariable logistic-regression model was established based on 284 patients with minor morbidity and 706 patients without any morbidity to identify the risk factors for minor morbidity. The data for major morbidity (Clavien-Dindo Grade ≥III complications) were excluded. This multivariable logistic-regression model got a Hosmer–Lemeshow P=0.13 and a C-statistic of 0.75 (95% CI: 0.72-0.79; P<0.001). FEV1/FVC, FEV1 to FVC ratio.
Table S2.
Multivariable analysis of predictors for major morbidity (Clavien-Dindo Grade ≥III complications)a
| Estimated factors | Odds ratio | 95% CI | P-value |
|---|---|---|---|
| Age (year) (per 1-year increase) | 0.992 | 0.97–1.02 | 0.53 |
| Gender (male vs female) | 1.45 | 0.83–2.54 | 0.20 |
| Body mass index (kg/m2) (per unit decrease) | 1.07 | 1.005–1.14 | 0.036 |
| FEV1% (per unit decrease) | 1.01 | 0.994–1.03 | 0.19 |
| FEV1/FVC (%) (per unit decrease) | 1.02 | 0.98–1.05 | 0.34 |
| Systemic inflammation score (2 vs 0-1) | 1.87 | 1.09–3.20 | 0.023 |
| Neutrophil-to-lymphocyte ratio (per unit increase) | 0.92 | 0.79–1.08 | 0.29 |
| Platelet-to-lymphocyte ratio (per unit increase) | 1.006 | 1.001–1.01 | 0.017 |
| Smoking history | 1.44 | 0.79–2.63 | 0.23 |
| Respiratory comorbidity | 3.37 | 2.19–5.19 | <0.001 |
| Cardio-cerebrovascular comorbidity | 1.58 | 1.01–2.45 | 0.044 |
| Renal insufficiency | 1.17 | 0.60–2.27 | 0.64 |
| Pulmonary fissure completeness (incomplete vs complete) | 2.40 | 1.58–3.66 | <0.001 |
| Dense pleural adhesion (present vs absent) | 1.12 | 0.58–2.15 | 0.75 |
| Operation time (mins) (per 10 mins increase) | 1.02 | 1.01–1.03 | <0.001 |
| Estimated intraoperative blood loss (mL) (per 10 mL increase) | 1.001 | 0.998–1.003 | 0.67 |
| Tumor invasion (T2-3 vs T1) | 1.12 | 0.73–1.72 | 0.60 |
| Lymph node metastasis | 1.27 | 0.55–2.95 | 0.57 |
Notes: aThe multivariable logistic-regression model was established based on 152 patients with major morbidity and 706 patients without any morbidity to identify the risk factors for major morbidity. The data for minor morbidity (Clavien-Dindo Grade II complications) were excluded. This multivariable logistic-regression model got a Hosmer–Lemeshow P=0.10 and a C-statistic of 0.82 (95% CI: 0.78-0.85; P<0.001). FEV1/FVC, FEV1 to FVC ratio.
Discussion
Key results and interpretations
The SIS is established on 3 laboratorial biomarkers, namely sALB level, lymphocyte and monocyte counts, which can be easily and inexpensively measured in routine clinical practice. This scoring system is characterized by a simple and effective integration of the clinically meaningful cutoffs for both sALB and LMR, reflecting a balance between host inflammatory and nutritional conditions.9 Since its first introduction onto oncological prognosis prediction, the SIS, as a novel risk stratification model, has been validated in a variety of surgical specialties.7–10
Chang et al7 firstly developed the SIS by incorporating the sALB at 40 g/L and median LMR at 4.44 based on the survival data of 441 patients undergoing nephrectomy for ccRCC. A higher preoperative SIS was reported to be significantly associated with aggressive oncological behaviors and poor 5-year overall survival (OS) of ccRCC. In the subsequent retrospective analysis of 727 colorectal cancer cases, Suzuki et al8 adopted the Chang’s original SIS to stratify patients with regard to long-term prognosis. They also reported that a higher preoperative SIS was significantly associated with a worse 5-year OS. In another one smaller cohort of 271 hepatocellular carcinoma patients, Shi et al10 utilized a ROC-derived optimal cutoff of LMR (4.50) as the dichotomization criteria in their modified SIS system. Accordingly, a preoperative SIS=2 was found to be predictive of poor 5-year OS after hepatic surgery. The most recent study conducted by Sato et al9 firstly evaluated the effects of preoperative SIS on postoperative complications in 187 patients undergoing gastric cancer surgery. The authors finally concluded that the SIS could also act as an effective risk assessment tool for short-term outcomes. However, until recently, the efficacy of the SIS has never been evaluated in lung cancer surgery.
To the best of our knowledge, the present study was the first to demonstrate the clinical significance of the SIS scoring system for postoperative outcomes in a large cohort consisting of >1,000 NSCLC patients who had undergone VATS lobectomy. In our NSCLC series, we modified the Chang’s original SIS scoring criteria by applying preoperative sALB at 40 g/L and median LMR at 4.42 for dichotomization and integration. This modified SIS could not only reflect patient pro-inflammatory and undernourished conditions but also correspond to the clinical practice of VATS lobectomy in our department.
On the basis of grouping and analytical methods that had been validated in currently available studies, we divided all included patients into 3 groups according to their SISs and then compared the clinical data between these groups.7–10 Patients with SIS=2, who were considered to suffer from hypoalbuminemia, lymphocytopenia and monocytosis, had the worst baseline characteristics, including the advanced age, more active smokers, severe cardiopulmonary comorbidities and impaired lung function. That might be attributed to a significantly increased risk of systemic inflammatory response in the patients with insufficient immuno-nutritional reverse, especially in elderly people and those who were debilitated by chronic underlying comorbidities. This phenomenon showed a good validity of the SIS to reflect patients’ immune-nutritional status before surgery. Furthermore, the highest ratios of T2-3-stage tumors and LNM in the patients with SIS=2 might also support a strong relationship between suppression of immuno-nutritional characteristics and aggressive oncological progression.
The highlight of our study was to demonstrate that the SIS scoring system could serve as a novel risk stratification tool for patients undergoing VATS lobectomy. Patients with a preoperative SIS=2, which reflect the worst immune-nutritional status, had the highest probability to experience both minor and major morbidities postoperatively. No significant difference was found in the morbidity rates between patients with SIS=0 and with SIS=1. We also found a significant step-wise increase in the prevalence of PAL and pneumonia in proportion to the SIS, which was similar to previous results reported in gastrectomy.9 However, no association was thus observed between preoperative SIS and in-mortality rate. We speculated that a limited number of events, only 3 deaths among >1,000 patients, might cause a large decline of analytical power. Finally, our multivariable logistic-regression analyses highlighted that a higher preoperative SIS could be an excellent discriminator for morbidity risks after VATS lobectomy. As an integrated indicator based on sALB and LMR, the biological reasons underlying the potent predictive value of preoperative SIS may be elucidated by a combination of the following two plausible mechanisms.
First, lymphocytes can assist to enhance cancer immune-surveillance and inhibit tumor growth by secreting a series of cytokines that participate in the cellular immunity. A large decline of circulating lymphocyte counts can lead to a weak and insufficient immune response to oncological progression.6,7,9 Furthermore, recent evidence demonstrates that monocytes can be recruited in carcinomatous tissues and further differentiate into tumor-associated macrophages, which play a key role in the tumor microenvironment, cancer cell proliferation and encouraging metastasis.15 Thus, an elevated level of circulating monocytes may represent the increase of tumor-associated macrophages as a surrogate for high cancer burden. Given the above concerns, a significant decline of LMR conveying both monocytosis and lymphocytopenia plays a strong predictive role for adverse outcomes.7
Second, sALB is known as a negative acute-phase protein and routinely employed to reflect patient nutritional status. Hypoalbuminemia represents not only an undernourished condition but also a sustained systemic inflammatory response as the synthesis of sALB could be suppressed by malnutrition and inflammation.16 Preoperative malnutrition has shown evident effects in terms of tissue vulnerability, delayed wound healing and increased susceptibility to infectious complications.9 Besides, the excessive protein catabolism induced by surgery can further impair global immune-nutritional function and physiological homeostasis. Thus, patients with a lower sALB level are less likely to have an adequate response to operative stress because of their compromised ability to withstand an acute injury, resulting in a dramatically increased risk of adverse events.
Generalizability
Our findings can facilitate thoracic surgeons to better distinguish the patients with high surgical risk by incorporating a preoperative SIS into traditional assessment models to complement morbidity prediction in VATS lobectomy. Of course, the SIS system might also be very helpful for risk stratification in octogenarians and patients undergoing major lung resections through conventional thoracotomy. In order to enhance the surgical tolerability and limit the adverse effects induced by major morbidities, nutritional supportive care and intensive symptomatic therapies will be quite necessary when patients have a higher preoperative SIS. An early enteral alimentation, which has been reported to elevate the sALB level and lymphocyte counts, may improve postoperative outcomes of patients with poor immune-nutritional reserve.9
Limitations
Several limitations in this study must be acknowledged.
First, our study was subject to the inherent limitations of any single-center retrospective analysis without external validation. Potential selection bias might complicate our findings, although our cohort included >1,000 patients in accordance with fairly strict eligibility criteria, which had been made to eliminate potential confounding influence from neoadjuvant/adjuvant therapy, concomitant malignancy, additional surgical procedures, intraoperative events and blood sampling time before surgery. We recommend that more prospective validating studies with much better control of potential confounders from patient characteristics are needed to verify the SIS efficacy in lung cancer surgery.
Second, putative inflammatory indicators such as the C-reactive protein, fibrinogen and cytokines, were not gathered in the present analysis because they might not be routinely measured in our institutional clinical practice. Several common risk scales involving these biomarkers could not be estimated to help to interpret our findings. Therefore, further studies are highly desirable to estimate the clinical significance of SIS in combination with such specific laboratory markers.
Third, we utilized the median LMR of the entire NSCLC series to modify the Chang’s original SIS scoring criteria since no international consensus had been recommended on the reference values for LMR. The dichotomized cutoffs of LMR varied across studies.
Fourth, some of pulmonary diffusion function indexes, such as the carbon monoxide diffusing capacity, had unfortunately missed from our database maintained during the study period. So these parameters were not evaluated in all included patients.
Fifth, overall morbidity included various types of individual complications. Predictive roles of preoperative SIS for each complication remained unclear. Thus, the relationship between the SIS and individual complications should be clarified in the future studies.
Finally, the potential influence of the SIS scoring system on long-term survival of surgical patients with NSCLC still remains unclear in this study since a 5-year follow-up for all included patients was not completed. Maybe we will further validate the prognostic value of SIS for both OS and disease-free survival in patients undergoing lung cancer surgery in the future.
Conclusions
The present study demonstrates that a higher preoperative SIS acts as an excellent discriminator for morbidity risks following VATS lobectomy for NSCLC. The SIS scoring system can be employed as a simplified, effective and routinely operated risk stratification tool to provide readily available and objective information for morbidity prediction. Owing to several inherent limitations of the retrospective design, more large-scale prospective validating analyses with a long-term follow-up are warranted to substantiate and validate our findings in the future.
Acknowledgments
We give special thanks to Dr Yan Wang, from Department of Thoracic Surgery, West China Hospital, Sichuan University, Chengdu, China, for his great assistance to this manuscript. This study was supported by Foundation of Science and Technology support plan Department of Sichuan Province (No. 2015SZ0158).
Disclosure
The authors report no conflicts of interest in this work.
Supplementary materials
References
- 1.Zhang SM, Zhu QG, Ding XX, et al. Prognostic value of EGFR and KRAS in resected non-small cell lung cancer: a systematic review and meta-analysis. Cancer Manag Res. 2018;10:3393–3404. doi: 10.2147/CMAR.S167578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang H, Li X, Shi J, et al. A nomogram to predict prognosis in patients undergoing sublobar resection for stage IA non-small-cell lung cancer. Cancer Manag Res. 2018;10:6611–6626. doi: 10.2147/CMAR.S182458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li S, Zhou K, Wang M, Lin R, Fan J, Che G. Degree of pulmonary fissure completeness can predict postoperative cardiopulmonary complications and length of hospital stay in patients undergoing video-assisted thoracoscopic lobectomy for early-stage lung cancer. Interact Cardiovasc Thorac Surg. 2018;26(1):25–33. doi: 10.1093/icvts/ivx261 [DOI] [PubMed] [Google Scholar]
- 4.Laursen LØ, Petersen RH, Hansen HJ, Jensen TK, Ravn J, Konge L. Video-assisted thoracoscopic surgery lobectomy for lung cancer is associated with a lower 30-day morbidity compared with lobectomy by thoracotomy. Eur J Cardiothorac Surg. 2016;49(3):870–875. doi: 10.1093/ejcts/ezv205 [DOI] [PubMed] [Google Scholar]
- 5.Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg. 2010;139(2):366–378. doi: 10.1016/j.jtcvs.2009.08.026 [DOI] [PubMed] [Google Scholar]
- 6.Chechlinska M, Kowalewska M, Nowak R. Systemic inflammation as a confounding factor in cancer biomarker discovery and validation. Nat Rev Cancer. 2010;10(1):2–3. doi: 10.1038/nrc2782 [DOI] [PubMed] [Google Scholar]
- 7.Chang Y, An H, Xu L, et al. Systemic inflammation score predicts postoperative prognosis of patients with clear-cell renal cell carcinoma. Br J Cancer. 2015;113(4):626–633. doi: 10.1038/bjc.2015.241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki Y, Okabayashi K, Hasegawa H, et al. Comparison of preoperative inflammation-based prognostic scores in patients with colorectal cancer. Ann Surg. 2018;267(3):527–531. doi: 10.1097/SLA.0000000000002115 [DOI] [PubMed] [Google Scholar]
- 9.Sato B, Kanda M, Tanaka C, et al. Significance of preoperative systemic inflammation score in short-term and long-term outcomes of patients with pathological T2-4 gastric cancer after radical gastrectomy. World J Surg. 2018;42(10):3277–3285. doi: 10.1007/s00268-018-4597-7 [DOI] [PubMed] [Google Scholar]
- 10.Shi S, Chen Q, Ye L, et al. Prognostic value of systemic inflammation score in patients with hepatocellular carcinoma after hepatectomy. Oncotarget. 2017;8(45):79366–79375. doi: 10.18632/oncotarget.18121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349. doi: 10.1016/j.jclinepi.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 12.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez FG, Falcoz PE, Kozower BD, Salati M, Wright CD, Brunelli A. The Society of Thoracic Surgeons and the European Society of Thoracic Surgeons general thoracic surgery databases: joint standardization of variable definitions and terminology. Ann Thorac Surg. 2015;99(1):368–376. doi: 10.1016/j.athoracsur.2014.05.104 [DOI] [PubMed] [Google Scholar]
- 14.Gao K, Yu PM, Su JH, et al. Cardiopulmonary exercise testing screening and pre-operative pulmonary rehabilitation reduce postoperative complications and improve fast-track recovery after lung cancer surgery: a study for 342 cases. Thorac Cancer. 2015;6(4):443–449. doi: 10.1111/1759-7714.12199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. doi: 10.1016/j.cell.2010.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12(3):223–226. doi: 10.1097/MCO.0b013e32832a7902 [DOI] [PubMed] [Google Scholar]