Abstract
The achievements of cell-based therapeutics have galvanized efforts to bring cell therapies to the market. To address the demands of the clinical and eventual commercial-scale production of cells, and with the increasing generation of large clinical datasets from chimeric antigen receptor T-cell immunotherapy, from transplants of engineered haematopoietic stem cells and from other promising cell therapies, an emphasis on biomanufacturing requirements becomes necessary. Robust infrastructure should address current limitations in cell harvesting, expansion, manipulation, purification, preservation and formulation, ultimately leading to successful therapy administration to patients at an acceptable cost. In this Review, we highlight case examples of cutting-edge bioprocessing technologies that improve biomanufacturing efficiency for cell therapies approaching clinical use.
Cell therapeutics — which entails the use of human cells as medicines — promise to transform the treatment of a wide range of diseases, such as cancer, neurodegenerative disorders and autoimmune disorders, by enabling sophisticated mechanisms of action that small chemical compounds cannot provide. For example, the differentiation of stem cells into specialized cells, such as hormone-secreting endocrine cells, cytotoxic lymphocytes or tissue-regenerating cells, can be exploited for therapeutic properties. Also, cells can be genetically engineered to perform a wide range of functions1–4 and, because of cell-homing properties5, can deliver drug payloads.
Academic and industrial research and development efforts are typically focused on understanding how cell therapies can treat a diverse set of indications, as highlighted by the recent rise of phase I-III trials6 (Fig. 1a). In fact, to date, commercial wins have been achieved at a relatively lower standard than expected from the pharmacological industry. On the basis of a semiquantitative analysis, the cell-therapy conversion rate from a phase III study to regulatory approval is estimated to be at 14.3%, which is considerably lower than the conversion rate (48.7%) of mature pharmaceutical drug classes showing new-drug-application success with the United States Food and Drug Administration (FDA; Supplementary Table 1). Future market analyses are encouraged by groups such as the Alliance for Regenerative Medicine, to further quantify and track trends as more studies and regulatory approvals proceed. Recent approvals in the United States and the European Union for GSK, Tigenix, Novartis and Kite (a Gilead company) are bringing new enthusiasm for better-defined success criteria that help move more cell therapies to the marketplace.
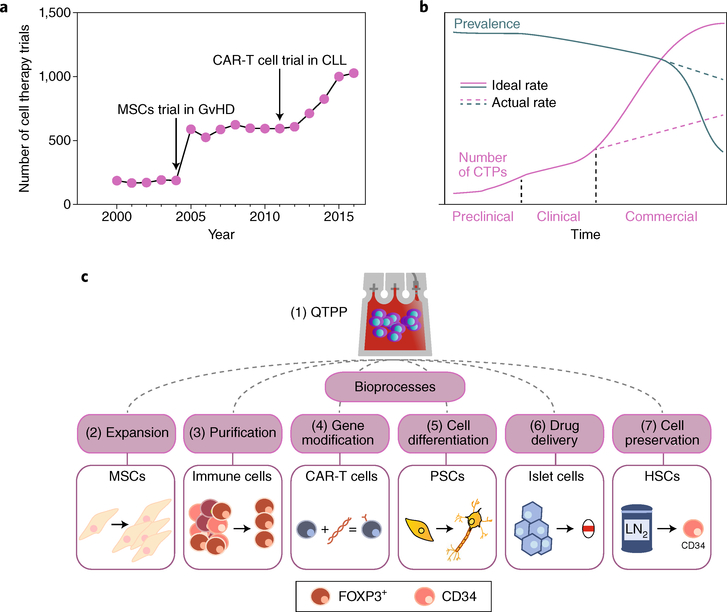
Fig. 1 |. Cell-therapy pharmacoeconomics and manufacture.
a, Number of cell-therapy clinical trials started yearly in the United States, from 2000 to 2016. The two inflection points correlate with the publication of two phase I human trials: MSCs to treat graft-versus-host disease (GvHD)19 and CAR-T cells against chronic lymphocytic leukaemia (CLL)163. b, Schematic of the supply-and-demand curve for a hypothetical CTP as it evolves from preclinical testing to commercialization. Disease prevalence, or demand, is shown by the green line; CTP production, or supply, is shown by the pink line. The dashed lines represent trajectories for which the scale of CTP production does not match clinical needs. The y axis represents an arbitrary number of units. c, The bioprocesses for the manufacturing of CTPs discussed in this Review, with the boxes illustrating the case studies used. The scalability of each bioprocess, which is designed to meet a quality target-product profile (QTPP), can improve the production efficiency of a specific CTP towards meeting clinical and commercial-scale demands. LN2, liquid nitrogen.
The promise of cell therapeutics comes with new challenges in reproducibly manufacturing and in administering cells to thousands of patients7. It is important to recognize that methods that are sufficient for generating products on the scale of early pivotal clinical studies may not directly translate to commercial-scale yields and efficiencies. Therefore, beyond the success rate of current clinical trials, commercial-scale demands for cell therapeutics in common diseases will hamstring the supply of a cell therapy product (CTP) if not assessed at an early developmental stage (Fig. 1b). This gap in supply and demand will ultimately affect patients who may not be served by a CTP, simply due to unfilled prescriptions. The associated logistical and economic factors involved are not trivial: physical space, production time, human resources, consumables, waste generation (environmental impact) and other direct costs — all these factors must be integrated into the long view of a manufacturing blueprint.
At their core, cell-manufacturing processes are not new. For example, the process of fermentation established infrastructure to produce large batches of chemical products derived from bacteria and yeast cells. Engineering tools such as stirred tank reactors, liquid-chromatography systems and cross-filtration technologies all matured during the development of new biochemicals. Similar tools were then repurposed for the development of biopharmaceuticals; indeed, cells are now engineered to produce a purified biological agent, such as a monoclonal antibody. Unlike in the use of cells for the production of a molecular agent, in cell therapy, the final manufactured product is the cells themselves. The production of a CTP thus requires additional processing steps, such as cell selection, purification, formulation, preservation and distribution. These processes pose different technical challenges from those required for the production of a molecular agent, especially in light of the number of modifications that cells need to undergo. Past manufacturing tools are nevertheless still valuable for the development of CTP bioprocesses with both scale up and cost reduction in mind. Biomanufacturing represents an important thrust of the Advanced Manufacturing Partnership initiative in the United States7,8.
For example, the handling of blood products and bone-marrow products over the past few decades has set precedents for the development of human cell therapies, and created a foundation for the basic quality specifications that need to be met9. Therefore, regulations, standards and guidance previously established for drugs and molecular biologics can now also be described for cell therapy. Although specific regulations will vary by region, each will require biomanufacturing solutions that support compliance with current good manufacturing practices (GMPs) to assure the quality and safety of products for human use through proper design, monitoring and control of the manufacturing processes and facilities. Supplementary Table 2 provides a summary of regulatory references for the United States and European Union jurisdictions. Japan has created a regulatory framework for regenerative medicine that has been partially adopted by the United States via the 21st Century Cures Act, with a new Regenerative Medicine and Advanced Therapies designation for expedited regulatory review for resolution of product-development questions. A discussion of regulatory considerations is however beyond the scope of this Review.
A critical decision point in the evaluation of CTP manufacturing is the distinction between autologous and allogeneic cell therapies. Although they share the same end goal — producing a high-quality cell therapeutic — there are stark differences between both modalities when it comes to manufacturing. Autologous therapies have considerable additional logistical challenges because a closed-distribution model means that a product inventory for broad distribution cannot be created. The input of variable patient-derived cells into an autologous pipeline requires a robust process to ensure the consistency of the final product; yet a lower bar for patient testing is arguably required, due to the material returning to the same patient. Patient screening and release testing are also required for each CTP, given the individualized treatment modality. Automated point-of-care technologies for autologous treatments may enable on-site preparation of products at hospitals and would then require medical-device (510k US) classification. Current trends show that autologous therapies are routinely conducted at an ~1 l scale, whereas bioprocesses for allogeneic cell therapies are planned to operate at a >100 l scale. Allogeneic therapies need to include extensive testing of cell banks, yet will not require all patients to be extensively screened to produce a bulk material. Although bulk production can help decrease costs through economies of scale, a larger facility with generally more expensive equipment is necessary. Operating a manufacturing site to fulfil incoming CTP prescriptions, where the site must manufacture a certain amount of product within a specified timeframe, brings in further concerns around overhead costs, especially of personnel.
In this Review, we discuss the manufacturing of cell products for clinically advanced cell therapies, and highlight bioprocesses that may face issues before achieving commercial production scalability (Fig. 1c). We first describe current understanding of product quality. We then review critical bioprocesses, and highlight bottlenecks in cell expansion, cell engineering, cell differentiation, cell purification, cell-biomaterial formulation, cell preservation and cell transportation. Each bioprocess is presented as a case study paired with a specific cell-therapy example, chosen to give context to real-world applications. Emerging engineering solutions that can maintain or improve the control of these bioprocesses are also discussed.
Quality target-product profile
As advanced cell therapies emerge, a regulatory framework for product development and management is required. An example is the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), which established a framework for quality by design (ICH Q8 — Pharmaceutical Development, August 2009)10. A quality target-product profile (QTPP) defines the critical attributes of a CTP through metrics that bioprocess engineers can work towards in a stage-specific development programme. Supplementary Table 3 provides a descriptive summary for the range of attributes that typically need to be controlled for CTPs. Such attributes form the basis of product specifications, which are measured following a broad range of analytical methods (a thorough summary of which is provided by the Publicly Available Specification (PAS) 93:2011 — Characterization of human cells for clinical applications). Bioprocessing-engineering controls can then be developed and applied to assure that manufacturing performance will result in products that meet or exceed these specifications while recognizing the inherent complexity, significant heterogeneity and batch-to-batch variation that is typical of cell-based products. This will require, for example, innovative cell-processing solutions that are suitable for variable incoming cellular starting material from one stage to the next and that yield a standardized output with dampened variability.
Potency should be prioritized early in CTP development because it ultimately confirms CTP utility. Potency is the bar by which key decisions are made, including product-lot release, shelf life, comparability between products manufactured within or between sites, and validation of clinical preparation. However, potency can be challenging to define and measure due to the complex nature of many CTPs, and their poorly understood mechanisms of action and natural lot-to-lot variability11,12. In an ideal product, a series of causation studies connects the (pre)clinical efficacy of CTPs to a mechanism of action and its relevant measurable bioactivities and to quantitative assays for the laboratory measurement of potency (these aspects complicate international standardization). Supplementary Table 4 summarizes example approaches to potency quantification that have been pursued for a range of CTPs.
Potency is also directly associated with the composition of a CTP, defined by a coupled set of quality attributes: purity and identity. Identity defines the ‘active pharmaceutical ingredient(s)’, whereas purity distinguishes CTPs from any non-pharmacological cells (which are considered impurities). Typically, it is not immediately practical to produce pure cell compositions with precise frequencies of each cell type. The challenge is rather to dissect the positive and negative roles played by different cells. For example, in immunotherapy with chimeric antigen receptor (CAR)-T cells where CD19-CAR-T cells are the active agent (Supplementary Table 4), CD4+ cells are a subset of CD3+ T cells that may be considered a part of the product or an impurity. A process to isolate, transduce and expand T cells can result in uncontrolled variable proportions of the constituent T cells because of proportions that are variable from one patient to another and because of variable expansion characteristics between the T-cell types. Increased control in cell-mixture composition requires additional manufacturing steps and results in an increased cost of goods, yet motivates the engineering of innovative process design for each of the unit operations to establish a favourable cost-benefit trade-off.
Test methods for quantifying product attributes relevant to a QTPP can also be an engineering challenge. For example, an in vitro potency bioassay may need development to quantify potency for product release. Also, CTPs must be certified to be free of microbes and other manufacturing residuals, such as culture reagents. In this regard, treatments that reduce pathogens through selective destruction13,14 for blood products and their components, such as rapid and online test methods (for example, for sterility) and closed microfluidic systems and other technologies that inherently eliminate microbial contamination risk, are being explored. Engineering solutions are therefore needed to establish sensitive, timely and cost-efficient test methods that reflect the unique nature of CTPs, yet deliver the robustness to consistently meet critical quality attributes.
Adherent cell expansion for large-scale cell manufacturing
Cell therapy can at times be the result of a ‘mass effect’, that is, cells necessary for a regenerative or immune process in the body need to be present in significant numbers. Therefore, by increasing cell numbers during a pathological condition, the balance of therapy can tilt towards repair. In these instances, growing sufficient numbers of cells becomes essential for the delivery of an effective dose.
The cell-expansion method can be used to classify CTPs; for example, suspension culture versus adherent culture. Suspension cultures have the benefit of high yields in a spatially efficient format. In contrast, traditional adherent cell-culture methods require vast and logistically impractical planar surface areas for cell growth, when developed at commercial scales. Mesenchymal stromal cells (MSCs) are one adherent cell therapy. MSCs are promising for the treatment of haematopoietic failure15–17, graft-versus-host disease18–20, and disease and injury of the gastrointestinal tract21–25, skin26, heart27,28, lung29–32, liver33,34 and kidneys35, because of their ability to release a cascade of trophic factors after cell infusion33,36,37. Given their ease of isolation and relatively low immunogenicity, allogeneic MSC products have entered advanced stages of clinical trials. The need for large-scale production is now critical, as single doses are in the range of 1–10 million MSCs kg−1. MSCs are traditionally cultured on two-dimensional (2D) surfaces, such as flasks or multilayer cell factories (Fig. 2a), and lot sizes of about 10–50 billion cells are expected from a large-scale run38; however, 2D methods have proven difficult to scale up and cost-effectively operate at the commercial scale. It is clear that current 2D culture systems will be insufficient to meet the need for future commercial viability; rather, scalable, closed-loop and potentially automated technology is needed.
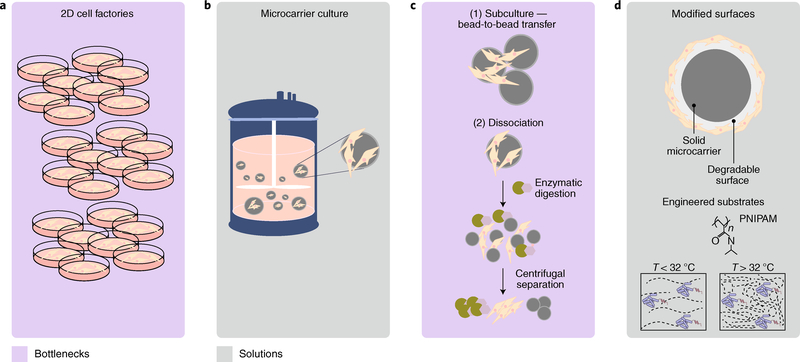
Fig. 2 |. Process optimization for the expansion of cells and for cell collection from microcarriers.
a, Production of clinical lots by using adherent MSCs in 2D cell-culture plates. Issues with the scaling of costs and labour efficiency make 2D culture unlikely to meet an estimated demand of >1012 viable cells per year, necessary for treating prevalent adult indications. b, Suspension culture systems for MSCs use microcarriers and stirred tank bioreactors and are a scalable and sustainable approach for cell expansion at high density. c, Unit operations identified as major bioprocessing bottlenecks: (1) bead-to-bead transfer for MSC subculturing and expansion; (2) the need for enzymatic digestion and centrifugal separation to isolate the MSCs from the microcarriers. d, Materials-science innovations in microcarrier substrates can improve product purity, identity and potency through degradable and temperature (T)-sensitive materials (such as poly(N-isopropylacrylamide), PNIPAM) that remove the need for additional enzymatic dissociation processes.
For example, the 3D adherent bioreactor Quantum Cell Expansion System (Terumo BCT) is a GMP-compliant, functionally closed, automated hollow-fibre system for clinical MSC expansion. This system can generate about 1 × 108 cells in 2 weeks, thus fulfilling the International Society of Cell Therapies’ minimum criteria for defining MSCs39. The cells exhibit normal metaphase karyotype40, and show comparable efficacy in different animal models41. The system, however, requires precoating of the bioreactor with fibronectin, which is a shortcoming if more than one passage is needed. Nevertheless, the Quantum Cell Expansion System does not require access to clean rooms, and eliminates the need for multiple incubators. At the time of writing, one initial system has been cleared by the FDA for clinical use in early-phase trials.
Transitioning from adherent 2D to adherent 3D cultures is the most viable path forward for the commercial production of cells for thousands of patients. Another option is the use of microcarriers — that is, supporting microparticulate matrices that allow for cell expansion in 3D conditions (Fig. 2b). MSCs adhere to microcarriers and are cultured in stir tank bioreactors (from 300 ml to 1,000 l in volume) for large-scale expansion42. Despite the advantages of microcarrier technology for scale up, a number of challenges remain regarding the optimization of the bioreactor culture environment (Fig. 2c and Supplementary Table 4); for example, the need to supply metabolic substrates, such as oxygen, is critical for commercially viable manufacturing. Indeed, less-than-ideal cell densities and the low specific oxygen-uptake rate of human MSCs are issues that must be addressed before any scale-up attempts43. Therefore, the ability of bioreactor systems to closely select and control process parameters such as dissolved oxygen can be used to improve product quality and increase yields44 over those of the largely non-controlled 2D methods. Owing to its limited solubility, oxygen needs to be supplied continuously; yet as cell density increases, it may become necessary to provide oxygen by sparging, typically with air, which also strips out the carbon dioxide produced45. However, sparging requires the inclusion of protective agents into the medium, most commonly the surfactant Pluronic F68, which can induce damaging cavitation bubbles that lead to reduced cell viability46. An opportunity for the reduction of cost of goods per dose is the use of serum-free media. In combination with microcarriers, serum-free media can increase yields47 and reduce sustainability risks associated with serum-based culture48.
Several advances in microcarrier process development have been achieved using GMP-ready technology and xeno-free media48. For example, 1.1 × 108 bone-marrow MSCs (BM-MSCs) and 4.5 × 107 adipose-tissue MSCs (AT-MSCs) were produced after 7 days of culture by using a non-porous plastic microcarrier-based 1 l stirred tank bioreactor (SoloHill Engineering). More recently, a comparison of a 2 l single-use bioreactor using synthetic microcarriers (Synthemax-II microcarriers) and xeno-free culture medium with static cultures and with spinner flasks showed the scalability advantages of bioreactors49. Human BM-MSCs grown in stirred-tank 5 l bioreactors using plastic microcarriers for 12 days retained key qualities, with matched viable cell counts in both day-8 and day-12 cell samples50. These studies cumulatively support the feasibility of scale-up approaches, and are useful for the identification of remaining obstacles.
Microcarriers can be made of different materials, support different sizes, present different porosities and different chemical properties, and hence be optimized for specific cell-growth conditions. Choosing the optimal conditions for cell growth is not trivial, and tends to be a major caveat of this technology. Approaches from materials science, such as biodegradable microcarriers or temperature-sensitive microcarriers51 (Fig. 2d), will alleviate the need for downstream detachment and separation methods to isolate MSCs for clinical indications where engraftment directly into a tissue is required, such as in bone formation52. For clinical indications that require intravenous administration of the MSC product, it is possible to remove microcarriers entirely by using a two-phase liquid/liquid system to form a temporary microcarrier surface for the MSCs to expand on53. Following culture, the temporary microcarrier surface can then be dispersed, resulting in a liquid/liquid interface where the MSCs are collected and from which the MSCs can be collected as single cells, without the need for enzymatic solutions to detach the MSCs (as is required in plastic microcarrier-based processes). Another method for removing the microcarriers from the bioreactor manufacturing process and to alleviate downstream challenges involves the culture of MSCs in suspension via the formation of spheroids. This expansion method has demonstrated improved critical quality attributes for MSCs and preclinical efficacy; however, major development is required to maintain the level of MSC expansion potential compared with current adherent expansion methods54. The introduction of standards that would bring industrial robustness to these materials-science solutions will help establish high-density MSC suspension cultures with ease of collection and separation, ultimately leading to a high-purity MSC product.
Purification via high-throughput cell sorting
In cell therapy, specific cell populations have to be isolated and enriched, with optimal purity, throughput and yield (typically, these are interdependent parameters). Separation technologies in cell therapy can be broken down into two categories: cell-cell separation, where the aim is to isolate one phenotype of cells away from another phenotype of cells; and cell-solution separation, where a population of cells is washed and the media replaced (or where a reduction in volume achieved). Cell-cell separations are commonly achieved through both chemical and mechanical means. Common methods include Ficoll to separate red blood cells, platelets and mononuclear cells by density separation, counterflow centrifugal elutriation to separate cells on the basis of both size and density, and fluorescence-activated or magnetic-activated cell sorting (FACS or MACS) to separate cells on the basis of specific properties. Devices have standardized the process of eliminating manufacturing bottlenecks, particularly in autologous-cell-therapy preparations.
To limit biological variation, isolating a cell population is one of the key steps of cell manufacturing. As an example, CD3+ leukocytes must be purified from a blood derivative, most commonly by apheresis. It is important to remove non-target components, such as monocytes, granulocytes, red blood cells and platelets, which may have detrimental downstream effects. For instance, maintaining a population that includes monocytes can result in these cells out-growing the CD3+ cells. Also, platelets have been shown to negatively impact selection criteria55 through reductions in both recovery and purity. The purity of CD3+ cells is thus important to establish successful downstream processing, with yield being less of a concern. A typical apheresis unit contains ~40 × 103 leukocytes μl−1 (ref. 56). This large population enables the trading of yield for higher purity, as ~200 × 106 CD3+ cells are needed to begin culture. The most common enrichment method used is anti-CD3-coated magnetic beads (Fig. 3a). These beads bind to target cells, which are then purified by placing the cell suspension in a magnetic field. This method is low-throughput, requires costly reagents, and frequently relies on a sole supplier of consumables (which bears supply-chain risk). The use of magnetically labelled cells in vivo carries some safety concerns57, with Dynabeads requiring an accepted release criteria of ≤100 beads per 3 × 106 cells58. Miltenyi have a commercial-use license from the FDA for their CD34 microbeads when operating under the Investigational New Drug programme. Other approaches to isolate cells from binding surfaces that are under consideration and are based on materials science operate at research grade. For example, in commercial products such as MagCloudz from Quad Technologies (Fig. 3b), magnetic beads are physically separated from the cell by a dissolvable, antibody-coated hydrogel; this results in a bead-free downstream product after substrate dissolution59.
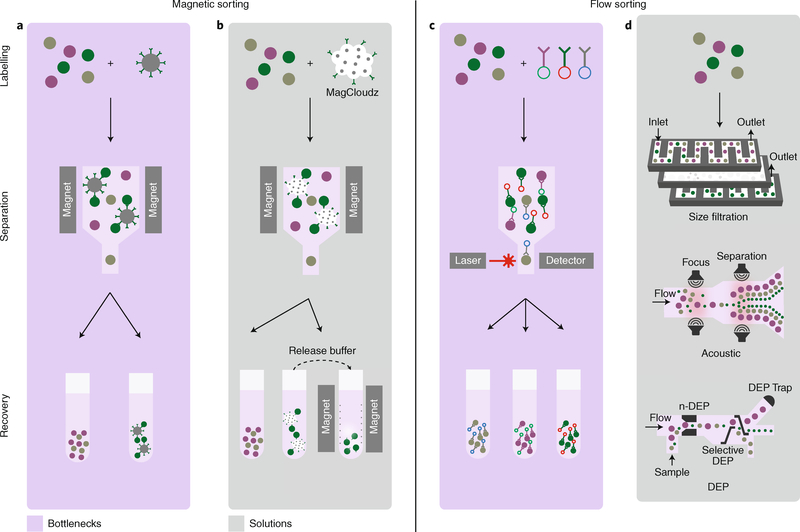
Fig. 3 |. Towards high-throughput label-free purification.
Cell-separation techniques, in which cells are first identified and labelled, and then separated and recovered, can currently be broken down into two main categories: magnetic sorting and flow sorting. a, Magnetic sorting uses magnetic beads coated with an antibody to separate cells from a mixed population (green, purple and grey). Current magnetic methods result in a positively selected population (green) that still has magnetic beads attached, which is undesirable (bottom right, recovered population). b, This issue has been circumvented by the MagCloudz QuadGel technology, which embeds magnetic particles into a hydrogel coated by antibodies, thereby effectively eliminating direct contact between the cells and the magnetic beads by using a release buffer that separates the magnetic particles from the hydrogel, which can then be recovered by magnets. c, Flow-cytometry sorting is a widely used method, based on fluorescently tagged antibodies (top right), that allows populations of cells to be selected according to antibody binding. The method is expensive and costly to set up in parallel; hence, it is typically used at low throughput. d, In microfluidic methods, which increase throughput for unlabelled cells, a mixed population of cells is passed through microchannels, where cell separation is driven by a sequence of events, such as size filtering, acoustic separation and dielectrophoresis (DEP) sorting. A DEP trap can be set to collect the desired cells. n-DEP, negative dielectrophoresis.
FACS is often used in combination with antibodies bound to fluorochromes to label cells and select them on the basis of their degree of laser-triggered excitation (Fig. 3c). In contrast to MACS, FACS is best used in cases where several markers are needed to identify and purify a CTP. This is needed in, for example, the purification of human regulatory T (Treg) cells for use in autoimmune diseases. Peripheral blood T cells are isolated and expanded, and subsequently sorted by FACS via extracellular staining, using a panel of antibodies that identifies cells with a surface marker phenotype of CD4+CD25HiCD127−/Low. This method is used clinically for Treg-cell purification even though it remains a challenge to sustain this workflow in commercial manufacturing. A number of solutions for the label-free purification of cells (Fig. 3d) are under investigation, notably the use of microfluidic technologies in combination with acoustics, size separation or Raman scattering59–65. SonoSep uses acoustic-wave separation technology, but has so far only been demonstrated at laboratory scale. Dielectrophoresis has shown promise at separating cells on the basis of membrane capacitance, but currently operates at 150,000 cells h−1 (ref. 66), suggesting that apheresis for an individual patient would take nearly 50 hours to carry out. Hence, despite substantial technological developments, the challenge to reach CTP scalability, quality and safety for clinical implementation remains.
Production of CAR-T cells and haematopoietic stem cells
Cell engineering — the application of methods to modify cells through genetic manipulation (Fig. 4a) — enables the study of disease mechanisms, the identification of new drugs, and the manipulation of cell function to derive biologics and cell-based therapies. There are numerous methods for introducing genetic changes into a cell, and they are typically classified as either viral or non-viral. Examples of the latter class are transfection techniques that introduce nucleic acids into cells via chemically based products such as liposomes or through non-chemical methods such as electroporation — where an electric field is applied to cells to increase membrane permeability, allowing chemicals or genetic material to pass into the cell67. Electroporation has been used to introduce RNA to transiently modify cell function, with the full expectation that only short-lived expression of the new genetic material will occur68. To achieve stable expression of transgenes, transfection has been used to introduce, in the host cell, plasmid combinations that encode transposon elements and the transposase enzyme (such as the Sleeping Beauty and piggyBac transposon systems); this method is relatively cost effective and is in early clinical development in CART-cell therapy for leukaemia patients69–72. Most frequently, however, primary cells and stem cells require viral transduction methods, rather than electroporation, to efficiently and stably transfer genetic material. A detailed history of viral vector manufacture and improvements in vector design and vector production73 is beyond the scope of this Review; yet it is worth noting that methods based on ex vivo viral gene therapy are often incorporated into bioprocessing workflows for the production of cell therapeutics.
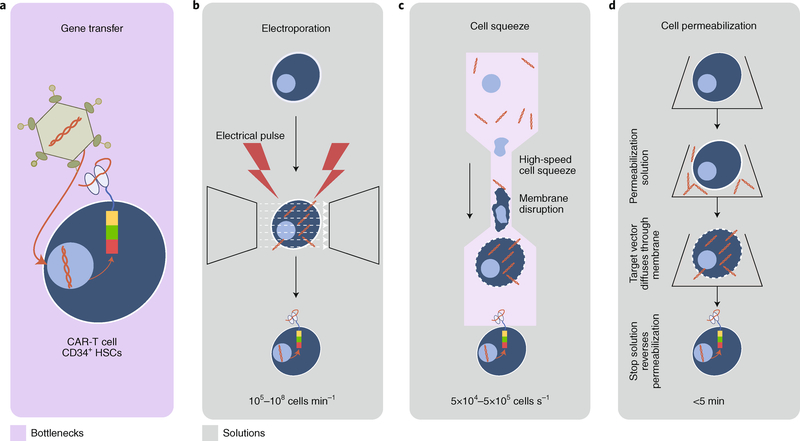
Fig. 4 |. Streamlining the genetic modification of cells for therapy.
a, An overview of the traditional method used for the genetic modification of T cells for CAR-T-cell transformation. Cells are incubated (at conditions for optimal multiplicity of infection) with viruses carrying an engineered vector that enables the expression of a desired antigen receptor, providing the CAR-T cell with recognition specificity. b-d, More efficient methods in various states of use and development to generate CAR-T cells include flow through electroporation (MaxCyte STX), where pores in individual cells are generated by an electrical pulse (b), mechanical membrane disruption by forcing cells through a narrow pore (CellSqueeze, SQZ Biotechnologies; c), and the use of permeabilization solutions where the target vector diffuses through the cell membrane and a stop solution then reverses the permeabilization (Avectas; d). Engineered vectors are displayed in orange and the CAR expressed on the membrane surface is displayed in multiple colours.
A prominent example of cell engineering is the use of T-cell immunotherapy to target tumour-associated antigens74. CAR-T cells expressing receptors for CD19 (ref. 75) have recently received FDA approval for the treatment of paediatric patients with relapsed or refractory acute lymphocytic leukaemia and of adults with refractory large B-cell lymphoma76. First, resting polyclonal T cells are collected from the peripheral blood of patients, typically by apheresis, and activated with CD3 or CD28 beads, to induce a population expansion, before being transduced and purified. As artificial immune receptors, CARs impart specificity, activation and co-stimulation to the cell. Additional transgenes can be included in the vector, including cytokines, immunomodulatory proteins, surface membrane tags and suicide genes. Although there are examples of the transient expression of CARs (ref. 77), long-term efficacy in patients remains to be demonstrated. Rather, stable chimeric-receptor expression and the persistence of these cells is the current standard, and may be required for efficacy; hence, the use of retroviral vectors for stable introduction of CARs into cells. Efficiency for CAR constructs has increased significantly through improvements in vector design78, manufacturing, as well as in the use of additive reagents such as retronectin to help create a cationic charge for facilitating viral transport into a cell (although this remains an expensive process). Also, the vector-manufacturing process is difficult to standardize. Batch-to-batch variations using the same multiplicity of infection — that is, the number of vector particles transfected per cell — are measured by comparing vector titration with transgene expression. Vector titre in turn is determined by a nonlinear vector-mediated transduction process that controls for suspension volume, length of incubation, temperature and cell concentration79, making the vector-manufacturing process a highly variable and expertise-dependent step that requires a flexible manufacturing process with associated high costs. Although it is possible to use small amounts of vector and thereby reduce costs, this is offset by the need to prolong the culturing step of newly engineered cells, to achieve a therapeutic dose of engineered T cells.
Cell engineering has also been successfully applied to haematopoietic gene therapy for a variety of monogenic diseases, including severe combined immunodeficiency, Wiskott-Aldrich syndrome, chronic granulomatous disease, cerebral adrenoleukodystrophy and hemoglobinopathies80–82. In these settings, CD34+ autologous haematopoietic stem cells (HSCs) are isolated from either the bone marrow or peripheral blood, stimulated with a cocktail of haematopoietic growth factors, and transduced with either a retroviral or lentiviral vector carrying a normal copy of the defective gene under the control of a constitutively active promoter. Genetically modified HSCs are administered intravenously and then migrate to the bone marrow, where they engraft and produce differentiated cells expressing the normal protein. Vector integration into long-lived HSCs is required to produce normal differentiated cells for the duration of the patient’s lifespan. In most cases, conditioning therapy is needed to eliminate at least a fraction of the patient’s abnormal HSCs and to facilitate engraftment and persistence of genetically modified cells. However, excitement towards early promising clinical results was tempered by the development of acute leukaemia in several patients as a direct consequence of insertional mutagenesis caused by γ-retroviral vectors. Since then, many alternative vectors, including lentiviruses and adenoviruses, have been explored, as well as improvements in vector design and in the use of self-inactivating vectors; and recent studies have not observed a preferential integration of modern vectors into potential oncogenic regions83. Nevertheless, gene-editing approaches might replace gene transfer84; in addition to avoiding potential insertional mutagenesis events, the correction of endogenous mutated sequences by gene editing should produce normal gene products under the control of endogenous promoters capable of controlling gene expression in physiological conditions. This will likely enhance the potency of the engineered cells and normalize the function of corrected HSCs after transplantation.
Improving the efficiency of cell-transfection methods is expected to make a large impact on scalability and cost, with electroporation being the most common method for cell-based therapies. Modern electroporators are designed to significantly reduce cell damage while maintaining high efficiency. This proven technology is used in clinical applications despite poor cell recovery and scalability. Technologies such as MaxCyte (Fig. 4b), Nucleofector (Lonza) and Gene Pulsar (Biorad) have effectively increased the scale of transfection. Lonza’s Nucleofector Portfolio allows the efficient transfection of hard-to-transfect cell lines and primary cells with different substrates, including DNA vectors and short hairpin RNA, microRNA and short interfering RNA oligonucleotides. The versatility of the system allows the transfection of adherent cells and flexibility in cell numbers and cell status, whereas the ability to add-on a 96-well module or an independent reaction system (such as the high-throughput nucleofection system) can enable high-throughput transfection with as low as 2 × 104 cells in less than 5 min or transfection of as many as 1 × 109 cells with the 4D-Nucleofector LV Unit85. Transfection efficiency is cell-type dependent, with achievable efficiencies of approximately 70% for peripheral blood mononuclear cells and 90% for unstimulated human T cells. MaxCyte provides post-transfection cell viabilities of >90% and transfection efficiencies >90% for most commonly used cell types, allowing scalability between 0.5 × 106 and 0.7 × 108 cells in seconds and up to 2 × 1010 cells in less than 30 min (refs 86–88). The electroporation buffer and workflow for transfection are still problematic, because glucose-free and protein-free buffers can cause the deterioration of cells. Nevertheless, some clinical trials have used electroporation methods for CAR-T-cell manufacturing89,90, as well as gene-editing strategies such as clustered regularly interspaced short palindromic repeats (CRISPR)-CRISPR-associated protein-9 nuclease (Cas9) to further modify or improve engineered T-cell products91.
Throughput parallel processing is an important step towards automated solutions and decentralized manufacturing for cell engineering. For example, the Miltenyi Prodigy is a semiautomated closed system to manufacture CAR-T cells. The entire process, including T-cell stimulation, transduction and expansion, is carried out in one instrument and can electroporate up to 50 ml of cell suspensions in less than 30 min. SQZ Biotech are developing a microfluidic channel that squeezes cells (CellSqueeze), creating small pores in the membrane by which delivery of material can occur while maintaining good cell viability and function92 (Fig. 4c) and handling one million cells per second. The Neon Transfection System by ThermoFisher Scientific promises efficiency of up to 90% in many cell types, with the ability to transfect from 2 × 104 to 6 × 106 cells per reaction. Specifically, peripheral blood mononuclear cells can be transfected with 23% efficiency and 95% viability by using this technology. The main advantage of this design is the better maintenance of physiological conditions, which results in high cell survival compared with what is achieved in conventional electroporation93; however, it comes with high running costs of the gold-plated electrodes, with a proprietary transfection buffer, and with the costs of Neon pipette tips and tubes. Avectas have developed a proprietary delivery solution that initially permeabilizes the cell membrane, allowing for diffusion of the delivery material into the cell, and then reverses the permeabilization (Fig. 4d), effectively controlling loading. Avectas has a delivery efficiency of ~53% and a cell survival of 78% (for comparison, the efficiency and cell survival are 93% and 73%, respectively for electroporation)94. For most methods, effective delivery must be balanced with maintenance of cell viability to provide a scalable solution (Avectas is also developing a closed continuous system for GMP manufacturing). A further avenue for efficient cell engineering is rooted in nanoparticle research; in fact, it has been shown that biodegradable polymeric nanoparticles can transfect large quantities of cells95. Although each approach is often dependent on cell type and purpose, the transfection utility of physiologically relevant cells remains a challenge, with critical bottlenecks being the achievement of reproducibly efficient transfection, low cytotoxicity and high-throughput output.
Reducing the cost of goods for cell manufacturing
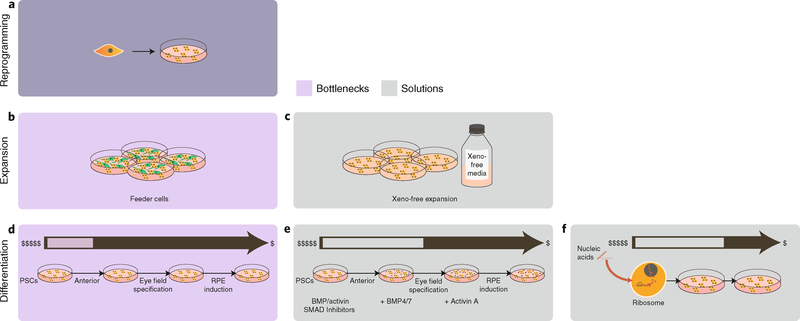
In 2007, human fibroblasts were genetically reprogrammed to induced pluripotent stem cells (iPSCs) by using a combination of transcription factors96. iPSCs are similar to embryonic stem cells (ESCs) in growth characteristics and requirements, in pluripotency and in ability to be reprogrammed into a variety of adult somatic cells, and have significant advantages regarding ethical considerations, ease of derivation and, perhaps, ultimate utility. However, on the one hand, each ESC line derived from an embryo has a unique presentation of major histocompatibility complexes and other antigens; therefore, from the perspective of regenerative medicine and transplantation, the use of somatic cells differentiated from third-party ESCs can raise the chances of rejection by recipients of the cells. On the other hand, because GMP-grade iPSCs can be derived from autologous somatic cells, rejection issues arising from differentiated cells from iPSCs can be mitigated97,98 (Fig. 5a). There have been proposals to establish ESC cell banks (haplobanks) that are representative of the major histocompatibility antigens observed in different populations. This could represent a solution to transplant-rejection issues in large numbers of people and to concerns about the immediate availability of ESCs for differentiation into various tissue types99. Differentiated iPSCs may also prove highly useful in drug screening; for instance, collections of iPSC-derived differentiated cell lines could be assembled into subsets based on ethnicity and sex, two of the several factors that often confound drug development. And for diseases where genetic variation primarily determines disease occurrence and progression, rather than grouping samples on the basis of potentially imprecise clinical diagnoses, genotypically distinct groups of iPSCs could also be assembled. Such an approach may be especially useful for neurological and neuropsychiatric disorders, given that fresh brain tissue is generally unavailable and that these conditions often arise from multiple and genetically distinct causes100–102.
Fig. 5 |. Overview of current tools for differentiating PSCs into retinal and neuronal lineages.
a, PSCs are initially seeded in cell-culture plates. b, Feeder cells, required to maintain proper PSC propagation, are constrained by having to maintain adequate media nutrients (which usually results in frequent media changes). c, The use of partially defined xeno-free media with the required growth factors necessary for lineage-specific cell culture removes the need for the feeder cells. d, Current state-of-the-art processes for differentiation (for example, PSCs that undergo anterior neuroectoderm differentiation and that need to undergo eye field specification before being induced as retinal pigment epithelial (RPE) cells) are expensive and time-consuming, requiring frequent media changes with specific growth factors for several months. e, The use of small molecules (bone morphogenetic protein (BMP), activin and SMAD inhibitors) greatly reduces costs at each differentiation step compared with the use of growth factors, and improves culture efficiency. f, The use of regulatory RNAs could further reduce culture costs by reducing the number of differentiation steps.
These applications all speak to the potential of iPSCs. Yet the cost of goods sold for producing CTPs via derivation of fully differentiated cells can be large. Generating a cell bank for iPSCs is a manual and labour-intensive task that would involve large human-capital costs. Each reprogrammed and clinically useful iPSC population can take several months to develop from a collection of primary cells. Approximately 200–300 vials are recommended at approximately 2 × 106 cells per vial to have a sufficient number of iPSCs to amplify the starting material and produce final products. A few public reports have stated that each cell line costs approximately US$10,000–20,000 to produce and validate103, with additional costs of meeting current GMP requirements ranging between US$50,000 and US$100,000 per cell line104. Product-development costs are even higher (~US$800,000) for generating an iPSC-derived tissue product that is suitable for clinical use103. These numbers all exclude the large start-up costs of personnel, facility and specialized equipment for iPSC manufacture. Although iPSCs for autologous therapy may seem like an alternative approach, the same issues of capped scale and individualized screening make autologous iPSC-based therapy difficult.
The costs of goods in cell manufacturing via derivation from iPSCs are strongly associated with the amount of cell processing and purification required, beginning with the derivation and maintenance of the iPSCs themselves. In contrast to the manufacturing of CAR-T cells, the generation of iPSCs only requires the transient expression of reprogramming genes. iPSCs have been derived from many adult cell types through the introduction of reprogramming factors by Sendai virus vectors (a cytoplasmic RNA virus vector), episomal vectors, messenger RNA transfection105–107 and other methods, none of which require vector integration into the host genomic DNA, thus avoiding the threat of insertional mutagenesis. However, costs associated with producing these transgenes, in addition to the media conditions needed to engineer adult cells to iPSCs, are high. Once transfected, earlier protocols for initial propagation of iPSCs and ESCs used feeder layers (such as mouse embryonic fibroblasts) to support cell self-renewal. Creating a defined media (Fig. 5b,c) based on GMP and xeno-free reagents (such as mTeSR1 media, StemCell Tech) have minimized the labour used for manual colony picking and subculture108, although the media necessitates growth factors, which can rebalance those cost gains. Although iPSCs can be grown in defined media, the need for growth substrata (such as Matrigel) makes large-scale culture a challenge. Three-dimensional cultures in cellular aggregates or microcarriers — and by using rotating Erlenmeyer flasks, wave reactors, rotating wall bioreactors or stirred tank bioreactors, for example — are a logical evolution in stem cell cultivation methods. Although these systems allow for the large-scale expansion of cells, they also present new culture variables that must be taken into account to maintain stemness.
The differentiation process from either iPSCs or ESCs also needs optimizing for the use of defined cell lineages as CTPs. Knowledge from developmental biology can now be translated into practice with specific, defined growth media that recapitulates a lineage-specific differentiation process (Fig. 5d). Protocols for the production of differentiated cellular derivatives are in their infancy, requiring inordinate amounts of time and labour109, and often producing cells that are relatively immature. Current strategies typically involve supplying the starting population of pluripotent stem cells (PSCs) with the necessary factors for a specified lineage commitment. This can be achieved indirectly by recreating the properties of the 3D niche of the PSCs, or directly by feeding the required cytokines and growth factors to the cultured PSCs. Both these differentiation methods require long and expensive culture protocols, need an extensive list of cytokines and growth factors and long culture times (50–100 days) and demand frequent human handling before the cells become CTPs110. Apart from the direct tumorigenic risk of an undifferentiated PSC, the continuous culturing of iPSC and ESCs lines might introduce the accumulation of gross chromosomal alterations111 and small mutations in specific genes112,113. The derivation, characterization, differentiation and purification of iPSCs are not easily automated114.
Small molecules designed for directing PSC differentiation could offer a significant cost advantage over biologics and make the economics of PSC manufacturing and scale-up more compelling. However, the design of suitable small molecules requires an understanding of the regulatory networks governing lineage commitment (Fig. 5e). One way of tackling such wide-angled approaches to generating candidate targets involves the use of large-scale genomics and proteomics115. In fact, there have already been some successes in the use of small molecules for the directed differentiation of PSCs into committed cell lineages116,117. Also, the increasing ease of genetic manipulation enabled by CRISPR and transcription activator-like effector nucleases opens up the possibility of replacing the extrinsic control of directed differentiation with an intrinsic sequence of events118. For example, the introduction of tunable gene cassettes regulating developmental pathways reduces the need for the continuous supply of exogenous factors and their associated costs of goods sold. The exploitation of regulatory RNAs, which can be manufactured at low cost (Fig. 5f), is another exciting avenue towards realizing feasible CTP scale-up through reduced costs. MicroRNAs show promise in achieving directed differentiation119–121, and large-intergenic-non-coding RNAs are strongly implicated in the induction of pluripotency122. The efficiency of gene-delivery technology will definitely accelerate the introduction of these approaches in standard practice. Next-generation synthetic biology and gene-editing tools can also be aimed at creating new bioprocessing tools115. Improved methods and reagents should foster the creation of reproducible protocols for the continuous derivation of human differentiated cell types.
Cell-material composites for cell delivery
Cells can benefit from a delivery system that controls their introduction to, and interaction with, the human body. This can range from the use of extracorporeal cell bioreactors for continuous cell-blood interaction33,123,124 to the packaging of CTPs within implantable protective devices before patient administration125. One purpose of these devices is placing a protective barrier, typically permeable to small molecules and proteins yet impermeable to the host’s immune cells (Fig. 6a), between the cell therapeutic and the surrounding biological environment126. These barriers aim to improve in situ survival and function and reduce immune cell clearance. The scalability of the final product then becomes also contingent on device manufacturing. In what follows, we explore this point through a case study of immunoisolation and delivery of CTPs for type I diabetes.
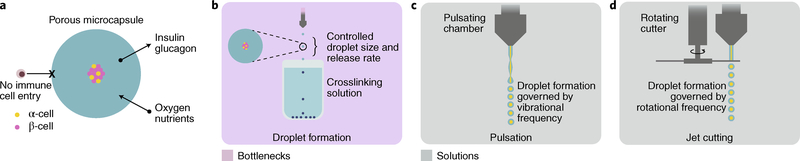
Fig. 6 |. Islet encapsulation.
a, Islet-entrapment devices, such as crosslinked alginate capsules, are semipermeable, protecting pancreatic islet cells from immune cells while still permitting oxygen and nutrients to enter, and insulin and glucagon to escape. b, A common method of encapsulation is the formation of droplets of polymer material and islet cells via extrusion dripping into a crosslinking solution. Although effective, this method is relatively low throughput. c, Pulsating extrusion heads increase the frequency of droplet formation proportionally to pulsation frequency. d, Jet-cutting technology mechanically creates droplets by passing a blade through a continuous extrusion stream, whereby droplet formation is proportional to the rotation frequency and the separation between blades.
Insulin replacement therapy for type I diabetes — an autoimmune disease in which insulin-producing β-cells in the pancreas are destroyed, resulting in pathologic blood glucose control127 — provides inefficient glycaemic control, with the risk of overdosing. An alternative strategy is to implant β-cells that respond to fluctuations in blood glucose levels and that secrete insulin in real time128–130. Humans require approximately a minimum of 7,000 islet equivalents (IEQs) per kg of recipient (hence, 490,000 IEQs for a recipient weighing 70 kg) to restore glycaemic control131. One limitation so far has been the source of islet cells, which have come from animal or cadaveric harvests with varying number and quality. Recent advances in controlled differentiation of PSCs into glucose-responsive β-like cells may provide a source for a limitless supply on the basis of small-scale production studies132,133. To date, several cell-formulation strategies for β-cell replacement therapy have been explored134; however, long-term clinical utility has not yet been demonstrated128,129,134. DIABECELL, an alginate-encapsulated neonatal porcine islet implant from Living Cell Technologies has been tested in phase I/II clinical trials with 10,000 IEQs kg−1, and has shown limited statistically significant efficacy in reducing glycated haemoglobin (HbA1c) levels in small sample populations135,136. A subcutaneously implanted, miniaturized (about the size of an ice-hockey puck and measuring 2.5 inches across) bioreactor with an oxygen-supply compartment and islets in an alginate-filled immune-protected compartment137–140 (βair, produced by Beta O2) has been used in early human trials with reported multi-month xenogeneic islet viability141. To maintain cell viability, the oxygen-supply compartment is refilled on a daily basis by connecting it to an external oxygen port through a polyurethane tube. Two other devices employing a prevascularization approach have been studied for subcutaneous implantation. Encaptra (produced by ViaCyte), which is currently undergoing phase I/II clinical trials62, has an immunoisolating barrier and a single chamber with Viacyte’s human ESC-derived pancreatic endoderm cells62, introduced after prevascularization of the implantation site. The Sernova Cell Pouch also needs a prevascularized implantation site, but not an immunoisolating membrane142. However, the Sernova Cell Pouch study recruited only three patients, and phase I/II clinical trials were terminated143 without results or patient complications being reported. It is likely that the study termination resulted from either a lack of efficacy, or from potential implementation challenges. These early clinical studies were conducted on small numbers of patients, and therefore scalability remains to be tested.
New polymers for cell encapsulation that improve cell-therapy performance can lead to scale-up challenges. In particular, implanted materials can induce a foreign-body response, resulting in fibrous encapsulation and in isolation of the cellular device144,145. A combinatorial chemistry approach developed to engineer new biomaterials led to a modified alginate that mitigated the foreign-body response and maintained islet viability for months146. The modified alginate entraps islets derived from stem cells and has shown long-term glycaemic control in immunocompetent murine models147. The alginate is first mixed with cells, and the solution can then be slowly extruded as droplets into a solution of divalent cations, which crosslink the gel network, entrapping the cells within it (Fig. 6b). Proof-of-concept studies with these laboratory-scale methods serve as initial-quality benchmarking tools in the effort to produce several GMP batches at higher scale. Synthesizing the modified alginate at high purity, volume and stable quality will be necessary to move forward into large clinical studies, with assurance that producing the material will not be a rate-limiting bottleneck in the manufacture of the final product.
Although the efficacy and performance of microencapsulated β-cell formulations continues to be investigated, their clinical translation will ultimately require advanced methods for formulation, shipping and storage. Ideally, high-throughput production of microencapsulated β-cell formulations should be developed to produce monodisperse and homogenous microcapsules within a narrow size distribution with high encapsulation efficiency and production rates, under mild and sterile conditions, and at low costs. Current candidate lead materials that can be integrated with high-throughput methods for the production of encapsulated cells are summarized in Supplementary Table 6. One potentially high-throughput approach for producing small monodisperse capsules involves pulsation of a laminar jet (Fig. 6c), nozzle vibration (laminar-jet breakup) or rotating-disk-and-jet-cutter technology (Fig. 6d). The laminar-jet-breakup technique uses axisymmetric disturbances to break the jet from the nozzle into equally sized droplets, and achieves production rates as high as 104 particles per second148. Vibration frequency, diameter of the nozzle, viscosity and flow rate of the polymer-cell suspension determine the size and production rate of the microcapsules. The jet-cutter technology provides a higher production rate, generating 500 μm particles with the encapsulated material at a rate of 104 particles per second (60 ml min−1; ref. 148). Compared with extrusion-drip methods, high-throughput capsule production by emulsification techniques is easier because they are not limited by scale. Appropriate dispersion devices and operating conditions (such as mixing rates and type of surfactants used) can lead to reductions in capsule size. Overall, both the high-throughput production of capsules and the synthesis of materials are bottlenecks to the development of scalable platforms.
Preservation and supply-chain management
Although shelf life in many early-phase trials conducted in hospital or academic settings is of limited concern, biopreservation will play a role in late-phase clinical trials and eventually in commercialization. Quality-by-design principles and preservation solutions that allow the product to meet critical quality attributes, such as potency, will have to be applied in late-phase trials, because starting and final product materials must be transported between clinical sites and manufacturing facilities. Biopreservation solutions that extend product shelf life also facilitate logistics, by maximizing manufacturing scheduling and patient-treatment possibilities.
Cryopreservation — the storage of cells at extremely low temperatures (−196 °C for liquid nitrogen and −156 °C for vapour nitrogen; storage in vapour is preferred so as to avoid contamination through a liquid phase149) — drastically minimizes metabolic activity and therefore preserves cell health. However, intracellular ice formation must be minimized to prevent cell rupture. The most-common cryoprotectant, dimethyl sulfoxide (DMSO), has been injected in patients at a dose of 1 g kg−1 day−1, and has been proven to be safe150,151. Although DMSO minimizes ice formation, it is also toxic to cells. Therefore, steps must be taken to minimize both contact time and osmotic shock152,153. Alternative cryoprotectant agents, including sugars such as trehalose154,155, polyvinylpyrrolidine156, methylcellulose, sucrose and glycerol, are less efficient than DMSO in sustaining viability157. However, sericin, a protein hydrolysate from the silk worm, has shown promising results in promoting encapsulated cell viability and cryopreservation as an alternative to serum components158. After DMSO has been added to the product, it should be frozen in a controlled manner to prevent loss of cell viability through undesired temperature gradients. In general, a rate of 1–2 °C min−1 is used in a controlled-rate freezer, although significant development work can be done to optimize the freezing curve for a specific product or container. Once a CTP is frozen, it is usually stored in liquid nitrogen, where it is tracked and stored. When needed, the product can be transported in a dry shipper, which can usually maintain the low temperatures of liquid nitrogen for a few days and up to two weeks.
Although cryopreservation has been used for years, challenges with post-stability cell function remain. The longest-standing example is the cryopreservation of CD34+ cells, which began with fetal-cord-blood banking, where cells are stored for long periods of time (decades) for potential use in future treatments. Although CD34+ cells have been cryopreserved for years, the impact of many factors remain to be understood, such as the time cells are in DMSO before cryopreservation, and the specific manner in which the DMSO is added to the cells. Also, many early-phase studies may be conducted with DMSO-containing cryomedia, usually made differently in each lab, which adds variability and cannot be well controlled. Non-standardized cryomedia may contain additives, such as plasma or serum, that are not sustainable. A cryoprotectant containing DMSO that is frequently used in cell therapies is CryoStor (BioLife Solutions). It is important to note that any singlesource supplier of a reagent in this relatively infant industry may be considered a supply-chain risk. As gene-modified CD34+ cells are now in clinical trials for a variety of haematological applications (such as sickle cell disease and beta thalassemia), the fragile nature of engineered cells must be recognized relative to cryopreservation to ensure an effective product. The thawing procedure then requires minimizing osmotic shock as much as possible. However, it is usually performed manually and therefore hard to control and standardize. Once thawed, cells are fragile and must be administered as soon as possible to avoid loss of viability. In cases where cryoinjury is a concern, as has been suggested for MSCs, cells are being thawed and re-cultured before administration to improve post-thaw function159. Yet in an ideal clinical setting, cells should not require washing or further handling before administration.
As a number of issues exist with cryopreservation, alternative solutions for preservation or storage of cell therapies, such as lyophilization, should be considered. Lyophilization has shown little success, yet it is claimed that Prestige Lyotechnology (Osiris) preserves cells at ambient temperatures, and that the Petaka culture plate (Celartia) maintains viability for up to two weeks of shipping or storage at ambient temperature. Another potential remedy is DMSO-free cryoprotectant, such as PRIME-XV FreezIS DMSO-Free (Irvine Scientific; marketed for MSCs), especially for cell types that have been shown to have reduced potency due to DMSO exposure. However, these cryoprotectants may be less effective in terms of cell recovery and must be tested for any specific CTP Hypothermic cell pausing, in which cells are held at ambient temperatures for short time periods, may also provide increased cell recovery with less logistical issues and lower cost compared with cryopreservation, and will depend on the hold period that meets demands160. Advances such as controlled thawing devices, such as ThawSTAR (BioCision) or VIA freeze (Asymptote), are automated systems for vials and bags that standardize the thawing process. As sustainability and standardization of biopreservation techniques increase, it is also important to recognize the strides made in shipping and storage of CTPs. So-called smart shippers (EVO Shipper, BioLife Solutions) can have a variety of sensors and make use of the global positioning system to track products. Automated liquid-nitrogen freezers can quickly retrieve products (Biostore III, Brooks), which can ensure traceability and remove any accidental thawing period associated with searching for products. There has been an emergence of new biopreservation solutions for CTPs, yet many concerns about cryopreservation methods remain.
Integration of the ever-increasing biopreserved CTPs into routine clinical use presents new supply-chain and logistical challenges. Although the need for environment and temperature-controlled systems for CTP preservation cannot be denied, efficient distribution, handling and chain-of-custody documentation is critical for large-scale commercialization. For all CTPs, a quality system must document the chain of custody, beginning with the collection of cellular material, followed by shipment and transit to the manufacturing facility, and ending with administration of the CTP to the patient (Fig. 7). The supply chain of CTPs is challenging because the processes occur in different facilities and are performed and handled by multiple individuals at different organizations. A robust procedure that standardizes the compilation and collection of the required data is therefore needed. For example, the EVO Shipper (BioLife Solutions) includes a cloud-based service that integrates thermal stability and precise data management for the end-to-end protection and visibility of CTPs between patient and manufacturing logistics, and the company Vineti (joint venture of GE and Mayo Clinic) provides transparency and visibility throughout the chain of custody, simplifying the process from collection and scheduling to infusion; it consolidates logistics, manufacturing and clinical data to improve patient safety and product performance.
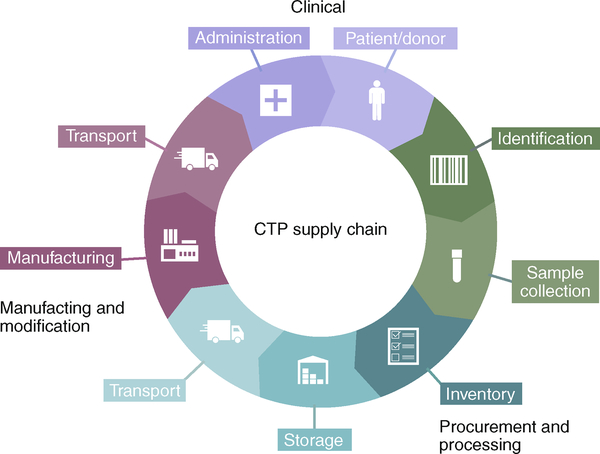
Fig. 7 |. Supply chain for CTPs.
The CTP supply chain is a complicated flow process comprising a series of dynamic components starting in a clinical environment, going through bioprocessing and then returning to the clinic. Initial seeding products are derived from patients or donors. These are screened for health and for safety (identification). Once cleared, sample collection begins. Proper inventory must then be made for tracking purposes. The cells are then put into storage (either short term or long term, depending on whether they are meant for banking or for immediate use). Transportation of the cells proceeds to the manufacturing facility, where purification, modification and/or expansion can take place. Once processing is complete, the product is moved onto the end location, for administration to the end patient. Supply-chain logistics are crucial to the overall success of CTPs.
Outlook
The first two commercial CAR-T cell products available in the United States — Kymriah (Novartis) and Yescarta (Gilead) — were approved in 2017 and followed in the footsteps of previously approved cell therapies, such as the stem cell gene therapy Strimvelis (GSK) for a rare disease (severe combined immunodeficiency due to adenosine deaminase deficiency) and the immunotherapy Provenge (Valeant) for prostate cancer. For these therapies and for other promising upcoming cell therapies, bioprocess engineering will make a significant impact in terms of product quality and costs. Scalability, with the hope that wide (commercial) dissemination and subsequently economies of scale make treatments affordable, should be considered, even for early-stage therapies undergoing proof-of-concept testing in humans. The costs of goods sold are becoming more visible, especially as new approvals have set initial price points ranging from US$93,000 to US$665,000 per treatment. As more commercial products are being placed on the market, reimbursement agencies are starting to paint a picture of cost against value. The industry is still immature with regard to pricing161. Reducing cost of goods sold is generally approached as an engineering exercise for improving the efficiency of existing bioprocesses, and is only heavily emphasized when a therapy is approaching pivotal clinical studies. A standard approach would involve deconstructing the manufacturing process into unit operations, and then analysing each unit operation against three sets of parameters: input materials, labour, and facility overheads and time (Fig. 8). In early manufacturing stages using inefficient bioprocesses, such as PSC differentiation, a primary component of costs lies in labour and facility overheads162. At later stages, significant reductions in costs of goods sold can be accomplished by using automated handling platforms and by defining process tolerances. A linear expansion of existing technology to meet commercial components of a target product profile is driven by reductions in cost of goods. Although innovations in bioprocess engineering could create new intellectual-property barriers, these could also significantly impact therapeutic adoption by offering a competitive cost/treatment equation.
Fig. 8 |. Segmented costs for translating cell therapeutics.
Costs associated with the development of cell therapies, broken down in steps, from manufacturing to commercialization.
Many current methods are immediately suitable for clinical testing, but will buckle under the burden of a full post-clinical-trial patient load. For successful manufacturing, four key attributes need to be considered: quality, cost of goods sold, scalability and sustainability. These four parameters should be worked into cell-therapy development to alleviate future issues associated with the scaling-up process. Automated processes can then amplify the gains made by the development of bioprocessing tools for reaching scalability and sustaining it while minimizing costs and the chances of error.
Supplementary Material
Acknowledgements
We thank a number of colleagues for feedback on a draft of the manuscript, specifically B. Hampson and T. Heathman from Hitachi Chemical Advanced Therapeutics Solutions. This work was supported in part by the Shriners Hospitals for Children (B.P.) and by the National Institutes of Health Grant R01EB012521 (B.P.).
Footnotes
Competing interests
A.A., M.L., O.S.F., D.K., M.V.M., J.R., J.T., R.M.O. and S.L. declare no competing interests. D.S., C.L. and R.P. are employees of Hitachi Chemical Advanced Therapeutics Solutions. R.D. owns equity in BlueRock Therapeutics. D.G.A. is a founder and equity shareholder in Siglion Therapeutics. R.N.B. is an employee and equity shareholder of Sentien Biotechnologies, Inc. B.P. is a founder and equity shareholder of Sentien Biotechnologies, Inc.
Additional information
Supplementary information is available for this paper at https://doi.org/10.1038/s41551-018-0246-6.
Reprints and permissions information is available at www.nature.com/reprints.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bianchi M et al. Restoration of NET formation by gene therapy in CGD controls aspergillosis. Blood 114, 2619 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grossman M et al. Successful ex vivo gene therapy directed to liver in a patient with familial hypercholesterolaemia. Nat. Genet 6, 335–341 (1994). [DOI] [PubMed] [Google Scholar]
- 3.Bainbridge JWB et al. Long-term effect of gene therapy on Leber’s congenital amaurosis. N. Engl. J. Med 372, 1887–1897 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett J et al. Safety and durability of effect of contralateral-eye administration of AAV2 gene therapy in patients with childhood-onset blindness caused by RPE65 mutations: a follow-on phase 1 trial. Lancet 388, 661–672 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark MA, Jepson MA & Hirst BH Exploiting M cells for drug and vaccine delivery. Adv. Drug Deliv. Rev 50, 81–106 (2001). [DOI] [PubMed] [Google Scholar]
- 6.Culme-Seymour EJ, Davie NL, Brindley DA, Edwards-Parton S & Mason C A decade of cell therapy clinical trials (2000–2010). Regen. Med 7, 455–462 (2012). [DOI] [PubMed] [Google Scholar]
- 7.National Cell Manufacturing Consortium Achieving Large-Scale, Cost- Effective, Reproducible Manufacturing of High-Quality Cells: A Technology Road Map to 2025 (Office of Science, Technology, and Policy, 2016); http://www.cellmanufacturingusa.org/sites/default/files/NCMC_Roadmap_021816_high_res-2.pdf
- 8.National Science and Technology Council Advanced Manufacturing: A Snapshot of Priority Technology Areas Across the Federal Government (Office of Science and Technology Policy, 2016). [Google Scholar]
- 9.Human Cells, Tissues, and Cellular and Tissue-based Products, FDA 21 CFR § 1271 (US Government Publishing Office, 2006). [Google Scholar]
- 10.Lipsitz YY, Timmins NE & Zandstra PW Quality cell therapy manufacturing by design. Nat. Biotechnol 34, 393–400 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Pritchett T & Little L Hard cell: potency testing for cellular therapy products. BioProcess Int. 10, 36–48 (2012). [Google Scholar]
- 12.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research Guidance for Industry: Potency Tests for Cellular and Gene Therapy Products (January, 2011).
- 13.Castrillo A, Cardoso M & Rouse L Treatment of buffy coat platelets in platelet additive solution with the mirasol® pathogen reduction technology system. Transfus. Med. Hemother 40, 44–48 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marschner S & Goodrich R Pathogen reduction technology treatment of platelets, plasma and whole blood using riboflavin and UV light. Transfus. Med. Hemother 38, 8–18 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang JF, Wu YF, Harrintong J & McNiece IK Ex vivo expansions and transplantations of mouse bone marrow-derived hematopoietic stem/ progenitor cells. J. Zhejiang Univ. Sci 5, 157–163 (2004). [DOI] [PubMed] [Google Scholar]
- 16.Angelopoulou M et al. Cotransplantation of human mesenchymal stem cells enhances human myelopoiesis and megakaryocytopoiesis in NOD/ SCID mice. Exp. Hematol 31, 413–420 (2003). [DOI] [PubMed] [Google Scholar]
- 17.Ball LM et al. Cotransplantation of ex vivo expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical hematopoietic stem-cell transplantation. Blood 110, 2764–2767 (2007). [DOI] [PubMed] [Google Scholar]
- 18.Le Blanc K et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 371, 1579–1586 (2008). [DOI] [PubMed] [Google Scholar]
- 19.Le Blanc K et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 363, 1439–1441 (2004). [DOI] [PubMed] [Google Scholar]
- 20.Ringden O et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation 81, 1390–1397 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Parekkadan B et al. Aire controls mesenchymal stem cell-mediated suppression in chronic colitis. Mol. Ther 20, 178–186 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parekkadan B, Tilles AW & Yarmush ML Bone marrow-derived mesenchymal stem cells ameliorate autoimmune enteropathy independently of regulatory T cells. Stem Cells 26, 1913–1919 (2008). [DOI] [PubMed] [Google Scholar]
- 23.Parekkadan B et al. Bone marrow stromal cell transplants prevent experimental enterocolitis and require host CD11b+ splenocytes. Gastroenterology 140, 966–975 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duijvestein M et al. Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn’s disease: results of a phase I study. Gut 59, 1662–1669 (2010). [DOI] [PubMed] [Google Scholar]
- 25.Semont A et al. Mesenchymal stem cells increase self-renewal of small intestinal epithelium and accelerate structural recovery after radiation injury. Adv. Exp. Med. Biol 585, 19–30 (2006). [DOI] [PubMed] [Google Scholar]
- 26.Shumakov VI, Onishchenko NA, Rasulov MF, Krasheninnikov ME & Zaidenov VA Mesenchymal bone marrow stem cells more effectively stimulate regeneration of deep burn wounds than embryonic fibroblasts. Bull. Exp. Biol. Med. 136, 192–195 (2003). [DOI] [PubMed] [Google Scholar]
- 27.Huang J et al. Genetic modification of mesenchymal stem cells overexpressing CCR1 increases cell viability, migration, engraftment, and capillary density in the injured myocardium. Circ. Res 106, 1753–1762 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noiseux N et al. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol. Ther 14, 840–850 (2006). [DOI] [PubMed] [Google Scholar]
- 29.Ortiz LA et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc. Natl Acad. Sci. USA 100, 8407–8411 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta N et al. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J. Immunol 179, 1855–1863 (2007). [DOI] [PubMed] [Google Scholar]
- 31.Lee JW et al. Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. Am. J. Respir. Crit. Care Med 187, 751–760 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matthay MA et al. Therapeutic potential of mesenchymal stem cells for severe acute lung injury. Chest 138, 965–972 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parekkadan B et al. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS ONE 2, e941 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Poll D et al. Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology 47, 1634–1643 (2008). [DOI] [PubMed] [Google Scholar]
- 35.Togel F et al. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am. J. Physiol. Ren. Physiol 289, F31–42 (2005). [DOI] [PubMed] [Google Scholar]
- 36.Elman JS et al. Pharmacokinetics of natural and engineered secreted factors delivered by mesenchymal stromal cells. PLoS ONE 9, e89882 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parekkadan B & Milwid JM Mesenchymal stem cells as therapeutics. Annu. Rev. Biomed. Eng 12, 87–117 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rowley J, Abraham E, Campbell A, Brandwein H & Oh S Meeting lot-size challenges of manufacturing adherent cells for therapy. BioProcess Int. 10, 7 (2012). [Google Scholar]
- 39.Lechanteur C et al. Clinical-scale expansion of mesenchymal stromal cells: a large banking experience. J. Transl. Med 14, 145 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones M et al. Genetic stability of bone marrow-derived human mesenchymal stromal cells in the quantum system. Cytotherapy 15, 1323–1339 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanley PJ et al. Efficient manufacturing of therapeutic mesenchymal stromal cells with the use of the quantum cell expansion system. Cytotherapy 16, 1048–1058 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schnitzler AC et al. Bioprocessing of human mesenchymal stem/stromal cells for therapeutic use: current technologies and challenges. Biochem. Eng. J 108, 3–13 (2016). [Google Scholar]
- 43.Rafiq QA, Coopman K & Hewitt CJ Scale-up of human mesenchymal stem cell culture: current technologies and future challenges. Curr. Opin. Chem. Eng 2, 8–16 (2013). [Google Scholar]
- 44.Estrada JC et al. Culture of human mesenchymal stem cells at low oxygen tension improves growth and genetic stability by activating glycolysis. Cell Death Differ. 19, 743–755 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sieblist C et al. Insights into large-scale cell-culture reactors: II. Gas-phase mixing and CO2 stripping. Biotechnol. J 6, 1547–1556 (2011). [DOI] [PubMed] [Google Scholar]
- 46.Nienow AW Reactor engineering in large scale animal cell culture. Cytotechnology 50, 9–33 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan KY, Reuveny S & Oh SKW Recent advances in serum-free microcarrier expansion of mesenchymal stromal cells: parameters to be optimized. Biochem. Biophys. Res. Commun 473, 769–773 (2016). [DOI] [PubMed] [Google Scholar]
- 48.dos Santos F et al. Toward a clinical-grade expansion of mesenchymal stem cells from human sources: a microcarrier-based culture system under xeno-free conditions. Tissue Eng. Part C Methods 17, 1201–1210 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cunha B et al. Exploring continuous and integrated strategies for the up- and downstream processing of human mesenchymal stem cells. J. Biotechnol 213, 97–108 (2015). [DOI] [PubMed] [Google Scholar]
- 50.Rafiq QA, Brosnan KM, Coopman K, Nienow AW & Hewitt CJ Culture of human mesenchymal stem cells on microcarriers in a 5 l stirred-tank bioreactor. Biotechnol. Lett 35, 1233–1245 (2013). [DOI] [PubMed] [Google Scholar]
- 51.Yang HS, Jeon O, Bhang SH, Lee SH & Kim BS Suspension culture of mammalian cells using thermosensitive microcarrier that allows cell detachment without proteolytic enzyme treatment. Cell Transplant. 19, 1123–1132 (2010). [DOI] [PubMed] [Google Scholar]
- 52.Shekaran A et al. Biodegradable ECM-coated PCL microcarriers support scalable human early MSC expansion and in vivo bone formation. Cytotherapy 18, 1332–1344 (2016). [DOI] [PubMed] [Google Scholar]
- 53.Hanga MP et al. Expansion of bone marrow-derived human mesenchymal stem/stromal cells (hMSCs) using a two-phase liquid/liquid system. J. Chem. Technol. Biotechnol 92, 1577–1589 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sart S, Tsai A-C, Li Y & Ma T Three-dimensional aggregates of mesenchymal stem cells: cellular mechanisms, biological properties, and applications. Tissue Eng. Part B Rev 20, 365–380 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dykes J, Lenshof A, Astrand-Grundstrom IB, Laurell T & Scheding S Efficient removal of platelets from peripheral blood progenitor cell products using a novel micro-chip based acoustophoretic platform. PLoS ONE 6, e23074 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lupu M, Gooley T, Zellmer E, Graves SS & Storb R Principles of peripheral blood mononuclear cell apheresis in a preclinical canine model of hematopoietic cell transplantation. J. Vet. Intern. Med 22, 74–82 (2008). [DOI] [PubMed] [Google Scholar]
- 57.The dose makes the poison. Nat. Nanotech 6, 329 (2011). [DOI] [PubMed] [Google Scholar]
- 58.Perin EC et al. A phase II dose-escalation study of allogeneic mesenchymal precursor cells in patients with ischemic or nonischemic heart failure. Circ. Res 117, 576–584 (2015). [DOI] [PubMed] [Google Scholar]
- 59.Hatch A, Hansmann G & Murthy SK Engineered alginate hydrogels for effective microfluidic capture and release of endothelial progenitor cells from whole blood. Langmuir 27, 4257–4264 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang BL et al. Microfluidic high-throughput culturing of single cells for selection based on extracellular metabolite production or consumption. Nat. Biotechnol 32, 473–478 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chabert M & Viovy J-L Microfluidic high-throughput encapsulation and hydrodynamic self-sorting of single cells. Proc. Natl Acad. Sci. USA 105, 3191–3196 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.A safety, tolerability, and efficacy study of VC-01™ combination product in subjects with type I diabetes mellitus. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/results/NCT02239354 (2015).
- 63.Hsu CH, Chen C, Irimia D & Toner M Fast sorting of CD4+ T cells from whole blood using glass microbubbles. Technology (Singap. World Sci.) 3, 38–44 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Toner M & Irimia D Blood-on-a-chip. Annu. Rev. Biomed. Eng 7, 77–103 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sethu P et al. Microfluidic isolation of leukocytes from whole blood for phenotype and gene expression analysis. Anal. Chem 78, 5453–5461 (2006). [DOI] [PubMed] [Google Scholar]
- 66.Simon MG et al. Increasing label-free stem cell sorting capacity to reach transplantation-scale throughput. Biomicrofluidics 8, 064106 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nakamura H & Funahashi J Electroporation: past, present and future. Dev. Growth Differ 55, 15–19 (2013). [DOI] [PubMed] [Google Scholar]
- 68.Zhao Y et al. Multiple injections of electroporated autologous T cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Cancer Res. 70, 9053–9061 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jin Z et al. The hyperactive Sleeping Beauty transposase SB100X improves the genetic modification of T cells to express a chimeric antigen receptor. Gene Ther. 18, 849–856 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Raja Manuri PV et al. piggyBac transposon/transposase system to generate CD19-specific T cells for the treatment of B-lineage malignancies. Hum. Gene Ther 21, 427–437 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saito S et al. Anti-leukemic potency of piggyBac-mediated CD19-specific T cells against refractory Philadelphia chromosome-positive acute lymphoblastic leukemia. Cytotherapy 16, 1257–1269 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ramanayake S et al. Low-cost generation of Good Manufacturing Practice-grade CD19-specific chimeric antigen receptor-expressing T cells using piggyBac gene transfer and patient-derived materials. Cytotherapy 17, 1251–1267 (2015). [DOI] [PubMed] [Google Scholar]
- 73.van der Loo JC & Wright JF Progress and challenges in viral vector manufacturing. Hum. Mol. Genet 25, R42–R52 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lim WA & June CH The principles of engineering immune cells to treat cancer. Cell 168, 724–740 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sadelain M CD19 CAR T cells. Cell 171, 1471 (2017). [DOI] [PubMed] [Google Scholar]
- 76.Neelapu SS et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med 377, 2531–2544 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lynn RC et al. High-affinity FRβ-specific CAR T cells eradicate AML and normal myeloid lineage without HSC toxicity. Leukemia 30, 1355–1364 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maus MV & June CH Making better chimeric antigen receptors for adoptive T-cell therapy. Clin. Cancer Res. 22, 1875–1884 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang B et al. The significance of controlled conditions in lentiviral vector titration and in the use of multiplicity of infection (MOI) for predicting gene transfer events. Genet. Vaccin. Ther 2, 6 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Eichler F et al. Hematopoietic stem-cell gene therapy for cerebral adrenoleukodystrophy. N. Engl. J. Med 377, 1630–1638 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thrasher AJ & Williams DA Evolving gene therapy in primary immunodeficiency. Mol. Ther 25, 1132–1141 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hacein-Bey-Abina S et al. A modified gamma-retrovirus vector for X-linked severe combined immunodeficiency. N. Engl. J. Med 371, 1407–1417 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu C & Dunbar CE Stem cell gene therapy: the risks of insertional mutagenesis and approaches to minimize genotoxicity. Front. Med 5, 356–371 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Singh N, Shi J, June CH & Ruella M Genome-editing technologies in adoptive T cell immunotherapy for cancer. Curr. Hematol. Malig. Rep 12, 522–529 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moore JC et al. Efficient, high-throughput transfection of human embryonic stem cells. Stem Cell Res. Ther 1, 23 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li LH et al. Rapid and efficient nonviral gene delivery of CD154 to primary chronic lymphocytic leukemia cells. Cancer Gene. Ther 13, 215–224 (2006). [DOI] [PubMed] [Google Scholar]
- 87.Li LH et al. Highly efficient, large volume flow electroporation. Technol. Cancer Res. Treat 1, 341–350 (2002). [DOI] [PubMed] [Google Scholar]
- 88.Fratantoni JC, Dzekunov S, Singh V & Liu LN A non-viral gene delivery system designed for clinical use. Cytotherapy 5, 208–210 (2003). [DOI] [PubMed] [Google Scholar]
- 89.Beatty GL et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol. Res 2, 112–120 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kebriaei P et al. Phase I trials using Sleeping Beauty to generate CD19-specific CAR T cells. J. Clin. Invest. 126, 3363–3376 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ren J et al. Multiplex genome editing to generate universal CAR T cells resistant to PD1 inhibition. Clin. Cancer Res. 23, 2255–2266 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sharei A et al. Cell squeezing as a robust, microfluidic intracellular delivery platform. J. Vis. Exp 81, e50980 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim JA et al. A novel electroporation method using a capillary and wire-type electrode. Biosens. Bioelectron 23, 1353–1360 (2008). [DOI] [PubMed] [Google Scholar]
- 94.O’Dea S et al. Vector-free intracellular delivery by reversible permeabilization. PLoS ONE 12, e0174779 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang F et al. Genetic engineering of human stem cells for enhanced angiogenesis using biodegradable polymeric nanoparticles. Proc. Natl Acad. Sci. USA 107, 3317–3322 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Takahashi K et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007). [DOI] [PubMed] [Google Scholar]
- 97.Scudellari M How iPS cells changed the world. Nature 534, 310 (2016). [DOI] [PubMed] [Google Scholar]
- 98.Di Foggia V, Makwana P, Ali RR & Sowden JC Induced pluripotent stem cell therapies for degenerative disease of the outer retina: disease modeling and cell replacement. J. Ocul. Pharmacol. Ther 32, 240–252 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Focosi D et al. Induced pluripotent stem cells in hematology: current and future applications. Blood Cancer J. 4, e211 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Imaizumi Y & Okano H Modeling human neurological disorders with induced pluripotent stem cells. J. Neurochem 129, 388–399 (2014). [DOI] [PubMed] [Google Scholar]
- 101.Vaccarino FM et al. Annual research review: the promise of stem cell research for neuropsychiatric disorders. J. Child Psychol. Psychiatry 52, 504–516 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Haggarty SJ, Silva MC, Cross A, Brandon NJ & Perlis RH Advancing drug discovery for neuropsychiatric disorders using patient-specific stem cell models. Mol. Cell. Neurosci 73, 104–115 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bravery CA Do human leukocyte antigen-typed cellular therapeutics based on induced pluripotent stem cells make commercial sense? Stem Cells Dev. 24, 1–10 (2015). [DOI] [PubMed] [Google Scholar]
- 104.Jacquet L et al. Strategy for the creation of clinical grade hESC line banks that HLA-match a target population. EMBO Mol. Med 5, 10–17 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fusaki N, Ban H, Nishiyama A, Saeki K & Hasegawa M Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc. Jpn Acad. Ser. B Phys. Biol. Sci 85, 348–362 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yu J et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science 324, 797–801 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sridhar A, Ohlemacher SK, Langer KB & Meyer JS Robust differentiation of mRNA-reprogrammed human induced pluripotent stem cells toward a retinal lineage. Stem Cells Transl. Med 5, 417–426 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jenkins MJ & Farid SS Human pluripotent stem cell-derived products: advances towards robust, scalable and cost-effective manufacturing strategies. Biotechnol. J 10, 83–95 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lin H, Li Q & Lei Y An integrated miniature bioprocessing for personalized human induced pluripotent stem cell expansion and differentiation into neural stem cells. Sci. Rep 7, 40191 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Serra M, Brito C, Correia C & Alves PM Process engineering of human pluripotent stem cells for clinical application. Trends Biotechnol. 30, 350–359 (2012). [DOI] [PubMed] [Google Scholar]
- 111.Hong SG, Dunbar CE & Winkler T Assessing the risks of genotoxicity in the therapeutic development of induced pluripotent stem cells. Mol. Ther 21, 272–281 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nguyen HT, Geens M & Spits C Genetic and epigenetic instability in human pluripotent stem cells. Hum. Reprod. Update 19, 187–205 (2013). [DOI] [PubMed] [Google Scholar]
- 113.Lund RJ, Narva E & Lahesmaa R Genetic and epigenetic stability of human pluripotent stem cells. Nat. Rev. Genet 13, 732–744 (2012). [DOI] [PubMed] [Google Scholar]
- 114.Trainor N, Pietak A & Smith T Rethinking clinical delivery of adult stem cell therapies. Nat. Biotechnol. 32, 729 (2014). [DOI] [PubMed] [Google Scholar]
- 115.Lipsitz YY, Timmins NE & Zandstra P W. Quality cell therapy manufacturing by design. Nat. Biotechnol 34, 393–400 (2016). [DOI] [PubMed] [Google Scholar]
- 116.Choudhary P et al. Directing differentiation of pluripotent stem cells toward retinal pigment epithelium lineage. Stem Cells Transl. Med 6, 490–501 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ding S et al. Synthetic small molecules that control stem cell fate. Proc. Natl Acad. Sci. USA 100, 7632–7637 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Saxena P et al. A programmable synthetic lineage-control network that differentiates human IPSCs into glucose-sensitive insulin-secreting beta-like cells. Nat. Commun 7, 11247 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kamat V et al. MicroRNA screen of human embryonic stem cell differentiation reveals miR-105 as an enhancer of megakaryopoiesis from adult CD34+ cells. Stem Cells 32, 1337–1346 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yu Z, Li Y, Fan H, Liu Z & Pestell R miRNAs regulate stem cell self-renewal and differentiation. Front. Genet 3, 191 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jiang C et al. MicroRNA-184 promotes differentiation of the retinal pigment epithelium by targeting the AKT2/mTOR signaling pathway. Oncotarget 7, 52340–52353 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Loewer S et al. Large intergenic non-coding RNA-RoR modulates reprogramming of human induced pluripotent stem cells. Nat. Genet. 42, 1113–1117 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li M et al. Phenotypic and functional characterization of human bone marrow stromal cells in hollow-fibre bioreactors. J. Tissue Eng. Regen. Med 6, 369–377 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yagi H et al. Long-term superior performance of a stem cell/hepatocyte device for the treatment of acute liver failure. Tissue Eng. Part A 15, 3377–3388 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.de Vos P et al. Multiscale requirements for bioencapsulation in medicine and biotechnology. Biomaterials 30, 2559–2570 (2009). [DOI] [PubMed] [Google Scholar]
- 126.Rokstad AMA, Lacik I, de Vos P & Strand BL Advances in biocompatibility and physico-chemical characterization of microspheres for cell encapsulation. Adv. Drug Deliv. Rev 67–68, 111–130 (2014). [DOI] [PubMed] [Google Scholar]
- 127.National Diabetes Statistics Report, 2014: Estimates of Diabetes and its Burden in the United States (Center for Disease Control and Prevention, 2014). [Google Scholar]
- 128.Qi M Transplantation of encapsulated pancreatic islets as a treatment for patients with type 1 diabetes mellitus. Adv. Med 2014, 429710 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Desai T & Shea LD Advances in islet encapsulation technologies. Nat. Rev. Drug Discov 16, 338–350 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Scharp DW & Marchetti P Encapsulated islets for diabetes therapy: history, current progress, and critical issues requiring solution. Adv. Drug Deliv. Rev 67–68, 35–73 (2014). [DOI] [PubMed] [Google Scholar]
- 131.Shapiro A et al. Islet transplantation in type 1 diabetes: ongoing challenges, refined procedures, and long-term outcome. Rev. Diabet. Stud 9, 385–406 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hrvatin S et al. Differentiated human stem cells resemble fetal, not adult, β cells. Proc. Natl Acad. Sci. USA 111, 3038–3043 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pagliuca FW et al. Generation of functional human pancreatic β cells in vitro. Cell 159, 428–439 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.O’Sullivan ES, Vegas A, Anderson DG & Weir GC Islets transplanted in immunoisolation devices: a review of the progress and the challenges that remain. Endocr. Rev 32, 827–844 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Open-label investigation of the safety and effectiveness of DIABECELL(R) in patients with type I diabetes mellitus. ClinicalTrials.govClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT00940173 (2014).
- 136.Open-label investigation of the safety and efficacy of DIABECELL in patients with type 1 diabetes mellitus. ClinicalTrials.govClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT01736228 (2015).
- 137.Barkai U et al. Enhanced oxygen supply improves islet viability in a new bioartificial pancreas. Cell Transplant. 22, 1463–1476 (2013). [DOI] [PubMed] [Google Scholar]
- 138.Neufeld T et al. The efficacy of an immunoisolating membrane system for islet xenotransplantation in minipigs. PLoS ONE 8, e70150 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ludwig B et al. Transplantation of human islets without immunosuppression. Proc. Natl Acad. Sci. USA 110, 19054–19058 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ludwig B et al. A novel device for islet transplantation providing immune protection and oxygen supply. Horm. Metab. Res 42, 918–922 (2010). [DOI] [PubMed] [Google Scholar]
- 141.Ludwig B et al. Islet transplantation at the Dresden diabetes center: five years’ experience. Horm. Metab. Res 47, 4–8 (2015). [DOI] [PubMed] [Google Scholar]
- 142.Pepper AR et al. Diabetes is reversed in a murine model by marginal mass syngeneic islet transplantation using a subcutaneous cell pouch device. Transplantation 99, 2294–2300 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.A phase I/II study of the safety and efficacy of Sernova’s Cell Pouch™ for therapeutic islet transplantation ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT01652911 (2016).
- 144.Tuch BE et al. Safety and viability of microencapsulated human islets transplanted into diabetic humans. Diabetes Care 32, 1887–1889 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.de Groot M, Schuurs TA & van Schilfgaarde R Causes of limited survival of microencapsulated pancreatic islet grafts. J. Surg. Res 121, 141–150 (2004). [DOI] [PubMed] [Google Scholar]
- 146.Vegas AJ et al. Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates. Nat. Biotechnol 34, 345–352 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Vegas AJ et al. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat. Med 22, 306–311 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Martin-Banderas L et al. Making drops in microencapsulation processes. Lett. Drug Des. Discov. 7, 300–309 (2010). [Google Scholar]
- 149.Hunt CJ Cryopreservation of human stem cells for clinical application: a review. Transfus. Med. Hemother 38, 107–123 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sauer-Heilborn A, Kadidlo D & McCullough J Patient care during infusion of hematopoietic progenitor cells. Transfusion 44, 907–916 (2004). [DOI] [PubMed] [Google Scholar]
- 151.Khera N et al. Limiting the daily total nucleated cell dose of cryopreserved peripheral blood stem cell products for autologous transplantation improves infusion-related safety with no adverse impact on hematopoietic engraftment. Biol. Blood Marrow Transplant 18, 220–228 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Best BP Cryoprotectant toxicity: facts, issues, and questions. Rejuvenation Res 18, 422–436 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Song YS et al. Microfluidics for cryopreservation. Lab Chip 9, 1874–1881 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Buchanan SS et al. Cryopreservation of stem cells using trehalose: evaluation of the method using a human hematopoietic cell line. Stem Cells Dev. 13, 295–305 (2004). [DOI] [PubMed] [Google Scholar]
- 155.Eroglu A et al. Intracellular trehalose improves the survival of cryopreserved mammalian cells. Nat. Biotechnol 18, 163–167 (2000). [DOI] [PubMed] [Google Scholar]
- 156.Thirumala S, Wu X, Gimble JM & Devireddy RV Evaluation of polyvinylpyrrolidone as a cryoprotectant for adipose tissue-derived adult stem cells. Tissue Eng. Part C Methods 16, 783–792 (2010). [DOI] [PubMed] [Google Scholar]
- 157.Shivakumar SB et al. Cryopreservation of human Wharton’s jelly-derived mesenchymal stem cells following controlled rate freezing protocol using different cryoprotectants; a comparative study. Int. J. Stem Cells 8, 155–169 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Miyamoto Y et al. Cryopreservation of human adipose tissue-derived stem/progenitor cells using the silk protein sericin. Cell Transplant. 21, 617–622 (2012). [DOI] [PubMed] [Google Scholar]
- 159.Moll G et al. Do cryopreserved mesenchymal stromal cells display impaired immunomodulatory and therapeutic properties? Stem Cells 32, 2430–2442 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Robinson NJ, Picken A & Coopman K Low temperature cell pausing: an alternative short-term preservation method for use in cell therapies including stem cell applications. Biotechnol. Lett 36, 201–209 (2014). [DOI] [PubMed] [Google Scholar]
- 161.Lipsitz YY et al. A roadmap for cost-of-goods planning to guide economic production of cell therapy products. Cytotherapy 19, 1383–1391 (2017). [DOI] [PubMed] [Google Scholar]
- 162.Hassan S et al. Allogeneic cell therapy bioprocess economics and optimization: downstream processing decisions. Regen. Med 10, 591–609 (2015). [DOI] [PubMed] [Google Scholar]
- 163.Porter DL, Levine BL, Kalos M, Bagg A & June CH Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med 365, 725–733 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.