Abstract
Background: Intramedullary spinal cord metastases (ISCM) in malignancies is a devastating issue with limited research. This study aims to identify the clinical features, management, prognostic factors, and outcomes of this special entity.
Methods: A retrospective review of 61 patients of ISCM diagnosed and treated in our institute from June 2010 to March 2018 was conducted (lost to follow-up: 3). Data were retrieved according to the items including age, gender, primary tumor, interval to the ISCM occurrence, ISCM segments, and other synchronous metastases. The interventions, response, prognostic factors, and outcomes of ISCM were systematically analyzed.
Results: Lung cancer (67.21%) was the commonest ISCM source, followed by breast cancer (14.75%). In total, 9.84% of patients presented with ISCM initially. The mean span from the primaries to ISCM was 18.77 months (range=0–10 years). The thoracic segment was most commonly involved (77.05%), followed by cervical (39.34%), lumbar level (34.43%), and conus medullaris (6.56%). The management of ISCM was challenging, since 55.74% of individuals had a poor physical condition (PS=3–4) and 72.41% had widespread dissemination synchronously (≥2 organs). Radiotherapy (RT) attained an objective response rate (ORR) of 61.90% or 62.50% and a local control rate (LCR) of 90.48% or 87.50% for symptoms used alone or with other strategies, respectively. ISCM bears a dismal prognosis, with a median overall survival (OS) of 4 months. Patients with only one segment involved had an apparently better prognosis than those with 2–4 involved segments (median OS=7.0 vs 3.0 months) (P<0.01). The OS of patients treated was remarkably superior to those without any intervention (median OS=5.0 vs 2.0 months) (P<0.01).
Conclusion: ISCM is a distinct entity needing more attention for high cancer incidence, prolonged survival, and lack of research. RT is the mainstay with satisfactory effect. Multiple spinal cord segments involvement and no treatment are poor prognostic factors of OS.
Keywords: intramedullary spinal cord metastasis, radiotherapy, combined treatment
Introduction
Intramedullary spinal cord metastases (ISCM) is rarely encountered in the clinical setting, and easily ignored by clinicians, owing to a lack of awareness and related research1–11 (Table 1). In fact, as the diagnosis and treatment of cancer improve and more cancer patients survive, the incidence of ISCM keeps rising. ISCM is often associated with rapid deterioration of neurological function and devastating outcome. Prompt identification and appropriate intervention is urgent to prevent neurological deficits and prolong patients’ survival.12 Therefore, we carried out this retrospective research of ISCM, aiming to clarify the clinicopathological features and explore the optimal management of this special entity.
Table 1.
Summary of prior studies of ISCM treatment and outcome
| References | Date | Number of pts | Sex | Median age (years) | Primary tumor | Location of ISCM | Presence of other metastases | Treatment strategy | Outcome of neurological status post management | Overall median survival (range)(days) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Cervical | Thoracic | Lumbar to Conus | Brain | Other systemic | Improved | Unchanged | Deteriorated | |||||||
| Sung et al1 | 2013 | 301 | 143 | 116 | 56 (4–82) | Lung 144 (47.8%) | 122 (41%) | 102 (34%) | 113 (38%) | n=214 | n=198 | Surgery 89 (40%) | 51 (33%) | 66 (43%) | 36 (24%) | 120 (4–1800) |
| Breast 48 (15.9%) | 131 (61%) | 127 (64%) | Surgery 36 | Surgery 19 | Surgery 7 | Surgery | ||||||||||
| Melanoma 18 (5.9%) | Conservative treatment 107 (48%) | Conservative treatment 15 | Conservative treatment 45 | Conservative treatment 12 | 180 (14–720) | |||||||||||
| Renal cell 17 (5.6%) | Palliative treatment | Palliative treatment 0 | Palliative treatment 2 | Palliative treatment 17 | Conservative treatment | |||||||||||
| Colorectal 16 (5.3%) | 27 (12%) | 150 (14–1800) | ||||||||||||||
| Lymphoma 14 (4.7%) | Palliative treatment | |||||||||||||||
| CNS (drop metastasis) 11 (3.7%) | 30 (4–120) | |||||||||||||||
| Unknown 10 (3.3%) | ||||||||||||||||
| Sarcoma 6 (2.0%) | ||||||||||||||||
| Ovarian 5 (1.7%) | ||||||||||||||||
| Endometrial 2 (0.7%) | ||||||||||||||||
| Esophageal 2 (0.7%) | ||||||||||||||||
| Gastric 2 (0.7%) | ||||||||||||||||
| Others 6 (2.0%) | ||||||||||||||||
| Dam-Hieu et al2 | 2009 | 19 | 10 | 9 | 56 (35–75) | Lung 13 (68%) | 4 (21%) | 5 (26.3%) | 11 (58%) | 5 (26.3%) | 55 (26.3%) | Surgery 13 (68%) | 9 (52.6%) | 7 (36.8%) | 3 (15.8%) | 183 (4−720) |
| Breast 3 (16%) | Radiotherapy 11 (57.9%) | Surgery+Radiotherapy 9 | Surgery 2 | Surgery 3 | ||||||||||||
| Colorectal 1 (5.5%) | Chemotherapy 1 (5.2%) | Chemotherapy 0 | Radiotherapy 0 | Radiotherapy 0 | ||||||||||||
| Esophageal 1 (5.5%) | Abstention 5 (26.3%) | Abstention 0 | Chemotherapy 1 | Chemotherapy 0 | ||||||||||||
| Thyroid carcinoma 1 (5.5%) | Abstention 4 | Abstention 0 | ||||||||||||||
| Shin et al3 | 2009 | 9 | 3 | 6 | 50 (14–71) | Lung 2 (22.2%) | 6 (66.7%) | 2 (22.2%) | 2 (22.2%) | 8 (88.9%) | 2 (22.2%) | Radiosurgery 9 | 8 | 1 | 1 | 240 (60–570) |
| Breast 3 (33.3%) | ||||||||||||||||
| Renal cell carcinoma 1 (11.1%) | ||||||||||||||||
| Melanoma 1 (11.1%) | ||||||||||||||||
| Choroid plexus carcinoma 1 (11.1%) | ||||||||||||||||
| Glioma 1 (11.1%) | ||||||||||||||||
| Flanagan et al4 | 2012 | 7 | 5 | 2 | 61 (41–81) | non-Hodgkin’s lymphoma 7 (100%) | 4 (57.1%) | 4 (57.1%) | 0 | NA | NA | Radiotherapy 1 | 6 (100%) | 0 | 0 | 345 (30–840) |
| Chemotherapy 3 Chemotherapy + Radiotherapy 2 | ||||||||||||||||
| Unknown 1 | ||||||||||||||||
| Hashii et al5 | 2011 | 18 | 8 | 10 | 55 (37–76) | Lung 8 (44.4%) | NA | NA | NA | 14 (77.8%) | NA | Radiotherapy | 8 (44.4%) | 10 (55.6%) | 0 | 120 |
| Breast 6 (33.3%) | ||||||||||||||||
| Melanoma 2 (11.1%) | ||||||||||||||||
| Renal cell carcinoma 1 (5.6%) | ||||||||||||||||
| Rectal cancer 1 (5.6%) | ||||||||||||||||
| Veeravagu et al6 | 2012 | 9 | 4 | 5 | 63 (33–77) | Lung 2 (22.2%) | 7 (77.8%) | 3 (33.3%) | 1 (11.1%) | NA | NA | Radiosurgery | 1 (20%) | 4 (80%) | 0 | 123 (33–273) |
| Breast 5 (55.6%) | ||||||||||||||||
| Cystic adenocarcinoma 1 (11.1%) | ||||||||||||||||
| Epithelioid hemangioepithelioma 1 (11.1%) | ||||||||||||||||
| Wilson et al7 | 2012 | 9 | 3 | 6 | 56 (38–68) | Lung 3 (33.3%) | 4 (44.4%) | 5 (55.6%) | 0 | NA | NA | Surgery | 1 (11.1%) | 7 (77.8%) | 1 (11.1%) | 192 |
| Breast 4 (44.4%) | ||||||||||||||||
| Melanoma 2 (22.2%) | ||||||||||||||||
| Hoover et al8 | 2012 | 15 | 9 | 6 | 55 (38–74) | Lung 1 (6.7%) | 3 (20%) | 2 (13%) | 10 (67%) | 3 (20%) | NA | Surgery | 8 (53.3%) | 2 (13.3%) | 5 (33.3%) | 150 |
| Breast 2 (13.3%) | ||||||||||||||||
| Melanoma 3 (20%) | ||||||||||||||||
| Renal cell carcinoma 3 (20%) | ||||||||||||||||
| Carcinoid tumor 1 (6.7%) | ||||||||||||||||
| Mesenchymal chondrosarcoma 2 (13.3%) | ||||||||||||||||
| Gastric adenocarcinoma 1 (6.7%) | ||||||||||||||||
| Chondrosarcoma 1 (6.7%) | ||||||||||||||||
| Diffuse large B-cell lymphoma 1 (6.7%) | ||||||||||||||||
| Diehn et al9; Rykken et al10 | 2015 | 49 | 23 | 26 | 57.7 (7–80) | Lung carcinoma 24 (49%) | 18 (26%) | 40 (57%) | 12 (17%) | NA | NA | NA | NA | NA | NA | 104 (95% CI=48–156) |
| Breast carcinoma 7 (14%) | ||||||||||||||||
| Melanoma 5 (10%) | ||||||||||||||||
| CNS origin 4 (8%) | ||||||||||||||||
| Renal cell carcinoma 3 (6%) | ||||||||||||||||
| Other 6 (12%) | ||||||||||||||||
| Payer et al11 | 2015 | 22 | 13 | 9 | 55 (21–86) | Lung carcinoma 6 (27.2%) | 9 (41%) | 14 (63.6%) | 5 (22.7%) | 9 (41%) | 6 (27.2%) | Surgery 22 (100%) | 4 (21%) | 11 (58%) | 4 (21%) | 348 |
| Breast carcinoma 3 (13.6%) | Surgery+Radiotherapy 6 (27.2%) | |||||||||||||||
| Melanoma 2 (9%) | Surgery+Chemotherapy 7 (31.8%) | |||||||||||||||
| CNS origin 3 (13.6%) | Surgery+Radiotherapy | |||||||||||||||
| Bladder carcinoma 1 (4.5%) | +Chemotherapy 3 (13.6%) | |||||||||||||||
| Prostate carcinoma 1 (4.5%) | ||||||||||||||||
| Ovarian carcinoma 1 (4.5%) | ||||||||||||||||
| Kidney carcinoma 1 (4.5%) | ||||||||||||||||
| Unknown 4 (18.1%) | ||||||||||||||||
Abbreviations: ISCM, intramedullary spinal cord metastases; NA, not available; pts, patients.
Materials and methods
Study population
From June 2010 to March 2018, 61 patients diagnosed as ISCM with a history of malignancy in the First Hospital, Jilin University were retrospectively analyzed. All the patients had a definitive MRI manifestation of ISCM. Besides, four patients underwent surgery which confirmed ISCM pathologically. Only three individuals were lost to follow-up. Data were retrieved according to characteristics such as age, gender, primary pathology, diagnostic methods, interval from primary cancer to ISCM, performance status (PS) at diagnosis of ISCM, involved segment and number of spinal cord, synchronous metastasis, interventions, treatment response, outcomes, and overall survival (OS). OS was defined as the period from the diagnosis of ISCM to the death or latest follow-up. The end date for last follow-up was July 8, 2018.
Ethical considerations
This study was approved by the Ethics Committee of the First Hospital of Jilin University (no 2018–358) and conducted in accordance with the Declaration of Helsinki. Patient consent to review their medical records was waived due to the retrospective nature of the study. Patient data were reviewed confidentially.
Statistical analysis
The cumulative survival curve was generated by Kaplan-Meier method. Log-rank (Mantel-Cox) Test and Gehan-Breslow-Wilcoxon Test were used in univariate analysis of prognostic factors for OS. P<0.05 was considered as statistically significant.
Results
Patient characteristics
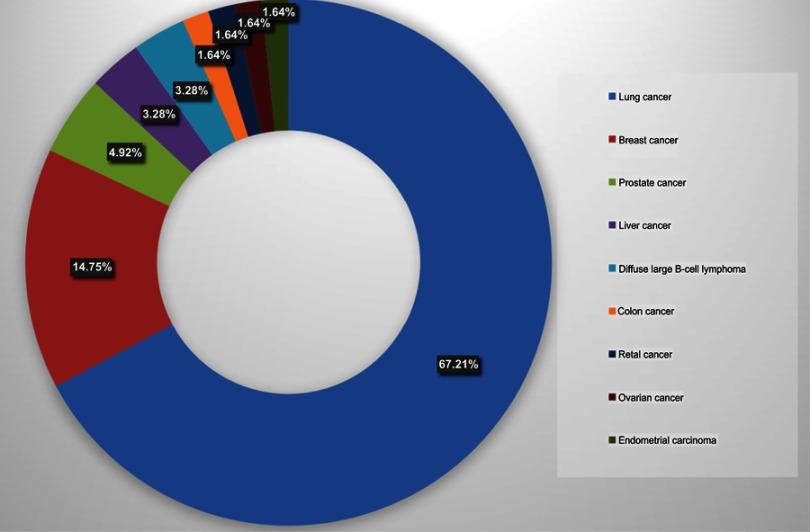
Among the 61 ISCM patients enrolled in this review, there was no predominance of gender (M:F=1.03). The mean age at diagnosis of ISCM was 57.90 years (range=35–78 years). Lung cancer (67.21%) constituted the majority of primary malignancies. Additionally, small cell lung carcinoma (SCLC) (39.34%) was the most common subtype of lung cancer in ISCM. Other offenders with less frequencies included breast cancer (14.75%), prostate cancer (4.92%), liver cancer (3.28%), diffuse large B-cell lymphoma (DLBCL, 3.28%), colon cancer (1.64%), retal cancer (1.64%), ovarian cancer (1.64%), and endometrial carcinoma (1.64%) (Figure 1). All the patients had definite MRI findings suggestive of ISCM. Four patients conducted local resection which confirmed ISCM pathologically besides the typical MRI manifestation of spinal cord parenchymal involvement. Notably, in six individuals, ISCM was the first sign of primary cancer outside the central nervous system (CNS). The mean interval from primary cancer developed to ISCM was 18.77 months (range=0–10 years). Generally, patients of ISCM had a poor physical function. Over half of individuals (55.74%) had a PS score of 3 or 4. Twenty-six patients developed into complete paraplegia, and the average interval to paraplegia was 22.85 days. At the diagnosis of ISCM, in terms of the control of primary cancer, 20 and 28 patients were evaluated as progressive disease (PD) and stable disease (SD), respectively. Eight candidates underwent complete resection of the primaries (Table 2).
Figure 1.
Histologic types of primary cancer in the development of ISCM (n=61).
Abbreviation: ISCM, intramedullary spinal cord metastases.
Table 2.
Clinical features of ISCM patients
| n | % | |
|---|---|---|
| Sex (n=61) | ||
| Male | 31 | 50.82 |
| Female | 30 | 49.18 |
| Age (years) (n=61) | ||
| Mean at diagnosis of ISCM (years) | 57.90 | |
| Range (years) | 35-78 | |
| Primary Cancer Pathology (n=61) | ||
| Lung cancer | 41 | 67.21 |
| SCLC | 24 | 39.34 |
| Lung adenocarcinoma | 8 | 13.11 |
| Lung squamous carcinoma | 1 | 1.64 |
| Lung large cell carcinoma | 1 | 1.64 |
| Lung poorly differentiated adenocarcinoma with neuroendocrine carcinoma | 1 | 1.64 |
| NSCLC | 1 | 1.64 |
| Not specified | 5 | 8.20 |
| Breast Cancer | 9 | 14.75 |
| Prostate Cancer | 3 | 4.92 |
| Liver Cancer | 2 | 3.28 |
| DLBCL | 2 | 3.28 |
| Colon Cancer | 1 | 1.64 |
| Retal Cancer | 1 | 1.64 |
| Ovarian cancer | 1 | 1.64 |
| Endometrial carcinoma | 1 | 1.64 |
| MRI (n=61) | ||
| Yes | 61 | 100 |
| No | 0 | 0 |
| ISCM pathology (n=61) | ||
| Yes (surgery) | 4 | 6.56 |
| No | 57 | 93.44 |
| Interval from primary cancer to ISCM (n=61) | ||
| At diagnosis of primary cancer | 6 | 9.84 |
| Mean (months) | 18.77 | |
| Range (years) | 0-10 | |
| PS at diagnosis of ISCM-ECOG (n=61) | ||
| 0 | 1 | 1.64 |
| 1 | 13 | 21.31 |
| 2 | 13 | 21.31 |
| 3 | 21 | 34.43 |
| 4 | 13 | 21.31 |
| Occurrence of paraplegia (n=60) | ||
| Yes | 26 | 43.33 |
| No | 34 | 56.67 |
| Speed until paraplegia (n=26) | ||
| Average interval to paraplegia (day) | 22.85 | |
| Control of the primary cancer at the diagnosis of ISCM (n=58) | ||
| PD | 20 | 34.48 |
| SD | 28 | 48.28 |
| PR or CR | 2 | 3.45 |
| CR (complete resection) | 8 | 13.79 |
Abbreviations: DLBCL, diffuse large B-cell lymphoma; ISCM, intramedullary spinal cord metastases; NSCLC, non-small cell lung carcinoma; PD, progressive disease; SD, stable disease, SCLC, small cell lung carcinoma; ECOG, eastern cooperative oncology group; MRI, magnetic resonance imaging; PR, partial remission; CR, complete remission.
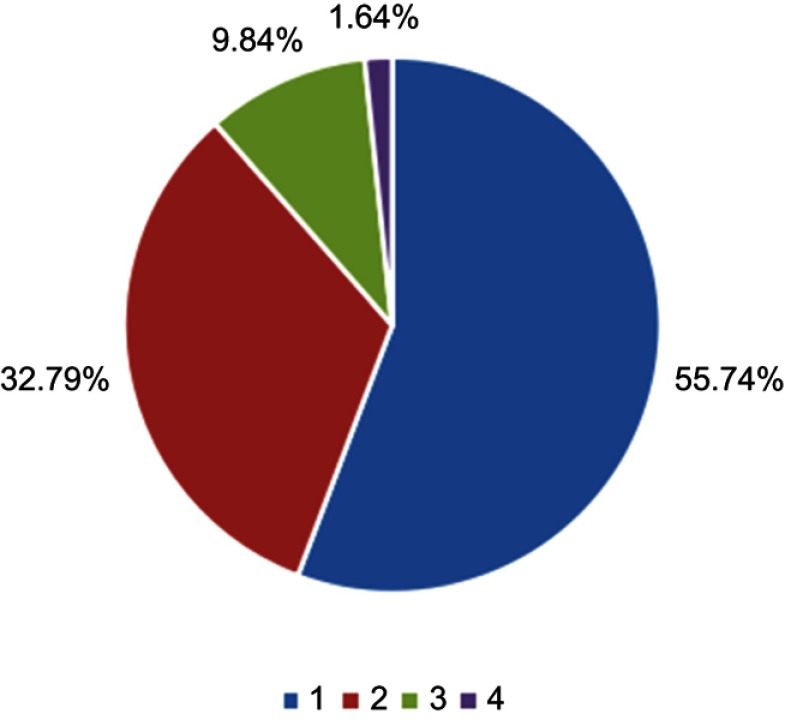
In terms of the involved spinal cord segments of ISCM, the thoracic segment was most common (77.05%), followed by cervical (39.34%), lumbar level (34.43%), and conus medullaris (6.56%) (Table 3). In total, 55.74% patients involved one spinal cord level among C/T/L/Conus medullaris, while 32.79% individuals had two levels of ISCM, 9.84% patients had three levels of involvement, while only one patient (1.64%) had whole spinal cord involvement (Figure 2).
Table 3.
Involved spinal cord segments of ISCM patients (n=61)
| Involved spinal cord segments | n | % |
|---|---|---|
| C | 24 | 39.34 |
| T | 47 | 77.05 |
| L | 21 | 34.43 |
| Conus medullaris | 4 | 6.56 |
Abbreviation: ISCM, intramedullary spinal cord metastases.
Figure 2.
The number of involved spinal cord segments at the diagnosis of ISCM (n=61).
Abbreviation: ISCM, intramedullary spinal cord metastases.
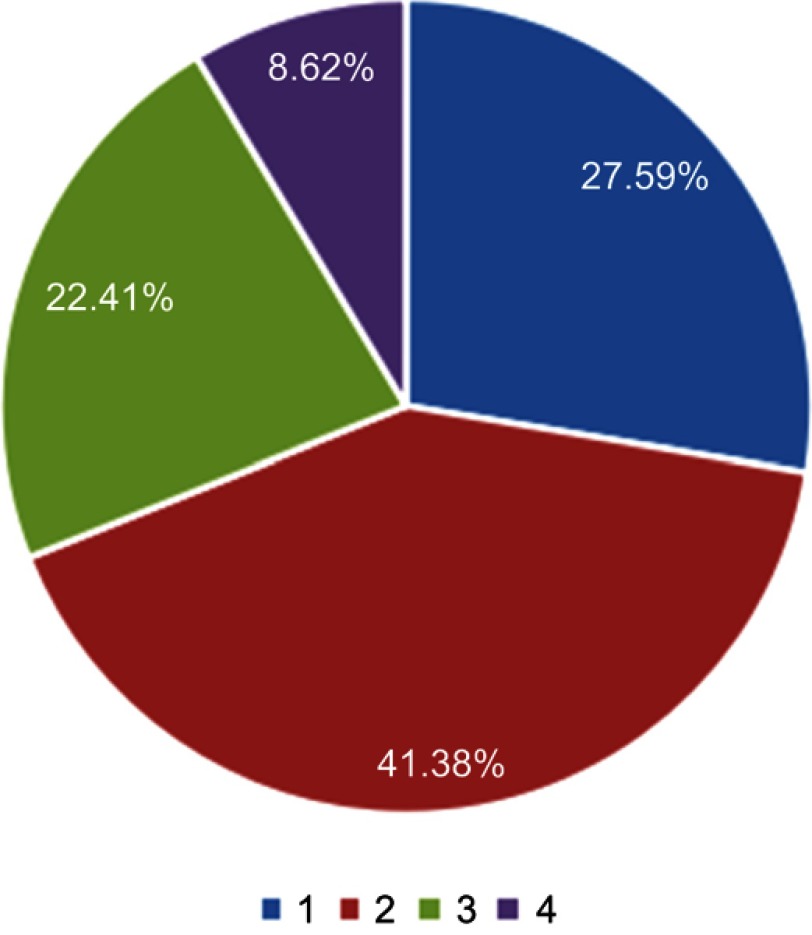
Synchronous metastases in other organs or tissues of ISCM patients were found in 58 patients. Bone was the most common metastatic sites concurrently (75.86%), followed by brain (62.07%), leptomeninges (24.14%), liver (17.24%), adrenal gland (12.07%), lung (5.17%), kidney (3.45%), marrow (3.45%), muscle (3.45%), non-regional lymph node (3.45%), and spleen (1.72%) (Table 4). Unsurprisingly, 72.41% patients had multiple metastases in other sites synchronously when diagnosed as ISCM (two organs: 41.38%; three organs: 22.41%; four organs: 8.62%), indicating ISCM was generally end-stage disease (Figure 3).
Table 4.
Other synchronous metastatic sites of ISCM patients (n=58)
| Sites of metastases | n | % |
|---|---|---|
| Lung | 3 | 5.17 |
| Liver | 10 | 17.24 |
| Bone | 44 | 75.86 |
| Brain | 36 | 62.07 |
| leptomeninges | 14 | 24.14 |
| Adrenal gland | 7 | 12.07 |
| Kidney | 2 | 3.45 |
| Spleen | 1 | 1.72 |
| Marrow | 2 | 3.45 |
| Muscle | 2 | 3.45 |
| Non-regional lymph node | 2 | 3.45 |
Abbreviation: ISCM, intramedullary spinal cord metastases.
Figure 3.
The number of concurrent metastases in other organs at the diagnosis of ISCM (n=58).
Abbreviation: ISCM, intramedullary spinal cord metastases.
Treatment
After diagnosis of ISCM, 13 individuals refused any interventions, and the treatment information was unavailable in one patient. Due to the poor general status and heavy tumor burden, a small proportion of patients underwent surgery (8.51%), while 82.98% performed local irradiation, 36.17% undertook chemotherapy, and only one patient took Crizotinib for ALK gene rearrangement (Table 5). As the first therapeutic option of ISCM, RT was essential for definite efficacy and acceptable toxicity. RT achieved an ORR of 61.90% or 62.50% and LCR of 90.48% or 87.50% for symptoms used singly or combined with other strategies, respectively (Table 6). Only one patient underwent RT with 5 Gy in a single fraction. Thirty-six patients conducted conventional external beam RT with 1.8/2.0/2.5/3.0 Gy per fraction.
Table 5.
Treatment strategies of the ISCM patients (n=47)
| n | % | |
|---|---|---|
| Surgery | ||
| Yes | 4 | 8.51 |
| No | 43 | 91.49 |
| Radiotherapy | ||
| Yes | 39 | 82.98 |
| No | 8 | 17.02 |
| Chemotherapy | ||
| Yes | 17 | 36.17 |
| No | 30 | 63.83 |
| Target therapy | ||
| Yes | 1 | 2.13 |
| No | 46 | 97.87 |
Abbreviation: ISCM, intramedullary spinal cord metastases.
Table 6.
Evaluation for symptoms control by RT (n=37)
| n | ORR (%) | LCR (%) | |
|---|---|---|---|
| RT | 21 | 61.90 | 90.48 |
| Complete remission | 2 | ||
| Improved | 11 | ||
| Unchanged | 6 | ||
| Deteriorated | 2 | ||
| Multiple modality including RT | 16 | 62.50 | 87.50 |
| RT+CT Improved |
8 6 |
||
| Unchanged | 2 | ||
| RT+CT+nerve blocking | 1 | ||
| Improved | 1 | ||
| RT+IT | 4 | ||
| Improved | 2 | ||
| Unchanged | 1 | ||
| Deteriorated | 1 | ||
| RT+Crizotinib | 1 | ||
| Unchanged | 1 | ||
| RT+S+CT | 2 | ||
| Improved | 1 | ||
| Deteriorated | 1 |
Abbreviations: RT, radiotherapy; LCR, local control rate; IT, intrathecal chemotherapy; S, surgery; CT, chemotherapy; ORR, objective response rate.
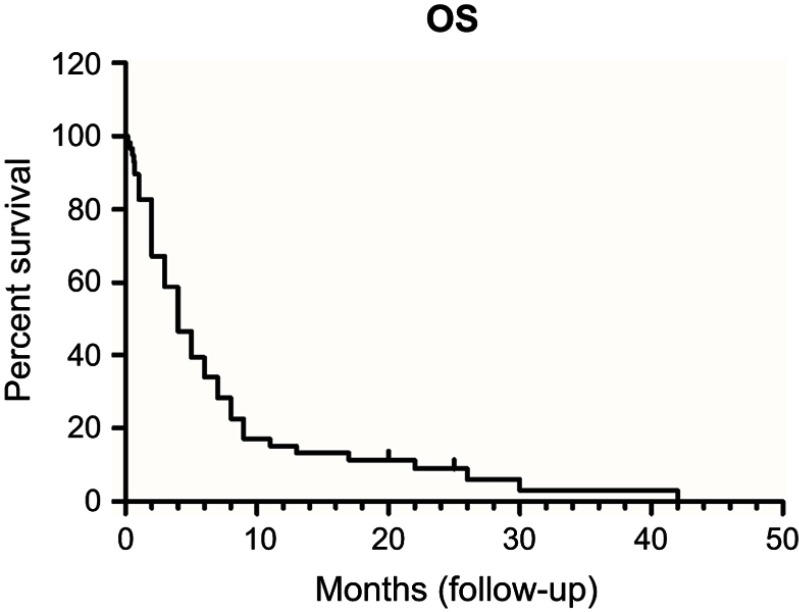
Survival analysis
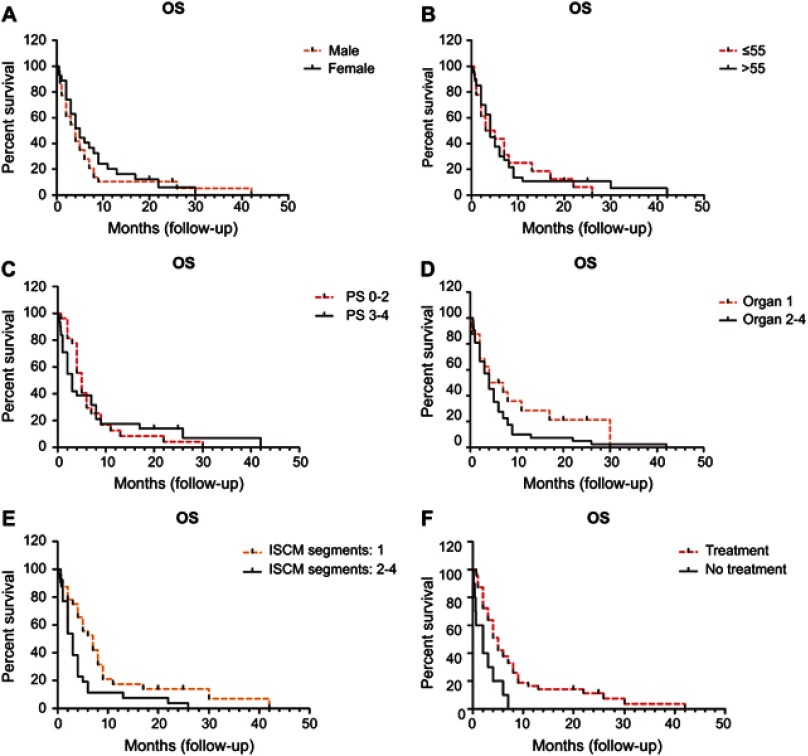
ISCM patients usually bear a rather dismal prognosis. The median OS of ISCM was only 4 months (Figure 4).
Figure 4.
Overall survival (OS) curve (n=58).
Gender and age were not prognostic factors of OS in ISCM patients (M: 4.0 vs F: 5.0 months; ≤55: 4.0 vs >55: 4.0 months). The median OS for patients with a PS score of 0–2 was longer than its counterpart with a PS score 3–4 (5.0 vs 3.0 months) but failed to reach statistical difference. The median OS for candidates with one synchronous metastatic site appear to be better than those with 2–4 sites (5.5 vs 4.0 months), but didn’t reach statistical difference. The number of involved spinal cord segments had a strong association with OS. Patients with only one segment involved (C/T/L/Conus medullaris) had an apparently better prognosis than those with two-to-four involved segments (median OS: 7.0 vs 3.0 months) (P<0.01). The OS of patients treated was remarkably superior to those without any management (median OS: 5.0 vs 2.0 months) (P<0.01) (Table 7, Figure 5).
Table 7.
Univariate analysis of prognostic factors for OS
| Prognostic factor | Hazard ratio (95% CI) |
P-value Log-rank (Mantel-Cox) test |
P-value Gehan-Breslow-Wilcoxon test |
|---|---|---|---|
| Gender (M: F) | 1.258 (0.7081–2.235) | 0.4338 | 0.2450 |
| Age (years) (≤55: >55) | 1.055 (0.5666–1.963) | 0.8664 | 0.9522 |
| PS (0–2: 3–4) | 0.9207 (0.5171–1.639) | 0.7789 | 0.1220 |
| Number of concurrent metastases in other organs (1: 2–4) |
0.6413 (0.3473–1.184) | 0.1557 | 0.3649 |
| Number of involved spinal cord segments (1: 2–4) | 0.4199 (0.2254–0.7823) | 0.0063 | 0.0063 |
| Treatment (yes: no) | 0.1987 (0.06931–0.5697) | 0.0026 | 0.0024 |
Abbreviations: OS, overall survival; PS, performance status.
Figure 5.
Number of ISCM segments and treatment affect OS of ISCM while gender, age, PS and number of synchronous metastatic sites fail to show a defined correlation with OS.
Abbreviations: ISCM, intramedullary spinal cord metastases; OS, overall survival; PS, performance status.
Discussion
ISCM from malignancies is underestimated in clinical practice, especially in the pre-MRI era.13 With the increasing morbidity of cancer and the prolongation of patients’ survival, the actual incidence of ISCM is rising. However, due to the relative low occurrence of ISCM and general setting of end-stage of cancer, no prospective studies have been conducted to date.13 As for the retrospective study, the largest research included 49 patients.9,10 Sung et al1 performed a 20-year retrospective study in the Royal Hobart Hospital of eight ISCM patients and reviewed 291 ISCM cases published in the literature since 1960. We conducted a review of related English literature with the key word “intramedullary spinal cord metastases” in Pubmed from June 2009 to January 2019, and found 10 case series of small samples.1–11 The clinicopathological characteristics, managements, and subsequent outcomes of this special entity are summarized in Table 1. To the best of our knowledge, our study is the largest single center research currently, and will help a lot to expand our understanding and aid practitioners in treatment of this unique situation.
Epidemiologically, in our research, ISCM occurred in a wide spectrum of patients, aged from 35 to 78 years, with a mean age at diagnosis of 57.90 years. ISCM was more likely to affect elderly individuals, similar to previous reports.1–11,14 There was no predominance of gender, different from the male or female predominance described by other series.6,7,11,15,16 Notably, ISCM can herald an underlying neoplasm. 9.84% of patients presented with ISCM initially in our study, lower than the 18.75–25% reported previously.13,17,18 The interval from primary cancer to ISCM spanned up to 10 years, reminding clinicians of the possibility of ISCM in cancer populations presented with typical neurological deficits and MRI findings, even after a long latency. The thoracic segment appeared to be the most common involved (77.05%), similar to the reports by Diehn et al,9 Rykken et al10 and Payer et al.11 Rades and Schiff13 while Findlay et al19 found that the cervical segment was the most common site. Unsurprisingly, lung cancer was the main offender of ISCM, especially the subset of SCLC tending to have ISCM, followed by breast cancer, the same as previous reports.1,2,5,9,10 This might be partly explained by the fact that lung and breast cancer have the highest incidence with a large population.
Three possible routes have been posed for the occurrence of ISCM.20 Haematogeneous dissemination via arterial and/or venous pathways is considered as the main contributor. The coexistence of brain and lung (62.07% and 5.17% in our series,respectively) metastases confirmed spread by the arterial system. In the reports by Sung et al1 and Hashii et al,5 the brain metastasis coexisted in 61% and 77.8% of the cohort respectively. Leptomeningeal seeding via cerebrospinal fluid circulation is another vital mechanism. In our study, 24.14% of candidates coexisted with definite meningeal metastasis, supporting the above idea. Besides, the direct invasion from metastases of adjacent structures accounts for a considerable proportion of patients. Bone metastasis resulted in epidural spinal cord compression, then invaded through the dura and into the spinal cord parenchyma. Bone was the most common metastatic site concurrently (75.86%) in our report, suggesting direct invasion was an important route in ISCM.
Generally, ISCM patients bear an extremely grim outcome with a median OS of 4 months in our report, similar to 3–4 months by previous research.14,21 In the analyses summarized in Table 1, the median OS of ISCM ranged from 104–348 days.1–11 Therefore, the optimal intervention paradigm for ISCM has not been established.
RT has been proved to be critical in maintenance of quality-of-life (QoL) in ISCM individuals.5 However, for radioresistant tumors such as renal cell carcinoma and melanoma, conventional external RT (30 Gy in 10 fractions or 40 Gy in 20 fractions) is hard for ideal control of ISCM and relief of symptoms. Stereotactic radiosurgery (SRS) is promising for limited, oligometastatic disease.17 Parikh and Heron22 documented Cyberknife SRS (15 Gy in three fractions) contributed to a 26-month OS and nearly fully functional recovery in an ISCM patient of renal cell carcinoma. A retrospective research of nine patients with 11 ISCM conducted by Veeravagu et al6 noted that Cyberknife SRS delivered 14–27 Gy (median 21 Gy) in 1–5 fractions (median=3 fractions) was safe and effective. The neurological status post-management was improved and unchanged in 20% and 80% of patients, respectively.6 Garcia et al23 reported a dose of 14 Gy in one fraction to an ISCM lesion in a heavily-treated breast cancer patient who attained long-term local control of 37 months without obvious toxicity. The majority of patients in our research performed conventional fractionated RT with a single dose of 1.8/2.0/2.5/3.0 Gy and also gained satisfactory improvement of symptoms. This might be partly explained by the fact that radiosensitive tumors such as SCLC and breast cancer constituted the mainstay of the cohort.
For a long period, surgery has no role in the management of ISCM. With the development of intraoperative imaging guidance and microsurgical technique, surgery can exert a certain effect for the selective cohort. For those highly selected patients such as limited tumor burden, satisfactory PS and non-lymphoma primary, radical resection can bring OS benefit and neurological function improvement. In the document described by Sung et al,1 36 out of 89 patients (40.45%) who underwent surgery attained improved neurological status. In the cohort presented by Payer et al,11 22 ISCM patients performed surgery and gained the longest median OS of 348 days. The median OS was beyond 9.4 months in the surgery group vs 5 months in the conservative intervention group for ISCM.24 The surgery indication in ISCM should be highly selective, since the benefits and risks of surgery need to be fully evaluated. It had been reported that radical tumor resection of ISCM brought no survival benefits, but the deterioration of patients’ function.25 However, in our retrospective research, surgery was unfeasible for the majority of patients, since 55.74% of the candidates had a poor physical status (PS=3–4), 44.26% had multiple involvements of spinal cord segments, and 72.41% of patients had widespread metastases in other sites synchronously when diagnosed as ISCM. Multimodal local intervention including surgery and irradiation might attain survival benefit. Minomo et al26 reported an ISCM patient of squamous cell lung cancer who experienced repeated recurrences, surgery, and radiotherapy twice contributed a long-term survival of 25 months.
Due to the existence of blood–spinal barrier, chemotherapy has little effect on the treatment of ISCM and failed to extend the survival for ISCM patients.24 Nowadays, chemotherapy is reserved for chemotherapy-sensitive tumors (such as small cell lung cancer and hematological neoplasms) and as an adjuvant therapy for radiotherapy or surgery.18
For patients with rapidly developing spinal cord compression symptoms, steroid can quickly relieve pain and delay neurological deterioration by reducing local tissue edema and promoting the normality of the blood–spinal barrier without prolonging the patient’s survival.24 Nowadays, for ISCM, the steroid is generally combined with other treatment strategies.18
In the modern era of immunotherapy, checkpoint inhibitors shed new light for recurrent, refractory, and metastatic circumstances. Phillips et al27 documented regression of an ISCM with Nivolumab, an anti-programmed cell death-1 (PD-1) antibody in pulmonary adenocarcinoma. This dramatic response exerted by Nivolumab might be for tiny lesions such as 4 mm of ISCM described in the case mentioned above.27 Since the majority of ISCM is large with multiple segment involvements, the exact effect of checkpoint inhibitors is still unclear and needs to be further explored.
The treatment-related adverse events have been seldom reported previously, probably owing to the quite short survival of patients. Long-term adverse events, such as radiation myelitis, have not yet occurred in patients who have died. Further deterioration of neurological function postoperatively has been revealed by some studies, partly due to spinal cord edema and intraoperative nerve injury.1,2,8,11,25
In terms of factors influencing therapeutic activity, ISCM originated from lung/breast carcinomas having a poorer survival than other pathological types. Besides, multiple ISCMs is also a worse prognostic indicator.9 Multiple spinal cord levels involvement and no treatment were unveiled to be poor prognostic factors of OS. Thus, early identification and active management is paramount for this unique entity. Multidisciplinary approach should be available and individualized according to the primary pathology, systemic spread of tumor, PS, and economic status of patient.
Conclusion
ISCM is a special entity needing more attention with increasing incidence and still grim prognosis. Early diagnosis and multidisciplinary approach are critical for a better outcome. RT remains the mainstay of management nowadays. In the near future, multicentral or even national data is needed to clarify the clinicopathological features, prognostic factors, and optimal intervention for this unique disease.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Sung WS, Sung MJ, Chan JH, et al. Intramedullary spinal cord metastases: a 20-year institutional experience with a comprehensive literature review. World Neurosurg. 2013;79(3–4):576–584. [DOI] [PubMed] [Google Scholar]
- 2.Dam-Hieu P, Seizeur R, Mineo JF, Metges JP, Meriot P, Simon H. Retrospective study of 19 patients with intramedullary spinal cord metastasis. Clin Neurol Neurosurg. 2009;111(1):10–17. doi: 10.1016/j.clineuro.2008.06.019 [DOI] [PubMed] [Google Scholar]
- 3.Shin DA, Huh R, Chung SS, Rock J, Ryu S. Stereotactic spine radiosurgery for intradural and intramedullary metastasis. Neurosurg Focus. 2009;27(6):E10. doi: 10.3171/2009.9.FOCUS09194 [DOI] [PubMed] [Google Scholar]
- 4.Flanagan EP, O’Neill BP, Habermann TM, Porter AB, Keegan BM. Secondary intramedullary spinal cord non-Hodgkin’s lymphoma. J Neurooncol. 2012;107(3):575–580. doi: 10.1007/s11060-011-0781-4 [DOI] [PubMed] [Google Scholar]
- 5.Hashii H, Mizumoto M, Kanemoto A, et al. Radiotherapy for patients with symptomatic intramedullary spinal cord metastasis. J Radiat Res. 2011;52(5):641–645. [DOI] [PubMed] [Google Scholar]
- 6.Veeravagu A, Lieberson RE, Mener A, et al. CyberKnife stereotactic radiosurgery for the treatment of intramedullary spinal cord metastases. J Clin Neurosci. 2012;19(9):1273–1277. doi: 10.1016/j.jocn.2012.02.002 [DOI] [PubMed] [Google Scholar]
- 7.Wilson DA, Fusco DJ, Uschold TD, Spetzler RF, Chang SW. Survival and functional outcome after surgical resection of intramedullary spinal cord metastases. World Neurosurg. 2012;77(2):370–374. doi: 10.1016/j.wneu.2011.07.016 [DOI] [PubMed] [Google Scholar]
- 8.Hoover JM, Krauss WE, Lanzino G. Intradural spinal metastases: a surgical series of 15 patients. Acta Neurochir (Wien). 2012;154(5):871–877. discussion 877. doi: 10.1007/s00701-012-1313-5 [DOI] [PubMed] [Google Scholar]
- 9.Diehn FE, Rykken JB, Wald JT, et al. Intramedullary spinal cord metastases: prognostic value of MRI and clinical features from a 13-year institutional case series. AJNR Am J Neuroradiol. 2015;36(3):587–593. doi: 10.3174/ajnr.A4160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rykken JB, Diehn FE, Hunt CH, et al. Intramedullary spinal cord metastases: MRI and relevant clinical features from a 13-year institutional case series. AJNR Am J Neuroradiol. 2013;34(10):2043–2049. doi: 10.3174/ajnr.A3526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Payer S, Mende KC, Westphal M, Eicker SO, Eicker SO. Intramedullary spinal cord metastases: an increasingly common diagnosis. Neurosurg Focus. 2015;39(2):E15. doi: 10.3171/2015.5.FOCUS15149 [DOI] [PubMed] [Google Scholar]
- 12.Osawa H, Okauchi S, Ohara G, Kagohashi K, Satoh H. A long-term control of intramedullary thoracic spinal cord metastasis from small cell lung cancer. Acta Medica (Hradec Kralove). 2018;61(2):57–59. doi: 10.14712/18059694.2018.52 [DOI] [PubMed] [Google Scholar]
- 13.Rades D, Schiff D. Epidural and intramedullary spinal metastasis: clinical features and role of fractionated radiotherapy. Handb Clin Neurol. 2018;149:227–238. doi: 10.1016/B978-0-12-811161-1.00015-3 [DOI] [PubMed] [Google Scholar]
- 14.Lee SS, Kim MK, Sym SJ, et al. Intramedullary spinal cord metastases: a single-institution experience. J Neurooncol. 2007;84(1):85–89. doi: 10.1007/s11060-007-9345-z [DOI] [PubMed] [Google Scholar]
- 15.Costigan DA, Winkelman MD. Intramedullary spinal cord metastasis. A clinicopathological study of 13 cases. J Neurosurg. 1985;62(2):227–233. doi: 10.3171/jns.1985.62.2.0227 [DOI] [PubMed] [Google Scholar]
- 16.Potti A, Abdel-Raheem M, Levitt R, Schell DA, Mehdi SA. Intramedullary spinal cord metastases (ISCM) and non-small cell lung carcinoma (NSCLC): clinical patterns, diagnosis and therapeutic considerations. Lung Cancer. 2001;31(2–3):319–323. [DOI] [PubMed] [Google Scholar]
- 17.Majmundar N, Shao B, Assina R. Lung adenocarcinoma presenting as intramedullary spinal cord metastasis: case report and review of literature. J Clin Neurosci. 2018;52:124–131. doi: 10.1016/j.jocn.2018.03.030 [DOI] [PubMed] [Google Scholar]
- 18.Kalayci M, Cagavi F, Gul S, Yenidunya S, Acikgoz B. Intramedullary spinal cord metastases: diagnosis and treatment - an illustrated review. Acta Neurochir (Wien). 2004;146(12):1347–1354. discussion 1354. doi: 10.1007/s00701-004-0386-1 [DOI] [PubMed] [Google Scholar]
- 19.Findlay JM, Bernstein M, Vanderlinden RG, Resch L. Microsurgical resection of solitary intramedullary spinal cord metastases. Neurosurgery. 1987;21(6):911–915. [DOI] [PubMed] [Google Scholar]
- 20.Hrabalek L. Intramedullary spinal cord metastases: review of the literature. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2010;154(2):117–122. [DOI] [PubMed] [Google Scholar]
- 21.Schiff D, O’Neill BP. Intramedullary spinal cord metastases: clinical features and treatment outcome. Neurology. 1996;47(4):906–912. [DOI] [PubMed] [Google Scholar]
- 22.Parikh S, Heron DE. Fractionated radiosurgical management of intramedullary spinal cord metastasis: A case report and review of the literature. Clin Neurol Neurosurg. 2009;111(10):858–861. doi: 10.1016/j.clineuro.2009.06.008 [DOI] [PubMed] [Google Scholar]
- 23.Garcia R, Sallabanda K, Santa-Olalla I, et al. Robotic radiosurgery for the treatment of intramedullary spinal cord metastases: a case report and literature review. Cureus. 2016;8(5):e609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalita O. Current insights into surgery for intramedullary spinal cord metastases: a literature review. Int J Surg Oncol. 2011;2011:989506. doi: 10.1155/2011/989506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gasser T, Sandalcioglu IE, El Hamalawi B, van de Nes JA, Stolke D, Wiedemayer H. Surgical treatment of intramedullary spinal cord metastases of systemic cancer: functional outcome and prognosis. J Neurooncol. 2005;73(2):163–168. doi: 10.1007/s11060-004-4275-5 [DOI] [PubMed] [Google Scholar]
- 26.Minomo S, Tokoro A, Utsumi T, Ishihara M, Akira M, Atagi S. A case of long-term survival after multimodal local treatments of intramedullary spinal cord metastasis of squamous cell lung cancer. J Thorac Dis. 2016;8(8):E681–683. doi: 10.21037/jtd.2016.06.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phillips KA, Gaughan E, Gru A, Schiff D. Regression of an intramedullary spinal cord metastasis with a checkpoint inhibitor: a case report. CNS Oncol. 2017;6(4):275–280. doi: 10.2217/cns-2017-0007 [DOI] [PMC free article] [PubMed] [Google Scholar]