Abstract
Fat-specific protein 27 (FSP27) is highly expressed in the fatty liver of genetically obese ob/ob mice and promotes hepatic triglyceride (TG) accumulation. The nuclear hormone receptor liver X receptor α (LXRα) also plays a critical role in the control of TG levels in the liver. The present study demonstrated transcriptional regulation of Fsp27a and Fsp27b genes by LXRα. Treatment with the LXR ligand T0901317 markedly increased Fsp27a and Fsp27b mRNAs in wild-type C57BL/6J and ob/ob mouse livers. A reporter assay indicated that two LXR-responsive elements (LXREs) are necessary for LXRα-dependent induction of Fsp27a and Fsp27b promoter activities. Furthermore, the LXRα/retinoid X receptor α complex is capable of directly binding to the two LXREs both in vitro and in vivo. These results suggest that LXRα positively regulates Fsp27a and Fsp27b expression through two functional LXREs. Fsp27a/b are novel LXR target genes in the ob/ob fatty liver.
Keywords: PPAR, LXR, Fatty liver, Nuclear receptor
1. Introduction
Fat-specific protein 27 (FSP27) was initially isolated by a differential screening approach using mouse adipocyte TA cell lines expressing a mature adipocyte-specific gene (Williams et al., 1992; Danesch et al., 1992). FSP27 belongs to the cell death-inducing DNA fragmentation factor 45-like effector (CIDE) family, which consists of three proteins: CIDEA, CIDEB, and FSP27. The human homolog of mouse FSP27 was also reported as CIDEC (Liang et al., 2003). FSP27 was found to be highly expressed in white and brown adipose tissue and localized to lipid droplets (LDs) in adipocytes (Puri et al., 2007). FSP27 promotes the formation of LD–LD fusions on adipocytes (Gong et al., 2011; Jambunathan et al., 2011) and enlarged unilocular LDs in cooperation with perilipin 1, another LD-associated protein (Sun et al., 2013). Fsp27-null mice showed protection from diet-induced obesity and insulin resistance, and exhibited a small mass of white adipose tissue and the presence of multilocular LDs (Toh et al., 2008; Nishino et al., 2008). Adipocyte-specific Fsp27-null mice also exhibited small white adipose tissue masses and hepatic steatosis (Tanaka et al., 2015).
Previous studies demonstrated that hepatic peroxisome proliferator-activated receptor γ (PPARγ) promoted triglyceride (TG) accumulation and fatty liver development in ob/ob mice, a well-characterized model of type 2 diabetes, obesity, and fatty liver because of its mutated leptin gene (Matsusue et al., 2003). Furthermore, FSP27 was established as the direct mediator of PPARγ-dependent hepatic steatosis in ob/ob mice. Interestingly, FSP27 expression showed the highest levels in ob/ob fatty liver, but lower levels in normal liver and led to an increase in hepatic TG levels (Matsusue et al., 2008). Recently, FSP27 isoforms were identified as FSP27α and FSP27β. FSP27β contains an additional 10 amino acids at the N-terminus of the original FSP27 identified in ob/ob fatty liver (named as FSP27α) and shows higher intracellular stability than FSP27α. It was also demonstrated that both isoforms directly promote hepatic TG accumulation (Xu et al., 2015).
Liver X receptor (LXR) α and β are members of a family of ligand-dependent nuclear receptors (Mangelsdorf et al., 1995). LXRs heterodimerize with retinoid X receptors (RXR) and regulate transcription by binding to LXR-responsive elements (LXRE) of target genes. LXRs play important roles in regulating genes associated with lipogenesis in the liver (Baranowski, 2008). Indeed, activation of LXRs by the LXR ligand, T0901317, caused a marked increase in hepatic TG levels and aggravation of fatty liver in ob/ob or other fatty liver model mice (Matsusue et al., 2014; Chisholm and Chisholm, 2003). Regarding hepatic fat accumulation, two transcription factors, sterol regulatory element-binding protein 1c (SREBP1c) and carbohydrate response element-binding protein (ChREBP), play crucial roles in LXR-mediated hepatic lipogenesis (Baranowski, 2008; Matsusue et al., 2014). SREBP1c and ChREBP are direct targets of LXRs and control the expression of nearly all genes integral to lipogenesis, including fatty acid synthase (Fas), acetyl-CoA carboxylase, and stearoyl-CoA desaturase 1 (Scd1) (Baranowski, 2008; Matsusue et al., 2014). Thus, LXR signaling mediated by SREBP1c and ChREBP is thought to contribute to an increase in hepatic TG content by upregulating these lipogenic genes. Whether LXRs induce not only lipogenic genes but also Fsp27a/b in ob/ob fatty liver is unknown. Additionally, the transcriptional regulation of hepatic Fsp27a/b by LXRs remains unclear.
In the present study, administration of T0901317 to C57BL/6J mice wild-type for the leptin gene (OB/OB) and ob/ob mice showed markedly increased Fsp27a and Fsp27b expression. Furthermore, two functional LXREs were identified within 5′-upstream regions of Fsp27a and Fsp27b. These findings suggest that Fsp27a and Fsp27b are directly regulated by LXRα and a novel LXRα target gene in the liver.
2. Materials and methods
2.1. Animals and treatment
All animal protocols and studies were performed according to guidelines from the Center for Experimental Animals at Fukuoka University. Eight-week-old male mice (n = 4) on an ob/ob background or C57BL/6J mice with a wild-type leptin gene (OB/OB) were fed an ad libitum diet (MF, Oriental Yeast, Fukuoka, Japan) with or without 0.025% (w/w) T0901317 (Sigma Aldrich, St. Louis, MO, USA) for 2 weeks, as previously described (Matsusue et al., 2014). GW3965 (Selleck, Japan) was administered with 20 mg/day/kg for 3 days by oral gavage (Laffitte et al., 2003). As a positive control for oral gavage administration, T0901317 was administered at 20 mg/day/kg for 3 days (Jakel et al., 2004). Vehicle alone was administered as a negative control (0.5% methyl-cellulose).
2.2. Total RNA isolation and qPCR
RNA was extracted using TRIzol reagent (Thermo Fisher Scientific, Waltham, MA, USA), and quantitative polymerase chain reaction (qPCR) was performed using cDNA generated from 1 μg of total RNA with an AffinityScript qPCR cDNA Synthesis kit (Agilent Technologies, Santa Clara, CA, USA). The primer sequences used were described previously for the following genes: Fsp27a and Fsp27b, (Xu et al., 2015); Lxra, Srebp1c, Fas, Scd1, Gpat, and Pparg (Matsusue et al., 2014).
2.3. Cell culture
HEK293FT cells were cultured at 37 °C under 5% CO2 in Dulbecco's Modified Eagle's Medium with high glucose and pyruvate (Thermo Fisher Scientific), supplemented with 10% fetal bovine serum (Biowest, Nuaillé, France) and 1% Antibiotic-Antimycotic (Thermo Fisher Scientific).
2.4. Construction of reporter and expression plasmids
The transcriptional start sites of mouse Fsp27a and Fsp27b were determined previously (Danesch et al., 1992; Xu et al., 2015). The −1698 (A1), −1426 (A2), −1267 (A3), and −139 (A4) base pair (bp) fragments from the transcriptional start site (+1) of the mouse Fsp27a 5′-upstream region, containing KpnI and MluI sites at the 5′- and 3′-end of the primers, were amplified by PCR and cloned into the luciferase reporter vector pGL3 basic (Promega, Madison, WI, USA) as previously described (Matsusue et al., 2008).
Internal deletion constructs for Fsp27a were prepared by inverse PCR. The Fsp27a A4 construct was used as a template. The primer sequences were as follows: A4-1forward, 5′-GGAGCTGGGGTATATGGC-3′ and reverse, 5′-CCCAGCCTCCTGGCAATA-3′; A4-2 forward, 5′-ATGGCTGAGGTCGCAGTT-3′ and reverse, 5′-CCATGTCCCTTATATACC-3’; A4-3 forward, 5′-ATAAGGGACATGGTTGGA-3′ and reverse, 5′-ATATACCCCAGCTCCTCA-3′.
The −2647 (B1), −2375 (B2), −1382 (B3), and −806 (B4) bp fragments from the transcriptional start site (+1) of the mouse Fsp27b 5′-upstream region, containing CACC sites at the 5′-end of the primers, were amplified by PCR and cloned into the Gateway entry vector pENTR/D-TOPO (Thermo Fisher Scientific), and then recombined into the destination vector pGL4.17 (Promega), which was prepared using the Gateway Vector Conversion System according to the manufacturer's instructions (Thermo Fisher Scientific). The primer sequences were as follows: B1 forward, 5′-CACCCTCCCATTGCTCATTCG-3’; B2 forward, 5′-CACCATCAGCTGTGCCTACGGATG-3′; B3 forward, 5′-CACCTGAGACAGGGCCAACTCT-3′; B4 forward, 5′-CACCAGTGTTGGGTTGTGGTGAGG-3′; reverse for all constructs, 5′-TGTTTCTCCGACCCAAGCTG-3′.
The LXRα and RXRα expression vectors were prepared as described previously (Matsusue et al., 2006). The complete open reading frame of mouse LXRβ was amplified by PCR from a mouse liver cDNA library by using gene-specific primers and cloned into the Gateway entry vector, pENTR/D-TOPO (Thermo Fisher Scientific). This sequence was then recombined into the destination vector, pcDNA3.1 (Thermo Fisher Scientific), which was prepared using the Gateway Vector Conversion System (Thermo Fisher Scientific). The primer sequences were as follows: forward, 5′-CACCACCATGGCTTCCCCCACAAGTTCTCTGG-3′ and reverse, 5′-CATCTTCAAGAAGACACCACCAAG-3′.
2.5. Transient transfection and reporter assay
HEK293FT cells were seeded at a density of 2.0 × 105 cells/well in 24-well plates at 24 h prior to transfection. Cells were transfected with plasmids using jetPEI DNA transfection reagent (Polyplus-transfection, Illkirch, France) according to the manufacturer's instructions. Typically, each well contained 2 μL of jetPEI DNA transfection reagent, 0.15 μg of LXRα (or LXRβ) and RXRα expression plasmids, 0.4 μg of luciferase reporter constructs containing the 5′-region of mouse Fsp27a/b, and 0.05 μg of phRL/TK (Promega) as an internal control for transfection efficiency. After adding the reagents, cells were transfected for 6 h at 37 °C in an atmosphere of 5% CO2. The cells were then incubated for 42 h in fresh medium containing 10 μM T0901317, 10 μM GW3965, 0.1 μM LG100268 (Sigma-Aldrich), or DMSO. The luciferase assay was performed using the Dual-Luciferase Reporter Assay System (Promega). Luciferase activity was measured using a GENE LIGHT 55A luminometer (Nition, Microtec Co., Ltd., Chiba, Japan).
2.6. In vitro transcription/translation and EMSA
Mouse LXRα and human RXRα proteins were synthesized in vitro from LXRα and RXRα expression plasmids using the TNT Quick Coupled Transcription/Translation System (Promega) according to the manufacturer's protocols. Electrophoretic mobility shift assay (EMSA) was performed as described previously (Matsusue et al., 2006). For the supershift assay, 0.5 μg of anti-LXRα IgG (Perseus Proteomics, Tokyo, Japan) was included for 30 min after the binding reactions. The gels were exposed to an imaging plate. The gel images were visualized using an FLA-7000 imaging analyzer (Fujifilm, Tokyo, Japan). Probe sequences for the EMSA were as follows: mouse phosphofructokinase-2 (pfk2) LXRE (Zhao et al., 2012), 5′-CTCTCCTGACCTCTCCCAACCTCTGCGG-3′; non-LXRE, 5′-GCACACACCTTTAGTCCCGGCTCTCTGG-3′; LXRE-1, 5′-CCGGACAGGTGACTACAGGACAGAAAGG-3′; LXRE-2, 5′-CCACGGAGGCCTGACGAGGTGACAGATC-3′; LXRE-3, 5′-GGTATATGGCTACTAGAGAGCAGATGGT-3′.
2.7. ChIP assay
Chromatin immunoprecipitation (ChIP) assays were performed using a SimpleChIP Enzymatic Chromatin IP Kit-Magnetic Beads (Cell Signaling Technology, Danvers, MA, USA) according to the manufacturer's protocols. Briefly, T0901317-treated ob/ob mouse livers were treated with 1% formaldehyde for 10 min. The isolated nuclei were digested with micrococcal nuclease and further sonicated. Anti-LXRα IgG (Perseus Proteomics) was used to immunoprecipitate LXRα-binding DNA fragments. The precipitated chromatin was digested with proteinase K for 2 h at 65 °C. Finally, DNA was purified using a spin column and subjected to qPCR. Primer sequences for ChIP-qPCR were as follows; non-PPRE forward, 5′-CTGACGTGTGGTTAGAGACATGG-3′ and reverse, 5′-GTGTATTTGTGCACGTGAGAGC-3′; LXRE-1 forward, 5′-GAAGGAATGTCGCCTCTTCCAG-3′ and reverse, 5′-CTAAGGCAGCAACACGAAGC-3′; LXRE-3′ forward, 5′-GCTGGGGTATATGG CTACTAGAGAG-3′ and reverse, 5′- GATTAACGCAGGTTCTCCTTGG-3'.
2.8. Statistical analysis
Experimental values are expressed as the mean ± standard error of the mean (S.E.M.). Statistical analysis was performed using Student's t-test for unpaired data, with P < 0.05 considered to indicate statistical significance.
3. Results
3.1. Fsp27a and Fsp27b expression in OB/OB and ob/ob mouse livers is induced by T0901317
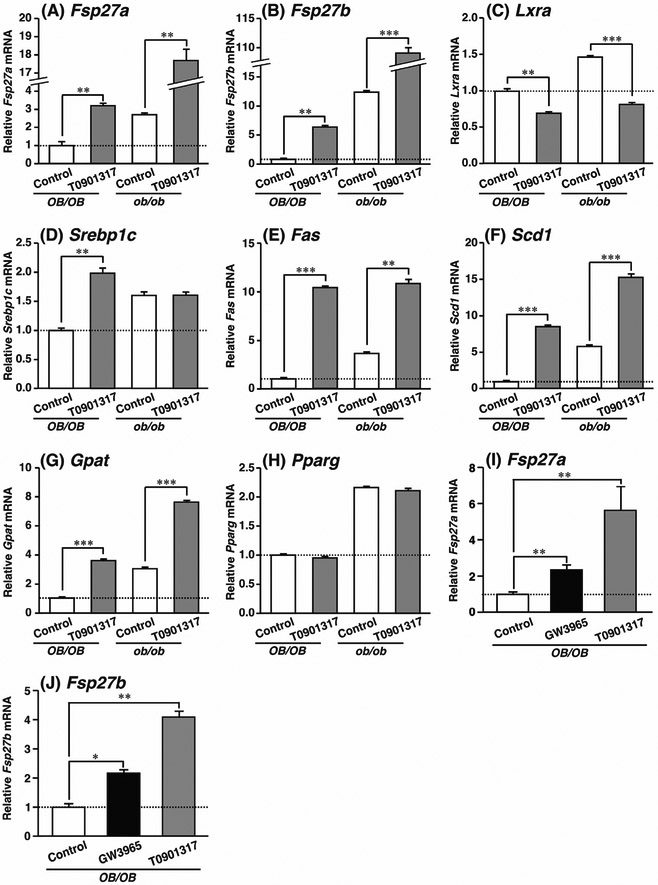
Treatment with T0901317 led to a marked increase in liver weight and hepatic TG levels in ob/ob mice (Matsusue et al., 2014). The same effect of the ligand was observed in another obese model, db/db mice (Chisholm and Chisholm, 2003). To examine the expression of hepatic Fsp27a and Fsp27b in LXR-dependent fatty liver, the mRNA of both genes was measured in ligand-treated OB/OB and ob/ob mice. The expression of Fsp27a mRNA in OB/OB and ob/ob mouse livers was induced by approximately 3.0- and 6.0-fold with T0901317 compared to that in the control (Fig. 1A). Furthermore, the expression of Fsp27b mRNA in T0901317-treated OB/OB and ob/ob mouse livers was 6.0- and 9.0-fold higher than in each control liver (Fig. 1B).
Fig. 1. T0901317 increases the expression levels of Fsp27a and Fsp27b in OB/OB and ob/ob mouse livers.
T0901317 was administered at 0.025% (w/w) for 2 weeks in ligand-mixed diets. qPCR analyses of (A) Fsp27a, (B) Fsp27b, (C) Lxra, (D) Srebp1c, (E) Fas, (F) Scd1, (G) Gpat, and (H) Pparg mRNAs were examined using liver samples from T0901317-treated OB/OB and ob/ob mice. Effect of GW3965 on Fsp27a (I) or Fsp27b (J) mRNA was examined using liver samples from T0901317- or GW3965-treated OB/OB mice. T0901317 or GW3965 was administered at 20 mg/day/kg for 3 days by oral gavage. Gene expression was normalized to 36b4 mRNA. Each bar represents the average ± S.E.M. of four individual experiments. OB/OB, C57BL/6 mice wild-type for the leptin gene; ob/ob, leptin-deficient mice; Control, no T0901317 treatment. Significant differences from OB/OB or ob/ob mice with Control: *P < 0.05, **P < 0.01, ***P < 0.001.
Although the expression of LXRα by T0901317 slightly decreased in OB/OB and ob/ob livers, the expression of LXR-target genes, Srebp1c, Fas, Scd1, and glycerol-3-phosphate acyltransferase (Gpat) mRNAs significantly increased in both livers (Fig. 1C–G). The expression of hepatic PPARγ was induced in ob/ob fatty liver (Matsusue et al., 2003) and activated PPARγ induced Fsp27a mRNA expression in fatty liver (Matsusue et al., 2008). T0901317 had no significant effect on the expression of hepatic Pparg mRNA in OB/OB and ob/ob livers (Fig. 1H). T0901317 has been reported to activate the farnesoid X receptor (FXR) or pregnane X receptor (PXR) (Mitro et al., 2007). To evaluate the LXR-specific regulation of Fsp27a (Fig. 1I) or Fsp27b (Fig. 1J) mRNA, the expression of both genes by an LXR-specific ligand, GW3965, was examined in the OB/OB liver. The results revealed that both Fsp27a and Fsp27b mRNAs were significantly induced approximately 2.0-fold with GW3965 as compared to that in the control. As a positive control for the same oral gavage administration, the expression of Fsp27a and Fsp27b mRNAs was induced by approximately 5.0- and 4.0-fold with T0901317. These results suggest that LXRα positively regulates Fsp27a and Fsp27b expression in OB/OB and ob/ob livers.
3.2. Three putative LXREs on 5′-upstream and exon 1 of Fsp27a and Fsp27b
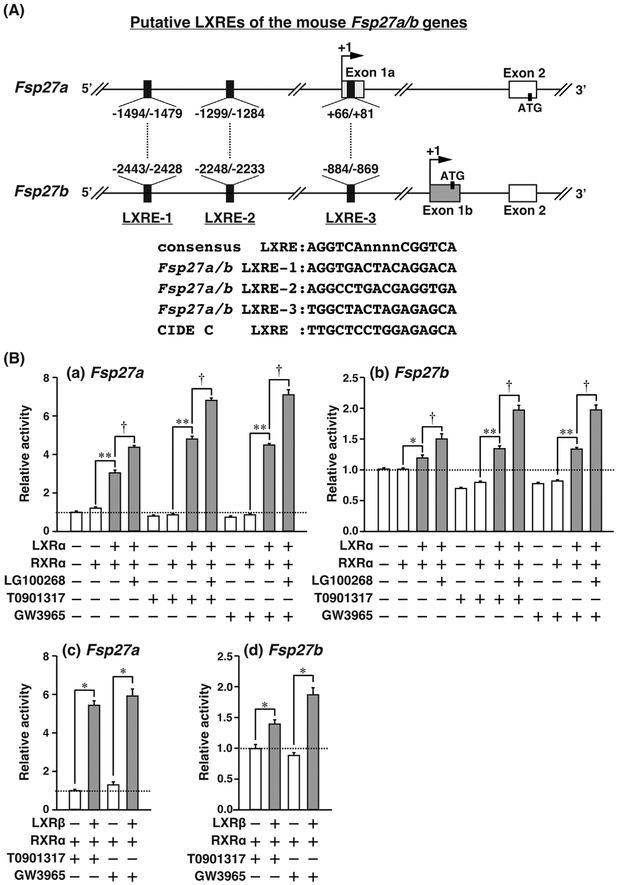
Fsp27a and Fsp27b show differences in exon 1, termed as a and b, respectively, while exons 2–6 are the same (Fig. 2A) (Xu et al., 2015). Thus, the promoter regions for Fsp27a or Fsp27b differ. To detect functional LXRE in the Fsp27a/b 5′-upstream region, database analysis was performed. By searching the JASPAR database (http://jaspar.genereg.net/), three putative LXREs were identified (Fig. 2A). Interestingly, LXRE-3 of Fsp27a was located in a 5′-untranslated region (5′-UTR) of exon 1a. LXRE-1, LXRE-2, and LXRE-3 showed 83%, 75%, and 58% homology with a typical LXRE consensus sequence (Willy et al., 1995), respectively. Human CIDEC as the homolog of mouse FSP27 also contained LXRE at a position corresponding to LXRE-3.
Fig. 2. LXRα promotes the promoter activity of Fsp27a and Fsp27b.
(A) Location and sequences of three putative LXREs in the 5′-region or exon 1 of mouse Fsp27a and Fsp27b genes. Arrow and ATG indicate transcription and translation start sites, respectively. (B) Fsp27a A1 including −1698/+257 region (a) and Fsp27b B1 including −2647/+77 region (b) reporter plasmids were transfected into HEK293FT cells with or without the expression plasmids for LXRα. Fsp27a A1 (c) and Fsp27b B1 (d) reporter plasmids were transfected into HEK293FT cells with or without the expression plasmids for LXRβ. Six hours after transfection, the medium was changed to fresh medium containing 10 μM T0901317, 10 μM GW3965, or 0.1 μM LG100268. The cells were harvested at 42 h after transfection and luciferase activity was measured. Each bar represents the average ± S.E.M. of three individual experiments. Significant differences from cells without LXRα or LXRβ expression plasmid: *P < 0.01, **P < 0.001. Significant differences from cells without LG100268: † P < 0.001.
To determine how LXRα regulates the promoter activity of Fsp27a and Fsp27b, Fsp27a (−1698/+257 bp) or Fsp27b (−2647/+77 bp), luciferase reporter plasmids containing the three putative LXREs, were constructed. Fsp27a or Fsp27b reporter and the LXRα and RXRα expression plasmids were transfected into HEK293FT cells. The relative fold-inductions of Fsp27a promoter activity with LXRα and RXRα, compared to that without LXRα, were as follows: non-LXR ligand, 3.0-fold; with T0901317, 5.0-fold; and with GW3965, 5.0-fold (Fig. 2Ba). Similarly, the relative fold-inductions of Fsp27b activity were as follows: non-LXR ligand, 1.2-fold; with T0901317, 1.5-fold; and with GW3965, 1.5-fold (Fig. 2Bb). The effects of the RXR-specific ligand LG100268 in the presence of LXRα, RXRα and T0901317 or GW3965 showed a similar tendency for Fsp27a and Fsp27b promoter activities. The relative fold-inductions of Fsp27a/b promoter activity with LG100268, compared to without LG100268, were as follows: non-LXR ligand, 1.3-fold; with T0901317, 1.5-fold; and with GW3965, 1.5-fold (Fig. 2Ba and b). Furthermore, the effect of LXRβ expression on the promoter activity of Fsp27a and Fsp27b was examined. In the presence of RXRα and T0901317 or GW3965, the Fsp27a promoter activity with LXRβ was induced by approximately 5.5-fold with T0901317 and 6.0-fold with GW3965 compared to that without LXRβ (Fig. 2Bc). In contrast, Fsp27b promoter activity with LXRβ was induced approximately 1.5-fold with T0901317 and 2.0-fold with GW3965 (Fig. 2Bd).
3.3. Identification of a functional LXRE of Fsp27a and Fsp27b
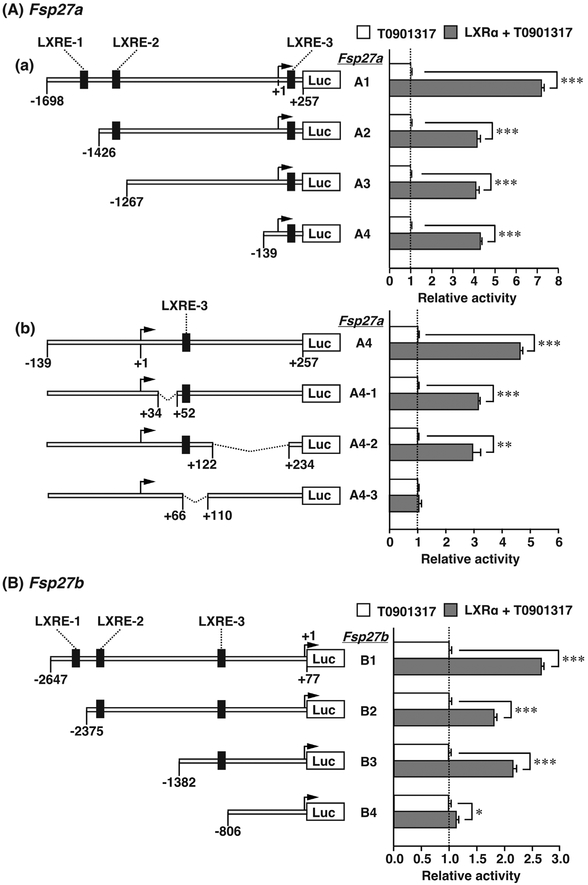
To identify the cis-element responsible for ligand-dependent promotion by LXRα, reporter constructs with serial deletions of the 5′-flanking DNA regions of Fsp27a and Fsp27b were prepared. The luciferase activity of the Fsp27a A1 construct was induced by approximately 7.0-fold with LXRα, whereas the activity of the A2 construct lacking LXRE-1 decreased by approximately 60% compared to the A1 construct. The activity of the A3 and A4 constructs lacking LXRE-2 was not different from that of the A2 construct (Fig. 3Aa). LXRE-3 is in the 5′-UTR of exon 1a of Fsp27a (Fig. 2A). Thus, to determine the role of LXRE-3 in LXR ligand-dependent promotion, an internal deletion mutant of LXRE-3 using the Fsp27a A4 construct was prepared. The Fsp27a A4 construct including LXRE-3 was induced by approximately 4.5-fold with LXRα (Fig. 3Ab). Although upstream or downstream deletion of LXRE-3 led to a slight decrease in the induction by LXRα (A4-1 or A4-2), the induction was completely lost in the A4-3 construct lacking LXRE-3 (Fig. 3Ab). Furthermore, LXR ligand-dependent promotion of Fsp27b also depended on LXRE-1 and LXRE-3. Luciferase activity of the Fsp27b B1 construct was induced by approximately 2.5-fold with LXRα, whereas the activity of the B2 construct lacking LXRE-1 decreased by approximately 60%. This induction was nearly lost in the B4 construct lacking LXRE-3 (Fig. 3B). These results suggest that LXRα induces the promoter activity of Fsp27a and Fsp27b through LXRE-1 and LXRE-3.
Fig. 3. Induction of the promoter activity of Fsp27a and Fsp27b genes by T0901317 depends on two LXREs.
(A) (a) Serially deleted Fsp27a A1–A4 and (b) internally deleted Fsp27a A4-1–A4-3 reporter plasmids were transfected into HEK293FT cells with or without LXRα expression plasmid. (B) Serially deleted Fsp27b B1–B4 reporter plasmids were transfected into HEK293FT cells with or without LXRα expression plasmid. In (A) and (B), 6 h after transfection, the medium was changed to fresh medium containing 10 μM T0901317. The cells were harvested at 42 h after transfection and luciferase activity was measured. Each bar represents the average ± S.E.M. of three individual experiments. Significant differences from cells without LXRα expression plasmid: *P < 0.01, **P < 0.001.
3.4. LXRα/RXRα heterodimer directly binds to LXRE-1 and LXRE-3
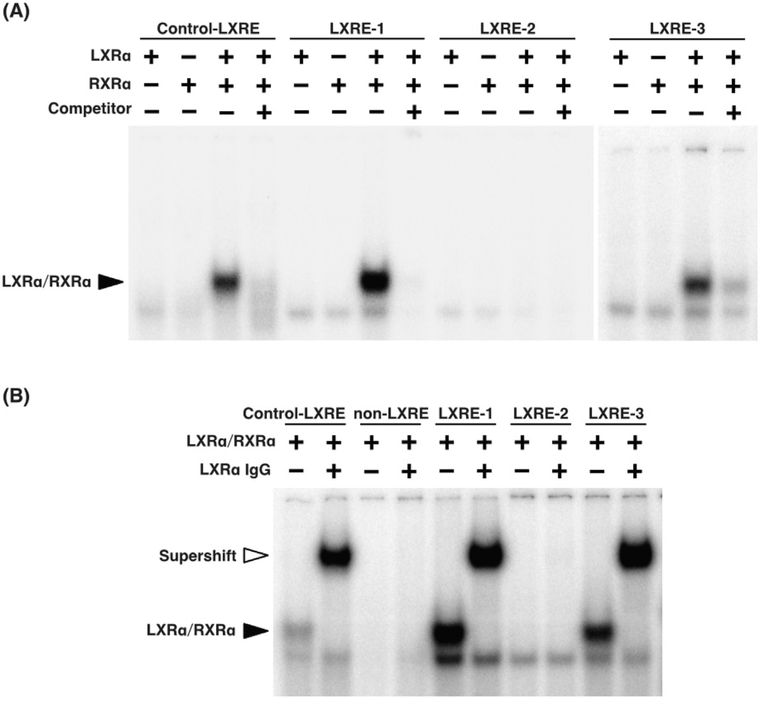
To examine whether LXRα directly binds to LXRE-1 and LXRE-3, electrophoretic mobility shift assays using LXRE probes for Fsp27a and Fsp27b, with Pfk2 as a positive control probe (Zhao et al., 2012), were performed. Although LXRα or RXRα did not bind to all LXRE probes, the LXRα/RXRα heterodimer directly bound to control-LXRE, LXRE-1, and LXRE-3, but not LXRE-2 (Fig. 4A) in vitro. The binding of LXRα/RXRα to LXRE-1 and LXRE-3 was nearly lost by adding a 200-fold molar excess of each unlabeled probe. Furthermore, the binding of LXRα/RXRα to LXRE-1 and LXRE-3 were supershifted by the addition of anti-LXRα IgG (Fig. 4B). These results suggest that LXRα/RXRα heterodimers directly bind to LXRE-1 and LXRE-3 of Fsp27a and Fsp27b.
Fig. 4. LXRα/RXRα heterodimers directly bind to LXRE-1 and LXRE-3 in the Fsp27a/b 5′-upstream or 5′-UTR region.
(A) EMSAs were performed using 32P-labeled oligonucleotide probes. Probes were incubated with LXRα/RXRα produced by in vitro translation with or without an excess of unlabeled probe as competitor. (B) Supershift assays were performed using anti-LXRα IgG. The location of the LXRα/RXRα complex is indicated by the filled arrowhead, while the supershifted complex is indicated by the open arrowhead.
3.5. Endogenous LXRα in ob/ob fatty liver interacts with LXRE-1 and LXRE-3
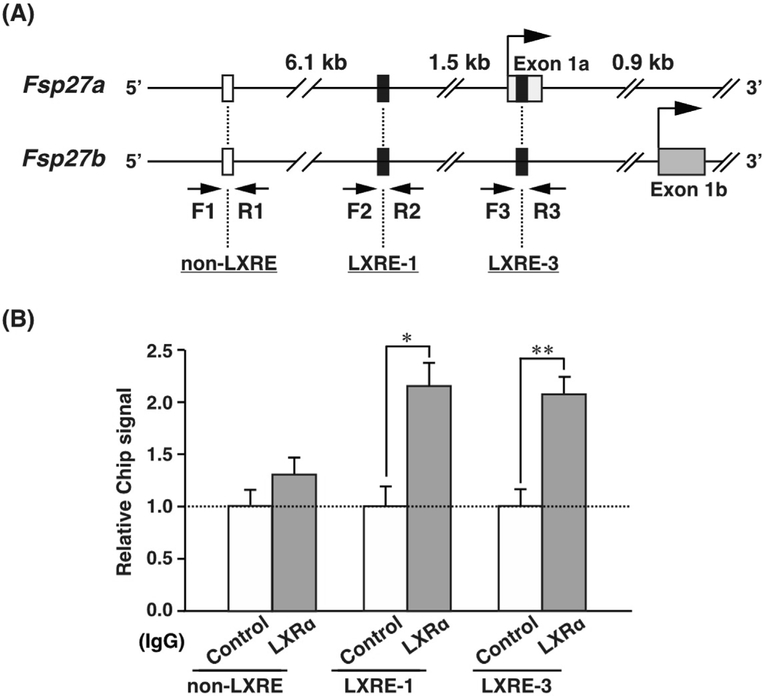
To examine the interactions between endogenous LXRα and LXRE-1 or LXRE-3 under physiological conditions, a ChIP assay was performed with chromatin from T0901317-treated ob/ob mouse liver. Three primer sets were designed to amplify LXRE-1, LXRE-3, and non-LXRE (negative control) (Fig. 5A). The chromatin from cross-linked liver was fragmented and incubated with anti-LXRα IgG (LXRα) or anti-rabbit IgG (Control) as a negative control. Precipitated LXREs were detected by qPCR using each ChIP primer. The results revealed an association of endogenous LXRα in ob/ob fatty liver with LXRE-1 and LXRE-3. The addition of anti-LXRα IgG led to an increase of approximately 2.0-fold in LXRE-1 and LXRE-3 as compared to the control IgG, although the non-LXRE signal slightly increased (Fig. 5B). These results suggest that endogenous LXRα also interacts with LXRE-1 and LXRE-3 in ob/ob mouse fatty liver.
Fig. 5. Endogenous LXRα associates with LXRE-1 and LXRE-3.
(A) The positions of ChIP primer pairs are shown by arrows. F1 and R1 primer sets located >6.1 kb upstream of Fsp27a/b were also used as negative controls. (B) ChIP-qPCR analysis was performed using chromatin samples from the livers of T0901317-treated ob/ob mouse liver with anti-rabbit IgG (control) or anti-LXRα IgG. Each bar represents the average ± S.E.M. of three individual experiments. Significant differences from Control: *P < 0.01, **P < 0.001.
4. Discussion
The present study demonstrated a novel regulation mechanism of Fsp27a and Fsp27b by LXRα. An LXRα ligand, T0901317, markedly increased Fsp27a and Fsp27b mRNAs in OB/OB and ob/ob mouse livers. Fsp27a and Fsp27b contain two functional LXREs, LXRE-1 and LXRE-3. LXRα directly binds to these LXREs in vitro and in vivo. Interestingly, LXRE-3 of Fsp27a is located in the 5′-UTR of exon 1a, which is also observed for the LXREs of the ATP-binding cassette transporter 1 (Schwartz et al., 2000) or organic anion transporting polypeptide 1B1 (Meyer Zu Schwabedissen et al., 2010) genes. The LXR family consists of two members, LXRα and LXRβ, which are encoded by individual genes (Alberti et al., 2000). Mouse LXRα is highly expressed in the liver and kidney, while LXRβ is ubiquitously expressed (Seol et al., 1995). The present study revealed induction of Fsp27a and Fsp27b promoter activities by LXRβ. Therefore, LXRβ also appears to contribute to regulating hepatic Fsp27a and Fsp27b.
T0901317 potentially activates not only LXRs, but also FXR or PXR (Houck et al., 2004). Thus, the involvement of T0901317-activated FXR or others in regulating hepatic Fsp27a and Fsp27b cannot be ruled out. However, Fxr-null mice exhibited elevated hepatic TG levels as opposed to the phenotype displayed by Lxr-null mice (Sinal et al., 2000; Beaven et al., 2013). Another study found no significant difference in the expression of Fsp27a mRNA in white adipose tissue between wild-type and Fxr-null mice (Abdelkarim et al., 2010). Thus, FXR likely does not or only slightly contributes to regulating Fsp27a and Fsp27b in the liver. Notably, the LXR-specific ligand GW3965 significantly induced Fsp27a or Fsp27b mRNA in the OB/OB liver. These results support that LXRα/β positively regulates the transcriptional expression of Fsp27a or Fsp27b in the liver.
It has reported that Lxra mRNA in human macrophages or mouse adipose tissue is induces by T0901317-activated LXR through LXRE located in the 5′-upstream region (Whitney et al., 2001; Ulven et al., 2004). However, we found that Lxra mRNA was slightly decreased by T0901317 administration. The discrepancy may be related to the tissue or cell-specific manner of self-regulation of LXRα by LXR (Whitney et al., 2001; Ulven et al., 2004), although the mechanism of the specificity remains unclear. Indeed, a study showed that the levels of hepatic Lxra mRNA in T0901317-administered mice were lower than in untreated mice, which agrees with our results (Matsusue et al., 2014; Ulven et al., 2004). Further, we examined whether Lxra protein levels between control and T0901317-treated livers remain unchanged (data not shown).
The fatty liver of ob/ob mice was found to result from PPARγ induction of FSP27 through a typical PPRE in the Fsp27 promoter region (Matsusue et al., 2008). It was reported that Fsp27 mRNA is upregulated by PPARα (Langhi and Baldan, 2015) or cAMP-response element-binding protein (Vila-Brau et al., 2013) in OB/OB livers. In these earlier reports, Fsp27 mRNA was not distinguished between Fsp27a and Fsp27b mRNAs and was used as the primer set for the common exon sequence in Fsp27a/b. Additionally, cyclic AMP-responsive element-binding protein H (CREBH) was also reported as a critical factor for Fsp27b mRNA induction in the fasting normal liver or ob/ob fatty liver (Xu et al., 2015). Interestingly, induction by CREBH occurred in an Fsp27b-specific manner because the CREBH response element is located in the promoter region of Fsp27b. In contrast to CREBH, activated LXRα upregulates both Fsp27a and Fsp27b in fatty liver and upregulates hepatic lipogenesis by controlling lipogenic genes. Because the expression of all LXR targets without T0901317 in the ob/ob fatty liver was higher than that in the normal liver, LXRs appear to be constitutively active in the fatty liver. The hepatic TG levels increased by activated LXRs are thought to be accompanied by elevated SREBP1c, ACC, FAS, and SCD1 (Baranowski, 2008). Notably, FSP27α and FSP27β may promote hepatic TG accumulation (Matsusue et al., 2008; Xu et al., 2015). Therefore, LXRs likely promote not only TG synthesis by lipogenic enzymes, but also TG accumulation through FSP27α and FSP27β. TG synthesis and accumulation by LXRs appear to largely contribute to the development and aggregation of fatty liver.
In summary, the present study demonstrated that hepatic Fsp27a and Fsp27b are positively regulated by LXRα through two functional LXREs. CIDEC, the human homolog of mouse Fsp27, also has an LXRE in the 5′-upstream region. Therefore, elucidating the mechanism of TG-accumulating effects by LXRs through FSP27α and FSP27β may lead to new therapeutic opportunities for controlling TG accumulation in non-alcoholic fatty liver disease and its associated pathologies.
Acknowledgments
Funding
This work was supported by a Grant from KAKENHI (17K08799) and funds (No. 147015) from the Central Research Institute of Fukuoka University.
Abbreviations:
- FSP27
fat-specific protein 27
- CIDE
cell death-inducing DNA fragmentation factor 45-like effector
- LD
lipid droplet
- TG
triglyceride
- PPAR
peroxisome proliferator-activated receptor
- CREBH
cyclic AMP-responsive element binding protein H
- LXR
liver X receptor
- LXRE
LXR response element
- FAS
fatty acid synthase
- SCD1
stearoyl-coenzyme A desaturase 1
- PFK2
phosphofructokinase-2
- SREBP1c
sterol regulatory element-binding protein-1c
- ChREBP
carbohydrate response element-binding protein
- ACC
acetyl CoA carboxylase
- GPAT
glycerol-3-phosphate acyltransferase
- UTR
untranslated region
- ob/ob
leptin-deficient mice
- OB/OB
C57BL/6J wild-type for leptin gene
References
- Abdelkarim M, Caron S, Duhem C, Prawitt J, Dumont J, Lucas A, Bouchaert E, Briand O, Brozek J, Kuipers F, Fievet C, Cariou B, Staels B, 2010. The farnesoid X receptor regulates adipocyte differentiation and function by promoting peroxisome proliferator-activated receptor-gamma and interfering with the Wnt/beta-catenin pathways,. J. Biol. Chem 285, 36759–36767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberti S, Steffensen KR, Gustafsson JA, 2000. Structural characterisation of the mouse nuclear oxysterol receptor genes LXRalpha and LXRbeta. Gene 243, 93–103. [DOI] [PubMed] [Google Scholar]
- Baranowski M, 2008. Biological role of liver X receptors. J. Physiol. Pharmacol 59 (Suppl 7), 31–55. [PubMed] [Google Scholar]
- Beaven SW, Matveyenko A, Wroblewski K, Chao L, Wilpitz D, Hsu TW, Lentz J, Drew B, Hevener AL, Tontonoz P, 2013. Reciprocal regulation of hepatic and adipose lipogenesis by liver X receptors in obesity and insulin resistance. Cell Metabol. 18, 106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm JW, Chisholm JW, 2003. The LXR ligand T0901317 induces severe lipogenesis in the db/db diabetic mouse. J. Lipid Res 44, 2039–2048. [DOI] [PubMed] [Google Scholar]
- Danesch U, Hoeck W, Ringold GM, 1992. Cloning and transcriptional regulation of a novel adipocyte-specific gene, FSP27. CAAT-enhancer-binding protein (C/EBP) and C/EBP-like proteins interact with sequences required for differentiation-dependent expression. J. Biol. Chem 267, 7185–7193. [PubMed] [Google Scholar]
- Gong J, Sun Z, Wu L, Xu W, Schieber N, Xu D, Shui G, Yang H, Parton RG, Li P, 2011. Fsp27 promotes lipid droplet growth by lipid exchange and transfer at lipid droplet contact sites. J. Cell Biol 195, 953–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houck KA, Borchert KM, Hepler CD, Thomas JS, 2004. T0901317 is a dual LXR/FXR agonist. Mol. Genet. Metabol. 83, 184–187. [DOI] [PubMed] [Google Scholar]
- Jakel H, Nowak M, Moitrot E, Dehondt H, Hum DW, Pennacchio LA, Fruchart-Najib J, Fruchart J-C, 2004. The liver X receptor ligand T0901317 down-regulates APOA5 gene expression through activation of SREBP-1c. J. Biol. Chem 279, 45462–45469. [DOI] [PubMed] [Google Scholar]
- Jambunathan S, Yin J, Khan W, Tamori Y, Puri V, 2011. FSP27 promotes lipid droplet clustering and then fusion to regulate triglyceride accumulation. PLoS One 6, e28614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffitte BA, Chao LC, Li J, Walczak R, Hummasti S, Joseph SB, Castrillo A, Wilpitz DC, Mangelsdorf DJ, Collins JL, Saez E, Tontonoz P, 2003. Activation of liver X receptor improves glucose tolerance through coordinate regulation of glucose metabolism in liver and adipose tissue. Proc. Natl. Acad. Sci. U.S.A 100, 5419–5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhi C, Baldan A, 2015. CIDEC/FSP27 is regulated by peroxisome proliferator-activated receptor alpha and plays a critical role in fasting- and diet-induced hepatosteatosis. Hepatology 61, 1227–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L, Zhao M, Xu Z, Yokoyama KK, Li T, 2003. Molecular cloning and characterization of CIDE-3, a novel member of the cell-death-inducing DNA-fragmentation-factor (DFF45)-like effector family. Biochem. J 370, 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM, 1995. The nuclear receptor superfamily: the second decade. Cell 83, 835–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsusue K, Haluzik M, Lambert G, Yim SH, Gavrilova O, Ward JM, Brewer B Jr., Reitman ML, Gonzalez FJ, 2003. Liver-specific disruption of PPARgamma in leptin-deficient mice improves fatty liver but aggravates diabetic phenotypes. J. Clin. Invest 111, 737–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsusue K, Miyoshi A, Yamano S, Gonzalez FJ, 2006. Ligand-activated PPAR-beta efficiently represses the induction of LXR-dependent promoter activity through competition with RXR. Mol. Cell. Endocrinol. 256, 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsusue K, Kusakabe T, Noguchi T, Takiguchi S, Suzuki T, Yamano S, Gonzalez FJ, 2008. Hepatic steatosis in leptin-deficient mice is promoted by the PPARgamma target gene Fsp27. Cell Metabol. 7, 302–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsusue K, Aibara D, Hayafuchi R, Matsuo K, Takiguchi S, Gonzalez FJ, Yamano S, 2014. Hepatic PPARγ and LXRα independently regulate lipid accumulation in the livers of genetically obese mice. FEBS Lett. 588, 2277–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer Zu Schwabedissen HE, Bottcher K, Chaudhry A, Kroemer HK, Schuetz EG, Kim RB, 2010. Liver X receptor alpha and farnesoid X receptor are major transcriptional regulators of OATP1B1. Hepatology 52, 1797–1807. [DOI] [PubMed] [Google Scholar]
- Mitro N, Vargas L, Romeo R, Koder A, Saez E, 2007. T0901317 is a potent PXR ligand: implications for the biology ascribed to LXR. FEBS Lett. 581, 1721–1726. [DOI] [PubMed] [Google Scholar]
- Nishino N, Tamori Y, Tateya S, Kawaguchi T, Shibakusa T, Mizunoya W, Inoue K, Kitazawa R, Kitazawa S, Matsuki Y, Hiramatsu R, Masubuchi S, Omachi A, Kimura K, Saito M, Amo T, Ohta S, Yamaguchi T, Osumi T, Cheng J, Fujimoto T, Nakao H, Nakao K, Aiba A, Okamura H, Fushiki T, Kasuga M, 2008. FSP27 contributes to efficient energy storage in murine white adipocytes by promoting the formation of unilocular lipid droplets. J. Clin. Invest 118, 2808–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri V, Konda S, Ranjit S, Aouadi M, Chawla A, Chouinard M, Chakladar A, Czech MP, 2007. Fat-specific protein 27, a novel lipid droplet protein that enhances triglyceride storage. J. Biol. Chem 282, 34213–34218. [DOI] [PubMed] [Google Scholar]
- Schwartz K, Lawn RM, Wade DP, 2000. ABC1 gene expression and ApoA-I-mediated cholesterol efflux are regulated by LXR. Biochem. Biophys. Res. Commun 274, 794–802. [DOI] [PubMed] [Google Scholar]
- Seol W, Choi HS, Moore DD, 1995. Isolation of proteins that interact specifically with the retinoid X receptor: two novel orphan receptors. Mol. Endocrinol 9, 72–85. [DOI] [PubMed] [Google Scholar]
- Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ, 2000. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 102, 731–744. [DOI] [PubMed] [Google Scholar]
- Sun Z, Gong J, Wu H, Xu W, Wu L, Xu D, Gao J, Wu JW, Yang H, Yang M, Li P, 2013. Perilipin1 promotes unilocular lipid droplet formation through the activation of Fsp27 in adipocytes. Nat. Commun 4, 1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N, Takahashi S, Matsubara T, Jiang C, Sakamoto W, Chanturiya T, Teng R, Gavrilova O, Gonzalez FJ, 2015. Adipocyte-specific disruption of fat-specific protein 27 causes hepatosteatosis and insulin resistance in high-fat diet-fed mice. J. Biol. Chem 290, 3092–3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh SY, Gong J, Du G, Li JZ, Yang S, Ye J, Yao H, Zhang Y, Xue B, Li Q, Yang H, Wen Z, Li P, 2008. Up-regulation of mitochondrial activity and acquirement of brown adipose tissue-like property in the white adipose tissue of fsp27 deficient mice. PLoS One 3, e2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulven SM, Dalen KT, Gustafsson J-Å, Nebb HI, 2004. Tissue-specific autoregulation of the LXRalpha gene facilitates induction of apoE in mouse adipose tissue. J. Lipid Res 45, 2052–2062. [DOI] [PubMed] [Google Scholar]
- Vila-Brau A, De Sousa-Coelho AL, Goncalves JF, Haro D, Marrero PF, 2013. Fsp27/CIDEC is a CREB target gene induced during early fasting in liver and regulated by FA oxidation rate. J. Lipid Res 54, 592–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney KD, Watson MA, Goodwin B, Galardi CM, Maglich JM, Wilson JG, Willson TM, Collins JL, Kliewer SA, 2001. Liver X receptor (LXR) regulation of the LXRalpha gene in human macrophages. J. Biol. Chem 276, 43509–43515. [DOI] [PubMed] [Google Scholar]
- Williams PM, Chang DJ, Danesch U, Ringold GM, Heller RA, 1992. CCAAT/enhancer binding protein expression is rapidly extinguished in TA1 adipocyte cells treated with tumor necrosis factor. Mol. Endocrinol 6, 1135–1141. [DOI] [PubMed] [Google Scholar]
- Willy PJ, Umesono K, Ong ES, Evans RM, Heyman RA, Mangelsdorf DJ, 1995. LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev. 9, 1033–1045. [DOI] [PubMed] [Google Scholar]
- Xu X, Park JG, So JS, Lee AH, 2015. Transcriptional activation of Fsp27 by the liver-enriched transcription factor CREBH promotes lipid droplet growth and hepatic steatosis. Hepatology 61, 857–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao LF, Iwasaki Y, Nishiyama M, Taguchi T, Tsugita M, Okazaki M, Nakayama S, Kambayashi M, Fujimoto S, Hashimoto K, Murao K, Terada Y, 2012. Liver X receptor alpha is involved in the transcriptional regulation of the 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase gene. Diabetes 61, 1062–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]