Abstract
Background
The Automated Self-administered 24-hour Dietary Assessment Tool (ASA24) includes a highly standardized multi-pass web-based recall that, like the Automated Multiple Pass Method (AMPM), captures detailed information about dietary intake using multiple probes and reminders to enhance recall of intakes. The primary distinction between ASA24 and AMPM is that the ASA24 user interface guides participants, thus removing the need for interviewers.
Objective
The objective of this study was to compare dietary supplement use reported on self-administered (ASA24-2011) versus interviewer-administered (AMPM) 24-hour recalls.
Design
The Food Reporting Comparison Study (FORCS) was an evaluation study designed to compare self-reported intakes captured using the self-administered ASA24 versus data collected via interviewer-administered AMPM recalls. Between 2010–2011, 1081 women and men were enrolled from three integrated health care systems that belong to the National Cancer Institute funded Cancer Research Network: Security Health Plan: the Marshfield Clinic, Wisconsin; Henry Ford Health System, Michigan; and Kaiser Permanente Northern California, California. Quota sampling was used to ensure a balance of age, sex, and race/ethnicity. Participants were randomized into four groups and asked to complete two dietary recalls: 1) two ASA24s; 2) two AMPMs; 3) ASA24 first, AMPM second; 4) AMPM first, ASA24 second. Dietary supplements were coded using the 2007–2008 NHANES Dietary Supplement Database. Analyses used the two one-sided test to assess equivalence of reported supplement use between methods.
Results
Complete 24-hour dietary recalls that included both dietary and supplement intake data were available for 1076 participants (507 men and 569 women). The proportions reporting supplement use via ASA24 and AMPM were 46% and 43%, respectively. These proportions were equivalent, with a small effect size of less than 20%. There were two exceptions in subgroup analyses: reported use among those 40-59 years of age and non-Hispanic blacks was higher for ASA24 than AMPM.
Conclusions
This study provides evidence that there is little difference in reported supplement use by mode of administration (i.e. interview- vs. self-administered recall).
Keywords: Dietary supplement, ASA24, dietary assessment, evaluation, adults
Introduction
Approximately half of US adults report consuming a dietary supplement in the previous 30 days based on data collected in the National Health and Nutrition Examination Survey (NHANES).1,2 Close to one third of adults report regular use of multivitamin-mineral supplements for the purpose of improving overall health, and nearly 20% of women report regular use of calcium supplements for bone health.2,3 The contribution of dietary supplements to total nutrient intake among users can be considerable, even to the point of being potentially excessive.4-6 It is important to measure and include the contribution of supplements in dietary assessment because their exclusion leads to error in estimates of total nutrient intakes and of the proportions of sample populations meeting or exceeding thresholds of nutrient intakes.
Food frequency questionnaires (FFQs) have traditionally been the typical method of choice for collecting food intake data, especially in large studies. Dietary supplement intake data are also commonly collected on frequency-type questionnaires that query respondents about supplement types (e.g. multi-vitamin mineral, calcium containing, B-complex) and frequency of use.7 Although valuable information regarding supplement use has been obtained using frequency questionnaires, such as most frequently used types and demographic characteristics of users, it is also useful to collect supplement data using more detailed dietary assessment instruments, such as 24-hour recalls (24-HR) to obtain total nutrient intakes for a given day.
Using interview administered 24-HRs in large studies, with or without questions regarding dietary supplement use, can be costly due to the reliance on trained staff to conduct and code recalls.8 The Automated Self-Administered 24-hour Dietary Assessment Tool (ASA24), a freely available web-based tool, was developed to make it feasible to collect multiple high-quality recalls from large samples, eliminating the need for an interviewer.9 The Food Reporting Comparison Study (FORCS) was designed to compare reported intakes using ASA24-2011, a self-administered recall, to intakes collected using the Automated Multiple Pass Method (AMPM), an interview-administered recall, in a large sample of US adults.10 The main analysis examined differences in reported intakes of foods and beverages and showed that for energy and most nutrients and food groups reported, intakes were equivalent between ASA24 and AMPM. 10 The purpose of this secondary analysis is to evaluate whether reporting of dietary supplement use is comparable between ASA24 and AMPM recalls.
Methods
Sample
Methods of FORCS have been described in detail by Thompson et al.10 In brief, in 2010–2011, 1081 men and women were enrolled from three integrated health care systems belonging to the National Cancer Institute funded Cancer Research Network — Security Health Plan: the Marshfield Clinic, Wisconsin; Henry Ford Health System, Michigan; and Kaiser Permanente Northern California, California. Sites identified current users of their online system and drew pools of age-eligible users into sampling strata defined by sex, age (20–34, 35–54, and 55–70 years), and race/ethnicity (non-Hispanic white; non-Hispanic black; Hispanic). Quota sampling was used to ensure balance of sex, age, and race/ethnicity.
The institutional review boards of the National Cancer Institute, Westat, Marshfield Clinic, the Henry Ford Health System, and Kaiser Permanente Northern California, as well as the US Office of Management and Budget approved all study procedures and informed consent forms for this study.
Dietary intake and supplement data
All eligible participants who provided written consent were asked to complete two 24-HRs, four to six weeks apart. Each participant was randomly assigned to one of four study groups: Group 1 completed two ASA24 self-administered recalls; Group 2 completed two AMPM telephone-administered interviews; Group 3 completed one ASA24 followed by one AMPM and Group 4 completed one AMPM followed by one ASA24. Study group assignment was balanced for sex, age, and race/ethnicity.10 All recalls were conducted without prior scheduling to avoid potential reactivity, which can arise when participants know at the time of eating that they will be reporting their consumption.
Since dietary patterns tend to vary between week and weekend days, participants were asked to complete recalls on a combination of these days. Approximately a third of participants completed recalls on two week days; another third completed two weekend days; and the final third reported their intakes for one week day and one weekend day.
ASA24, which was developed by the National Cancer Institute under contract with Westat, a survey research company in Rockville, MD, is a freely available web-based tool for the collection of dietary intake data.9 ASA24 includes a progression of passes based on the interviewer-administered AMPM, developed by the US Department of Agriculture and used to collect 24-HR in “What We Eat in America”, the dietary interview component of the NHANES.
AMPM is a highly standardized multi-pass interviewer-administered 24-HR that captures detailed information about dietary intake by using multiple probes and reminders to enhance memory and recall of reported intakes.11 Although ASA24 adapts this multiple-pass approach, the primary distinction between it and AMPM is that the ASA24 user interface guides participants through self-completion of a recall.
In addition to foods and beverages, both AMPM and ASA24 allowed for collection of dietary supplement intakes for the prior 24 hours. In both cases, reported dietary supplements were coded to the NHANES-Dietary Supplement Database 2007–08 (NHANES-DSD)12, a comprehensive database with nutrient information for approximately 5000 products reported by respondents in NHANES since 199913.
Within ASA24-2011 recalls, supplements were reported by browsing through categories (e.g. calcium-containing) or searching for user-entered supplement names (e.g. calcium). Included in the supplement descriptions are brand names and doses. Respondents were prompted to select the exact or closest match to the supplement actually taken and to report the quantity taken on the reporting day. When a respondent could not find a supplement he or she consumed, a text box for “unfound supplements” was available, allowing the person to type in the name or type of the supplement as well as details such as the brand name and amount taken.
Output files for ASA24 included a free text field used to report “unfound supplements.” These entries were reviewed. “Unfound supplements” were reported 116 times by 50 unique participants completing ASA24 recalls. Sixty-nine supplements unfound by the user were matched with NHANES-DSD12 codes based on the description provided. The remaining 26 could not be matched to an NHANES-DSD12 code and were determined to have no known nutrient content (e.g. herbal mixtures). Twenty-one prescription or over-the-counter medications (e.g., aspirin, Lipitor) were also excluded.
During AMPM telephone administered recalls, supplement intake was reported to the interviewer who queried the participant regarding information on the supplement label and matched this to an existing NHANES-DSD12 code. Known medications were not recorded by interviewers and all supplements were coded.
Statistical analysis
Analyses focused on comparing differences in frequency of reported dietary supplement use between 24-HR methods (ASA24 vs. AMPM) using all recalls of each method, regardless of order of completion by a given participant. Recalls collected using AMPM were defined as the standard by which to compare recalls collected using ASA24 because the AMPM method is an established and validated tool used in nutrition surveillance14. Analyses used the two one-sided test15 for assessing equivalence between the two recall methods. To account for the fact that some individuals completed their recalls using both methods while others completed two recalls using one or the other method, standard errors of the differences in proportion of reported supplement use were estimated with the delete-one jackknife procedure, operating at the individual level. To assess the effect size, or equivalence margin, of the difference between the AMPM and ASA24 proportion of reported supplement use, each reporting proportion was scaled using a variance stabilizing transformation.16 Effect sizes were defined as small (20%), medium (50%) and large (80%)10 operationally, this is the same as examining the 90% confidence interval for the difference between the two-scaled proportions to determine if the confidence interval falls between ± 20%, ± 50% or ± 80% of the difference10.
In addition, to assess the overall prevalence of total supplement usage, four mutually exclusive supplement categories were created using a scheme similar to that of Bailey et al2 to explore reporting of commonly reported supplement subgroups: 1) multivitamin-minerals: supplements with at least 3 vitamins combined with at least 1 mineral; 2) vitamin C: supplements containing vitamin C and no other vitamins or minerals; 3) calcium-containing: supplements containing calcium, but not defined as a multivitamin-mineral; and 4) vitamin D-containing: supplements containing vitamin D, but not defined as a multivitamin-mineral. Multivitamin-mineral supplements are an important category because they are the most commonly consumed supplements2, calcium and vitamin D are of interest because dietary intakes of these nutrients are low and they are thus considered nutrients of concern for the U.S. population,17 and vitamin C is noteworthy because it is a commonly-reported single-nutrient supplement.2 Exploratory analyses examined equivalence between the two recall methods in the proportions of participants reporting their use of any supplement as well as specific supplement types. These analyses were stratified by demographic variables including sex, age, and race/ethnicity.
All statistical analyses were performed using SAS software.18
Results
Table 1 includes results for participants who provided at least one complete recall, defined for these analyses as those for which both food/beverage and dietary supplement intake data were complete. Complete dietary supplement intake data includes those who completed the module and reported that no supplements were taken. The observed demographic characteristics of the sample reflect the intended effects of quota sampling, with a diverse sample in terms of sex, age, and race/ethnicity. Eighty-eight percent (n=950) of participants completed a second recall, a completion rate for the second recall that varied little by demographic characteristics (range= 85-92%). The total number of recalls included in analyses from both the first and second administrations was 2,026, with 1,004 ASA24 and 1,022 AMPM recalls.
Table 1.
Demographic Characteristicsa and Response Rate for 2nd Dietary Recall, Food Reporting Comparison Study, 2011–2012 (n=1076)
| All participants | ||
|---|---|---|
| Sample n (%) |
Response Rate for Dietary Recall 2 (%) | |
| Total | 1076 | 88 |
| Sex | ||
| Men | 507 (47) |
85 |
| Women | 569 (53) |
91 |
| Age range (yr) | ||
| 20–39 | 403 (37) |
86 |
| 40–59 | 439 (41) |
88 |
| ≥60 | 234 (22) |
92 |
| Race/ethnicity | ||
| Non-Hispanic white | 471 (44) |
90 |
| Non-Hispanic black | 366 (34) |
87 |
| Hispanic | 230 (21) |
86 |
| Other | 9 (1) | 89 |
Based only on first dietary recall
Table 2 shows the prevalence of dietary supplement use by recall method and participant characteristics. Equivalence testing indicated the proportion of reported supplement use between ASA24 and AMPM are equivalent with a small effect size of less than 20%, with two exceptions. Reported use among those 40–59 years of age and non-Hispanic blacks was higher for ASA24 than AMPM, and the effect size slightly exceeded the definition for small. The effect size for each sub-group is illustrated in Figure 1. The small effect sizes indicate that if AMPM or ASA24 are used for collecting reported dietary supplement use, effects due to mode of recall administration are likely to be small.
Table 2.
Observed prevalence of reported dietary supplement use by participant characteristics and recall method, Food Reporting Comparison Study, 2011–2012 (n=1076)
| Observed reporting prevalence (%) | ||
|---|---|---|
| ASA24a | AMPMb | |
| Total | 46 | 43 |
| Sex | ||
| Men | 46 | 43 |
| Women | 51 | 51 |
| Age Category (y) | ||
| 20–39 | 34 | 34 |
| 40–59 | 56 | 50 |
| >60 | 62 | 62 |
| Race/ethnicityc | ||
| Non-Hispanic | ||
| white | 54 | 56 |
| Non-Hispanic | ||
| black | 44 | 38 |
| Hispanic | 45 | 46 |
Automated Self-Administered 24-hour Dietary Assessment Tool (ASA24)
Automated Multiple Pass Method (AMPM)
Due to the small sample size, other race/ethnicity has not been included for comparison.
Figure 1.
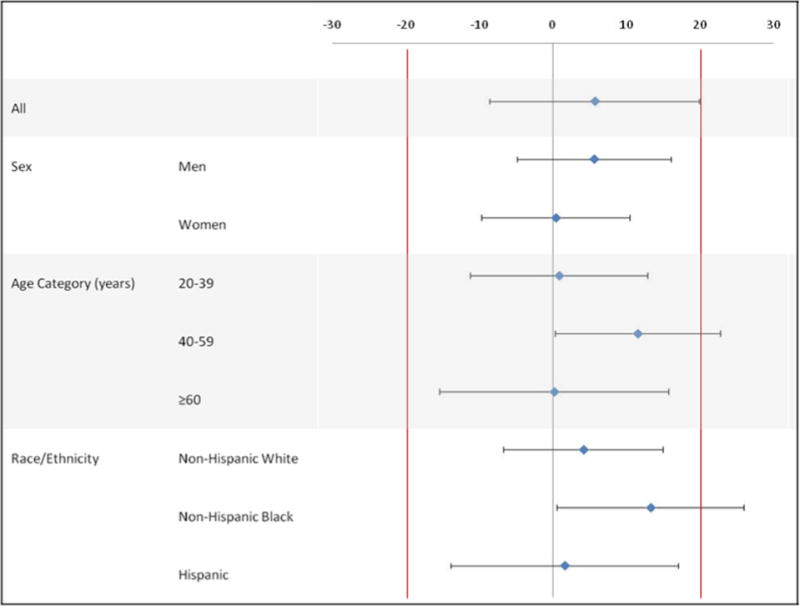
Difference and effect size between scaled prevalencea of reported dietary supplement use on ASA24b vs. AMPMc methods, Food Reporting Comparison Study, 2011–2012 (n=1076)
aPrevalence of reported dietary supplement use was scaled using variance stabilizing transformation.15
bAutomated Self-Administered 24-hour Dietary Assessment Tool (ASA24)
cAutomated Multiple Pass Method (AMPM)
A similar proportion of participants reported supplement use on the first and second recall. Fifty percent of those completing ASA24 and 47% of those completing AMPM for the first recall reported taking any dietary supplement (data not shown). For the second recall and for both methods of recall, 47% of participants reported taking any supplement. Among supplement users for both methods of recall, about 80% included three or fewer individual supplements. A small proportion of recalls included five or more supplements (10% with AMPM and 11% with ASA24).
Exploratory results for frequency of reported use by supplement subgroups types are shown in Table 3 for the first recall (findings for the second recall were similar; data not shown). Among those who reported supplement use, the most commonly reported supplement subgroup type was multivitamin-minerals, reported by a majority of participants using both recall methods. For both recall methods; reported use of supplement, especially calcium containing supplements, was higher among women than men, for participants aged 60 years of age or older versus other age groups, and for non-Hispanic whites versus non-Hispanic blacks or Hispanics.
Table 3.
Prevalence (n (%)) of dietary supplement use by supplement type and participant characteristics among participants who reported any supplement use on the first recall, Food Reporting Comparison Study, 2011–2012 (n=1076)
| Characteristic | Multivitamin-mineral | Vitamin C | Calcium | Vitamin D | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| ASA24a | AMPMb | ASA24a | AMPMb | ASA24a | AMPMb | ASA24a | AMPMb | |
| Total | 156 (59.1) | 171 (65.8) | 21 (7.9) | 28 (10.8) | 92 (34.9) | 77 (29.6) | 111 (42.1) | 103 (39.6) |
| Sex | ||||||||
| Men | 69 (56.48) | 76 (68.47) | 6 (5.1) | 11 (9.9) | 29 (25.0) | 20 (18.0) | 32 (27.6) | 32 (28.8) |
| Women | 87 (58.8) | 95 (63.8) | 15 (10.1) | 17 (11.4) | 63 (42.6) | 57 (38.3) | 79 (53.4) | 71 (47.7) |
| Age range (yr) | ||||||||
| 20–39 | 51 (68.9) | 46 (64.8) | 4 (5.4) | 9 (12.7) | 9 (12.2) | 9 (12.7) | 18 (24.3) | 17 (23.9) |
| 40–59 | 66 (55.5) | 73 (65.8) | 8 (6.7) | 7 (6.3) | 50 (42.0) | 35 (31.5) | 56 (47.1) | 43 (38.7) |
| >60 | 39 (54.9) | 52 (66.7) | 9 (12.7) | 12 (15.4) | 33 (46.5) | 33 (42.3) | 37 (52.1) | 43 (55.1) |
| Race/ethnicityc | ||||||||
| Non-Hispanic white | 80 (61.1) | 85 (68.6) | 13 (9.9) | 15 (12.1) | 56 (42.8) | 46 (37.1) | 54 (41.2) | 51 (41.1) |
| Non-Hispanic black | 52 (59.8) | 47 (65.3) | 6 (6.9) | 6 (8.3) | 20 (23.0) | 18 (25.0) | 38 (43.7) | 27 (37.5) |
| Hispanic | 23 (52.3) | 37 (59.7) | 1 (2.3) | 7 (11.3) | 16 (36.4) | 12 (19.4) | 19 (43.2) | 24 (38.7) |
Automated Self-Administered 24-hour Dietary Assessment Tool (ASA24)
Automated Multiple Pass Method (AMPM)
Due to the small sample size, other race/ethnicity has not been included for comparison.
Discussion
Previous findings from FORCS showed that for energy and most nutrients and food groups reported, intakes were equivalent between ASA24 and AMPM.10 This secondary analysis extends those results to the reporting of dietary supplements, finding that the proportions of supplement users as assessed by ASA24 and AMPM were also equivalent with a small effect size.
Although exploratory in nature, demographic patterns in the types of dietary supplements reported using both recall methods were consistent with those observed for adults in NHANES 2007-2010.2 For example, for both ASA24 and AMPM, the proportion of participants reporting supplement use was higher among women than men, and higher among those 60 years of age and older than those <60 years of age. This consistency suggests that supplement intakes collected using ASA24 are comparable to other studies, providing reasonable face validity. Although the results may inform hypotheses for future work, they should be interpreted cautiously as the study was not powered to support these tests.
The current study highlights a distinction between interviewer-administered recalls and the ASA24-2011. Direct communication between an interviewer and participant in AMPM and post-data collection coding greatly reduced the prevalence of “unknown supplements.” In ASA24, 116 reports of “unknown supplements” suggest that some ASA24 respondents had trouble finding the supplements they consumed or distinguishing dietary supplements from other types of medications. Knowledgeable research staff assigned appropriate codes to these unknown supplements using the NHANES-DSD, a time-consuming task that might not be feasible in a large-scale study. This coding helps improve frequency estimates for specific supplement types, but the effects on estimated distributions of total nutrient intakes in a large sample needs further investigation. In ASA24-2011 and ASA24 2014, the typing of text into the “unknown supplement” box does not lead to the assignment of a default supplement code, unlike the case for reporting an unfound food or beverage. The identification of this limitation led to a new development in ASA24-2016, such that reporting of an “unknown supplement” is followed up with questions to collect further details (e.g. categories), which mimics the unfound foods pass and allows for default auto-coding.
Nutrient adequacy can be achieved through the appropriate intakes of a variety of foods and beverages recommended in dietary guidelines. Nevertheless, it is clear that a large proportion of US adults consume dietary supplements. As a result, ignoring supplement intakes will lead researchers to underestimate total nutrient intakes, which are critical for informing policies and programs as well as examining nutrient intakes relative to disease risk, incidence, and/or treatment. Including the dietary supplement module as part of an ASA24 dietary recall has the potential to improve our understanding of behaviors related to dietary supplement use, the contributions supplements have on total nutrient intakes, and their associations with chronic disease.
From its release in 2009 until February of 2018, ASA24 has been widely adopted for use in more than 3,000 studies to collect close to 365,000 recall and record days. In this study, all participants had access to the internet. An interview-administered dietary recall may be a more suitable tool for some study designs where, for example, literacy is low or access to the internet is a challenge. Though designed to be self-administered, the ASA24 can be used as an interview administered tool.
FORCS is one of two studies conducted to formally evaluate the impact of mode of administration for an interview- vs. a self-administered recall. A feeding study of 81 participants evaluated both recall modes against observed intakes and found good agreement between reported and true intakes for ASA24 and few differences between ASA24 and AMPM recalls.19
Conclusion
The current analysis provides evidence that there is little difference in reported supplement use due to the effect of the mode of administration (i.e. interview- vs. self-administered recall). This finding supports the use of ASA24 as an affordable and efficient tool for the collection of supplement intakes that contribute to the collection of total nutrient intakes from food, beverages, and supplements.
Acknowledgments
The authors would like to acknowledge the Cancer Research Network, a consortium of nine nonprofit research centers based in integrated health care delivery organizations, for its facilitation of the study (NIH grant numbers U19 CA079689 and U24 CA171524). We acknowledge Victor Kipnis for his insight regarding the statistical analysis.
Please note, Victor Kipnis has granted permission to be acknowledged for his contributions.
The Cancer Research Network, a consortium of nine nonprofit research centers based in integrated health care delivery organizations, helped facilitate the study (NIH grant numbers U19 CA079689 and U24 CA171524).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Funding Disclosure
The FORCS Study was completed under contract with Westat (Contract NO: NO2-PC-64406).
Conflict of Interest Disclosure
The authors have no conflicts of interest to declare.
Contributor Information
TusaRebecca E. Pannucci, Email: tusarebecca.pannucci@cnpp.usda.gov, Lead Nutritionist, Nutrition and Economic Analysis, Center for Nutrition Policy and Promotion, United States Department of Agriculture, 3101 Park Center Dr., Room 1044, Alexandria, VA 22302, W: 703-305-4363, F: 703-305-3300.
Frances E. Thompson, Email: thompsof@mail.nih.gov, Epidemiologist, Division of Cancer Control and Population Sciences, National Cancer Institute, National Institutes of Health, Department of Health and Human Services, 9609 Medical Center Drive, Room 3E432, Bethesda, MD 20814-9692, T: 240-276-6781, F: 240-276-7906.
Regan L. Bailey, Email: reganbailey@purdue.edu, Associate Professor, Department of Nutrition Science, Purdue University, Stone Hall, Room 143A, 700 West State Street, West Lafayette, IN 47907, T: (765) 494-2829.
Kevin W. Dodd, Email: doddk@mail.nih.gov, Mathematical Statistician, Division of Cancer Prevention, National Cancer Institute, Department of Health and Human Services, 9609 Medical Center Drive, Room 5E120, Bethesda, MD 20814-9692, T: 240-276-7021, F: 240-276-7906.
Nancy Potischman, Email: potischn@mail.nih.gov, Director, Population Studies Program, Office of Dietary Supplements, National Institutes of Health, 6100 Executive Boulevard, Room 3B01, MSC 7517, Bethesda, MD 20892-7517, T:301-496-0187, F:301-480-1845.
Sharon I. Kirkpatrick, Email: haron.kirkpatrick@uwaterloo.ca, Assistant Professor, School of Public Health and Health Systems, University of Waterloo, 200 University Avenue West, Waterloo, Ontario N2L 3G1, T: (519) 888-4567.
Gwen L. Alexander, Email: galexan2@hfhs.org, Investigator, Public Health Sciences Research, Henry Ford Health System, One Ford Place, 5C, Detroit, MI, 48202, T: 313-874-6737.
Laura A. Coleman, Email: laura.coleman@abbott.com, Medical Science Liaison, Research and Development, Abbot Nutrition, 3300 Stelzer Rd, Columbusm, OH 43219, T: 715-316-3361.
Lawrence H. Kushi, Email: Larry.Kushi@nsmtp.kp.org, Principal Investigator, Population Sciences and Health Disparities, University of California, Davis School of Medicine and Kaiser Permanente Division of Research, 2000 Broadway, Oakland, CA, 94612, T: 510-891-3837.
Michelle Groesbeck, Email: mgroesb1@hfhs.org, Special Projects Coordinator, Public Health Sciences Research, Henry Ford Health System, One Ford Place, 5C, Detroit, MI, 48202, T: 800-436-7936.
Maria Sundaram, Email: mariasundaram@gmail.com, Research Epidemiologist, Center for Clinical Epidemiology & Population Health, Marshfield Clinic Research Foundation, 1000 N Oak Avenue, ML-2, Marshfield, WI 54449, T: 7152216060.
Heather Clancy, Email: heather.a.clancy@kp.org, CRN Project Director, Kaiser Permanente Division of Research, 2101 Webster, 20th Floor, Oakland, CA 94607, T: 510-627-2293.
Stephanie M. George, Email: stephanie.george@nih.gov, Senior Epidemiologist, Office of Disease Prevention, Office of the Director, National Institutes of Health, 6100 Executive Blvd, Suite 2B03, Rockville, MD 20852, T: 301-451-9416.
Lisa Kahle, Email: kahle@imsweb.com, Senior Systems Analyst, Information Management Systems, 12501 Prosperity Dr., Silver Spring, MD 20904, T: 717-486-3315.
Amy F. Subar, Nutritionist, Division of Cancer Control and Population Sciences, National Cancer Institute, National Institutes of Health, Department of Health and Human Services, 9609 Medical Center Drive, Room 4E140, Bethesda, MD 20814-9763.
References
- 1.Kantor ED, Rehm CD, Du M, White E, Giovannucci EL. Trends in Dietary Supplement Use Among US Adults From 1999–2012. JAMA. 2016;316(14):1464–1474. doi: 10.1001/jama.2016.14403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey RL, Gahche JJ, Miller PE, Thomas PR, Dwyer JT. Why US adults use dietary supplements. JAMA Intern Med. 2013 Mar 11;173(5):355–361. doi: 10.1001/jamainternmed.2013.2299. [DOI] [PubMed] [Google Scholar]
- 3.Nicastro HL, Bailey RL, Dodd KW. Using 2 Assessment Methods May Better Describe Dietary Supplement Intakes in the United States. J Nutr. 2015 Jul;145(7):1630–1634. doi: 10.3945/jn.115.211466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rock CL. Multivitamin-multimineral supplements: who uses them? The American Journal of Clinical Nutrition. 2007 Jan 1;85(1):277S–279S. doi: 10.1093/ajcn/85.1.277S. 2007. [DOI] [PubMed] [Google Scholar]
- 5.Bailey RL, Dodd KW, Goldman JA, et al. Estimation of Total Usual Calcium and Vitamin D Intakes in the United States. The Journal of Nutrition. 2010 Apr 1;140(4):817–822. doi: 10.3945/jn.109.118539. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bailey RL, McDowell MA, Dodd KW, Gahche JJ, Dwyer JT, Picciano MF. Total folate and folic acid intakes from foods and dietary supplements of US children aged 1–13 y. The American Journal of Clinical Nutrition. 2010 Aug 1;92(2):353–358. doi: 10.3945/ajcn.2010.29652. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller MF, Bellizzi KM, Sufian M, Ambs AH, Goldstein MS, Ballard-Barbash R. Dietary supplement use in individuals living with cancer and other chronic conditions: a population-based study. J Am Diet Assoc. 2008 Mar;108(3):483–494. doi: 10.1016/j.jada.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Thompson FS, AF . Chapter 1 Dietary assessment methodology. In: Coultson AMB, CJ, Ferruzzi MG, editors. Nutrition in the Prevention and Treatment of Disease. Third. San Diego, CA: Elsevier Press; 2012. [Google Scholar]
- 9.Subar AF, Kirkpatrick SI, Mittl B, et al. The Automated Self-Administered 24-hour dietary recall (ASA24): a resource for researchers, clinicians, and educators from the National Cancer Institute. J Acad Nutr Diet. 2012 Aug;112(8):1134–1137. doi: 10.1016/j.jand.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson FE, Dixit-Joshi S, Potischman N, et al. Comparison of Interviewer-Administered and Automated Self-Administered 24-Hour Dietary Recalls in 3 Diverse Integrated Health Systems. Am J Epidemiol. 2015 Jun 15;181(12):970–978. doi: 10.1093/aje/kwu467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blanton CA, Moshfegh AJ, Baer DJ, Kretsch MJ. The USDA Automated Multiple-Pass Method accurately estimates group total energy and nutrient intake. J Nutr. 2006;136(10):2594–2599. doi: 10.1093/jn/136.10.2594. [DOI] [PubMed] [Google Scholar]
- 12.U.S. Department of Healthy and Human Services. National Health and Nutrition Examination Survey 1999–2014 Data Documentation, Codebook, and Frequencies. Dietary Supplement Database – Ingredient Information. 2002 https://wwwn.cdc.gov/nchs/nhanes/1999-2000/DSII.htm. Accessed June, 2017.
- 13.Dwyer J, Picciano MF, Raiten DJ, Committee aMotS Food and Dietary Supplement Databases for What We Eat in America–NHANES. The Journal of Nutrition. 2003 Feb 1;133(2):624S–634S. doi: 10.1093/jn/133.2.624S. 2003. [DOI] [PubMed] [Google Scholar]
- 14.Moshfegh AJ, Rhodes DG, Baer DJ, et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am J Clin Nutr. 2008 Aug;88(2):324–332. doi: 10.1093/ajcn/88.2.324. [DOI] [PubMed] [Google Scholar]
- 15.Schuirmann DJ. A comparison of the two one-sided tests procedure and the power approach for assessing the equivalence of average bioavailability. Journal of pharmacokinetics and biopharmaceutics. 1987 Dec;15(6):657–680. doi: 10.1007/BF01068419. [DOI] [PubMed] [Google Scholar]
- 16.Cohen J. Statistical power analysis for the behavioral sciences. 2nd. Hillsdale, N.J.: L. Erlbaum Associates; 1988. [Google Scholar]
- 17.U.S. Department of Agriculture USDoHaHS. 2015–2020 Dietary Guidelines for Americans. (8th) 2015 Dec; Available at http://health.gov/dietaryguidelines/2015/guidelines/. 2015.
- 18.SAS version 9.2; SAS Institute, 2010.
- 19.Kirkpatrick SI, Subar AF, Douglass D, et al. Performance of the Automated Self-Administered 24-hour Recall relative to a measure of true intakes and to an interviewer-administered 24-h recall. Am J Clin Nutr. 2014 Apr 30; doi: 10.3945/ajcn.114.083238. [DOI] [PMC free article] [PubMed] [Google Scholar]


