ABSTRACT
Objective
Hypertensive disorders affect 3–10% of pregnancies. Delayed delivery carries maternal risks, while early delivery increases fetal risk, so appropriate timing is important. The aim of this study was to compare immediate delivery with expectant management for prevention of adverse maternal and neonatal outcomes in women with hypertensive disease in pregnancy.
Methods
CENTRAL, PubMed, MEDLINE and ClinicalTrials.gov were searched for randomized controlled trials comparing immediate delivery to expectant management in women presenting with gestational hypertension or pre‐eclampsia without severe features from 34 weeks of gestation. The primary neonatal outcome was respiratory distress syndrome (RDS) and the primary maternal outcome was a composite of HELLP syndrome and eclampsia. The PRISMA‐IPD guideline was followed and a two‐stage meta‐analysis approach was used. Relative risks (RR) and numbers needed to treat or harm (NNT/NNH) with 95% CI were calculated to evaluate the effect of the intervention.
Results
Main outcomes were available for 1724 eligible women. Compared with expectant management, immediate delivery reduced the composite risk of HELLP syndrome and eclampsia in all women (0.8% vs 2.8%; RR, 0.33 (95% CI, 0.15–0.73); I 2 = 0%; NNT, 51 (95% CI, 31.1–139.3)) as well as in the pre‐eclampsia subgroup (1.1% vs 3.5%; RR, 0.39 (95% CI, 0.15–0.98); I 2 = 0%). Immediate delivery increased RDS risk (3.4% vs 1.6%; RR, 1.94 (95% CI 1.05–3.6); I 2 = 24%; NNH, 58 (95% CI, 31.1–363.1)), but depended upon gestational age. Immediate delivery in the 35th week of gestation increased RDS risk (5.1% vs 0.6%; RR, 5.5 (95% CI, 1.0–29.6); I 2 = 0%), but immediate delivery in the 36th week did not (1.5% vs 0.4%; RR, 3.4 (95% CI, 0.4–30.3); I 2 not applicable).
Conclusion
In women with hypertension in pregnancy, immediate delivery reduces the risk of maternal complications, whilst the effect on the neonate depends on gestational age. Specifically, women with a‐priori higher risk of progression to HELLP, such as those already presenting with pre‐eclampsia instead of gestational hypertension, were shown to benefit from earlier delivery. © 2019 The Authors. Ultrasound in Obstetrics & Gynecology published by John Wiley & Sons Ltd on behalf of the International Society of Ultrasound in Obstetrics and Gynecology.
Keywords: expectant management, HELLP syndrome, immediate delivery, pre‐eclampsia, RDS
INTRODUCTION
Hypertensive disorders are present in 3–10% of all pregnancies1, 2, 3. They are among the main causes of maternal and perinatal morbidity and mortality4, 5, 6, 7. Worldwide, between 80 and 120 women with a pregnancy complicated by hypertension die each day8.
Delivery of the placenta remains the only definitive treatment for pregnancy hypertensive disorders. However, early iatrogenic delivery potentially affects perinatal outcome. Preterm birth is associated with increased perinatal mortality and additional morbidity in the short and long term9, 10, 11, 12, 13, 14. Although induction of labor was considered previously to result in higher Cesarean section rates15, 16, 17, 18, 19, 20, 21, recent studies demonstrate lower or equivalent rates22, 23, 24, 25.
On the other hand, prolonging pregnancies complicated by hypertensive disorders may increase maternal risk26, 27. Managed expectantly, gestational or chronic hypertension may progress to pre‐eclampsia28, 29 or to more severe complications such as eclampsia, placental abruption or HELLP syndrome.
Management strategies for hypertensive disorders of pregnancy have been evaluated at various gestational ages27, 29, 30, 31. In the HYPITAT‐I trial, women with gestational hypertension or pre‐eclampsia without severe features from 36 + 0 weeks of gestation were randomized to either immediate delivery or expectant management27. The Deliver or Deliberate trial evaluated immediate delivery vs expectant management (until the 37th week of gestation) for women with pre‐eclampsia between 34 + 0 and 36 + 6 weeks of gestation30. The same management strategies and gestational age range were studied in the HYPITAT‐II trial31.
These trials evaluated different outcomes, gestational ages and hypertensive disorders and used composite outcome to overcome the rarity of severe outcomes. They also had different inclusion and exclusion criteria and intervention protocols. Therefore, general conclusions regarding optimal timing of delivery, when the benefits of immediate delivery outweigh the consequences of early delivery, are difficult to draw.
Combining individual participant data from these trials has the potential to overcome some of these drawbacks and provide stronger evidence to guide clinical practice and future research. With this aim, we performed an individual participant data meta‐analysis (IPDMA) comparing immediate delivery to expectant monitoring for the prevention of adverse maternal and neonatal outcomes in pregnancies from 34 weeks of gestation complicated by a hypertensive disorder.
SUBJECTS AND METHODS
This IPDMA was registered on PROSPERO (CRD42017083348) and its protocol was published after peer‐review32. It is reported in accordance with the PRISMA‐IPD statement33.
Search strategy
An electronic search of CENTRAL, PubMed, MEDLINE and ClinicalTrials.gov was performed for published or registered randomized controlled trials (RCT) comparing immediate delivery with expectant management in women presenting with gestational hypertension or pre‐eclampsia without severe features from 34 weeks of gestation. The following search strategy was used: (‘hypertensive disorders of pregnancy’ OR ‘pregnancy induced hypertension’ OR ‘gestational hypertension’ OR (‘pre‐eclampsia’ OR ‘preeclampsia’) OR (‘hypertension’ AND (‘chronic’ OR ‘chronical*’ OR ‘pre‐existent’ OR ‘preexistent’)) AND ‘pregnancy’), with the limits ‘human’ and ‘randomized controlled trial’. The search period was from database inception to 31 December 2017. Cluster‐randomized trials and quasirandom design studies were not eligible. Authors of eligible trials were asked whether they were aware of relevant studies that had not been identified in the search.
Data collection
Authors of eligible studies were approached to participate in the IPDMA, comment on the protocol draft and provide data. Supplied data were assessed for missing data, internal consistency and randomization. Summary statistics of relevant variables were checked against published results. Investigators were asked for clarification on discrepancies and a final summary was sent for verification. After resolution, individual study datasets were merged into the IPDMA dataset. All included trials were approved by the relevant committees. Details can be found in the original manuscripts.
Inclusion and exclusion criteria
Women were included with singleton or multiple pregnancy presenting from 34 weeks of gestation onwards with gestational hypertension, pre‐eclampsia, deteriorating pre‐existing hypertension or superimposed pre‐eclampsia.
Hypertension was defined as blood pressure (BP) levels ≥ 140 mmHg systolic or ≥ 90 mmHg diastolic and pre‐eclampsia as hypertension and proteinuria (300 mg or more total protein in a 24‐h urine sample, recurrent positive protein dipstick test or protein/creatinine ratio of 30 mg/mmol or more). Deteriorating pre‐existing hypertension was defined as the need for new or additional antihypertensive drugs after 34 weeks of gestation in women with pre‐existing hypertension. Superimposed pre‐eclampsia was defined as new onset proteinuria in women with pre‐existing hypertension.
Participants were excluded with signs of severe disease (BP ≥ 160 mmHg systolic or ≥ 110 mmHg diastolic, proteinuria ≥ 5 g/24 h, oliguria, cerebral/visual disturbances, pulmonary edema/cyanosis, epigastric or right upper quadrant pain, impaired liver function and thrombocytopenia), as well as with diabetes mellitus, gestational diabetes requiring insulin treatment, kidney or heart disease, HELLP syndrome or HIV. Pregnancies with suspected or confirmed major structural or chromosomal abnormality were also excluded.
Risk of bias assessment
Two investigators (H.G. and T.P.B.) independently evaluated the included trials for risk of bias. This assessment was based on criteria found in the Cochrane Handbook for Systematic Reviews of Interventions34. The criteria were as follows: random sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessment; incomplete outcome data; selective reporting and other bias. Each was characterized as low, unclear or high for each trial. Disagreements were resolved by consensus.
Outcome measures
The primary neonatal outcome was respiratory distress syndrome (RDS) and the primary maternal outcome was a composite of HELLP syndrome and eclampsia (hereafter HELLP or eclampsia). Secondary outcomes were stroke, cardiac arrest, pulmonary edema, renal failure, liver failure, disseminated intravascular coagulation (DIC), placental abruption/antenatal hemorrhage, thromboembolic disease, severe postpartum hemorrhage (> 1000 mL), Cesarean section, neonatal intensive care unit (NICU) admission, small‐for‐gestational age (SGA; birth weight < 10th percentile), 5‐min Apgar score < 7, arterial cord pH < 7.05, bronchopulmonary dysplasia, seizures, intracerebral hemorrhage, intraventricular hemorrhage Grade III or IV, cerebral infarction, periventricular leukomalacia, hypoxic ischemic encephalopathy, necrotizing enterocolitis Grade II or higher and culture‐proven sepsis. A composite adverse maternal outcome was evaluated, consisting of eclampsia, stroke, cardiac arrest, pulmonary edema, renal failure, liver failure, HELLP, DIC, placental abruption/antenatal hemorrhage and/or thromboembolic disease. A composite adverse neonatal outcome was also evaluated, consisting of RDS, bronchopulmonary dysplasia, seizures, intracerebral hemorrhage, intraventricular hemorrhage Grade III or IV, cerebral infarction, periventricular leukomalacia, hypoxic ischemic encephalopathy, necrotizing enterocolitis Grade II or higher and culture‐proven sepsis.
Quality of evidence
To assess systematically the quality of the evidence provided by the included studies, the approach of the GRADE Working Group was followed35. Scoring points were attributed according to type of evidence, quality, consistency, directness and effect size. The final score was then used to categorize evidence quality as high, moderate, low or very low.
Data analysis
Outcomes were analyzed on an intention‐to‐treat basis using a two‐stage IPDMA approach. Aggregate outcomes were recalculated at the trial level and then standard meta‐analysis techniques were used to evaluate the overall effect of the intervention (pooled relative risk (RR) with 95% CI)36, 37, 38. Heterogeneity was assessed using the I 2 statistic. Mantel–Haenszel fixed‐effect model was used if statistical heterogeneity was acceptable (I 2 ≤ 30%) and trial‐specific interventions were deemed sufficiently similar. Random‐effects models were used otherwise. Descriptive comparisons were performed to assess between‐study differences.
Predefined subgroup analyses were performed by hypertensive disorder type, gestational age, obstetric history (previous hypertensive disorder of pregnancy, Cesarean section, abortion, parity), ethnicity, multiple pregnancy, maternal age, body mass index, transvaginal sonography cervical length and Bishop score. Interactions between the intervention and subgroups were evaluated using the chi‐square test and resulting interaction P‐values.
Statistical analyses were performed using IBM SPSS Statistics 23 software (version 23.0.0; IBM Corporation, Armonk, NY, USA) and Review Manager (Version 5.3; Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).
RESULTS
Data were collected on five RCTs: HYPITAT‐I, DIGITAT, Deliver or Deliberate, HYPITAT‐II and GRIT27, 28, 30, 31, 39. A summary of the search can be found in Figure 1. The total number of participants in the trials was 2825. Of these, 1778 were eligible for this study. Information on non‐eligible participants as well as a summary of each of the five included studies can be found in Table 1. Baseline characteristics of the 1724 women for whom the primary outcomes were available can be found in Table 2. The HYPITAT‐I, HYPITAT‐II and Deliver or Deliberate studies evaluated immediate delivery vs expectant management for pregnancies between 34 + 0 and 41 + 0 weeks of gestation complicated by hypertensive disorders. HYPITAT‐II and Deliver or Deliberate protocols mandated delivery in the expectant management group by 37 weeks.
Figure 1.
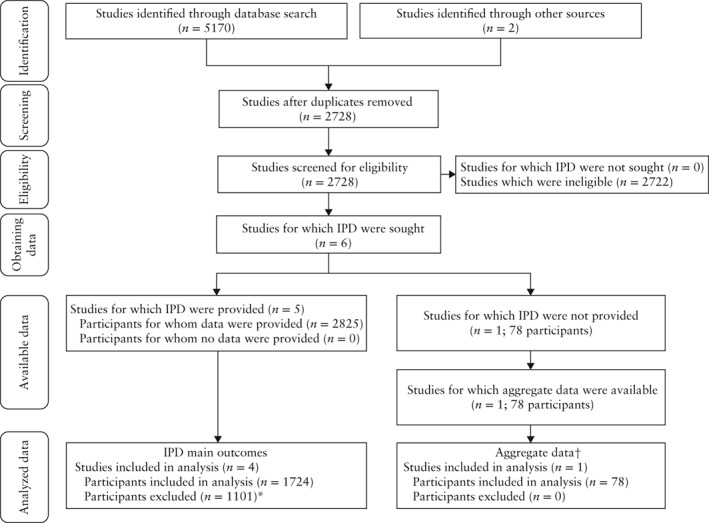
Flowchart summarizing search for, and analysis of, individual patient data from randomized controlled trials reporting on management of near‐term women with hypertensive disorder of pregnancy. *1047 excluded due to individual participant data (IPD) meta‐analysis inclusion/exclusion criteria and 54 from GRIT study39 for which main outcomes were not collected. †Placental abruption, intrauterine growth restriction, neonatal intensive care unit admission and perinatal mortality.
Table 1.
Summary of randomized controlled trials on management of near‐term women with hypertensive disorder of pregnancy included in individual patient data meta‐analysis
| Study | Trial enrolment | Trial participants | Non‐eligible participants (n) | Eligible participants (n) |
|---|---|---|---|---|
| GRIT; | ||||
| GRIT Study Group (2003)39 | Sixty‐nine hospitals in 13 European countries | 547 pregnant women with fetal compromise between 24 + 0 and 36 + 0 weeks, umbilical artery Doppler waveform recorded and clinical uncertainty whether immediate delivery was indicated | Randomized before 34 weeks: 493 | 54 |
| HYPITAT‐I; | ||||
| Koopmans (2009)27 | Six academic and 32 non‐academic hospitals in The Netherlands | 756 women with singleton pregnancy between 36 + 0 and 41 + 0 weeks and who had gestational hypertension or pre‐eclampsia without severe features | None | 756 |
| DIGITAT; | ||||
| Boers (2010)28 | Eight academic and 44 non‐academic hospitals in The Netherlands | 650 women with singleton pregnancy between 36 + 0 and 41 + 0 weeks with suspected intrauterine growth restriction | Randomized without hypertensive disorder: 540 | 110 |
| Deliver or Deliberate; | ||||
| Owens (2014)30 | Single center in USA | 169 women who met ACOG 2002 criteria45 for pre‐eclampsia without severe features and gestational dating 34 + 0 to 36 + 6 weeks | Randomized before 34 weeks: 4; HIV: 2; diabetes: 7; major congenital abnormality: 1 | 155 |
| HYPITAT‐II; | ||||
| Broekhuijsen (2015)31 | Seven academic hospitals and 44 non‐academic hospitals in The Netherlands | 703 women with non‐severe hypertensive disorders of pregnancy between 34 + 0 and 36 + 6 weeks of gestation | None | 703 |
Only first author of each study is given.
Table 2.
Baseline characteristics of eligible trial participants with main outcomes available, according to management
| Characteristic | Delivery (n = 861) | Expectant management (n = 863) | Difference (in median or %) | P |
|---|---|---|---|---|
| Maternal age (years) | 29.0 (25.0–33.0) | 29.0 (26.0–33.0) | 0.0 | 0.082 |
| GA at randomization (weeks) | 36.0 (35.0–38.0) | 36.0 (35.0–38.0) | 0.0 | 0.655 |
| BMI at booking (kg/m2)* | 25.8 (22.8–30.5) | 25.7 (22.8–29.8) | 0.1 | 0.709 |
| Cervical length (mm)† | 32.0 (24.0–40.0) | 31.0 (23.0–38.8) | 1.0 | 0.344 |
| Bishop score at randomization‡ | 3.0 (2.0–4.0) | 3.0 (2.0–4.0) | 0.0 | 0.167 |
| Study | 0.186 | |||
| HYPITAT‐I27 | 377 (43.8) | 379 (43.9) | –0.1 | |
| DIGITAT28 | 46 (5.3) | 64 (7.4) | –2.1 | |
| Deliver or Deliberate30 | 86 (10.0) | 69 (8.0) | 2.0 | |
| HYPITAT‐II31 | 352 (40.9) | 351 (40.7) | 0.2 | |
| Hypertensive disease | 0.763 | |||
| Gestational hypertension | 355 (41.2) | 365 (42.3) | –1.1 | |
| Pre‐eclampsia | 392 (45.5) | 378 (43.8) | 1.7 | |
| Chronic hypertension | 114 (13.2) | 120 (13.9) | –0.7 | |
| Nulliparous | 593 (68.9) | 581 (67.3) | 1.6 | 0.502 |
| Caucasian§ | 671 (81.2) | 665 (80.9) | 0.3 | 0.900 |
| Multiple pregnancy | 18 (2.1) | 26 (3.0) | –0.9 | 0.285 |
Data presented as median (interquartile range) or n (%).
P‐values calculated using Mann–Whitney U‐test or chi‐square test.
Data available in delivery and expectant management groups, respectively, in:
694 and 718 cases;
721 and 723 cases;
700 and 695 cases;
826 and 822 cases.
BMI, body mass index; GA, gestational age.
Chronologically, HYPITAT‐I ran parallel with DIGITAT. Women with hypertension as well as suspected intrauterine growth restriction were preferentially included in the DIGITAT study. The GRIT trial evaluated the intervention in pregnancies with fetal compromise between 24 + 0 and 36 + 0 weeks of gestation. GRIT collected data on neonatal outcome only and its main respiratory outcome was ventilation for 24 h or more, not RDS.
Randomization to immediate delivery before 37 weeks of gestation resulted in preterm delivery in 86.0% (435/506) of women. In the expectant management group, this occurred in 61.0% (303/497). In the former group, median time to delivery after randomization was 2 days (interquartile range (IQR), 1.0–3.0) vs 7 days (IQR, 4.0–12.0) in the latter. To avoid selection bias because of fetal compromise, GRIT data were not used to calculate preterm delivery rates and median time to delivery.
Primary outcomes
Figure 2 presents the primary outcome results by study and Table 3 presents the pooled results for all outcomes. Immediate delivery reduced the risk of HELLP or eclampsia (0.8% vs 2.8%; RR, 0.33 (95% CI, 0.15–0.73); I 2 = 0%; numbers needed to treat (NNT), 51 (95% CI, 31.1–139.3)). Seven of the 861 (0.8%) women in the immediate delivery group developed HELLP vs 22 of the 863 (2.5%) women in the expectant management group (RR, 0.36 (95% CI, 0.16–0.80); I 2 = 0%; NNT, 58 (95% CI, 33.9–190.3)). Three expectantly managed women progressed to eclampsia, one of whom also presented HELLP. No women in the immediate delivery group presented eclampsia. There were 29 (3.4%) neonates with RDS following the 861 immediate deliveries and 14 (1.6%) in the 863 pregnancies managed expectantly (RR, 1.94 (95% CI, 1.05–3.59); I 2 = 24%; numbers needed to harm, 58 (95% CI, 31.1–363.1)).
Figure 2.
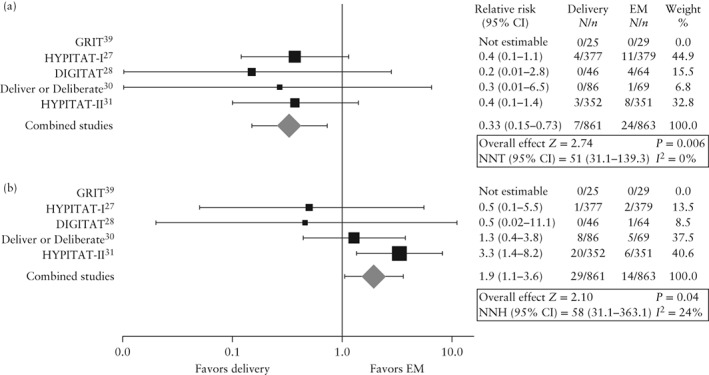
Forest plot showing relative risk of HELLP syndrome and/or eclampsia (a) and neonatal respiratory distress syndrome (b) in women presenting with gestational hypertension or pre‐eclampsia without severe features from 34 weeks of gestation who underwent immediate delivery vs those managed expectantly. Mantel–Haenszel fixed‐effect model used. NNT/NNH, numbers needed to treat/harm; EM, expectant management.
Table 3.
Pooled risk of maternal and neonatal outcomes in women presenting with gestational hypertension or pre‐eclampsia without severe features from 34 weeks of gestation who underwent immediate delivery vs those managed expectantly
| Outcome | Delivery | Expectant management | RR (95% CI) | Refs* | I 2 (%) | Model | Data missing | Quality of evidence (GRADE35) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Events | Total | Events | Total | |||||||
| HELLP syndrome or eclampsia | 7 | 861 | 24 | 863 | 0.33 (0.15–0.73) | 27, 28, 30, 31 | 0 | FE | 0 | High |
| HELLP syndrome | 7 | 861 | 22 | 863 | 0.36 (0.16–0.80) | 27, 28, 30, 31 | 0 | FE | 0 | High |
| Eclampsia | 0 | 861 | 3 | 863 | 0.23 (0.03–2.04) | 27, 28, 30, 31 | 0 | FE | 0 | Moderate |
| PPH | 69 | 861 | 90 | 863 | 0.77 (0.57–1.04) | 27, 28, 30, 31 | 2 | FE | 0 | High |
| Cesarean section | 233 | 886 | 245 | 892 | 1.02 (0.83–1.26) | 27, 28, 30, 31, 39 | 52 | RE | 0 | Moderate |
| Pulmonary edema | 0 | 756 | 2 | 776 | 0.20 (0.01–4.17) | 27, 28, 31 | N/A | FE | 0 | Moderate |
| Placental abruption | 0 | 756 | 2 | 776 | 0.20 (0.01–4.14) | 27, 28, 31 | N/A | FE | 0 | Moderate |
| Placental abruption† | 3 | 794 | 5 | 814 | 0.72 (0.18–2.83) | 27, 28, 29, 31 | 0 | FE | 0 | Moderate |
| Thromboembolic disease | 2 | 756 | 1 | 776 | 1.60 (0.25–12.99) | 27, 28, 31 | 0 | FE | 0 | Moderate |
| CAMO‡ | 9 | 861 | 28 | 863 | 0.35 (0.17–0.72) | 27, 28, 30, 31 | 0 | FE | 0 | Moderate |
| RDS | 29 | 861 | 14 | 863 | 1.94 (1.05–3.59) | 27, 28, 30, 31 | 24 | FE | 0 | High |
| NICU admission | 53 | 808 | 40 | 798 | 1.21 (0.69–2.12) | 27, 28, 30, 31 | 40 | RE | 118/1724 | High |
| NICU admission† | 65 | 846 | 43 | 836 | 1.42 (0.78–2.59) | 27, 28, 29, 30, 31 | 51 | RE | 118/1800 | High |
| SGA | 122 | 815 | 146 | 798 | 0.84 (0.63–1.13) | 27, 30, 31 | 32 | RE | 1/1614 | High |
| SGA† | 128 | 853 | 150 | 836 | 0.87 (0.66–1.15) | 27, 29, 30, 31 | 23 | FE | 1/1690 | High |
| 5‐min Apgar < 7 | 29 | 886 | 20 | 891 | 1.43 (0.83–2.48) | 27, 28, 30, 31, 39 | 0 | FE | 1/1778 | High |
| Seizures | 5 | 728 | 2 | 727 | 2.49 (0.48–12.82) | 27, 31 | 0 | FE | 0 | Moderate |
| IVH Grade III or IV | 3 | 364 | 0 | 363 | 4.23 (0.49–36.72) | 31, 39 | 0 | FE | 0 | Moderate |
| NEC ≥ Grade II | 4 | 377 | 0 | 376 | 5.31 (0.64–43.79) | 31, 39 | 0 | FE | 0 | Moderate |
| Arterial cord pH < 7.05 | 20 | 790 | 28 | 774 | 0.70 (0.40–1.24) | 27, 28, 30, 31 | 5 | FE | 160/1724 | High |
| PVL | 4 | 303 | 2 | 284 | 1.89 (0.34–10.38) | 31 | N/A | FE | 0 | Moderate |
| Culture‐proven sepsis | 5 | 775 | 1 | 794 | 2.79 (0.65–11.88) | 27, 28, 31 | 15 | FE | 0 | Moderate |
| Perinatal mortality | 1 | 886 | 1 | 892 | 1.16 (0.08–17.60) | 27, 28, 30, 31, 39 | N/A | FE | 0 | Moderate |
| Perinatal mortality† | 3 | 924 | 2 | 930 | 1.60 (0.27–9.34) | 27, 28, 29, 30, 31, 39 | 0 | FE | 0 | Moderate |
| CANO§ | 47 | 886 | 20 | 892 | 2.30 (1.38–3.82) | 27, 28, 30, 31, 39 | 11 | FE | 0 | Moderate |
| BPD | 0 | 886 | 0 | 892 | — | 27, 28, 30, 31, 39 | — | — | — | N/A |
| ICH | 0 | 886 | 0 | 892 | — | 27, 28, 30, 31, 39 | — | — | — | N/A |
| Cerebral infarction | 0 | 886 | 0 | 892 | — | 27, 28, 30, 31, 39 | — | — | — | N/A |
| Hypoxic ischemic encephalopathy | 0 | 886 | 0 | 892 | — | 27, 28, 30, 31, 39 | — | — | — | N/A |
| Stroke | 0 | 861 | 0 | 863 | — | 27, 28, 30, 31 | — | — | — | N/A |
| Cardiac arrest | 0 | 861 | 0 | 863 | — | 27, 28, 30, 31 | — | — | — | N/A |
| DIC | 0 | 861 | 0 | 863 | — | 27, 28, 30, 31 | — | — | — | N/A |
| Renal failure | 0 | 861 | 0 | 863 | — | 27, 28, 30, 31 | — | — | — | N/A |
| Liver failure | 0 | 861 | 0 | 863 | — | 27, 28, 30, 31 | — | — | — | N/A |
Data given as n unless otherwise stated.
Only first author of each study is given.
References (Refs): HYPITAT‐I, Koopmans (2009)27; DIGITAT, Boers (2010)28; Hamed (2014)29; Deliver or Deliberate, Owens (2014)30; HYPITAT‐II, Broekhuijsen (2015)31; GRIT, GRIT Study Group (2003)39.
Includes aggregate data from Hamed et al.29.
Eclampsia, stroke, cardiac arrest, pulmonary edema, renal failure, liver failure, HELLP, disseminated intravascular coagulation, placental abruption/antenatal hemorrhage and/or thromboembolic disease.
Respiratory distress syndrome, bronchopulmonary dysplasia, seizures, intracerebral hemorrhage, intraventricular hemorrhage Grade III or IV, cerebral infarction, periventricular leukomalacia, hypoxic ischemic encephalopathy, necrotizing enterocolitis Grade II or higher and culture‐proven sepsis.
BPD, bronchopulmonary dysplasia; CAMO, composite adverse maternal outcome; CANO, composite adverse neonatal outcome; DIC, disseminated intravascular coagulation; FE, fixed‐effects; ICH, intracerebral hemorrhage; IVH, intraventricular hemorrhage; N/A, not applicable; NEC, necrotizing enterocolitis; NICU, neonatal intensive care unit; PPH, postpartum hemorrhage; PVL, periventricular leukomalacia; RDS, respiratory distress syndrome; RE, random‐effects; RR, relative risk; SGA, small‐for‐gestational age.
Secondary outcomes
Table 3 presents the pooled secondary outcome results. Severe postpartum hemorrhage occurred in 8.0% of women after immediate delivery and in 10.4% of women in the expectant management group (RR, 0.77 (95% CI, 0.57–1.04); I 2 = 2%). The Deliver or Deliberate and HYPITAT‐II studies tracked but did not (plan to) report on this outcome. Consequently, rates may have been underestimated in the former, as only three (1.9%) occurrences were recorded in 155 pregnancies. In the latter, severe postpartum hemorrhage occurred fewer times after immediate delivery vs expectant management (8.5% vs 13.7%; RR, 0.62 (95% CI, 0.40–0.96)).
Cesarean section rates were 26.3% in the immediate delivery group vs 27.5% in those managed expectantly (RR, 1.02 (95% CI, 0.83–1.26); I 2 = 52%). The heterogeneity present is likely derived from the elevated rate of Cesarean section in the GRIT study; 92% and 75% in the immediate delivery and expectant management groups, respectively. Comparison restricted to non‐elective Cesarean section showed comparable results (22.0% vs 22.1%; RR, 1.08 (95% CI, 0.8–1.4); I 2 = 56%).
GRIT and DIGITAT data were not used in the analysis of SGA because of their inclusion criteria. SGA pooled rates from the other three studies were 15% for immediate delivery and 18.3% for expectant management (RR, 0.84 (95% CI, 0.63–1.13); I 2 = 32%). The corresponding rates when GRIT and DIGITAT data were included were comparable, at 20.3% and 25.1%, respectively (RR, 0.92 (95% CI, 0.79–1.06); I 2 = 36%). Deliver or Deliberate and HYPITAT‐II did not report on SGA in their respective papers. In the individual participant data of the former study, rates were 11.6% vs 8.6% (RR, 1.34 (95% CI, 0.51–3.50)) and in the latter they were 17.6% vs 25.1% (RR, 0.70 (95% CI, 0.52–0.94)).
The rate of 5‐min Apgar score < 7 was 3.3% after immediate delivery vs 2.2% in pregnancies managed expectantly (RR, 1.43 (95% CI 0.83–2.48); I 2 = 0%), while NICU admission rates were 6.6% and 5.0%, respectively (RR, 1.21 (95% CI, 0.69–2.12); I 2 = 40%). Rate of infants presenting arterial cord pH < 7.05 was 2.5% after immediate delivery vs 3.6% in the expectant management group (RR, 0.70 (95% CI, 0.40–1.24); I 2 = 5%).
Seizures occurred in five infants in the immediate delivery group and in two in the expectant management group (0.7% vs 0.3%; RR, 2.49 (95% CI, 0.48–12.82)). Culture‐proven sepsis rates were 0.6% and 0.1%, respectively (RR, 2.8 (95% CI, 0.65–11.88)). Three neonates in the immediate delivery group presented intraventricular hemorrhage Grade III or IV and four presented necrotizing enterocolitis; none in the expectant management group presented these outcomes. There were four cases of periventricular leukomalacia in the immediate delivery group and two in the expectant management group. There were no cases of bronchopulmonary dysplasia, intracerebral hemorrhage, cerebral infarction or hypoxic ischemic encephalopathy.
There were two perinatal deaths in the included trials, both from GRIT, one in each group. Inclusion of aggregate data from Hamed et al.29 on placental abruption, intrauterine growth restriction, NICU admission, and perinatal mortality did not change these results significantly (Table 3).
Subgroup analyses
Figure 3 shows the results of the subgroup analyses of the primary maternal and neonatal outcomes. There was no evidence that the intervention effect was different for any of the subgroups.
Figure 3.
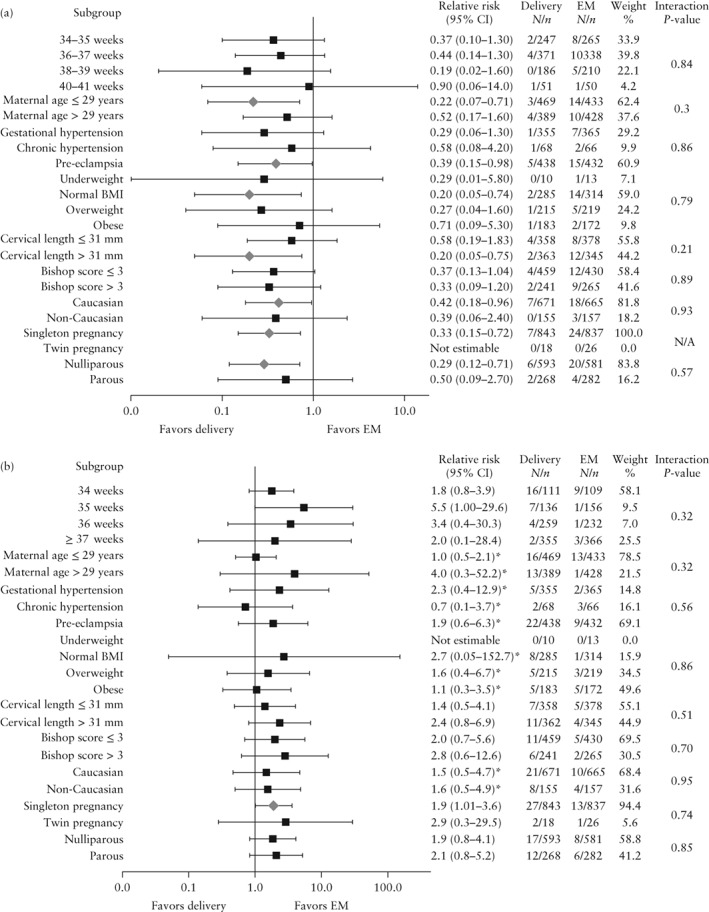
Forest plot showing pooled relative risk of HELLP syndrome and/or eclampsia (a) and neonatal respiratory distress syndrome (b) in women presenting with gestational hypertension or pre‐eclampsia without severe features from 34 weeks of gestation who underwent immediate delivery vs those managed expectantly, according to subgroup. Mantel–Haenszel fixed‐effect or *random‐effects model used. BMI, body mass index; EM, expectant management; N/A, not applicable.
The subgroups of women presenting with pre‐eclampsia, those at or below the median age of 29 years, those who were nulliparous and those with cervical length greater than the median of 31 mm were at increased risk of HELLP or eclampsia when managed expectantly.
Infants born after randomization to immediate delivery at 35 weeks of gestation were at higher risk of RDS (5.1% vs 0.6%; RR, 5.5 (95% CI, 1.0–29.6); I 2 = 0%). Of those randomized to expectant management at 35 weeks, 18 of 34 (52.9%) were born at term if the mother presented with gestational hypertension at randomization. The rate was similar for women with pre‐eclampsia, as 53 of 100 reached term. For those randomized with gestational hypertension at 34 weeks and those randomized with gestational hypertension at 36 weeks, 68.8% (11/16) and 88.3% (68/77), respectively, reached term. In the case of pre‐eclampsia, the respective rates were 17.1% (14/82) and 84.1% (111/132).
Median time to delivery after randomization to expectant management at 34 weeks of gestation was 16 (IQR, 12.8–19.0) days in cases of gestational hypertension and 9.5 (IQR, 5.0–16.0) days in cases of pre‐eclampsia. The equivalent medians for 35 weeks were 9.5 (IQR, 5.5–13.0) days and 10 (IQR, 6.0–12.0) days, respectively, and for 36 weeks they were 7 (IQR, 4.0–13.5) days and 5 (IQR, 4.0–8.0) days, respectively. Pregnancies selected because of fetal compromise were not included in the subgroup analysis of term birth rates and median days to delivery for those randomized preterm.
Risk of bias and quality of evidence
The results of our risk of bias evaluation of the studies based on Cochrane guidelines can be found in Table 4. GRADE assessment of quality of evidence and further data for each outcome are available in Table 3.
Table 4.
Risk of bias in randomized controlled trials on management of near‐term women with hypertensive disorder of pregnancy
| Study | Random sequence generator | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias |
|---|---|---|---|---|---|---|---|
| GRIT; GRIT Study Group (2003)39 | Low | Unclear | High | Unclear | Unclear | Low | Low |
| HYPITAT‐I; Koopmans (2009)27 | Low | Low | High | Unclear | Low | Low | Unclear |
| DIGITAT; Boers (2010)28 | Low | Low | High | Unclear | Low | Low | Low |
| Deliver or Deliberate; Owens (2014)30 | Low | Unclear | High | Unclear | Unclear | Low | Unclear |
| HYPITAT‐II; Broekhuijsen (2015)31 | Low | Low | High | Unclear | Low | Low | Low |
Only first author of each study is given.
DISCUSSION
Primary findings
While immediate delivery decreases the risk of a composite outcome of HELLP and eclampsia, it also increases the risk of RDS, especially if delivery occurs prior to 36 weeks of gestation.
Strengths and limitations
For this IPDMA, we collected and reanalyzed individual data from five previous RCT on the management of near‐term hypertensive disorders in pregnancy. By harmonizing inclusion/exclusion criteria and using individual participant data, we were able to include hypertensive women from the DIGITAT study.
Unfortunately, we sought but did not receive, data from Hamed et al.29. Their trial included 76 women with chronic hypertension, which could have facilitated our evaluation of this subgroup. Furthermore, as HYPITAT‐II was the only included trial that had data allowing distinction between pre‐eclampsia and superimposed pre‐eclampsia, we were unable to differentiate between the two in the pooled results. RDS incidence is affected by corticosteroid administration prior to delivery; of the included studies, only HYPITAT‐II had available data on its use. Consequently, no adjustments were possible to account for this.
Even in our combined dataset, the low incidence of most severe adverse outcomes remained a difficult challenge. The results from two currently ongoing trials, i.e. the PHOENIX trial (ISRCTN01879376) and the WILL trial (NIHR‐HTA ‐ 16/167/123) will have to be awaited to re‐evaluate risks of severe outcomes.
Clinical meaning of findings
The American College of Obstetricians and Gynecologists (ACOG) currently suggests delivery at 37 weeks of gestation in the presence of gestational hypertension or pre‐eclampsia40. On the other hand, in the UK, The National Institute for Health and Care Excellence (NICE) guidelines recommend this only for women with pre‐eclampsia. For women with gestational hypertension, timing of delivery is left to mutual agreement between patient and obstetrician41. Our evaluation of pooled data may contribute to further sophistication of this advice. Women with gestational hypertension more often developed HELLP syndrome after expectant management as compared to immediate delivery. Since the Deliver or Deliberate and HYPITAT‐II trials allowed expectant management only until 37 weeks of gestation, occurrence of progression to HELLP or eclampsia was precluded. If allowed to continue beyond this gestational age, these pregnancies would likely have contributed to an even larger difference in our primary maternal outcome. Therefore, our findings strengthen the evidence base for the ACOG recommendation.
A recently published Cochrane review pooled aggregate results from the two HYPITAT studies and concluded that immediate delivery is associated with less composite maternal morbidity and mortality for women with a hypertensive disorder after 34 weeks' gestation42. However, the review authors pooled different composite outcomes, highlighting the relevance of this IPDMA. They also found that immediate delivery lowers HELLP risk, a result that is in accordance with our IPDMA. On the other hand, they found more NICU admissions after immediate delivery, which we could not confirm. From 34 weeks of gestation to term, current guidelines concur that management should be expectant as long as no severe features are present. Our results favor maintaining this recommendation.
Subgroup analyses
We found no evidence of statistically significant interaction effects present in particular subgroups. This implies that the relative effects of the intervention do not appear to differ between subgroups. However, there were subgroups with increased risks of RDS or progression to HELLP or eclampsia.
Women with a‐priori higher risk of progression to HELLP, such as those already presenting with pre‐eclampsia instead of with gestational hypertension, were shown to benefit from earlier delivery. For gestational and chronic hypertension, we were not able to demonstrate a statistically significant difference in HELLP syndrome and/or eclampsia between the management groups. Even in our substantial dataset, conclusions remain difficult to draw for hypertensive disorders other than pre‐eclampsia due to their low prevalence.
The higher rates of the composite outcome of HELLP syndrome and/or eclampsia found in nulliparous women and in those with high cervical lengths managed expectantly are biologically plausible, as both risk factors contribute to a longer peripartum period and therefore more opportunity for deterioration.
Immediate delivery at 35 weeks was the only gestational age subgroup with a significantly higher risk of RDS. This is unlikely to be a false‐positive finding because of the higher prior probability of RDS at this gestational age when compared to 36 and 37 weeks. Incidence of RDS stabilizes at around 0.3% from the 38th week of gestation onwards43, 44. RDS risk was not elevated for those randomized at 34 weeks, which could be a false‐negative finding or because of insufficient power. On the other hand, expectant management initiated in the 34th week of gestation, i.e. between 2 + 1 weeks and 3 + 0 weeks before term, did not often result in term delivery. This was particularly apparent in those with pre‐eclampsia randomized to expectant management, as only 17.1% reached term. Progression to severe disease or fetal distress before term triggered preterm iatrogenic delivery as per protocol, potentially raising RDS rates to resemble those in the immediate delivery subgroup. Similar considerations are valid for the subgroup randomized at 36 weeks with two caveats: (1) RDS rates and severity at this gestational age are lower than at 34 weeks and (2) the period of opportunity to deteriorate was, at most, 1 week because of protocol‐mandated delivery at 37 weeks44.
In addition to women with pre‐eclampsia, the HYPITAT‐II trial included women with gestational hypertension. This may explain the contrast with results from Deliver or Deliberate, which did not include gestational hypertension. The lower RDS occurrence with expectant management of preterm gestational hypertension in the former study possibly occurred because more of these women were able to reach the 37th week of gestation without clinical deterioration compared to those with pre‐eclampsia.
Secondary outcome analyses
In agreement with previous RCT‐based assessments, we did not find a higher Cesarean section rate when delivery was immediate23, 24. We found no difference in other secondary maternal and neonatal outcomes. Although SGA was reduced by delivery in the HYPITAT‐II study, this was not observed in the Deliver or Deliberate study, and our pooled results were not conclusive. Whether immediate delivery sufficiently alleviates prolonged fetal exposure to a hypertensive environment to decrease SGA merits further investigation.
Conclusion
Our study can inform women and clinicians in decision making on the timing of delivery. To reduce the risk of progression to HELLP or eclampsia, we recommend immediate delivery of pregnancies complicated by gestational hypertension or pre‐eclampsia by 37 weeks of gestation. Despite our large database, uncertainty remains regarding effects on rare, severe outcomes. Moreover, long‐term consequences of the intervention need to be investigated and more, larger trials are needed.
REFERENCES
- 1. Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre‐eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol 2011; 25: 391–403. [DOI] [PubMed] [Google Scholar]
- 2. Abalos E, Cuesta C, Grosso AL, Chou D, Say L. Global and regional estimates of pre‐eclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol 2013; 170: 1–7. [DOI] [PubMed] [Google Scholar]
- 3. Mol BWJ, Roberts CT, Thangaratinam S, Magee LA, De Groot CJM, Hofmeyr GJ. Pre‐eclampsia. Lancet 2016; 387: 999–1011. [DOI] [PubMed] [Google Scholar]
- 4. Dahlstrøm B, Ellström Engh M, Bukholm G, Øian P. Changes in the prevalence of pre‐eclampsia in Akershus County and the rest of Norway during the past 35 years. Acta Obstet Gynecol Scand 2006; 85: 916–921. [DOI] [PubMed] [Google Scholar]
- 5. Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of pre‐eclampsia, eclampsia, and gestational hypertension, United States, 1987–2004. Am J Hypertens 2008; 21: 521–526. [DOI] [PubMed] [Google Scholar]
- 6. Khan KS, Wojdyla D, Say L, Gülmezoglu AM, van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet 2006; 367: 1066–1074. [DOI] [PubMed] [Google Scholar]
- 7. Duley L. The global impact of pre‐eclampsia and eclampsia. Semin Perinatol 2009; 33: 130–137. [DOI] [PubMed] [Google Scholar]
- 8. von Dadelszen P, Magee LA. Preventing deaths due to the hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol 2016; 36: 83–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang M, Dorer D, Fleming M, Catlin E. Clinical outcomes of near‐term infants. Pediatrics 2004; 114: 372–376. [DOI] [PubMed] [Google Scholar]
- 10. Engle WA, Tomashek KM, Wallman C. “Late‐Preterm” infants: a population at risk. Pediatrics 2007; 120: 1390–1401. [DOI] [PubMed] [Google Scholar]
- 11. Leone A, Ersfeld P, Adams M, Schiffer PM, Bucher HU, Arlettaz R. Neonatal morbidity in singleton late preterm infants compared with full‐term infants. Acta Paediatr 2012; 101: 6–10. [DOI] [PubMed] [Google Scholar]
- 12. Teune MJ, Bakhuizen S, Bannerman CG, Opmeer BC, van Kaam AH, van Wassenaer AG, Morris JM, Mol BWJ. A systematic review of severe morbidity in infants born late preterm. Am J Obstet Gynecol 2011; 205: 374.e1–e9. [DOI] [PubMed] [Google Scholar]
- 13. Katz J, Lee ACC, Kozuki N, Lawn JE, Cousens S, Blencowe H, Ezzati M, Bhutta ZA, Marchant T, Willey BA, Adair L, Barros F, Baqui AH, Christian P, Fawzi W, Gonzalez R, Humphrey J, Huybregts L, Kolsteren P, Mongkolchati A, Mullany LC, Ndyomugyenyi R, Nien JK, Osrin D, Roberfroid D, Sania A, Schmiegelow C, Silveira MF, Tielsch J, Vaidya A, Velaphi SC, Victora CG, Watson‐Jones D, Black RE. Mortality risk in preterm and small‐for‐gestational‐age infants in low‐income and middle‐income countries: a pooled country analysis. Lancet 2013; 382: 417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Seikku L, Gissler M, Andersson S, Rahkonen P, Stefanovic V, Tikkanen M, Paavonen J, Rahkonen L. Asphyxia, neurologic morbidity, and perinatal mortality in early‐term and post‐term birth. Pediatrics 2016; 137: e20153334. [DOI] [PubMed] [Google Scholar]
- 15. Yeast JD, Jones A, Poskin M. Induction of labor and the relationship to Cesarean delivery: a review of 7001 consecutive inductions. Am J Obstet Gynecol 1999; 180: 628–633. [DOI] [PubMed] [Google Scholar]
- 16. Seyb ST, Berka RJ, Socol ML, Dooley SL. Risk of Cesarean delivery with elective induction of labor at term in nulliparous women. Obstet Gynecol 1999; 94: 600–607. [DOI] [PubMed] [Google Scholar]
- 17. Maslow AS, Sweeny A L. Elective induction of labor as a risk factor for Cesarean delivery among low‐risk women at term. Obstet Gynecol 2000; 95: 917–922. [DOI] [PubMed] [Google Scholar]
- 18. Dublin S, Lydon‐Rochelle M, Kaplan RC, Watts DH, Critchlow CW. Maternal and neonatal outcomes after induction of labor without an identified indication. Am J Obstet Gynecol 2000; 183: 986–994. [DOI] [PubMed] [Google Scholar]
- 19. van Gemund N, Hardeman A, Scherjon SA, Kanhai HH. Intervention rates after elective induction of labor compared to labor with a spontaneous onset. Gynecol Obstet Invest 2003; 56: 133–138. [DOI] [PubMed] [Google Scholar]
- 20. Heffner L. Impact of labor induction, gestational age, and maternal age on Cesarean delivery rates. Obstet Gynecol 2003; 102: 287–293. [DOI] [PubMed] [Google Scholar]
- 21. Vahratian A, Zhang J, Troendle JF, Sciscione AC, Hoffman MK. Labor progression and risk of Cesarean delivery in electively induced nulliparas. Obstet Gynecol 2005; 105: 698–704. [DOI] [PubMed] [Google Scholar]
- 22. Tajik P, van der Tuuk K, Koopmans CM, Groen H, van Pampus MG, van der Berg PP, van der Post JA, van Loon AJ, de Groot CJM, Kwee A, Huisjes AJM, van Beek E, Papatsonis DNM, Bloemenkamp KW, van Unnik GA, Porath M, Rijnders RJ, Stigter RH, de Boer K, Scheepers HC, Zwinderman AH, Bossuyt PM, Mol BW. Should cervical favourability play a role in the decision for labour induction in gestational hypertension or mild pre‐eclampsia at term? An exploratory analysis of the HYPITAT trial. BJOG 2012; 119: 1123–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wood S, Cooper S, Ross S. Does induction of labour increase the risk of Cesarean section? A systematic review and meta‐analysis of trials in women with intact membranes. BJOG 2014; 121: 674–685. [DOI] [PubMed] [Google Scholar]
- 24. Mishanina E, Rogozinska E, Thatthi T, Uddin‐Khan R, Khan KS, Meads C. Use of labour induction and risk of Cesarean delivery: a systematic review and meta‐analysis. CMAJ 2014; 186: 665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bernardes TP, Broekhuijsen K, Koopmans CM, Boers KE, van Wyk L, Tajik P, van Pampus MG, Scherjon SA, Mol BW, Franssen MT, van den Berg PP, Groen H. Caesarean section rates and adverse neonatal outcomes after induction of labour versus expectant management in women with an unripe cervix: a secondary analysis of the HYPITAT and DIGITAT trials. BJOG 2016; 123: 1501–1508. [DOI] [PubMed] [Google Scholar]
- 26. Martin JN, Thigpen BD, Moore RC, Rose CH, Cushman J, May W. Stroke and severe pre‐eclampsia and eclampsia: a paradigm shift focusing on systolic blood pressure. Obstet Gynecol 2005; 105: 246–254. [DOI] [PubMed] [Google Scholar]
- 27. Koopmans C, Bijlenga D, Groen H. Induction of labour versus expectant monitoring for gestational hypertension or mild pre‐eclampsia after 36 weeks' gestation (HYPITAT): a multicentre, open‐label randomised controlled trial. Lancet 2009; 374: 979–988. [DOI] [PubMed] [Google Scholar]
- 28. Boers KE, Vijgen SMC, Bijlenga D, van der Post JAM, Bekedam DJ, Kwee A, van der Salm PCM, van Pampus MG, Spaanderman MEA, de Boer K, Duvekot JJ, Bremer HA, Hasaart THM, Delemarre FMC, Bloemenkamp KWM, Van Meir CA, Willekes C, Wijnen EJ, Rijken M, Le Cessie S, Roumen FJME, Thornton JG, van Lith JMM, Mol BWJ, Scherjon SA. Induction versus expectant monitoring for intrauterine growth restriction at term: randomised equivalence trial (DIGITAT). BMJ 2010; 341: c7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hamed HO, Alsheeha MA, Abu‐Elhasan AM, Abd Elmoniem AE, Kamal MM. Pregnancy outcomes of expectant management of stable mild to moderate chronic hypertension as compared with planned delivery. Int J Gynecol Obstet 2014; 127: 15–20. [DOI] [PubMed] [Google Scholar]
- 30. Owens MY, Thigpen B, Parrish MR, Keiser SD, Sawardecker S, Wallace K, Martin JNJ. Management of pre‐eclampsia when diagnosed between 34–37 weeks' gestation: deliver now or deliberate until 37 weeks? J Miss State Med Assoc 2014; 55: 208–211. [PubMed] [Google Scholar]
- 31. Broekhuijsen K, van Baaren GJ, van Pampus MG, Ganzevoort W, Sikkema JM, Woiski MD, Oudijk MA, Bloemenkamp KWM, Scheepers HCJ, Bremer HA, Rijnders RJP, van Loon AJ, Perquin DAM, Sporken JMJ, Papatsonis DNM, van Huizen ME, Vredevoogd CB, Brons JTJ, Kaplan M, van Kaam AH, Groen H, Porath MM, van den Berg PP, Mol BWJ, Franssen MTM, Langenveld J. Immediate delivery versus expectant monitoring for hypertensive disorders of pregnancy between 34 and 37 weeks of gestation (HYPITAT‐II): an open‐label, randomised controlled trial. Lancet 2015; 385: 2492–2501. [DOI] [PubMed] [Google Scholar]
- 32. Broekhuijsen K, Bernardes T, van Baaren G‐J, Tajik P, Novikova N, Thangaratinam S, Boers K, Koopmans CM, Wallace K, Shennan AH, Langenveld J, Groen H, van den Berg PP, Mol BWJ, Franssen MTM. Relevance of individual participant data meta‐analysis for studies in obstetrics: delivery versus expectant monitoring for hypertensive disorders of pregnancy. Eur J Obstet Gynecol Reprod Biol 2015; 191: 80–83. [DOI] [PubMed] [Google Scholar]
- 33. Stewart LA, Clarke M, Rovers M, Riley RD, Simmonds M, Stewart G, Tierney JF. Preferred reporting items for a systematic review and meta‐analysis of individual participant data: The PRISMA‐IPD statement. JAMA 2015; 313: 1657–1665. [DOI] [PubMed] [Google Scholar]
- 34. Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies In: Cochrane Handbook for Systematic Reviews of Interventions , Version 5.1.0, JPT Higgins, Green S. (eds). The Cochrane Collection, 2011. Available from www.handbook.cochrane.org. [Google Scholar]
- 35. Schünemann H, Brozek J, Guyatt G, Oxman A. (eds). GRADE Handbook for Grading quality of evidence and strength of recommendations , The GRADE Working Group, 2013. Available from www.guidelinedevelopment.org/handbook. [Google Scholar]
- 36. Talge NM, Mudd LM, Sikorskii A, Basso O. United States birth‐weight reference corrected for implausible gestational age estimates. Pediatrics 2014; 133: 844–853. [DOI] [PubMed] [Google Scholar]
- 37. Cole TJ, Williams AF, Wright CM; RCPCH Growth Chart Expert Group. Revised birth centiles for weight, length and head circumference in the UK – WHO growth charts. Ann Hum Biol 2011; 38: 7–11. [DOI] [PubMed] [Google Scholar]
- 38. Perined . Geboortegewichtcurven. https://www.perined.nl/producten/geboortegewichtcurven.
- 39. van Bulck B, Kalakoutis GM, Sak P, Schneider KTM, Major T, Karpathios SE, Todros T, Arduini D, Tranquilli A, Tenore AC, Roncaglia N, Frusca T, Groningen AZ, Weinans MJN, Leiden AZ, van Roosmalen J, De Heel, Zaandam, van der Slikke JW , van Geijn H, Pernet PJM, Wolf H, Utrecht AZ, Stigter RH, Wilczynski J, Vasco E, Rashid M, Novak‐Antolic Z, Danielian P, Jenkinson SD, Welch CR, Griffin C, Gee H, Tuffnell D, Cresswell J, Tariq T, Sengupta B, Tydeman G, Kumarendran MK, Churchill D, Bewley S, Fusi L, Lindow SW, Johal W, Fairlie FM, Neales K, Thornton JG, Scudamore I, Konje J, Walkinshaw SA, Griffiths M, Dawson A, Mires G, Johanson R, Fraser RB, Hendy Ibbs P, Steel SA, Ramsay M, Robins JB, Heard MJ, Tonge HM, Manyonda IT, Walker J, Maresh M, Yoong A, Soothill P, Cameron H, Byrne D, Beattie B, Bober S, Felber B, Isaac E, Liddle L, McGhee T, Rowsell P, Howie P, Field D, Levene M, Lilford RJ, Grant A, Steer P, Breart G, Torgeson D, Hornbuckle J, Vail A, Spiegelhalter D; The GRIT Study Group . A randomised trial of timed delivery for the compromised preterm fetus: Short term outcomes and Bayesian interpretation. BJOG 2003; 110: 27–32. [DOI] [PubMed] [Google Scholar]
- 40. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy . Obstet Gynecol 2013; 122: 1122–1131. [DOI] [PubMed] [Google Scholar]
- 41. National Collaborating Centre for Women's and Children's Health (UK) . Hypertension in Pregnancy: The Management of Hypertensive Disorders During Pregnancy. London: RCOG Press, 2010. [PubMed] [Google Scholar]
- 42. Cluver C, Novikova N, Koopmans CM, West HM. Planned early delivery vs expectant management for hypertensive disorders from 34 weeks' gestation to term. Cochrane Database Syst Rev 2017; 1: CD009273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, Hale EC, Newman NS, Schibler K, Carlo WA, Kennedy KA, Poindexter BB, Finer NN, Ehrenkranz RA, Duara S, Sánchez PJ, O'Shea TM, Goldberg RN, van Meurs KP, Faix RG, Phelps DL, Frantz ID 3rd, Watterberg KL, Saha S, Das A, Higgins RD; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Neonatal outcomes of extremely preterm infants from the NICHD. Pediatrics 2010; 126: 443–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hibbard JU, Wilkins I, Sun L, Gregory K, Haberman S, Hoffman M, Kominiarek MA, Reddy U, Bailit J, Branch DW, Burkman R, Gonzalez Quintero VH, Hatjis CG, Landy H, Ramirez M, van Veldhuisen P, Troendle J, Zhang J; Consortium on safe labor. Respiratory morbidity in late preterm births. JAMA 2010; 304: 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. ACOG Practice Bulletin. Diagnosis and management of preeclampsia and eclampsia. No. 33. Int J Gynaecol Obstet 2002; 77: 67–75. [PubMed] [Google Scholar]


