Summary
Background
Tegoprazan is a novel potassium‐competitive acid blocker that has a fast onset of action and can control gastric pH for a prolonged period, which could offer clinical benefit in acid‐related disorders.
Aim
To confirm the non‐inferiority of tegoprazan to esomeprazole in patients with erosive oesophagitis (EE).
Methods
In this multicentre, randomised, double‐blind, parallel‐group comparison study, 302 Korean patients with endoscopically confirmed EE (Los Angeles Classification Grades A‐D) were randomly allocated to either tegoprazan (50 or 100 mg) or esomeprazole (40 mg) treatment groups for 4 or 8 weeks. The primary endpoint was the cumulative proportion of patients with healed EE confirmed by endoscopy up to 8 weeks from treatment initiation. Symptoms, safety and tolerability were also assessed.
Results
The cumulative healing rates at week 8 were 98.9% (91/92), 98.9% (90/91) and 98.9% (87/88) for tegoprazan 50 mg, tegoprazan 100 mg and esomeprazole 40 mg, respectively. Both doses of tegoprazan were non‐inferior to esomeprazole 40 mg. The incidence of adverse events was comparable among the groups, and tegoprazan was well‐tolerated.
Conclusion
Once daily administration of tegoprazan 50 or 100 mg showed non‐inferior efficacy in healing EE and tolerability to that of esomeprazole 40 mg.
1. INTRODUCTION
Gastro‐oesophageal reflux disease (GERD) is a prevalent digestive disease that results from reflux of gastric contents into the oesophagus.1, 2 The prevalence of GERD in East Asian countries is increasing, and is reported to be 4.5%‐15.7%3, 4 Population‐based studies have shown that the prevalence of symptom‐based GERD in East Asia was 5.2%‐8.5% from 2005 to 2010. According to the Korean National Health Insurance claim, data also show that the prevalence of GERD in Korea is increasing rapidly from 4.6% to 7.3% between 2005 and 2008.5, 6
The spectrum of GERD includes erosive oesophagitis (EE) and non‐erosive reflux disease (NERD). EE is characterised by the presence of oesophageal mucosal erosions induced by the reflux of gastric contents from the stomach, which can be diagnosed by endoscopy. Currently, proton‐pump inhibitors (PPIs) are the first‐line drug for treating EE and controlling symptoms.7 Studies in patients with EE have shown high healing rates (88%‐96%) after 8‐week treatment with a PPI once daily.8, 9, 10 However, some patients may have endoscopic evidence of oesophagitis and/or reflux symptoms despite PPI therapy.11, 12, 13
Tegoprazan, (S)‐4‐((5,7‐difluorochroman‐4‐yl)oxy)‐N,N,2‐trimethyl‐1H‐benzo[d]imidazole‐6‐carboxamide, was developed in South Korea by CJ Healthcare Corp. It is a novel, potent, and highly selective potassium‐competitive acid blocker (P‐CAB) with a mechanism of action distinct from that of the PPIs.14
Unlike PPIs, that require a chemical transformation into their active form and bind covalently to the gastric H+ /K+‐ATPase, tegoprazan inhibits H+ /K+‐ATPase in a reversible and K+‐competitive manner without a need for any conversion. It is an acid‐resistant weak base which can remain in a highly acidic canaliculi of gastric parietal cells.
Nonclinical studies have shown that this compound suppresses gastric acid secretion faster and more potently than esomeprazole treated group.14 The therapeutic potential of tegoprazan may be derived from its ability to accumulate at high concentrations in the canaliculi of gastric parietal cells. Consequently, it is slowly cleared from the gastric glands and exerts its effects independent of acid levels, leading to a strong and sustained effect.14 Tegoprazan has been demonstrated a strong antisecretory potency and a fast onset of action in several phase I studies.15 Its suppressive effect on acid secretion reaches a maximum plateau within 0.5‐1 hour after administration at a dose of 50, 100 or 200 mg.15 The food effect on pharmacodynamics and pharmacokinetics study indicated that the efficacy of tegoprazan is independent of food intake.16 The pharmacodyniamc data of tegoprazan indicate that the 50 and 100 mg doses increase intragastric pH to ≥4 significantly more than the dexlansoprazole 60 mg with evening dosing.17 In a phase II dose‐ranging study, the proportions of patients with EE who were healed up to week 8 were comparable between tegoprazan (50‐200 mg, once daily) and esomeprazole (40 mg, once daily).18
On the basis of these phase I pharmacodynamics data and phase II dose finding study in EE patients, it was hypothesised that treatment with tegoprazan could produce at least comparable clinical efficacy in healing of oesophagitis and symptom control compared with the treatment of PPIs.
Esomeprazole has been shown to have better acid control than lansoprazole, omeprazole and rabeprazole,19, 20 and higher oesophagitis healing rates than lansoprazole and omeprazole.21 Therefore, esomeprazole 40 mg was considered to be the most appropriate comparator to tegoprazan.
The purpose of this phase III study was to verify the non‐inferiority of safety and efficacy of tegoprazan 50 and 100 mg to those of esomeprazole 40 mg in patients with EE.
2. MATERIALS AND METHODS
2.1. Study design and treatments
Adult patients with endoscopically confirmed EE at screening were eligible for enrolment into the 8‐week, double‐blind, three‐armed, active‐controlled comparison study (ClinicalTrials.gov identifier NCT03006874). The study was designed to verify the non‐inferiority of tegoprazan 50 and 100 mg to esomeprazole 40 mg in patients with EE. The study was conducted at 27 sites in South Korea from October 2016 to August 2017.
The study protocol was approved by the Institutional Review Board at each study site. The study was conducted in accordance with the Declaration of Helsinki and the International Harmonisation on Conference Harmonised Tripartite Guideline for Good Clinical Practice. All participants provided written informed consent before they were enrolled. The participants were required to discontinue PPI use during the screening period and throughout the study period. The screening period was up to 14 days. Endoscopy was performed to evaluate the presence and severity of EE based on the Los Angeles (LA) Classification system during the screening period, and then at the week 4, and week 8 or final visit (if not healed by week 4).
Following the screening period, patients were randomised 1:1:1 to receive either tegoprazan (50 or 100 mg) or esomeprazole (40 mg). The doses were administered once daily for 4 or 8 weeks via the oral route. Treatment was completed after 4 or 8 weeks if healing of mucosal lesions was endoscopically confirmed.
2.2. Study subjects
Male or female patients aged ≥20 years with endoscopically confirmed EE (LA Classification Grades A‐D) were eligible for inclusion in the study. The major exclusion criteria included complications associated with oesophagitis, active gastric or duodenal ulcer, gastrointestinal bleeding, Zollinger‐Ellison syndrome, malignancy, AIDS, hepatitis, eosinophilic oesophagitis, a history of acid‐lowering surgery, previous oesophageal or gastric surgery, malignancy within 5 years prior to enrolment, primary oesophageal motility disorders, irritable bowel syndrome, inflammatory bowel disease or any of the following abnormal laboratory test values at the screening (blood urea nitrogen and serum creatinine level, >1.5 upper limit of normal [ULN]; total bilirubin level and serum levels of alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase and gamma glutamyltransferase, >2ULN). Pregnant and lactating subjects and those who needed treatment with nonsteroidal anti‐inflammatory drugs or surgery requiring hospitalisation were excluded from the study. Any female of child‐bearing potential who was sexually active was required to use adequate contraceptive measures during the study period. Patients were not allowed to concomitantly use any medications that could affect efficacy evaluation. These included proton pump inhibitors (within 2 weeks prior to enrolment), histamine receptor 2 blocking agents, antidepressants, antipsychotics and antianxiety drugs.
2.3. Study protocol
The patients were randomised to receive either tegoprazan or esomeprazole. All randomisation information was securely stored and could be accessed by authorised personnel only. A double‐dummy method, using matching tegoprazan and esomeprazole placebo tablets, was employed to ensure that the study was double‐blinded. All medications were provided in sealed boxes and supplied by the medication supervisor to ensure blinded allocation.
At the start of the screening period, patient demographics and other baseline characteristics were recorded, including medical history, concurrent medical conditions, medication history and concomitant medications. At the start of the screening period, clinical laboratory tests (complete blood count, serum chemistry and urinalysis), physical examination, pregnancy test, electrocardiogram test and Helicobacter pylori test were performed. In addition, vital signs were assessed. At week 4 and 8 (or upon early termination), physical examination, clinical laboratory tests and pregnancy test were performed. Additionally, vital signs (including electrocardiogram [ECG]), adverse events, concomitant medication, patients’ diary and treatment compliance were checked.
Endoscopy was performed by principal investigators with at least 3 years of endoscopy experience at the start of the screening period and at weeks 4 and 8 (or upon early termination). Severities of EE were defined based on the endoscopic findings according to the LA Classification from grade A to D. Healed EE was defined as the absence of oesophageal mucosal erosions or ulcers on oesophagogastroduodenoscopy. Changes in symptoms were assessed using the Reflux Disease Questionnaire (RDQ), which is a 12‐item self‐administered questionnaire designed to assess the frequency and severity of heartburn, acid regurgitation and dyspepsia. Mean RDQ score change was checked from baseline to week 4 or 8. Other patient‐reported outcome measures included GERD Health‐Related Quality of Life (GERD‐HRQL) scores, GERD symptoms and compliance with treatment. The GERD‐HRQL scale has 11 items that focus on heartburn symptoms, dysphagia, medication effects and the patient's health condition. Each item is scored from 0 to 5, with a higher score indicating a worse quality of life. Patients rated the severity of heartburn and regurgitation at daytime and night‐time according to the four‐point scale (0: none; 1: mild; 2: moderate; 3: severe) using daily diaries.
2.4. Outcome evaluation
The primary endpoint was the proportion of EE patients with healed EE confirmed by endoscopy up to week 8. The secondary endpoints included subjective symptoms, such as changes in RDQ scores, the percentage of days without major symptoms as reported in the patient's diary, GERD‐HRQL score, and the proportion of EE patients with healed EE confirmed by endoscopy at week 4.
Safety was assessed by physical examinations and the analysis of adverse events, laboratory test values, ECG findings and vital signs. The frequency and severity of adverse events and concomitant medications were monitored throughout the study. Treatment‐emergent adverse event (TEAE) was defined as an adverse event occurred during treatment, representing a change from baseline. All TEAEs were graded based on severity as severe, moderate or mild by the investigator. A drug‐related TEAE was an adverse event that was deemed by the investigator as possibly related or as related to the study drug. A serious TEAE was defined as an adverse event that could cause death, hospitalisation, disability, congenital anomaly or a life‐threatening adverse event.
2.5. Statistical analysis
Based on the results of a phase II study in which the proportion of EE patients showing healed EE up to week 8 was 96.0% for tegoprazan 50 mg, 97.9% for tegoprazan 100 mg, and 96.1% for esomeprazole 40 mg, the sample size in the present study was calculated 100 subjects per treatment group. A power of ≥90% was used to detect non‐inferiority of tegoprazan to esomeprazole with a non‐inferiority margin of 10%.
For the primary endpoint, the proportion of patients with healed EE up to week 8 was calculated in the per protocol set (PPS) population. The non‐inferiority of tegoprazan to esomeprazole was tested by comparing the lower bound of two‐sided 95% confidence intervals with a non‐inferiority margin of 10%. The same analyses were performed for the proportion of patients with healed EE up to week 4. Wilcoxon's rank sum test was performed to compare the change of symptom scores between groups.
Besides the PPS analysis (n = 271), the intention‐to‐treat (ITT) analysis (n = 300) was also performed for efficacy analysis.
3. RESULTS
3.1. Study subjects
Out of the 452 patients enrolled in the study, 302 eligible patients were randomised to receive either tegoprazan (50 mg, n = 100; 100 mg, n = 102) or esomeprazole (n = 100). A total of 286 subjects completed the study (Figure 1).
Figure 1.
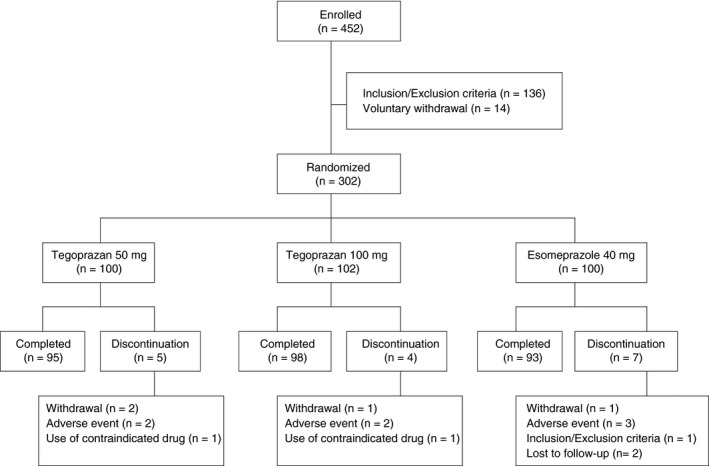
Randomisation protocol and patient disposition
The treatment groups were comparable regarding the baseline characteristics except the history of smoking. EE severity at baseline was similar between treatment groups (Table 1).
Table 1.
Demographic characteristics of the intention‐to‐treat population
| Demographics | Tegoprazan | Esomeprazole | |
|---|---|---|---|
| 50 mg (n = 99) | 100 mg (n = 102) | 40 mg (n = 99) | |
| Age (y), mean (range) | 52.7 (21.0‐74.0) | 52.8 (20.0‐74.0) | 50.4 (21.0‐75.0) |
| Males, n (%) | 62 (62.6) | 66 (64.7) | 53 (53.5) |
| Height (cm), mean (range) | 165.2 (142.5‐187.0) | 166.1 (150.2‐183.0) | 165.0 (145.3‐189.0) |
| Weight (kg), mean (range) | 66.8 (43.0‐110.8) | 66.4 (42.5‐112.5) | 65.5 (40.7‐99.5) |
| Alcohol consumption, n (%) | 42 (42.4) | 45 (44.1) | 39 (39.4) |
| Smoking, n (%) | 27 (27.3) | 17 (16.7) | 13 (13.1) |
| Helicobacter pylori infection status positive, n (%) | 23 (23.2) | 21 (20.6) | 21 (21.2) |
| Baseline LA Classification grade A, n (%) | 66 (66.7) | 67 (65.7) | 66 (66.7) |
| Baseline LA Classification grade B, n (%) | 29 (29.3) | 30 (29.4) | 29 (29.3) |
| Baseline LA Classification grades C/D, n (%) | 4 (4.0) | 5 (4.9) | 4 (4.0) |
LA, Los Angeles.
The overall compliance rate exceeded 95% in all the treatment groups. The mean compliance rates were 98.1%, 97.9% and 97.1% in the tegoprazan 50 mg, tegoprazan 100 mg and esomeprazole 40 mg, respectively.
Sixteen patients (5.3%) did not complete the study: five in the tegoprazan 50 mg group, four in the tegoprazan 100 mg group and seven in the esomeprazole group. The details of each treatment group are summarised in the flow chart in Figure 1.
3.2. Healing rate of EE
In the PPS population, the proportion of patients with healed EE over the 8‐week treatment period was 98.9%, 98.9% and 98.9% in the tegoprazan 50 and 100 mg and esomeprazole 40 mg groups, respectively. The lower bound of the two‐sided 95% confidence interval of the treatment difference (tegoprazan‐esomeprazole) met the prespecified non‐inferiority criteria (Table 2). Both doses of tegoprazan were non‐inferior to esomeprazole 40 mg (P < 0.0001). In the ITT analysis, the healing rates up to week 8 were comparable between the tegoprazan (50 and 100 mg) and esomeprazole (40 mg) groups. There were no statistically significant differences between the treatment groups (Table 2).
Table 2.
Healing rates (%) of erosive oesophagitis up to week 8
| Treatment | % Patients healed | Difference from esomeprazole | [95% CIs] | P‐valuea non‐inferiority |
|---|---|---|---|---|
| Week 8 PPS | ||||
| Tegoprazan 50 mg | 98.9 | 0.1 | [−3.0, 3.1] | <.0001 |
| Tegoprazan 100 mg | 98.9 | 0.0 | [−3.0, 3.1] | <.0001 |
| Esomeprazole 40 mg | 98.9 | |||
| ITT | ||||
| Tegoprazan 50 mg | 96.0 | 3.0 | [−3.3, 9.4] | <.0001 |
| Tegoprazan 100 mg | 95.1 | 2.2 | [−4.4, 8.7] | 0.0001 |
| Esomeprazole 40 mg | 92.9 | |||
| Week 4 PPS | ||||
| Tegoprazan 50 mg | 91.3 | −3.0 | [−10.5, 4.5] | 0.0343 |
| Tegoprazan 100 mg | 93.4 | −0.9 | [−7.9, 6.1] | 0.0056 |
| Esomeprazole 40 mg | 94.3 | |||
| ITT | ||||
| Tegoprazan 50 mg | 87.9 | 0.0 | [−9.1, 9.1] | 0.0156 |
| Tegoprazan 100 mg | 90.2 | 2.3 | [−6.3, 11.0] | 0.0026 |
| Esomeprazole 40 mg | 87.9 | |||
CIs, confidence intervals; ITT, intention‐to‐treat; PPS, per protocol set.
Non‐inferiority test at the significant level 0.05 (two‐sided).
In the PPS population, the proportion of patients with healed EE over the 4‐week treatment period was 91.3% and 93.4% in the tegoprazan 50 and 100 mg groups, respectively, which were similar to that of the esomeprazole group (94.3%). The healing rate at week 4 in the ITT analysis was 87.9% in the tegoprazan 50 mg group (n = 99), 90.2% in the tegoprazan 100 mg group (n = 102) and 87.9% in the esomeprazole 40 mg group (n = 99). In the ITT analysis, tegoprazan 50 and 100 mg were non‐inferior to esomeprazole 40 mg (P = 0.0156 and 0.0026, respectively).
3.3. Symptom response
Patients in all the three treatment groups reported significant improvement in the severity and frequency of heartburn, dyspepsia and regurgitation, which were assessed at weeks 4 and 8 using RDQ scores (P < 0.0001). Mean RDQ severity and frequency scores significantly decreased after the 4‐ and 8‐week treatments with the investigational drugs. There were no significant differences between the treatment groups regarding mean changes in RDQ total scores at either week 4 or 8. Mean reductions in the severity/frequency of heartburn and dyspepsia did not show statistically significant differences between the treatment groups at week 8.
Reductions in the regurgitation severity and frequency were statistically higher in the tegoprazan 50 mg group at week 4 and 8 than in the esomeprazole 40 mg group. However, significant difference (P‐value) of regurgitation at baseline was observed between the treatment groups, and the least‐squares mean change in the regurgitation score from baseline to week 4 or 8 was analysed additionally. There was no statistically significant difference between the three treatment groups after adjustment of baseline (Table 3).
Table 3.
Mean RDQ symptom scores at baseline, week 4 and 8 (per protocol set)
| Mean RDQ | Tegoprazan | Esomeprazole | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 50 mg (n = 92) | 100 mg (n = 91) | 40 mg (n = 88) | |||||||
| Severity | Baseline | Week 4 | Week 8 | Baseline | Week 4 | Week 8 | Baseline | Week 4 | Week 8 |
| Major symptom | 2.00 | 0.58 | 0.58 | 1.87 | 0.58 | 0.58 | 1.72 | 0.50 | 0.48 |
| Heartburn | 1.76 | 0.53 | 0.56 | 1.86 | 0.62 | 0.62 | 1.84 | 0.48 | 0.47 |
| Dyspepsia | 1.43 | 0.41 | 0.40 | 1.47 | 0.38 | 0.37 | 1.47 | 0.45 | 0.45 |
| Regurgitation | 2.24 | 0.62 | 0.60 | 1.88 | 0.54 | 0.54 | 1.61 | 0.52 | 0.50 |
| Frequency | |||||||||
| Major symptom | 2.02 | 0.88 | 0.87 | 2.01 | 0.85 | 0.84 | 1.82 | 0.78 | 0.75 |
| Heartburn | 1.75 | 0.79 | 0.79 | 1.97 | 0.92 | 0.90 | 1.89 | 0.76 | 0.74 |
| Dyspepsia | 1.54 | 0.52 | 0.51 | 1.49 | 0.52 | 0.49 | 1.57 | 0.61 | 0.62 |
| Regurgitation | 2.29 | 0.97 | 0.95 | 2.05 | 0.77 | 0.77 | 1.75 | 0.79 | 0.76 |
RDQ, Reflux Disease Questionnaire.
There was a significant increase in the percentage of days without major symptoms (heartburn and regurgitation) in all the treatment groups, but there were no statistically significant differences between the treatment groups (data not shown). GERD‐HRQL total scores over the 4‐ and 8‐week treatment periods reduced significantly in all the treatment groups. The mean reduction in GERD‐HRQL scores of the tegoprazan‐treated groups was not significantly different from that in the esomeprazole‐treated group (Table 4).
Table 4.
GERD‐HRQL score changes from baseline at week 4 and week 8 (per protocol set)
| GERD‐HRQL | Tegoprazan | Esomeprazole | ||||
|---|---|---|---|---|---|---|
| 50 mg (n = 92) | 100 mg (n = 91) | 40 mg (n = 88) | ||||
| Week 4 | Week 8 | Week 4 | Week 8 | Week 4 | Week 8 | |
| Change from baseline | −7.9 | −8.1 | −7.3 | −7.3 | −6.9 | −7.1 |
| P‐valuea | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Difference from esomeprazole | −1.0 | −1.0 | −0.4 | −0.2 | — | — |
| P‐valueb | 0.1858 | 0.1920 | 0.7560 | 0.8216 | — | — |
GERD‐HRQL, Gastro‐oesophageal Reflux Disease Health‐Related Quality of Life.
Wilcoxon's signed rank test.
Wilcoxon's rank sum test.
3.4. Tolerability and safety
Three hundred patients received at least one dose of a study medication and were included in the safety analyses. Most TEAEs (136/149, 91.3%) were mild in intensity. Two severe TEAEs that were joint injury and breast cancer in the tegoprazan 50 mg group that were “definitely not related” to the investigational drug. There were no severe TEAEs in the tegoprazan and esomeprazole groups. The percentages of patients with more than one TEAE were 28.3%, 23.5% and 30.3% in the tegoprazan 50 mg, tegoprazan 100 mg and esomeprazole 40 mg groups, respectively (Table 5). Drug‐related TEAEs (≥2%) are shown in Table 6. Dyspepsia (2.0%) and chest discomfort (2.0%) in the tegoprazan groups, and headache (4.0%) in the esomeprazole group were the most frequently reported drug‐related TEAEs (Table 6). All the TEAEs disappeared spontaneously without any treatment. The percentage of patients with TEAEs leading to premature discontinuation of treatment was 2.9% in the tegoprazan 50 mg group, 2.9% in the esomeprazole 40 mg group and 3.0% in the tegoprazan 100 mg group. Headache (2% in the esomeprazole 40 mg group) was the most common adverse event that led to discontinuation. The rate of serious TEAEs was 2.0% in the tegoprazan 50 mg group, 2.0% in the tegoprazan 100 mg group and 1.0% in the esomeprazole 40 mg group. All serious TEAEs were considered to be unrelated to the study medication by the investigators. No significant changes in vital signs or ECG findings were observed during the study period.
Table 5.
Summary of treatment‐emergent adverse events (TEAEs)
| Tegoprazan | Esomeprazole | ||||||
|---|---|---|---|---|---|---|---|
| 50 mg (n = 99) | 100 mg (n = 102) | 40 mg (n = 99) | |||||
| n (%) | [F] | n (%) | [F] | n (%) | [F] | ||
| Any TEAE | 28 (28.3) | [43] | 24 (23.5) | [55] | 30 (30.3) | [51] | |
| Drug‐related TEAE | 10 (10.1) | [17] | 5 (4.9) | [14] | 11 (11.1) | [14] | |
| Serious TEAE | 2 (2.0) | [2] | 2 (2.0) | [2] | 1 (1.0) | [1] | |
| Death | 0 (0.0) | [0] | 0 (0.0) | [0] | 0 (0.0) | [0] | |
| Most frequently reported TEAEs by system organ class and preferred term a | P‐value | ||||||
| Gastrointestinal disorders | 10 (10.1) | 9 (8.8) | 10 (10.1) | 0.9390b | |||
| –Diarrhoea | 3 (3.0) | 5 (4.9) | 1 (1.0) | 0.3093b | |||
| Nervous system disorders | 0 (0.0) | 2 (2.0) | 7 (7.1) | 0.0087c | |||
| –Headache | 0 (0.0) | 2 (2.0) | 6 (6.1) | 0.0220c | |||
| Infections and infestations | 6 (6.0) | 8 (7.8) | 9 (9.1) | 0.7229b | |||
| –Viral upper respiratory tract infection | 4 (4.0) | 6 (5.9) | 6 (6.1) | 0.7818b | |||
[F] = Frequency of TEAEs
N‐dash represents preferred term.
≥3%
Chi‐square test
Fisher's exact test.
Table 6.
Drug‐related treatment‐emergent adverse events (TEAEs) reported by at least 2% of patients in any treatment group
| System organ class/Preferred term | Tegoprazan | Esomeprazole | P‐valuea | |
|---|---|---|---|---|
| 50 mg (n = 99) n (%) | 100 mg (n = 102) n (%) | 40 mg (n = 99) n (%) | ||
| Dyspepsia | 2 (2.0) | 1 (1.0) | 0 (0.0) | 0.5467 |
| Chest discomfort | 2 (2.0) | 0 (0.0) | 1 (1.0) | 0.3245 |
| Headache | 0 (0.0) | 1 (1.0) | 4 (4.0) | 0.0196 |
Fisher's exact test.
4. DISCUSSION
The findings of the current randomised controlled study in 302 patients with EE demonstrated that tegoprazan, when administered at 50 or 100 mg once daily, was non‐inferior to esomeprazole 40 mg regarding the healing rate of EE. Both doses of tegoprazan were highly effective in healing EE. Tegoprazan 100 mg did not provide additional clinical benefit over tegoprazan 50 mg. However, we did not observe any superior results of tegoprazan over esomeprazole since the healing rates in all treatment groups were too high to detect any significant differences.
The healing rates after 4 weeks of treatment were also comparable between the treatment groups, and higher than 90% in all treatment groups. ITT analyses of the healing rate after the 4‐week treatment demonstrated the non‐inferiority of tegoprazan 50 mg to esomeprazole 40 mg.
All these results are consistent with those of a previous phase II dose‐ranging study in EE patients.18 Subgroup analysis according to the baseline LA grade of oesophagitis revealed no significant differences in healing rates between the two doses of tegoprazan and esomeprazole 40 mg at week 4 and 8 (data not shown).
Treatment with tegoprazan also showed high healing rates (100%) in EE patients with LA grade C/D, although the number of those patients was small (n = 3‐4/group). In South Korea, most of patients with EE have LA grade A or B. Patients with LA grade C or D are relatively rare.22, 23
Currently, two P‐CABs including tegoprazan have been approved for the treatment of GERD. Revaprazan, the first P‐CAB was approved for treating peptic ulcers.24, 25 The second P‐CAB clinically available was vonoprazan, which was recently approved for the treatment of EE in Japan.26, 27, 28 Tegoprazan was approved in South Korea in July, 2018 for the treatment of EE and NERD, and it became the first P‐CAB clinically available for treating NERD.29, 30
The present study showed that RDQ scores assessing the severity and frequency of regurgitation significantly decreased more in the tegoprazan 50 mg group than in the esomeprazole group (P < 0.05, Wilcoxon's rank sum test). However, there was significant difference in the baseline score at the baseline between treatment groups. We analysed the regurgitation score change from baseline to 4 or 8 week using Least square mean (data now shown), and found that there was no significant difference of the regurgitation score change among the three treatment groups.
Both tegoprazan and esomeprazole were well tolerated in the current study. Two patients in the tegoprazan 50 mg group, two in the tegoprazan 100 mg group and three in the esomeprazole group discontinued treatment because of TEAEs. Dyspepsia and chest discomfort were drug‐related TEAEs with an incidence of ≥2% in the tegoprazan 50 mg group (Table 6). Headache was the most frequently reported drug‐related TEAE in the esomeprazole 40 mg group, that was the only drug‐related TEAE showing a significant difference compared to the other group (P = 0.0196). Diarrhoea was the most frequent TEAE in the tegoprazan 50 and 100 mg groups. However, there was no significant difference in the occurrence rate of diarrhoea between the three treatment groups (P = 0.3093).
Furthermore, there were no newly identified safety signals or any significant changes in vital signs, ECG findings or other safety signals. Overall, all the treatments were well tolerated. Moreover, the incidences of TEAEs, drug‐related TEAEs, serious TEAEs and TEAEs leading to drug discontinuation were similar between the tegoprazan‐ and esomeprazole‐treated groups. The majority (91.3%) of TEAEs were considered mild in severity and not related to the study drug.
In conclusion, once daily administration of tegoprazan 50 or 100 mg shows non‐inferior efficacy in healing EE and tolerability to that of esomeprazole 40 mg.
ACKNOWLEDGEMENTS
Declaration of personal interests: The authors would like to thank all the investigators who contributed to this study. Statistical analysis was supported by Jae Hoon Kwon. Hyo Ju Park, Min Ja Kang, Chi Hye Park, Bong Tae Kim, Sangjun Youn and Geun Seog Song are employees of CJ Healthcare Corp., Seoul, Korea.
AUTHORSHIP
Guarantor of the article: Poong‐Lyul Rhee.
Author contributions: All authors were involved in the acquisition of data and interpretation of study results. Kwang Jae Lee and Poong‐Lyul Rhee were involved in the study design, drafting of the manuscript and critical revision of the manuscript. All authors approved the final version of the manuscript, including the authorship list.
APPENDIX 1. AUTHORS’ COMPLETE AFFILIATIONS
1.1.
Kwang Jae Lee, Department of Gastroenterology, Ajou University Hospital, Suwon, Korea; Byoung Kwan Son, Department of Internal Medicine, Eulji University Eulji Hospital, Seoul, Korea; Gwang Ha Kim, Department of Internal Medicine, Pusan National University School of Medicine, and Biomdical Research Institute, Pusan National University Hospital, Busan, Korea; Hye‐Kyung Jung, Department of Internal Medicine, College of Medicine, Ewha Womans University, Seoul, Korea; Hwoon‐Yong Jung, Department of Internal Medicine, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea; Il‐Kwun Chung, Department of Internal Medicine, Soonchunhyang University Cheonan Hospital, Cheonan, Korea; In‐Kyung Sung, Department of Internal Medicine, Konkuk University Medical Center, Seoul, Korea; Jin Il Kim, Department of Internal Medicine, Yeouido St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea; Jong Hyeok Kim, Department of Internal Medicine, Hallym University Sacred Heart Hospital, Anyang, Korea; Joon Seong Lee, Department of Internal Medicine, Soonchunhyang University Seoul Hospital, Seoul, Korea; Joong Goo Kwon, Department of Internal Medicine, Daegu Catholic University Medical Center, Daegu, Korea, Jung Ho Park, Department of Internal Medicine, Kangbuk Samsung Hospital, Seoul, Korea; Kyu Chan Huh, Department of Internal Medicine, Konyang University Hospital, Daejeon, Korea; Kyung Sik Park, Department of Internal Medicine, Keimyung University School of Medicine, Daegu, Korea; Moo‐In Park, Department of Internal Medicine, Kosin University Gospel Hospital, Busan, Korea; Nayoung Kim, Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea; Oh Young Lee, Department of Internal Medicine, Hanyang University Hospital, Seoul, Korea, Sam Ryong Jee, Department of Internal Medicine, Inje University Busan Paik Hospital, Busan, Korea; Sang Kil Lee, Department of Internal Medicine, Yonsei University College of Medicine, Seoul Korea; Sei Jin Youn, Department of Internal Medicine, Chungbuk National University Hospital, Cheongju, Korea; Sung Kook Kim, Department of Internal Medicine, Kyungpook National University Hospital, Daegu, Korea; Soo Teik Lee, Department of Internal Medicine, Chonbuk National University Hospital, Jeonju, Korea; Su Jin Hong, Digestive Disease Center and Research Institute, Department of Internal Medicine, Soonchunhyang University College of Medicine, Bucheon, Korea; Suck Chei Choi, Department of Internal Medicine, Wonkwang University Hospital, Iksan, Korea; Tae Nyeun Kim, Department of Internal Medicine, Yeungnam University College of Medicine, Daegu, Korea; Young Hoon Youn, Department of Internal Medicine, Gangnam Severance Hospital, Seoul, Korea; Hyo Ju Park, Min Ja Kang, Chi Hye Park, Bong Tae Kim, Sangjun Youn, Geun Seog Song, Clinical Development Division, CJ Healthcare Corp., Seoul, Korea; Poong‐Lyul Rhee, Department of Medicine, Sungkyunkwan University, Samsung Medical Center, Seoul, Korea.
Lee KJ, Son BK, Kim GH, et al. Randomised phase 3 trial: tegoprazan, a novel potassium‐competitive acid blocker, vs. esomeprazole in patients with erosive oesophagitis. Aliment Pharmacol Ther. 2019;49:864‐872. 10.1111/apt.15185
Funding Information
This study was funded in full by CJ Healthcare Corp, Seoul, Korea.
The complete list of affiliations is listed in Appendix 1.
The Handling Editor for this article was Professor Jonathan Rhodes, and it was accepted for publication after full peer‐review.
REFERENCES
- 1. Vakil N, van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence‐based consensus. Am J Gastroenterol. 2006;101:1900‐1920. [DOI] [PubMed] [Google Scholar]
- 2. Goh KL. Gastroesophageal reflux disease in Asia: a historical perspective and present challenges. J Gastroenterol Hepatol. 2011;26(Suppl. 1):2‐10. [DOI] [PubMed] [Google Scholar]
- 3. El‐Serag HB, Sweet S, Winchester CC, et al. Update on the epidemiology of gastro‐oesophageal reflux disease: a systematic review. Gut. 2014;63:871‐880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hung LJ, Hsu PI, Yang CY, et al. Prevalence of gastroesophageal reflux disease in a general population in Taiwan. J Gastroenterol Hepatol. 2011;26:1164‐1168. [DOI] [PubMed] [Google Scholar]
- 5. Kim KM, Cho YK, Bae SJ, et al. Prevalence of gastroesophageal reflux disease in Korea and associated health‐care utilization: a national population‐based study. J Gastroenterol Hepatol. 2012;27:741‐745. [DOI] [PubMed] [Google Scholar]
- 6. Jung HK. Epidemiology of gastroesophageal reflux disease in Asia: a systematic review. J Neurogastroenterol Motil. 2011;17:14‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108:308‐328. [DOI] [PubMed] [Google Scholar]
- 8. Bardhan KD, Hawkey CJ, Long RG, et al. Lansoprazole versus ranitidine for the treatment of reflux oesophagitis. UK Lansoprazole Clinical Research Group. Aliment Pharmacol Ther. 1995;9:145‐151. [DOI] [PubMed] [Google Scholar]
- 9. Castell DO, Richter JE, Robinson M, Sontag SJ, Haber MM. Efficacy and safety of lansoprazole in the treatment of erosive reflux esophagitis. The Lansoprazole Group. Am J Gastroenterol. 1996;91:1749‐1757. [PubMed] [Google Scholar]
- 10. Sharma VK, Leontiadis GI, Howden CW. Meta‐analysis of randomized controlled trials comparing standard clinical doses of omeprazole and lansoprazole in erosive oesophagitis. Aliment Pharmacol Ther. 2001;15:227‐231. [DOI] [PubMed] [Google Scholar]
- 11. Chiba N, De Gara CJ, Wilkinson JM, Hunt RH. Speed of healing and symptom relief in grade II to IV gastroesophageal reflux disease: a meta‐analysis. Gastroenterology. 1997;112:1798‐1810. [DOI] [PubMed] [Google Scholar]
- 12. Fass R, Shapiro M, Dekel R, Sewell J. Systematic review: proton‐pump inhibitor failure in gastro‐oesophageal reflux disease – where next? Aliment Pharmacol Ther. 2005;22:79‐94. [DOI] [PubMed] [Google Scholar]
- 13. Mermelstein J, Mermelstein AC, Chait MM. Proton pump inhibitor‐refractory gastroesophageal reflux disease: challenges and solutions. Clin Exp Gastroenterol. 2018;11:119‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Takahashi N, Take Y. Tegoprazan, a novel potassium‐competitive acid blocker to control gastric acid secretion and motility. J Pharmacol Exp Ther. 2018;364:275‐286. [DOI] [PubMed] [Google Scholar]
- 15. Han S, Choi HY, Kim YH, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of escalating single and multiple oral doses of tegoprazan, a novel potassium‐competitive acid blocker (P‐CAB) in healthy male subjects. Abstract presentation at Korea Digestive Week 2017.
- 16. Han S, Choi HY, Kim YH, et al. Pharmacokinetics, pharmacodynamics and food‐effect of single oral dose of tegoprazan, a novel potassium‐competitive acid blocker (P‐CAB) in healthy male subjects. Abstract presentation at Korea Digestive Week 2017.
- 17. Han S, Choi HY, Kim YH, et al. Pharmacodynamics of tegoprazan, a novel potassium‐competitive acid blocker (P‐CAB) compared to dexlansoprazole with evening dosing in healthy male subjects. Abstract presentation at Korea Digestive Week 2017.
- 18. Jung HC, Lee DH, Chun HJ, et al. A Randomized, dose‐ranging, phase II study of tegoprazan, a novel potassium‐competitive acid blocker, for the treatment of erosive reflux disease (ERD). Abstract presentation at Korea Digestive Week 2017.
- 19. Miner P Jr, Katz PO, Chen Y, Sostek M. Gastric acid control with esomeprazole, lansoprazole, omeprazole, pantoprazole, and rabeprazole: a five‐way crossover study. Am J Gastroenterol. 2003;98:2616‐2620. [DOI] [PubMed] [Google Scholar]
- 20. Wilder‐Smith C, Lind T, Lundin C, et al. Acid control with esomeprazole and lansoprazole: a comparative dose‐ response study. Scand J Gastroenterol. 2007;42:157‐164. [DOI] [PubMed] [Google Scholar]
- 21. Zheng RN. Comparative study of omeprazole, lansoprazole, pantoprazole and esomeprazole for symptom relief in patients with reflux esophagitis. World J Gastroenterol. 2009;15:990‐995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim BJ, Cheon WS, Oh H, Kim JW, Park JD, Kim JG. Prevalence and risk factor of erosive esophagitis observed in Korean National Cancer Screening Program. J Korean Med Sci. 2011;26:642‐646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Song JH, Chung SJ, Lee JH, et al. Relationship Between Gastroesophageal Reflux Symptoms and Dietary Factors in Korea. J Neurogastroenterol Motil. 2011;17:54‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yu KS, Bae KS, Shon JH, Cho JY, Yi SY, Chung JY, et al. Pharmacokinetic and pharmacodynamic evaluation of a novel proton pump inhibitor, YH1885, in healthy volunteers. J Clin Pharmacol. 2004;44:73‐82. [DOI] [PubMed] [Google Scholar]
- 25. Chang R, Chung IS, Park SH, Kim SK, Choi SR, Song GA, et al. Phase III clinical trial of revaprazan (revanex R) for gastric ulcer. Korean J Gastrointest Endosc. 2007;34:312‐319. [Google Scholar]
- 26. Ashida K, Sakurai Y, Hori T, et al. Randomised clinical trial: vonoprazan, a novel potassium competitive acid blocker, vs. lansoprazole for the healing of erosive oesophagitis. Aliment Pharmacol Ther. 2016;43:240‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jenkins H, Sakurai Y, Nishimura A, Okamoto H, Hibberd M, Jenkins R, et al. Randomised clinical trial: safety, tolerability, pharmacokinetics and pharmacodynamics of repeated doses of TAK‐438 (vonoprazan), a novel potassium‐competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther. 2015;41:636‐648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Inatomi N, Matsukawa J, Sakurai Y, Otake K. Potassium‐competitive acid blockers: advanced therapeutic option for acid‐related diseases. Pharmacol Ther. 2016;168:12‐22. [DOI] [PubMed] [Google Scholar]
- 29. Kinoshita Y, Sakurai Y, Shiino M, et al. Evaluation of the efficacy and safety of vonoprazan in patients with nonerosive gastroesophageal reflux disease: a phase III, randomized, double‐blind, placebo‐controlled, multicenter study. Curr Ther Res Clin Exp. 2016;81‐82:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tajimi M, Nii T, Takahashi N, Yamamoto T, Koizumi S. Firstin‐human study of the novel acid pump antagonist, RQ‐00000004, demonstrated a rapid elevation of gastric pH following single oral administration in healthy subjects. Gastroenterology. 2011;140:S‐80. [Google Scholar]


