Abstract
Peptides and small molecules that bind to peptide receptors are important classes of drugs that are used for a wide variety of different applications. The search for novel neuropeptides traditionally involved a time-consuming approach to purify each peptide to homogeneity and determine its amino acid sequence. The discovery in the 1980s of enkephalin convertase/carboxypeptidase E (CPE), and the observation that this enzyme was involved in the production of nearly every known neuropeptide led to the idea for a one-step affinity purification of CPE substrates. This approach was successfully used to isolate hundreds of known neuropeptides in mouse brain, as well as over a dozen novel peptides. Some of the novel peptides found using this approach are among the most abundant peptides present in brain, but had not been previously identified by traditional approaches. Recently, receptors for two of the novel peptides have been identified, confirming their role as neuropeptides that function in cell-cell signaling. Small molecules that bind to one of these receptors have been developed and found to significantly reduce food intake and anxiety-like behavior in an animal model. This review describes the entire project, from discovery of CPE to the novel peptides and their receptors.
Keywords: Neuropeptide, peptidomics, carboxypeptidase, enkephalin, proSAAS
Introduction: Peptides and peptide receptors as pharmacological targets
Peptides have many diverse functions and are found in virtually all organisms (Chung & Civelli, 2006; Fricker, 2012; Husson et al., 2007; Sobrino Crespo et al., 2014; Strand, 2003). In multicellular organisms, a large number of peptides are known to signal between cells, either between tissues as peptide hormones or between neurons as neuropeptides. Because of their important roles in signaling, peptides are used as drugs (Fosgerau & Hoffmann, 2015; Kaspar & Reichert, 2013). In some cases the peptides used as therapeutics are identical in sequence and post-translational modification (PTM) as the endogenous neuropeptides or peptide hormone; examples include insulin and vasopressin. In other cases the peptides used as therapeutic agents are modified to enhance their stability; such modifications include the introduction of D-amino acids or PTMs that block the action of peptidases. There are currently more than 60 peptides that have been approved by the Food and Drug Administration and marketed as therapeutic agents, and many more peptides are in clinical testing (Fosgerau & Hoffmann, 2015; Kaspar & Reichert, 2013).
In addition to peptides themselves, small molecules that bind to peptide receptors are important drugs for many diverse applications. Examples include drugs for treatment of pain (mu opioid receptor agonists such as morphine, oxycodone, and many others), nausea and vomiting (aprepitant, a neurokinin receptor antagonist), and narcolepsy (suvorexant, an orexin receptor antagonist) (Levy, 2001; Preskorn, 2014). Additional drugs targeting other peptide receptors are in various stages of the development pipeline (Fosgerau & Hoffmann, 2015; Kaspar & Reichert, 2013).
Hundreds of bioactive peptides have been identified since the first peptide hormone was discovered and characterized in the early 1900s (Bayliss, 1902; Fricker, 2012; Strand, 2003). In addition to the known neuropeptides and peptide hormones, there are many peptides of unknown function—these are termed “orphan peptides” (Chung & Civelli, 2006; Fricker, 2010; Fricker, 2012; Ozawa et al., 2010). It is possible that some of these orphan peptides bind to receptors and function as neurotransmitters or peptide hormones. There are dozens of orphan G protein-coupled receptors (GPRCs) and it is likely that some of these bind peptides (Chung & Civelli, 2006; Civelli, 2008; Civelli et al., 2013; Ozawa et al., 2010). Thus, the identification of novel peptides and “de-orphanization” of their receptors is an area with potential therapeutic applications.
Biosynthesis of Neuropeptides: Discovery of Enkephalin Convertase/Carboxypeptidase E
In the mid-1970s, endogenous peptides with opiate-like properties were discovered in mammalian tissue extracts, including Met- and Leu-enkephalin, beta-endorphin, and various dynorphin peptides (Goldstein et al., 1979; Hughes et al., 1975; Lazarus, Ling & Guillemin, 1976; Li, Chung & Doneen, 1976; Roberts & Herbert, 1977; Stern et al., 1979). Precursors of these peptides were identified and in most cases, the precursors contained lysine and/or arginine residues on either side of the bioactive peptides (Kimura et al., 1980; Lewis et al., 1980). This led to the hypothesis that a trypsin-like endopeptidase initially cleaved the neuropeptide precursor protein into intermediates that contained C-terminal Lys/Arg residues, and these were subsequently removed by a carboxypeptidase B-like enzyme (Roberts et al., 1979). Because trypsin and carboxypeptidase B were produced in the exocrine pancreas and not known to be produced in brain or other endocrine tissues, it was likely that novel enzymes were involved in neuropeptides production. When I joined Dr. Solomon Snyder’s laboratory as a student in 1980, he proposed that I identify the unknown carboxypeptidase that produced enkephalin, the so-called “enkephalin convertase.”
If enkephalin convertase was specific for producing enkephalin, it would recognize more than the C-terminal basic residues (i.e. Lys or Arg). Because the existing assays for pancreatic carboxypeptidase B used very short peptides such as hippuryl-Arg (Folk et al., 1960), these substrates were not likely to be useful to detect an enzyme with specificity for a longer peptide. A new assay was developed that used the C-terminal sequence of an enkephalin precursor: Phe-Leu-Arg (Fricker & Snyder, 1982). A fluorescent group was attached to the N-terminus of this peptide so that it could be readily detected, and enzymatic conversion of substrate (dansyl-Phe-Leu-Arg) into product (dansyl-Phe-Leu) monitored by extraction of the product into chloroform. Using this assay, enkephalin convertase was detected in bovine adrenal chromaffin granules (Fricker & Snyder, 1982). Chromaffin granules were previously reported to contain enkephalin as well as enkephalin precursors, implying that they contain the carboxypeptidase that generates enkephalin (Lewis et al., 1979; Stern et al., 1979). The enzyme detected in the chromaffin granules was a metallopeptidase with pH optimum around 5.5 (Fricker & Snyder, 1982). These properties were similar to those of an enzyme associated with pancreatic beta cells (Zuhlke et al., 1977; Zuhlke et al., 1976), but distinct from an enzyme that was claimed to be the enkephalin-producing carboxypeptidase (Hook, Eiden & Brownstein, 1982). The chromaffin granule metallopeptidase was purified to homogeneity from adrenal, brain, and pituitary (Fricker & Snyder, 1983). Upon characterization, the enzyme was shown to convert enkephalin precursors into enkephalin and was named enkephalin convertase in the initial publication (Fricker & Snyder, 1982). However, enkephalin convertase was not specific for enkephalin precursors, cleaving basic residues from a wide range of peptides containing C-terminal Lys or Arg, although not from dipeptides such as hippuryl-Arg which are too short to be efficient substrates of this enzyme (Fricker & Snyder, 1983; Supattapone, Fricker & Snyder, 1984). The finding that enkephalin convertase was not specific for the sequence of the enkephalin precursor matched the broad distribution of the enzyme in all neuroendocrine tissues, with no correlation with the distribution of enkephalin. Taken together, it appeared that enkephalin convertase was a common enzyme for the production of all neuropeptides that were produced from precursors that required removal of basic amino acids, including the synthesis of insulin and other peptide hormones (Davidson & Hutton, 1987; Docherty & Hutton, 1983; Mains & Eipper, 1984). Therefore, the name enkephalin convertase was considered inaccurate and the enzyme was renamed carboxypeptidase E (CPE) (Fricker, 1985; Fricker, 1988). Other names have been used for this enzyme, such as carboxypeptidase H, but CPE is most commonly used.
CPE and the Discovery of Novel Neuropeptides
If CPE functions in the biosynthesis of a large number of known neuropeptides, then it was predicted that this enzyme would also function in the production of novel neuropeptides. At the time of the discovery of CPE in the early 1980s, novel neuropeptides were being discovered every year or two, and it was assumed that many unknown neuropeptides awaited discovery (Tatemoto, Carlquist & Mutt, 1982; Tatemoto et al., 1986; Tatemoto et al., 1984a; Tatemoto et al., 1984b; Tatemoto et al., 1985; Tatemoto & Mutt, 1980; Tatemoto et al., 1983). One major limitation to the discovery of neuropeptides was the purification approach, which usually started with many kilograms of tissue and took years to separate the bioactive neuropeptide from all other proteins and peptides present in the extracts (Burgus & Guillemin, 1970; Hughes et al., 1975; Tatemoto, Carlquist & Mutt, 1982; Tatemoto & Mutt, 1980).
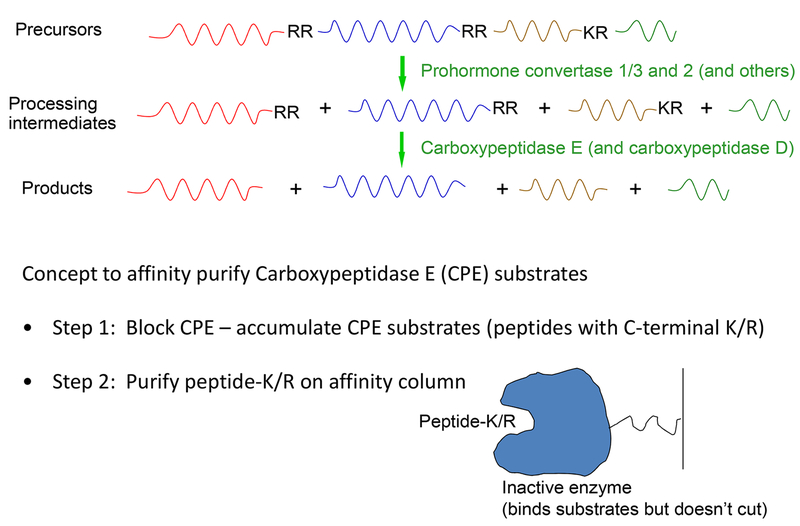
Because CPE seemed to be specific for neuropeptide production, based on the enzyme’s neuroendocrine distribution and localization to peptide-containing secretory vesicles, CPE could be used to rapidly identify novel neuropeptides. The idea was to use affinity chromatography to isolate CPE substrates in a single step, instead of using conventional chromatography that only afforded a 5–10-fold purification in each step and therefore required multiple steps to achieve sufficient purity for sequence determination. This idea consisted of two steps (Figure 1). The first involved inhibition of CPE to accumulate the Lys/Arg-extended CPE substrates—these are normally present at extremely low levels within brain and other neuroendocrine tissues. The second step involved purification on an affinity column, using an inactive enzyme that could bind substrates but not cleave them.
Figure 1: Neuropeptide processing scheme and concept for the affinity purification of CPE substrates. Top:
Neuropeptides are produced from precursors by selective cleavages at sites that usually contain basic amino acids lysine (K) and arginine (R). Initially, an endopeptidase cleaves the precursor to generate intermediates that contain basic residues on their C-terminus except for the C-terminal peptide, which does not require addition processing to generate the mature form. Endopeptidases include prohormone convertase 1 (PC1, also known as PC3) and prohormone convertase 2 (PC2). For some neuropeptides, the processing of their precursors starts in the late Golgi and is mediated by endopeptidases in this compartment such as furin and other furin-like enzymes. Following the endopeptidase, a carboxypeptidase removes the C-terminal basic residue. Carboxypeptidase E (CPE) is the major neuropeptide-processing carboxypeptidase that is present within secretory vesicles. For neuropeptide precursors initially cleaved in the Golgi by furin or furin-like enzymes, carboxypeptidase D is present in this compartment and is able to remove the basic residues from the intermediates to produce the mature peptides. For some peptides, additional post-translational modifications are required, such as C-terminal amidation, N-terminal acetylation, or phosphorylation of Ser or Thr residues (not shown). Bottom: The general concept for the affinity purification of CPE substrates. Step 1 requires inhibition of CPE to enrich for CPE substrates—otherwise the peptides containing C-terminal lysine or arginine are present at extremely low levels in brain and other tissues. As the scheme was originally envisioned, step 2 required purification on a column of inactive CPE, which could bind substrates but not cleave them. In practice, anhydrotrypsin has the required specificity for peptides with C-terminal basic residues, and does not bind with high affinity to peptides with internal basic residues. Following purification of the CPE substrates, the column eluate contains a mixture of hundreds of peptides. These are fractionated on reverse phase liquid chromatography columns and analyzed by on-line electrospray ionization mass spectrometry, which can often determine the sequence of peptides in complex mixtures.
For step 1 of the scheme, potent inhibitors of CPE were identified from a screen of compounds previously developed as inhibitors of carboxypeptidase B and related enzymes (Fricker, Plummer & Snyder, 1983; McKay & Plummer, 1978; Plummer & Ryan, 1981). However, none of these compounds was able to penetrate the secretory vesicles where the production of neuropeptides occurred, presumably due to the highly-charged nature of the compounds. Without the essential first step of in vivo inhibition of CPE activity, it was not possible to pursue the idea of affinity purification of neuropeptides precursors.
In the late-1980s, techniques to generate mice with a targeted disruption of a specific gene (termed “knock-out” mice) were developed (Capecchi, 1989; Capecchi, 2001; Evans, 2001; Smithies, 2001). Knock-out of the CPE gene would lead to elevated levels of CPE substrates, a requirement for the peptide purification scheme to succeed (Figure 1). However, it was assumed that the knockout would completely eliminate the mature forms of most bioactive peptides and this would be embryonic lethal. At the time, CPE was incorrectly thought to be the only neuropeptide-producing carboxypeptidase in the secretory pathway (Fricker, 1988; Fricker, 1993; Fricker & Devi, 1994). Fortunately, mice lacking CPE activity were viable. In fact, such mice already existed—a spontaneous mutation in the CPE gene occurred in the early 1970s at The Jackson Laboratory in a colony of inbred mice, and because the mutant mouse was notably overweight, the mutation was named fat (Coleman & Eicher, 1990). Mice homozygous for the fat mutation (i.e. fat/fat mice) were extremely overweight and also sterile, but mice heterozygous for the fat mutation were fertile and had normal body weights. Geneticists mapped the location of the fat gene to chromosome 8 near the Cpe gene, and my laboratory helped determine that these mice had a mutation in the protein-coding region of the gene that caused CPE to be completely inactive (Naggert et al., 1995). The mutation was renamed Cpefat to reflect the altered gene.
Analysis of peptides in brain and neuroendocrine tissues of Cpefat/Cpefat mice revealed that mature fully-processed neuropeptides were present, albeit at extremely low levels for most peptides (Cain, Wang & Beinfeld, 1997; Fricker et al., 1996; Naggert et al., 1995; Rovere et al., 1996). This led to the discovery of carboxypeptidase D, a CPE-like enzyme that is mainly present in the trans Golgi network of the secretory pathway where it primarily functions in the processing of proteins and peptides that transit to the cell surface via the constitutive secretory pathway (Song & Fricker, 1995; Varlamov & Fricker, 1998; Xin et al., 1997). Although carboxypeptidase D appears to be excluded from the mature secretory vesicles where CPE is enriched and the majority of the neuropeptide processing occurs, the presence of carboxypeptidase D in the trans Golgi network and immature secretory vesicles is sufficient to produce enough of the mature bioactive forms of neuropeptides to permit the animals to live a fairly normal lifespan (Varlamov et al., 1999a; Varlamov et al., 1999b).
As predicted from earlier studies on the substrate specificity and distribution of CPE, studies on Cpefat/Cpefat mice confirmed that CPE is the major carboxypeptidase involved in the production of nearly every neuropeptide (Che, Biswas & Fricker, 2005; Che & Fricker, 2002; Zhang et al., 2008). The only exceptions are secretory pathway peptides that do not require removal of C-terminal basic residues, and peptides that are not produced within the secretory pathway. For example, secretory pathway peptides that are present in the Cpefat/Cpefat mice include peptides located on the C-terminus of their precursor such as beta-endorphin which only require endopeptidase cleavage, or peptides in which the C-terminal basic residues are not removed such as alpha-neoendorphin which terminates in -Pro-Arg and is not an efficient substrate of CPE (Zhang et al., 2008).
In addition to the secretory pathway peptides, a large number of other peptides are detected in brains of both wild-type and Cpefat/Cpefat mice as well as all other genotypes of mice that have been studied (Fricker, 2010; Zhang et al., 2008). These other peptides are mainly derived from proteins known to be localized to the cell cytoplasm, mitochondria, and/or nucleus; these are termed “intracellular peptides” to distinguish them from neuropeptides derived from secretory pathway proteins (Ferro et al., 2014; Fricker, 2010). Quantitative peptidomics studies indicated that the intracellular peptides are present at comparable levels in wild-type and Cpefat/Cpefat mice brains, and therefore are not substrates of CPE, consistent with their intracellular location as well as the absence of basic amino acid cleavage sites (Zhang et al., 2008). Some of these intracellular peptides have been found to be secreted via an unknown mechanism from brain slices and these secreted peptides may function as non-classical neuropeptides (Gelman et al., 2013). For example, the peptide RVD-hemopressin is secreted from mouse brain slices and binds to cannabinoid CB1 receptors (Gomes et al., 2009; Heimann et al., 2007). Intracellular peptides that are not secreted from cells may bind to intracellular proteins and alter their folding and/or interactions with other proteins (Ferro et al., 2014). However, these potential functions of peptides are difficult to identify and the targets are not easily druggable because of their intracellular location, as opposed to the cell surface location of neuropeptide receptors.
Using mice lacking CPE to identify novel neuropeptides: Discovery of proSAAS-derived peptides
Once the Cpefat/Cpefat mutation was found to inactivate CPE activity and lead to the accumulation of neuropeptides, which is step 1 of the scheme shown in Figure 1, my laboratory began working on step 2; the affinity column to purify peptides with C-terminal Lys/Arg residues in a single step. Mutagenesis of a critical active site residue in CPE (Glu300Gln) rendered the enzyme inactive but still able to bind substrates (Qian, Varlamov & Fricker, 1999). (This is not the same point mutation as found in the Cpefat/Cpefat mice, which is Ser202Pro and causes the protein to be unstable and rapidly degraded.) Alternatively, treatment of the wild-type enzyme with chelating agents to remove the active site zinc ion also eliminated enzyme activity without compromising substrate binding. Either of these approaches were able to produce the intended result; a protein that bound CPE substrates without cleaving them. However, it was difficult to produce large amounts of CPE and couple it to a solid-phase matrix (e.g. agarose) in high yield. Instead, the commercially available anhydrotrypsin-agarose was found to be comparable in terms of binding specificity (Che et al., 2001; Fricker et al., 2000). This was unexpected, in that trypsin typically cleaves peptides with internal Lys/Arg residues, and peptides with these residues on the C-terminus represent trypsin products, not substrates. Usually, enzymes bind substrates with much higher affinity than products. However, in testing it was found that the anhydrotrypsin-agarose bound poorly to peptides with internal Lys/Arg residues and with high affinity to peptides with C-terminal Lys/Arg residues (Che et al., 2001; Fricker et al., 2000). Thus, the commercially-available resin was an ideal resource for step 2 of the scheme to purify neuropeptide precursors from brains of Cpefat/Cpefat mice.
Using the anhydrotrypsin affinity column, hundreds of peptide were purified from Cpefat/Cpefat mouse brains and identified using mass spectrometry (Che et al., 2001; Fricker et al., 2000). Many of these peptides corresponded to known neuropeptides with C-terminal Lys/Arg, as predicted. This finding confirmed that CPE is involved in the production of the vast majority of neuropeptides. In addition to the predicted peptides from known neuropeptide precursors, several novel peptides were found from a new precursor (Che et al., 2001; Fricker et al., 2000). Because the functions of the peptides were not known at the time, and there was no amino acid sequence homology to known peptides, it was impossible to name the novel peptides with a functional name. Thus, the peptides were named based on amino acid sequence motifs found within the peptides. The first peptide identified had a Ser-Ala-Ala-Ser sequence in the middle of the peptide, and so the peptide was named SAAS based on the single letter amino acid nomenclature (Fricker et al., 2000). Because two forms of this peptide were found, the larger was named Big SAAS and the smaller was named Little SAAS. The precursor was therefore named proSAAS, using the tradition of naming neuropeptide precursors. Other peptides identified in the affinity column eluate that were subsequently found to arise from different regions of proSAAS include peptides named GAV (Big and Little), PEN, and LEN (Big and Little). The big and little forms of the proSAAS-derived peptides arose from partial cleavage by the prohormone convertases (PCs) at basic amino acid-containing sites that are not efficiently cleaved (e.g., single basic residues)—this is also found with many other neuropeptide precursors that undergo differential processing into big and little forms (Fricker, 2012).
The initial report on the discovery of proSAAS raised the possibility that the proSAAS-derived peptides functioned as neuropeptides but did not directly test this, which can take years to prove (Fricker et al., 2000). Another function for proSAAS was found and described in the initial report—that of PC1/3 inhibition (Fricker et al., 2000). The enzyme PC2 has an endogenous inhibitor, named 7B2, but 7B2 is specific for PC2 and does not inhibit PC1/3 (Braks & Martens, 1994; Martens et al., 1994). Because the distribution of proSAAS in mouse neuroendocrine tissue was very broad and overlapped with the distribution of PC1/3, it was logical to test proSAAS as a PC1/3 inhibitor (Fricker et al., 2000). While proSAAS is a potent inhibitor of PC1/3, further studies mapped the inhibitory region to the junction of PEN and LEN and not the mature peptides, raising the possibility that the mature forms of these peptides had additional functions as neuropeptides (Basak et al., 2001; Cameron, Fortenberry & Lindberg, 2000; Qian et al., 2000).
To explore functions of proSAAS and/or proSAAS derived peptides, transgenic mice that overexpress proSAAS and knock-out mice lacking proSAAS were generated (Morgan et al., 2010; Wei et al., 2004). The transgenic mice were slightly overweight, and the knock-out mice were leaner than wild-type littermates, suggesting a role of proSAAS-derived peptides in body weight regulation. ProSAAS-derived peptides may also contribute to rewarding behavior and anxiety, based on the behavior of transgenic and/or knock-out mice (Morgan et al., 2010; Wei et al., 2004). Although proSAAS is present in most neuroendocrine cells, levels in some cell types are much higher than in others; very high levels of proSAAS expression are found in the amygdala and the hypothalamus, especially the arcuate nucleus (Fricker et al., 2000). Within the arcuate nucleus, proSAAS peptides are most abundant in neurons that express neuropeptide Y (NPY), and are not abundant in neurons that express peptides derived from proopiomelanocortin (POMC) (Wardman et al., 2011). NPY neurons are orexigenic while POMC neurons are anorexigenic (Crown, Clifton & Steiner, 2007). The co-localization of proSAAS peptides and NPY is consistent with a role for these peptides in body weight regulation, possibly as neuropeptides.
Evidence that a proSAAS-derived peptide functioned in cell-cell signaling was provided from whole-cell patch clamp recordings of parvocellular neurons in the hypothalamic paraventricular nucleus (Wardman et al., 2011). Application of the peptide Big LEN to these cells produced a rapid and reversible inhibition of synaptic glutamate release that was spike independent and dependent on postsynaptic G protein activity (Wardman et al., 2011). This result strongly suggested that Big LEN is a neuropeptide that activates a G protein-coupled receptor (GPCR) expressed in the hypothalamus.
Matching “orphan” peptides and receptors
Direct evidence that proSAAS-derived peptides function as neuropeptides was recently provided from studies that sought to match these peptides with receptors. Rather than take a high throughput approach, the Devi laboratory took a targeted approach and focused on orphan GPCRs that were expressed at relatively high levels in brain regions that matched the distribution of proSAAS and its peptides. Using this targeted approach, Gomes et al screened a dozen receptors and found that Big LEN activated the orphan GPCR named GPR171 (Gomes et al., 2013). The shorter form of this peptide, Little LEN, did not bind to the receptor, but the C-terminal tetrapeptide of Big LEN that is cleaved to generate little LEN (i.e. Leu-Leu-Pro-Pro) was a weak agonist at GPR171 (Gomes et al., 2013). Thus, binding of Big LEN to GPR171 is mediated through the C-terminal portion of this peptide, and the processing step that generates Little LEN and the tetrapeptide affects its biological activity.
Using a similar approach, Devi and colleagues found that PEN activates the orphan GPCR named GPR83 (Gomes et al., 2016). GPR83 is also known as the Glucocorticoid-Induced Receptor, JP05, and GPR72, and was previously been implicated in body weight regulation and other functions (Adams et al., 2003; Muller et al., 2013; Wang et al., 2001). GPR83 knock-out mice are resistant to diet-induced obesity when placed on a high-fat diet, and have normal body weight on a regular chow diet (Muller et al., 2013). Interestingly, GPR83 and GPR171 are colocalized in some brain regions, as are their peptide ligands; PEN and Big LEN. Coexpression of GPR83 and GPR171 in cultured cell lines alters the signaling properties of each receptor, suggesting functional interactions of these receptors (Gomes et al., 2016).
Recently, a small molecule GPR171 agonist was identified and tested in mice (Wardman et al., 2016). As predicted from the previous studies on proSAAS, this agonist elevated food intake and body weight (Wardman et al., 2016). This effect was attenuated in mice in which GPR171 expression was reduced using short hairpin RNA-mediated knockdown, indicating that the effect was mediated by GPR171 (Wardman et al., 2016). While there is a limited commercial market for pharmaceuticals that elevate food intake (e.g., to treat anorexia), if GPR171 antagonists have the opposite effect and reduce feeding and body weight, this would be a novel therapeutic approach for a multi-billion dollar market. Very recently, a small molecule GPR171 antagonist was identified (Bobeck et al., 2017). This compound significantly reduced food intake in an animal model in which NPY/AgRP neurons were stimulated, but did not affect baseline feeding in unstimulated mice (Bobeck et al., 2017). In addition, injection of the GPR171 antagonist into the basolateral amygdala reduced anxiety-like behavior (Bobeck et al., 2017). Taken together, these results are a promising new direction in the development of pharmaceuticals for treatment of disorders that have a large global market.
Conclusion/Future Directions:
An important step in pharmacology is target identification. As a class, peptide receptors are a validated target, with numerous small molecules and peptide-based drugs on the market and more in development. There are dozens of orphan GPCRs that have defied attempts at deorphanization, potentially because their peptide ligands are novel and were not tested with the orphan GPCR. This appears to have been the case for GPR171 and GPR83, which bind peptides that are not commonly studied even though they are among the most abundant peptides in brain (Fricker, 2012).
Decades ago, the slow step in the discovery of new neuropeptides was the purification and identification of peptides using bioassays. Now, with mass spectrometry-based peptidomic approaches, peptide discovery is relatively easy and the hard part is finding functions for all of the novel peptides.
Based on the phenotype of mice lacking CPE activity, it is clear that peptides play important roles in many processes for which there is a major therapeutic demand. In addition to obesity and infertility, mice lacking CPE activity are anxious, depressed, and have memory problems (Rodriguiz et al., 2013; Woronowicz et al., 2008). Some of these same problems have been reported in a human patient lacking CPE activity due to a point mutation within the CPE gene that caused a frameshift and truncation of the protein (Alsters et al., 2015). Thus, further studies to identify the peptide(s) responsible for each of these behaviors in mice may be translatable to novel therapeutic approaches for major clinical problems. Studies on conditional CPE knock-out mice in which the Cpe gene is disrupted in specific cell types may reveal novel roles for peptides in anxiety, depression, memory, fertility, and body weight regulation; such studies are currently in progress.
Acknowledgments:
The US National Institute of Drug Abuse (DA-004494) supported much of the work described in this review that was performed in the author’s laboratory. The author wishes to thank Prof. Lakshmi Devi for helpful discussions, and all collaborators on the publications cited in the references for their contributions to the project.
Nonstandard Abbreviations:
- CPE
carboxypeptidase E
- GPCR
G protein-coupled receptors
- PC
prohormone convertase
- PTM
post-translational modification
- NPY
neuropeptide Y
- POMC
proopiomelanocortin
Footnotes
Conflict of Interest Statement:
The author has no conflicts of interest to declare.
References
- Adams F, Grassie M, Shahid M, Hill DR, & Henry B (2003). Acute oral dexamethasone administration reduces levels of orphan GPCR glucocorticoid-induced receptor (GIR) mRNA in rodent brain: potential role in HPA-axis function. Brain Res Mol Brain Res 117, 39. [DOI] [PubMed] [Google Scholar]
- Alsters SI, Goldstone AP, Buxton JL, Zekavati A, Sosinsky A, Yiorkas AM et al. (2015). Truncating Homozygous Mutation of Carboxypeptidase E (CPE) in a Morbidly Obese Female with Type 2 Diabetes Mellitus, Intellectual Disability and Hypogonadotrophic Hypogonadism. PLoS One 10, e0131417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basak A, Koch P, Dupelle M, Fricker LD, Devi LA, Chretien M et al. (2001). Inhibitory specificity and potency of proSAAS-derived peptides toward proprotein convertase 1. J.Biol.Chem 276, 32720. [DOI] [PubMed] [Google Scholar]
- Bayliss WM, Starling EH (1902). The mechanism of pancreatic secretion. J Physiol 28, 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobeck EN, Gomes I, Pena D, Cummings KA, Clem RL, Mezei M et al. (2017). The BigLEN-GPR171 Peptide Receptor System Within the Basolateral Amygdala Regulates Anxiety-Like Behavior and Contextual Fear Conditioning. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braks JAM, & Martens GJM (1994). 7B2 is a neuroendocrine chaperone that transiently interacts with prohormone convertase PC2 in the secretory pathway. Cell 78, 263. [DOI] [PubMed] [Google Scholar]
- Burgus R, & Guillemin R (1970). Hypothalamic releasing factors. Annu Rev Biochem 39, 499. [DOI] [PubMed] [Google Scholar]
- Cain BM, Wang W, & Beinfeld MC (1997). Cholecystokinin (CCK) levels are greatly reduced in the brains but not the duodenums of Cpe fat /Cpe fat mice: A regional difference in the involvement of carboxypeptidase E (Cpe) in pro-CCK processing. Endocrinol. 138, 4034. [DOI] [PubMed] [Google Scholar]
- Cameron A, Fortenberry Y, & Lindberg I (2000). The SAAS granin exhibits structural and functional homology to 7B2 and contains a highly potent hexapeptide inhibitor of PC1. FEBS Lett. 473, 135. [DOI] [PubMed] [Google Scholar]
- Capecchi MR (1989). Altering the genome by homologous recombination. Science 244, 1288. [DOI] [PubMed] [Google Scholar]
- Capecchi MR (2001). Generating mice with targeted mutations. Nat Med 7, 1086. [DOI] [PubMed] [Google Scholar]
- Che FY, Biswas R, & Fricker LD (2005). Relative quantitation of peptides in wild type and Cpe<fat/fat> mouse pituitary using stable isotopic tags and mass spectrometry. J.Mass Spectrom. 40 227. [DOI] [PubMed] [Google Scholar]
- Che FY, & Fricker LD (2002). Quantitation of neuropeptides in Cpe fat /Cpe fat mice using differential isotopic tags and mass spectrometry. Anal.Chem 74, 3190. [DOI] [PubMed] [Google Scholar]
- Che FY, Yan L, Li H, Mzhavia N, Devi L, & Fricker LD (2001). Identification of peptides from brain and pituitary of Cpe fat /Cpe fat mice Proc.Natl.Acad.Sci.USA 98, 9971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, & Civelli O (2006). Orphan neuropeptides. Novel neuropeptides modulating sleep or feeding. Neuropeptides 40, 233. [DOI] [PubMed] [Google Scholar]
- Civelli O (2008). The orphanin FQ/nociceptin (OFQ/N) system. Results Probl.Cell Differ. 46, 1. [DOI] [PubMed] [Google Scholar]
- Civelli O, Reinscheid RK, Zhang Y, Wang Z, Fredriksson R, & Schioth HB (2013). G protein-coupled receptor deorphanizations. Annu Rev Pharmacol Toxicol 53, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman DL, & Eicher EM (1990). Fat (fat) and tubby (tub), two autosomal recessive mutations causing obesity syndromes in the mouse. J.Hered. 81, 424. [DOI] [PubMed] [Google Scholar]
- Crown A, Clifton DK, & Steiner RA (2007). Neuropeptide signaling in the integration of metabolism and reproduction. Neuroendocrinology 86, 175. [DOI] [PubMed] [Google Scholar]
- Davidson HW, & Hutton JC (1987). The insulin secretory granule carboxypeptidase H: purification and demonstration of involvement in proinsulin processing. Biochem.J 245, 575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty K, & Hutton JC (1983). Carboxypeptidase activity in the insulin secretory granule. FEBS Lett. 162, 137. [DOI] [PubMed] [Google Scholar]
- Evans MJ (2001). The cultural mouse. Nat Med 7, 1081. [DOI] [PubMed] [Google Scholar]
- Ferro ES, Rioli V, Castro LM, & Fricker LD (2014). Intracellular peptides: From discovery to function. EuPA Open Proteomics 3, 143. [Google Scholar]
- Folk JE, Piez KA, Carroll WR, & Gladner JA (1960). Carboxypeptidase B: Purification and characterization of the porcine enzyme. J.Biol.Chem 235, 2272. [PubMed] [Google Scholar]
- Fosgerau K, & Hoffmann T (2015). Peptide therapeutics: current status and future directions. Drug Discov Today 20, 122. [DOI] [PubMed] [Google Scholar]
- Fricker LD (1985). Neuropeptide biosynthesis: focus on the carboxypeptidase processing enzyme. Trends.Neurosci 8, 210. [Google Scholar]
- Fricker LD (1988). Carboxypeptidase E. Annu.Rev.Physiol 50, 309. [DOI] [PubMed] [Google Scholar]
- Fricker LD (1993). Opioid peptide processing enzymes In Handbook of Experimental Pharmacology, Vol 104/I; Opioids Herz A, pp. 529 Springer-Verlag, Berlin. [Google Scholar]
- Fricker LD (2010). Analysis of mouse brain peptides using mass spectrometry-based peptidomics: implications for novel functions ranging from non-classical neuropeptides to microproteins. Mol.Biosyst 6, 1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker LD (2012). Neuropeptides and other bioactive peptides Morgan & Claypool Life Sciences, Charleston, S.C. [Google Scholar]
- Fricker LD, Berman YL, Leiter EH, & Devi LA (1996). Carboxypeptidase E activity is deficient in mice with the fat mutation: Effect on peptide processing. J.Biol.Chem 271, 30619. [DOI] [PubMed] [Google Scholar]
- Fricker LD, & Devi L (1994). Enzymes involved in opioid peptide biosynthesis In Pharmacology of Opioid Peptides Tseng L, pp. 87 Harwood Academic Publishers, Switzerland. [Google Scholar]
- Fricker LD, McKinzie AA, Sun J, Curran E, Qian Y, Yan L et al. (2000). Identification and characterization of proSAAS, a granin-like neuroendocrine peptide precursor that inhibits prohormone processing. J.Neurosci. 20, 639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker LD, Plummer THJ, & Snyder SH (1983). Enkephalin convertase: Potent, selective, and irreversible inhibitors. Biochem.Biophys.Res.Commun 111, 994. [DOI] [PubMed] [Google Scholar]
- Fricker LD, & Snyder SH (1982). Enkephalin convertase: Purification and characterization of a specific enkephalin-synthesizing carboxypeptidase localized to adrenal chromaffin granules. Proc.Natl.Acad.Sci.USA 79, 3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker LD, & Snyder SH (1983). Purification and characterization of enkephalin convertase, an enkephalin-synthesizing carboxypeptidase. J.Biol.Chem 258, 10950. [PubMed] [Google Scholar]
- Gelman JS, Dasgupta S, Berezniuk I, & Fricker LD (2013). Analysis of peptides secreted from cultured mouse brain tissue. Biochim Biophys Acta 1834, 2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A, Tachibana S, Lowney LI, Hunkapiller M, & Hood L (1979). Dynorphin-(1–13), an extraordinarily potent opioid peptide. Proc.Natl.Acad.Sci.U.S.A 76, 6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes I, Aryal DK, Wardman JH, Gupta A, Gagnidze K, Rodriguiz RM et al. (2013). GPR171 is a hypothalamic G protein-coupled receptor for BigLEN, a neuropeptide involved in feeding. Proc Natl Acad Sci U S A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes I, Bobeck EN, Margolis EB, Gupta A, Sierra S, Fakira AK et al. (2016). Identification of GPR83 as the receptor for the neuroendocrine peptide PEN. Sci Signal 9, ra43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes I, Grushko JS, Golebiewska U, Hoogendoorn S, Gupta A, Heimann AS et al. (2009). Novel endogenous peptide agonists of cannabinoid receptors. FASEB J. 23, 3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimann AS, Gomes I, Dale CS, Pagano RL, Gupta A, de Souza LL et al. (2007). Hemopressin is an inverse agonist of CB1 cannabinoid receptors. Proc.Natl.Acad.Sci.U.S.A 104, 20588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook VYH, Eiden LE, & Brownstein MJ (1982). A carboxypeptidase processing enzyme for enkephalin precursors. Nature (Lond.). 295, 341. [DOI] [PubMed] [Google Scholar]
- Hughes J, Smith TW, Kosterlitz HW, Fothergill LA, Morgan BA, & Morris HR (1975). Identification of two related pentapeptides from the brain with potent opiate agonist activity. Nature 258, 577. [DOI] [PubMed] [Google Scholar]
- Husson SJ, Mertens I, Janssen T, Lindemans M, & Schoofs L (2007). Neuropeptidergic signaling in the nematode Caenorhabditis elegans. Prog.Neurobiol 82, 33. [DOI] [PubMed] [Google Scholar]
- Kaspar AA, & Reichert JM (2013). Future directions for peptide therapeutics development. Drug Discov Today 18, 807. [DOI] [PubMed] [Google Scholar]
- Kimura S, Lewis RV, Stern AS, Rossier J, Stein S, & Udenfriend S (1980). Probable precursors of [Leu]enkephalin and [Met]enkephalin in adrenal medulla: peptides of 3–5 kilodaltons. Proc.Natl.Acad.Sci.U.S.A 77, 1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus LH, Ling N, & Guillemin R (1976). beta-Lipotropin as a prohormone for the morphinomimetic peptides endorphins and enkephalins. Proc Natl Acad Sci U S A 73, 2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy MH (2001). Advancement of opioid analgesia with controlled-release oxycodone. Eur J Pain 5 Suppl A, 113. [DOI] [PubMed] [Google Scholar]
- Lewis RV, Stern AS, Kimura S, Rossier J, Brink L, Gerber LD et al. (1980). Opioid peptides and precursors in the adrenal medulla. Adv.Biochem.Psychopharmacol 22, 167. [PubMed] [Google Scholar]
- Lewis RV, Stern AS, Rossier J, Stein S, & Udenfriend S (1979). Putative enkephalin precursors in bovine adrenal medulla. Biochem.Biophys.Res.Commun 89, 822. [DOI] [PubMed] [Google Scholar]
- Li CH, Chung D, & Doneen BA (1976). Isolation, characterization and opiate activity of beta-endorphin from human pituitary glands. Biochem Biophys Res Commun 72, 1542. [DOI] [PubMed] [Google Scholar]
- Mains RE, & Eipper BA (1984). Secretion and regulation of two biosynthetic enzyme activities, peptidyl-glycine alpha-amidating monooxygenase and a carboxypeptidase, by mouse pituitary corticotropic tumor cells. Endocrinol. 115, 1683. [DOI] [PubMed] [Google Scholar]
- Martens GJ, Braks JA, Eib DW, Zhou Y, & Lindberg I (1994). The neuroendocrine polypeptide 7B2 is an endogenous inhibitor of prohormone convertase PC2. Proc.Natl.Acad.Sci.USA 91, 5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay TJ, & Plummer THJ (1978). By-product analogues for bovine carboxypeptidase B. Biochem. 17, 401. [DOI] [PubMed] [Google Scholar]
- Morgan DJ, Wei S, Gomes I, Czyzyk T, Mzhavia N, Pan H et al. (2010). The propeptide precursor proSAAS is involved in fetal neuropeptide processing and body weight regulation. J.Neurochem. 113, 1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller TD, Muller A, Yi CX, Habegger KM, Meyer CW, Gaylinn BD et al. (2013). The orphan receptor Gpr83 regulates systemic energy metabolism via ghrelin-dependent and ghrelin-independent mechanisms. Nat Commun 4, 1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naggert JK, Fricker LD, Varlamov O, Nishina PM, Rouille Y, Steiner DF et al. (1995). Hyperproinsulinemia in obese fat/fat mice associated with a point mutation in the carboxypeptidase E gene and reduced carboxypeptidase E activity in the pancreatic islets. Nature Genetics 10, 135. [DOI] [PubMed] [Google Scholar]
- Ozawa A, Lindberg I, Roth B, & Kroeze WK (2010). Deorphanization of novel peptides and their receptors. AAPS.J. 12, 378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer THJ, & Ryan TJ (1981). A potent mercapto bi-product analogue inhibitor for human carboxypeptidase N. Biochem.Biophys.Res.Commun 98, 448. [DOI] [PubMed] [Google Scholar]
- Preskorn SH (2014). CNS drug development: lessons from the development of ondansetron, aprepitant, ramelteon, varenicline, lorcaserin, and suvorexant. Part I. J Psychiatr Pract 20, 460. [DOI] [PubMed] [Google Scholar]
- Qian Y, Devi LA, Mzhavia N, Munzer S, Seidah NG, & Fricker LD (2000). The C-terminal region of proSAAS is a potent inhibitor of prohormone convertase 1. J.Biol.Chem 275, 23596. [DOI] [PubMed] [Google Scholar]
- Qian Y, Varlamov O, & Fricker LD (1999). Glu300 of rat carboxypeptidase E is essential for enzymatic activity but not substrate binding or routing to the regulated secretory pathway. J.Biol.Chem 274, 11582. [DOI] [PubMed] [Google Scholar]
- Roberts JL, & Herbert E (1977). Characterization of a common precursor to corticotropin and beta-lipotropin: identification of beta-lipotropin peptides and their arrangement relative to corticotropin in the precursor synthesized in a cell-free system. Proc.Natl.Acad.Sci.U.S.A 74, 5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JL, Phillips M, Rosa PA, Budarf M, & Herbert E (1979). Processing of common precursor forms of adrenocorticotropin and endorphins in cultures of mouse pituitary cells and in mouse pituitary. Prog.Clin.Biol.Res 31, 761. [PubMed] [Google Scholar]
- Rodriguiz RM, Wilkins JJ, Creson TK, Biswas R, Berezniuk I, Fricker AD et al. (2013). Emergence of anxiety-like behaviours in depressive-like Cpe(fat/fat) mice. Int J Neuropsychopharmacol 16, 1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovere C, Viale A, Nahon J, & Kitabgi P (1996). Impaired processing of brain proneurotensin and promelanin-concentrating hormone in obese fat/fat mice. Endocrinol. 137, 2954. [DOI] [PubMed] [Google Scholar]
- Smithies O (2001). Forty years with homologous recombination. Nat Med 7, 1083. [DOI] [PubMed] [Google Scholar]
- Sobrino Crespo C, Perianes Cachero A, Puebla Jimenez L, Barrios V, & Arilla Ferreiro E (2014). Peptides and food intake. Front Endocrinol (Lausanne) 5, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, & Fricker LD (1995). Purification and characterization of carboxypeptidase D, a novel carboxypeptidase E-like enzyme, from bovine pituitary. J.Biol.Chem 270, 25007. [DOI] [PubMed] [Google Scholar]
- Stern AS, Lewis RV, Kimura S, Rossier J, Gerber LD, Brink L et al. (1979). Isolation of the opioid heptapeptide Met-enkephalin [Arg6,Phe7] from bovine adrenal medullary granules and striatum. Proc.Natl.Acad.Sci.U.S.A 76, 6680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand FL (2003). Neuropeptides: general characteristics and neuropharmaceutical potential in treating CNS disorders. Prog.Drug Res 61, 1. [DOI] [PubMed] [Google Scholar]
- Supattapone S, Fricker LD, & Snyder SH (1984). Purification and characterization of a membrane-bound enkephalin-forming carboxypeptidase, “enkephalin convertase”. J.Neurochem. 42, 1017. [DOI] [PubMed] [Google Scholar]
- Tatemoto K, Carlquist M, & Mutt V (1982). Neuropeptide Y--a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature 296, 659. [DOI] [PubMed] [Google Scholar]
- Tatemoto K, Efendic S, Mutt V, Makk G, Feistner GJ, & Barchas JD (1986). Pancreastatin, a novel pancreatic peptide that inhibits insulin secretion. Nature 324, 476. [DOI] [PubMed] [Google Scholar]
- Tatemoto K, Jornvall H, McDonald TJ, Carlquist M, Go VL, Johansson C et al. (1984a). Isolation and primary structure of human PHI (peptide HI). FEBS Lett. 174, 258. [DOI] [PubMed] [Google Scholar]
- Tatemoto K, Jornvall H, Siimesmaa S, Hallden G, & Mutt V (1984b). Isolation and characterization of cholecystokinin-58 (CCK-58) from porcine brain. FEBS Lett. 174, 289. [DOI] [PubMed] [Google Scholar]
- Tatemoto K, Lundberg JM, Jornvall H, & Mutt V (1985). Neuropeptide K: isolation, structure and biological activities of a novel brain tachykinin. Biochem.Biophys.Res.Commun 128, 947. [DOI] [PubMed] [Google Scholar]
- Tatemoto K, & Mutt V (1980). Isolation of two novel candidate hormones using a chemical method for finding naturally occurring polypeptides. Nature 285, 417. [DOI] [PubMed] [Google Scholar]
- Tatemoto K, Rokaeus A, Jornvall H, McDonald TJ, & Mutt V (1983). Galanin - a novel biologically active peptide from porcine intestine. FEBS Lett. 164, 124. [DOI] [PubMed] [Google Scholar]
- Varlamov O, Eng FJ, Novikova EG, & Fricker LD (1999a). Localization of metallocarboxypeptidase D in AtT-20 cells: Potential role in prohormone processing. J.Biol.Chem 274, 14759. [DOI] [PubMed] [Google Scholar]
- Varlamov O, & Fricker LD (1998). Intracellular trafficking of metallocarboxypeptidase D in AtT-20 cells: Localization to the trans-Golgi network and recycling from the cell surface. J.Cell Sci. 111, 877. [DOI] [PubMed] [Google Scholar]
- Varlamov O, Wu F, Shields D, & Fricker LD (1999b). Biosynthesis and packaging of carboxypeptidase D into nascent secretory vesicles in pituitary cell lines. J.Biol.Chem 274, 14040. [DOI] [PubMed] [Google Scholar]
- Wang D, Herman JP, Pritchard LM, Spitzer RH, Ahlbrand RL, Kramer GL et al. (2001). Cloning, expression, and regulation of a glucocorticoid-induced receptor in rat brain: effect of repetitive amphetamine. J Neurosci 21, 9027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardman JH, Berezniuk I, Di S, Tasker JG, & Fricker LD (2011). ProSAAS-Derived Peptides are Colocalized with Neuropeptide Y and Function as Neuropeptides in the Regulation of Food Intake. PLoS One 6, e28152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardman JH, Gomes I, Bobeck EN, Stockert JA, Kapoor A, Bisignano P et al. (2016). Identification of a small-molecule ligand that activates the neuropeptide receptor GPR171 and increases food intake. Sci Signal 9, ra55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S, Feng Y, Che FY, Pan H, Mzhavia N, Devi L et al. (2004). Obesity and diabetes in transgenic mice expressing proSAAS. J.Endocrinol. 180 357. [DOI] [PubMed] [Google Scholar]
- Woronowicz A, Koshimizu H, Chang SY, Cawley NX, Hill JM, Rodriguiz RM et al. (2008). Absence of carboxypeptidase E leads to adult hippocampal neuronal degeneration and memory deficits. Hippocampus 18, 1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin X, Varlamov O, Day R, Dong W, Bridgett MM, Leiter EH et al. (1997). Cloning and sequence analysis of cDNA encoding rat carboxypeptidase D. DNA Cell Biol. 16, 897. [DOI] [PubMed] [Google Scholar]
- Zhang X, Che FY, Berezniuk I, Sonmez K, Toll L, & Fricker LD (2008). Peptidomics of Cpe(fat/fat) mouse brain regions: implications for neuropeptide processing. J.Neurochem. 107, 1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuhlke H, Kohnert KD, Jahr H, Schmidt S, Kirscke H, & Steiner DF (1977). Proteolytic and transhydrogenolytic activites in isolated pancreatic islets of rats. Acta.Biol.Med.Germ 36, 1695. [PubMed] [Google Scholar]
- Zuhlke H, Steiner DF, Lernmark A, & Lipsey C (1976). Carboxypeptidase B-like and trypsin-like activities in isolated rat pancreatic islets. Ciba Found Symp 41, 183. [DOI] [PubMed] [Google Scholar]