Abstract
Psoriasis is a chronic autoimmune disease affecting skin which may also manifest in nails and joints. Several biologic treatments have been approved in Japan for psoriasis. Each biologic has a different profile for efficacy and safety, including different dosing regimens and co‐payment considerations which may complicate treatment decisions made by patients and physicians during short consultations. Elucidating patient preference is expected to contribute to shared decision‐making between patients and physicians to optimize treatment satisfaction and outcomes. However, the number of studies investigating this in Japan is very limited. The study used a discrete choice experiment methodology to elicit patient preferences for hypothetical options in an experimental framework. Participants were asked to choose their preferred treatment option from two hypothetical choices, defined by different levels of six attributes (i.e. early onset of efficacy, long‐term efficacy, sustained efficacy after drug withdrawal, dosing convenience, co‐payment and risk of serious infection). The survey included 16 treatment choice scenarios and was completed by 395 participants. Across all participants, the attribute regarded as most important was sustained efficacy after drug withdrawal, followed by dosing convenience, co‐payment, long‐term efficacy, early onset of efficacy and risk of serious infection. The study found that patients prefer treatments which have durable efficacy and lower treatment burden characterized as fewer injection frequency and lower co‐payment. These results may be helpful to understand patient preference for biologic treatments used for psoriasis in Japan and contribute to shared decision‐making between patients and physicians to improve patient satisfaction and treatment outcomes.
Keywords: biologics, discrete choice experiment, Japan, patient preference, psoriasis
Introduction
Psoriasis is a chronic autoimmune disease affecting skin which may also present with manifestations in the nails and joints. Skin lesions of psoriasis can be localized or generalized and appear in many different forms, with distinct subtypes that vary in severity, course and duration. As it is a chronic condition with visible characteristics and no cure, patients sometimes endure social stigma.1
Based on prevalence of 0.34%, it is estimated that there are over 400 000 people living with psoriasis in Japan.2 The prevalence of psoriasis is higher in males than in females in Japan,3 which is different than what is reported in Western countries where prevalence is similar between males and females.4
Current treatments for psoriasis are categorized as topical therapies, phototherapy, and conventional and biologic systemic therapies. Patients with mild disease or limited pathology are managed with topical therapies. Patients with moderate to severe disease may require phototherapy and systemic therapies including oral medications and biologics.5 Since 2010, several biologic treatments have been approved in Japan for moderate to severe psoriasis. The patient satisfaction with these biologics was reported to be higher among topical, phototherapy, oral agents and biologic treatments.6
Biologics inhibit the action of specific types of immune‐mediated cells, or inhibit the binding of proteins which play a role in the immune system in developing psoriasis, including pathways such as tumor necrosis factor (TNF)‐α, interleukin (IL)‐17A or IL‐23.7 Among those targets, IL‐23/IL‐17 was detected as an immune axis and the discovery has brought further understanding of the role of cellular immunology in psoriasis.8 Biologics specifically inhibiting either of these two targets have been able to demonstrate superior improvements in clinical outcomes of psoriasis compared with TNF inhibitors and an IL‐12/23 p40 inhibitor.9 The Psoriasis Area and Severity Index (PASI) is a well‐accepted scoring system10 used to evaluate the severity of psoriasis. A PASI‐90, interpreted as a 90% or more improvement from baseline PASI score,7 was adopted as the primary efficacy endpoint in recent clinical trial programs of biologics, and it was achieved in the majority of patients with the new biologics.11, 12, 13, 14, 15 Some biologics can demonstrate the attainment of even higher efficacy of PASI‐100 (i.e. total clearance of all skin lesions).9 These biologic treatments have different properties in terms of dosing convenience, co‐payment implications, and demonstrated safety and efficacy profiles; thus, the treatment options that may fit the needs of each patient have been expanded.
In addition to considering the different characteristics of the biologics, consultation time with physicians is often limited and this also hampers decision‐making for patients and physicians. Understanding patient preference improves the quality of care that physicians are able to provide6 because integrating patient preference into decision‐making may increase the patient's treatment adherence and the overall success of treatment.16 However, one study pointed out time pressures and difficulties in eliciting patient preference as challenges for physicians.6 A patient's behavior survey conducted by the Ministry of Health, Labour and Welfare in 2017 showed that 66.6% of outpatients spent less than 10 min for a consultation at medical institutions and 28.6% of patients spent less than 5 min with their physician.17 This consultation time would be insufficient for both patients and physicians to make mutually satisfactory treatment decisions.
Evidence reporting treatment preference directly elicited from patients is expected to contribute to improved decision‐making to optimize treatment satisfaction which will lead to higher adherence and better outcomes.18 However, the number of studies investigating patient preference when selecting a biologic therapy for psoriasis in Japan is very limited. Therefore, the objective of this study was to identify factors that affect patient preference when choosing a biologic therapy and the priority placed on each factor.
To investigate patient preference, the study adopted a discrete choice experiment (DCE). The method was introduced as a way to measure stated preference.19 It has been used by economists to investigate an individual's preference and trade‐off between product characteristics,20 and gained increasing popularity in health care to quantify preferences of patients and caregivers, physicians and other stakeholders.21 DCE is described as “a scientifically rigorous alternative to traditional patient‐centered outcomes research methods” with a theoretical basis, and can evaluate not only the patient's physical state but also treatment processes such as timing and location of administration. In this study, the research method was therefore employed to reveal the preferences of patients with psoriasis as one of the most scientific assessments for patient‐reported outcome.19
Methods
Participants
All participants were invited from a patient panel in a general Internet panel in Japan (n = 614 490) which is maintained by a contracted market research company. The patient panel consisted of patients reported to have psoriasis and received any treatments for psoriasis in the previous year (n = 2248). In the patient panel, 1465 patients were undergoing any treatment at the time of planning the study, and those were recruited for this study by the market research company.
Those who agreed to participate in the study proceeded to the survey. Participants were eligible if they met the following criteria: (i) aged 20 years or older; (ii) were willing to sign the informed consent; (iii) self‐reported a diagnosis of psoriasis; and (iv) were currently receiving treatment for psoriasis with non‐biologics or biologics. There were no exclusion criteria.
A minimum sample size was estimated that at least 300 valid responses would be necessary for robust quantitative research and a minimum of 65 valid responses were considered to be necessary for an adequately powered study.22 The equation (n × t × a) / c ≥ 500 was used for the calculation of minimum sample size which adopts projected sample size (n) of the main study, number of choice scenarios to be selected (t), number of alternatives per scenario (a) and maximum number of levels for any one attribute (c). The minimum sample size was calculated as 62.5 and rounded up to 65.
Study design
The DCE methodology is “designed to test hypotheses regarding strength of preference, relative importance of, and acceptable trade‐offs among attributes that define the research question”.21 The methodology involves conducting a survey using questionnaires “to elicit subjects' preferences for hypothetical options in an experimental framework” and provides “experimental control over the choice context, which makes it possible to estimate the relative importance of each factor in the experiment design”.19
In this study, the survey asked all participants with any severity, regardless of treatment, to assume a situation where they were going to start a biologic treatment as a patient with moderate to severe psoriasis. The questionnaire presented two hypothetical treatment options and the participants chose a preferred one from the two treatment options. Each option consisted of six attributes, constructed from a combination of outcome and process attributes similar to prior published studies,23, 24, 25, 26, 27 investigating preference for biologic treatments amongst patients with psoriasis. The attributes and their levels are shown in Table 1.
Table 1.
Attributes and levels in discrete choice experiment
| Attributes | Levels |
|---|---|
| The rate that psoriasis (e.g. redness and dandruff) disappears by more than 90% 1 month after starting the treatment | 10 patients in 100 20 patients in 100 30 patients in 100 |
| The rate that psoriasis (e.g. redness and dandruff) disappears by more than 90% and the efficacy is sustained 1 year after starting the treatment | 50 patients in 100 65 patients in 100 80 patients in 100 |
| The rate that psoriasis (e.g. redness and dandruff) disappears by more than 90% and the efficacy is sustained approximately half year after stopping the treatment | 20 patients in 100 40 patients in 100 60 patients in 100 |
| Frequency of the treatment | Every 2 weeks, at home Every 4 weeks, at home Every 8 weeks, at hospital Every 12 weeks, at hospital |
| Monthly average co‐payment of 1st year and 2nd year and thereafter | 1st year / 2nd year and thereafter |
| ¥26 000 / ¥15 000 | |
| ¥35 000 / ¥22 000 | |
| The annual rate of serious infectious events requiring hospitalization including serious infections such as pneumonia requiring hospitalization | 1 patient in 100 2 patients in 100 |
Attributes included early onset of efficacy, long‐term efficacy, sustained efficacy after drug withdrawal and safety outcomes. It was considered that these factors were important matters affecting the choice of treatment for patients with psoriasis. All three attributes for efficacy were set and explained to participants assuming PASI‐90 because it was considered to be “the measure of optimal response”28 of treatments for psoriasis. Additionally, the use of a consistent efficacy level for the three efficacy attributes could prevent participants from misunderstanding the questionnaire.
“Early response” was defined as efficacy at Week 4, indicating 4 weeks after initiation of the treatment. Week 4 was described by the European Medicines Agency (EMA) as the earliest time point among minimum “pre‐specified time points for assessment”29 when a clinically meaningful response to biologics for psoriasis could be observed.30 “Week 4” was translated to “1 month” in the survey for participants to make the questionnaire easier to understand. “Long‐term efficacy” was used to indicate efficacy at Week 52. This parameter was described in the EMA guidelines29 as persisting response of maintenance therapy after achieving maximum efficacy, noting it could be decreased due to loss of efficacy or adverse effects. The guidelines also recommended evaluating long‐term efficacy at 1 year.29 “Week 52” was translated to “1 year” in the survey for ease of participants' understanding. “Sustained efficacy after drug withdrawal” referred to efficacy that was sustained for approximately 6 months after stopping the treatment. Treatment guidelines currently recommend continuous therapy for moderate to severe psoriasis;31, 32 however, discussion on how long a successful therapy should be continued is ongoing.33 As psoriasis is a chronic disease requiring long‐term treatment, patients may need to stop their treatments occasionally due to adverse events, pregnancy or other lifestyle reasons. Therefore, sustained efficacy after drug withdrawal was considered an important attribute when selecting the treatment from a long‐term perspective. “Serious infection” was selected as the safety outcome and defined as per 100 patient‐years of serious infectious events requiring hospitalization including serious infections such as pneumonia requiring hospitalization. Serious infectious events were considered to be a common concern amongst the biologics as they are immunosuppressant products.34 Further, Japanese guidance for use of biologics for psoriasis advises caution to prevent this adverse event.35
Regarding process attributes, “dosing convenience” and “cost” were selected. Dosing convenience was defined as a combination of dosing frequency and location of administration. There are several patterns of dosing frequency among biologic treatments for moderate to severe psoriasis in Japan: every 2 weeks, monthly, every 2 months and every 3 months. Location of administration changes depending on dosing frequency in Japan. If the frequency is at least once per month, the biologics can be administrated at home. If the dosing frequency is every 2 or 3 months, they are administrated at hospitals.
The cost borne by the patient (i.e. co‐payment) was determined assuming a situation where a patient starts a biologic treatment for psoriasis under Japanese Universal Health Coverage. As co‐payment of biologic treatments for psoriasis often exceeds a pre‐fixed ceiling amount, High‐Cost Medical Expense Benefit can be applied for the treatments. Using this benefit, patients are only required to pay a pre‐fixed ceiling amount if monthly co‐payment exceeds a specified amount calculated by their annual income and age. This benefit provides further reduction in co‐payment applied from the fourth time in 12 months after the first application; therefore, the monthly co‐payment under the Japanese health insurance system would be different in the first and subsequent years. In this study, the cost attribute consisted of co‐payment in the first year and the second year and thereafter. The two attribute levels represented the highest and the lowest co‐payment for the median annual household income in Japan when biologics were prescribed every 3 months.36
Good practice for conjoint analysis including DCE released by the ISPOR task force in 2011, limits attribute levels to two to four per attribute.37 In this study, each level was derived from publicly released profiles of currently marketed or in‐development biologic treatments for moderate to severe psoriasis. Therefore, the levels were realistic and did not include extreme values such as no infectious event risk. An example of a scenario is shown in Figure 1.
Figure 1.
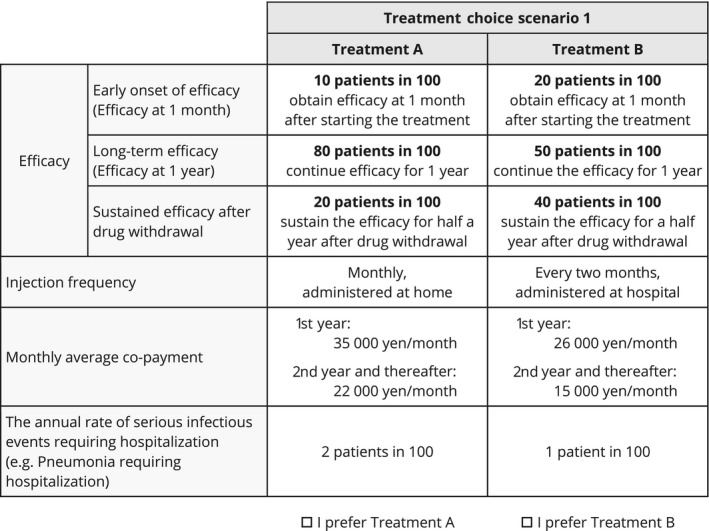
Example of treatment choice scenario for discrete choice experiment.
Sixteen choice scenarios were selected based on orthogonal array in order to reduce respondent burden.38 Accordingly, the study was designed to allow completion of the survey in approximately 20 min. Each choice scenario included two hypothetical treatment options. Participants were repeatedly requested to select one preferred option from the alternatives assuming that the two options were the only available treatments. Sixteen choice scenarios were randomly presented to each participant to avoid possible bias due to the order of scenarios.
Statistical analysis
A conditional logit model was used to identify factors that affect patient preference. Patient preference was evaluated by preference weights and relative importance. The relative importance (%) was calculated based on distance between the highest and lowest preference weights for each attribute divided by the sum of all preference weight differences. In addition, descriptive statistics were used to summarize the sample in terms of sociodemographic and clinical characteristics described in Table 2. Data collected from the main study was used for this analysis. Subgroup analyses were also conducted for patient characteristics and clinical characteristics which were considered to affect patient preference in choice of therapy. All statistical tests were two‐sided and used a significance level of 0.05. In preference weights, non‐overlapping error bars indicate statistically significant differences across levels within attributes. No adjustments for multiple comparisons were performed. STATA version 15 (College Station, TX, USA) was used as statistical analysis software.
Table 2.
Patient demographics
| Characteristics | n | % |
|---|---|---|
| Sex (n, %) | ||
| Female | 98 | 24.8 |
| Male | 297 | 75.2 |
| Age (n, %) | ||
| 20–29 years | 3 | 0.8 |
| 30–39 years | 30 | 7.6 |
| 40–49 years | 99 | 25.1 |
| 50–59 years | 124 | 31.4 |
| 60–69 years | 100 | 25.3 |
| 70–79 years | 39 | 9.9 |
| ≥80 years | 0 | 0.0 |
| Age at which psoriasis developed (n, %) | ||
| <16 years | 35 | 8.9 |
| 16–39 years | 173 | 43.8 |
| 40–59 years | 146 | 37.0 |
| ≥60 years | 41 | 10.4 |
| Years since psoriasis diagnosis (n, %) | ||
| <1 year | 13 | 3.3 |
| 1 to <6 years | 90 | 22.8 |
| 6 to <11 years | 66 | 16.7 |
| 11 to <21 years | 102 | 25.8 |
| ≥21 years | 124 | 31.4 |
| Percentage of body surface area affected by psoriasis (n, %)a | ||
| <3% | 147 | 37.2 |
| 3% | 111 | 28.1 |
| 6% | 76 | 19.2 |
| ≥10% | 61 | 15.4 |
| Affected areas by psoriasis (n, %) | ||
| Scalp | 256 | 64.8 |
| Palms | 58 | 14.7 |
| Nail | 98 | 24.8 |
| Back of hand | 113 | 28.6 |
| Soles | 44 | 11.1 |
| Top of feet | 105 | 26.6 |
| Groin | 83 | 21.0 |
| Genital area | 70 | 17.7 |
| Armpit | 48 | 12.2 |
| Other | 282 | 71.4 |
| No areas are affected by psoriasis | 5 | 1.3 |
| Frequency of visit for treatment of psoriasis (n, %) | ||
| Once or a few times a week | 13 | 3.3 |
| Once every 2 weeks | 16 | 4.1 |
| Once a month | 136 | 34.4 |
| Once every 2 months | 96 | 24.3 |
| Once every 3 months | 69 | 17.5 |
| Once every 4 months or more | 36 | 9.1 |
| None of the above | 29 | 7.3 |
| Presence of joint pain (n, %) | ||
| Yes | 72 | 18.2 |
| No | 323 | 81.8 |
| Current treatment for psoriasis (n, %) | ||
| Topical medication | 371 | 93.9 |
| Oral medication | 94 | 23.8 |
| Phototherapy | 36 | 9.1 |
| Periodically administrated injection (biologics) | 36 | 9.1 |
| Other | 7 | 1.8 |
| No treatment received currently | 0 | 0.0 |
| Presence of knowledge of the High‐Cost Medical Expense Benefit | ||
| Yes | 332 | 84.1 |
| No | 63 | 15.9 |
| Level of annual income or household income (n, %) | ||
| Exclusion from residential taxation | 32 | 8.1 |
| Annual income <¥3.7 million | 142 | 35.9 |
| Annual income from ¥3.7 to <¥7.7 million | 139 | 35.2 |
| Annual income from ¥7.7 to <¥11.6 million | 37 | 9.4 |
| Annual income ≥¥11.6 million | 18 | 4.6 |
| I do not want to answer | 27 | 6.8 |
| Presence of knowledge of biologic drugs (only patients who had no experience of periodically administrated injection answered; n, %) | ||
| Yes | 163 | 41.3 |
| No | 188 | 47.6 |
The participant selected a category that best described his/her condition.
Informed consent was obtained from all participants prior to them commencing the survey and eligible participants were determined based on inclusion criteria. The participants accessed an online survey website from a computer‐connectable device and answered the questionnaire. Participants completed sociodemographic and clinical questionnaires and then completed the DCE. The study was performed according to the principles of the Declaration of Helsinki and approved by the Non‐Profit Organization MINS Institutional Review Board.
Prior to conducting the main study, a pilot study was executed (n = 31) to confirm the sample size required for the main study and to verify the questionnaire's understandability.
Results
Demographics
A total of 395 participants with psoriasis completed the survey. Table 2 summarizes the sociodemographic and clinical characteristics of the participants. The online survey was designed to disallow incomplete survey responses; hence, there were no missing data.
Overall, more than three‐quarters (75.2%) of the participants were male. The largest portion of participants (31.4%) were 50–59 years old, followed by 60–69 years old (25.3%) and 40–49 years old (25.1%). The participants aged 60 years and older accounted for approximately 30%. In terms of disease duration after diagnosis of psoriasis, only 3.3% of patients were for less than a year. The remaining participants were from 1 to 5 years (22.8%), 6 to 10 years (16.7%), 11 to 20 years (25.8%) and more than 21 years (31.4%). The participants reported their severity as expressed in body surface area of skin lesion (BSA), with 37.2% of these having mild psoriasis (BSA: <3%), 47.3% moderate psoriasis (BSA: ≥3% and <10%) and 15.4% severe psoriasis (BSA: ≥10%). Almost all the participants (98.7%) stated that they had active psoriasis. Among participants with psoriasis in visible or sensitive areas, psoriasis in scalp was the most frequently reported manifestation (64.8%). At the time of the survey, the participants were on the following treatments: topical treatments (93.9%), oral medication (23.8%), phototherapy (9.1%) and biologic therapies (9.1%). Current frequencies of visits to clinics and hospitals were as follows: a few times in a month (7.3%), monthly (34.4%), every 2 months (24.3%), every 3 months (17.5%) and every 4 months or more (9.1%). More than 80% of participants (84.1%) knew of the High‐Cost Medical Expense Benefit which offers fixed monthly co‐payments to reduce their out‐of‐pocket expenditure.
Overall results
Across all participants, the attribute regarded as most important was sustained efficacy after drug withdrawal (relative importance [RI]: 25.4%), followed by dosing convenience (RI: 19.0%), co‐payment (RI: 18.2%), long‐term efficacy (RI: 17.5%) and early onset of efficacy (RI: 13.0%). The attribute of least relative importance was risk of serious infection (RI: 6.8%). Preference weights derived from the attribute levels showed that the participants favored the higher probability in any type of efficacy parameter, dosing convenience with fewer administration frequencies, lower probability of serious infection and lower co‐payment (Figs 2, 3).
Figure 2.
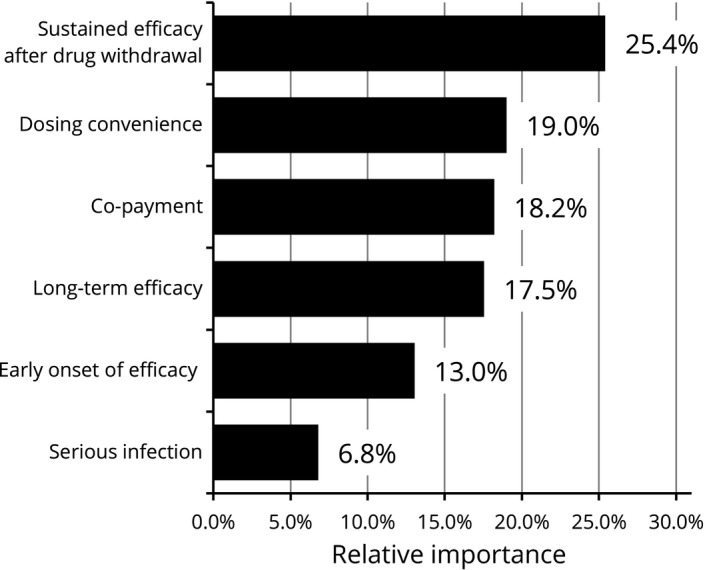
Relative importance of attributes (overall results, n = 395). Relative importance is relatively described values calculated by the distance between the highest and the lowest attribute levels. For example, the distance of sustained efficacy after drug withdrawal is 0.66, addition of the highest level of 0.316 and the lowest level of 0.344. This value is converted to percentage of total which is the sum of all attributes' distance. The higher percentages indicate the more preferred attribute.
Figure 3.
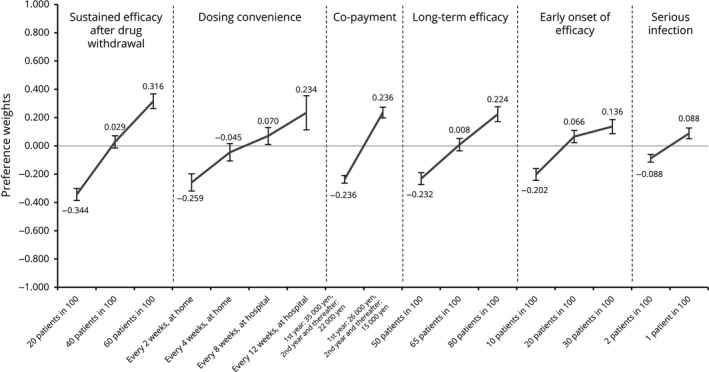
Preference weights for attribute levels (overall results, n = 395). Preference weights are showed on the vertical scale, describing how much each level was selected within one attribute. Regarding risk of serious infection, one patient in 100 was the highest and therefore the most preferred level in this attribute. Non‐overlapping error bars indicate statistically significant differences across levels within attributes.
Results from subgroup analyses
A number of additional analyses were undertaken to assess whether any particular subpopulations held different preferences. In most subgroup analyses, sustained efficacy after drug withdrawal was chosen as the most important attribute.
A subgroup analysis conducted by age showed that participants aged both 60 years and older (35.2% of the overall participants) and under 60 years old prioritized sustained efficacy after drug withdrawal the most while the elderly group presented higher importance on serious infection when selecting the treatment (RI: 11.0%) compared with the overall results (RI: 6.8%) (Figs 4, 5, 6, 7). Results stratified by sex demonstrated a difference in preference of females compared with the overall results. Female participants (24.8% of the overall participants) regarded dosing convenience as most important (RI: 28.5%), followed by sustained efficacy after drug withdrawal (RI: 26.9%) and long‐term efficacy (RI: 17.6%) (Figs 8, 9, 10, 11). When analyzed by severity using BSA, all three subgroups put the greatest value on sustained efficacy after drug withdrawal while the participants with severe psoriasis whose BSA was 10% and more attached higher relative importance to dosing convenience (RI: 23.3%) compared with the overall results (RI: 19.0%). Examining disease duration from diagnosis, no marked difference in RI was observed regardless of disease duration compared with the overall results. In regard to current treatments received by participants at the time of the survey, those treated with biologics presented a similar trend to the overall results and placed the highest importance on sustained efficacy after drug withdrawal while the importance of co‐payment was lower (RI: 8.0%) compared with overall results (RI: 18.2%). Results from subgroups according to current visit frequencies showed that participants currently visiting with less frequency placed more importance on dosing convenience. For those visiting a clinician every 3 or 4 months, dosing convenience was the highest preference with RI of 22.9% and 23.0%, respectively. Whereas in those who visited a clinician a few times in a month, dosing convenience was ranked fourth (RI: 16.0%), third in the monthly visit group (RI: 17.2%) and second in the every 2 months group (RI: 17.6%). Stratified by annual income level based on the High‐Cost Medical Expense Benefit, participants in the highest income level of more than ¥11.6 million representing less than 5% (n = 18) showed the strongest preference for dosing convenience (RI: 39.2%) and RI of the other five attributes ranged 9.5–15.5%. Those who were in the four other income levels presented comparable findings with the overall results.
Figure 4.
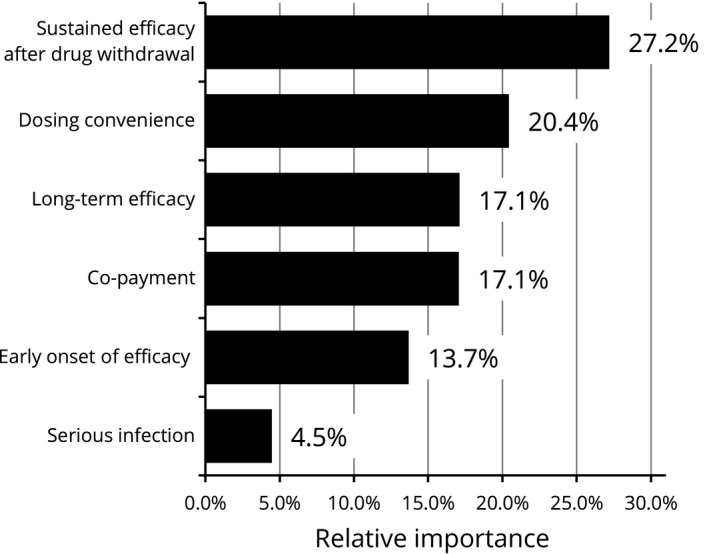
Relative importance of attributes (<60 years, n = 256). Relative importance is relatively described values calculated by the distance between the highest and the lowest attribute levels. The higher percentages indicate the more preferred attribute.
Figure 5.
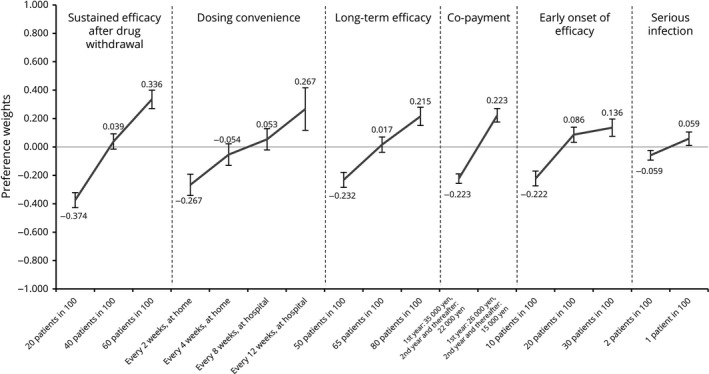
Preference weights for attribute levels (<60 years, n = 256). Preference weights are showed on the vertical scale, describing how much each level was selected within one attribute. The highest value indicates the most preferred level and the lowest value indicates the least preferred level in each attribute. Non‐overlapping error bars indicate statistically significant differences across levels within attributes.
Figure 6.
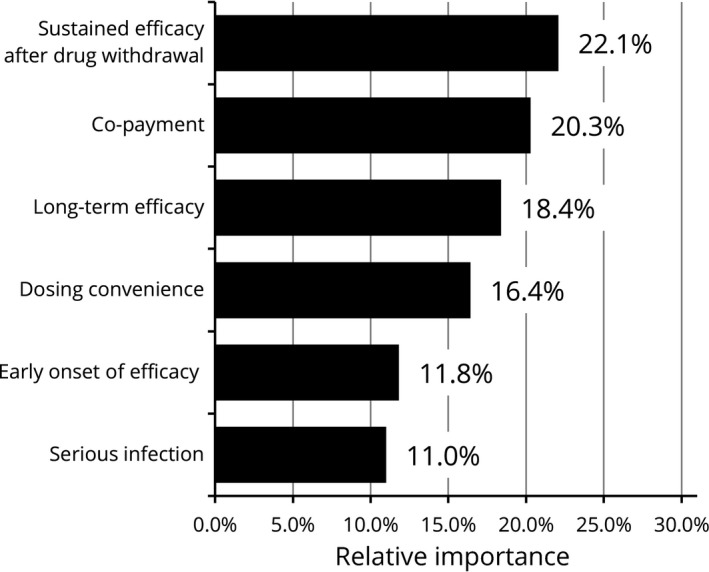
Relative importance for attributes (≥60 years, n = 139). Relative importance is relatively described values calculated by the distance between the highest and the lowest attribute levels. The higher percentages indicate the more preferred attribute.
Figure 7.
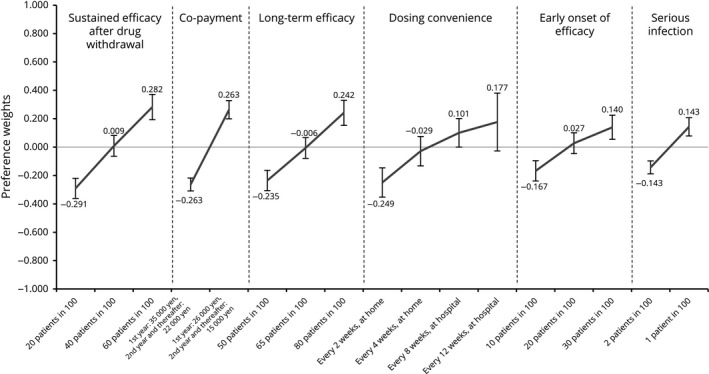
Preference weights for attribute levels (≥60 years, n = 139). Preference weights are showed on the vertical scale, describing how much each level was selected within one attribute. The highest value indicates the most preferred level and the lowest value indicates the least preferred level in each attribute. Non‐overlapping error bars indicate statistically significant differences across levels within attributes.
Figure 8.
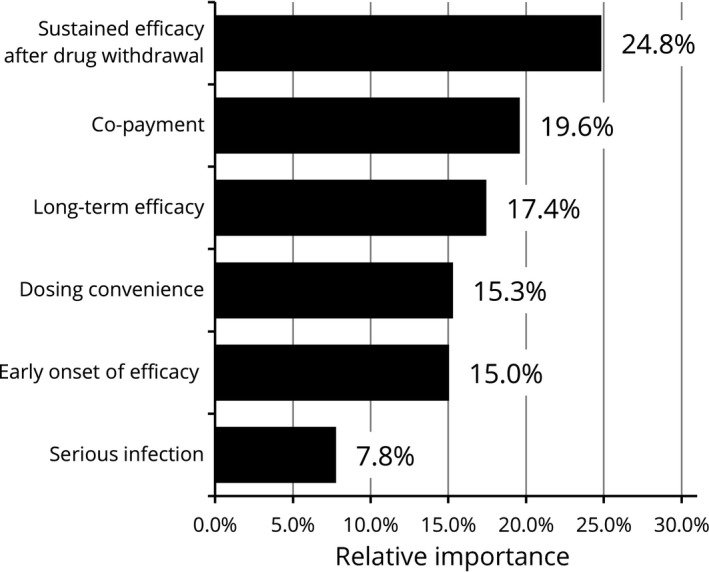
Relative importance for attributes (males, n = 297). Relative importance is relatively described values calculated by the distance between the highest and the lowest attribute levels. The higher percentages indicate the more preferred attribute.
Figure 9.
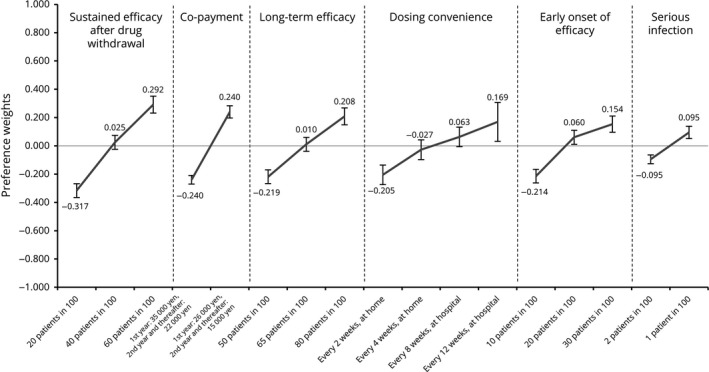
Preference weights for attribute levels (males, n = 297). Preference weights are showed on the vertical scale, describing how much each level was selected within one attribute. The highest value indicates the most preferred level and the lowest value indicates the least preferred level in each attribute. Non‐overlapping error bars indicate statistically significant differences across levels within attributes.
Figure 10.
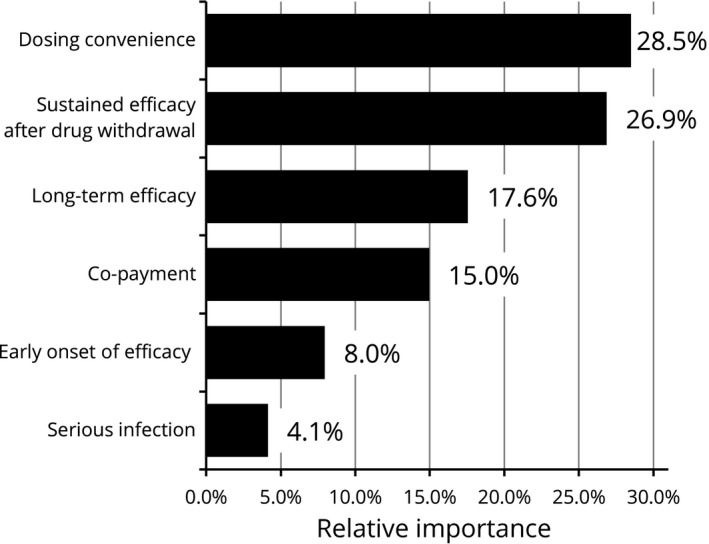
Relative importance for attributes (females, n = 98). Relative importance is relatively described values calculated by the distance between the highest and the lowest levels in each attribute. The larger values indicate the more preferred options.
Figure 11.
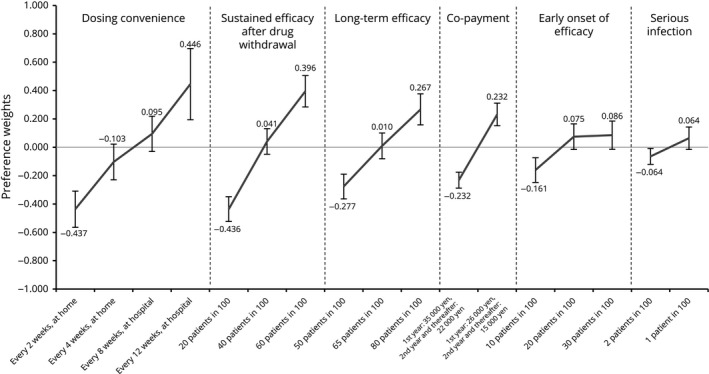
Preference weights for attribute levels (females, n = 98). Preference weights are showed on the vertical scale, describing how much each level was selected within one attribute. The highest value indicates the most preferred level and the lowest value indicates the least preferred level in each attribute. Non‐overlapping error bars indicate statistically significant differences across levels within attributes.
Discussion
This study demonstrates the preference of Japanese patients with psoriasis in selecting a biologic treatment based on profiles of approved or in‐development biologics for psoriasis. Most patients, regardless of their individual characteristics, attached the highest importance to sustained efficacy after drug withdrawal. This is a new insight revealed in the study. The similar trend was also identified as “bio‐holiday” in a recent study investigating preferences of patients with psoriasis in Japan.39 Additionally, the majority of patients prioritized long‐term efficacy compared with early onset of efficacy. These results indicate that patients with psoriasis favored durability rather than onset speed of efficacy in psoriasis treatment. It is interesting that “sustained efficacy after drug withdrawal” was chosen as the most preferred element in spite of no availability of those drugs in the current clinical setting. This reveals that there is an unmet patient desire that can potentially be addressed by new treatment options in the future.
Dosing convenience was the second preferred attribute by patients. In this study, dosing convenience was defined as a combination of frequency and location of the treatment and set as one of the process attributes. Tendency towards high preference of process attributes was also observed in prior studies conducted in foreign countries investigating treatment preference of patients with moderate to severe psoriasis. One previous research stated that process attributes related to drug administration may outweigh outcome attributes.23 In contrast, there was a study in Japan reporting that injection frequency was of lower priority.39 Each existing study had their own definitions such as frequency and route of administration. In this study, dosing convenience was defined as a combination of frequency and location of treatment, assuming realistic options in Japan and excluding unrealistic ones. The result, therefore, may be one of practical findings in the actual clinical setting. Treatment convenience for patients with psoriasis may be an important factor when selecting the treatment as it is a chronic disease and patients need to manage treatment over the longer term. Additionally, given a subgroup analysis by disease duration showing no marked difference in relative importance compared with the overall results, dosing convenience would be important not only for patients living with psoriasis for more than 10 years but also for patients with psoriasis for a shorter period of time.
Furthermore, patients taking treatments with less visit frequencies assigned greater importance to dosing convenience. It was suggested that these patients may be prioritizing the dosing convenience to manage and adjust the treatment to suit their lifestyle. Moreover, when conducting a subgroup analysis by sex, it was found that dosing frequency was the most important attribute for females. In a prior study, it was reported that females put more importance on treatment frequency than males.25 Because this study adopted dosing convenience as a combination of frequency and location of administration, it was unclear whether either factor affected high preference of dosing frequency. However, considering that dosing convenience with fewer administration frequency was preferred, and that more than 80% of participants were aged 40–69 years who may play an important role in household duties and jobs, it indicates an unmet need for patients, especially female patients with psoriasis, to reduce the frequency of treatment as much as possible.
Patients prioritized co‐payment as the third attribute in overall results, following sustained efficacy after drug withdrawal and dosing convenience. In a subgroup analysis, however, patients treated with biologics showed lower relative importance of co‐payment and ranked as the fifth attribute. A similar trend was observed in prior studies demonstrating that the cost was the least important attribute among process attributes.23, 27 The reason for this variance may be due to differences in individual characteristics. The majority of patients in this study were treated with non‐biologics while the prior studies enrolled patients with moderate to severe psoriasis and included patients being treated with biologics. Therefore, this would indicate that patients currently treated with biologics may be less sensitive about co‐payment because they pay the current treatment cost. On the other hand, co‐payment may become a hurdle for patients treated with non‐biologics when initiating treatment with biologics.
The study adopted efficacy using PASI‐90, as it has been commonly used in contemporary clinical trials as a more stringent efficacy endpoint.11, 12, 13, 14, 15 This measurement is also considered “the new standard of care”40 in actual clinical practice for moderate to severe psoriasis treated with biologics in Japan. Moreover, the study showed that the participants always preferred a higher probability in any type of efficacy parameter, dosing convenience with fewer administration frequency, a lower probability of serious infection and lower co‐payment. Such results confirm the internal validity of the study because the participants consistently made rational decisions by selecting better treatment options in the survey adopting the methodology of DCE.
Limitation
The number of patients with psoriasis treated with biologics in the current patient panel was very limited, and the study could not focus solely on patients with moderate to severe psoriasis due to insufficient numbers. As the study recruited psoriatic patients with any severity, regardless of treatment, 90% of the study population were treated with non‐biologics and 84.6% were reported to have BSA of less than 10%. This may affect the subgroup analyses of this study. Because the treatment choice scenarios were prepared assuming biologic treatments for moderate to severe psoriasis, it might have been difficult for some participants to personally relate to the selection based on their past experience. However, the study showed similar trends consistent with previous studies presenting greater preference for dosing convenience,25 higher prescription rate of treatments with longer dosing frequencies in females,41 more concern about severe adverse event occurrence in the elderly patients,23, 25 and lower importance of co‐payment or cost in patients currently treated with biologics.23, 27 These results may partially support the external validity of the study.
A bias might have occurred due to the study being conducted solely online, which might have limited the accessibility of this survey to the general population. However, the higher participation of males and the age distribution in the study are consistent with existing epidemiology data in Japan.3
In conclusion, the attribute that influenced psoriatic patients most was sustained efficacy after drug withdrawal, followed by dosing convenience, co‐payment, long‐term efficacy, early onset of efficacy and safety. The study found that patients preferred treatments which have durable efficacy and lower treatment burden characterized by fewer injection frequency and lower co‐payment. These results may be helpful to understand patient preference for biologics used to treat psoriasis in Japan and contribute to shared decision‐making between patients and physicians which will ultimately improve patient satisfaction and treatment outcomes. As further research on treatments for psoriasis will continue, it may be useful to consider patient preference when conducting future research and examine how treatment characteristics may benefit patients living with the disease.
Conflict of Interest
Y. T. has received honoraria for research from Maruho, LEO Pharma, Eisai, AbbVie, Kyowa Hakko Kirin, Celgene, Meiji‐Seika‐pharma, Taiho and Eli Lilly & Co., and for lecturing from Maruho, Kyowa Hakko Kirin, LEO Pharma, Eli Lilly & Co. and Janssen Pharmaceutical. K. I., J. K. and I. K. are full‐time employees of AbbVie GK and may own AbbVie stock. Keigo Hanada is an employee of CRECON Medical Assessment Inc. that was paid to conduct analyses for this article.
Acknowledgments
The design, study conduct, and financial support for the study were provided by AbbVie GK. AbbVie participated in the interpretation of data, review and approval of the publication.
References
- 1. Griffiths CEM, van der Walt JM, Ashcroft DM et al The global state of psoriasis disease epidemiology: a workshop report. Br J Dermatol 2017; 177(1): e4–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kubota K, Kamijima Y, Sato T et al Epidemiology of psoriasis and palmoplantar pustulosis: a nationwide study using the Japanese national claims database. BMJ Open 2015; 5(1): e006450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Takahashi H, Nakamura K, Kaneko F, Nakagawa H, Iizuka H, Japanese Society for Psoriasis Research . Analysis of psoriasis patients registered with the Japanese Society for Psoriasis Research from 2002‐2008. J Dermatol 2011; 38(12): 1125–1129. [DOI] [PubMed] [Google Scholar]
- 4. Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol 2017; 31(2): 205–212. [DOI] [PubMed] [Google Scholar]
- 5. Boehncke W‐H, Schön MP. Psoriasis. Lancet 2015; 386(9997): 983–994. [DOI] [PubMed] [Google Scholar]
- 6. van Cranenburgh OD, de Korte J, Sprangers MA, de Rie MA, Smets EM. Satisfaction with treatment among patients with psoriasis: a web‐based survey study. Br J Dermatol 2013; 169(2): 398–405. [DOI] [PubMed] [Google Scholar]
- 7. Ronholt K, Iversen L. Old and new biological therapies for psoriasis. Int J Mol Sci 2017; 18(11): E2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gaffen SL, Jain R, Garg AV, Cua DJ. The IL‐23‐IL‐17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol 2014; 14(9): 585–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. No DJ, Amin M, Bhutani T, Wu JJ. A systematic review of active comparator controlled clinical trials in patients with moderate‐to‐severe psoriasis. J Dermatolog Treat 2018; 29(5): 467–474. [DOI] [PubMed] [Google Scholar]
- 10. Puig L. PASI90 response: the new standard in therapeutic efficacy for psoriasis. J Eur Acad Dermatol Venereol 2015; 29(4): 645–648. [DOI] [PubMed] [Google Scholar]
- 11. Thaci D, Blauvelt A, Reich K et al Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol 2015; 73(3): 400–409. [DOI] [PubMed] [Google Scholar]
- 12. Reich K, Pinter A, Lacour JP et al Comparison of ixekizumab with ustekinumab in moderate‐to‐severe psoriasis: 24‐week results from IXORA‐S, a phase III study. Br J Dermatol 2017; 177(4): 1014–1023. [DOI] [PubMed] [Google Scholar]
- 13. Blauvelt A, Papp KA, Griffiths CE et al Efficacy and safety of guselkumab, an anti‐interleukin‐23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double‐blinded, placebo‐ and active comparator‐controlled VOYAGE 1 trial. J Am Acad Dermatol 2017; 76(3): 405–417. [DOI] [PubMed] [Google Scholar]
- 14. Reich K, Armstrong AW, Foley P et al Efficacy and safety of guselkumab, an anti‐interleukin‐23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double‐blind, placebo‐ and active comparator‐controlled VOYAGE 2 trial. J Am Acad Dermatol 2017; 76(3): 418–431. [DOI] [PubMed] [Google Scholar]
- 15. Papp KA, Blauvelt A, Bukhalo M et al Risankizumab versus ustekinumab for moderate‐to‐severe plaque psoriasis. N Engl J Med 2017; 376(16): 1551–1560. [DOI] [PubMed] [Google Scholar]
- 16. Umar N, Yamamoto S, Loerbroks A, Terris D. Elicitation and use of patients' preferences in the treatment of psoriasis: a systematic review. Acta Derm Venereol 2012; 92(4): 341–346. [DOI] [PubMed] [Google Scholar]
- 17. Ministry of Health Labour and Welfare . Patient's behavior survey in 2017. Published 2018. [Cited 25 September 2018]. Available from URL: https://www.mhlw.go.jp/toukei/saikin/hw/jyuryo/17/dl/kekka-gaiyo.pdf.
- 18. Delestras S, Roustit M, Bedouch P et al Comparison between two generic questionnaires to assess satisfaction with medication in chronic diseases. PLoS ONE 2013; 8(2): e56247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Patient preference methods ‐ a patient centered evaluation paradigm. ISPOR CONNECTIONS. Published 2007. [Cited 25 September 2018]. Available from URL: https://www.ispor.org/docs/default-source/sig-documents/Patient-Preference-Methods.pdf.
- 20. Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes, 4th edn Oxford, UK: Oxford University Press, 2015. [Google Scholar]
- 21. Hauber AB, Gonzalez JM, Groothuis‐Oudshoorn CG et al Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR Conjoint analysis Good Research Practices Task Force. Value Health 2016; 19(4): 300–315. [DOI] [PubMed] [Google Scholar]
- 22. Orme BK. Getting Started with Conjoint Analysis: Strategies for Product Design and Pricing Research. Madison, WI: Research Publishers, 2006. [Google Scholar]
- 23. Schaarschmidt M‐L, Astrid Schmieder M, Nasir Umar M et al Patient preferences for psoriasis treatments. Process characteristics can outweigh outcome attributes. Arch Dermatol 2011; 147(11): 1285–1294. [DOI] [PubMed] [Google Scholar]
- 24. Schaarschmidt ML, Umar N, Schmieder A et al Patient preferences for psoriasis treatments: impact of treatment experience. J Eur Acad Dermatol Venereol 2013; 27(2): 187–198. [DOI] [PubMed] [Google Scholar]
- 25. Kromer C, Schaarschmidt ML, Schmieder A, Herr R, Goerdt S, Peitsch WK. Patient preferences for treatment of psoriasis with biologicals: a discrete choice experiment. PLoS ONE 2015; 10(6): e0129120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alcusky M, Lee S, Lau G et al Dermatologist and patient preferences in choosing treatments for moderate to severe psoriasis. Dermatol Ther (Heidelb) 2017; 7(4): 463–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rigopoulos D, Ioannides D, Chaidemenos G et al Patient preference study for different characteristics of systemic psoriasis treatments (Protimisis). Dermatol Ther 2018; 31(3): e12592. [DOI] [PubMed] [Google Scholar]
- 28. Ryan C, Korman NJ, Gelfand JM et al Research gaps in psoriasis: opportunities for future studies. J Am Acad Dermatol 2014; 70(1): 146–167. [DOI] [PubMed] [Google Scholar]
- 29. European Medicines Agency . Guideline on clinical investigation of medicinal products indicated for the treatment of psoriasis. Published 2004. [Cited 25 September 2018]. Available from URL: http://www.ema.europa.eu/ema/pages/includes/document/open_document.jsp?webContentId=WC500003329.
- 30. Papp KA, Lebwohl MG. Onset of action of biologics in patients with moderate‐to‐severe psoriasis. J Drugs Dermatol 2017; 17(3): 247–250. [PubMed] [Google Scholar]
- 31. Brezinski EA, Armstrong AW. Off‐label biologic regimens in psoriasis: a systematic review of efficacy and safety of dose escalation, reduction, and interrupted biologic therapy. PLoS ONE 2012; 7(4): e33486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ramirez‐Fort MK, Levin AA, Au SC, Gottlieb AB. Continuous versus intermittent therapy for moderate‐to‐severe psoriasis. Clin Exp Rheumatol 2013; 31(4 Suppl 78): S63–S70. [PubMed] [Google Scholar]
- 33. Mrowietz U, de Jong EM, Kragballe K et al A consensus report on appropriate treatment optimization and transitioning in the management of moderate‐to‐severe plaque psoriasis. J Eur Acad Dermatol Venereol 2014; 28(4): 438–453. [DOI] [PubMed] [Google Scholar]
- 34. Yiu ZZN, Smith CH, Ashcroft DM et al Risk of serious infection in patients with psoriasis receiving biologic therapies: a prospective cohort study from the British Association of Dermatologists Biologic Interventions Register (BADBIR). J Invest Dermatol 2018; 138(3): 534–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ohtsuki M, Terui T, Ozawa A et al Japanese guidance for use of biologics for psoriasis (the 2013 version). J Dermatol 2013; 40(9): 683–695. [DOI] [PubMed] [Google Scholar]
- 36. Ministry of Health Labour and Welfare . Comprehensive survey of living conditions in 2017. Published 2018. [Cited 25 September 2018]. Available from URL: https://www.mhlw.go.jp/toukei/saikin/hw/k-tyosa/k-tyosa17/dl/10.pdf.
- 37. Bridges JF, Hauber AB, Marshall D et al Conjoint analysis applications in health–a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health 2011; 14(4): 403–413. [DOI] [PubMed] [Google Scholar]
- 38. Addelman S. Symmetrical and asymmetrical fractional factorial plans. Technometrics 1962; 4(1): 47–58. [Google Scholar]
- 39. Bolt T, Kobayashi H, Mahlich J. Patient and physician preferences for therapy characteristics for psoriasis: a discrete choice experiment in Japan. Pharmacoecon Open 2018; 10.1007/s41669-018-0104-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Okubo Y, Tsuruta D, Tang AC et al Analysis of treatment goal alignment between Japanese psoriasis patients and their paired treating physicians. J Eur Acad Dermatol Venereol 2018; 32(4): 606–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sruamsiri R, Iwasaki K, Tang W, Mahlich J. Persistence rates and medical costs of biological therapies for psoriasis treatment in Japan: a real‐world data study using a claims database. BMC Dermatol 2018; 18(1): 5. [DOI] [PMC free article] [PubMed] [Google Scholar]


