Abstract
Objectives
To examine the impact of apathy on cognitive performance in the elderly following the conceptual principles proposed by Marin1 and Stuss et al2 and to determine the role of the symptoms of apathy in different cognitive domains.
Methods
Cross‐sectional study with a cohort of healthy elderly subjects over 55 years old (n = 140). One hundred forty healthy‐elderly subjects (aged 79.24 ± 8.6 years old) were recruited from 12 day centers in Northern Spain. Participants underwent a neuropsychological battery, which evaluated Mini Mental State Examination (MMSE), attention, processing speed, verbal fluency, visual and verbal memory, working memory, and executive functioning. Apathy was assessed by the Lille Apathy Rating Scale (LARS), which is composed of four factors: intellectual curiosity, emotion, action initiation, and self‐awareness. Correlation and linear regression analyses were performed.
Results
In the correlational analysis, the LARS total score correlated negatively with global cognition, verbal fluency, and visual and verbal memory. The intellectual curiosity factor correlated negatively with all cognitive domains except attention. The emotion factor correlated negatively with visual memory. No correlation was found between the action initiation and self‐awareness factors or any of the cognitive variables. Multiple stepwise regression analysis showed that symptoms of apathy explained cognitive performance in attention, processing speed, verbal fluency, visual and verbal memory, working memory, executive functioning, and MMSE.
Conclusions
Apathy was significantly associated with cognitive performance, especially with the intellectual curiosity factor. Our results suggest that specific symptoms of apathy contribute differently to individual cognitive domains.
Keywords: apathy, cognition, cognitive performance, elderly, motivation, neuropsychology
Key points.
This is the first study that shows that each symptom of apathy bears a different influence on cognitive performance in a healthy elderly sample
General apathy and apathy symptoms were associated with poorer cognitive performance in healthy adults
Symptoms of intellectual curiosity showed a predominant role in the explanation of apathy in cognitive performance
It is necessary to take into account which specific apathy symptoms are present in the elderly in order to make more accurate diagnosis and to implement personalized and effective treatments
1. INTRODUCTION
According to the original classification proposed by Marin,1 apathy is a complex neurobehavioral syndrome characterized by a lack of motivation that affects behavior, cognition, and emotion that is not attributable to an alteration of consciousness, a disturbance of intellect or emotion. On the contrary, Stuss et al2 proposed the reduction of initiative as a consequence of the lack of response to stimuli as the main symptom of apathy. They also proposed that apathy differs in different states: affective, emotional, behavioral, and self‐awareness. In the review by Levy and Dubois3 based on the studies of Marin1 and Stuss et al,2 the authors determined that apathy was related to an auto‐activation deficit (corresponding to the behavior factor), disruption of cognitive processing (corresponding to the cognitive factor), and disruption of emotional‐affective processing (corresponding to the emotion factor) and that each of them affected different areas of the brain. Apathy is a common syndrome and represents one of the major problems in public health.4 In the elderly population, apathy symptoms affect 49% of people over 77 years old.5 In agreement with the criteria proposed by Marin and Stuss et al,1, 2 a multidimensional evaluation is necessary due to the complexity of the syndrome.
Currently, there are only three tests that assess apathy in a multidimensional way: the Apathy Scale for Institutionalized Patients with Dementia Nursing Home version (APADEM‐NH),6 the Apathy Inventory (AI),7 and the Lille Apathy Rating Scale (LARS).8 The first one is specific for institutionalized Alzheimer's disease (AD) patients, and AI is composed of only three items, which offer a general estimation of the emotional, behavioral, and cognitive components of apathy. Some authors have considered the LARS as the best adapted scale for evaluating apathy in noninstitutionalized elderly people.4 LARS is composed of four dimensions, three of which represent those proposed by Marin1 (action initiation, emotion, and intellectual curiosity) for the diagnosis of apathy, and the other is the dimension proposed by Stuss et al,2 “self‐awareness,” which refers to the lack of criticism and behavioral adjustment derived from apathy.
Previous studies have related apathy to cognitive performance in the elderly, in particular with inefficient integration of information, poor verbal fluency, naming, reduced performance in constructional praxis, executive functioning, and a lower score in the Mini Mental State Examination (MMSE; see Supporting Information Table S1 to see review).3, 9, 10, 11 In addition, apathy has been suggested to be a risk factor for progression to AD.12, 13, 14
Apathy has been studied under other conditions, such as mild cognitive impairment (MCI),15, 16 AD and other dementias,13, 17, 18 Parkinson's Disease (PD),19, 20, 21 post stroke,22 HIV‐1 infection,23 and in neuropsychiatric disorders, such as schizophrenia24 or depression.25 The frequency of apathy reported in these studies was 14% in MCI,13 60% in AD,12 and 36% in PD.26 In MCI, apathy has been associated with total recall in memory15 and impairment in executive functioning.13, 27 In AD, apathy has been related to lower scores in the Buschke Selective Reminding Test, the Boston Naming Test,18 and to very low MMSE scores.13 In PD, apathy has been associated with lower performance in Controlled Word Association and Trail Making Test B28 and with a higher number of errors in the Stroop test.29 However, in other studies, no direct relationship has been found between apathy and loss of cognitive functioning in community‐dwelling adults,30, 31, 32 AD,10, 14 or PD patients.28
Additionally, evidence in different studies has suggested that apathy in neurodegenerative diseases was associated with difficulties in daily living activities,10, 13 poor insight,7 worse quality of life,33 and stress in caregivers.34 To date, seven studies have researched the relationship between apathy and cognition in healthy elderly samples with different outcomes and methodological differences.9, 10, 11, 30, 31, 32, 35 However, none of them evaluated apathy in a multidimensional way. There are advantages in considering apathy as a complex neurobehavioral and multidimensional syndrome, since it will allow us to implement targeted treatments taking into account the apathy predominant component: behavioral, emotional, or cognitive.
To our knowledge, no previous studies have described the independent symptoms of apathy that specifically affect cognitive performance in healthy elderly adults. Therefore, the aim of the present study was, firstly, to investigate the relationship between the level of apathy and cognitive performance in healthy elderly people following the conceptual principles proposed by Marin1 and Stuss et al2 by means of the LARS and, secondly, to analyze which symptoms of apathy best explain cognitive performance with the four LARS factors.
2. METHODS
2.1. Participants
One hundred and forty participants were recruited from 12 day centers in the Biscay and Alava regions in northern Spain. The inclusion criteria were the following: (a) age over 55 years old; (b) independence in basic activities of daily living according to the Likert‐type semistructured interview carried out by the clinicians responsible for the day centers. Exclusion criteria were the following: (a) history of neurologic (neurodegenerative disease, dementia, traumatic brain injury, or cerebrovascular disease); (b) psychiatric illness or Neuropsychiatric Inventory Questionnaire36 (NPI‐Q > 4) (delusions, depression, anxiety, or psychiatric conditions); (c) illiteracy; (d) MMSE37 ≤ 21 adjusted by age and education level, following recommendations by Manubens et al38 and Bermejo et al39; (e) severe physical constraints. Sixteen participants were disqualified because of exclusion criteria, and the sample was therefore made up of 124 participants (43 males and 81 females) ranging in age from 56 to 95 years old (M = 79.09, SD = 8.93 years). The mean of cognitive reserve was measured by The Cognitive Reserve Questionnaire,40 9.97 (SD = 3.32 CI, 9.10‐10.55). Participants were taking medication (42.7 % for hypertension and 21.8% for diabetes). The sociodemographic variables are given in Table 1.
Table 1.
Sociodemographic, neuropsychological, and clinical‐test scores of the sample (n = 124)
|
Mean (SD) n % |
95% CI | |||
|---|---|---|---|---|
| Age | 79.09 (8.93) | 77.50‐80.68 | ||
| Gender | Male | 43 (34.7) | ||
| Female | 81 (65.3) | |||
| Years of education | 8.41 (2.4) | 7.98‐8.84 | ||
| Cognitive Reserve | 9.97 (3.32) | 9.10‐10.55 | ||
| MMSE | MMSE | 26.19 (2.27) | 25.75‐26.56 | |
| Attention | BTA | 11.33 (3.87) | 11.34‐12.93 | |
| Processing Speed | Salthouse | 11.39 (6.12) | 11.33‐14.13 | |
| TMT‐A | 77.15 (16.58) | 70.58‐78.36 | ||
| Verbal fluency | CIFA | Letter “p” | 8.03 (4.21) | 7.40‐9.34 |
| Animals | 12.56 (4.43) | 11.50‐13.48 | ||
| Foods | 12.98 (4.63) | 11.74‐13.79 | ||
| Visual memory | BVMT | Learn | 10.20 (6.50) | 9.64‐12.46 |
| Total recall | 3.45 (3.05) | 3.65‐4.98 | ||
| Verbal memory | HVLT | Learn | 16.03 (4.72) | 15.44‐17.60 |
| Total recall | 3.14 (2.75) | 2.50‐3.79 | ||
| Working memory | BD‐WAIS‐III | 3.70 (1.01) | 3.43‐4.05 | |
| Executive functioning | Stroop Color‐Word | 19.99 (9.76) | 19.65‐24.16 | |
| Stroop Interference | −0.01 (8.22) | −2.07 to 1.87 | ||
| TMT‐B | 236.6 (78.21) | 208.80‐245.89 | ||
| GDS‐12D | 1.12 (1.48) | 0.73‐1.34 | ||
| LARS | Total | −21.62 (1.71) | −23.44 to −20.51 | |
| Intellectual curiosity | −0.65 (0.38) | −0.73 to −0.58 | ||
| Emotion | −0.53 (0.32) | −0.59 to −0.47 | ||
| Action initiation | −0.83 (0.33) | −0.89 to −0.77 | ||
| Self‐awareness | −0.79 (0.36) | −0.86 to −0.72 | ||
Abbreviations: BD‐WAIS‐III, Backward Digits WAIS‐III; BTA, Brief Test of Attention; BVMT, Brief Visual Memory Test; CIFA, Calibrated Ideational Fluency Assessment; Cognitive Reserve, Cognitive Reserve Questionnaire; GDS‐12D, Geriatric Depression Scale; HVLT, Hopkins Verbal Learning Test; LARS, Lille Apathy Rating Scale; MMSE, Mini Mental State Examination; TMT‐A, Trail Making Test‐A; TMT‐B, Trail Making Test‐B.
2.2. Neuropsychological assessment
Participants underwent a comprehensive neuropsychological battery including MMSE37 to determine global cognitive status. The Brief Test of Attention (BTA)41 was used to measure attention. The Calibrated Ideational Fluency Assessment (CIFA)42 was used to measure verbal fluency. The Brief Visual Memory Test (version‐1) (BVMT)43 was used to evaluate performance in visual memory, learning, and long‐term recall. The Hopkins Verbal Learning Test (version‐2) (HVLT)44 was used to measure performance in verbal memory, learning, and long‐term recall. Processing speed was evaluated through Trail Making Test‐A (TMT‐A)45 and the Salthouse Letter Comparison Test.46 The Stroop Test47 (color‐word and interference) and Trail Making Test‐B (TMT‐B)45 were used to assess executive functioning. Backward Digits for the the Wechsler Adult Intelligence Scale—Third Edition (WAIS‐III)48 was used to measure working memory.
2.3. Clinical assessment
Apathy was evaluated through the Spanish version of the LARS.8, 49 This scale is composed of nine subscales grouped into four factors: (a) intellectual curiosity: reduction in everyday productivity and lack of interests and initiative; (b) emotion: extinction of novelty seeking and motivation and poor social life; (c) action initiation: lack of concern; (d) self‐awareness: extinction of self‐awareness and blunting of emotional responses. The Geriatric Depression Scale (GDS‐15) was used to measure depression.50, 51 Factorial analysis performed by Mitchell et al52 and Ligthart et al11 discovered that three apathy items are included in the GDS‐15 (Have you dropped many of your activities and interests?, Do you prefer to stay at home, rather than going out and doing new things?, and Do you feel full of energy?). Therefore, we excluded these three items, and the GDS‐12D was used in the analysis for monitoring depression without the apathetic component.11, 52 The Neuropsychiatric Inventory Questionnaire (NPI‐Q)36 was used to measure neuropsychiatric behavior such as delusions, hallucinations, disinhibition, agitation/aggression, or depression/dysphoria.
2.4. Procedure
Clinical interviews were conducted in order to collect sociodemographic, clinical, and cognitive reserve data. The neuropsychological battery included global cognitive status, attention, processing speed, verbal fluency, visual and verbal learning and memory, working memory, and executive functioning. All measurements were converted into z scores. Some measurements were adjusted so that higher scores indicated better cognitive performance. The z scores were pooled into composite cognitive domains. All of them reached satisfactory internal consistency: verbal fluency (Cronbach's α = 0.76); visual learning and memory (α = 0.95); verbal learning and memory (α = 0.67); processing speed (α = 0.84); and executive functioning (α = 0.70). Attention and working memory were identified with a single test. Reliability analysis of the LARS and its four factors was performed. The LARS showed satisfactory internal consistency (α = 0.77). Three of the four factors reached satisfactory internal consistency: intellectual curiosity (α = 0.74), emotion (α = 0.61), action initiation (α = 0.60), and self‐awareness (α = 0.37). In order to improve the reliability of the self‐awareness factor, items 18, 19, 20, and 30 were removed from the analysis. The internal consistency of the self‐awareness factor was (α = 0.56).
2.5. Statistical analysis
The statistical analyses were carried out using IBM SPSS statistics v23.53 The Kolmogorov‐Smirnov test was used to establish the normal distribution of the variables studied. Pearson's correlation was used to determine the relationship between the LARS total score, four factors (self‐awareness, action initiation, emotion, and intellectual curiosity), cognitive domains, and clinical variables. Bivariate correlation analysis adjusted by Bonferroni's correction 0.0026 for controlling type 1 error rate was used. Multiple stepwise regression analysis was conducted to analyze the role of symptoms of apathy (four factors) in the cognitive domains. Analysis was adjusted in step 1 (enter method) by confounding variables.
3. RESULTS
The sociodemographic, neuropsychological, and clinical test scores of the sample are provided in Table 1. The LARS total scores for the sample were −21.62 (1.71) (95% CI, −23.44 to −20.51). With a cutoff point of −10, up to 6.1% of the sample was severely apathetic. The bivariate correlations between LARS total scores and clinical and cognitive variables showed that apathy scores were significant for MMSE, verbal fluency, and visual and verbal memory. The higher the level of apathy, the lower the cognitive performance. This same analysis was repeated for each of the LARS factors. Intellectual curiosity and emotion were also related to cognitive performance. The intellectual curiosity factor showed a predominant role in the correlation of apathy in all cognitive domains except attention. The emotion factor was only related to visual memory. The action initiation and self‐awareness factor did not show any significant correlation. The bivariate correlations are provided in Table 2. A visual representation of these results can be observed in Figure 1.
Table 2.
Pearson's Correlation between cognitive domains, LARS Total Score and Factors
| LARS Total | IC | E | AI | SA | |
|---|---|---|---|---|---|
| Age | 0.18 | 0.22 | 0.09 | 0.01 | 0.09 |
| Sex | −0.09 | −0.07 | 0.06 | 0.07 | −0.15 |
| Cognitive reserve | −0.27 | −0.26 | 0.25 | 0.01 | −0.15 |
| GDS‐12D | −0.26 | 0.32** | 0.25 | 0.05 | 0.18 |
| Hypertension | −0.23 | −0.26 | −0.08 | −0.16 | −0.18 |
| Diabetes | −0.13 | −0.07 | −0.18 | −0.05 | 0.07 |
| MMSE | −0.31** | −0.33** | −0.20 | −0.02 | −0.23 |
| Attention | −0.25 | −0.17 | −0.12 | −0.16 | −0.21 |
| Processing speed | −0.21 | −0.40** | −0.03 | 0.07 | 0.06 |
| Verbal fluency | −0.39** | −0.49** | −0.25 | −0.05 | −0.13 |
| Visual memory | −0.47** | −0.49** | −0.34** | −0.06 | 0.29 |
| Verbal memory | −0.32** | −0.36** | −0.26 | 0.09 | 0.16 |
| Working memory | −0.28 | −0.35** | −0.11 | −0.05 | 0.15 |
| Executive functioning | −0.23 | −0.38** | −0.09 | 0.13 | 0.15 |
Abbreviations: AI, action initiation; E, emotion; GDS‐12D, Geriatric Depression Scale; IC, intellectual curiosity; LARS, Lille Apathy Rating Scale; MMSE, Mini Mental State Examination; SA, self‐awareness. Values were adjusted by Bonferroni's correction 0.0026.
P ≤ 0.0026.
Figure 1.
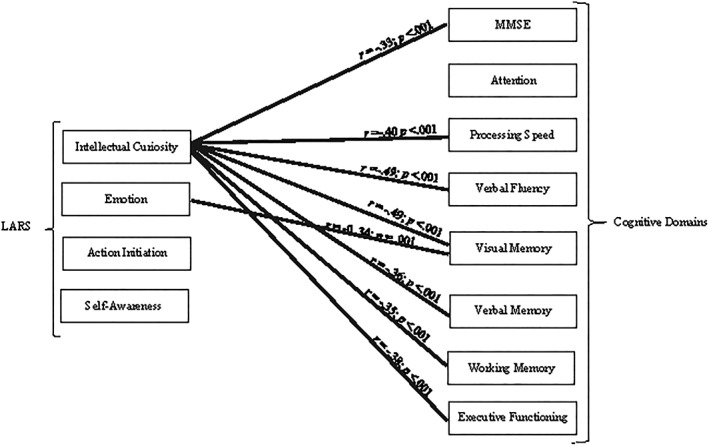
Pearson's correlation between cognitive domains and apathy subscales
The influence of symptoms of apathy on cognitive domains was estimated by means of multiple stepwise regression. Analysis was adjusted in step 1 by significantly correlated variables and with variables that may affect the results according to the literature: age, cognitive reserve, hypertension, and diabetes has been associated with higher levels of apathy.31 The influence of symptoms of apathy for each cognitive domain was not the same, so the intellectual curiosity (∆ R 2 = 0.029, F = 3.83, P = 0.002, IC 95% [−0.59, −0.01] of β1 = −0.21) and self‐awareness factors (∆R 2 = 0.02, F = 3.9, P = 0.001, IC 95% [−0.48, − 0.01] of β1 = 0.19) accounted for 5.6% of the variance for MMSE adjusted by confounding variables. The intellectual curiosity factor was statistically significant for attention (∆ R 2 = 0.096, F = 5.93, P < 0.001, IC 95% [−0.89, −0.26] of β1 = −0.36) and accounted for 9.6% of the variance. The processing speed with the intellectual curiosity factor (∆ R 2 = 0.088, F = 12.56, P < 0.001, IC 95% [−0.83, −0.27] of β1 = −0.34) accounted for 8.6% of the variance. The intellectual curiosity factor was statistically significant (∆ R 2 = 0.094, F = 12.88, P < 0.001, IC 95% [−0.71, −0.25] of β1 = −0.35) and accounted for 9.4% of the variance for verbal fluency. For visual memory, the intellectual curiosity (∆ R 2 = 0.049, F = 12.95, P < 0.001, IC 95% [−0.79, −0.15] of β1 = −0.27) and self‐awareness factors (∆ R 2 = 0.022, F = 12.18, P < 0.001, IC 95% [−0.48, −0.01] of β1 = −0.17) were statistically significant and accounted for 7.1% of the variance. The emotion (∆ R 2 = 0.034, F = 10.06, P < 0.001, IC 95% [−0.76, −0.08] of β1 = −0.21) and action initiation factors (∆ R 2 = 0.025, F = 9.63, P < 0.001, IC 95% [0.02, 0.45] of β1 = −0.18) accounted for 5.9% of the variance for verbal memory. Working memory with the intellectual curiosity factor (∆ R 2 = 0.087, F = 3.48, P = 0.004, IC 95% [−0.85, −0.15] of β1 = −0.30) accounted for 6.2% of the variance. For executive functioning, intellectual curiosity was statistically significant (∆ R 2 = 0.058, F = 10.0, P < 0.001, IC 95% [−0.66, −0.14] of β1 = −0.29) and accounted for 5.8% of the variance. The intellectual curiosity factor showed a predominant role in the explanation for apathy in cognitive performance in all cognitive domains except in verbal memory (see Table S2 to see multiple stepwise regression analysis, also see Figure 2).
Figure 2.
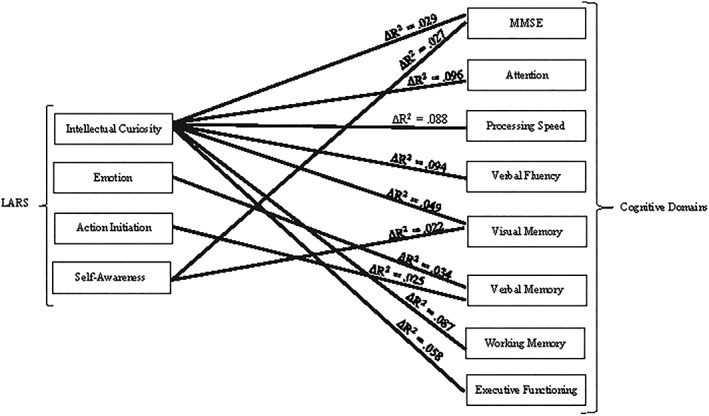
Multiple stepwise regression analysis of cognitive domains and apathy subscales
4. DISCUSSION
To our knowledge, this is the first study that analyzes the specific symptoms of apathy that explain cognitive performance in healthy elderly people. The main findings show that general apathy, and specifically the intellectual curiosity factor, is associated with poorer cognitive performance. Additionally, multiple stepwise regression analysis shows that the symptoms of apathy (intellectual curiosity, emotion, action initiation, and self‐awareness) have a different degree of influence on cognitive performance in each cognitive domain, ranging from 5.6% to 9.6%. All cognitive domains assessed in this study were affected by symptoms of apathy. Lack of intellectual curiosity was the most representative symptom of apathy in relation to cognitive performance.
Although the literature relating apathy to cognitive performance in the elderly is limited, our findings are in line with previous studies in which apathy was associated with lower MMSE scores.9, 11 The study conducted by Onyike et al10 did not find any significant relationship between apathy and memory in contrast to our results. However, these authors found that executive functioning, naming, and verbal fluency were associated with apathy in their elderly population. In our study, the cognitive domains most affected by apathy were also verbal fluency and attention. From a neuropsychological and neuroanatomical perspective, verbal fluency and attention are two cognitive functions highly associated with executive functions.54 In this sense, Levy and Dubois3 proposed apathy as a dysfunction of executive functioning. The severity of cognitive impairment, including the verbal fluency, attentional and executive components, associated with apathy has also been found in some neurodegenerative diseases, such as PD and AD.14, 28 In other diseases, apathy has been associated with poorer performance in slowed processing, poorer integration of information,22, 55 and poorer performance in verbal fluency, for example, in poststroke patients with lesions of the anterior cingulate cortex.56
A strong point of this study is the extensive neuropsychological assessment that was carried out, which has provided an overall view of the influence of apathy on cognitive performance, even after checking for confounding variables such as age, hypertension, diabetes, cognitive reserve, and depression. However, the relationship between vascular risk factors and apathy was only found in our sample for diabetes and not for hypertension, in contrast to other studies.11, 31
One of the causes of discrepancies in study results may be related to methodological issues due to a lack of consensus on the definition and measurement of apathy. Today there are 15 instruments for evaluating apathy in clinical populations. Two systematic reviews57, 58 have studied the reliability and validity of these 15 scales. The authors concluded that the Apathy Evaluation Scale (AES),59 NPI‐Q,60 LARS,8 and the Dementia Apathy Interview and Rating are the most valid instruments to assess apathy. The Starkstein Apathy Scale (SAS)28 has also been used although it is defined as less comprehensive than the AES,57 NPI‐Q,60 and AI,7 which have been used in clinical research. However, these scales depend exclusively on the caregivers' opinion. By contrast, the LARS is a multidimensional scale composed of 33 items, subdivided into nine subscales, grouped into four factors. Moreover, this scale is consistent with the recent international consensus criteria for the diagnosis of apathy.4, 8, 61 The LARS is supported by the principles of Marin1 and Stuss et al2 and takes the pathophysiological processes of apathy into account.49 This scale has been validated for testing in PD and very mild to moderate dementia, demonstrating high internal consistency.4, 8 In our study, the LARS also obtained a good level of internal consistency in three of its four factors.
Another strong point is that the LARS allowed us to analyze symptoms of apathy separately by means of its four factors and therefore facilitates deeper analysis of the components of apathy that may be relevant for cognition. The intellectual curiosity factor composed of lack of initiative and interests and reduction in everyday productivity subscales was the strongest factor related to cognitive domains in our study. These results may be a key to understanding the influence of apathy on cognitive performance in elderly people and in the design of focused rehabilitation strategies. The results are related to the conceptualization of Stuss et al,2 Levy and Dubois,3 and Pagonabarraga et al26 on apathy. The authors define apathy as a disorder of initiative that can be divided into three subtypes: affective, behavioral, and cognitive, which depend on different neural systems. In particular, the cognitive subtype is characterized by impairments in executive functions. By contrast, in the definitions proposed by Marin,1 Robert et al,7 and Starkstein et al62, the central axis of the apathy syndrome is the lack of motivation reflected by emotional, behavioral, and cognitive symptoms but without cognitive impairment. In our study, the presence of the intellectual curiosity factor in all cognitive domains suggests the importance of the lack of initiative, lack of interest, and reduction in everyday productivity in cognitive performance. Moreover, our results support the idea that lack of initiative, rather than lack of motivation, is a key point in cognitive type apathy in our sample of elderly population without dementia. The presence of these symptoms may be essential in understanding why apathy is a risk factor for cognitive impairment and subsequently for later dementia.
Self‐awareness was another factor related to MMSE and visual memory. In these two cognitive functions, poor performance was related to blunting of emotional response and extinction of self‐awareness. This factor was proposed by Stuss et al2 and included by Sockeel et al8 in the LARS. Stuss et al2 defined self‐awareness as the ability to mediate between present and future information. He determined that this ability was altered in people with apathy. The results of this study suggest that worse performance in general cognition and in visual memory is determined by a poorer self‐awareness ability. These results are in line with a previous study in which the authors related cognitive performance to metacognition in aging.63 The action initiation factor formed by the concern subscale was positively related to performance in verbal memory, which indicates that concern increases in relation to performance. This relationship is consistent with the literature in which loss of memory is the most frequent concern in aging.64 Another factor related to worse performance in verbal memory was the emotion factor. This factor is also related to lack of novelty seeking, motivation, and social life. Some research has shown a relationship between the development of cognitive impairment and social isolation.65 Apathy has been associated with lack of motivation in the literature although in this study, the emotion factor made up of motivation, novelty seeking, and social life was only associated with verbal memory. This result supports the aforementioned idea that lack of initiative, rather than lack of motivation, is the factor that relates most to the cognitive symptoms in apathy. It is also interesting to see how, when carrying out the analysis with the LARS total score, information is lost if compared with performing the analysis by subscales. This is how it can be demonstrated that specific symptoms of apathy affect mental resources differently. According to our results, it would be advisable to study apathy factors rather than apathy in general when analyzing its influence on cognitive performance.
Some limitations should be taken into account. Firstly, our results are based on cross‐sectional data. Some longitudinal studies have associated apathy with a faster cognitive and functional decline13, 30, 31; for example, the appearance of apathy has been related to a rapid progression to AD66, 67 in MCI. Second, medium to low reliability of the action initiation and self‐awareness factors makes it necessary to address these findings with caution. The third limitation is the low educational level of the sample, which is representative of the Spanish population for people over 71 years of age. The fourth limitation is the absence of severe apathy in our sample; it would be useful if future research measured severe levels of apathy compared with a control group. Finally, another limitation of this study is the absence of brain measurements. Some recent studies associate the presence of symptoms of apathy with smaller total brain volume and grey‐matter lesions and load, especially in the frontal lobe.5, 32 Levy and Dubois3 associated the three apathy components proposed by Marin1 with the prefrontal cortex: the emotional‐affective component was associated with the orbital‐medial prefrontal cortex, cognition was associated with the dorsolateral prefrontal cortex, and auto‐activation was associated with basal ganglial lesions. Interestingly, these regions have been related to attentional regulation and effort, inhibitory control of interference, working memory, episodic and recent memory, narrative expression, and verbal fluency.68 These findings are in line with the present study that found a relationship between apathy symptomatology and lower performance in global cognitive status, attention, processing speed, verbal fluency, visual learning and memory, verbal learning and memory, working memory, and executive functioning. Future neuroimaging analysis may help to better understand whether apathy affects all brain areas or is exclusively a frontal lobe pathology, as suggested by some studies.5, 32
In conclusion, this study provides new evidence demonstrating that symptoms of apathy affect mental resources differently, which concurs with Stuss et al,2 Levy and Dubois3 and Pagonabarraga et al.26 Assessment of apathy with the LARS in combination with an extensive neuropsychological battery allowed us to examine the influence of symptoms of apathy on cognitive performance. To our knowledge, this study is the first to determine that intellectual curiosity, emotion, action initiation and self‐awareness factors are associated with a worse performance in attention, processing speed, verbal fluency, visual and verbal memory, working memory, and executive functioning in healthy elderly people. Considering the high prevalence of apathy across different diseases9 it is essential to make a more accurate diagnosis taking into account what symptoms of apathy are present. This will allow us to implement more personalized and effective treatments focused on behavior, emotion, or cognition. Personalized treatments could reduce intervention times and improve associated symptoms such as depressive symptoms, irritability, and caregiver's distress34 and also reduce functional disability improving the quality of life. The authors suggest that when cognitive symptoms are present, it would be advisable to implement neurorehabilitation strategies, as proposed by Van Reekum et al.69
ETHICS STATEMENT
The study was approved by the Ethics Committee of the University of Deusto (Bilbao). All participants were volunteers and gave their informed consent for participation in the study.
FUNDING INFORMATION
Montoya‐Murillo received a collaborative predoctoral grant from the University of Deusto. Ibarretxe‐Bilbao, Peña, and Ojeda received found from the Education Department of the Basque Government (Equipo A) (IT946‐16).
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Supporting information
Table S1 Cognitive Performance in the Elderly with Apathy
Table S2 Multiple Stepwise Regression Analysis of Cognitive Domains and Apathy Subscales
ACKNOWLEDGEMENTS
The authors would like to thank all of the different day centers for their contributions and all the participants for their collaboration.
Montoya‐Murillo G, Ibarretxe‐Bilbao N, Peña J, Ojeda N. The impact of apathy on cognitive performance in the elderly. Int J Geriatr Psychiatry. 2019;34:657‐665. 10.1002/gps.5062
REFERENCES
- 1. Marin RS. Apathy: A neuropsychiatric syndrome. J Neuropsychiatry Clin Neurosci. 1991;3(3):243‐254. [DOI] [PubMed] [Google Scholar]
- 2. Stuss DT, Van Reekum R, Murphy KJ. Differentiation of states and causes of apathy In: Borod JC, ed. Series in affective science. The neuropsychology of emotion. New York, NY, US: Oxford University Press; 2000:340‐363. [Google Scholar]
- 3. Levy R, Dubois B. Apathy and the functional anatomy of the prefrontal cortex‐basal ganglia circuits. Cereb Cortex. 2006;16(7):916‐928. [DOI] [PubMed] [Google Scholar]
- 4. Fernández‐Matarrubia M, Matías‐Guiu JA, Moreno‐Ramos T, et al. Validation of the lille's apathy rating scale in very mild to moderate dementia. Am J Geriatr Psychiatry. 2016;24(7):517‐527. [DOI] [PubMed] [Google Scholar]
- 5. Grool AM, Geerlings MI, Sigurdsson S, et al. Structural MRI correlates of apathy symptoms in older persons without dementia: AGES‐reykjavik study. Neurol. 2014;82(18):1628‐1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Agüera‐Ortiz L, Gil‐Ruiz N, Cruz‐Orduña I, Ramos‐García M, Osorio‐Suárez R, Valentí‐Soler M. Creación de una escala de medición de la apatía en pacientes con demencia tipo alzheimer institucionalizados: La escala APADEM‐NH‐66. Psicogeriatría. 2010;2(4):207‐219. [Google Scholar]
- 7. Robert P, Clairet S, Benoit M, et al. The apathy inventory: assessment of apathy and awareness in alzheimer's disease, parkinson's disease and mild cognitive impairment. Int J Geriatr Psychiatry. 2002;17(12):1099‐1105. [DOI] [PubMed] [Google Scholar]
- 8. Sockeel P, Dujardin K, Devos D, Deneve C, Destee A, Defebvre L. The lille apathy rating scale (LARS), a new instrument for detecting and quantifying apathy: validation in parkinson's disease. J Neurol Neurosurg Psychiatry. 2006;77(5):579‐584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clarke DE, Ko JY, Lyketsos C, Rebok GW, Eaton WW. Apathy and cognitive and functional decline in community‐dwelling older adults: results from the baltimore ECA longitudinal study. Int Psychogeriatr. 2010;22(05):819‐829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Onyike CU, Sheppard JE, Tschanz JT, et al. Epidemiology of apathy in older adults: the cache county study. Am J Geriatr Psychiatry. 2007;15(5):365‐375. [DOI] [PubMed] [Google Scholar]
- 11. Ligthart SA, Richard E, Fransen NL, et al. Association of vascular factors with apathy in community‐dwelling elderly individuals. Arch Gen Psychiatry. 2012;69(6):636‐642. [DOI] [PubMed] [Google Scholar]
- 12. Robert PH, Berr C, Volteau M, et al. Apathy in patients with mild cognitive impairment and the risk of developing dementia of alzheimer's disease: a one‐year follow‐up study. Clin Neurol Neurosurg. 2006;108(8):733‐736. [DOI] [PubMed] [Google Scholar]
- 13. Starkstein SE, Jorge R, Mizrahi R. Robinson RG. A prospective longitudinal study of apathy in alzheimer's disease. J Neurol Neurosurg Psychiatry. 2006;77(1):8‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lechowski L, Benoit M, Chassagne P, et al. Persistent apathy in alzheimer's disease as an independent factor of rapid functional decline: The REAL longitudinal cohort study. Int J Geriatr Psychiatry. 2009;24(4):341‐346. [DOI] [PubMed] [Google Scholar]
- 15. Robert PH, Berr C, Volteau M, et al. Neuropsychological performance in mild cognitive impairment with and without apathy. Dement Geriatr Cogn Disord. 2006;21(3):192‐197. [DOI] [PubMed] [Google Scholar]
- 16. Teng E, Lu PH, Cummings JL. Neuropsychiatric symptoms are associated with progression from mild cognitive impairment to alzheimer's disease. Dement Geriatr Cogn Disord. 2007;24(4):253‐259. [DOI] [PubMed] [Google Scholar]
- 17. Clarke DE, van Reekum R, Simard M, et al. Apathy in dementia: clinical and sociodemographic correlates. J Neuropsychiatry Clin Neurosci. 2008;20(3):337‐347. [DOI] [PubMed] [Google Scholar]
- 18. Kuzis G, Sabe L, Tiberti C, Dorrego F, Starkstein SE. Neuropsychological correlates of apathy and depression in patients with dementia. Neurol. 1999;52(7):1403‐1407. [DOI] [PubMed] [Google Scholar]
- 19. Dujardin K, Sockeel P, Delliaux M, Destée A, Defebvre L. Apathy may herald cognitive decline and dementia in parkinson's disease. Mov Disord. 2009;24(16):2391‐2397. [DOI] [PubMed] [Google Scholar]
- 20. Martínez‐Horta S, Pagonabarraga J, de Bobadilla RF, García‐Sanchez C, Kulisevsky J. Apathy in parkinson's disease: more than just executive dysfunction. J Int Neuropsychol Soc. 2013;19(05):571‐582. [DOI] [PubMed] [Google Scholar]
- 21. Pluck GC, Brown RG. Apathy in parkinson's disease. J Neurol Neurosurg Psychiatry. 2002;73(6):636‐642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brodaty H, Sachdev PS, Withall A, Altendorf A, Valenzuela MJ, Lorentz L. Frequency and clinical, neuropsychological and neuroimaging correlates of apathy following stroke—the sydney stroke study. Psychol Med. 2005;35(12):1707‐1716. [DOI] [PubMed] [Google Scholar]
- 23. Castellon SA, Hinkin CH, Wood S, Yarema KT. Apathy, depression, and cognitive performance in HIV‐1 infection. J Neuropsychiatry Clin Neurosci. 1998;10(3):320‐329. [DOI] [PubMed] [Google Scholar]
- 24. Hartmann MN, Kluge A, Kalis A, Mojzisch A, Tobler PN, Kaiser S. Apathy in schizophrenia as a deficit in the generation of options for action. J Abnorm Psychol. 2015;124(2):309. [DOI] [PubMed] [Google Scholar]
- 25. Feil D, Razani J, Boone K, Lesser I. Apathy and cognitive performance in older adults with depression. Int J Geriatr Psychiatry. 2003;18(6):479‐485. [DOI] [PubMed] [Google Scholar]
- 26. Pagonabarraga J, Kulisevsky J, Strafella AP, Krack P. Apathy in parkinson's disease: clinical features, neural substrates, diagnosis, and treatment. Lancet Neurol. 2015;14(5):518‐531. [DOI] [PubMed] [Google Scholar]
- 27. Adams KB. Depressive symptoms, depletion, or developmental change? Withdrawal, apathy, and lack of vigor in the geriatric depression scale. Gerontologist. 2001;41(6):768‐777. [DOI] [PubMed] [Google Scholar]
- 28. Starkstein SE, Mayberg HS, Preziosi T, Andrezejewski P, Leiguarda R, Robinson R. Reliability, validity, and clinical correlates of apathy in parkinson's disease. J Neuropsychiatry Clin Neurosci. 1992;4(2):134‐139. [DOI] [PubMed] [Google Scholar]
- 29. Aarsland D, Larsen JP, Lim NG, et al. Range of neuropsychiatric disturbances in patients with parkinson's disease. J Neurol Neurosurg Psychiatry. 1999;67(4):492‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brodaty H, Altendorf A, Withall A, Sachdev P. Do people become more apathetic as they grow older? A longitudinal study in healthy individuals. Int Psychogeriatr. 2010;22(03):426‐436. [DOI] [PubMed] [Google Scholar]
- 31. Van der Mast R, Vinkers D, Stek M, et al. Vascular disease and apathy in old age. The leiden 85‐plus study. Int J Geriatr Psychiatry. 2008;23(3):266‐271. [DOI] [PubMed] [Google Scholar]
- 32. Yan H, Onoda K, Yamaguchi S. Gray matter volume changes in the apathetic elderly. Front Hum Neurosci. 2015;9:318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gerritsen D, Jongenelis K, Steverink N, Ooms M, Ribbe M. Down and drowsy? Do apathetic nursing home residents experience low quality of life. Aging Ment Health. 2005;9(2):135‐141. [DOI] [PubMed] [Google Scholar]
- 34. Benoit M, Andrieu S, Lechowski L, Gillette‐Guyonnet S, Robert P, Vellas B. Apathy and depression in alzheimer's disease are associated with functional deficit and psychotropic prescription. Int J Geriatr Psychiatry. 2008;23(4):409‐414. [DOI] [PubMed] [Google Scholar]
- 35. Yao H, Takashima Y, Mori T, et al. Hypertension and white matter lesions are independently associated with apathetic behavior in healthy elderly subjects: the sefuri brain MRI study. Hypertens Res. 2009;32(7):586‐590. [DOI] [PubMed] [Google Scholar]
- 36. Boada M, Cejudo JC, Tarraga L, Lopez OL, Kaufer D. Neuropsychiatric inventory questionnaire (NPI‐Q): Spanish validation of an abridged form of the neuropsychiatric inventory (NPI). Neurologia. 2002;17(6):317‐323. [PubMed] [Google Scholar]
- 37. Lobo A, Saz P, Marcos G. Grupo de trabajo ZARADEMP. Examen cognoscitivo MINI MENTAL.Madrid: TEA Eds . 2002.
- 38. Manubens J, Martínez‐Lage P, Martínez‐Lage J, et al. Variacion de las puntuaciones en el mini‐mental‐state con la edad y el nivel educativo. datos normalizados en la poblacion mayor de 70 años de pamplona. Neurologia. 1998;13:111‐119. [PubMed] [Google Scholar]
- 39. Bermejo Pareja F, Morales González J, Valerga C, Ser TD, Artolazábal J, Gabriel Sánchez R. Comparación entre dos versiones españolas abreviadas de evaluación del estado mental en el diagnóstico de demencia: Datos de un estudio en ancianos residentes en la comunidad. Med Clín. 1999;112(9):330‐334. [PubMed] [Google Scholar]
- 40. Rami L, Valls Pedret C, Bartrés Faz D, et al. Cuestionario de reserva cognitiva. valores obtenidos en población anciana sana y con enfermedad de alzheimer. Rev Neurol. 2011;52(4):195‐201. [PubMed] [Google Scholar]
- 41. Schretlen D, Bobholz JH, Brandt J. Development and psychometric properties of the brief test of attention. Clin Neuropsychol. 1996;10(1):80‐89. [Google Scholar]
- 42. Schretlen D, Vannorsdall T. Calibrated ideational fluency assessment (CIFA) professional manual. Lutz, FL: Psychological Assessment Resources; 2010. [Google Scholar]
- 43. Benedict RH, Schretlen D, Groninger L, Dobraski M, Shpritz B. Revision of the brief visuospatial memory test: studies of normal performance, reliability, and validity. Psychol Assess. 1996;8(2):145‐153. [Google Scholar]
- 44. Brandt J, Benedict RH. Hopkins verbal learning test, revised: professional manual. Lutz, FL: Psychological Assessment Resources; 2001. [Google Scholar]
- 45. Reitan RM, Wolfson D. The Halstead–Reitan neuropsycholgical test battery: therapy and clinical interpretation. Tucson, AZ: Neuropsychological Press; 1985. [Google Scholar]
- 46. Salthouse TA. The processing‐speed theory of adult age differences in cognition. Psychol Rev. 1996;103(3):403‐428. [DOI] [PubMed] [Google Scholar]
- 47. Golden CJ. STROOP: Test de colores y palabras. Madrid, España: TEA ediciones; 2001. [Google Scholar]
- 48. Wechsler D. WAIS‐III: Administration and scoring manual: Wechsler adult intelligence scale. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 49. Garcia‐Ramos R, Villanueva Iza C, Catalan MJ, Reig‐Ferrer A, Matias‐Guiu J. Validation of a spanish version of the lille apathy rating scale for parkinson's disease. The Scientific World Journal. 2014;2014(849834):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Martínez de La Iglesia J, Onís Vilches M, Dueñas Herrero R, Albert Colomer C, Aguado Taberné C, Luque Luque R. Versión española del cuestionario de yesavage abreviado (GDS) para el despistaje de depresión en mayores de 65 años: Adaptación y validación. Medifam. 2002;12(10):26‐40. [Google Scholar]
- 51. Yesavage JA, Sheikh JI. 9/geriatric depression scale (GDS) recent evidence and development of a shorter version. Clin Gerontol. 1986;5(1‐2):165‐173. [Google Scholar]
- 52. Mitchell J, Mathews HF, Yesavage JA. A multidimensional examination of depression among the elderly. Res Aging. 1993;15(2):198‐219. [Google Scholar]
- 53. IBM SPSS Statistics for Window (Version 23.0) [Computer Software].
- 54. Verdejo‐García A, Bechara A. Neuropsicología de las funciones ejecutivas. Psicothema. 2010;22(2):227‐235. [PubMed] [Google Scholar]
- 55. Jonsson M, Edman Å, Lind K, Rolstad S, Sjögren M, Wallin A. Apathy is a prominent neuropsychiatric feature of radiological white‐matter changes in patients with dementia. Int J Geriatr Psychiatry. 2010;25(6):588‐595. [DOI] [PubMed] [Google Scholar]
- 56. Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4(6):215‐222. [DOI] [PubMed] [Google Scholar]
- 57. Radakovic R, Harley C, Abrahams S, Starr JM. A systematic review of the validity and reliability of apathy scales in neurodegenerative conditions. Int Psychogeriatr. 2015;27(06):903‐923. [DOI] [PubMed] [Google Scholar]
- 58. Clarke DE, Ko JY, Kuhl EA, van Reekum R, Salvador R, Marin RS. Are the available apathy measures reliable and valid? A review of the psychometric evidence. J Psychosom Res. 2011;70(1):73‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the apathy evaluation scale. Psychiatry Res. 1991;38(2):143‐162. [DOI] [PubMed] [Google Scholar]
- 60. Cummings JL, Mega M, Gray K, Rosenberg‐Thompson S, Carusi DA, Gornbein J. The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurol. 1994;44(12):2308‐2314. [DOI] [PubMed] [Google Scholar]
- 61. Robert P, Onyike C, Leentjens A, et al. Proposed diagnostic criteria for apathy in alzheimer's disease and other neuropsychiatric disorders. Eur Psychiatry. 2009;24(2):98‐104. [DOI] [PubMed] [Google Scholar]
- 62. Starkstein SE, Petracca G, Chemerinski E, Kremer J. Syndromic validity of apathy in alzheimer's disease. Am J Psychiatry. 2001;158(6):872‐877. [DOI] [PubMed] [Google Scholar]
- 63. Charlton RA, Barrick TR, Markus HS, Morris RG. Theory of mind associations with other cognitive functions and brain imaging in normal aging. Psychol Aging. 2009;24(2):338‐348. [DOI] [PubMed] [Google Scholar]
- 64. Junqué C, Jurado MA. Envejecimiento y demencias. Barcelona: Martínez Roca; 1994. [Google Scholar]
- 65. World Health Organization . Dementia. http://www.who.int/mediacentre/factsheets/fs362/en/. Updated 2017.
- 66. Alberca RA. Manifestaciones psicológicas y conductuales de la enfermedad de alzheimer. 3rd ed. Barcelona: Editorial Glosa, SL; 2011. [Google Scholar]
- 67. Vicini Chilovi B, Conti M, Zanetti M, Mazzu I, Rozzini L, Padovani A. Differential impact of apathy and depression in the development of dementia in mild cognitive impairment patients. Dement Geriatr Cogn Disord. 2009;27(4):390‐398. [DOI] [PubMed] [Google Scholar]
- 68. Siddiqui SV, Chatterjee U, Kumar D, Siddiqui A, Goyal N. Neuropsychology of prefrontal cortex. Indian J Psychiatry. 2008;50(3):202‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. van Reekum R, Stuss DT, Ostrander L. Apathy: why care? J Neuropsychiatry Clin Neurosci. 2005;17(1):7‐19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Cognitive Performance in the Elderly with Apathy
Table S2 Multiple Stepwise Regression Analysis of Cognitive Domains and Apathy Subscales


