Abstract
Background
Patients with a pathological complete response (pCR) after neoadjuvant chemoradiotherapy (nCRT) for oesophageal cancer may benefit from non‐surgical management. The aim of this study was to determine the diagnostic performance of visual response assessment of the primary tumour after nCRT on T2‐weighted (T2W) and diffusion‐weighted (DW) MRI.
Methods
Patients with locally advanced oesophageal cancer who underwent T2W‐ and DW‐MRI (1·5 T) before and after nCRT in two hospitals, between July 2013 and September 2017, were included in this prospective study. Three radiologists evaluated T2W images retrospectively using a five‐point score for the assessment of residual tumour in a blinded manner and immediately rescored after adding DW‐MRI. Histopathology of the resection specimen was used as the reference standard; ypT0 represented a pCR. Sensitivity, specificity, area under the receiver operating characteristic (ROC) curve (AUC) and interobserver agreement were calculated.
Results
Twelve of 51 patients (24 per cent) had a pCR. The sensitivity and specificity of T2W‐MRI for detection of residual tumour ranged from 90 to 100 and 8 to 25 per cent respectively. Respective values for T2W + DW‐MRI were 90–97 and 42–50 per cent. AUCs for the three readers were 0·65, 0·66 and 0·68 on T2W‐MRI, and 0·71, 0·70 and 0·70 on T2W + DW‐MRI (P = 0·441, P = 0·611 and P = 0·828 for readers 1, 2 and 3 respectively). The κ value for interobserver agreement improved from 0·24–0·55 on T2W‐MRI to 0·55–0·71 with DW‐MRI.
Conclusion
Preoperative assessment of residual tumour on MRI after nCRT for oesophageal cancer is feasible with high sensitivity, reflecting a low chance of missing residual tumour. However, the specificity was low; this results in overstaging of complete responders as having residual tumour and, consequently, overtreatment.
Introduction
A standard therapy with curative intent for patients with locally advanced oesophageal cancer consists of neoadjuvant chemoradiotherapy (nCRT) followed by surgery. nCRT improves survival compared with surgery alone (5‐year survival rate 47 versus 33 per cent respectively)1. In 25–30 per cent of patients with oesophageal cancer, the resection specimen shows no residual tumour cells (ypT0) after nCRT2, 3, also known as a pathological complete response (pCR). Patients with a pCR have an excellent prognosis, with a 5‐year recurrence‐free survival rate of 62 per cent4, 5, which is better than that of patients with vital tumour cells in the resection specimen (no pCR). The high postoperative morbidity and mortality rates after oesophagectomy raise the question of whether non‐surgical management (watch and wait) is a safe alternative treatment option in patients with oesophageal cancer who have a clinical complete response (cCR)6.
To implement non‐surgical treatment for advanced oesophageal cancer it is critical to accurately identify pCR. CT and fluorodeoxyglucose (FDG) PET/CT are both inaccurate in discriminating residual disease from pCR owing to the presence of wall thickening and/or radiation oesophagitis7, 8, 9, 10, 11, 12, 13. The same is true for endoscopic ultrasonography (EUS), which has an accuracy of only 36 per cent for ypT determination14, 15. Endoscopic response evaluation after nCRT is hampered by the fact that it provides information only on the luminal side of the oesophagus16, whereas residual cancer cells are located beneath the mucosal layer in a subset of patients17, 18. Even deeper bite‐on‐bite biopsies combined with EUS and fine‐needle aspiration of suspicious lymph nodes yielded a negative predictive value (NPV) of only 45 per cent for detecting tumours with a tumour regression grade (TRG) of 2 or higher11. Previous retrospective cohort studies19, 20, 21, 22 reported on patients with a cCR after neoadjuvant treatment who did not have surgery but underwent serial response assessments including endoscopy, EUS, CT and/or FDG‐PET/CT. Among those assessed as having a cCR who did undergo oesophagectomy, residual tumour was found in 28–33 per cent of patients19, 20, 21. In a propensity‐based matching study22, after a median follow‐up of 51·1 months, local recurrence had developed more frequently among patients with a cCR who underwent a watch‐and‐wait approach than in those who had surgical treatment.
The limitations of the current response assessment tools warrant investigation of other imaging techniques. In rectal cancer, MRI can aid in the diagnosis of a cCR after nCRT23, 24. Although MRI of the oesophagus is technically more challenging, owing to oesophageal motility and motion of the surrounding heart and diaphragm, advances in technology now enable the acquisition of high‐resolution magnetic resonance (MR) images. Previous studies25, 26, 27, 28, 29 on response prediction with MRI in oesophageal cancer focused on quantitative diffusion‐weighted (DW) MRI parameters. However, visual response assessment on T2‐weighted (T2W) and functional DW‐MRI in rectal cancer yielded higher sensitivity for detecting residual tumour compared with quantitative assessment30. The performance of visual response assessment on MRI in oesophageal cancer is as yet unknown. The aim of this study was to determine the diagnostic performance of visual response assessment of the primary oesophageal tumour after nCRT on T2W‐MRI and functional DW‐MRI.
Methods
Patients diagnosed with locally advanced oesophageal cancer were enrolled prospectively and data were analysed retrospectively. The study was approved by the local medical ethics committees and registered at ClinicalTrials.gov (NCT02139488 and NCT02125448). Written informed consent to participate was obtained from the patients. Patients underwent MRI before and after nCRT between July 2013 and September 2017. Inclusion criteria were: biopsy‐proven locally advanced, non‐metastatic oesophageal cancer; 5 weeks of nCRT (total of 41·4 Gy in 23 fractions, with weekly administration of carboplatin and paclitaxel) followed by oesophagectomy; and maximum of 21 days between preoperative MRI and surgery. Patients were excluded if MRI quality was judged insufficient by at least two radiologists. Causes of insufficient image quality were severe motion artefacts leading to blurred T2W images, and lack of, or only slight, diffusion restriction in the spleen as a surrogate marker of inadequate DW images.
Image acquisition
Imaging was performed on a 1·5‐T MRI scanner (Achieva or Ingenia; Koninklijke Philips, Best, the Netherlands), using Torso‐XL (16 channel) or anterior/posterior (28 channel) receiver coils respectively (supplied by Koninklijke Philips). The MRI protocol consisted of T2W multislice turbo spin‐echo sequences in transverse (slice thickness 4 mm) and sagittal (slice thickness 3 mm) planes. A respiratory navigator was positioned on the diaphragm, and to reduce motion artefacts, images were acquired only during expiration31. A DW echo‐planar imaging sequence was acquired in the transverse direction with b = 0, b = 200 and b = 800 s/mm2, and a slice thickness of 4 mm. The transverse T2W and DW sequences were angled in identical planes. Detailed MRI sequence parameters are provided in Table S1 (supporting information).
Image evaluation
Images were analysed independently by three expert radiologists, who were blinded to tumour characteristics (location, histology, TNM stage32) and clinical outcomes. The radiologists first judged images of the primary tumour area acquired by T2W‐MRI and DW‐MRI before nCRT. They then scored the images obtained by T2W‐MRI after nCRT for the likelihood of residual tumour. Finally, still in the same reading session, DW‐MR images of b = 800 s/mm2 were added and the scoring was repeated. A five‐point confidence level score (CLS) was used, derived from previous studies in rectal cancer (CLS1, definitely complete response; 2, probably complete response; 3, inconclusive; 4, probably residual tumour; 5, definitely residual tumour)23.
MRI criteria
A complete response of the primary tumour on T2W‐MRI was defined by a normalized oesophageal wall or only a thin hypointense signal (indicating fibrosis) without distortion of the wall; on DW‐MRI, a complete response of the primary tumour was defined by the absence of high signal on images of b = 800 s/mm2 in the irradiated tumour bed. On T2W‐MRI, a residual mass with persistent isointense signal or the presence of mixed hyperintense and hypointense signals within the tumour bed were considered signs of residual tumour; on DW‐MRI, the presence of high signal within the tumour bed indicated residual tumour. These criteria are illustrated in Figs 1 and 2.
Figure 1.
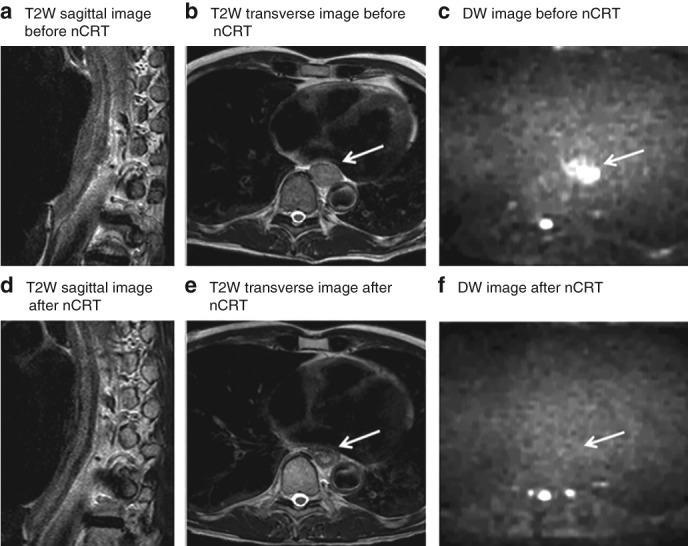
MRI of a patient with locally advanced oesophageal cancer that showed a pathological complete response to neoadjuvant chemoradiotherapy Images from a 55‐year‐old man with a cT3N0 lower oesophageal squamous cell carcinoma, and a complete pathological response after neoadjuvant chemoradiotherapy (nCRT) and oesophagectomy (tumour regression grade 1, ypT0 N0). a–c T2‐weighted (T2W) sagittal (a) and transverse (b) images before chemoradiotherapy show a hyperintense oesophageal wall, accompanied by a hyperintense signal on diffusion‐weighted (DW) imaging (c). d–f T2W sagittal (d) and transverse (e) images after nCRT show a hypointense oesophageal wall, indicating fibrosis; no high signal remained on the corresponding DW image (f). Arrows mark (initial) tumour location.
Figure 2.
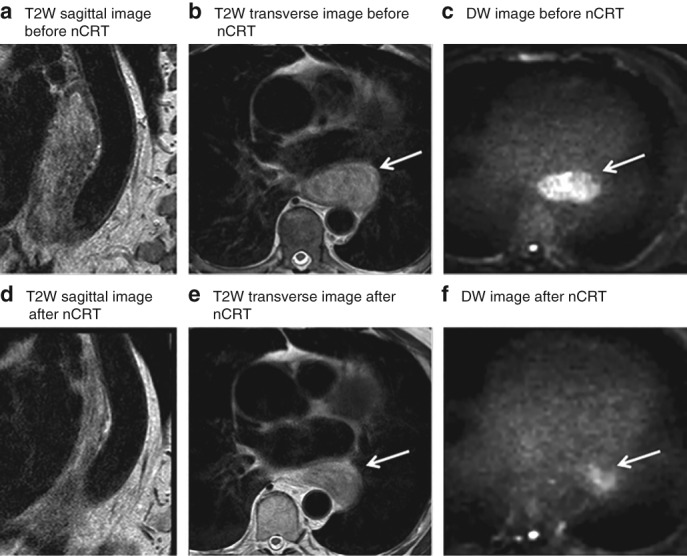
MRI of a patient with locally advanced oesophageal cancer that showed pathological residual tumour after chemoradiotherapy and surgery Images from a 78‐year‐old man with a cT2 N0 lower oesophageal adenocarcinoma, who had residual tumour after neoadjuvant chemoradiotherapy (nCRT) and oesophagectomy (tumour regression grade 5, ypT2 N0). T2‐weighted (T2W) sagittal (a,d) and transverse (b,e) images before (a,b) and after (d,e) nCRT both show a hyperintense oesophageal wall. The corresponding b = 800 diffusion‐weighted (DW) images before (c) and after (f) nCRT demonstrate a clear hyperintense signal, highly suspicious for tumour. Arrows mark tumour location.
Reference standard
Histopathological examination of the resection specimen was performed by dedicated gastrointestinal pathologists at the two centres. The resection specimen was evaluated in accordance with the seventh edition of the UICC protocol for ypTNM classification32. The TRG of the resected primary tumour was assessed according to Mandard and colleagues3. The tumour bed was embedded completely for histopathological analysis. A pCR was defined as ypT0 (TRG 1) and residual tumour as ypT1–4 (TRG 2–5).
Statistical analysis
Before undertaking the analyses, dichotomization between CLS2 (probably complete response) and CLS3 (inconclusive) was decided as the cut‐off, to minimize the risk of missing residual disease. Receiver operating characteristic (ROC) curves were constructed and areas under the curve (AUCs) calculated to evaluate the performance of the three radiologists on T2W‐MRI only and on T2W + DW‐MRI, with histologically confirmed residual tumour as the positive outcome. Sensitivities, specificities, positive predictive values (PPVs) and NPVs were calculated with 95 per cent confidence intervals. AUCs on T2W‐MRI and T2W + DW‐MRI were compared by means of the DeLong test33. P < 0·050 was considered statistically significant. The possible change in the number of uncertainties was analysed by comparing the number of equivocal scores (CLS3). Interobserver agreement between radiologists was calculated using quadratic weighted κ values (0–0·20, poor; 0·21–0·40, fair; 0·41–0·60, moderate; 0·61–0·80, good; and 0·81–1·00 excellent agreement)34. Statistical analyses were done using SPSS® version 22 (IBM, Armonk, New York, USA) and Stata® version 11 (StataCorp, College Station, Texas, USA).
Results
Six of 57 patients were excluded: four had insufficient MRI quality, one patient had distant metastases after completion of nCRT and therefore did not undergo surgical resection, and one patient was deemed to have unresectable disease at surgical exploration. Therefore, 51 patients were evaluated (Fig. 3). Of the 51 included patients, 42 (82 per cent) were diagnosed with adenocarcinoma and nine (18 per cent) with squamous cell carcinoma. Table 1 shows baseline patient and tumour characteristics at both institutes that participated in the study. Transhiatal oesophagectomy was performed in 24 patients (47 per cent) and transthoracic oesophagectomy in 27 (53 per cent), followed by gastric conduit reconstruction with cervical anastomosis in all patients. The median interval between the last radiation fraction and oesophagectomy was 59 (range 24–75) days. It was 47 (17–65) days between the last radiation fraction and MRI, and 11 (4–21) days from post‐nCRT MRI until oesophagectomy.
Figure 3.
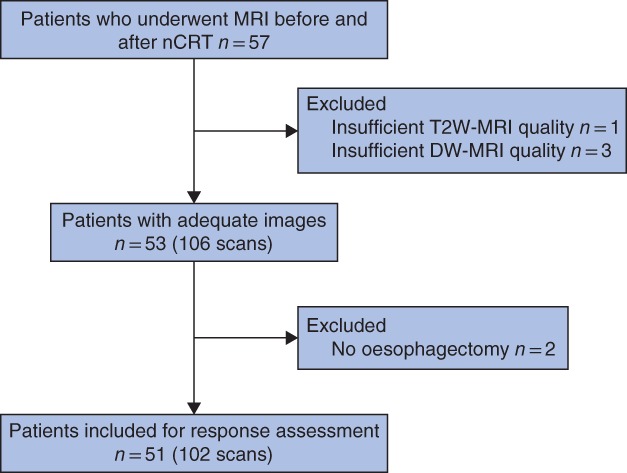
Study flow chart nCRT, neoadjuvant chemoradiotherapy; T2W, T2‐weighted; DW, diffusion‐weighted.
Table 1.
Patient and tumour characteristics
| Hospital 1 (n = 32) | Hospital 2 (n = 19) | Total (n = 51) | |
|---|---|---|---|
| Age (years) * | 64 (53–72) | 64 (60–68) | 64 (56–72) |
| Sex ratio (M : F) | 23 : 9 | 16 : 3 | 39 : 12 |
| Tumour location | |||
| Upper oesophageal | 0 | 1 | 1 (2) |
| Middle oesophageal | 3 | 3 | 6 (12) |
| Lower oesophageal | 15 | 11 | 26 (51) |
| Gastro‐oesophageal junction | 14 | 4 | 18 (35) |
| Histological tumour type | |||
| Adenocarcinoma | 28 | 14 | 42 (82) |
| Squamous cell carcinoma | 4 | 5 | 9 (18) |
| Grade of differentiation | |||
| Well differentiated | 2 | 0 | 2 (4) |
| Moderately differentiated | 15 | 10 | 25 (49) |
| Poorly differentiated | 14 | 3 | 17 (33) |
| Undifferentiated | 0 | 2 | 2 (4) |
| Unknown | 1 | 4 | 5 (10) |
| Clinical T category † | |||
| cT1 | 1 | 0 | 1 (2) |
| cT2 | 8 | 4 | 12 (24) |
| cT3 | 23 | 14 | 37 (73) |
| cT4a | 0 | 1 | 1 (2) |
| Clinical N category † | |||
| cN0 | 15 | 4 | 19 (37) |
| cN1 | 6 | 8 | 14 (27) |
| cN2 | 9 | 7 | 16 (31) |
| cN3 | 2 | 0 | 2 (4) |
| Radicality of resection | |||
| R0 | 32 | 18 | 50 (98) |
| R1 | 0 | 1 | 1 (2) |
| Mandard grade | |||
| TRG 1 | 6 | 6 | 12 (24) |
| TRG 2 | 10 | 7 | 17 (33) |
| TRG 3 | 12 | 4 | 16 (31) |
| TRG 4 | 3 | 1 | 4 (8) |
| TRG 5 | 1 | 1 | 2 (4) |
Values in parentheses are percentages unless indicated otherwise;
values are median (i.q.r.).
According to the seventh edition of the TNM classification32. Hospital 1, Netherlands Cancer Institute; hospital 2, University Medical Center Utrecht. TRG, tumour regression grade.
On histopathological assessment, a pCR of the primary tumour (ypT0, TRG 1) was found in 12 of 51 patients (24 per cent). In one of these patients, the disease was confirmed as ypT0N1, which was regarded a complete response of the primary tumour area (ypT0) in this study. The pCR rate was five of nine (56 per cent) for squamous cell carcinomas and seven of 42 (17 per cent) for adenocarcinomas. The remaining 39 patients had residual tumour, which was graded as TRG 2 in 17 of 51 patients (33 per cent), TRG 3 in 16 (31 per cent), TRG 4 in four (8 per cent) and TRG 5 in two patients (4 per cent).
Diagnostic performance
ROC curves for the assessment of residual tumour after nCRT are shown in Fig. 4. AUCs on T2W‐MRI were 0·65 for reader 1, 0·66 for reader 2 and 0·68 for reader 3. After addition of DW‐MRI, AUCs were 0·71, 0·70 and 0·70 respectively (P = 0·441, P = 0·611 and P = 0·828). The sensitivity for detection of residual tumour ranged from 90 to 100 per cent on T2W‐MRI alone, and from 90 to 97 per cent after addition of DW‐MRI. Specificity ranged from 8 to 25 per cent on T2W‐MRI alone, and from 42 to 50 per cent after addition of DW‐MRI (Table 2). The numbers of MRI‐positive and ‐negative tests per TRG stage for response assessment on T2W + DW‐MRI are shown in Table 3.
Figure 4.
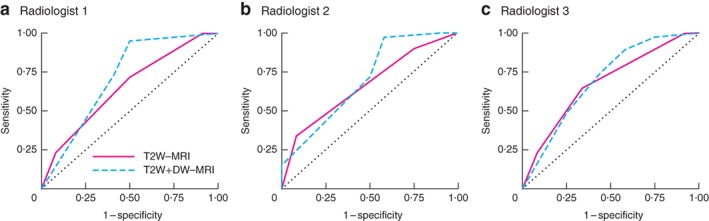
Receiver operating characteristic curves for assessment of residual tumour after neoadjuvant chemoradiotherapy using T2‐weighted MRI and T2‐weighted combined with diffusion‐weighted MRI a Reader 1, b reader 2 and c reader 3. T2W, T2‐weighted; DW, diffusion‐weighted. Comparison of areas under the curve for T2W‐MRI versus T2W + DW‐MRI: a P = 0·441, b P = 0·611, c P = 0·828 (DeLong test33).
Table 2.
Diagnostic performance for assessment of residual tumour
| T2W‐MRI | T2W + DW‐MRI | |||||
|---|---|---|---|---|---|---|
| Reader 1 | Reader 2 | Reader 3 | Reader 1 | Reader 2 | Reader3 | |
| Sensitivity (%) | 100 | 90 | 90 | 95 | 97 | 90 |
| (89, 100) | (75, 97) | (75, 97) | (81, 99) | (85, 100) | (75, 97) | |
| Specificity (%) | 8 | 25 | 25 | 50 | 42 | 42 |
| (0, 40) | (7, 57) | (7, 57) | (22, 78) | (17, 71) | (17, 71) | |
| PPV (%) | 78 | 80 | 80 | 86 | 84 | 83 |
| (64, 88) | (64, 90) | (64, 90) | (71, 94) | (70, 93) | (68, 92) | |
| NPV (%) | 100 | 43 | 43 | 75 | 83 | 56 |
| (6, 100) | (12, 80) | (12, 80) | (36, 96) | (37, 99) | (23, 85) | |
| True‐positive | 39 | 35 | 35 | 37 | 38 | 35 |
| False‐positive | 11 | 9 | 9 | 6 | 7 | 7 |
| True‐negative | 1 | 3 | 3 | 6 | 5 | 5 |
| False‐negative | 0 | 4 | 4 | 2 | 1 | 4 |
| Accuracy (%) | 78 | 75 | 75 | 84 | 84 | 78 |
| AUC* | 0·65 | 0·66 | 0·68 | 0·71 | 0·70 | 0·70 |
| (0·47, 0·83) | (0·49, 0·83) | (0·51, 0·86) | (0·52, 0·90) | (0·52, 0·88) | (0·51, 0·88) | |
Values in parentheses are 95 per cent confidence intervals. Residual tumour (tumour regression grade 2–5 in resected primary tumour) was considered the positive outcome. T2W, T2‐weighted; DW, diffusion‐weighted; PPV, positive predictive value; NPV, negative predictive value; AUC, area under the receiver operating characteristic curve.
Comparison of T2W‐MRI versus T2W + DW‐MRI: P = 0·441, P = 0·611 and P = 0·828 for readers 1, 2 and 3 respectively (DeLong test33).
Table 3.
Number of test‐positive and test‐negative patients according to tumour regression grade for response assessment on T2‐weighted combined with diffusion‐weighted MRI
| Mandard grade | No. of patients | |||||
|---|---|---|---|---|---|---|
| Reader 1 | Reader 2 | Reader 3 | ||||
| MRI‐positive | MRI‐negative | MRI‐positive | MRI‐negative | MRI‐positive | MRI‐negative | |
| TRG 1 | 6 | 6 | 7 | 5 | 7 | 5 |
| TRG 2 | 16 | 1 | 16 | 1 | 15 | 2 |
| TRG 3 | 15 | 1 | 16 | 0 | 14 | 2 |
| TRG 4 | 4 | 0 | 4 | 0 | 4 | 0 |
| TRG 5 | 2 | 0 | 2 | 0 | 2 | 0 |
TRG, tumour regression grade according to Mandard and colleagues3; MRI‐positive, clinical residual tumour; MRI‐negative, clinical complete response.
Equivocal (confidence level 3) scores
Readers 1, 2 and 3 assigned 16, 30 and 15 equivocal scores (CLS3) respectively on T2W‐MRI, which decreased to 9, 11 and 9 equivocal scores after the addition of DW‐MRI. Fig. 5 shows an example of a tumour for which all readers assigned an equivocal score on T2W‐MRI, whereas a correct diagnosis of residual tumour (CLS4 for all 3 readers) was made after addition of DW‐MRI.
Figure 5.
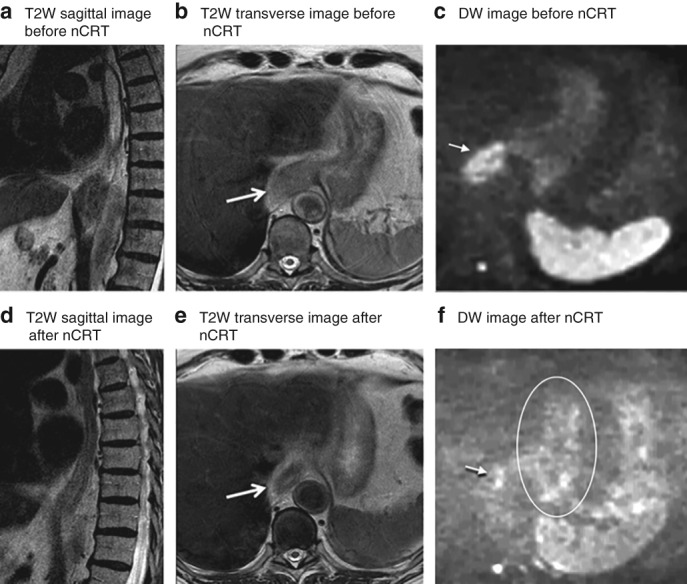
MRI of a patient with locally advanced oesophageal cancer located at the gastro‐oesophageal junction Images from an 80‐year‐old man with a cT3 N0 squamous cell carcinoma located at the gastro‐oesophageal junction. Histopathology after oesophagectomy showed residual tumour (tumour regression grade 2, ypT1a N0). a–c T2‐weighted (T2W) sagittal (a) and transverse (b) images before neoadjuvant chemoradiotherapy (nCRT) show a thick hyperintense wall, accompanied by a hyperintense signal on diffusion‐weighted (DW) imaging (c). d–f After nCRT, the T2W images (d,e) show shrinkage of the wall with a mixed hyperintense and hypointense signal, which was assigned a confidence level score of 3 by all readers. The DW image (f) shows spots of hyperintense signal in the primary tumour area (arrow), which is suspicious for residual tumour and was therefore assigned a confidence level score of 4 by all readers. The area within the circle indicates normal stomach wall, which also shows small hyperintense areas on DW imaging. Arrows indicate tumour location.
Interobserver agreement
Interobserver agreement was fair to moderate on T2W‐MRI alone (quadratic weighted κ = 0·24, 0·55 and 0·41), and increased to moderate to good on T2W + DW‐MRI (quadratic weighted κ = 0·55, 0·71 and 0·61).
Discussion
This study has shown that preoperative response assessment after nCRT for oesophageal cancer performed visually on (DW‐)MRI has promising overall diagnostic performance, with AUCs in the range 0·65–0·71. The sensitivity of DW‐MRI for detection of residual tumour was high (over 90 per cent), indicating that the chance of missing residual tumour was small. Addition of images obtained by functional DW‐MRI to the anatomical T2W‐MRI protocol did not influence the overall diagnostic performance to a great extent, but had a positive impact on the specificity and NPV for most readers. Moreover, addition of DW sequences led to improved interobserver agreement and a reduction in the number of equivocal scores, indicating increased confidence of the readers. MRI showed promising visualization of the primary oesophageal tumour bed after nCRT in oesophageal cancer and could thereby improve current response assessment strategies. The main drawback was the poor specificity of MRI in this unimodal approach, which in clinical practice would result in overstaging of complete responders as having residual tumour and, consequently, overtreatment. Therefore, exploration of response assessment including MRI, but also other diagnostic modalities, after nCRT for locally advanced oesophageal cancer is warranted.
The present study evaluated oesophageal MRI for the assessment of complete response of the primary tumour by visual interpretation of morphology on T2W‐MRI and restrictive signals on DW‐MRI. Previous studies26, 27, 29 on this subject did not perform visual assessment, but focused on quantitative DW‐MRI. These studies found that an increase in the apparent diffusion coefficient (ADC, a quantitative measure of the magnitude of diffusion) during nCRT, compared with before nCRT, is a predictor of response. Furthermore, an increase in ADC after nCRT compared with the baseline value seemed to be predictive of gross tumour response, defined as TRG 1–228, 29 or TRG 1–325. However, the results reported for the preoperative selection of complete response (TRG 1 only) using ADC values were poor26, 29. In contrast, in the present study, preoperative visual response assessment on DW‐MRI after nCRT had high sensitivity for the detection of residual tumour. Moreover, the cut‐off was predefined and can therefore be used prospectively, whereas in the aforementioned studies exploring ADC values, the optimal cut‐offs were defined retrospectively which limits their use.
A recent study11 of clinical assessment with endoscopy/EUS, in which bite‐on‐bite biopsies and fine‐needle aspirates were obtained after nCRT, yielded a specificity of 72 per cent for detection of residual tumour, which is higher than the specificity in the present study. However, the reported sensitivity of 77 per cent was lower than values of over 90 per cent in the present study. Combined with clinical examination and endoscopy, (DW‐)MRI has led to the safe selection of patients with rectal tumours for a watch‐and‐wait policy after nCRT35. Combining (DW‐)MRI and endoscopy/EUS will potentially result in accurate assessment of pCR after nCRT for oesophageal cancer without missing residual disease.
The specificity for detection of residual tumour improved from 8–25 to 42–50 per cent after adding DW‐MRI in the present study. This specificity is, however, still low. One potential explanation for the overstaging of a pCR as residual tumour is the occurrence of small punctate foci of hyperintensity at the former tumour bed on images obtained with b = 800 DW‐MRI (implying restricted diffusion, which raises the suspicion of tumour). These false‐positive foci were observed in patients with a tumour of the gastro‐oesophageal junction and may be explained by the fact that the normal stomach wall also shows small hyperintensities on DW‐MRI, or by the presence of radiation‐induced inflammation. Prolonging the interval between the end of radiotherapy and MRI may result in resolution of inflammation. Furthermore, prolonging the interval between radiation and surgery may lead to an increase in pCR rates. A recent analysis in oesophageal cancer36 showed that a longer interval between nCRT and surgery increased pCR rates, without increasing the frequency of postoperative complications.
This study had a relatively large sample size compared with previous response studies in oesophageal cancer; however, validation in a larger cohort is required. This will also allow subgroup analyses of squamous cell carcinoma and adenocarcinoma. Another potential limitation is that ADC maps were not included in the response evaluation, although the readers could always refer to the T2W images to rule out, for example, shine‐through effects caused by fluid in the oesophageal lumen. Furthermore, lymph node response was not assessed for two reasons. The differentiation between benign and malignant lymph nodes on oesophageal T2W‐MRI remains challenging as non‐enlarged nodes may harbour malignant cells, whereas reactive (benign) nodes may be enlarged37, 38. DW‐MRI can detect lymph nodes, but all lymph nodes have a high signal on DW images. In patients with T0 N1 rectal cancer, MRI showed poor performance for detection of lymphadenopathy39. Second, the field of view (FOV) of MRI in this study focused on the primary tumour and did not comprise the complete craniocaudal perioesophageal area owing to imaging time restrictions. Hence, lymph nodes outside this FOV could not be assessed. Therefore, this study focused on tumour detection at the primary tumour bed only (TRG 1, ypT0). Other response assessment tools are needed for the detection of lymph node metastases after nCRT, such as EUS.
To overcome the limitations of the present study and further increase specificity without decreasing sensitivity for the preoperative detection of residual tumour in oesophageal cancer, larger studies are needed. The multicentre observational PRIDE (Preoperative Image‐guided Identification of Response to Neoadjuvant Chemoradiotherapy in Esophageal Cancer) study40 was initiated to explore the combination of multiple diagnostic modalities in assessing the response of the primary tumour and lymph nodes to chemoradiotherapy. This study aims to develop an optimal multimodal response prediction model focusing on clinical (endoscopy and EUS) and radiological (MRI and FDG‐PET/CT) assessment combined with patient‐specific parameters (such as circulating tumour DNA) for oesophageal cancer.
Supporting information
Table S1 MRI sequence parameters
Acknowledgements
S.E.V. and F.E.M.V. contributed equally to this article. The study was registered with ClinicalTrials.gov (NCT02139488 and NCT02125448).
Disclosure: The authors declare no conflict of interest.
References
- 1. Shapiro J, van Lanschot JJB, Hulshof MCCM, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL et al; CROSS study group. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long‐term results of a randomised controlled trial. Lancet Oncol 2015; 16: 1090–1098. [DOI] [PubMed] [Google Scholar]
- 2. van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP et al; CROSS Group. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012; 366: 2074–2084. [DOI] [PubMed] [Google Scholar]
- 3. Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry‐Amar M, Petiot JF et al Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 1994; 73: 2680–2686. [DOI] [PubMed] [Google Scholar]
- 4. Berger AC, Farma J, Scott WJ, Freedman G, Weiner L, Cheng JD et al Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated with significantly improved survival. J Clin Oncol 2005; 23: 4330–4337. [DOI] [PubMed] [Google Scholar]
- 5. Donahue JM, Nichols FC, Li Z, Schomas DA, Allen MS, Cassivi SD et al Complete pathologic response after neoadjuvant chemoradiotherapy for esophageal cancer is associated with enhanced survival. Ann Thorac Surg 2009; 87: 392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van der Wilk BJ, Eyck BM, Spaander MCW, Valkema R, Lagarde SM, Wijnhoven BPL et al Towards an organ‐sparing approach for locally advanced esophageal cancer. Dig Surg 2018: 1–8. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Konieczny A, Meyer P, Schnider A, Komminoth P, Schmid M, Lombriser N et al Accuracy of multidetector‐row CT for restaging after neoadjuvant treatment in patients with oesophageal cancer. Eur Radiol 2013; 23: 2492–2502. [DOI] [PubMed] [Google Scholar]
- 8. Kwee RM. Prediction of tumor response to neoadjuvant therapy in patients with esophageal cancer with use of 18F FDG PET: a systematic review. Radiology 2010; 254: 707–717. [DOI] [PubMed] [Google Scholar]
- 9. van Rossum PS, Fried DV, Zhang L, Hofstetter WL, van Vulpen M, Meijer GJ et al The incremental value of subjective and quantitative assessment of 18F‐FDG PET for the prediction of pathologic complete response to preoperative chemoradiotherapy in esophageal cancer. J Nucl Med 2016; 57: 691–700. [DOI] [PubMed] [Google Scholar]
- 10. Yip C, Cook GJ, Landau DB, Davies A, Goh V. Performance of different imaging modalities in assessment of response to neoadjuvant therapy in primary esophageal cancer. Dis Esophagus 2016; 29: 116–130. [DOI] [PubMed] [Google Scholar]
- 11. Noordman BJ, Spaander MCW, Valkema R, Wijnhoven BPL, van Berge Henegouwen MI, Shapiro J et al; SANO study group. Detection of residual disease after neoadjuvant chemoradiotherapy for oesophageal cancer (preSANO): a prospective multicentre, diagnostic cohort study. Lancet Oncol 2018; 19: 965–974. [DOI] [PubMed] [Google Scholar]
- 12. Heneghan HM, Donohoe C, Elliot J, Ahmed Z, Malik V, Ravi N et al Can CT–PET and endoscopic assessment post‐neoadjuvant chemoradiotherapy predict residual disease in esophageal cancer? Ann Surg 2016; 264: 831–838. [DOI] [PubMed] [Google Scholar]
- 13. van Heijl M, Omloo JM, van Berge Henegouwen MI, Hoekstra OS, Boellaard R, Bossuyt PM et al Fluorodeoxyglucose positron emission tomography for evaluating early response during neoadjuvant chemoradiotherapy in patients with potentially curable esophageal cancer. Ann Surg 2011; 253: 56–63. [DOI] [PubMed] [Google Scholar]
- 14. Griffin JM, Reed CE, Denlinger CE. Utility of restaging endoscopic ultrasound after neoadjuvant therapy for esophageal cancer. Ann Thorac Surg 2012; 93: 1855–1860. [DOI] [PubMed] [Google Scholar]
- 15. Jost C, Binek J, Schuller JC, Bauerfeind P, Metzger U, Werth B et al Endosonographic radial tumor thickness after neoadjuvant chemoradiation therapy to predict response and survival in patients with locally advanced esophageal cancer: a prospective multicenter phase II study by the Swiss Group for Clinical Cancer Research (SAKK 75/02). Gastrointest Endosc 2010; 71: 1114–1121. [DOI] [PubMed] [Google Scholar]
- 16. van Rossum PS, Goense L, Meziani J, Reitsma JB, Siersema PD, Vleggaar FP et al Endoscopic biopsy and EUS for the detection of pathologic complete response after neoadjuvant chemoradiotherapy in esophageal cancer: a systematic review and meta‐analysis. Gastrointest Endosc 2016; 83: 866–879. [DOI] [PubMed] [Google Scholar]
- 17. Chao YK, Chang Y, Yeh CJ, Chang HK, Tseng CK, Chuang WY. Characterization of residual tumours at the primary site in patients with a near pathological complete response after neoadjuvant chemoradiotherapy for oesophageal cancer. Br J Surg 2016; 103: 1874–1879. [DOI] [PubMed] [Google Scholar]
- 18. Shapiro J, ten Kate FJ, van Hagen P, Biermann K, Wijnhoven BP, van Lanschot JJ. Residual esophageal cancer after neoadjuvant chemoradiotherapy frequently involves the mucosa and submucosa. Ann Surg 2013; 258: 678–688. [DOI] [PubMed] [Google Scholar]
- 19. Castoro C, Scarpa M, Cagol M, Alfieri R, Ruol A, Cavallin F et al Complete clinical response after neoadjuvant chemoradiotherapy for squamous cell cancer of the thoracic oesophagus: is surgery always necessary? J Gastrointest Surg 2013; 17: 1375–1381. [DOI] [PubMed] [Google Scholar]
- 20. Furlong H, Bass G, Breathnach O, O'Neill B, Leen E, Walsh TN. Targeting therapy for esophageal cancer in patients aged 70 and over. J Geriatr Oncol 2013; 4: 107–113. [DOI] [PubMed] [Google Scholar]
- 21. Ohkura Y, Shindoh J, Ueno M, Iizuka T, Udagawa H. Comparison of outcome of esophagectomy versus nonsurgical treatment for resectable esophageal cancer with clinical complete response to neoadjuvant therapy. Ann Surg Oncol 2018; 25: 2428–2433. [DOI] [PubMed] [Google Scholar]
- 22. Taketa T, Xiao L, Sudo K, Suzuki A, Wadhwa R, Blum MA et al Propensity‐based matching between esophagogastric cancer patients who had surgery and who declined surgery after preoperative chemoradiation. Oncology 2013; 85: 95–99. [DOI] [PubMed] [Google Scholar]
- 23. Lambregts DM, Vandecaveye V, Barbaro B, Bakers FC, Lambrecht M, Maas M et al Diffusion‐weighted MRI for selection of complete responders after chemoradiation for locally advanced rectal cancer: a multicenter study. Ann Surg Oncol 2011; 18: 2224–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maas M, Lambregts DM, Nelemans PJ, Heijnen LA, Martens MH, Leijtens JW et al Assessment of clinical complete response after chemoradiation for rectal cancer with digital rectal examination, endoscopy, and MRI: selection for organ‐saving treatment. Ann Surg Oncol 2015; 22: 3873–3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. De Cobelli F, Giganti F, Orsenigo E, Cellina M, Esposito A, Agostini G et al Apparent diffusion coefficient modifications in assessing gastro‐oesophageal cancer response to neoadjuvant treatment: comparison with tumour regression grade at histology. Eur Radiol 2013; 23: 2165–2174. [DOI] [PubMed] [Google Scholar]
- 26. Fang P, Musall BC, Son JB, Moreno AC, Hobbs BP, Carter BW et al Multimodal imaging of pathologic response to chemoradiation in esophageal cancer. Int J Radiat Oncol Biol Phys 2018; 102: 996–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Imanishi S, Shuto K, Aoyagi T, Kono T, Saito H, Matsubara H. Diffusion‐weighted magnetic resonance imaging for predicting and detecting the early response to chemoradiotherapy of advanced esophageal squamous cell carcinoma. Dig Surg 2013; 30: 240–248. [DOI] [PubMed] [Google Scholar]
- 28. Li QW, Qiu B, Wang B, Wang DL, Yin SH, Yang H et al Prediction of pathologic responders to neoadjuvant chemoradiotherapy by diffusion‐weighted magnetic resonance imaging in locally advanced esophageal squamous cell carcinoma: a prospective study. Dis Esophagus 2018; 31. [DOI] [PubMed] [Google Scholar]
- 29. van Rossum PS, van Lier AL, van Vulpen M, Reerink O, Lagendijk JJ, Lin SH et al Diffusion‐weighted magnetic resonance imaging for the prediction of pathologic response to neoadjuvant chemoradiotherapy in esophageal cancer. Radiother Oncol 2015; 115: 163–170. [DOI] [PubMed] [Google Scholar]
- 30. Foti PV, Privitera G, Piana S, Palmucci S, Spatola C, Bevilacqua R et al Locally advanced rectal cancer: qualitative and quantitative evaluation of diffusion‐weighted MR imaging in the response assessment after neoadjuvant chemo‐radiotherapy. Eur J Radiol Open 2016; 3: 145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lever FM, Lips IM, Crijns SP, Reerink O, van Lier AL, Moerland MA et al Quantification of esophageal tumor motion on cine‐magnetic resonance imaging. Int J Radiat Oncol Biol Phys 2014; 88: 419–424. [DOI] [PubMed] [Google Scholar]
- 32. Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol 2010; 17: 1721–1724. [DOI] [PubMed] [Google Scholar]
- 33. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988; 44: 837–845. [PubMed] [Google Scholar]
- 34. Lowry R. Cohen's Unweighted Kappa, Kappa with Linear Weighting, Kappa with Quadratic Weighting, Frequencies and Proportions of Agreement; 2001. (updated 2018). http://vassarstats.net/kappa.html [accessed 3 July 2018]. [Google Scholar]
- 35. van der Valk MJM, Hilling DE, Bastiaannet E, Meershoek‐Klein Kranenbarg E, Beets GL, Figueiredo NL et al; IWWD Consortium. Long‐term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet 2018; 391: 2537–2545. [DOI] [PubMed] [Google Scholar]
- 36. van der Werf LR, Dikken JL, van der Willik EM, van Berge Henegouwen MI, Nieuwenhuijzen GAP, Wijnhoven BPL; Dutch Upper Gastrointestinal Cancer Audit (DUCA) group. Time interval between neoadjuvant chemoradiotherapy and surgery for oesophageal or junctional cancer: a nationwide study. Eur J Cancer 2018; 91: 76–85. [DOI] [PubMed] [Google Scholar]
- 37. Alper F, Turkyilmaz A, Kurtcan S, Aydin Y, Onbas O, Acemoglu H et al Effectiveness of the STIR turbo spin‐echo sequence MR imaging in evaluation of lymphadenopathy in esophageal cancer. Eur J Radiol 2011; 80: 625–628. [DOI] [PubMed] [Google Scholar]
- 38. Mizowaki T, Nishimura Y, Shimada Y, Nakano Y, Imamura M, Konishi J et al Optimal size criteria of malignant lymph nodes in the treatment planning of radiotherapy for esophageal cancer: evaluation by computed tomography and magnetic resonance imaging. Int J Radiat Oncol Biol Phys 1996; 36: 1091–1098. [DOI] [PubMed] [Google Scholar]
- 39. Loftås P, Sturludóttir M, Hallböök O, Almlöv K, Arbman G, Blomqvist L. Assessment of remaining tumour involved lymph nodes with MRI in patients with complete luminal response after neoadjuvant treatment of rectal cancer. Br J Radiol 2018; 91: 20170938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Borggreve AS, Mook S, Verheij M, Mul VEM, Bergman JJ, Bartels‐Rutten A et al; PRIDE study group. Preoperative image‐guided identification of response to neoadjuvant chemoradiotherapy in esophageal cancer (PRIDE): a multicenter observational study. BMC Cancer 2018; 18: 1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 MRI sequence parameters


