Abstract
Objective
The aim of this study was to compare health related quality of life (HRQOL) in infants and children with avoidant restrictive food intake disorder (ARFID) to healthy and chronically ill controls.
Method
A cross‐sectional study was conducted in children who meet ARFID criteria at our tertiary care pediatric feeding clinic (September 2014 to July 2016). Before consultation, parents of patients (n = 100) were asked to complete questionnaires to determine HRQOL: the TNO‐AZL Preschool Children Quality of Life (0–5 years), and “Pediatric Quality of Life Inventory” (6–7 and 8–10 years). HRQOL of ARFID patients was compared to both healthy (0–5 years n = 241; 6–7 years n = 61; 8–10 years n = 192) and chronically ill (0–5 years n = 79; 6–7 years n = 11; 8–10 years n = 26) controls.
Results
The prevalence of ARFID was 64%. HRQOL of ARFID patients aged 0–5 years (n = 37) was significantly lower on 6/12 scales (appetite, lungs, stomach, motor functioning, positive mood and liveliness) compared to healthy controls (P < .01), and on 4/12 scales (appetite, stomach, motor functioning, and liveliness) compared to chronically ill controls (P < .01). The ARFID patients scored significantly better on the problem behavior scale compared to healthy and chronically ill controls (P < .01). ARFID patients aged 6–7 (n = 9) had significantly lower scores in 3/6 scales (total score, psychosocial health, and school functioning) (P < .01), and aged 8–10 (n = 2) had a significantly lower school functioning scale (P < .01) compared to healthy controls.
Conclusion
HRQOL of children with ARFID is decreased on multiple scales. The effect on HRQOL should be incorporated in clinical practice, and clinical studies should add HRQOL as an outcome measure.
Keywords: avoidant restrictive food intake disorder, enteral nutrition, health related quality of life, pediatrics, tube feeding
Abbreviations
- ARFID
avoidant restrictive food intake disorder
- DSM‐5
Diagnostic and Statistical Manual of Mental Disorders fifth edition
- HRQOL
health related quality of life
- PedsQL
Pediatric Quality of Life Inventory
- TAPQOL
TNO‐AZL Preschool Children Quality of Life
1. INTRODUCTION
The diagnosis “avoidant restrictive food intake disorder (ARFID)” was introduced in the Diagnostic and Statistical Manual of Mental Disorders fifth edition (DSM‐5) in 2013. It extended and replaced the formerly used DSM‐IV diagnosis “feeding disorder of infancy and early childhood” (American Psychiatric Association & DSM‐5 Task Force, 2013; Bryant‐Waugh, 2013; Kerzner et al., 2015). ARFID describes an eating/feeding disturbance (e.g., lack of interest in eating/food; avoidance based on the sensory characteristics of food; concern about aversive consequences of eating) as manifested by persistent failure to meet appropriate nutritional/energy needs associated with ≥1 of the following: significant weight loss/failure to achieve expected weight gain/faltering growth in children, significant nutritional deficiency, dependence on enteral feeding/oral nutritional supplements, or a marked interference with psychosocial functioning (American Psychiatric Association & DSM‐5 Task Force, 2013).
ARFID is a common clinical entity, with a point prevalence rate of 3.2% in a general pediatric population (Kurz, van Dyck, Dremmel, Munsch, & Hilbert, 2015). Earlier studies found ARFID patients to be younger, the proportion of males to be higher, and to be more commonly diagnosed with comorbid psychiatric and/or medical symptoms compared to other DSM‐5 eating disorders (Norris, Spettigue, & Katzman, 2016). However, ARFID is a heterogeneous clinical group including all age groups from young children to adults. In a study including ARFID patients aged 10–18 years, 30% were male, 55% had comorbid medical conditions and 58% anxiety disorders. This was significantly more common compared to children with other DSM‐5 eating disorders (Fisher et al., 2014). While more is known about general feeding disorders in younger patients, relatively few studies have been published describing clinical features of ARFID in children younger than 10 years of age.
Feeding problems caused by ARFID can lead to poor nutrition, which can result in malnourishment, faltering in growth and nutritional deficiencies (Kerzner, 2009; Silverman, 2010). For this reason, dietary supplements or tube feeding can be administered (Gottrand & Sullivan, 2010). ARFID may have an impact on the child's life due to hospital visits, feeding tube replacements, feeding therapy, stress during mealtimes and related parent–child interaction problems. A method that is commonly used to assess the impact of a disorder on a child's life is evaluating the health related quality of life (HRQOL) (Haverman et al., 2012), which incorporates measures of physical symptoms, functional status, and disease impact on psychological and social functioning (Eiser & Morse, 2001a; Haverman et al., 2012; Linscheid, 2006; Payot & Barrington, 2011). A study measuring HRQOL in adults with ARFID found lower mental HRQOL scores compared to the general population (Hay et al., 2017). To the best of our knowledge, however, the HRQOL of children with ARFID, and in particular, in those who are tube‐dependent, has not yet been studied. It is known, however, that tube feeding can result in loss of appetite, food aversion, delayed transition to oral feeding, and impact the psychosocial functioning of the child and it's family (Benoit, Wang, & Zlotkin, 2000; Hartdorff et al., 2015; Krom et al., 2018; Krom, de Winter, & Kindermann, 2017; Wilken, Bartmann, Dovey, & Bagci, 2018).
Therefore, the primary aim of our study is to compare the HRQOL of infants and children with ARFID to both healthy and chronically ill controls. The secondary aim is to compare the HRQOL of children with ARFID receiving tube feeding to those without tube feeding.
2. METHOD
2.1. Participants and procedure
A cross‐sectional study was conducted at the Diagnostic Center for Feeding Problems in the Emma Children's Hospital/Amsterdam UMC (tertiary care) in Amsterdam, the Netherlands, between September 12, 2014 and July 1, 2016. Patients aged 0–10 years were referred by pediatricians or general practitioners because of feeding difficulties, and evaluated by our multidisciplinary feeding team, consisting of a pediatric gastroenterologist, dietician, psychologist, and speech language pathologist.
Prior to the scheduled outpatient multidisciplinary consultation, a letter with information about the aims and procedure of the study was sent to the parents of the patients. Thereafter, the parents were called to check whether they received the letter and to give them the opportunity to ask questions. After parental consent, a user name and password, enabling to login on our web‐based method (www.hetklikt.nu; Haverman et al., 2014) were sent by e‐mail, and the appropriate questionnaires (depending on child's age) for assessing socio‐demographics and HRQOL (see paragraphs “Socio‐demographics” and “HRQOL”) were automatically selected.
Patients fulfilling the DSM‐5 criteria for ARFID were considered eligible for participation and were included in the analyses for the present study. Following the DSM‐5 criteria, these children suffer from a feeding disturbance. This is evident from the failure to meet nutritional and/or energy needs, resulting in faltering growth, poor weight gain, weight loss, nutritional deficiencies, tube feeding or nutritional supplement dependence, and/or problems in psychosocial functioning. If the feeding disturbance is better explained by a medical condition, another mental disorder, anorexia or bulimia nervosa, body shape/weight related, lack of food, or cultural/religious practices, children are excluded from the diagnosis. If, however, the feeding disturbance occurs in the context of another condition, and is more severe than general, the children may be diagnosed with ARFID (American Psychiatric Association & DSM‐5 Task Force, 2013). Eligibility was checked retrospectively by a research physician, who also works as a medical doctor diagnosing and treating children with feeding disorders in our tertiary center. Extensive chart reports of the multidisciplinary team members were consulted, before analyzing the data (see paragraph “Medical and paramedical data”).
Eligible patients of whom the parents completed the questionnaires were defined as participants, and those of whom the parents did not, were defined as nonparticipants.
The Committee for Medical Ethics at the Academic Medical Center in Amsterdam, the Netherlands confirmed that the Medical Research Involving Human Subjects Act did not apply to our study.
2.2. Measures
2.2.1. Socio‐demographics
Online baseline questionnaires were completed by the fathers or mothers of the participating patients to obtain information regarding both patients and parents. Incomplete socio‐demographic data were completed during psychological consultation.
2.2.2. Medical and paramedical data
Medical and paramedical data of both the participants and nonparticipants were collected during the standardized consultations by our multidisciplinary team.
The pediatric gastroenterologist assessed medical data with respect to history (age, sex, gestational age, birth weight, and age onset of the feeding problems), underlying diseases (or alarm symptoms for underlying diseases such as oral motor skill difficulties, dysphagia, odynophagia, vomiting, recurrent ear nose throat or airway disease, abnormal defecation, and neurological symptoms) and medication. At physical examination the pediatric gastroenterologist checked for the general condition and abnormalities, which could indicate underlying diseases. The speech language pathologist collected data concerning feeding skills and speech language development. The dietician obtained data according to type of feeding (orally of by tube) and nutritional intake including dietary supplements. The psychologist assessed social–emotional and psychosocial functioning, development, behavioral problems, traumatic experiences related to feeding, parental coping with feeding problems, and the psychological history of parents.
During consultation, weight and height were assessed by a digital scale and height chart. TNO growth‐charts for height for age, weight for age and weight for height (The Netherlands, 2010) were available for measuring standard deviation scores. SD scores for height and weight were corrected when the gestational age was <37 weeks until a patients' calendar age of 24 months. Disease specific secondary reference curves for SD scores for height (for patients with Down syndrome, Noonan syndrome, and Silver‐Russell syndrome) and weight (Down syndrome) were used as appropriate.
2.3. Definitions
We classified medical diagnoses into categories used by the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD‐10 Version: 2016; World Health Organization [WHO], 2016).
Prematurity was defined as gestational age < 37 weeks. Small for gestational age was defined as birth weight < p10.
We used following definitions for the DSM‐5 criteria “Significant weight loss or failure to achieve expected weight gain or faltering growth” (American Psychiatric Association & DSM‐5 Task Force, 2013): Acute malnourishment was defined as weight for age < −2 SD in children <1 year, and weight for height < −2 SD in children >1 year, or a faltering in growth >1 SD within 3 months on TNO growth charts. Chronic malnourishment was defined as height for age <−2 SD for all age groups. Growth delay was defined as deflection of >1 SD in 3 months in all age groups, a deflection of 0.5–1 SD per year in children aged <4 and of 0.25 SD/year in children aged ≥4 years (Joosten, 2012).
Demographics of the participants were compared to the nonparticipants to check for sample representativeness.
2.3.1. HRQOL
The TNO‐AZL Preschool Children Quality of Life (TAPQOL) and Pediatric Quality of Life Inventory (PedsQL) questionnaires were completed by parents of the patients to assess HRQOL. The TAPQOL and PedsQL scores were computed according to the manual, and subscales were constructed. Cronbach's alpha was calculated to assess the internal consistency of the questionnaires. Cronbach's alpha was considered low <0.50, moderate 0.50–0.70, and good >0.70 (Cronbach, 1951; Field, 2009).
2.3.2. TAPQOL
We used the TAPQOL, a reliable and valid instrument for measuring parents' perception of HRQOL in preschool children (aged 0–5 years) (Fekkes et al., 2000). The TAPQOL is a generic, multidimensional HRQOL questionnaire based on 43 proxy reported questions. It assesses the child's functioning in the past 4 weeks on 12 multi‐item scales: Stomach (three items), skin (three items), lungs (three items), sleeping (four items), appetite (three items), motor functioning (four items), positive mood (three items), anxiety (three items), liveliness (three items), problem behavior (seven items), social functioning (three items), and communication (four items). Higher scores on these subscales indicate higher HRQOL. The subscales motor functioning, social functioning, and communication only apply for children older than 18 months. Dutch norm data for healthy and chronically ill patients were used as control groups. These data were retrieved from a Dutch general population of children 1–5 years of age visiting six well‐baby clinics, and were described previously (2000) (Fekkes et al., 2000).
Besides the lung subscale (0.510), Cronbach's alpha in our sample was between 0.714 and 0.929, indicating a good internal consistency for the TAPQOL.
2.3.3. PedsQL
The PedsQL was used to assess the HRQOL for patients aged 6–7 years (by proxy version) and 8–10 years (self‐report version). The PedsQL is a 23‐item questionnaire covering four subscales of the child's functioning in the past week: Physical (eight items), emotional (five items), social (five items), and school functioning (five items). Higher scores on these subscales indicate higher HRQOL. The by proxy version is a parallel version of the self‐report version. The person tense (first at the self‐report and third at the by proxy version) and language (age appropriate) were different. Dutch norm data for healthy and chronically ill children from the general population, retrieved from urban and suburban elementary schools in Amsterdam, described previously (2009), were used as control groups (Engelen, Haentjens, Detmar, Koopman, & Grootenhuis, 2009).
Cronbach's alpha for the PedsQL (6–7) in our population indicated a low internal consistency for the psychosocial health (0.434), and school functioning (0.331) subscales. The other subscales showed a good internal consistency (Cronbach's alpha 0.737–0.866).
2.4. Statistical analysis
The Statistical Package for Social Science (IBM SPSS Statistics) version 23 was used to manage and analyze the data. To determine the prevalence of ARFID in our population we generated a bootstrapped confidence interval (Bca CI95%) based on 1,000 samples. Normality was tested through the Shapiro–Wilk test in addition to eyeballing. Differences between participants and nonparticipants were analyzed using T‐tests for normally distributed variables (SD scores for height, weight, and weight for height), Mann–Whitney U tests for not normally distributed variables (age, age of onset feeding disorder, gestational age, and birth weight), and Fischer Exact tests for binary variables (gender, underlying diseases, medication, and tube feeding). The HRQOL subscales of the children with ARFID were compared to both a healthy and a chronically ill Dutch norm population, and within the largest age group (0–5 years), the HRQOL of children with ARFID receiving tube feeding were compared to those without tube feeding using Mann–Whitney U tests.
Effect sizes (r) were measured by using the following formula: r = Z/(√N), where Z is the Z‐value and N is the total number of samples. The standard values for small, medium, and large effect sizes were 0.1, 0.3, and 0.5, respectively (Field, 2009). Due to multiple testing we defined P < .01 as statistically significant.
3. RESULTS
A total of 100 patients were evaluated by our multidisciplinary team between September 12, 2014 and July 1, 2016. The prevalence of new patients fulfilling the ARFID criteria was 64% (95% Bs CI 54.1–73.3). The most important criteria for ARFID in these patients were the failure of meeting nutritional and/or energy needs, shown by tube feeding (62.5%) or supplement (10.9%) dependence, growth faltering/limited weight gain/weight loss (9.4%), nutritional deficiencies (3.1%), or problems in psychosocial functioning (14.1%). Seventy‐five percent of these eligible patients participated in the study (see Figure 1).
Figure 1.
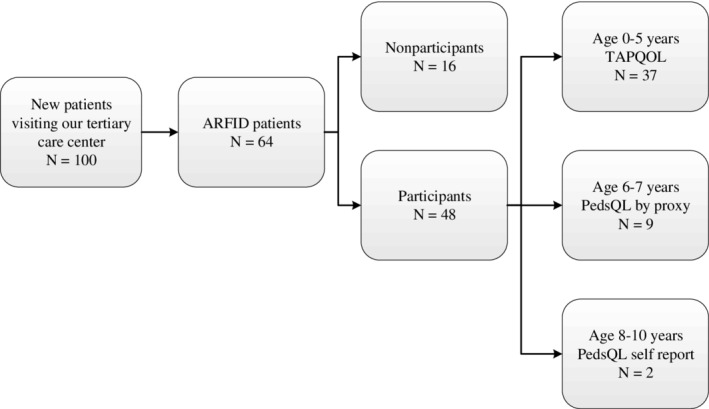
Flowchart inclusion, participants, and nonparticipants
3.1. Socio‐demographic and medical characteristics
The median age (corrected for prematurity) of the ARFID patients was 1.85 years (IQR 1.19–4.61), and 64.1% were females. No significant differences for baseline patient's characteristics were found between participants and nonparticipants (Table 1).
Table 1.
Baseline characteristics of the patients
| Participants (n = 48) | Nonparticipants(n = 16) | ||||
|---|---|---|---|---|---|
| Children | N | Median (IQR 25–75) | N | Median (IQR25‐75) | P a |
| History | |||||
| Age (years) | 48 | 1.84 (1.19–4.61) | 16 | 2.32 (0.85–2.58) | 1.000 |
| Gestational age (weeks) | 453 | 38.14 (36.00–39.43) | 142 | 37.5 (36.25–38.72) | 0.748 |
| Birth weight (grams) | 435 | 2,700 (1895–3,305) | 133 | 2,760 (2413–3,017) | 0.966 |
| Age of onset feeding disorder (months) | 462 | 0 (0–1.75) | 151 | 0 (0–2) | 0.545 |
| N | Mean (SD) | N | Mean (SD) | P b | |
| Physical examination | |||||
| Height (SD)c | 461 | −0.78 (1.24) | 16 | −1.07 (1.49) | 0.460 |
| Weight (SD)c | 461 | −1.31 (1.18) | 16 | −1.45 (1.61) | 0.726 |
| Weight for height (SD) | 461 | −0.90 (1.11) | 16 | −0.85 (1.13) | 0.879 |
| N | % | N | % | P d | |
| Sex (female) | 31 | 64.6 | 10 | 62.5 | 1.000 |
| History | |||||
| Underlying diseases | 43 | 89.6 | 14 | 87.5 | 1.000 |
| Prematurity | 173 | 35.4 | 62 | 42.9 | 0.762 |
| Small for gestational age | 98 | 18.8 | 23 | 15.4 | 0.711 |
| Diseases of the circulatory system | 81 | 16.7 | 5 | 31.3 | 0.286 |
| Diseases of the respiratory system/Infections | 21 | 43.8 | 3 | 18.8 | 0.084 |
| Congenital malformations, deformations and chromosomal abnormalities | 11 | 22.9 | 3 | 18.8 | 1.000 |
| Diseases of the digestive system | 21 | 43.8 | 9 | 56.3 | 0.405 |
| Endocrine, nutritional and metabolic diseases | 1 | 2.1 | 0 | 0 | 1.000 |
| Diseases of the skin and subcutaneous tissue | 53 | 10.4 | 2 | 12.5 | 1.000 |
| Mental and behavioral disorders | 7 | 14.6 | 0 | 0 | 0.178 |
| Other diseases | 28 | 58.3 | 12 | 75 | 0.372 |
| Medication | 36 | 75.0 | 8 | 50 | 0.117 |
| Tube feeding | 28 | 58.3 | 10 | 62.5 | 1.000 |
1One missing data; 2Two missing data; 3 Three missing data; 4 Four missing data; 5Five missing data; 8Eight missing data; 9Nine missing data.
Significant difference (P < .01).
Mann–Whitney U tests.
T‐tests.
SD scores were corrected when gestational age was <37 weeks until a patients' calendar age of 24 months. Disease specific secondary reference curves for SD scores for height (for patients with Down syndrome, Noonan syndrome, and Silver–Russell syndrome) and weight (Down syndrome) were used as appropriate (TNO growth‐charts, The Netherlands, 2010).
Fischer Exact tests.
Baseline characteristics of the parents of 48 participants and 16 nonparticipants are shown in Supporting Information Table S1.
3.2. HRQOL–TAPQOL
TAPQOL scores for HRQOL were available for 37 ARFID patients aged 0–5 years (see Table 2). The age of patients included in this study did not differ significantly with the norm group (P = .046).
Table 2.
HRQOL in children with ARFID compared to Dutch norm groups
| TAPQOL(0–5 years) | Children with ARFED | Reference group healthy population | Reference group chronically ill children | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subscale | N | Median | IQR 25–75 | N | Median | IQR 25–75 | P | r | N | Median | IQR 25–75 | P | r |
| Sleeping | 37 | 81.25 | 50.00–93.75 | 241 | 87.50 | 75.00–100,00 | .0181 | −.14 | 79 | 81.25 | 75.00–100.00 | .163 | −.078 |
| Appetite | 37 | 75.00 | 50.00–75.00 | 240 | 83.33 | 75.00–100.00 | .0001 | −.42 | 79 | 83.33 | 75.00–91.67 | .000* | −.275 |
| Lungs | 37 | 83.33 | 66.67–100.00 | 241 | 100.00 | 100.00–100.00 | .000* | −.42 | 79 | 100.00 | 66.61–100.00 | .297 | −.059 |
| Stomach | 37 | 66.67 | 50–100 | 239 | 100.00 | 83.33–100.00 | .000* | −.34 | 79 | 100.00 | 83.33–100.00 | .000* | −.236 |
| Skin | 37 | 91.76 | 83.33–100 | 241 | 100.00 | 91.67–100.00 | .440 | −.05 | 78 | 91.67 | 83.33–100.00 | .418 | −.045 |
| Motor functioning | 21 | 100.00 | 78.125–100.00 | 202 | 100.00 | 100.00–100.00 | .000* | −.30 | 71 | 100.00 | 100.00–100.00 | .000* | −.212 |
| Social functioning | 21 | 100.00 | 83.33–100.00 | 205 | 100.00 | 83.33–100.00 | .452 | −.05 | 71 | 100.00 | 83.33–100.00 | .249 | −.070 |
| Problem behavior | 37 | 78.57 | 64.29–85.71 | 241 | 71.43 | 57.14–78.57 | .002* | −.19 | 79 | 71.43 | 57.14–78.57 | .007* | −.150 |
| Communication | 21 | 93.75 | 81.25–100.00 | 201 | 93.75 | 87.50–100.00 | .622 | −.03 | 68 | 93.75 | 87.50–100.00 | .588 | −.033 |
| Anxiety | 37 | 66.67 | 50.00–100.00 | 241 | 83.33 | 66.61–100.00 | .102 | −.10 | 79 | 66.67 | 66.61–100.00 | .434 | −.044 |
| Positive mood | 37 | 100.00 | 100.00–100.00 | 241 | 100.00 | 100.00–100.00 | .006* | −.16 | 79 | 100.00 | 100.00–100.00 | .079 | −.107 |
| Liveliness | 37 | 100.00 | 83.33–100.00 | 240 | 100.00 | 100.00–100.00 | .000* | −.24 | 79 | 100.00 | 100.00–100.00 | .004* | −.151 |
P < .05.
P < .01.
Note. Higher scores represent higher quality of life (scores 0–100).
The HRQOL of patients with ARFID was significantly lower in six of 12 subscales (appetite, lungs, stomach, motor functioning, positive mood, and liveliness) compared to healthy controls (P < .01). The subscale problem behavior was significantly higher compared to the healthy controls (P < .01).
The HRQOL of patients with ARFID was significantly lower in four of 12 subscales (appetite, stomach, motor functioning, and liveliness), and higher in the subscale problem behavior compared to children with other chronic health conditions (P < .01).
3.3. HRQOL—PedsQL
The HRQOL of patients aged 6–7 years with ARFID (n = 9) was significantly lower in three of six subscales (total score, psychosocial health, and school functioning) compared to healthy controls (P < .01). Comparing the ARFID patients to chronically ill children, no significant differences were found in any scale (see Table 3).
Table 3.
HRQOL of ARFID patients (aged 6–7 and 8–10 years) compared to healthy and chronically ill controls
| PEDSQOL | Children with ARHD | Reference group healthy population | Reference group chronically ill children | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subscale*: | N | Median | IQR 25–75 | N | Median | IQR 25–75 | P | r | N | Median | IQR 25–75 | P | r |
| 6–7 year old | |||||||||||||
| Total score | 9 | 71.74 | 69.02 – 78.80 | 61 | 88.04 | 81.52 – 91.85 | .000* | −.4011 | 11 | 80.34 | 73.91 – 86.96 | .09 | −.383 |
| Physical health | 9 | 75.00 | 68.75 – 89.06 | 61 | 90.63 | 84.38 – 96.88 | .016** | −.28 | 11 | 81.25 | 71.88 – 93.75 | .42 | −.188 |
| Psychosocial health | 9 | 71.67 | 65.00 – 75.83 | 61 | 85 | 79.17‐ 91.67 | .000* | −.415 | 11 | 83.33 | 73.33 – 86.67 | .048 | −.443 |
| Emotional functioning | 9 | 65.00 | 62.50 – 77.50 | 61 | 80 | 70–90 | .033** | −.2527 | 11 | 80 | 60–85 | .506 | −.156 |
| Social functioning | 9 | 75.00 | 70.00 – 96.25 | 61 | 95 | 80–100 | .080 | −.21 | 11 | 85 | 75–90 | 0.56 | −.137 |
| School functioning | 9 | 65.00 | 55.00–75.00 | 61 | 85 | 79.17‐ 91.67 | .000* | −.4042 | 11 | 80 | 70–90 | .047** | −.445 |
| 8–12 years old | |||||||||||||
| Total score | 2 | 65.22 | 192 | 82.61 | 77.17–89.13 | .357 | −.0719 | 26 | 79.89 | 73.37–88.59 | .516 | −.135 | |
| Physical health | 2 | 60.94 | 192 | 87.5 | 81.25 – 90.63 | .968 | −.0027 | 26 | 84.38 | 75–88.28 | .966 | −.017 | |
| Psychosocial health | 2 | 67.50 | 192 | 81.67 | 73.33 | .126 | −.11 | 26 | 79.17 | 71.25 – 88.33 | .167 | −.279 | |
| Emotional functioning | 2 | 70.00 | 192 | 75 | 66.25 – 85.00 | .67 | −.0315 | 26 | 77.5 | 70–90 | .622 | −.102 | |
| Social functioning | 2 | 80.00 | 192 | 90 | 80–95 | .213 | −.092 | 26 | 82.5 | 78.75 – 91.25 | .63 | −.121 | |
| School functioning | 2 | 52.50 | 192 | 80 | 70–85 | .006* | −.16 | 26 | 75 | 68.75–85 | .021** | −.401 | |
P < .01.
P < .05.
Note. higher scores represent higher HRQOL (scores 0–100).
The HRQOL of patients with ARFID aged 8–10 was lower in the subscale school functioning compared to healthy controls (P < .01; see Table 3). Comparing ARFID patients to chronically ill children, no significant differences were found in any scale.
Due to small numbers for the PedsQL in both age groups (6–7 and 8–10 years), and a low internal consistency in some of the subscales (see Method section), these results are explorative.
3.4. HRQOL of ARFID patients with and without tube feeding
In total, 28 participating ARFID patients received tube feeding by nasogastric tube (n = 21), percutaneous endoscopic gastrostomy (n = 6), or button (n = 1). The tube feeding started at a median age of 1 (IQR 0‐6) month. Twenty‐seven of these patients were in the age group 0–5 years. The TAPQOL scores of these 27 patients were compared with patients in the same age group not receiving tube feeding (n = 10).
The HRQOL of ARFID patients receiving tube feeding did not differ significantly compared to ARFID patients without tube feeding (see Supporting Information Table S2).
4. DISCUSSION
The prevalence rate of ARFID in our tertiary care multidisciplinary center for pediatric feeding problems is 64%. This study shows that the HRQOL of young ARFID patients is decreased on multiple subscales. ARFID patients aged 0–5 years showed a significantly lower HRQOL on the subscales appetite, lungs, stomach, motor functioning, positive mood and liveliness compared to healthy controls, and on the subscales appetite, stomach, motor functioning, and liveliness compared to chronically ill patients. Parents of ARFID patients reported significantly less problem behavior compared to both healthy and chronically ill controls. The HRQOL of ARFID patients 0–5 years receiving tube feeding did not differ compared to those without tube feeding. ARFID patients aged 6–7 had significantly lower scores on the subscales total score, psychosocial health, and school functioning compared to healthy controls. ARFID patients aged 8–10 had a lower school functioning subscale compared to healthy controls.
The prevalence rate of ARFID in our Diagnostic Center for Feeding Problems is higher than described in other studies: 3.2% in a general pediatric population, 1.5% in a pediatric gastroenterology setting, 15% in an adolescent medicine setting, 13.8% in an adolescent medicine eating disorder program (patients 8–18 years), 22.5% in a day program for children and adolescents with eating disorders and comorbid psychopathology (patients 7–17 years), and 5% in another pediatric tertiary care hospital evaluating all patients with eating disorders (mean age 13.7 ± 2.5 years). All studies used the DSM‐5 criteria to determine if patients had ARFID (Eddy et al., 2015; Fisher et al., 2014; Kurz et al., 2015; Nicely, Lane‐Loney, Masciulli, Hollenbeak, & Ornstein, 2014; Ornstein et al., 2013). There might be several explanations for the higher prevalence rate of ARFID in our study. The first reason is our specialized tertiary care setting for feeding problems in which patients with severe feeding problems from the whole country are seen as a second or third opinion. In addition, our hospital is known to perform the clinical hunger provocation program to wean children from tube feeding and initiate oral feeding. For this particular reason, tube‐fed children are often referred to our hospital. Then, our patients are younger than those of earlier mentioned studies, and it is known that children with ARFID tend to be younger compared to other eating disorders (Norris et al., 2014). Furthermore, the fact that our tertiary care center has a large NICU ward, and is an expert center for certain genetic diseases and syndromes contributed to our population consisting of medically fragile children with almost 90% of ARFID patients showing comorbidities. When comparing ARFID patients to other DSM‐5 eating disorders or in various clinical settings, ARFID patients are found to be a heterogeneous group with varying ages and comorbidities. They are more often diagnosed with medical comorbidities/conditions, including but not limited to congenital malformations, very low birth weight, cerebral palsy, and diseases of the gastrointestinal tract that may be related or unrelated to the ARFID diagnosis. They can be tube dependent as well (Eddy et al., 2015; Fisher et al., 2014; Norris et al., 2016). Additionally, the diversity within the ARFID population may be attributed to the chronic medical conditions and/or development delays associated with feeding issues (Bryant‐Waugh, Markham, Kreipe, & Walsh, 2010; Rommel, de Meyer, Feenstra, & Veereman‐Wauters, 2003; Wilken et al., 2018). As such, the DSM‐5 criteria do not exclude patients with the aforementioned comorbidities from the ARFID diagnosis.
Tube feeding or symptoms of the medical condition may have triggered the food aversion and refusal in the beginning (Hartdorff et al., 2015; Levy et al., 2009). Therefore, it can be difficult to distinguish between medical conditions and ARFID. However, the diagnosis ARFID can be made if the feeding disturbance is more severe, and therefore warrants additional attention, than which can be expected of the medical condition only, or when the feeding problem does not resolve after treating the medical disorder (American Psychiatric Association & DSM‐5 Task Force, 2013; Eddy et al., 2015; Nicely et al., 2014). Considering the high rate of medical comorbidities in our population of children with ARFID, differences in HRQOL compared to healthy children may also originate from the medical comorbidity, and not just from the feeding problems alone. Therefore, the HRQOL of ARFID patients was compared to chronically ill controls in addition to healthy controls.
The largest age group of our sample consisted of ARFID patients aged 0–5 years old. Their decreased HRQOL subscales appetite and stomach might be explicable both as cause and consequence of the feeding disorder. We believe the impaired HRQOL on the lung subscale, which had a moderate internal consistency, could be better explained by the high rate of respiratory conditions in our population and as a side effect of tube feeding, than by the feeding disorder itself. These children showed a decreased score for the motor functioning and liveliness subscales compared to both healthy and chronically ill children. We hypothesize that this might be due to a lower activity index, associated with a lower energy intake and nutritional status, or due to restriction of movements in tube fed children and less exploration consequently. The severity of feeding problems in this tertiary care sample could have influenced HRQOL. Therefore, these results may not be generalized to the whole pediatric ARFID population. No differences were found on the subscales sleeping, skin, social functioning, communication and anxiety. Especially the latter is remarkable, considering longitudinal studies showing that children with early food refusal were at risk for anxiety disorders (Ammaniti, Lucarelli, Cimino, D'Olimpio, & Chatoor, 2012). A possible explanation for this difference could be, that anxiety problems occur later in life. The ARFID patients scored better on the subscale problem behavior (measuring difficult and aggressive behavior of the child with following seven questionnaires roughly translated: “My child was … (1) bad tempered, (2) aggressive, (3) agitated, (4) angry, (5) impatient, (6) stubborn, (7) unmanageable”), both compared to healthy and chronically ill controls. In contrast to our results, another study revealed infants with feeding problems to show more signs of difficult temperament, heightened emotional reactivity, and aggressive behavior, still present at the age of 5 and 8 years, measured with the child behavior checklist (CBCL 1½–5 and 6–18) (Ammaniti et al., 2012). On the other hand, another study showed that children who refused to eat at an early age (3–12 months) had more eating problems at home and primary school at follow‐up (9.6 ± 0.3 years), but their general behavior, measured by the total Rutter score and the hyperactivity score calculated from the Rutters's “Children's behavior questionnaire for completion by teachers” and “Children's behavior questionnaire for completion by parents”, was not different (Dahl, Rydell, & Sundelin, 1994). Since the literature is not consistent, problem behavior among children with ARFID needs to be further elucidated in future studies.
Explorative results showed that the children with ARFID aged 6–7 years had lower HRQOL on the total score and on the subscales, psychosocial health, and school functioning, compared to healthy controls, and ARFID patients aged 8–10 had lower HRQOL in the subscale school functioning compared to healthy controls. Prior to the definition ARFID, children with other feeding disorders were also described to have an impaired cognition, show more social and school difficulties, have emotional regulation problems, and show more anxiety and obsessive–compulsive symptoms (Bryant‐Waugh et al., 2010; Chatoor et al., 2004; Lukens & Silverman, 2014; Rommel et al., 2003). Our explorative results of impaired HRQOL in the older age categories of ARFID patients in addition to the known psychosocial problems in children with other feeding disorders, might indicate that HRQOL in older children could also be impaired, but larger groups are necessary to investigate and confirm this speculation.
In our study approximately 60% of the children were tube fed. In the previous DSM versions, however, these children would probably not have been recognized to have a ‘feeding disorder of infancy and early childhood’, because they often lack the criteria ‘failure to meet appropriate nutritional and/or energy needs’. Patients who do meet appropriate nutritional and/or energy needs, but whom rely on enteral feeding or oral nutritional supplements, may now fulfill the relatively new diagnosis ARFID in contrast to the former DSM‐diagnosis ‘feeding disorder of infancy and early childhood’ (Bryant‐Waugh et al., 2010). Studies analyzing the HRQOL in children with feeding disorders other than ARFID receiving tube feeding have scarcely been performed. Davis (2016) measured the HRQOL in tube dependent children at baseline and after treatment to wean children off tube to oral feeding with the Infant Toddler Quality of Life. The HRQOL scores improved significantly on the subscales growth and development, behavior, general health perceptions, parental health: emotional, and parental health: time. They also report the HRQOL to be generally low compared to other studies, but it is unclear how this was measured exactly (Davis et al., 2016). Dunitz‐Scheer et al. (2009) also reported an impaired quality of life, but did not mention if and how the quality of life had been measured. Since tube feeding can impact health and psychosocial functioning in children, we suspected that ARFID patients receiving tube feeding, would also have lower HRQOL subscales (Benoit et al., 2000; Hartdorff et al., 2015; Krom et al., 2017; Krom et al., 2018; Wilken et al., 2018). However, no significant differences were found in our study. This might be due to the small patient sample. Further studies assessing the health related quality of life in a larger sample size of tube fed children are necessary.
4.1. Strengths and Limitations
This is the first study reporting HRQOL in children with ARFID. The high response rate of 75% is the result of several phone calls to remind the parents of the patients to complete the questionnaires, which led to obtaining a relatively large sample. Another strength of the study was the use of validated questionnaires of representative healthy Dutch norm groups (Engelen et al., 2009; Fekkes et al., 2000). Considering so many children had medical comorbidities, we compared them with a Dutch reference group of chronically ill children as well.
Some limitations, however, have to be considered. Due to the cross‐sectional design of the study, causality cannot be determined. Questionnaire studies are always at risk for selection bias. This, however, was not objected and likely, as we found no significant differences between participants and nonparticipants. The by proxy questionnaires were also a limitation, since the parental perception of the HRQOL of their child might be influenced by their own mental health and concerns (Eiser & Morse, 2001b). As the diagnosis ARFID was not used in our clinical setting at the start of the study, the diagnosis was made retrospectively by the research physician. As the reference group of chronically ill children was gathered from a general population, it could be possible that comorbidity of our patients was more severe, which also may have contributed to the lower HRQOL scores of ARFID patients. The small sample size for the PedsQL in both age groups (6–7 and 8–10 years), and a low internal consistency in some of the subscales were limitations of the study.
5. CONCLUSION
HRQOL of children with ARFID is lower compared to both healthy and chronically ill controls. The subgroup of ARFID patients receiving tube feeding did not differ significantly from those not receiving tube feeding. We recommend to add HRQOL as an outcome measure in clinical studies. Assessing the HRQOL of children with ARFID in daily practice can detect problems on the affected subscales, and personalized care from appropriate disciplines can focus on these problems to improve the HRQOL. Especially the psychologist may play a key role in evaluating and treating psychosocial problems and the HRQOL. Further studies, however, should determine if this will lead to an improvement of HRQOL by measuring the HRQOL in a standardized way before and after treatment. The HRQOL of children with tube feeding should be measured in a larger sample size. Furthermore, the parental HRQOL of children with ARFID compared to healthy and chronically ill children should be measured. Future studies should also assess the behavior and psychopathology of children with ARFID and their parents in order to target treatment, and improve care.
Supporting information
Table S1 Baseline characteristics of participating and nonparticipating parents
Table S2: ARFID patients with and without tube feeding
ACKNOWLEDGMENTS
The authors would like to acknowledge Stephanie Louise Kiel‐Clayton for revising our manuscript carefully as a native English speaker.
Krom H, van der Sluijs Veer L, van Zundert S, et al. Health related quality of life of infants and children with avoidant restrictive food intake disorder. Int J Eat Disord. 2019;52:410–418. 10.1002/eat.23037
REFERENCES
- American Psychiatric Association , & DSM‐5 Task Force . (2013). Diagnostic and statistical manual of mental disorders : DSM‐5. Washington, D.C.: American Psychiatric Association. [Google Scholar]
- Ammaniti, M. , Lucarelli, L. , Cimino, S. , D'Olimpio, F. , & Chatoor, I. (2012). Feeding disorders of infancy: A longitudinal study to middle childhood. The International Journal of Eating Disorders, 45(2), 272–280. [DOI] [PubMed] [Google Scholar]
- Benoit, D. , Wang, E. E. , & Zlotkin, S. H. (2000). Discontinuation of enterostomy tube feeding by behavioral treatment in early childhood: A randomized controlled trial. The Journal of Pediatrics, 137(4), 498–503. [DOI] [PubMed] [Google Scholar]
- Bryant‐Waugh, R. (2013). Feeding and eating disorders in children. Current Opinion in Psychiatry, 26(6), 537–542. [DOI] [PubMed] [Google Scholar]
- Bryant‐Waugh, R. , Markham, L. , Kreipe, R. E. , & Walsh, B. T. (2010). Feeding and eating disorders in childhood. The International Journal of Eating Disorders, 43(2), 98–111. [DOI] [PubMed] [Google Scholar]
- Chatoor, I. , Surles, J. , Ganiban, J. , Beker, L. , Paez, L. M. , & Kerzner, B. (2004). Failure to thrive and cognitive development in toddlers with infantile anorexia. Pediatrics, 113(5), e440–e447. [DOI] [PubMed] [Google Scholar]
- Cronbach, L. (1951). Coefficient alpha and the internal structure of tests. Psychometrika, 16, 297–334. [Google Scholar]
- Dahl, M. , Rydell, A. M. , & Sundelin, C. (1994). Children with early refusal to eat: Follow‐up during primary school. Acta Paediatrica, 83(1), 54–58. [DOI] [PubMed] [Google Scholar]
- Davis, A. M. , Dean, K. , Mousa, H. , Edwards, S. , Cocjin, J. , Almadhoun, O. , … Hyman, P. E. (2016). A randomized controlled trial of an outpatient protocol for transitioning children from tube to Oral feeding: No need for amitriptyline. The Journal of Pediatrics, 172, 136–141.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunitz‐Scheer, M. , Levine, A. , Roth, Y. , Kratky, E. , Beckenbach, H. , Braegger, C. , … Scheer, P. J. (2009). Prevention and treatment of tube dependency in infancy and early childhood. Infant, Child, and Adolescent Nutrition, 1, 73–82. [Google Scholar]
- Eddy, K. T. , Thomas, J. J. , Hastings, E. , Edkins, K. , Lamont, E. , Nevins, C. M. , … Becker, A. E. (2015). Prevalence of DSM‐5 avoidant/restrictive food intake disorder in a pediatric gastroenterology healthcare network. The International Journal of Eating Disorders, 48(5), 464–470. [DOI] [PubMed] [Google Scholar]
- Eiser, C. , & Morse, R. (2001a). Quality‐of‐life measures in chronic diseases of childhood. Health Technology Assessment, 5(4), 1–157. [DOI] [PubMed] [Google Scholar]
- Eiser, C. , & Morse, R. (2001b). Can parents rate their child's health‐related quality of life? Results of a systematic review. Quality of Life Research, 10(4), 347–357. [DOI] [PubMed] [Google Scholar]
- Engelen, V. , Haentjens, M. M. , Detmar, S. B. , Koopman, H. M. , & Grootenhuis, M. A. (2009). Health related quality of life of Dutch children: Psychometric properties of the PedsQL in The Netherlands. BMC Pediatrics, 9, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekkes, M. , Theunissen, N. C. M. , Brugman, E. , Veen, S. , Verrips, E. G. H. , Koopman, H. M. , … Verloove‐Vanhorick, S. P. (2000). Development and psychometric evaluation of the TAPQOL: A health‐related quality of life instrument for 1‐5‐year‐old children. Quality of Life Research, 9(8), 961–972. [DOI] [PubMed] [Google Scholar]
- Field, A. (2009). Discovering statistics using SPSS. London: SAGE publications. [Google Scholar]
- Fisher, M. M. , Rosen, D. S. , Ornstein, R. M. , Mammel, K. A. , Katzman, D. K. , Rome, E. S. , … Walsh, B. T. (2014). Characteristics of avoidant/restrictive food intake disorder in children and adolescents: A "new disorder" in DSM‐5. The Journal of Adolescent Health, 55(1), 49–52. [DOI] [PubMed] [Google Scholar]
- Gottrand, F. , & Sullivan, P. B. (2010). Gastrostomy tube feeding: When to start, what to feed and how to stop. European Journal of Clinical Nutrition, 64(Suppl 1), S17–S21. [DOI] [PubMed] [Google Scholar]
- Hartdorff, C. M. , Kneepkens, C. M. F. , Stok‐Akerboom, A. M. , van Dijk‐Lokkart, E. M. , Engels, M. A. H. , & Kindermann, A. (2015). Clinical tube weaning supported by hunger provocation in fully‐tube‐fed children. Journal of Pediatric Gastroenterology and Nutrition, 60(4), 538–543. [DOI] [PubMed] [Google Scholar]
- Haverman, L. , Grootenhuis, M. A. , van den Berg, J. M. , van Veenendaal, M. , Dolman, K. M. , Swart, J. F. , … van Rossum, M. A. J. (2012). Predictors of health‐related quality of life in children and adolescents with juvenile idiopathic arthritis: Results from a web‐based survey. Arthritis Care Res (Hoboken), 64(5), 694–703. [DOI] [PubMed] [Google Scholar]
- Haverman, L. , van Oers, H. A. , Limperg, P. F. , Hijmans, C. T. , Schepers, S. A. , Sint Nicolaas, S. M. , … Grootenhuis, M. A. (2014). Implementation of electronic patient reported outcomes in pediatric daily clinical practice: The KLIK experience. Clinical Practice in Pediatric Psychology, 2(1), 50–67. [Google Scholar]
- Hay, P. , Mitchison, D. , Collado, A. E. L. , González‐Chica, D. A. , Stocks, N. , & Touyz, S. (2017). Burden and health‐related quality of life of eating disorders, including avoidant/restrictive food intake disorder (ARFID), in the Australian population. Journal of Eating Disorders, 5, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joosten, K. F. M. (2012). Signalering van somatische oorzaken van afwijkend voedingsgedrag. Utrecht: NVK. [Google Scholar]
- Kerzner, B. (2009). Clinical investigation of feeding difficulties in young children: A practical approach. Clinical Pediatrics (Phila), 48(9), 960–965. [DOI] [PubMed] [Google Scholar]
- Kerzner, B. , Milano, K. , MacLean, W. C. , Berall, G. , Stuart, S. , & Chatoor, I. (2015). A practical approach to classifying and managing feeding difficulties. Pediatrics, 135(2), 344–353. [DOI] [PubMed] [Google Scholar]
- Krom, H. , de Winter, J. P. , & Kindermann, A. (2017). Development, prevention, and treatment of feeding tube dependency. European Journal of Pediatrics, 176(6), 683–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krom, H. , van Zundert, S. M. C. , Otten, M. A. G. M. , van der Sluijs Veer, L. , Benninga, M. A. , & Kindermann, A. (2018). Prevalence and side effects of pediatric home tube feeding. Clinical Nutrition, 38(1), 234–239. [DOI] [PubMed] [Google Scholar]
- Kurz, S. , van Dyck, Z. , Dremmel, D. , Munsch, S. , & Hilbert, A. (2015). Early‐onset restrictive eating disturbances in primary school boys and girls. European Child & Adolescent Psychiatry, 24(7), 779–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy, Y. , Levy, A. , Zangen, T. , Kornfeld, L. , Dalal, I. , Samuel, E. , … Levine, A. (2009). Diagnostic clues for identification of nonorganic vs organic causes of food refusal and poor feeding. Journal of Pediatric Gastroenterology and Nutrition, 48(3), 355–362. [DOI] [PubMed] [Google Scholar]
- Linscheid, T. R. (2006). Behavioral treatments for pediatric feeding disorders. Behavior Modification, 30(1), 6–23. [DOI] [PubMed] [Google Scholar]
- Lukens, C. T. , & Silverman, A. H. (2014). Systematic review of psychological interventions for pediatric feeding problems. Journal of Pediatric Psychology, 39(8), 903–917. [DOI] [PubMed] [Google Scholar]
- Nicely, T. A. , Lane‐Loney, S. , Masciulli, E. , Hollenbeak, C. S. , & Ornstein, R. M. (2014). Prevalence and characteristics of avoidant/restrictive food intake disorder in a cohort of young patients in day treatment for eating disorders. Journal of Eating Disorders, 2(1), 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris, M. L. , Robinson, A. , Obeid, N. , Harrison, M. , Spettigue, W. , & Henderson, K. (2014). Exploring avoidant/restrictive food intake disorder in eating disordered patients: A descriptive study. The International Journal of Eating Disorders, 47(5), 495–499. [DOI] [PubMed] [Google Scholar]
- Norris, M. L. , Spettigue, W. J. , & Katzman, D. K. (2016). Update on eating disorders: Current perspectives on avoidant/restrictive food intake disorder in children and youth. Neuropsychiatric Disease and Treatment, 12, 213–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornstein, R. M. , Rosen, D. S. , Mammel, K. A. , Callahan, S. T. , Forman, S. , Jay, M. S. , … Walsh, B. T. (2013). Distribution of eating disorders in children and adolescents using the proposed DSM‐5 criteria for feeding and eating disorders. Journal of Adolescent Health, 53(2), 303–305. [DOI] [PubMed] [Google Scholar]
- Payot, A. , & Barrington, K. J. (2011). The quality of life of young children and infants with chronic medical problems: Review of the literature. Current Problems in Pediatric and Adolescent Health Care, 41(4), 91–101. [DOI] [PubMed] [Google Scholar]
- Rommel, N. , de Meyer, A. M. , Feenstra, L. , & Veereman‐Wauters, G. (2003). The complexity of feeding problems in 700 infants and young children presenting to a tertiary care institution. Journal of Pediatric Gastroenterology and Nutrition, 37(1), 75–84. [DOI] [PubMed] [Google Scholar]
- Silverman, A. H. (2010). Interdisciplinary care for feeding problems in children. Nutrition in Clinical Practice, 25(2), 160–165. [DOI] [PubMed] [Google Scholar]
- Wilken, M. , Bartmann, P. , Dovey, T. M. , & Bagci, S. (2018). Characteristics of feeding tube dependency with respect to food aversive behaviour and growth. Appetite, 123, 1–6. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) . (2016). International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD‐10 Version). Geneva: WHO; Retrieved from: http://apps.who.int/classifications/icd10/browse/2016/en Access 7juli2016 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Baseline characteristics of participating and nonparticipating parents
Table S2: ARFID patients with and without tube feeding


