Abstract
Objective
This study examined the prospective, potentially bidirectional association of aggressive behavior with BMI and body composition across childhood in three population‐based cohorts.
Methods
Repeated measures of aggression and BMI were available from the Generation R Study between ages 6 and 10 years (N = 3,974), the Netherlands Twin Register (NTR) between ages 7 and 10 years (N = 10,328), and the Swedish Twin Study of Child and Adolescent Development (TCHAD) between ages 9 and 14 years (N = 1,462). In all samples, aggression was assessed with the Child Behavior Checklist. Fat mass and fat‐free mass were available in the Generation R Study. Associations were examined with cross‐lagged modeling.
Results
Aggressive behavior at baseline was associated with higher BMI at follow‐up in the Generation R Study (β = 0.02, 95% CI: 0.00 to 0.04), in NTR (β = 0.04, 95% CI: 0.02 to 0.06), and in TCHAD (β = 0.03, 95% CI: −0.02 to 0.07). Aggressive behavior was prospectively associated with higher fat mass (β = 0.03, 95% CI: 0.01 to 0.05) but not fat‐free mass. There was no evidence that BMI or body composition preceded aggressive behavior.
Conclusions
More aggressive behavior was prospectively associated with higher BMI and fat mass. This suggests that aggression contributes to the obesity problem, and future research should study whether these behavioral pathways to childhood obesity are modifiable.
Introduction
Childhood obesity is a worldwide public health problem, with an increasing prevalence expected to reach 9% of children in 2020 1. Although accumulating evidence suggests that children with overweight show more externalizing behavioral problems (e.g., aggressive, oppositional behaviors) than children with normal weight 2, 3, the directionality of the association between high BMI and aggressive behavioral problems in childhood remains unclear.
Four longitudinal population‐based studies have previously examined the potential bidirectional association between externalizing behavior problems—consisting of aggressive behavior and/or attention‐deficit/hyperactivity disorder (ADHD) problems 4—and high BMI in childhood 5, 6, 7, 8. These studies yielded mixed results, raising questions about the directionality of effects. Two prospective studies reported that early externalizing behavior problems predicted higher BMI in early childhood 8 and early adolescence 6, whereas no association between childhood BMI and subsequent increases in externalizing behavior was found. Conversely, two other prospective population‐based studies observed no longitudinal associations between externalizing behavior and BMI in children aged 2 years at baseline and 12 years at follow‐up 5 or in toddlers aged 18 months at baseline and 36 months at follow‐up 7. These conflicting findings call for further investigations into the potential bidirectionality of this association. Although it is well known that ADHD poses a risk for developing obesity 9, there is a particular need to elucidate the association between aggressive behavior and BMI in childhood. Aggressive behaviors were shown to be the most common reasons for referral to child and adolescent mental health services, and they are substantially predictive of poorer long‐term functioning and high societal costs 10. And although ADHD is often comorbid with aggression, no study has yet examined the independent prospective associations with BMI, which could provide further insight into the specific behavioral mechanisms of the development of obesity in childhood. Furthermore, previous studies have focused on BMI only, although adequately distinguishing fat mass from fat‐free mass may benefit determining specificity of associations 11.
The aim of the current study was to examine the prospective, potentially bidirectional, associations between aggressive behavior and BMI across childhood. Insights obtained from this study will contribute to a better understanding of the behavioral—and potentially modifiable—pathways to obesity risk in childhood. Here, we assessed the direction of association between aggressive behavior and BMI in three large population‐based cohorts from early childhood (ages 6‐7 to 10 years) to early adolescence (ages 9 to 14 years). Furthermore, in one cohort, we also assessed the directionality of associations of aggressive behavior with fat mass and lean mass in order to examine more in‐depth weight‐related obesity indicators. Sensitivity analyses were conducted with additional adjustment for co‐occurring attention, social, and internalizing problems at baseline. We hypothesized that aggressive behavior would be associated with higher BMI and fat mass at later ages rather than vice versa, as behavioral inhibition deficits associated with aggression might increase the risk for unhealthy lifestyles and, therefore, higher BMI 12.
Methods
Study design and population
The three population‐based cohort samples included in the current study also collaborate under the European Union Seventh Framework Program–Aggression in Children: Unravelling Gene‐Environment Interplay to Inform Treatment and Intervention Strategies (ACTION) consortium 13. First, primary analyses were conducted in the Generation R Study, a prospective cohort from fetal life onward in Rotterdam, the Netherlands. The study is designed to investigate early environmental and genetic pathways leading to normal and abnormal growth, development, and health 14. The study was approved by the medical ethical committee of the Erasmus Medical Center, Rotterdam. For the current study, children with data on BMI, body composition, and aggressive behavior at ages both 6 and 10 years were included, resulting in a study sample of 3,974 children.
Independent replication of the relationship between BMI and aggressive behavior was performed in the Netherlands Twin Register (NTR; N = 10,328, assessed at ages 7 and 10 years) 15, 16 and the Swedish Twin Study of Child and Adolescent Development (TCHAD; N = 1,462, assessed at ages 9 and 14 years) 17. Both twin cohorts are designed to investigate the genetic and environmental effects on children's cognitive functioning, health, and emotional and behavioral problems during development. For the current analyses, both twins from each twin pair were included in the analyses, and these analyses were adjusted for family relatedness. Written informed consent and assent were obtained for all participants from all cohorts. Previous research in both twin cohorts has shown higher twin correlations for aggressive behavior in monozygotic (range, r = 0.48 to 0.84) versus dizygotic (range, r = 0.35 to 0.78) twins 18, 19, indicating moderate to high twin heritability. Similarly, the twin heritability for BMI was estimated to be moderately high in childhood 20.
Measurements
Aggressive behavior
The aggressive behavior subscale of the Child Behavior Checklist (CBCL) was employed in all three studies. The CBCL was completed by the mothers and rated on a 3‐point Likert scale (0 = not true, 1 = somewhat true, sometimes true, 2 = very true, often true). The CBCL is a reliable, valid measurement of emotional and behavior problems 21, including affective, anxiety, attention, and aggression problems, and is generalizable across societies worldwide 22. Moreover, the CBCL has been shown to be a valid screening instrument for Diagnostic and Statistical Manual of Mental Disorders (4th Edition) externalizing disorders 23. In the Generation R Study, the CBCL/1.5‐5 was used to measure aggressive behavior at a mean age of 6 years 24. This version of the CBCL was chosen because it was expected that most children would be younger than 6 years at assessment. Indeed, 57.4% of the children were 5 years old at the assessment wave, the remainder were 6 years (37.7%) or 7 years or older (4.9%), and we used the CBCL/1.5‐5 version for all children during this assessment wave to enhance comparability across all children, as recommended in the Achenbach System of Empirically Based Assessment manual 24. The items of the aggressive behavior problems scale largely overlap with the CBCL/6‐18 (e.g., “physically attacks” and “stubborn, sullen or irritable”). Earlier work from the Generation R Study demonstrated that the internal consistency (Cronbach α) was similar for all syndrome scales for children aged 5 years versus children older than 5 years 25, indicating that the CBCL/1.5‐5 assesses aggressive behavior problems similarly in 5‐year‐old children and 6‐ to 7‐year‐old children. At the age of 10 years, aggressive behavior was measured with the CBCL/6‐18, which was also used in the NTR cohort at both waves. In the TCHAD sample, the CBCL/4‐18, which is an earlier version of the CBCL but with identical items that assess aggressive behavior problems, was used to assess aggressive behavior.
Child BMI and body composition
At the ages of 6 and 10 years, child weight and height were measured at the Generation R research center. The obtained BMIs (weight in kilograms divided by height in meters squared) were standardized into BMI standard deviation (SD) scores by correcting for sex and age, using the Dutch national reference in the Growth Analyser program (http://www.growthanalyser.org). In the NTR and TCHAD cohorts, height (meters) and weight (kilograms) data were based on mother reports, which were used to calculate BMI.
In the Generation R Study, body composition at both ages was measured with a dual‐energy x‐ray absorptiometry scanner (iDXA, 2008; GE Lunar, Madison, Wisconsin). Fat mass index (FMI) and fat‐free mass index (FFMI) were converted into sex‐ and age‐adjusted standardized scores.
Covariates
Based on prior studies 2, 3, the following sociodemographic covariates were included in the analyses. With respect to Generation R, sex and birth weight were determined using data from medical records. Maternal age, maternal education, and ethnicity of the child were assessed using questionnaires. Ethnicity of the child was categorized as Western or non‐Western national origin. Highest attained maternal educational level was categorized into low, medium, and high educational level. Maternal psychopathology symptoms were determined through a self‐reported questionnaire using the reliable and validated Brief Symptom Inventory 26, which includes 53 items encompassing a spectrum of psychiatric symptoms, comprising all subscales such as depression, anxiety, and hostility. Maternal BMI was measured at the research center when children were 6 years old.
In the NTR and TCHAD samples, similar covariates were available for adjustment of the models, namely gender, age at baseline, ethnicity of the child, birth weight, maternal educational level, maternal BMI (NTR only), and gestational age (NTR only). These data were derived from parent‐reported questionnaires.
Statistical analyses
Cross‐lagged structural equation modeling in Mplus 7.0 (Muthén & Muthén, Los Angeles, California) was used to examine the bidirectional relation between aggressive behavior and BMI/body composition over time. The cross‐lagged model consisted of stability paths across two consecutive time points for each variable, cross‐sectional paths between aggressive behavior and BMI, and cross‐lagged paths between behavior and BMI over two time points. The cross‐lagged paths indicated the extent to which aggressive behavior or BMI/body composition at time point 1 predicted scores on the other measure at time point 2, while accounting for stability and cross‐sectional paths. In all three cohorts, separate sensitivity analyses were conducted with additional adjustment for attention problems, internalizing problems, and both problems at baseline. Social problems are not assessed with the CBCL/1.5‐5, and therefore sensitivity analyses with additional adjustment for social problems at baseline were performed only in the NTR and TCHAD cohorts. Furthermore, we repeated our analyses in the Generation R Study after excluding all twin participants (n = 114) to ensure there was no overlap between this sample and the NTR. Finally, to increase the interpretability of results, the cross‐lagged model of aggression with BMI in the Generation R Study was repeated using weight status categories (underweight/normal weight vs. overweight/obesity) instead of BMI continuously at ages 6 and 10 years. For the twin cohorts, the “complex option” of Mplus (clustering corrected robust maximum likelihood estimation) was used to take family dependency of the observations into account. To determine the model fit, root mean square error of approximation (≤ 0.08), comparative fit index (≥ 0.95), and Tucker Lewis Index (≥ 0.95) were used as indices to determine good model fit 27. In all samples, full information maximum likelihood was used to account for missing data. Standardized estimates are presented throughout.
Results
Study sample demographics
The baseline characteristics of the three samples were comparable (Table 1). Of note, the Generation R Study included more children with a non‐Western ethnic background than the replication samples, reflecting the urban population base of the former. As expected, averages for gestational age and birth weight were lower for the twin cohorts than in the Generation R Study.
Table 1.
Demographic characteristics of the included study populations
| Generation R (N = 3,974) | NTR (N = 10,328) | TCHAD (N = 1,462) | ||||
|---|---|---|---|---|---|---|
| 6 years | 10 years | 7 years | 10 years | 9 years | 14 years | |
| Child characteristics | ||||||
| Sex, % female | 50.4 | 51.2 | 51.6 | |||
| Age, y, mean (SD) | 6.08 (0.40) | 9.75 (0.28) | 7.42 (0.40) | 10.05 (0.37) | 8.67 (0.47) | 13.67 (0.47) |
| Ethnicity, % | ||||||
| Dutch/Swedish | 67.0 | 94.3 | 92.2 | |||
| Other Western | 8.8 | 2.9 | 5.9 | |||
| Other Non‐Western | 24.2 | 2.9 | 1.9 | |||
| Birth weight, g, mean (SD) | 3,425.83 (567.20) | 2,508.97 (536.97) | 2,615.75 (526.39) | |||
| Gestational age, mean (SD) | 39.80 (1.85) | 36.73 (2.47) | N/A | |||
| BMI, kg/m2, mean (SD) | 15.99 (1.62) | 17.34 (2.52) | 15.35 (1.73) | 16.40 (2.16) | 16.25 (2.06) | 19.06 (2.74) |
| FMI, kg/m2, mean (SD) | 3.89 (1.20) | 4.68 (1.91) | N/A | N/A | N/A | N/A |
| FFMI, kg/m2, mean (SD) | 11.93 (0.89) | 12.55 (1.05) | N/A | N/A | N/A | N/A |
| Aggressive behavior score, median (IQR) | 4.00 (6.00) | 2.00 (4.00) | 4.00 (6.00) | 3.00 (5.00) | 3.00 (6.00) | 2.00 (5.00) |
| Maternal characteristics | ||||||
| Mother's age at baseline, mean (SD) | 37.98 (4.50) | N/A | N/A | |||
| Mother's BMI, kg/m2, mean (SD) | 25.27 (4.67) | 23.59 (3.66) | N/A | |||
| Maternal psychopathology symptoms, median (IQR) | 0.10 (0.19) | N/A | N/A | |||
| Maternal educational level, % | ||||||
| Low | 8.9 | 2.6 | 10.6 | |||
| Medium | 29.2 | 51.2 | 56.1 | |||
| High | 61.6 | 46.2 | 33.3 | |||
Values based on original, unimputed data. Missingness was highest for maternal psychopathology symptoms in Generation R (24.3%), for maternal BMI in NTR (22.6%), and for ethnicity in TCHAD (7.5%).
FFMI, fat‐free mass index; FMI, fat mass index; IQR, interquartile range; N/A, not applicable; NTR, Netherlands Twin Register; TCHAD, Swedish Twin Study of Child and Adolescent Development.
Bidirectional association of aggressive behavior with BMI
Figure 1 shows the results of the cross‐lagged model of aggressive behavior and BMI in the three cohorts. In the Generation R Study, BMI was highly stable over time (β = 0.80, 95% CI: 0.79 to 0.81), whereas aggressive behavior was moderately stable across the two time points (β = 0.56, 95% CI: 0.54 to 0.59). No cross‐sectional associations were observed between aggressive behavior and BMI. With regard to the longitudinal relationships, aggressive behavior at age 6 years was associated with higher BMI at age 10 years (β = 0.02, 95% CI: 0.00 to 0.04). No such association was observed in the opposite direction (i.e., BMI at age 6 years was not predictive of subsequent increases or decreases in aggressive behavior problems at age 10 years). In the NTR sample, aggressive behavior was associated with BMI at both time points in cross‐sectional analysis (β = 0.03, 95% CI: 0.01 to 0.06 and β = 0.03, 95% CI: 0.01 to 0.05, respectively). Even following adjustment for this cross‐sectional relationship, aggressive behavior at age 7 years was prospectively associated with subsequent higher BMI at age 10 years (β = 0.04, 95% CI: 0.02 to 0.06), but, conversely, BMI at age 7 years was not associated with subsequent more aggressive behavior at age 10 years. With respect to the TCHAD sample, no cross‐sectional association between aggressive behavior and BMI was observed at age 9 years; this association was observed only at age 14 years (β = 0.06, 95% CI: 0.01 to 0.11). Prospectively, aggressive behavior at age 9 years was not significantly associated with subsequent higher BMI at age 14 years, although the effect estimate had a magnitude (β = 0.03, 95% CI: −0.02 to 0.07) similar to those in the other two cohorts. An association in the reverse direction was not observed. Fit statistics of the cross‐lagged models indicated good model fit in all three samples.
Figure 1.
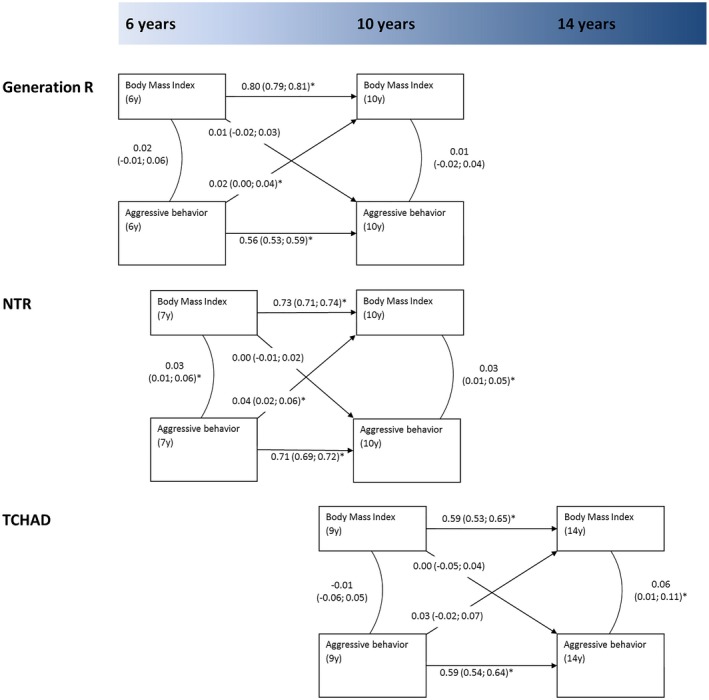
Cross‐lagged association model for the association between aggressive behavior and BMI in three population‐based samples across childhood. Estimates denote standardized β coefficients. All models were adjusted for sample‐specific covariates. Fit indices: Generation R sample (N = 3,974), root mean square error of approximation (RMSEA) = 0.06, comparative fit index (CFI) = 0.95, and Tucker Lewis Index (TLI) = 0.90; Netherlands Twin Register (NTR) sample (N = 10,328), RMSEA = 0.03, CFI = 0.98, and TLI = 0.95; Swedish Twin Study of Child and Adolescent Development (TCHAD) sample (N = 1,462), RMSEA = 0.02, CFI = 0.99, and TLI = 1.00. *Significant at P < 0.05; numbers in between brackets denote 95% CIs.
Bidirectional association of aggressive behavior with fat mass and fat‐free mass
In the Generation R Study, aggressive behavior at age 6 years was associated with subsequent higher FMI at age 10 years (Figure 2, β = 0.03, 95% CI: 0.01 to 0.05). Again, this association was not seen in the opposite direction. No associations were observed between aggressive behavior and FFMI in either direction. Fit indices for the cross‐lagged models pertaining to body composition both indicated good model fit.
Figure 2.
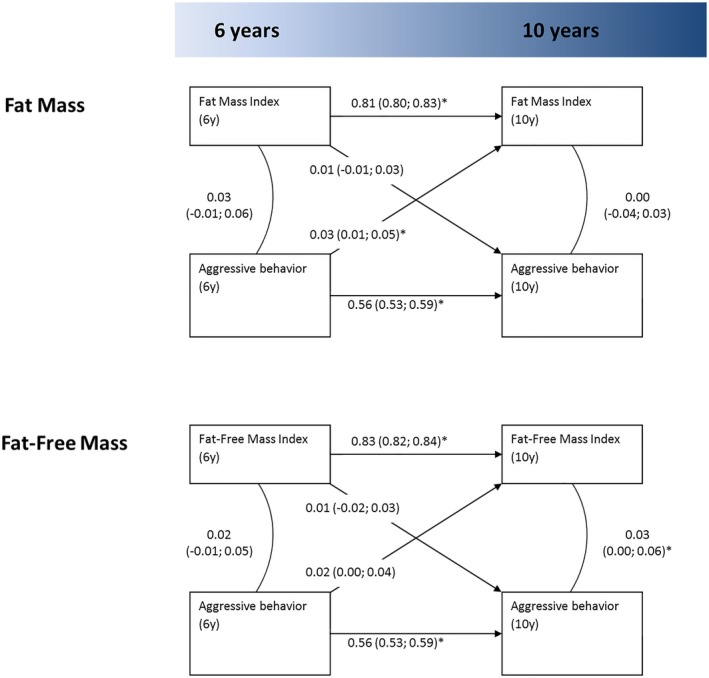
Cross‐lagged association model for the association of aggressive behavior with fat mass and fat‐free mass in the Generation R sample (N = 3,974). Estimates denote standardized β coefficients. All models were adjusted for sample‐specific covariates. Fit indices: fat mass, root mean square error of approximation (RMSEA) = 0.07, comparative fit index (CFI) = 0.94, and Tucker Lewis Index (TLI) = 0.86; fat‐free mass, RMSEA = 0.05, CFI = 0.96, and TLI = 0.92. *Significant at P < 0.05; numbers in between brackets denote 95% CIs.
Sensitivity analyses
Further analyses with additional adjustment for co‐occurring attention problems, internalizing problems, or both yielded results similar to the main findings (Supporting Information Figures S1‐S6). This was the case for the analyses pertaining to BMI and FMI, as well as FFMI. Similarly, analyses in NTR and TCHAD with additional adjustment for social problems resulted in similar associations (Supporting Information Figures S7‐S8). Analyses excluding twin participants from the Generation R Study yielded results comparable to the main findings (data not shown). Finally, in analyses using categories of normal weight versus overweight/obesity, a 1‐unit increase in aggressive behavior scores was associated with greater odds of overweight/obesity (odds ratio = 1.03, 95% CI: 1.01 to 1.05, data not shown).
Discussion
We observed that early aggressive behavior problems were associated with subsequent higher BMI later in childhood in three independent population‐based cohorts. We also demonstrated that aggressive behavior was specifically associated with subsequent higher fat mass but was not associated with higher fat‐free mass. No associations were observed in the opposite direction (i.e., higher BMI or fat mass at baseline did not predict more aggressive behavior problems at follow‐up). These observations were robust to additional adjustment for comorbid attention, social, and internalizing problems.
Although several studies have examined ADHD symptoms or the broader concept of externalizing problems in relation to BMI, to our knowledge, this is the first study focusing on the prospective relation between aggressive behavior and children's BMI. The estimates of the association between aggressive behavior—a component of externalizing problems—and increase in BMI were all relatively small in magnitude but were consistent. Small effect estimates were expected, given the relationship studied and the use of cross‐lagged model analyses, which are adjusted for cross‐sectional and longitudinal stability paths as well as covariates. Nonetheless, estimates were small to modest, indicating that higher levels of aggressive behavior are only marginally predictive of higher BMI at follow‐up, which is not surprising for composite complex phenotypes with many risk determinants such as BMI 28. Our findings in the Generation R sample of aggressive behavior predicting a higher BMI were replicated in the NTR sample of similar ages. Effect estimates in the older and smaller TCHAD sample were comparable in size to those observed in the other cohorts, although these associations were nonsignificant. Our findings extend previous studies showing associations between externalizing behavior and subsequent increases in BMI in children of younger and older ages 5, 6, 7, 8, but these previous studies, similar to our study, did not find an association in the direction from BMI to subsequent higher externalizing problems. Our present study extends these investigations by providing a specific focus on aggressive behavior, which is important given that aggressive behaviors were shown to predict substantial societal costs in terms of health and social service use 10. The results from the aforementioned studies, which examined externalizing problems more generally, could be clouded by the well‐established association between ADHD symptoms and obesity 9. Importantly, our findings remained in sensitivity analyses with additional adjustment for baseline attention problems, further suggesting a specific prospective link between aggressive behavior and higher BMI.
Thus, our findings suggest that aggressive behavior problems constitute one of the many contributing components of obesity in childhood. Although small in magnitude, the relatively modest effects obtained in the current study are of interest, as these associations might be indicative of one of the many likely pathways to increased weight and obesity later in life. Of note, the association between aggressive behavior and subsequently higher BMI was not statistically significant in the TCHAD sample of young adolescents. This lack of replication might potentially be due to the smaller sample size of the TCHAD cohort, which is not impossible considering the small estimates observed in the Generation R and NTR samples. These studies were larger in sample size, with corresponding lower standard errors and, hence, narrower CIs around regression estimates. Future examinations from childhood and adolescence into adulthood are required in order to determine specific developmentally sensitive periods, which are needed considering the stability of weight status across the life course 1.
We observed an association of aggressive behavior with subsequent higher fat mass but not with fat‐free mass, indicating that aggressive behavior is specifically associated with weight‐related physical health. This more in‐depth finding of body composition adds to the existing literature, which typically has focused on BMI more generally 5, 6, 7, 8. No associations were observed between baseline fat mass or lean mass and future changes in aggressive behavior problems, which further supported our main findings with regard to BMI. These findings lend support to the observation that aggressive behavior might also be considered in the multidisciplinary assessment of childhood obesity. Future research should focus on other behavioral indices of cardiometabolic health and the potential modifiability of these purported risk indicators.
A potential mechanism underlying the observed association might be that children who exhibit more aggressive behavior could also have more problems with behavioral self‐regulation and inhibitory control 29. Deficits in self‐regulation, specifically emotion regulation and behavioral inhibition, arise from executive functioning deficits 30. Children with deficits in self‐regulation potentially do not have the ability to respond adequately to their internal feelings of hunger or satiety cues, which in turn leads to overeating 12, resulting in weight gain and potentially obesity in the long term. Indeed, studies have indicated that the neural pathways controlling appetite and behavioral inhibition are interrelated 31. In addition, temperament, behavior traits, taste preferences, and appetite are regulated by the dopaminergic system 32, 33, 34. Moreover, evidence suggested that traits such as eating behavior 35, 36 and aggressive behavior 37 are both regulated via the same neurotransmitter pathways. Furthermore, aggressive behavior and BMI might share genetic vulnerabilities, which possibly explains the phenotypic associations identified in this study. Recent work has demonstrated common pathophysiological mechanisms for depressive symptoms and obesity 38, and this could potentially also apply to other psychiatric problems such as aggressive behavior, which are (genetically) related to depressed mood 39, 40.
Another potential mechanism that could explain the association between aggressive behavior and increased BMI and fat mass comprises inadequate coping mechanisms of parents in response to the challenging aggressive behavior of their child. Parents may allow their child to consume more sweets or unhealthy food, might accept more easily their child's refusal of healthy food, and may allow their children to exhibit sedentary activities such as watching television to avoid difficult behavior of their children 41. These actions may eventually, if performed regularly, result in a relatively high weight gain in children 42 and could thus mediate the relationship between aggressiveness and subsequent high BMI.
Strengths of the present study include the prospective study design of all three pediatric community cohorts with an identical instrument of aggressive behavior. Moreover, we were able to analyze body composition in addition to BMI. However, several limitations should be noted. First, our analyses relied solely on mother reports of the CBCL, which could be subject to reporter bias. Repeated multiple informant assessments of aggressive behavior would be preferred, but this was not achievable in the current population‐based design. Moreover, the CBCL is a valid and reliable measurement for aggressive behavior 22 and has good diagnostic accuracy for clinical disruptive behavior disorders 23. In addition, our analyses in the Generation R Study were adjusted for maternal BMI. Second, as in all population‐based studies, the included cohorts experienced attrition. However, although affecting prevalence, selective loss to dropout often does not influence the strength of association 43. Third, it is well‐established that not only obesity is important for determining physical health. Other factors, such as physical activity, also pose a significant risk for poorer physical health. In this study, we were not able to examine the directionality and possible mediation mechanisms among aggressive behavior, physical activity, and obesity, and further research is required to address these potentially modifiable pathways of risk. Fourth, measures of maternal psychopathology were unavailable in the NTR and TCHAD cohorts. However, adjustment for psychiatric symptoms of the mother only marginally affected our estimates in the Generation R Study. Fifth, the CBCL/1.5‐5 was also used for children aged 6 to 7 years in the Generation R Study, which might not be appropriate for this age. However, internal consistency of the aggressive behavior scale was similar for 5‐year‐olds versus 6‐ to 7‐year‐olds 25, and the multidimensional factor structure of the aggression behavior scale is also comparable to the CBCL/6‐18 44. Finally, we suggested a causal association from aggressive behavior to subsequent higher BMI and fat mass instead of vice versa. However, actual causal inference of composite phenotypes such as obesity is complex 28 and is restricted in this observational study.
Conclusion
The present study showed a small association between aggressive behavior and subsequent increased BMI and fat mass in childhood. This association indicates that aggressive behavior problems observed by mothers are part of one of the many composite risks for obesity in childhood, which also include other behavioral mechanisms such as coping strategies, self‐control, and impulsivity, as well as lifestyle‐related behaviors such as snacking, sedentary behaviors, and sports participation. Hence, it might be helpful to carefully screen for aggressive and other behavioral problems in children with increased risk of obesity. Moreover, our findings signal a need for conducting trials 45 assessing the extent to which treating behavior problems in children improves their physical health and well‐being. In general, professionals as well as parents and other people involved in the care of children with weight difficulties should be aware of the possible behavioral mechanisms associated with higher BMI and fat mass. Most importantly, future research should examine the extent to which these behavioral pathways to childhood obesity are modifiable, although they should bear in mind that effects might be small, as observed in the current study.
Supporting information
Acknowledgments
The authors gratefully acknowledge the contribution of all children and parents, general practitioners, hospitals, midwives, and pharmacies involved with all three cohorts of the present study. The Generation R Study is conducted by the Erasmus Medical Center (Rotterdam) in close collaboration with the School of Law and Faculty of Social Sciences of Erasmus University, Rotterdam; the Rotterdam area Municipal Health Service; the Rotterdam Homecare Foundation; and the Stichting Trombosedienst & Artsenlaboratorium Rijnmond, Rotterdam.
The data that support the findings of this study are available from the data management team of Generation R, but restrictions apply to the availability of these data, which were used under license for the current study and are therefore not publicly available. Data are, however, available from the authors upon reasonable request and with permission from Vincent Jaddoe. Interested researchers may contact Vincent Jaddoe (v.jaddoe@erasmusmc.nl). Similar procedures are in place for NTR (contact: di.boomsmsa@vu.nl) and TCHAD (contact: paul.lichtenstein@ki.se).
Funding agencies: This work was supported by the European Union Seventh Framework Program (2007‐2013): Aggression in Children: Unravelling Gene‐Environment Interplay to Inform Treatment and Intervention Strategies (ACTION; grant number 602768), the Dutch Diabetes Foundation (grant number 2013.81.1664 to PWJ), and the Netherlands Organization for Scientific Research (NWO; grant 016.VICI.170.200 to HT). The first phase of the Generation R Study was made possible by financial support from the Erasmus Medical Center, Rotterdam; the Erasmus University, Rotterdam; and The Netherlands Organization for Health Research and Development (ZonMw). The Netherlands Twin Register (NTR) is supported by the NWO (NWO 480‐04‐004; ZonMw 912‐10‐020; NWO 480‐15‐001). The Swedish Twin Study of Child and Adolescent Development (TCHAD) is supported by the Swedish Research Council for Health, Working Life, and Welfare and the Swedish Research Council.
Disclosure: HL has served as a speaker for Eli Lilly and Shire and has received research grants from Shire, all outside the submitted work. The other authors declared no conflict of interest.
Author contributions: IPMD, KB, and ZY conceptualized and designed the study, performed the statistical analyses, and drafted and revised the manuscript. IPMD and KB collected the data. SL, HL, PL, RG, CEMvB, MB, DIB, and MHJH coordinated and designed the study and critically reviewed the manuscript for important intellectual content. PWJ and HT conceptualized and designed the study, supervised data collection, coordinated and supervised the study, and critically reviewed the manuscript for important intellectual content.
References
- 1. de Onis M, Blossner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr 2010;92:1257‐1264. [DOI] [PubMed] [Google Scholar]
- 2. Sawyer MG, Miller‐Lewis L, Guy S, Wake M, Canterford L, Carlin JB. Is there a relationship between overweight and obesity and mental health problems in 4‐ to 5‐year‐old Australian children? Ambul Pediatr 2006;6:306‐311. [DOI] [PubMed] [Google Scholar]
- 3. Datar A, Sturm R. Childhood overweight and parent‐ and teacher‐reported behavior problems: evidence from a prospective study of kindergartners. Arch Pediatr Adolesc Med 2004;158:804‐810. [DOI] [PubMed] [Google Scholar]
- 4. Lahey BB, Waldman ID. Annual research review: phenotypic and causal structure of conduct disorder in the broader context of prevalent forms of psychopathology. J Child Psychol Psychiatry 2012;53:536‐557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bradley RH, Houts R, Nader PR, O'Brien M, Belsky J, Crosnoe R. The relationship between body mass index and behavior in children. J Pediatr 2008;153:629‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Anderson SE, He X, Schoppe‐Sullivan S, Must A. Externalizing behavior in early childhood and body mass index from age 2 to 12 years: longitudinal analyses of a prospective cohort study. BMC Pediatr 2010;10:49. doi: 10.1186/1471-2431-10-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garthus‐Niegel S, Hagtvet KA, Vollrath ME. A prospective study of weight development and behavior problems in toddlers: the Norwegian Mother and Child Cohort Study. BMC Public Health 2010;10:626. doi: 10.1186/1471-2458-10-626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Camfferman R, Jansen PW, Rippe RC, et al. The association between overweight and internalizing and externalizing behavior in early childhood. Soc Sci Med 2016;168:35‐42. [DOI] [PubMed] [Google Scholar]
- 9. Cortese S, Moreira‐Maia CR, St Fleur D, Morcillo‐Penalver C, Rohde LA, Faraone SV. Association between ADHD and obesity: a systematic review and meta‐analysis. Am J Psychiatry 2016;173:34‐43. [DOI] [PubMed] [Google Scholar]
- 10. Rivenbark JG, Odgers CL, Caspi A, et al. The high societal costs of childhood conduct problems: evidence from administrative records up to age 38 in a longitudinal birth cohort. J Child Psychol Psychiatry 2017;59:703‐710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wells JCK, Fewtrell MS. Measuring body composition. Arch Dis Child 2006;91:612‐617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Graziano PA, Calkins SD, Keane SP. Toddler self‐regulation skills predict risk for pediatric obesity. Int J Obes (Lond) 2010;34:633‐641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bartels M, Hendriks A, Mauri M, et al. Childhood aggression and the co‐occurrence of behavioural and emotional problems: results across ages 3‐16 years from multiple raters in six cohorts in the EU‐ACTION project. Eur Child Adolesc Psychiatry 2018;27:1105‐1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kooijman MN, Kruithof CJ, van Duijn CM, et al. The Generation R Study: design and cohort update 2017. Eur J Epidemiol 2016;31:1243‐1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van Beijsterveldt CE, Groen‐Blokhuis M, Hottenga JJ, et al. The Young Netherlands Twin Register (YNTR): longitudinal twin and family studies in over 70,000 children. Twin Res Hum Genet 2013;16:252‐267. [DOI] [PubMed] [Google Scholar]
- 16. Estourgie‐van Burk GF, Bartels M, Boomsma DI, Delemarre‐van de Waal HA. Body size of twins compared with siblings and the general population: from birth to late adolescence. J Pediatr 2010;156:586‐591. [DOI] [PubMed] [Google Scholar]
- 17. Lichtenstein P, Tuvblad C, Larsson H, Carlstrom E. The Swedish Twin study of CHild and Adolescent Development: the TCHAD‐study. Twin Res Hum Genet 2007;10:67‐73. [DOI] [PubMed] [Google Scholar]
- 18. Tuvblad C, Eley TC, Lichtenstein P. The development of antisocial behaviour from childhood to adolescence. A longitudinal twin study. Eur Child Adolesc Psychiatry 2005;14:216‐225. [DOI] [PubMed] [Google Scholar]
- 19. Porsch RM, Middeldorp CM, Cherny SS, et al. Longitudinal heritability of childhood aggression. Am J Med Genet B Neuropsychiatr Genet 2016;171:697‐707. [DOI] [PubMed] [Google Scholar]
- 20. Silventoinen K, Jelenkovic A, Sund R, et al. Genetic and environmental effects on body mass index from infancy to the onset of adulthood: an individual‐based pooled analysis of 45 twin cohorts participating in the COllaborative project of Development of Anthropometrical measures in Twins (CODATwins) study. Am J Clin Nutr 2016;104:371‐379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Achenbach TA, Rescorla LA. Manual for the ASEBA School‐Age Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2001. [Google Scholar]
- 22. Ivanova MY, Dobrean A, Dopfner M, et al. Testing the 8‐syndrome structure of the Child Behavior Checklist in 30 societies. J Clin Child Adolesc Psychol 2007;36:405‐417. [DOI] [PubMed] [Google Scholar]
- 23. Hudziak JJ, Copeland W, Stanger C, Wadsworth M. Screening for DSM‐IV externalizing disorders with the Child Behavior Checklist: a receiver‐operating characteristic analysis. J Child Psychol Psychiatry 2004;45:1299‐1307. [DOI] [PubMed] [Google Scholar]
- 24. Achenbach TM, Rescorla LA. Manual for the ASEBA Preschool Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2000. [Google Scholar]
- 25. Basten MM, Althoff RR, Tiemeier H, et al. The dysregulation profile in young children: empirically defined classes in the Generation R Study. J Am Acad Child Adolesc Psychiatry 2013;52:841‐850.e842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med 1983;13:595‐605. [PubMed] [Google Scholar]
- 27. Hooper D, Coughlan J, Mullen M. Evaluating model fit: a synthesis of the structural equation modelling literature In: Brown A, ed. 7th European Conference on Research Methodology for Business and Management Studies. London, UK: Academic Conferences and Publishing International; 2008:195‐200. [Google Scholar]
- 28. VanderWeele TJ. Commentary: on causes, causal inference, and potential outcomes. Int J Epidemiol 2016;45:1809‐1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eiden RD, Edwards EP, Leonard KE. A conceptual model for the development of externalizing behavior problems among kindergarten children of alcoholic families: role of parenting and children's self‐regulation. Dev Psychol 2007;43:1187‐1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bridgett DJ, Oddi KB, Laake LM, Murdock KW, Bachmann MN. Integrating and differentiating aspects of self‐regulation: effortful control, executive functioning, and links to negative affectivity. Emotion 2013;13:47‐63. [DOI] [PubMed] [Google Scholar]
- 31. McEwen BS. Understanding the potency of stressful early life experiences on brain and body function. Metabolism 2008;57(suppl 2):S11‐S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Peciña S, Berridge KC. Opioid site in nucleus accumbens shell mediates eating and hedonic 'liking' for food: map based on microinjection Fos plumes. Brain Res 2000;863:71‐86. [DOI] [PubMed] [Google Scholar]
- 33. Keskitalo K, Knaapila A, Kallela M, et al. Sweet taste preferences are partly genetically determined: identification of a trait locus on chromosome 16. Am J Clin Nutr 2007;86:55‐63. [DOI] [PubMed] [Google Scholar]
- 34. Krueger RF, Markon KE, Patrick CJ, Benning SD, Kramer MD. Linking antisocial behavior, substance use, and personality: an integrative quantitative model of the adult externalizing spectrum. J Abnorm Psychol 2007;116:645‐666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Leibowitz SF, Alexander JT. Hypothalamic serotonin in control of eating behavior, meal size, and body weight. Biol Psychiatry 1998;44:851‐864. [DOI] [PubMed] [Google Scholar]
- 36. Kuikka JT, Tammela L, Karhunen L, et al. Reduced serotonin transporter binding in binge eating women. Psychopharmacology 2001;155:310‐314. [DOI] [PubMed] [Google Scholar]
- 37. Stanley B, Molcho A, Stanley M, et al. Association of aggressive behavior with altered serotonergic function in patients who are not suicidal. Am J Psychiatry 2000;157:609‐614. [DOI] [PubMed] [Google Scholar]
- 38. Milaneschi Y, Lamers F, Peyrot WJ, et al. Genetic association of major depression with atypical features and obesity‐related immunometabolic dysregulations. JAMA Psychiatry 2017;74:1214‐1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rowe R, Rijsdijk FV, Maughan B, Eley TC, Hosang GM, Eley TC. Heterogeneity in antisocial behaviours and comorbidity with depressed mood: a behavioural genetic approach. J Child Psychol Psychiatry 2008;49:526‐534. [DOI] [PubMed] [Google Scholar]
- 40. Wertz J, Zavos H, Matthews T, et al. Why some children with externalising problems develop internalising symptoms: testing two pathways in a genetically sensitive cohort study. J Child Psychol Psychiatry 2015;56:738‐746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mamun AA, O'Callaghan MJ, Cramb SM, Najman JM, Williams GM, Bor W. Childhood behavioral problems predict young adults' BMI and obesity: evidence from a birth cohort study. Obesity (Silver Spring) 2009;17:761‐766. [DOI] [PubMed] [Google Scholar]
- 42. Hughes SO, Shewchuk RM, Baskin ML, Nicklas TA, Qu H. Indulgent feeding style and children's weight status in preschool. J Dev Behav Pediatr 2008;29:403‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wolke D, Waylen A, Samara M, et al. Selective drop‐out in longitudinal studies and non‐biased prediction of behaviour disorders. Br J Psychiatry 2009;195:249‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bolhuis K, Lubke GH, van der Ende J, et al. Disentangling heterogeneity of childhood disruptive behavior problems into dimensions and subgroups. J Am Acad Child Adolesc Psychiatry 2017;56:678‐686. [DOI] [PubMed] [Google Scholar]
- 45. Lumeng JC, Miller AL, Horodynski MA, et al. Improving self‐regulation for obesity prevention in Head Start: a randomized controlled trial. Pediatrics 2017;139:e20162047. doi: 10.1542/peds.2016-2047 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


