Abstract
Background & Aims
Remodelling of extracellular matrix is crucial in progressive liver fibrosis. Collagen type III desposition has been shown in acute decompensation. Extratracellular matrix is compiled of deposition of various components. The role of basement membrane collagen type IV in advanced cirrhosis and acute decompensation is unclear and investigated in this study.
Methods
Patients with decompensated cirrhosis from the prospective NEPTUN cohort (ClinicalTrials.gov Identifier: NCT03628807), who underwent transjugular intrahepatic portosystemic shunt procedure were included. Clinical and laboratory parameters, PRO‐C4 and C4M levels were measured in blood samples from portal and hepatic veins just before transjugular intrahepatic portosystemic shunt placement.
Results
Levels of C4M and PRO‐C4 are significantly lower in patients with massive ascites and impaired renal sodium excretion. C4M and PRO‐C4 show gender‐specific profiles with significantly lower levels in females compared to males. Females with higher C4M levels show higher mortality. By contrast, males with higher C4M levels show lower mortality. In multivariate Cox regression analysis, C4M is an independent predictor of survival in female patients.
Conclusion
This study shows that markers of collagen type IV remodelling do not accumulate in severe renal dysfunction. Although collagen type IV degradation markers derive from the liver, portal venous C4M levels are relevant for survival. Moreover, it demonstrates that circulating C4M shows gender‐specific profiles, which can independently predict survival in female patients with decompensated cirrhosis.
Keywords: ACLF, acute decompensation, acute‐on‐chronic liver failure, cirrhosis, collagen type IV, extracellular matrix remodelling, gender, liver, portal hypertension, transjugular intrahepatic portosystemic shunt
Abbreviations
- ACLF
acute‐on‐chronic liver failure
- AD
acute decompensation
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- CRP
C‐reactive protein
- HVPG
hepatic venous pressure gradient
- INR
international normalized ratio
- MELD
model for end‐stage liver disease
- OF
organ failure
- rs
Spearman's rank order correlation
- TIPS
transjugular intrahepatic portosystemic shunt
- YGT
γ‐glutamyl transferase
Key points.
Circulating markers of collagen type IV remodelling show gender‐specific profiles in decompensated cirrhosis. For female patients, they can predict survival. These markers do not accumulate in severe renal dysfunction.
1. INTRODUCTION
In the natural course of chronic liver disease, remodelling of extracellular matrix (ECM) leads to progressive fibrosis.1, 2, 3, 4, 5, 6 Matrix metalloproteinases (MMPs) cleave ECM molecules and generate small peptide fragments, known as neo‐epitopes. These neo‐epitopes are released into the circulation, and they can be measured with the Protein Fingerprint Technology using specific antibodies.6 The levels of these ECM markers reflect the degree of fibrosis,4, 7, 8, 9 success of anti‐fibrotic therapy as well as magnitude of portal hypertension.5, 10 The treatment of portal hypertension with transjugular intrahepatic portosystemic shunt implantation (TIPS) decompresses the portal venous system and might improve survival in selected patients.11, 12 Cirrhosis with portal hypertension can deteriorate with systemic inflammation and acute decompensation (AD). AD of cirrhosis might progress to multiple organ failure, defining acute‐on‐chronic liver failure (ACLF) with poor survival.15, 16 Interestingly, AD might boost the net deposition of collagen type III, a major component of the interstitial matrix, suggesting a new role of ECM in decompensated cirrhosis.3
The ECM can be subdivided in two compartments; the basement membrane and the underlying interstitial matrix. The basement membrane provides structural support and polarity to epithelial cells, controls growth, cell migration and epithelialization during tissue repair.17 In the liver, the ECM separating hepatocytes from sinusoidal endothelium is referred to as basement membrane‐like matrix, as it in addition contains non‐basement membrane constituents such as type I collagen and fibronectin.18
Type IV collagen is the major collagen of the basement membrane, increasing up to 14‐fold during cirrhosis, which is the highest relative increase among all collagens in the liver.19 The role of collagen type IV turnover in patients with advanced cirrhosis and AD20, 21 is still unclear and the interpretation of its turnover markers remains to be evaluated.
The present work investigates the role of collagen type IV turnover markers in the course of progression of liver fibrosis and gives insight to gender‐specific patterns in decompensated cirrhosis.
2. MATERIALS AND METHODS
Patients with decompensated cirrhosis from the prospective NEPTUN cohort (ClinicalTrials.gov Identifier: NCT03628807) who underwent TIPS procedure between 1996 and 2003 were included in this study. Inclusion criteria were age between 18 and 80, cirrhosis caused by alcohol or viral hepatitis, and decompensated cirrhosis with indication for TIPS. Exclusion criteria were contraindications for TIPS placement,22, 23 which were serum levels of bilirubin >5 mg/dL, spontaneous bacterial peritonitis, overt hepatic encephalopathy, pulmonary arterial hypertension and cardiac insufficiency. One to three weeks after TIPS insertion, an invasive control of the TIPS was performed as part of routine care.9 Biochemical blood analyses were performed using standard tests. The local ethics committee of the University of Bonn approved the study (029/13), and all patients agreed and signed an informed consent in accordance with the Helsinki Declaration for the procedures they underwent.
2.1. Transjugular intrahepatic portosystemic shunt insertion and haemodynamic measurements
TIPS (8‐10 mm Wallstent, Boston Scientific, Massachusetts, USA) placement was performed as previously described.22, 23 A single shot of antibiotic prophylaxis of cefuroxime (1.5 g) was administered at TIPS placement. Portal and hepatic venous pressures were measured invasively using a pressure transducer system (Combitrans, Braun Melsung, Germany) and a multichannel monitor (Sirecust, Siemens, Germany). The difference between portal and hepatic venous pressures was defined as portal hepatic pressure gradient (PHPG). The arterial pressure and heart rate were monitored noninvasively. Biochemical parameters as well as portal and systemic haemodynamics were recorded. The blood from the portal and the hepatic vein was collected as previously described.24 The blood sample from the portal vein was taken immediately after puncture of the vein. The hepatic venous sample was taken from the hepatic vein, which was used for the creation of the TIPS, right before puncturing portal vein. Immediately after entering the portal vein, but before dilation of the tract or insertion of the TIPS‐stent portal venous samples were taken. At invasive TIPS control after a median of 10 days range (1‐3 weeks), the catheter was advanced into the portal vein. Blood from the portal and hepatic vein were collected. CXCL9, CXCL10 and CXCL11 were measured as previously described.25, 26 Protein fingerprint markers were assessed in the portal and hepatic vein plasma samples.
2.2. Protein fingerprint marker assessment
Matrix metalloproteinases degraded type IV collagen (C4M8), and formation of type IV collagen (PRO‐C428) were examined. Briefly, each assay was run on a 96‐well streptavidin plate coated with the appropriate biotinylated synthetic peptide dissolved in an optimized assay and incubated 30 minutes at 20°C. Twenty microlitres of calibrator peptide or sample in appropriate dilution was added to the wells including 100 µL of horseradish peroxidase conjugated monoclonal antibody raised against the specific sequence of interest and incubated 1 hour at 20°C or overnight at 4°C. Following incubation, 100 µL tetramethylbenzidine (TMB) (Kem‐En‐Tec cat. 4380H) was added and the plate was incubated 15 minutes at 20°C in the dark. Hundred microlotres of stopping solution (1% H2SO4) was added in order to stop the reaction and measured at 450 nm with 650 nm as reference. All incubation steps included shaking at 300 rpm. After coating and antibody incubation, the plate was washed five times in washing buffer (20 mmol/L Tris, 50 mmol/L NaCl, pH 7.2). A calibration curve was plotted using a 4‐parametric fit model. Samples were measured within the detection range.
2.3. Statistical analysis
Plasma levels of the two Protein Fingerprint markers (PRO‐C4 and C4M) were logarithmically transformed to obtain normality and symmetry of variance. All correlations were calculated using Spearman's correlation. Comparison of the levels of Protein Fingerprints assessed in portal and hepatic venous blood at baseline and at control visit were analysed using paired samples t test. Patient survival was calculated by Kaplan‐Meier analysis, and survival curves were compared using log‐rank test. Univariate and multivariate analyses on patient's characteristics as predictors of mortality were calculated by Cox regression analysis. All data are shown as median with 95% confidence interval (CI) in squared brackets or as mean ± standard error of the mean (SEM). Differences between curves were calculated using analysis of variance (two‐way ANOVA). Inverse propensity score weighting was used to adjust for confounders between groups. P‐values less than 0.05 were considered statistically significant. Statistical analyses were performed using SPSS 23 with The Essentials for R plugin (IBM, Armonk, New York, USA). Data were plotted using GraphPad Prism v.5 (Graph Pad Software, La Jolla, CA, USA).
3. RESULTS
3.1. Patient characteristics
The study population included 105 cirrhosis patients, of those 67 (63.8%) were males. The median age was 59 (36‐80) years. Distribution of cirrhosis aetiology was alcohol in 75 (71%) patients, 12 (11%) chronic viral hepatitis (B and/or C) and 18 (17%) had other causes including cryptogenic cirrhosis, primary biliary cholangitis and patients suffering of autoimmune hepatitis. Median MELD score was 11 (6‐34), Child‐Pugh‐score was 8 (5‐12), CLIF‐C AD (acute decompensation) score was 50 (28‐68). Before TIPS implantation, most patients had oesophageal varices (I‐II° 65%; III‐IV° 22%) and ascites (mild 21%, severe 61%). The general characteristics of the study population are summarized in Table 1.
Table 1.
General data of all patients (n = 105). Data are shown as median and ranges for continuous variables or total number (%) for categorical variables
| Parameters | All patients |
|---|---|
| Gender (male) | 67 (64%) |
| Age (years) | 59 (36‐80) |
| Aetiology of cirrhosis (alcoholic/chronic viral hepatitis/other) | 75/12/18 (71/11/17%) |
| Indication for TIPS implementation (bleeding/ascites/both) | 37/56/12 (35/53/11%) |
| Child‐score | 8 (5‐12) |
| Child category (A/B/C) | 16/64/25 (15/61/24%) |
| MELD score | 11 (6‐34) |
| Varices (esophageal: absent/I‐II/III‐IV) | 14/68/23 (13/65/22%) |
| Variceal bleeding | 48 (46%) |
| Ascites (absent/mild/severe) | 19/22/64 (18/21/61%) |
| Hepatic encephalopathy (absent/history of) | 12 (11%) |
| CLIF‐C AD‐Score | 50 (28‐68) |
| Death | 94 (90%) |
| Follow‐up time (years) | 1.6 (0‐12.0) |
3.2. Association of collagen type IV remodelling markers with venous compartments and gender
Interestingly, male patients had significantly higher levels of C4M and PRO‐C4 than female patients (Figure 1A). The levels of degradation marker C4M in hepatic vein were significantly higher than in portal vein before TIPS placement (Figure 1B). Similarly, the levels of formation marker PRO‐C4 were significantly higher in hepatic vein compared to portal vein (Figure 1C). The liver therefore seems to be a main origin of these markers.
Figure 1.
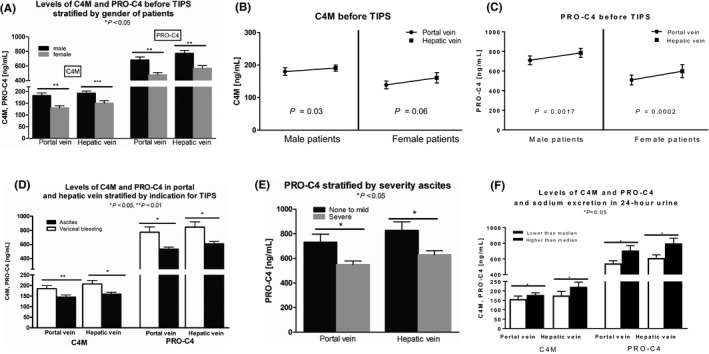
(A) shows levels of C4M and P4NP7S stratified by gender in hepatic and portal vein. Levels in females are significantly lower. (B) shows levels of C4M in portal and hepatic vein. Levels in hepatic vein are significantly higher. (C) shows levels of P4NP7S in portal and hepatic vein. Levels in hepatic vein are significantly higher. (C) shows that before TIPS procedure, the levels of C3M and P4NP7S in portal and hepatic vein stratified for indication for TIPS. Levels of patients with refractory ascites are significantly lower. (E) shows levels of P4NP7S stratified for severity of ascites in portal and hepatic vein. Levels in patients with severe ascites are significantly lower. (F) shows levels of P4NP7S and C4M stratified for sodium excretion in 24 h urine. Levels of P4NP7S are significantly higher in patients with higher than median sodium excretion. Levels of C4M are significantly higher in patients with higher than median sodium excretion. *P < 0.05, **P < 0.01, ***P < 0.001, ns not significant
3.3. Association of collagen type IV remodelling markers with ascites and renal function
Stratifying the patients for indication for TIPS, the levels of C4M in portal and hepatic vein are significantly lower in patients receiving TIPS for refractory ascites (n = 56) compared to those receiving TIPS for variceal bleeding (n = 37) (Figure 1D). We further stratified all patients according to the presence/absence and severity of ascites according to the International Club of Ascites. Indeed, patients with severe ascites showed significantly lower levels of PRO‐C4 in portal and hepatic vein compared to patients with less severe ascites (Figure 1E).
Patients with impaired sodium excretion (lower than median) showed significantly lower levels of PRO‐C4 in portal and hepatic vein (Figure 1F). C4M levels showed a similar pattern (Figure 1F). These data suggest that C4M and PRO‐C4 do not accumulate with renal failure but rather are independent of renal function.
3.4. Association of collagen type IV remodelling markers to mortality
Between male and female patients, only serum creatinine was statistically different with higher levels in male patients, the rate of severe ascites was not statistically different (Table 2).
Table 2.
Clinical parameters of all patients (n = 105) in comparison for gender. Data are shown as median and ranges
| Parameters | Male patients (n = 67) | Female patients (n = 38) | P‐value |
|---|---|---|---|
| PSPG (mmHg) | 20 [11‐35] | 20 [14‐32] | 0.859 |
| Ascites (absent/mild/severe) | 12/16/39 (18/24/58%) | 7/6/25 (18/16/66%) | 0.610 |
| Bilirubin (mg/dL) | 1.2 [0.4‐14.8] | 1.26 [0.3‐3.6] | 0.577 |
| gGT (IU/L) | 59 [9‐527] | 45 [8‐243] | 0.384 |
| AST (IU/L) | 20 [9‐73] | 18 [8‐46] | 0.365 |
| ALT (IU/L) | 18 [8‐94] | 18 [7‐55] | 0.249 |
| INR | 1.15 [0.95‐2.23] | 1.18 [0.36‐1.76] | 0.910 |
| Sodium (mEq/L) | 135 [119‐145] | 135 [120‐143] | 0.118 |
| Platelet count | 104 [27‐389] | 103 [37‐256] | 0.779 |
| Creatinine (mg/dL) | 1.2 [0.67‐8.2] | 0.95 [0.5‐5.7] | 0.039 |
| BUN (mg/dL) | 47 [9‐225] | 36 [12‐179] | 0.226 |
| Cholinesterasis (U/L) | 1650 [479‐3952] | 1635 [282‐4070] | 0.526 |
| White cell count (G/L) | 5.6 [1.4‐22.3] | 5.6 [1.5‐14.6] | 0.532 |
| Albumine (g/dL) | 30 [11‐52] | 33 [15‐56] | 0.193 |
| Child‐Pugh Score | 8 [5‐12] | 8 [5‐12] | 0.651 |
| Child‐Pugh category (A/B/C) | 10/41/16 (15/61/24%) | 6/23/9 (16/60/24%) | 0.936 |
| MELD score | 11 [6‐34] | 11 [6‐26,28] | 0.451 |
ALT, alanine transaminase; AST, aspartate transaminase; BUN, blood urea nitrogen; gGT, gamma glutamyl transpeptidase; INR, international normalized ratio; PSPG, portal systemic pressure gradient. Significance values are indicated in bold
Kaplan‐Meier survival curve for females showed that patients with higher than median levels of C4M either in portal or hepatic vein had significantly increased mortality (Figure 2C,D). Inversely, in male patients, lower levels of C4M were associated with increased 5‐year mortality (Figure 2E), whereas there was no association between C4M and mortality in the whole cohort (Figure 2A,B).
Figure 2.
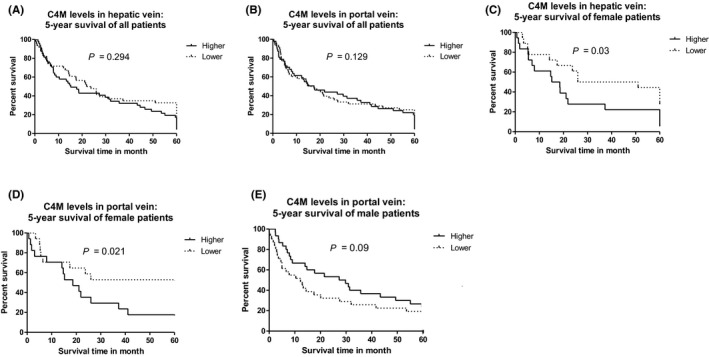
(A) Kaplan‐Meier survival curve of the whole cohort stratified for C4M in hepatic vein. (B) Kaplan‐Meier survival curve of the whole cohort stratified for C4M in portal vein. (C) Kaplan‐Meier survival curve of female patients stratified for C4M in hepatic vein. (D) Kaplan‐Meier survival curve of female patients stratified for C4M in portal vein. (E) Kaplan‐Meier survival curve of male patients stratified for C4M in hepatic vein
For female patients, C4M, serum sodium, variceal bleeding and PSPG were dependent predictors of survival. Multivariate analysis revealed high levels of C4M (above median) alongside variceal bleeding as TIPS indication and PHPG > 19 mmHg as independent predictors of survival (Table 3). To account for confounders between the high and low C4M group, we performed inverse propensity score weighting, adjusted for age and MELD score. Multivariate analysis after weighting shows serum creatinine and high levels of C4M as independent predictors of survival (Table 4).
Table 3.
Uni‐ and multivariate Cox regression analysis of female cohort
| Parameter | P‐value | Hazard ratio | 95% confidence interval |
|---|---|---|---|
| Sodium | 0.026 | 0.917 | 0.850‐0.990 |
| Creatinine | 0.058 | 1.455 | 0.988‐2.142 |
| MELD score | 0.330 | 1.041 | 0.960‐1.128 |
| Child score | 0.105 | 1.172 | 0.967‐1.420 |
| C4M levels in portal vein before TIPS | 0.095 | 1.004 | 0.999‐1.009 |
| C4M levels in portal vein after TIPS | 0.042 | 1.005 | 1.000‐1.010 |
| C4M levels in hepatic vein before TIPS | 0.04 | 1.004 | 1.000‐1.007 |
| C4M levels in hepatic vein after TIPS | 0.073 | 1.007 | 0.999‐1.014 |
| PRO‐C4 levels in hepatic vein before TIPS | 0.114 | 1.001 | 1.000‐1.002 |
| Delta C4M after TIPS | 0.069 | 0.994 | 0.988‐1.000 |
| Indication for TIPS: variceal bleeding | 0.035 | 0.172 | 0.034‐0.882 |
| Indication for TIPS: ascites | 0.056 | 0.228 | 0.050‐1.037 |
| Median PHPG | 0.04 | 0.472 | 0.231‐0.965 |
| PHPG higher than 19 mmHg | 0.014 | 0.394 | 0.188‐0.828 |
| Higher levels of C4M in portal vein before TIPS | 0.021 | 2.592 | 1.153‐5.827 |
| Indication for TIPS: variceal bleeding | 0.047 | 0.166 | 0.028‐0.980 |
| PHPG higher than 19 mmHg | 0.034 | 0.350 | 0.163‐0.932 |
| Higher levels of C4M in portal vein before TIPS | 0.002 | 2.758 | 1.175‐6.474 |
PHPG, portal hepatic pressure gradient; TIPS, transjugular intrahepatic portosystemic shunt.
Table 4.
Multivariate Cox regression analysis of female cohort after propensity score weighting (adjusted for age and MELD)
| Parameter | P‐value | Hazard ratio | 95% confidence interval |
|---|---|---|---|
| Creatinine | 0.001 | 1.653 | 1.242‐2.201 |
| Higher levels of C4M in portal vein before transjugular intrahepatic portosystemic shunt | <0.001 | 2.948 | 1.635‐5.283 |
3.5. Gender‐specific correlation of collagen type IV remodelling markers with prognostic scores and markers of inflammation and hepatic injury
In order to analyse the gender‐specific relationships of collagen type IV remodelling with systemic and hepatic inflammation, we performed correlation analysis stratified for male and female patients. Interestingly, MELD score is inversely correlated with markers of collagen type IV remodelling only in females. In males there is a different profile, where collagen type IV remodelling markers do not correlate with MELD score. Importantly, C4M levels in hepatic vein significantly correlate with serum levels of alanine aminotransferase (ALT) as a marker of liver injury in females, while they do inversely correlate in male patients (Table S1).
In male patients, C4M and Pro‐C4 in portal vein both positively correlate with markers of inflammation and immune activation CXCL‐9, CXCL‐10 and CXCL‐11.25, 26 In female patients, levels of C4M strongly positively correlate with CXCL‐9, CXCL‐10 and CXCL‐11 levels, while Pro‐C4 correlates inversely with CXCL‐10 and CXCL‐11 (Table S1).
4. DISCUSSION
This study covers a comprehensive analysis of the role of collagen type IV in decompensated cirrhosis. Its turnover markers do not accumulate with renal impairment and display a gender‐specific profile with portal venous C4M as an independent predictor of survival in female patients.
In chronic liver disease, ECM remodelling is a key factor in progressing fibrosis. Different components of ECM are described in ECM remodelling.29, 30 In a cohort of patients with alcoholic liver disease, various collagen types (I‐VI) were associated with increased hepatic venous pressure gradient (HVPG).9 Similarly, in compensated HIV/HCV‐co‐infected patients, the turnover marker of collagen type III, IV and V was associated with portal hypertension.4 Importantly, ECM is highly dynamic as shown in HIV patients receiving antiretroviral treatment, which led to attenuation of remodelling of collagen type IV and elastin, but not collagen type III and biglycan.5 Recently, collagen type III deposition was shown to be boosted in AD and ACLF.3 The present study suggests that the levels of collagen type IV remodelling markers do not accumulate with impaired renal function. In fact, in patients with severe ascites and low renal sodium excretion the levels are lower, suggesting that collagen type IV either might derive from skeletal muscle or other organs or that remodelling of the basement membrane occurs at an earlier stage of liver fibrosis, and may thus be an early marker of disease. This is in line with findings of type IV collagen genexpression, showing that the α1(IV)2α2(IV) isoform is the one almost only found in the liver,33 whereas the predominant isoform of type IV collagen in the kidney is α3(IV)α4(IV)α5(IV).34 Moreover, the C4M and PRO‐C4 markers target the alpha 1 chain of type IV collagen,7, 8 further emphasizing our findings that the liver may be a main origin of these markers.
In contrast to interstitial matrix collagens, which are predominantly produced by fibroblasts, the basement membrane collagens are predominantly produced by epithelial and endothelial cells such as sinusoids and hepatocytes with contribution from fibroblasts.35 Sinusoidal capillarization is significantly associated with chronic liver diseases, and involve the formation of basement membrane, defenestration and transformation of the sinusoidal endothelium into vascular type endothelium. In this study, collagen type IV turnover markers are significantly higher in patients with varices, suggesting an association with vascular formation and perisinusoidal fibrosis. In fact, angiogenesis has been shown to be dependent on increasing and subsequent extracellular deposition of collagen type IV.36 This could indicate a role of collagen type IV in angiogenesis of decompensated cirrhosis. Furthermore, angiogenesis is known to also be regulated by hormones in general, and estrogens specifically.37, 38 Additionally, estrogens have been shown to modulate fibrosis through the activity of collagen‐cleaving MMPs.39, 40 In fact, estradiol has been shown to reverse Transforming‐Growth‐Factor‐(TGF)‐beta1‐stimulated collagen type IV synthesis on the level of casein kinase 2 (CK2) activation.42 These mechanisms might explain our findings that the levels of collagen type IV remodelling markers are lower in females compared to males in our cohort. Moreover, it is possible that owing to estradiol‐related lower collagen type IV remodelling capacities in females, collagen type IV degradation (expressed by higher levels of C4M) cannot be compensated as sufficient as in males. This might explain our finding that higher levels of collagen type IV degradation are associated with higher mortality in females, while the opposite profile is found in male patients. The crucial role of collagen type IV degradation in females is further substantiated by being shown as an independent predictor of survival.
Considering these gender‐specific differences, it is not surprising that markers of collagen type IV remodelling have not been identified as non‐invasive markers for outcome in cirrhosis yet. Interestingly, MELD score is inversely correlated with markers of collagen type IV remodelling only in females, while it did not in males. This finding underlines the independent roles of collagen type IV remodelling for survival in female patients with cirrhosis and suggests a mechanism independent of the routine parameters calculated into the MELD score. Moreover, C4M levels in hepatic vein significantly correlate with serum levels of ALT as a marker of hepatic injury in females, while they do inversely correlate in males. This indicates the different roles of collagen type IV remodelling in both genders not only for survival but for hepatic injury in cirrhosis.
In male patients, C4M and Pro‐C4 in portal vein both positively correlate with markers of inflammation and immune activation CXCL‐9, CXCL‐10 and CXCL‐11, suggesting an association of increased collagen type IV turnover and inflammation in males. In female patients, levels of C4M strongly positively correlate with CXCL‐9, CXCL‐10 and CXCL‐11 levels, while Pro‐C4 correlates inversely with CXCL‐10 and CXCL‐11. These data point out that in females, these inflammation markers are correlated with less formation and higher degradation, therefore decreased collagen type IV deposition.
Collagen type IV is part of basement membrane, which contributes to intestinal barrier function from bacterial translocation.43, 44 Therefore, collagen type IV markers in portal vein might reflect remodelling of basement membrane and higher levels of the collagen type IV degradation marker C4M may indicate disruption of intestinal barrier function and consequently bacterial translocation, which could explain the independent predictive value for survival. Confirmation of this hypothesis however is beyond the scope of this study and further studies are needed.
This study has a well‐characterized patient cohort, but it has some limitations. Although all samples were obtained prospectively, the data collection and the measurements were carried out retrospectively. Moreover, liver biopsy was not performed. Finally, this study does not offer a pathophysiological mechanism to explain the gender‐specific differences in collagen type IV remodelling profiles.
In conclusion, this study shows for the first time that markers of collagen type IV remodelling derived from the liver, do not accumulate with renal dysfunction. Moreover, collagen type IV degradation markers display a gender‐specific profile, which can independently predict survival in female patients.
CONFLICT OF INTEREST
The authors do not have any disclosures to report.
AUTHORS’ CONTRIBUTIONS
JL, MP, MJN: study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, statistical analysis. RS, CM, DT: acquisition of data, analysis and interpretation of data, drafting of the manuscript, statistical analysis. FV, CPS, FB, SM, AK, MAK: interpretation of data, critical revision of the manuscript regarding important intellectual content. DJL: study concept and design, acquisition of data, analysis and interpretation of data, critical revision of the manuscript regarding important intellectual content, funding recipient, study supervision. JT: study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript regarding important intellectual content, funding recipient, administrative, technical and material support, study supervision.
Supporting information
ACKNOWLEDGEMENTS
We thank Gudrun Hack, Lise Larsen and Silke Bellinghausen for their excellent technical assistance.
Lehmann J, Praktiknjo M, Nielsen MJ, et al. Collagen type IV remodelling gender‐specifically predicts mortality in decompensated cirrhosis. Liver Int. 2019;39:885–893. 10.1111/liv.14070
Funding information
The authors were supported by grants from the Deutsche Forschungsgemeinschaft (SFB TRR57), European Union's Horizon 2020 research and innovation programme (No 668031) and Cellex Foundation. The funders had no influence on study design, data collection and analysis, decision to publish or preparation of the manuscript.
Handling Editor: Frank Tacke
REFERENCES
- 1. Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134(6):1655‐1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115(2):209‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Praktiknjo M, Lehmann J, Nielsen MJ, et al. Acute decompensation boosts hepatic collagen type III deposition and deteriorates experimental and human cirrhosis. Hepatol Commun. 2018;2(2):211‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jansen C, Leeming DJ, Mandorfer M, et al. PRO‐C3‐levels in patients with HIV/HCV‐Co‐infection reflect fibrosis stage and degree of portal hypertension. PLoS ONE. 2014;9(9):e108544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leeming DJ, Anadol E, Schierwagen R, et al. Combined antiretroviral therapy attenuates hepatic extracellular matrix remodeling in HIV patients assessed by novel protein fingerprint markers. AIDS Lond Engl. 2014;28(14):2081‐2090. [DOI] [PubMed] [Google Scholar]
- 6. Karsdal MA, Nielsen MJ, Sand JM, et al. Extracellular matrix remodeling: the common denominator in connective tissue diseases. Possibilities for evaluation and current understanding of the matrix as more than a passive architecture, but a key player in tissue failure. Assay Drug Dev Technol. 2013;11(2):70‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leeming DJ, Nielsen MJ, Dai Y, et al. Enzyme‐linked immunosorbent serum assay specific for the 7S domain of Collagen Type IV (P4NP 7S): A marker related to the extracellular matrix remodeling during liver fibrogenesis. Hepatol Res Off J Jpn Soc Hepatol. 2012;42(5):482‐493. [DOI] [PubMed] [Google Scholar]
- 8. Veidal SS, Karsdal MA, Nawrocki A, et al. Assessment of proteolytic degradation of the basement membrane: a fragment of type IV collagen as a biochemical marker for liver fibrosis. Fibrogenesis Tissue Repair. 2011;5(4):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Leeming DJ, Karsdal MA, Byrjalsen I, et al. Novel serological neo‐epitope markers of extracellular matrix proteins for the detection of portal hypertension. Aliment Pharmacol Ther. 2013;38(9):1086‐1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schierwagen R, Leeming DJ, Klein S, et al. Serum markers of the extracellular matrix remodeling reflect antifibrotic therapy in bile‐duct ligated rats. Front Physiol. 2013;4:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Salerno F, Cammà C, Enea M, Rössle M, Wong F. Transjugular intrahepatic portosystemic shunt for refractory ascites: a meta‐analysis of individual patient data. Gastroenterology. 2007;133(3):825‐834. [DOI] [PubMed] [Google Scholar]
- 12. García‐Pagán JC, Caca K, Bureau C, et al. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362(25):2370‐2379. [DOI] [PubMed] [Google Scholar]
- 13. Trebicka J. Emergency TIPS in a Child‐Pugh B patient: When does the window of opportunity open and close? J Hepatol. 2017;66(2):442‐450. [DOI] [PubMed] [Google Scholar]
- 14. Bureau C, Thabut D, Oberti F, et al. Transjugular intrahepatic portosystemic shunts with covered stents increase transplant‐free survival of patients with cirrhosis and recurrent ascites. Gastroenterology. 2017;152(1):157‐163. [DOI] [PubMed] [Google Scholar]
- 15. Moreau R, Jalan R, Gines P, et al. Acute‐on‐chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144(7):1426‐1437, 1437.e1‐9. [DOI] [PubMed] [Google Scholar]
- 16. Wong F. Acute kidney injury in liver cirrhosis: new definition and application. Clin Mol Hepatol. 2016;22(4):415‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mak KM, Mei R. Basement membrane type IV collagen and laminin: An overview of their biology and value as fibrosis biomarkers of liver disease. Anat Rec Hoboken NJ 2007. 2017;300(8):1371‐1390. [DOI] [PubMed] [Google Scholar]
- 18. Zeisberg M, Kramer K, Sindhi N, Sarkar P, Upton M, Kalluri R. De‐differentiation of primary human hepatocytes depends on the composition of specialized liver basement membrane. Mol Cell Biochem. 2006;283(1‐2):181‐189. [DOI] [PubMed] [Google Scholar]
- 19. Rojkind M, Ponce‐Noyola P. The extracellular matrix of the liver. Coll Relat Res. 1982;2(2):151‐175. [DOI] [PubMed] [Google Scholar]
- 20. Trebicka J. Predisposing factors in acute‐on‐chronic liver failure. Semin Liver Dis. 2016;36(2):167‐173. [DOI] [PubMed] [Google Scholar]
- 21. Chen W, Rock JB, Yearsley MM, Ferrell LD, Frankel WL. Different collagen types show distinct rates of increase from early to late stages of hepatitis C‐related liver fibrosis. Hum Pathol. 2014;45(1):160‐165. [DOI] [PubMed] [Google Scholar]
- 22. Brensing KA, Hörsch M, Textor J, et al. Hemodynamic effects of propranolol and nitrates in cirrhotics with transjugular intrahepatic portosystemic stent‐shunt. Scand J Gastroenterol. 2002;37(9):1070‐1076. [DOI] [PubMed] [Google Scholar]
- 23. Brensing KA, Textor J, Perz J, et al. Long term outcome after transjugular intrahepatic portosystemic stent‐shunt in non‐transplant cirrhotics with hepatorenal syndrome: a phase II study. Gut. 2000;47(2):288‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Trebicka J, Krag A, Gansweid S, et al. Endotoxin and tumor necrosis factor‐receptor levels in portal and hepatic vein of patients with alcoholic liver cirrhosis receiving elective transjugular intrahepatic portosystemic shunt. Eur J Gastroenterol Hepatol. 2011;23(12):1218‐1225. [DOI] [PubMed] [Google Scholar]
- 25. Berres M‐L, Asmacher S, Lehmann J, et al. CXCL9 is a prognostic marker in patients with liver cirrhosis receiving transjugular intrahepatic portosystemic shunt. J Hepatol. 2015;62(2):332‐339. [DOI] [PubMed] [Google Scholar]
- 26. Lehmann JM, Claus K, Jansen C, et al. Circulating CXCL10 in cirrhotic portal hypertension might reflect systemic inflammation and predict ACLF and mortality. Liver Int Off J Int Assoc Study Liver. 2018;38(5):875‐884. [DOI] [PubMed] [Google Scholar]
- 27. Berres M‐L, Lehmann J, Jansen C, et al. Chemokine (C‐X‐C motif) ligand 11 levels predict survival in cirrhotic patients with transjugular intrahepatic portosystemic shunt. Liver Int Off J Int Assoc Study Liver. 2016;36(3):386‐394. [DOI] [PubMed] [Google Scholar]
- 28. Nielsen MJ, Karsdal MA, Kazankov K, et al. Fibrosis is not just fibrosis ‐ basement membrane modelling and collagen metabolism differs between hepatitis B‐ and C‐induced injury. Aliment Pharmacol Ther. 2016;44(11‐12):1242‐1252. [DOI] [PubMed] [Google Scholar]
- 29. Liedtke C, Luedde T, Sauerbruch T, et al. Experimental liver fibrosis research: update on animal models, legal issues and translational aspects. Fibrogenesis Tissue Repair. 2013;6(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tsochatzis EA, Senzolo M, Germani G, Gatt A, Burroughs AK. Systematic review: portal vein thrombosis in cirrhosis. Aliment Pharmacol Ther. 2010;31(3):366‐374. [DOI] [PubMed] [Google Scholar]
- 31. Mehal W, To U. New approaches for fibrosis regression in alcoholic cirrhosis. Hepatol Int. 2016;10(5):773‐778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Buck M, Garcia‐Tsao G, Groszmann RJ, et al. Novel inflammatory biomarkers of portal pressure in compensated cirrhosis patients. Hepatol Baltim Md. 2014;59(3):1052‐1059. [DOI] [PubMed] [Google Scholar]
- 33. Ricard‐Blum S, Ruggiero F. The collagen superfamily: from the extracellular matrix to the cell membrane. Pathol Biol (Paris). 2005;53(7):430‐442. [DOI] [PubMed] [Google Scholar]
- 34. Miner JH. Glomerular basement membrane composition and the filtration barrier. Pediatr Nephrol Berl Ger. 2011;26(9):1413‐1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wells RG. Cellular sources of extracellular matrix in hepatic fibrosis. Clin Liver Dis. 2008;12(4):759‐768, viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bahramsoltani M, Slosarek I, De Spiegelaere W, Plendl J. Angiogenesis and collagen type IV expression in different endothelial cell culture systems. Anat Histol Embryol. 2014;43(2):103‐115. [DOI] [PubMed] [Google Scholar]
- 37. Barnabas O, Wang H, Gao X‐M. Role of estrogen in angiogenesis in cardiovascular diseases. J Geriatr Cardiol. 2013;10(4):377‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Trenti A, Tedesco S, Boscaro C, et al. Immunity and cell metabolism: Solving the puzzle. Int J Mol Sci. 2018;19(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang B, Zhang C‐G, Ji L‐H, Zhao G, Wu Z‐Y. Estrogen receptor β selective agonist ameliorates liver cirrhosis in rats by inhibiting the activation and proliferation of hepatic stellate cells. J Gastroenterol Hepatol. 2018;33(3):747‐755. [DOI] [PubMed] [Google Scholar]
- 40. Zhang B, Wu Z‐Y. Estrogen derivatives: novel therapeutic agents for liver cirrhosis and portal hypertension. Eur J Gastroenterol Hepatol. 2013;25(3):263‐270. [DOI] [PubMed] [Google Scholar]
- 41. Cengiz M, Ozenirler S, Yılmaz G. Estrogen receptor alpha expression and liver fibrosis in chronic hepatitis C virus genotype 1b: a clinicopathological study. Hepat Mon. 2014;14(9):e21885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zdunek M, Silbiger S, Lei J, Neugarten J. Protein kinase CK2 mediates TGF‐beta1‐stimulated type IV collagen gene transcription and its reversal by estradiol. Kidney Int. 2001;60(6):2097‐2108. [DOI] [PubMed] [Google Scholar]
- 43. Pozzi A, Yurchenco PD, Iozzo RV. The nature and biology of basement membranes. Matrix Biol J Int Soc Matrix Biol. 2017;57–58:885‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vllasaliu D, Falcone FH, Stolnik S, Garnett M. Basement membrane influences intestinal epithelial cell growth and presents a barrier to the movement of macromolecules. Exp Cell Res. 2014;323(1):218‐231. [DOI] [PubMed] [Google Scholar]
- 45. Mantaj J, Abu‐Shams T, Enlo‐Scott Z, Swedrowska M, Vllasaliu D. Role of the basement membrane as an intestinal barrier to absorption of macromolecules and nanoparticles. Mol Pharm. 2018. [DOI] [PubMed] [Google Scholar]
- 46. Biancheri P, Di Sabatino A, Corazza GR, MacDonald TT. Proteases and the gut barrier. Cell Tissue Res. 2013;351(2):269‐280. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


