Abstract
Scope
It is found in the previous study that egg‐white‐derived antihypertensive peptide Ile‐Arg‐Trp (IRW) upregulated angiotensin converting enzyme 2 (ACE2) in spontaneously hypertensive rats (SHRs). The objective of this study is to evaluate the contribution of ACE2 activation by IRW to blood‐pressure‐lowering activity in vivo.
Methods and results
Adult male SHRs (13–15 week old) are assigned into four groups: 1) untreated with saline infusion; 2) IRW administration (15 mg per kg body weight) with saline infusion; 3) Mas receptor (MasR) antagonist A779 (48 µg per kg body weight per h) infusion; 4) A779 infusion and IRW. Animals are implanted with telemetry transmitter first, and then an osmotic pump filled with saline or A779 is implanted. A779/saline is infused for 7 days, continued with an additional 7 days of treatments. Results indicate that blocking MasR abolished the blood‐pressure‐lowering effect of IRW. Akt/eNOS signaling in aorta is upregulated by IRW treatment but deactivated by A779 infusion. Circulating levels of interleukin 6 and monocyte chemoattractant protein 1, along with cyclooxygenase 2 in aorta are reduced by IRW but restored by A779 infusion.
Conclusion
IRW reduces blood pressure of SHR via the ACE2/Ang (1‐7)/MasR axis. Mechanisms pertaining to IRW as an ACE2 activator in vivo include enhanced endothelium‐dependent vasorelaxation and reduced vascular inflammation.
Keywords: ACE2, bioactive peptides, blood pressure, hypertension
1. Introduction
Hypertension is a risk factor for cardiovascular diseases, affecting about 25% of adults worldwide.1 In 2017, hypertension was redefined to be above 130/80 mmHg rather than 140/90 mmHg for systolic/diastolic blood pressure by the American Heart Association (AHA) and the American College of Cardiology (ACC),2 resulting in a higher prevalence. Essential hypertension, also known as primary hypertension, accounts for 95% of all hypertension cases around the world.3
Although the pathophysiology of essential hypertension is multifactorial and complicated, an over activated renin angiotensin system (RAS) is considered as a major factor, given the essential role of the RAS in regulating blood pressure.4 Angiotensin II (Ang II) is a vasoconstricting component in the RAS, which is converted from angiotensin I (Ang I) with the action of angiotensin converting enzyme (ACE).4 Indeed, Ang II is more than a vasoconstrictor; it also controls the structure and function of vascular wall. An excessive level of Ang II is associated with aberrant proliferation and migration of vascular cells, oxidative stress, and inflammation of vascular wall, which further promotes vascular remodelling and increases peripheral resistance.5 Hence, to reduce the generation of Ang II by inhibiting ACE activity is a common strategy for hypertension therapy.6
In 2000, angiotensin converting enzyme 2 (ACE2) was identified.7 As a homolog of ACE, ACE2 shares 42% of identical sequence with ACE but has opposing functions.7, 8 ACE2 cleaves the carboxyl‐terminal phenylalanine of Ang II to form angiotensin (1‐7) (Ang (1‐7)), which further counterbalances the harmful effects of Ang II via the mas receptor (MasR).9, 10 Contributions of increased ACE2 to modulating various cardiovascular functions have been recognized.11 In spontaneously hypertensive rats (SHRs), an animal model which mimetics human essential hypertension, overexpression of ACE2 was associated with decreased blood pressure, attenuated oxidative stress, and cardiac remodelling.12 Supplementation of ACE2 abolished Ang II‐induced hypertrophic response, confirming the beneficial roles of ACE2 in cardiovascular functions.13 Thus, targeting ACE2 is considered as a novel therapeutic strategy for hypertension.14
Given the side effects generated by prolonged use of antihypertensive drugs, food protein–derived antihypertensive peptides have attracted substantial interests as a safer alternative.15 Similar to antihypertensive pharmaceuticals, most of antihypertensive peptides were characterized as ACE‐inhibitory peptides.16 While peptide with the potential targeting ACE2 has been rarely reported. Our lab previously identified an antihypertensive peptide with the sequence of Ile‐Arg‐Trp (IRW) from egg white ovotransferrin.17 Blood pressure‐reducing effect of IRW was demonstrated in SHRs.18 Molecular mechanisms of IRW in modulating intracellular events of vascular cells as well as in reducing blood pressure of SHRs have also been explored.19, 20, 21 Interestingly, a transcriptomic study found that oral administration of IRW could upregulate mRNA of ACE2 in mesenteric artery of SHRs.22 Further study found IRW could activate in vitro ACE2 as well as cellular ACE2 activities.23 In addition, oral administration of IRW could upregulate protein expressions of ACE2 in aorta and kidney of SHRs.23 Taken together, IRW was shown the potential of an ACE2 activator. However, the contribution of ACE2 activation by IRW to reducing blood pressure is not known.
In the present study, we hypothesized that IRW activates the ACE2/Ang (1‐7)/MasR axis which contributes to reducing blood pressure in SHRs.
2. Experimental Section
2.1. Animal Model and Ethics Statement
Twelve to fourteen‐week‐old male SHRs (290.0 ± 10 g) were obtained from Charles River (Senneville, QC, Canada). Animals were kept in the University of Alberta animal core facility for a week of acclimatization, where they were exposed to 12:12 h of light:dark cycle with controlled humidity and temperature. All SHRs were given standard rat chow and water ad libitum. This protocol was approved by the University of Alberta Animal Welfare Committee (Protocol #AUP 00001571) in accordance with the guidelines issued by the Canada Council on Animal Care and also adhered to the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health.
2.2. Surgical Procedure
Thirteen to fifteen‐week‐old male SHRs were surgically implanted with telemetry transmitters (HD‐S10, DSI, St. Paul, MN, USA) for chronic blood pressure monitoring. The catheter of the telemetry probe was inserted into the left femoral artery of the rat and advanced up to the aorta. The transmitter was placed in a subcutaneous pocket in the left leg. After the surgery, the rats were allowed for a 7‐day post‐surgery recovery.
2.3. Dosage Information
The animals were randomly assigned into four groups: Untreated (n = 6), IRW (15 mg per kg body weight, n = 6), MasR antagonist A779 (48 µg per kg body weight per h, n = 6),24 and IRW+A779 (n = 6). The dosage of IRW was selected based on our previous study,18 which is equivalent to 2.43 mg per kg body weight in adult human according to the body surface area–based dosage translation equation.25 Basal blood pressure (Day‐7) was recorded, and then an osmotic pump (ALZET, Cupertino, USA) loaded with A779 was implanted subcutaneously into the animal. An osmotic pump (ALZET) filled with saline was implanted into animals without A779 treatment. A779 was infused for 7 days prior to the start of IRW treatment. IRW was dissolved in 20 mL of Ensure (Abbott Nutrition, QC, Canada) and administered orally once per day for 7 days.18
2.4. Telemetry Recording
Real‐time blood pressure was monitored during the IRW treatment period. Each rat was caged individually and placed on the top of a RPC‐1 receiver (ADI instruments, CO, USA). An atmospheric‐pressure monitor (Model APR‐1, ADI instruments) was also installed to normalize the pressure values received from the transmitters, which provided the actual values irrespective of changes in atmospheric pressure. Signals were recorded and output by Dataquest 4.3.6 (DSI). The average value of blood pressure/heart rate was calculated based on a 24 h recording (10 s of every 30 s).
2.5. Vascular Function
Endothelium‐dependent vasorelaxation using mesenteric artery was evaluated as we described previously.18 Mesenteric arteries (diameter: 150–250 µm, length: ≈2 mm) were mounted on two tungsten wires (Fine Wire Company, CA, USA) and attached to a wire‐myograph (DMT, Ann Arbor, MI, USA). After normalization and equilibration, arteries were exposed to 10 µm of phenylephrine (PE, Sigma‐Aldrich, St. Louis, MO, USA) twice, followed by a single dose of methacholine (MCh, 3 µm, Sigma‐Aldrich) to evaluate the integrity of the vessel. A cumulative concentration response curve to PE ranging 10−8 to 10−4 m was performed. Afterward, the vessel was pre‐constricted to 80% of maximum using PE. A cumulative concentration response curve to MCh (10−10 to 10−6 m) was performed to assess the endothelium‐dependent vasorelaxation.
2.6. Plasma Biomarker Analysis
Blood samples were collected at the end point, followed by centrifugation under 1000 × g for 15 min at 4 °C. Plasma was stored at −80 °C until analysis. Concentrations of Ang II, ACE2 and Ang (1‐7), and ACE were quantified by respective ELISA kit (CUSABIO TECHNOLOGY, Houston, TX, USA) according to the manual. The activity of circulating ACE was evaluated by a fluorometric kit (Sigma‐Aldrich) according to the instruction. Results were expressed as relevant fluorescent unit per min (RFU min−1). Estimations of cytokine samples were performed using a commercially available rat cytokine strips (Signosis, Santa Clara, CA, USA).18
2.7. Western Blotting
Total proteins were extracted from aortas by a protein extraction buffer (20 mm Tris, 5 mm EDTA, 10 mm Na4P2O7, 100 mm sodium fluoride, and 1% NP‐40) containing 1% v/v protease inhibitor cocktail (Sigma‐Aldrich). The homogenate was centrifuged at 15 000 × g for 15 min at 4 °C. Protein concentration was quantified by BCA assay (ThermoFisher, Waltham, MA, USA).
Total protein was loaded on a 9% SDS‐PAGE and then transferred to a nitrocellulose membrane. Bands of phospho‐Akt (p‐Akt, Cell Signalling, Danvers, MA, USA), phospho‐endothelial nitric oxide synthase (p‐eNOS, Cell Signalling), and phospho‐extracellular signal‐regulated kinases1/2 (p‐ERK1/2, Cell Signalling) were normalized to the corresponding “total” form (Akt, Cell Signalling; eNOS, BD Biosciences, Franklin Lakes, NJ, USA; ERK1/2, Cell Signalling). Bands of ACE2 (Abcam, Cambridge, MA, USA), MasR (NOVUS Biologicals, Littleton, CO, USA), ACE (Santa Cruz Biotechnology, Dallas, TX, USA), angiotensin type I receptor (AT1R, Invitrogen by ThermoFisher), angiotensin type II receptor (AT2R, Abcam), inhibitory κBα (IκBα, Cell Signalling), cyclooxygenase 2 (COX2, NOVUS Biologicals), and matrix metallopeptidase 9 (MMP9, NOVUS Biologicals) were normalized to β‐actin (Abcam) or glyceraldehyde 3‐phosphate dehydrogenase (GAPDH, Abcam). Bands were developed by Goat anti‐rabbit IRDye 800 CW or Donkey anti‐mouse 680 RD from Licor Biosciences (Lincoln, NE, USA) as the secondary antibody, and detected by Licor Odyssey BioImager (Licor Biosciences). Quantification of protein bands was performed using Image Studio Lite 5.2 (Licor Biosciences).
2.8. Statistics
Statistical analysis was performed using GraphPad Prism (La Jolla, CA, USA). All data were presented as mean ± SEM (standard error of mean) from 4–6 animals. Blood pressure and heart rate data were analyzed by two‐way ANOVA followed by Tukey's multiple comparisons test. MCh curves were fitted by nonlinear regression and analyzed by two‐way ANOVA coupled with Tukey's multiple comparisons test. Results of western blotting and plasma biomarker analysis were analyzed by one‐way ANOVA followed by Tukey's multiple comparisons test. A p value < 0.05 was considered as statistically significant.
3. Results
3.1. IRW Decreases Blood Pressure in SHRs via the ACE2/Ang (1‐7)/MasR Axis
The mean artery pressure of SHR was reduced from 147.82 to 126.85 mmHg by 7‐day IRW oral administration. Both systolic pressure and diastolic pressure were reduced during the treatment period (Figure 1 A–C), which was comparable as we previously reported.18 Infusion of MasR antagonist A779 for 7 days did not show a significant effect on blood pressure. However, we observed that the blood pressure‐reducing effect of IRW was blocked by A779 infusion, indicating the effect was via the ACE2/Ang (1‐7)/MasR axis. Regardless the decrease of blood pressure, heart rate was not altered by any of the treatment (Figure 1D).
Figure 1.
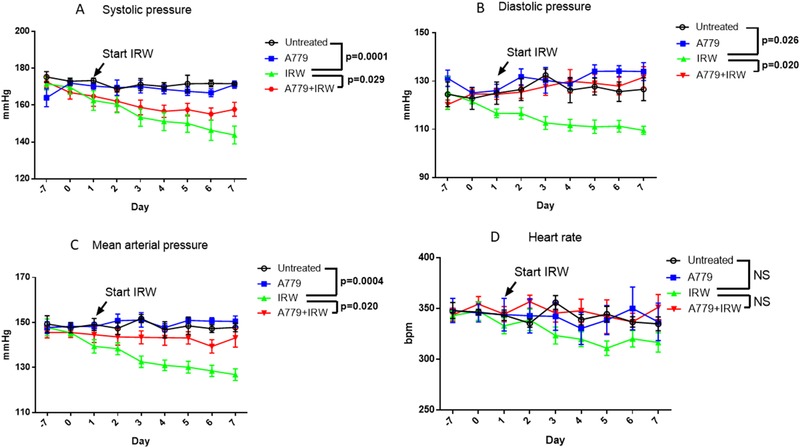
IRW decreases blood pressure of SHRs via the ACE2/Ang (1‐7)/MasR axis. A779 (48 µg per kg BW per h) or saline was infused 7 days prior to starting IRW treatment. The co‐treatment period of A779 and IRW was 7 days. A) Systolic pressure, B) diastolic pressure, C) mean arterial pressure, and D) heart rate were recorded over a 24‐h period. Data represented as mean ± SEM from six animals per treatment group. NS indicated no significant difference.
3.2. IRW Modulates the RAS Components
IRW treatment resulted in increased circulating ACE2 and Ang (1‐7) levels, but decreased the concentration of Ang II significantly (Figure 2A–C), which implicated the potential of IRW in increasing circulating ACE2 to decrease blood pressure in SHRs. On the contrary, IRW treatment did not show a significant effect on the concentration or activity of ACE (Figure 2D,E), which further demonstrated ACE2, instead of ACE, was the target of IRW in vivo.
Figure 2.
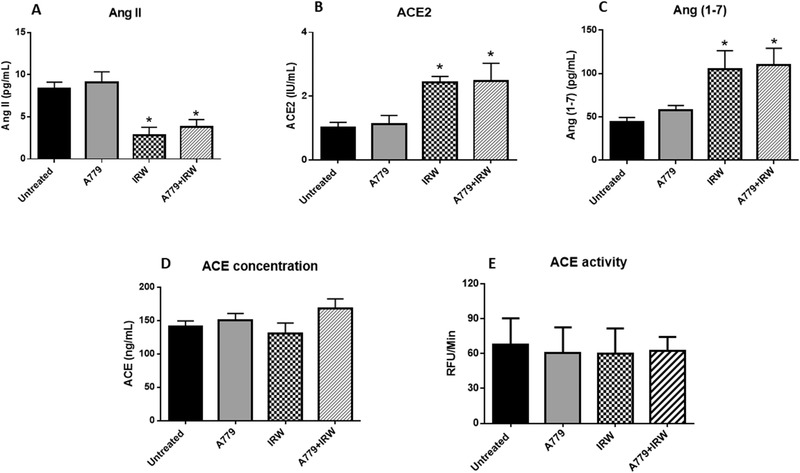
IRW modulates levels of circulating RAS components. Plasma from the rats were collected at the end point to evaluate the levels of circulating A) Ang II, B) ACE2, C) Ang (1‐7), D) ACE concentration, and E) ACE activity. Data represented as mean ± SEM from four to six animals per treatment group. *indicated p < 0.05, as compared with the untreated control.
Expressions of the RAS components in aorta were also examined. IRW showed a significant effect in upregulating expressions of ACE2 and MasR in aorta (Figure 3A,B), but not ACE, AT1R, or AT2R (Figure 3C–E). These results suggested IRW could not only activate the circulating ACE2/Ang (1‐7)/MasR axis, but also upregulate tissue ACE2/MasR expressions.
Figure 3.

IRW upregulates expressions of ACE2 and MasR in aorta of SHRs. Tissues were collected at the end point. Total proteins were extracted from aorta of SHR. Expressions of A) ACE2, B) MasR, C) ACE, D) AT1R, and E) AT2R were normalized to β‐actin. Data represented as mean ± SEM from four to six animals per treatment group.
3.3. Activation of the ACE2/Ang (1‐7)/MasR Axis by IRW Treatment Contributes to Improving Endothelium‐Dependent Vasorelaxation
MCh‐mediated vasodilation is associated with endothelium‐derived hyperpolarizing factor, which hence was used for assessing endothelium‐dependent vasorelaxation.26 As illustrated by Figure 4A, MCh‐mediated vasodilation was enhanced significantly by IRW treatment. However, the effect was abolished by A779 infusion. Akt/eNOS signaling plays a central role in regulating endothelium‐dependent vasodilation.27 It was found that the enhanced phosphorylation of Akt and eNOS in aorta by IRW treatment were mitigated by A779 infusion (Figure 4B,C). The above results indicated IRW activated the ACE2/Ang (1‐7)/MasR axis in SHRs and enhanced Akt/eNOS phosphorylation along with increased endothelium‐dependent vasorelaxation.
Figure 4.
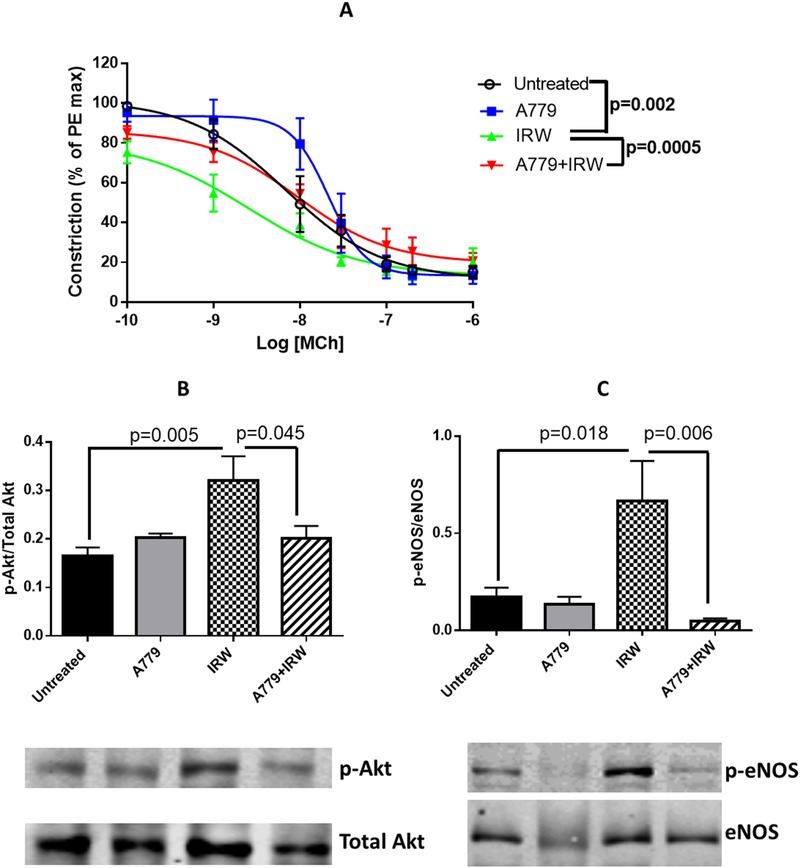
Activation of ACE2/Ang (1‐7)/MasR axis by IRW contributes to improving endothelium‐dependent vasorelaxation. A) Ex vivo cumulative response curve to MCh in isolated mesenteric arteries were performed. Tissues were collected at the end point. Total proteins were extracted from aorta of SHR. Expressions of B) p‐Akt and C) p‐eNOS were normalized to the corresponding total forms. Data represented as mean ± SEM from four to six animals per treatment group.
3.4. Activation of the ACE2/Ang (1‐7)/MasR Axis by IRW Treatment Contributes to Attenuating Vascular Inflammation
As we found in our previous study, amelioration of vascular inflammation was underlying the antihypertensive mechanism of IRW in SHRs.18 Whereas, results from the present study indicated levels of pro‐inflammatory cytokines interleukin 6 (IL‐6) and monocyte chemoattractant protein 1 (MCP1) in circulation were restored with A779 infusion (Figure 5A,B). In addition, upregulation of IκBα by IRW was abrogated and downregulation of COX2 was restored in aorta of SHRs with A779 infusion (Figure 5C,D). Taken together, activation of the ACE2/Ang (1‐7)/MasR axis by IRW treatment was associated with mitigating vascular inflammation in SHRs. Nuclear factor κB (NF‐κB) signaling was involved in the anti‐inflammatory effect of IRW as an ACE2 activator in SHRs.
Figure 5.
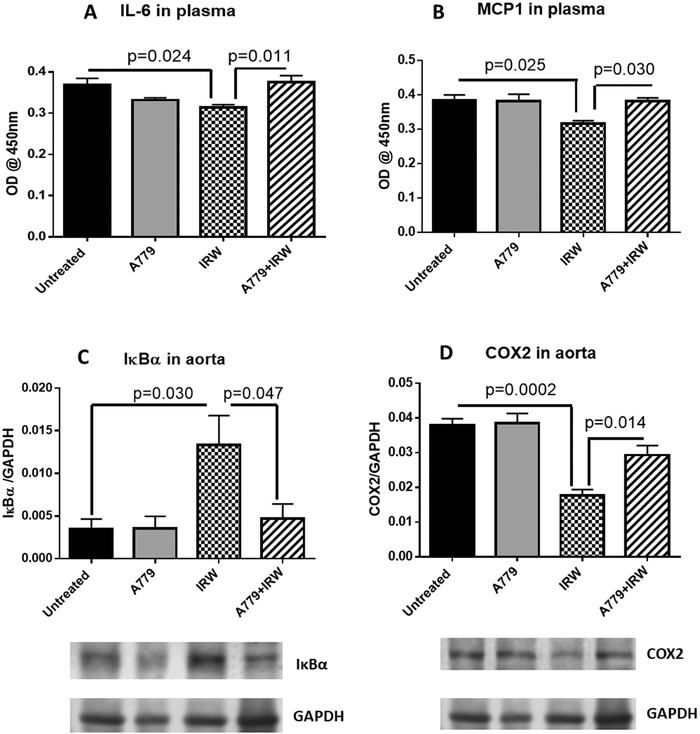
Activation of ACE2/Ang (1‐7)/MasR axis by IRW contributes to attenuating vascular inflammation. Plasma from the rats were collected at the end point to evaluate the levels of circulating A) IL‐6 and B) MCP1. Tissues were collected at the end point. Total proteins were extracted from aorta. Expressions of C) IκBα and D) COX2 were normalized to GAPDH. Data represented as mean ± SEM from four to six animals per treatment group.
3.5. IRW Treatment Did Not Affect ERK1/2 or MMP9 Expression in Aorta of SHRs
Downregulation of MMP9 via deactivating ERK1/2 mitogen‐activated protein kinases (MAPK) signalling is another beneficial effect of increased Ang (1‐7) level, which is further implicated in retarding atherosclerotic plaque formation.28, 29 However, IRW treatment showed no significant effect in regulating phosphorylation of ERK1/2 or expression of MMP9 in aorta of SHRs (Figure 6A,B).
Figure 6.
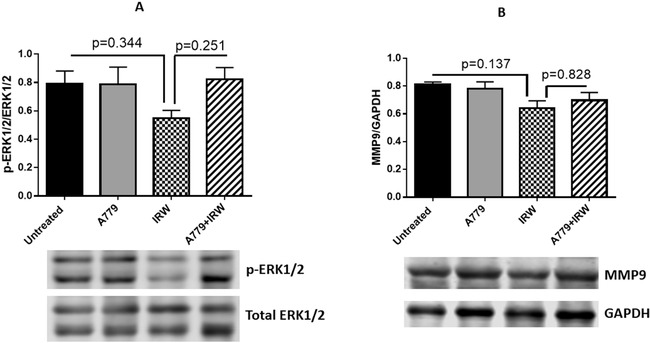
IRW treatment did not affect ERK1/2 MAPK or MMP9 expression in aorta of SHRs. Tissues were collected at the end point. Total proteins were extracted from the aorta of SHR. A) Expression of p‐ERK1/2 was normalized to total ERK1/2; B) expression of MMP9 was normalized to GAPDH. Data represented as mean ± SEM from four to six animals per treatment group.
4. Discussions
In this study, we found that blood pressure‐lowering effect of IRW could be blunt by infusion of MasR antagonist A779 in SHRs, indicating the mechanism of blood pressure‐lowering activity of IRW was via the ACE2/Ang (1‐7)/MasR axis. A direct activation of ACE2 by IRW treatment was evident as levels of circulating ACE2 and Ang (1‐7) were increased, but Ang II level was reduced. The infusion of A779 also abolished the IRW‐enhanced endothelium‐dependent vasorelaxation as well as the IRW‐mediated attenuation of vascular inflammation, suggesting the actions of IRW in vivo as an ACE2 activator were via modulations of endothelial dysfunction and vascular inflammation.
This study demonstrated the predominant role of the ACE2/Ang (1‐7)/MasR axis in mediating the antihypertensive effect of IRW in SHRs. Followed by our previous study, which showed the potential of IRW in activating ACE2 in vitro and in vascular smooth muscle cells,23 results from this study further indicated the in vivo efficacy of IRW as an ACE2 activator. Indeed, xanthenone (XNT), a drug identified from structure‐based screening, was claimed as ACE2 activator, blood pressure‐lowering effect of XNT was validated in SHRs.30 However, antihypertensive effect of XNT could not be achieved in ACE2 knockout mice,31 indicating the variability of the effect of ACE2 activator among different animal models. Thus, to investigate the ACE2‐activating effect of IRW in hypertensive models beyond SHR is expected in the future study.
We also found that the levels of circulating RAS components could be modulated by a 7 day IRW oral administration in SHRs. Specifically, levels of ACE2 and Ang (1‐7) were increased, coupled with a decreased level of Ang II, showing a direct in vivo ACE2‐activating evidence of IRW. As infusion of Ang (1‐7) contributed to mitigating blood pressure in SHRs,32 the reduction of blood pressure observed in this study was thus associated with the modulated circulating RAS. Casein‐derived tripeptide Ile‐Pro‐Pro (IPP) was previously reported with the activity of increasing circulating Ang (1‐7) in SHR,33 but the contribution of the increased Ang (1‐7) to blood pressure‐reducing effect of IPP was unknown. Results from this study reported for the first time about the in vivo ACE2‐activating activity of a food protein–derived bioactive peptide. Interestingly, the circulating ACE activity was unaffected upon IRW treatment, despite IRW was initially characterized as an ACE‐inhibitory peptide.17 The inconsistency between the results from the in vitro ACE‐inhibitory assay and this in vivo study further supported that ACE2 activation is the main mechanism of the observed blood pressure reduction by IRW administration. It was reported previously that ACE‐inhibitory peptide identified based on the in vitro assay might not show ACE‐inhibitory activity in vivo16; our study further reinforce this notion.
In terms of the local RAS components, IRW treatment was also found to upregulate expressions of ACE2 and MasR in aorta of SHRs, which was consistent with our previous finding that IRW upregulated ACE2 expression in aortic vascular smooth muscle cells.23 As overexpression of ACE2 in SHR was reported to reduce blood pressure,12 the upregulated ACE2 by IRW treatment, in addition to activating circulating ACE2, might also contribute to the blood pressure‐lowering effect of the peptide. IRW did not affect the expression levels of ACE, AT1R, or AT2R, further suggesting the distinguished role of the ACE2/Ang (1‐7)/MasR axis in mediating the antihypertensive effect of IRW. In addition, demonstrations of ACE2‐activation and ACE2 upregulation by IRW in SHR from this study suggest that targeting ACE2 represents a novel strategy to identify antihypertensive peptides from various food protein sources.
Further experiments were performed to elucidate the mechanisms of IRW as an ACE2 activator in vivo. Endothelial dysfunction manifests a common feature of hypertension cases.34 Our previous study showed that IRW could not only modulate tumor necrosis factor (TNF)‐stimulated endothelial dysfunction in vascular endothelial cells,19 but also enhance endothelium‐dependent vasorelaxation in SHRs.18 Here, we further found the improvement of endothelial dysfunction in SHRs by IRW treatment was mediated by the ACE2/Ang (1‐7)/MasR axis, which is consistent with the literature documenting that enhancement of endothelium‐dependent vasorelaxation is among one of major beneficial effects of increased Ang (1‐7).35, 36, 37, 38
Amelioration of vascular inflammation is another in vivo mechanism of IRW in reducing blood pressure in SHRs as we reported previously.18 Results from this study supported the relationship between the IRW‐activated ACE2/Ang (1‐7)/MasR axis and reduced vascular inflammation in SHRs. Moreover, such effect was found through NF‐κB signalling. Anti‐inflammatory effect of Ang (1‐7) was reported.39, 40 However, specific targets mediating such effect is unknown. In addition, we found reduced circulating Ang II level in this study; thus, further research elucidating if the ameliorated vascular inflammation is mainly through the increased Ang (1‐7) or decreased Ang II is warranted.
As documented, chronic infusion of Ang (1‐7) resulted in downregulated MMP9 expression via ERK1/2 MAPK signalling, which was implicated in inhibiting atherosclerotic lesion formation and enhancing plaque stability.28, 41 However, the increased Ang (1‐7) by IRW treatment did not show effect in inhibiting ERK1/2 MAPK phosphorylation or downregulating MMP9 expression in aorta of SHRs, which might be due to the short treatment period (7 days) we did in this study. Besides, differential responses among endothelium‐dependent vasorelaxation, vascular inflammation, and MMP9 expression toward the increased Ang (1‐7) by IRW treatment indicated selective roles of IRW as an ACE2 activator in SHRs.
5. Conclusion
IRW reduces blood pressure in SHRs via the ACE2/Ang (1‐7)/MasR axis, demonstrating the role of IRW as an ACE2 activator in vivo; activation of ACE2 contributes to enhanced endothelium‐dependent vasorelaxation and reduced vascular inflammation. Blood pressure‐lowering activity of numerous ACE‐inhibitory peptides is not due to the inhibition of ACE; the establishment of a role of the ACE2/Ang (1‐7)/MasR axis in this study thus sheds new lights into the molecular targets of food protein–derived antihypertensive peptides. While, only male SHR was used in this study, it would be of interest to investigate if there is gender difference, as female SHRs appear to be more dependent on Ang (1‐7) to mediate effects of antihypertensive agents than males.42
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgements
The authors would like to acknowledge Mrs. Sareh Panahi for providing technical support in doing animal surgery. This research was funded by a grant from the Natural Sciences and Engineering Research Council (NSERC) of Canada to J.W. The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript. S.T.D. is a Canada Research Chair in Maternal and Perinatal Cardiovascular Health. W.L. designed and performed the experiments, and wrote the manuscript; H.F. performed the experiments and analyzed the data; S.T.D. and J.W. designed the experiments.
Liao W., Fan H., Davidge S. T., Wu J., Egg White–Derived Antihypertensive Peptide IRW (Ile‐Arg‐Trp) Reduces Blood Pressure in Spontaneously Hypertensive Rats via the ACE2/Ang (1‐7)/Mas Receptor Axis. Mol. Nutr. Food Res. 2019, 63, 1900063 10.1002/mnfr.201900063
The copyright line for this article was changed on 13 May 2019 after original online publication.
References
- 1. Chockalingam A., Campbell N. R., G. Fodor J., Can. J. Cardiol. 2006, 22, 553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Whelton P. K., Carey R. M., Aronow W. S., Casey Jr D. E., Collins K. J., Himmelfarb C. D., S. M. DePalma, Gidding S., Jamerson K. A., Jones D. W., MacLaughlin E. J., Muntner P., Ovbiagele B., Smith S. C. Jr, Spencer C. C., Stafford R. S., Taler S. J., Thomas R. J., Williams K. A. Sr, Williamson J. D., Wright J. T., J. Am. Coll. Cardiol. 2018, 71, e127. [DOI] [PubMed] [Google Scholar]
- 3. Carretero O. A., Oparil S., Circulation 2000, 101, 329. [DOI] [PubMed] [Google Scholar]
- 4. Esteban V., Ruiz‐Ortega M., Hypertension 2001, 38, 1382. [DOI] [PubMed] [Google Scholar]
- 5. Touyz R. M., Schiffrin E. L., Pharmacol. Rev. 2000, 52, 639. [PubMed] [Google Scholar]
- 6. Te Riet L., van Esch J. H. M., Roks A. J. M., van den Meiracker A. H., Jan Danser A. H., Circ. Res. 2015, 116, 960. [DOI] [PubMed] [Google Scholar]
- 7. Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N., Donovan M., Woolf B., Robison K., Jeyaseelan R., Breitbart R. E., and Acton S., Circ. Res. 2000, 87, e1. [DOI] [PubMed] [Google Scholar]
- 8. Tipnis S. R., Hooper N. M., Hyde R., Karran E., Christie G., Turner A. J., J. Biol. Chem. 2000, 275, 33238. [DOI] [PubMed] [Google Scholar]
- 9. Santos R. A. S., e Silva A. C. S., Maric C., Silva D. M. R., Machado R. P., de Buhr I., Heringer‐Walther S., Pinheiro S. V. B., Lopes M. T., Bader M., Mendes E. P., Lemos V. S., Campagnole‐Santos M. J., Schultheiss H.‐P., Speth R., Walther T., Proc. Natl. Acad. Sci. U.S.A. 2003, 100, 8258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Re R. N., Nat. Clin. Pract. Cardiovasc. Med. 2004, 1, 42. [DOI] [PubMed] [Google Scholar]
- 11. Patel V. B., Zhong J. C., Grant M. B., Oudit G. Y., Circ. Res. 2016, 118, 1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yamazato M., Yamazato Y., Sun C., Diez‐Freire C., Raizada M. K., Hypertension, 2007, 49, 926. [DOI] [PubMed] [Google Scholar]
- 13. Zhong J. C., Guo D., Chen C. B., Wang W., Schuster M., Loibner H., Penninger J. M., Scholey J. W., Kassiri Z., Oudit G. Y., Hypertension, 2011, 57, 314. [DOI] [PubMed] [Google Scholar]
- 14. Ferreira A. J., Santos R. A. S., Bradford C. N., Mecca A. P., Sumners C., Katovich M. J., Raizada M. K., Hypertension 2010, 55, 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Torruco‐Uco J. G., Dominguez‐Magana M., Davila‐Ortiz G., Martinez‐Ayala A., Chel‐Guerrero L. A., Betancur‐Ancona D. A., Cienc. Tecnol. Aliment. 2008, 6, 158. [Google Scholar]
- 16. Wu J., Liao W., Udenigwe C. C., Trends Food Sci. Technol. 2017, 69, 214. [Google Scholar]
- 17. Majumder K., Wu J., Food Chem. 2011, 126, 1614. [DOI] [PubMed] [Google Scholar]
- 18. Majumder K., Chakrabarti S., Morton J. S., Panahi S., Kaufman S., Davidge S. T., Wu J., PLoS One 2013, 8, e82829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Majumder K., Chakrabarti S., Davidge S. T., Wu J., J. Agric. Food Chem. 2013, 61, 2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liao W., Chakrabarti S., Davidge S. T., Wu J., J. Agric. Food Chem. 2016, 64, 7342. [DOI] [PubMed] [Google Scholar]
- 21. Liao W., Fan H., Wu J., J. Agric. Food Chem. 2018, 66, 5133. [DOI] [PubMed] [Google Scholar]
- 22. Majumder K., Liang G., Chen Y., Guan L. L., Davidge S. T., Wu J., Mol. Nutr. Food Res. 2015, 59, 1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liao W., Bhullar K. S., Chakrabarti S., Davidge S. T., Wu J., J. Agric. Food Chem. 2018, 66, 11330. [DOI] [PubMed] [Google Scholar]
- 24. Sullivan J. C., Bhatia K., Yamamoto T., Elmarakby A. A., Hypertension 2010, 56, 658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reagan‐Shaw S., Nihal M., Ahmad N., FASEB J. 2008, 22, 659. [DOI] [PubMed] [Google Scholar]
- 26. Morton J. S., Reuda‐Clausen C. F., Davidge S. T., Am. J. Physiol.: Regul., Integr. Comp. Physiol. 2010, 298, R930. [DOI] [PubMed] [Google Scholar]
- 27. Shiojima I., Walsh K., Circ. Res. 2002, 90, 1243. [DOI] [PubMed] [Google Scholar]
- 28. Yang J. M., Dong M., Meng X., X. Zhao Y., Yang X. Y., Liu X. L., Hao P. P., Li J. J., Wang X. P., Zhang K., Gao F., Zhao X. Q., Zhang M. X., Zhang Y., Zhang C., Arterioscler., Thromb., Vasc. Biol. 2013, 33, 1978. [DOI] [PubMed] [Google Scholar]
- 29. R. Prestes T. R., Rocha N. P., Miranda A. S., Teixeira A. L., Simoes‐e Silva A. C., Curr. Drug Targets 2017, 18, 1301. [DOI] [PubMed] [Google Scholar]
- 30. Hernández Prada J. A., Ferreira A. J., Katovich M. J., Shenoy V., Qi Y., Santos R. A., Castellano R. K., Lampkins A. J., Gubala V., Ostrov D. A., Raizada M. K., Hypertension 2008, 51, 1312. [DOI] [PubMed] [Google Scholar]
- 31. Haber P. K., Ye M., Wysocki J., Maier C., Haque S. K., Battle D., Hypertension 2014, 63, 774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Benter I. F., Ferrario C. M., Morris M., Diz D. I., Am. J. Physiol.: Heart Circ. Physiol. 1995, 269, H313. [DOI] [PubMed] [Google Scholar]
- 33. Ehlers P. I., Nurmi L., Turpeinen A. M., Korpela R., Vapaatalo H., Life Sci. 2011, 88, 206. [DOI] [PubMed] [Google Scholar]
- 34. Giannotti G., Landmesser U., Herz Kardiovaskuläre Erkrankungen 2007, 32, 568. [DOI] [PubMed] [Google Scholar]
- 35. Lemos V. S., Silva D. M. R., Walther T., Alenina N., Bader M., Santos R., J. Cardiovasc. Pharmacol. 2005, 46, 274. [DOI] [PubMed] [Google Scholar]
- 36. Faria‐Silva R., Duarte F. V., Santos R. A. S., Hypertension 2005, 46, 948. [DOI] [PubMed] [Google Scholar]
- 37. Li G., Zhang H., Zhao L., Zhang Y., Yan D., Liu Y., J. Am. Soc. Hypertens. 2017, 11, 842. [DOI] [PubMed] [Google Scholar]
- 38. Sampaio W. O., dos Santos R. A., Faria‐Silva R., da Mata Machado L. T., Schiffrin E. L., Touyz R. M., Hypertension 2007, 49, 185. [DOI] [PubMed] [Google Scholar]
- 39. Simões e Silva A. C., Teixeira M. M., Pharmacol. Res. 2016, 107, 154. [DOI] [PubMed] [Google Scholar]
- 40. Simões e Silva A. C., Silveira K. D., Ferreira A. J., Teixeira M. M., Br. J. Pharmacol. 2013, 169, 477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dong B., Zhang C., Feng J. B., Zhao Y. X., Li S. Y., Yang Y. P., Dong Q. L., Deng B. P., Zhu L., Yu Q. T., Liu C. X., Liu B., Pan C. M., Song H. D., Zhang M. X., Zhang Y., Arterioscler., Thromb., Vasc. Biol. 2008, 28, 1270. [DOI] [PubMed] [Google Scholar]
- 42. Zimmerman M. A., Harris R. A., Sullivan J. C., Am. J. Physiol. Renal Physiol. 2014, 306, F1136. [DOI] [PMC free article] [PubMed] [Google Scholar]


