Abstract
To investigate the effect of antidiabetic agents on nonalcoholic fatty liver disease (NAFLD) in patients with type 2 diabetes mellitus (T2DM), 75 patients with T2DM and NAFLD under inadequate glycemic control by metformin were randomized (1:1:1) to receive add‐on liraglutide, sitagliptin, or insulin glargine in this 26‐week trial. The primary endpoint was the change in intrahepatic lipid (IHL) from baseline to week 26 as quantified by magnetic resonance imaging–estimated proton density fat fraction (MRI‐PDFF). Secondary endpoints included changes in abdominal adiposity (subcutaneous adipose tissue [SAT] and visceral adipose tissue [VAT]), glycated hemoglobin, and body weight from baseline to week 26. We analysed data from intent‐to‐treat population. MRI‐PDFF, VAT, and weight decreased significantly with liraglutide (15.4% ± 5.6% to 12.5% ± 6.4%, P < 0.001; 171.4 ± 27.8 to 150.5 ± 30.8, P = 0.003; 86.6 ± 12.9 kg to 82.9 ± 11.1 kg, P = 0.005, respectively) and sitagliptin (15.5% ± 5.6% to 11.7% ± 5.0%, P = 0.001; 153.4 ± 31.5 to 139.8 ± 27.3, P = 0.027; 88.2 ± 13.6 kg to 86.5 ± 13.2 kg, P = 0.005, respectively). No significant change in MRI‐PDFF, VAT, or body weight was observed with insulin glargine. SAT decreased significantly in the liraglutide group (239.9 ± 69.0 to 211.3 ± 76.1; P = 0.020) but not in the sitagliptin and insulin glargine groups. Changes from baseline in MRI‐PDFF, VAT, and body weight were significantly greater with liraglutide than insulin glargine but did not differ significantly between liraglutide and sitagliptin. Conclusion: Combined with metformin, both liraglutide and sitagliptin, but not insulin glargine, reduced body weight, IHL, and VAT in addition to improving glycemic control in patients with T2DM and NAFLD.
Abbreviations
- AE
adverse event
- ANCOVA
analysis of covariance
- BMI
body mass index
- FPG
fasting plasma glucose
- GLP‐1
glucagon‐like peptide‐1
- GLP‐1RA
glucagon‐like peptide‐1 receptor agonist
- HbA1c
glycated hemoglobin
- IDEAL IQ
iterative decomposition of water and fat with echo asymmetry and least‐squares estimation
- IHL
intrahepatic lipid
- IL‐6
interleukin‐6
- MRI
magnetic resonance imaging
- MRI‐PDFF
magnetic resonance imaging–estimated proton density fat fraction
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- PPG
postprandial plasma glucose
- PRL
prolactin
- SAT
subcutaneous adipose tissue
- T2DM
type 2 diabetes mellitus
- VAT
visceral adipose tissue
Nonalcoholic fatty liver disease (NAFLD) encompasses a spectrum of liver disease, ranging from excessive deposition of fat within the liver to progressive inflammation and fibrosis, resulting in nonalcoholic steatosis (NASH). NAFLD affects 17% to 46% of adults worldwide, with prevalence varying according to diagnostic method, age, sex, and ethnicity.1, 2, 3, 4 NAFLD has a high prevalence in patients with type 2 diabetes mellitus (T2DM), varying among different populations.1, 2, 5, 6 It has also been reported that T2DM was identified in 23% of patients with NAFLD and 47% of patients with NASH.4
According to clinical practice guidelines for NAFLD management,1, 2, 7 contemporary treatment of NAFLD is aimed at weight loss through diet and lifestyle modification. However, no pharmacotherapies are approved for the treatment of NAFLD, let alone for patients with T2DM and NAFLD.
Metformin, recommended as the first‐line therapy for patients with T2DM worldwide,8 does not show a detectable histological effect on NAFLD.2, 9 So far, there is no evidence regarding the efficacy of add‐on oral agents in patients with T2DM with NAFLD inadequately controlled on metformin monotherapy.
In a randomized, double‐blind, placebo‐controlled trial conducted in patients with prediabetes or diabetes with NAFLD, sitagliptin showed no effect on liver fat compared to placebo.10 In the Liraglutide Efficacy and Action in NASH (LEAN) study, treatment with liraglutide for 48 weeks induced a significantly greater resolution of NASH and attenuated the evolution to fibrosis compared with placebo in 26 patients with NASH, including 9 patients with T2DM.11 However, in two randomized studies, liraglutide treatment did not reduce liver fat in patients with T2DM.12, 13 Only a few studies have investigated the effect of basal insulin on liver fat in NAFLD, and these studies have had controversial results.12, 14, 15
Therefore, we designed this 26‐week comparative trial, aiming to evaluate the efficacy of intrahepatic lipid (IHL), abdominal adiposity, and glycemic control; and the safety of subcutaneous liraglutide (1.8 mg/day), sitagliptin (100 mg/day), and insulin glargine (initiated at 0.2I U/kg/day) as an add‐on treatment to metformin in patients with T2DM with NAFLD.
Patients and Methods
Study Design
This 26‐week, open‐label, active‐controlled, parallel‐group, multicenter trial was conducted at 10 centers in China between August 2014 and December 2016. This trial (Light‐On; NCT02147925) conformed to the Declaration of Helsinki and good clinical practice guidelines, and the trial was approved by independent ethics committees. All patients gave written informed consent prior to trial‐related activities.
Patients
Patients aged 30‐75 years with T2DM and glycated hemoglobin (HbA1c) levels between 6.5% and 10% (inclusive) were eligible for the study if they had been treated with metformin monotherapy at a stable dose of ≥1,500 mg/day for at least 3 months and were clinically diagnosed with NAFLD.7 Additional eligibility criteria included a magnetic resonance imaging–estimated proton density fat fraction (MRI‐PDFF) >10%, body mass index (BMI) between 20 and 35 kg/m2, and history of stable body weight (≤10% variation for at least 3 months).
Key exclusion criteria included a diagnosis of type 1 diabetes mellitus; treatment with any antidiabetic agent other than metformin, or treatment with any other drugs associated with hepatic steatosis, including but not limited to glucocorticoids, tamoxifen, amiodarone, or methotrexate, within 3 months of screening; a history or current episode of pancreatitis or other pancreatic diseases; plasma alanine transaminase level >2.5 times the upper limit of normal; estimated glomerular filtration rate <60 mL/min/1.73 m2; a diagnosis of congestive heart failure (New York Heart Association Functional Classification III‐IV); any history of liver disease, including autoimmune liver diseases or viral hepatitis; a weekly alcohol intake of >14 units for women or >21 units for men; and pregnancy or plans to become pregnant.
Procedures
After a 2‐week screening, eligible patients were randomized 1:1:1 to receive either subcutaneous liraglutide (Victoza; Novo Nordisk, Bagsvaerd, Denmark) 1.8 mg once daily, oral sitagliptin (Januvia; Merck & Co., Inc., Kenilworth, NJ) 100 mg once daily, or subcutaneous insulin glargine (Lantus; Sanofi, Bridgewater, NJ) at bedtime plus metformin (Glucophage; Bristol‐Myers Squibb Company, NJ) for 26 weeks (Fig. 1).
Figure 1.
Study design. Abbreviation: Met, metformin.
A randomization list was generated using Statistical Analysis System (SAS Institute, Inc., Cary, NC), and patients were allocated using a secure Oracle‐based interactive web response system (Jiaxing Taimei Medical Technology, Shanghai, China) in accordance with the sequence from the randomization list. After appropriate titrations, all dosages were sustained during the treatment period. All patients received diabetes education, which was routine clinical practice, including dietary and exercise suggestions according to China guidelines16 at enrollment, with reinforcement throughout the study.
Liraglutide was initiated at 0.6 mg/day and then increased by weekly forced titration to 1.8 mg/day or the maximum tolerated dose (at least 1.2 mg/day). Insulin glargine was started at 0.2 IU/kg/day and was then titrated by 2 to 6 units each day to achieve fasting plasma glucose (FPG) <7 mmol/L. Metformin was administered at a constant dose.
Assessments
At screening, all patients underwent a physical examination, ultrasound of the liver, and fasting blood sampling for biological measurements, including liver enzymes, FPG, plasma lipids, HbA1c, and 2‐hour postprandial glucose (PPG; after a mixed‐meal test, 162 kcal). All patients were tested for hepatitis B (HBsAg) and C (anti‐HCV).
At baseline (2 weeks after screening), eligible patients underwent abdominal magnetic resonance imaging (MRI) (GE Discovery 750 3.0T MR) to assess visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) in participant centers. IHL were assessed using MRI‐PDFF, accurately measured by MRI iterative decomposition of water and fat with echo asymmetry and least‐squares estimation (IDEAL IQ) (GE Discovery 750 3.0T MR), as a surrogate biomarker owing to its practicality, reliability, and transferability.17, 18, 19 Whole liver was covered during axial IDEAL IQ examination. The key protocol parameters were as follows: acquisition matrix = 160 × 160, echo time = 6, repetition time = 6 ms, flip angle = 3, field of vision = 400 mm, slice thickness = 10 mm, single breathhold with acquisition time = 19 seconds. Square regions of interest (ROIs) of 25 mm × 25 mm were manually placed on a single slice of the right posterior segment, right anterior segment, and left medial segment, respectively. Focal liver lesions, large vessels, artifacts, and bile duct were avoided. Measured fat fractions of the three ROIs were averaged to represent the fat fraction of the liver. In‐line postprocessing was automatically performed after IDEAL IQ scanning, and quantitative fat fraction map was generated in the image list. Images were then transferred to a workstation (AW 4.6, GE Medical System) for the measurement of hepatic fat. VAT and SAT were also measured by MRI IDEAL IQ. VAT and SAT were determined by measuring the mean areas of VAT and SAT of a region from 4 cm above to 4 cm below the fourth and fifth lumbar interspace. The images were also postprocessed on a GE workstation 4.6 (AW4.6, GE Medical System) with the Reformat software.
In addition to screening (week ‐2) and baseline (week 0) visits, patients visited the study centers at weeks 2, 4, 8, 12, 16, 20, and 26. At these visits, adverse events (AEs) were documented, and doses of liraglutide and insulin glargine were adjusted. After 26 weeks, treatment with the study drug (liraglutide, sitagliptin, or insulin glargine) was stopped for 2 days to avoid any acute drug effects on the collected data, and a final evaluation with a physical examination, fasting blood sampling for biological measurements, abdominal MRI to assess VAT and SAT, and MRI IDEAL IQ to accurately measure liver fat index was done.
Serum prolactin (PRL) was measured with radioimmunoassay method (kits from Beijing North Institute of Biological Technology, China, and XH6080 from Xi’an Nuclear Instrument Factory, China). Fasting insulin (FINS), adiponectin, and interleukin‐6 (IL‐6) were measured centrally at the Beijing North Institute of Biological Technology. Insulin resistance was measured by the homeostasis model assessment of insulin resistance (HOMA‐IR) index: HOMA‐IR = FINS (μIU/mL) × FBG (mmol/L)/22.5. Hepatic fibrosis was estimated at baseline and 26 weeks using validated formulae: FIB 4 index (FIB‐4) and NAFLD fibrosis score (NFS).1, 2, 20, 21
To ensure standardization, the same procedural instructions for MRI and IDEAL IQ were provided to all participating centers, and all scans were analyzed centrally by fully blinded specialists at the Third Affiliated Hospital of Sun Yat‐sen University (China).
Endpoints
The primary endpoint was the change in IHL from baseline to week 26 (end of treatment). Secondary endpoints included changes in abdominal adiposity (SAT and VAT), glycemia (HbA1c, FPG, and PPG), body weight, and BMI from baseline to week 26. Exploratory endpoints included changes in serum markers of fibrosis, inflammation, and PRL from baseline to week 26. Safety endpoints included hypoglycemic episodes, AEs, and serious AEs.
Statistical Analyses
The intent‐to‐treat population included all randomized patients, and the per‐protocol population included all eligible and treated patients without protocol violations that could potentially affect efficacy results. The safety population comprised all patients who received ≥1 dose of a study drug. The null hypothesis was that the treatment groups did not differ from each other with respect to the primary endpoint. A priori sample size calculations were based on the ability to detect an 18.6%, 18.6%, and 13.7% absolute clinical difference in liver fat before and after the intervention of liraglutide, sitagliptin, and insulin glargine, respectively.22 With a deviation estimate of 5.5% obtained from a similar study,22 we estimated that 22 patients in each group would be required, for a total of 66 patients (α = 0.05, β = 0.15). To account for a potential dropout rate of 10%, more than 74 patients should be enrolled. All 2‐sided tests were performed at a 5% significance level. Continuous endpoints were summarized by arithmetic means with SDs, and categorical endpoints were summarized by counts and percentages.
Efficacy endpoints were based on the intent‐to‐treat population, in which patients who did not have an end‐of‐treatment evaluation (including MRI IDEAL IQ to accurately measure IHL, VAT, and SAT) were included in the analysis and classified as having no improvement. Efficacy endpoints analyses were also repeated on the per‐protocol population. The primary and secondary endpoints (change from baseline after 26 weeks of treatment) were compared among three treatment groups using analysis of covariance (ANCOVA) with baseline values adjusted. Exploratory endpoints were compared among three treatment groups using analysis of variance (ANOVA). The primary endpoint (change from baseline in IHL after 26 weeks of treatment) was reanalyzed among three treatment groups using ANCOVA adjusting for change in body weight (Δweight) from baseline to the end of 26 weeks of treatment. The characteristics at baseline (Table 1) were compared among three treatment groups using ANOVA. A paired t test was used to compare the values between baseline and after treatment. Across treatment groups, continuous variables of baseline indices among groups and changes of values from baseline to endpoint were compared by ANCOVA with baseline values adjusted, respectively. Categorical variables (numbers of patients and AEs) were compared by the chi‐squared test. Spearman correlation was conducted to analyze the association of change of different variables. Multiple linear regression analysis was performed with the change of liver fat index (ΔMRI‐PDFF) as dependent variable and ΔHbA1c, Δweight, and the change of adipose tissue index (ΔSAT and ΔVAT) as independent variables to identify independent determinants of ΔMRI‐PDFF. Statistical analyses were performed using SPSS 23.0 software.
Table 1.
Baseline Characteristics of Trial Population
| Characteristic | Liraglutide (n = 24) | Sitagliptin (n = 27) | Insulin glargine (n = 24) |
|---|---|---|---|
| n (male/female) | 17/7 | 21/6 | 14/10 |
| Age (years) | 43.1 ± 9.7 | 45.7 ± 9.2 | 45.6 ± 7.6 |
| Duration of T2DM (years) | 3.3 ± 3.5 | 4.3 ± 3.8 | 5.8 ± 4.5 |
| Weight (kg) | 86.6 ± 12.9 | 88.2 ± 13.6 | 85.6 ± 14.2 |
| BMI (kg/m2) | 30.1 ± 3.3 | 29.7 ± 2.8 | 29.6 ± 3.5 |
| Waist (cm) | 101.7 ± 7.9 | 102.8 ± 8.3 | 102.9 ± 9.9 |
| SBP (mm Hg) | 125.2 ± 7.6 | 124.9 ± 10.7 | 126.9 ± 7.9 |
| DBP (mm Hg) | 78.1 ± 7.3 | 82.3 ± 7.6 | 83.5 ± 8.3 |
| AST (mmol/L) | 31.1 ± 11.7 | 34.4 ± 16.9 | 33.2 ± 17.4 |
| ALT (mmol/L) | 43.2 ± 21·2 | 46.0 ± 25.5 | 39.5 ± 25.7 |
| FPG (mmol/L) | 8.6 ± 2.8 | 8.4 ± 2.5 | 8.9 ± 2.2 |
| PPG (mmol/L) | 13.2 ± 3.1 | 13.7 ± 3.7 | 14.6 ± 3.9 |
| HbA1c | 7.8% ± 1.4% | 7.6% ± 0.9% | 7.7% ± 0.9% |
| TC (mmol/L) | 4.4 ± 0.9 | 4.9 ± 1.2 | 4.7 ± 1.2 |
| TG (mmol/L) | 2.3 ± 1.1 | 2.6 ± 1.4 | 2.9 ± 2.3 |
| LDL‐C (mmol/L) | 2.7 ± 0.8 | 3.1 ± 0.7 | 2.6 ± 1.0 |
| HDL‐C (mmol/L) | 1.1 ± 0.2 | 1.2 ± 0.6 | 1.1 ± 0.4 |
| MRI‐PDFF | 15.4% ± 5.6% | 15.5% ± 5.6% | 14.9% ± 5.5% |
| SAT (cm2) | 239.9 ± 69.0 | 239.5 ± 69.3 | 212.7 ± 57.7 |
| VAT (cm2) | 171.4 ± 27.8 | 153.4 ± 31.5 | 188.4 ± 74.7 |
| FIB‐4 | 0.7 9± 0.31 | 0.98 ± 0.42 | 1.10 ± 0.62 |
| NFS | −0.78 ± 0.81 | −1.55 ± 0.78 | −0.95 ± 0.85 |
Values are presented as mean ± SD.
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; BMI, body mass index; DBP, diastolic blood pressure; FIB‐4, FIB4 Index; FPG, fasting plasma glucose; HbA1c, glycated haemoglobin A1c; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; MRI‐PDFF, magnetic resonance imaging–estimated proton density fat fraction; NFS, NAFLD Fibrosis Score; PPG, postprandial plasma glucose; SAT, subcutaneous adipose tissue; SBP, systolic blood pressure; SD, standard deviation; T2DM, type 2 diabetes mellitus; TC, total cholesterol; TG, triglyceride; VAT, visceral adipose tissue.
Results
Of the 105 patients screened between August 2014 and May 2016, 75 were randomly assigned to receive a study drug (liraglutide, 24; sitagliptin, 27; and insulin glargine, 24); these patients made up the intent‐to‐treat population. The 65 patients who completed the study (18, 26, and 21, respectively; Fig. 2) were included in the per‐protocol efficacy analyses. Six patients in the liraglutide group withdrew from the study (four lost to follow‐up, one for protocol violations, and one for AEs), one patient in the sitagliptin group was lost to follow‐up, and three patients in the insulin glargine group withdrew for protocol violations.
Figure 2.

Patient disposition. Abbreviations: ITT, intent‐to‐treat; PP, per‐protocol.
In the intent‐to‐treat population, 69.3% of patients were male, and the mean (± SD) duration of T2DM was 4.7 ± 4.1 years. The baseline characteristics were similar across treatment groups (Table 1). At randomization, mean (± SD) dosages of metformin were similar across treatment groups (liraglutide, 1,608.7 ± 210.9 mg; sitagliptin, 1,648.6 ± 232.7 mg; and insulin glargine, 1,673.9 ± 243.5 mg) (P = 0.63). The mean daily dosages were 1.7 ± 0.3 mg and 21.7 ± 9.5 IU at the end of the study in the liraglutide group and in the insulin glargine group, respectively.
The efficacy results presented here were based on the intent‐to‐treat population; similar findings were obtained from the per‐protocol population (data not shown).
Efficacy Endpoints
Primary Efficacy Endpoint
In the liraglutide and sitagliptin groups, MRI‐PDFF significantly decreased from baseline to week 26 (liraglutide, 15.4% ± 5.6% to 12.5% ± 6.4%, P < 0.001; and sitagliptin, 15.5% ± 5.6% to 11.7% ± 5.0%, P = 0.001) (Table 2; Fig. 3A). Although this change (ΔMRI‐PDFF) was greater with liraglutide than sitagliptin, it was not significantly different between the two groups (−4.0 vs. −3.8; P = 0.911). In contrast, MRI‐PDFF did not change significantly from baseline in the insulin glargine group. ΔMRI‐PDFF in the liraglutide group was significantly higher than in the insulin glargine group (−4.0 vs. −0.8; P = 0.039), and ΔMRI‐PDFF in the sitagliptin group was also significantly higher than in the insulin glargine group (−3.8 vs. −0.8; P = 0.043).
Table 2.
Changes of Primary and Secondary Endpoint After 26 Weeks of Treatment With Liraglutide, Sitagliptin, or Insulin Glargine in Combination With Metformin
| Characteristic | Liraglutide (n = 24) | Sitagliptin (n = 27) | Insulin glargine (n = 24) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n (male/female) | 17/7 | 21/6 | 14/10 | ||||||
| Duration of T2DM (years) | 3.3 ± 3.5 | 4.3 ± 3.8 | 5.8 ± 4.5 | ||||||
| Before treatment | After treatment | Change (Δ) | Before treatment | After treatment | Change (Δ) | Before treatment | After treatment | Change (Δ) | |
| Weight (kg) | 86.6 ± 12.9* | 82.9 ± 11.1 | −3.6 ± 4.9† | 88.2 ± 13.6* | 86.5 ± 13.2 | −1.7 ± 2.9 | 85.6 ± 14.2 | 84.4 ± 14.6 | −1.2 ± 4.2 |
| BMI (kg/m2) | 30.1 ± 3.3* | 28.9 ± 2.9 | −1.1 ± 1.2 | 29.7 ± 2.9* | 29.2 ± 2.9 | −0.6 ± 0.9 | 29.6 ± 3.5 | 29.1 ± 3.8 | −0.4 ± 1.5 |
| Waist (cm) | 101.7 ± 7.9* | 99.0 ± 8.1 | −2.7 ± 3.9 | 102.8 ± 8.3* | 100.3 ± 8.0 | −2.5 ± 3.6 | 102.9 ± 9.9* | 100.7 ± 11.5 | −2.2 ± 4.7 |
| SBP (mm Hg) | 125.2 ± 7.6 | 121.7 ± 9.4 | −3.5 ± 8.6 | 124.9 ± 10.7 | 123.4 ± 13.0 | −1.6 ± 10.3 | 126.9 ± 7.9 | 127.5 ± 13.9 | 0.6 ± 11.1 |
| DBP (mm Hg) | 78.1 ± 7.3 | 78.7 ± 6.9 | 0.6 ± 7.5 | 82.3 ± 7.6* | 78.7 ± 7.9 | −3.6 ± 8.7 | 83.5 ± 8.3 | 83.9 ± 9.3 | 0.4 ± 7.7 |
| AST (mmol/L) | 31.1 ± 11.7 | 29.5 ± 13.2 | −1.8 ± 9.3 | 34.4 ± 16.9* | 25.7 ± 10.9 | −8.7 ± 19.2 | 33.2 ± 17.4 | 30.3 ± 18.9 | −2.9 ± 23.4 |
| ALT (mmol/L) | 43.2 ± 21.2 | 37.9 ± 20.7 | −5.2 ± 12.5 | 46.0 ± 25.5* | 34.8 ± 20.1 | −11.2 ± 25.7 | 39.5 ± 25.7 | 35.8 ± 20.6 | −0.8 ± 18.2 |
| FPG (mmol/L) | 8.6 ± 2.8* | 7.3 ± 2.6 | −1.3 ± 1.7 | 8.4 ± 2.5 | 7.6 ± 2.0 | −0.8 ± 2.2 | 8.9 ± 2.2 | 8.4 ± 2.2 | −0.4 ± 2.0 |
| PPG (mmol/L) | 13.2 ± 3.1* | 11.1 ± 3.2 | −2.2 ± 2.5† | 13.7 ± 3.7* | 11.9 ± 3.5 | −1.8 ± 2.9 | 14.6 ± 3.9 | 14.2 ± 4.0 | −0.3 ± 3.2 |
| HbA1c | 7.8% ± 1.4%‡ | 6.8% ± 1.7% | −1.0% ± 0.9% | 7.6% ± 0.9%* | 6.6% ± 1.1% | −1.0% ± 1.0% | 7.7% ± 0.9%* | 6.9% ± 1.1% | −0.7% ± 1.3% |
| TC (mmol/L) | 4.4 ± 0.9 | 4.5 ± 1.1 | 0.1 ± 0.8 | 4.9 ± 1.2 | 4.9 ± 1.2 | 0.03 ± 0.9 | 4.7 ± 1.2* | 5.3 ± 1.4 | 0.5 ± 1.1 |
| TG (mmol/L) | 2.3 ± 1.1 | 2.5 ± 1.4 | 0.2 ± 1.1 | 2.6 ± 1.4 | 2.6 ± 1.7 | −0.1 ± 1.9 | 2.9 ± 2.3 | 3.8 ± 3.6 | 0.9 ± 2.3 |
| LDL‐C (mmol/L) | 2.7 ± 0.8 | 2.7 ± 0.8 | −0.01 ± 0.6 | 3.1 ± 0.7 | 2.9 ± 0.8 | −0.1 ± 0.7 | 2.6 ± 1.0 | 2.9 ± 1.1 | 0.2 ± 0.7 |
| HDL‐C (mmol/L) | 1.1 ± 0.2 | 1.1 ± 0.2 | −0.01 ± 0.1 | 1.2 ± 0.6 | 1.1 ± 0.3 | −0.1 ± 0.6 | 1.1 ± 0.4 | 1.1 ± 0.4 | 0.1 ± 0.2 |
| MRI‐PDFF | 15.4% ± 5.6%‡ | 12.5% ± 6.4% | −4.0% ± 4.5%† | 15.5% ± 5.6%* | 11.7% ± 5.0% | −3.8% ± 5.0% | 14.9% ± 5.5% | 14.1% ± 7.3% | −0.8% ± 5.3% |
| SAT (cm2) | 239.9 ± 69.0* | 211.3 ± 76.1 | −28.6 ± 44.2† | 239.5 ± 69.3 | 230.2 ± 73.40 | −9.4 ± 29.1 | 212.7 ± 57.7 | 231.4 ± 65.6 | 18.6 ± 27.8 |
| VAT (cm2) | 171.4 ± 27.8* | 150.5 ± 30.8 | −20.9 ± 23.3† | 153.4 ± 31.5* | 139.8 ± 27.3 | −13.6 ± 21.2 | 188.4 ± 74.7 | 197.9 ± 73.5 | 9.5 ± 33.9 |
Values are presented as mean ± SD.
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; BMI, body mass index; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HbA1c, glycated haemoglobin A1c; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; MRI‐PDFF, magnetic resonance imaging–estimated proton density fat fraction; PPG, postprandial plasma glucose; SAT, subcutaneous adipose tissue; SBP, systolic blood pressure; T2DM, type 2 diabetes mellitus; TC, total cholesterol; TG, triglyceride; VAT, visceral adipose tissue.
P < 0.05, comparison of data between groups before treatment and after treatment.
P < 0.05, comparison among three groups.
P < 0.001, comparison of data between groups before treatment and after treatment.
Figure 3.
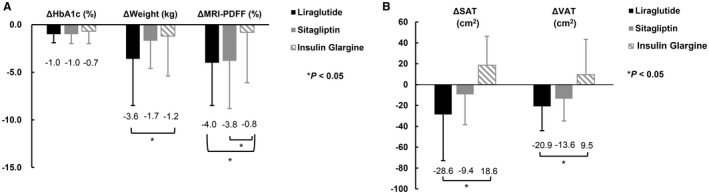
Changes of HbA1c, body weight, MRI‐PDFF, SAT and VAT from baseline to end of treatment. Change from baseline to the end of treatment in (A) HbA1c, body weight, and MRI‐PDFF and (B) SAT and VAT. Abbreviations: Δ, change from baseline to end of treatment; HbA1c, glycated hemoglobin; MRI‐PDFF, magnetic resonance imaging–estimated proton density fat fraction; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
After adjusting for Δweight, ΔMRI‐PDFF in the liraglutide group was significantly higher than in the insulin glargine group (P = 0.042), and ΔMRI‐PDFF in the sitagliptin group was also significantly higher than in the insulin glargine group (P = 0.027).
Secondary Efficacy Endpoints
Significant changes from baseline to week 26 were observed with liraglutide, except for lipid profile (Table 2). Similar changes were also observed with sitagliptin, except for FPG and lipid profile. In contrast, treatment with insulin glargine did not significantly affect any secondary efficacy parameters, except for HbA1c levels and total cholesterol (Table 2).
SAT decreased significantly in the liraglutide group (239.9 ± 69.0 cm2 to 211.3 ±7 6.1 cm2; P = 0.020) but not in the sitagliptin and insulin glargine groups (Table 2). ΔSAT (from baseline to week 26) was significantly greater in the liraglutide group than in the insulin glargine group (−28.6 ± 44.2 vs. 18.6 ± 27.8; P = 0.003; Fig. 3B); however, no significant difference was observed between the liraglutide and sitagliptin groups or between the sitagliptin and insulin glargine groups (Fig. 3B). VAT significantly decreased in the liraglutide and sitagliptin groups (171.4 ± 27.8 to 150.5 ± 30.8, P = 0.003; and 153.4 ± 31.5 to 139.8 ± 27.3, P = 0.027, respectively; Table 2) but not in the insulin glargine group. ΔVAT was significantly greater in the liraglutide group than in the insulin glargine group (−20.9 ± 23.3 vs. 9.5 ± 33.9; P = 0.020; Fig. 3B); but no significant difference was found between the liraglutide and sitagliptin groups or the sitagliptin and insulin glargine groups.
HbA1c levels improved significantly in all three treatment groups (liraglutide, 7.8% ± 1.4% to 6.8% ± 1.7%, P < 0.001; sitagliptin, 7.6% ± 0.9% to 6.6% ± 1.1%, P = 0.016; and insulin glargine, 7.7% ± 0.9% to 6.9% ± 1.1%, P = 0.013; Table 2). However, ΔHbA1c did not significantly differ across treatment groups (Fig. 3A). Liraglutide treatment resulted in significant improvement in FPG (8.6 ± 2.8 mmol/L to 7.3 ± 2.6 mmol/L; P = 0.001) and PPG (13.2 ± 3.1 mmol/L to 11.1 ± 3.2 mmol/L; P = 0.001), whereas sitagliptin significantly improved PPG (13.7 ± 3.7 mmol/L to 11.9 ± 3.5 mmol/L; P = 0.005) but not FPG (8.4 ± 2.5 mmol/L to 7.6 ± 2.0 mmol/L; P = 0.305). ΔPPG significantly differed between the liraglutide and insulin glargine groups (−2.2 ± 2.5 mmol/L vs. −0. 3 ± 3.2 mmol/L; P = 0.005) and between the sitagliptin and insulin glargine groups (−1.8 ± 2.9 mmol/L vs. −0.3 ± 3.2 mmol/L; P = 0.029), but no differences were found between the liraglutide and sitagliptin groups or the sitagliptin and insulin glargine groups. ΔFPG did not significantly differ among the three groups (Table 2).
In the liraglutide and sitagliptin groups, significant decreases in body weight (liraglutide, 86.6 ± 12.9 kg to 82.9 ± 11.1 kg, P = 0.005; and sitagliptin, 88.2 ± 13.6 kg to 86.5 ± 13.2 kg, P = 0.005) and BMI (liraglutide, 30.1 ± 3.3 kg/m2 to 28.9± 2.9 kg/m2, P = 0.001; and sitagliptin, 29.7 ± 2.9 kg/m2 to 29.2 ± 2.9 kg/m2, P = 0.006) were observed (Table 2). Neither body weight nor BMI showed a significant change with insulin glargine treatment (85.6 ± 14.2 kg to 84.4 ± 14.6 kg, P = 0.282; and 29.6 ± 3.5 kg/m2 to 29.1 ± 3.8 kg/m2, P = 0.254, respectively) (Table 2). The observed ∆weight was greater in the liraglutide group than in the insulin glargine group (−3.6 ± 4.9 kg vs. −1.2 ± 4.2 kg; P = 0.042), whereas no significant difference was seen between the liraglutide and sitagliptin groups or the sitagliptin and insulin glargine groups (Fig. 3A).
Correlation Analysis and Multiple Linear Regression Analysis
In intention‐to‐treat population, positive correlations were found between Δweight and ΔMRI‐PDFF (r = 0.31; P = 0.009), ΔSAT (r = 0.53; P < 0.001), and ΔVAT (r = 0.42; P = 0.006); between ΔMRI‐PDFF and ΔHbA1c (r = 0.37; P = 0.002); and between ΔMRI‐PDFF and ΔVAT (r = 0.49; P = 0.001); between ΔMRI‐PDFF and ΔSAT (r = 0.32; P = 0.044).
Multiple linear regression analysis revealed that Δweight was an independent determinant of ΔMRI–PDFF in T2DM with NAFLD (β = 0.488; 95% confidence interval, 0.046‐0.930; P = 0.031) after adjusting for the effects of antidiabetic agents, ΔHbA1c, ΔSAT, and ΔVAT, whereas the other indices did not show similar association with ΔMRI–PDFF after adjusting for other confounding factors (Table 3).
Table 3.
Multiple Linear Regression Analysis
| Factors | Partial regression coefficient (B) | 95% CI | P Value |
|---|---|---|---|
| ΔHbA1c | 0.799 | −0.506~2.103 | 0.222 |
| ΔWeight | 0.488 | 0.046~0.930 | 0.031 |
| ΔSAT | −0.044 | −0.091~0.004 | 0.073 |
| ΔVAT | 0.022 | −0.038~0.082 | 0.459 |
Multiple linear regression analysis adjusting for the other confounding factors.
Abbreviations: ΔHbA1c, change of glycated haemoglobin A1c; ΔSAT, change of subcutaneous adipose tissue; ΔVAT, change of visceral adipose tissue; ΔWeight, change of body weight; CI, confidence interval.
Exploratory Endpoints
Hepatic Fibrosis
There were no significant differences in FIB‐4 and NFS before and after treatment in the three groups (all P > 0.05) (Table 4).
Table 4.
Changes of Exploratory Endpoints After 26 Weeks of Treatment With Liraglutide, Sitagliptin, or Insulin Glargine in Combination With Metformin
| Exploratory endpoints | Liraglutide (n = 24) | Sitagliptin (n = 27) | Insulin glargine (n = 24) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Before treatment | After treatment | Change (Δ) | Before treatment | After treatment | Change (Δ) | Before treatment | After treatment | Change (Δ) | |
| PRL (μIU/mL) | 144.37 ± 56.58* | 220.15 ± 131.07 | 8.63 (−10.38, 90.35) | 143.14 ± 95.55* | 213.19 ± 179.65 | 4.09 (−14.29, 97.09) | 138.01 ± 44.85 | 148.08 ± 79.32 | 0 (−34.6, 29.47) |
| IL‐6 (pg/mL) | 2.61 ± 1.93* | 1.39 ± 1.30 | 0 (−3.78, 0.13) | 2.59 ± 2.45 | 1.39 ± 0.99 | 0 (−2.82, 0.51) | 2.39 ± 2.13 | 2.10 ± 1.78 | 0 (−0.68, 1.03) |
| Adiponectin (mg/mL) | 10.81 ± 9.89* | 17.77 ± 5.36 | 3.82 (0, 8.73) | 10.34 ± 10.40 | 13.39 ± 9.85 | 1.09 (−0.23, 8.28) | 14.75 ± 9.18 | 13.57 ± 9.34 | 0.33 (−1.71, 5.06) |
| HOMA‐IR | 9.23 ± 9.03* | 4.99 ± 3.31 | −2.19 (−5.04, −0.52) | 6.47 ± 6.43 | 5.25 ± 4.46 | −1.04 (−2.63, −0.35) | 7.29 ± 7.02 | 5.70 ± 3.36 | −0.77 (−3.40, 1.69) |
| FIB‐4 | 0.79 ± 0.31 | 0.81 ± 0.32 | 0 (−0.12, 0.17) | 0.98 ± 0.42 | 0.86 ± 0.32 | −0.12 (−0.24, 0.09) | 1.10 ± 0.62 | 1.11 ± 0.35 | 0 (−0.12, 0.19) |
| NFS | −0.78 ± 0.81 | −0.69 ± 0.68 | 0.03 (−0.26, 0.52) | −1.55 ± 0.78 | −1.57 ± 0.65 | −0.07 (−0.41, 0.35) | −0.95 ± 0.85 | −0.86 ± 0.84 | 0 (0.30, 0.51) |
Abbreviations: FIB‐4, fibrosis‐4 index; HOMA‐IR, homeostatic model assessment of insulin resistance; IL‐6, interleukin 6; NFS, NAFLD Fibrosis Score; PRL, prolactin.
P < 0.05, comparison of data between groups before treatment and after treatment.
PRL, Adiponectin, and IL‐6 Levels and Correlation Analysis
Serum PRL levels increased significantly in the liraglutide and sitagliptin groups (144.37 ± 56.58 μIU/mL to 220.15 ± 131.07 μIU/mL, P = 0.039; and 143.14 ± 95.55 μIU/mL to 213.19 ± 179.65 μIU/mL, P = 0.044, respectively) but not in the insulin glargine group (Table 4). ΔPRL did not significantly differ across treatment groups. Serum adiponectin levels increased significantly in the liraglutide group (10.81 ± 9.89 mg/mL to 17.77 ± 5.36 mg/mL; P = 0·038) but not in the sitagliptin and insulin glargine groups (Table 4). ΔAdiponectin was significantly greater in the liraglutide group than in the insulin glargine group (P = 0.045); however, no significant difference was observed between the liraglutide and sitagliptin groups or between the sitagliptin and insulin glargine groups. Serum IL‐6 levels decreased significantly in the liraglutide group (2.61 ± 1.93 pg/mL to 1.39 ± 1.30 pg/mL; P = 0.033) but not in the sitagliptin and insulin glargine groups (Table 4). ΔIL‐6 did not significantly differ across treatment groups.
In intention‐to‐treat patients, negative correlation was found between ΔPRL and ΔMRI‐PDFF (r = −0.419; P = 0.001), and positive correlation was found between ΔIL‐6 and ΔMRI‐PDFF (r = 0.662; P = 0.001). No significant correlations were observed between Δadiponectin and ΔMRI‐PDFF, ΔPRL and ΔSAT or ΔVAT, ΔIL‐6 and ΔSAT or ΔVAT, and Δadiponectin and ΔSAT or ΔVAT.
Safety
The rate of AEs varied across treatment groups (liraglutide, 20.8%; sitagliptin, 3.7%; and insulin glargine, 12.5%; P = 0.073; Table 5). Among the 24 patients treated with liraglutide, 4 reported nausea and vomiting, and 1 reported headache. Among the 27 patients treated with sitagliptin, 1 patient had an episode of nonsevere hypoglycemia, but continued the treatment. Of the 24 patients treated with insulin glargine, 2 had an episode of nonsevere hypoglycemia (neither discontinued treatment), and 1 reported toothache.
Table 5.
Adverse Events During Weeks 0‐26
| Adverse events | Liraglutide (n = 24) | Sitagliptin (n = 27) | Insulin glargine (n = 24) |
|---|---|---|---|
| Rate of adverse events* | 5 (20.8%)† | 1 (3.7%)† | 3 (12.5%)† |
| Gastrointestinal disorders | |||
| Nausea and vomiting | 4 (16.7%) | 0 (0%) | 0 (0%) |
| Nonsevere hypoglycemia | 0 (0%) | 1 (3.7%) | 2 (8.3%) |
| Others | |||
| Headache | 1 (4.2%) | 0 (0%) | 0 (0%) |
| Toothache | 0 (0%) | 0 (0%) | 1 (4.2%) |
Comparison among groups.
P = 0.073.
Discussion
In this randomized comparative study, we evaluated the efficacy of liraglutide, sitagliptin, or insulin glargine in patients with T2DM and NAFLD who experienced inadequate glycemic control with metformin alone. Combined with metformin, the three second‐line antidiabetic agents were able to improve glycemic control but showed different effects on IHL, SAT, VAT, and body weight. Our study provides evidence that, in combination with metformin, both liraglutide and sitagliptin could improve IHL in addition to glycemic control in patients with T2DM and NAFLD, but similar reduction in IHL was not observed with insulin glargine.
Results of this study are discordant with those from two prior studies.12, 13 A study reported by Tang et al. 12 showed no significant reduction in liver fat content with liraglutide treatment, whereas insulin glargine reduced total liver fat. In contrast, our study found that liraglutide treatment significantly reduced liver fat content, and insulin glargine showed no significant reduction in liver fat. Notably, in our study, there was significant reduction in body weight in the liraglutide group, but not in the insulin glargine group, whereas ΔMRI‐PDFF and Δweight were positively related in all three groups. Similar correlation was also reported in the Lira‐NAFLD Study.23 This suggests weight loss plays an important role in improvement of NAFLD. However, weight loss in the insulin glargine group was not evident, and the improvement in NAFLD was not associated with weight loss from the results reported by Tang et al. The shorter duration of the study by Tang et al. may have been insufficient to observe the effects of liraglutide on liver fat. In the study by Smits et al., 12 weeks of treatment with liraglutide 1.8 mg/day or sitagliptin 100 mg/day did not reduce hepatic fat in patients with T2DM, but it reports limited weight loss in both arms.13 Therefore, we think that the limited weight loss reported by Tang et al.12 and Smits et al.13 and the difference in treatment length between the studies (26 weeks vs. 12 weeks) may have contributed to the inconsistent findings.
It was reported that liraglutide improved NAFLD, quantified in the LEAN study by liver biopsy in 9 patients with T2DM and 17 normoglycemic patients.11 Results from two studies have also compared the effect of liraglutide in a self‐controlled design in 19 and 6 patients with T2DM, respectively,24, 25 and proved liraglutide could improve NAFLD. It should be noted that these studies used liraglutide as initial therapy, which was not recommended by most guidelines. In contrast, metformin is the first‐line antidiabetic agent worldwide; however, when metformin monotherapy fails to control glucose level as disease progresses, it is necessary to choose a second‐line antidiabetic agent.8 In this regard, our study results provides the first evidence for the add‐on treatment option to metformin for patients with T2DM and NAFLD.
Our study further compared the effects of metformin add‐on second‐line therapy on VAT and SAT using MRI IDEAL IQ, which is a criterion method for quantifying SAT and VAT. Intriguingly, both VAT and SAT were significantly reduced in the liraglutide group, whereas only VAT was significantly reduced in the sitagliptin group. This dissimilarity indicated that these two drugs might have different mechanisms on adipose tissue. Liraglutide was found to contribute to weight loss by inducing browning of white fat (inguinal adipose tissue, a type of SAT) through activating invariant natural killer T cells and inducing fibroblast growth factor 21, both in vivo and in vitro.26 Our previous research also demonstrated that exendin‐4, another GLP‐1 receptor agonist, could promote brown remodeling in white adipose tissue.27 However, there is no report showing any effect of sitagliptin on inducing browning of white fat thus far. Usually SAT, rather than VAT, is the site where browning of white fat happens.
In our study, it is a bit unexpected that metformin add‐on sitagliptin treatment reduced IHL, which was not reported in other studies.10, 13 The study by Cui J et al. reported that sitagliptin treatment for 24 weeks had no significant effect on liver fat measured by MRI‐PDFF compared with placebo control.10 The inconsistency with our study might be due to the different study population (patients with NAFLD with either prediabetes or controlled diabetes vs. patients with NAFLD and uncontrolled T2DM treated with metformin). Another probable explanation is the lack of weight loss observed under sitagliptin in the study by Cui J et al.
It is generally accepted that a change in lifestyle and diet would lead to weight loss, which would subsequently improve liver fat.1, 2 There were significant decreases in body weight in the liraglutide and sitagliptin groups, whereas a trend of body weight reduction (P = 0.282) was present in the insulin glargine group. We could not completely exclude the potential influence of lifestyle education on body weight change and thus IHL change. Therefore, we reanalyzed the change of IHL, adjusting for body weight change, and still found significant reduction of IHL in both the liraglutide and sitagliptin groups compared with the insulin glargine group. Based on these results, we could assume that improvement of liver fat is treatment specific. But future additional studies are needed to determine whether sitagliptin and liraglutide have a direct effect in the reduction of IHL or whether the reduction of liver fat content is associated with weight loss.
In the present trial, significant improvements in HbA1c were observed in all treatment groups. Significant improvements in FPG and PPG were observed with liraglutide, whereas sitagliptin treatment significantly improved PPG only. These findings are consistent with results from previous studies.13, 28
Main adipokines and cytokines involved in the pathogenesis of NAFLD include adiponectin, leptin, tumor necrosis factor‐α, and IL‐6. The previous studies found that patients with NASH had lower adiponectin levels compared with patients with NAFL, and hypoadiponectinemia might play an important pathophysiological role in the progression from NAFL to NASH.29 Our study found that serum adiponectin levels increased significantly (liraglutide group) or had an increasing trend (sitagliptin group); in these two groups, the improvement of IHL was also observed. It was also found that IL‐6 was higher in patients with NAFLD compared with non‐NAFLD controls.30 In our study, serum IL‐6 levels decreased significantly (liraglutide group) or had a decreasing trend (sitagliptin group), and positive correlation was found between ΔIL‐6 and ΔMRI‐PDFF. It was reported very recently by Bi et al. that PRL could improve hepatic steatosis through the CD36 pathway.31 CD36 was one of the receptors for free fatty acids (FFAs) that facilitated FFA uptake. The activation of signal transducer and activator of transcription 5 (STAT5) could improve hepatic steatosis by inhibiting CD36.32 Bi et al. found that PRL/PRL receptor improved hepatic steatosis by inhibiting STAT5/CD36. In our study, serum PRL levels increased significantly in the liraglutide and sitagliptin groups, and negative correlation was found between ΔPRL and ΔMRI‐PDFF.
The rate of AEs varied across treatment groups in the current study, with the highest rate in the liraglutide group. AEs associated with liraglutide were mostly gastrointestinal and mild to moderate in severity, a safety profile that is consistent with previous reports.11, 12, 28, 33, 34 Gastrointestinal AEs are known side effects of GLP‐1RAs and are usually mild and temporary, resolving without intervention after the initial few weeks to months of treatment. Sitagliptin was comparatively better tolerated, consistent with the reported safety profile for dipeptidyl peptidase‐4 inhibitors.13, 28 In the insulin glargine group, two cases of nonsevere hypoglycemia were reported. This is in line with previous studies, as hypoglycemia is a common AE associated with insulin use.12, 33
This study has several strengths. We used a randomized, active‐controlled, parallel‐study design and evaluated the effects of the most commonly used antidiabetic agents (representing three different drug classes), using an advanced method (MRI IDEAL IQ) as a surrogate for liver fat index. T2DM complicated with NAFLD is commonly seen in clinical practice, and there is potential synergistic effect between these two diseases. But lack of evidence results in a dilemma in decision making in choosing proper pharmacotherapy for such a common situation. Our study demonstrates clinical significance because it provides evidence on often‐used regimens for T2DM with NAFLD. However, the study also has certain limitations that must be acknowledged. First, the lack of a placebo control was a weakness of our study, and the open‐label trial design may have introduced bias. Second, our study lacks individual assessment of dietary changes. Additionally, MRI rather than liver biopsy as the reference standard to measure IHL was used. In general, histological data is the golden standard for liver disease research, and MRI is recommended by guidelines to be adopted in clinical trials for its noninvasive advantage and high‐quality diagnostic performance.1, 2, 19
Overall, the results of this study showed that second‐line add‐on treatment with both liraglutide and sitagliptin improved IHL in patients with T2DM and NAFLD under inadequate glycemic control by metformin monotherapy. Liraglutide improved glycemic profile to a greater degree than sitagliptin, albeit with a higher rate of AEs. Our study provided evidence of the effect of antidiabetic agents on NAFLD in patients with T2DM. The results may guide the pharmacotherapies for patients with T2DM and NAFLD.
Acknowledgment
We thank all the patients and investigators for their participation in this study. We also thank General Electric Company (China) for their help with the establishment of procedural instructions for MRI and IDEAL IQ. Editorial assistance of the first draft was provided by Urvashi Nikte, M.D.S., and Maribeth Bogush, M.C.I., Ph.D., of CACTUS Communications, and was funded by Novo Nordisk China. The authors take full responsibility for the content and conclusions stated in this manuscript. Novo Nordisk neither influenced the content of this publication nor was it involved in the study design, or data collection, analysis, or interpretation.
Supported by investigator‐initiated trial research funds from Novo Nordisk (to J.W.), National Natural Science Foundation of China (81770821, to W.X.), Pearl River S&T Nova Program of Guangzhou (201610010175 to F.X.) and Guangdong High‐Level Talents Special Support Program (2016TQ03R590 to F.X.).
Previously presented in part at the EASD 53rd Annual Meeting 2017 (Abstract #829) on September 13, 2017.
Potential conflict of interest: Nothing to report.
See Editorial on Page 2318
References
Author names in bold designate shared co‐first authorship.
- 1. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328‐357. [DOI] [PubMed] [Google Scholar]
- 2. European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO) . EASL‐EASD‐EASO Clinical Practice Guidelines for the management of non‐alcoholic fatty liver disease. J Hepatol 2016;64:1388‐1402. [DOI] [PubMed] [Google Scholar]
- 3. Fan JG, Farrell GC. Epidemiology of non‐alcoholic fatty liver disease in China. J Hepatol 2009;50:204‐210. [DOI] [PubMed] [Google Scholar]
- 4. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease‐meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73‐84. [DOI] [PubMed] [Google Scholar]
- 5. Diehl AM, Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med 2017;377:2063‐2072. [DOI] [PubMed] [Google Scholar]
- 6. Ratziu V, Bellentani S, Cortez‐Pinto H, Day C, Marchesini G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol 2010;53:372‐384. [DOI] [PubMed] [Google Scholar]
- 7. Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non‐alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012;55:2005‐2023. [DOI] [PubMed] [Google Scholar]
- 8. Standards of Medical Care in Diabetes‐2018 . Diabetes Care 2018;41(Suppl 1):S1‐S159. [DOI] [PubMed] [Google Scholar]
- 9. Li Y, Liu L, Wang B, Wang J, Chen D. Metformin in non‐alcoholic fatty liver disease: A systematic review and meta‐analysis. Biomed Rep 2013;1:57‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cui J, Philo L, Nguyen P, Hofflich H, Hernandez C, Bettencourt R, et al. Sitagliptin vs. placebo for non‐alcoholic fatty liver disease: A randomized controlled trial. J Hepatol 2016;65:369‐376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, et al. Liraglutide safety and efficacy in patients with non‐alcoholic steatohepatitis (LEAN): A multicentre, double‐blind, randomised, placebo‐controlled phase 2 study. Lancet 2016;387:679‐690. [DOI] [PubMed] [Google Scholar]
- 12. Tang A, Rabasa‐Lhoret R, Castel H, Wartelle‐Bladou C, Gilbert G, Massicotte‐Tisluck K, et al. Effects of insulin glargine and liraglutide therapy on liver fat as measured by magnetic resonance in patients with type 2 diabetes: A randomized trial. Diabetes Care 2015;38:1339‐1346. [DOI] [PubMed] [Google Scholar]
- 13. Smits MM, Tonneijck L, Muskiet MH, Kramer MH, Pouwels PJ, Pieters‐van den Bos IC, et al. Twelve week liraglutide or sitagliptin does not affect hepatic fat in type 2 diabetes: A randomised placebo‐controlled trial. Diabetologia 2016;59:2588‐2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Davies MJ, Russell‐Jones D, Selam JL, Bailey TS, Kerényi Z, Luo J, et al. Basal insulin peglispro vs insulin glargine in insulin‐naїve type 2 diabetes: IMAGINE 2 randomized trial. Diabetes Obes Metab 2016;18:1054‐1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buse JB, Rodbard HW, Trescoli Serrano C, Luo J, Ivanyi T, Bue‐Valleskey J, et al. Randomized clinical trial comparing basal insulin peglispro and insulin glargine in patients with type 2 diabetes previously treated with basal insulin: IMAGINE 5. Diabetes Care 2016;39:92‐100. [DOI] [PubMed] [Google Scholar]
- 16. Weng J, Ji L, Jia W, Lu J, Zhou Z, et al. Chinese Diabetes Society. Standards of care for type 2 diabetes in China. Diabetes Metab Res Rev 2016;32:442‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Idilman IS, Keskin O, Celik A, Savas B, Halil Elhan A, Idilman R, et al. A comparison of liver fat content as determined by magnetic resonance imaging‐proton density fat fraction and MRS versus liver histology in non‐alcoholic fatty liver disease. Acta Radiol 2016;57:271‐278. [DOI] [PubMed] [Google Scholar]
- 18. Kukuk GM, Hittatiya K, Sprinkart AM, Eggers H, Gieseke J, Block W, et al. Comparison between modified Dixon MRI techniques, MR spectroscopic relaxometry, and different histologic quantification methods in the assessment of hepatic steatosis. Eur Radiol 2015;25:2869‐2879. [DOI] [PubMed] [Google Scholar]
- 19. Caussy C, Reeder SB. Sirlin CB, Loomba R. Non‐invasive, quantitative assessment of liver fat by MRI‐PDFF as an endpoint in NASH trials. Hepatology 2018;68:763‐772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, et al; Nash Clinical Research Network . Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009;7:1104‐1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Angulo P, Bugianesi E, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Barrera F, et al. Simple noninvasive systems predict long‐term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2013;145:782‐789.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bi Y, Zhang B, Xu W, Yang H, Feng W, Li C, et al. Effects of exenatide, insulin, and pioglitazone on liver fat content and body fat distributions in drug‐naive subjects with type 2 diabetes. Acta Diabetol 2014;51:865‐873. [DOI] [PubMed] [Google Scholar]
- 23. Petit JM, Cercueil JP, Loffroy R, Denimal D, Bouillet B, Fourmont C, et al. Effect of liraglutide therapy on liver fat content in patients with inadequately controlled type 2 diabetes: The Lira‐NAFLD Study. J Clin Endocrinol Metab 2017;102:407‐415. [DOI] [PubMed] [Google Scholar]
- 24. Eguchi Y, Kitajima Y, Hyogo H, Takahashi H, Kojima M, Ono M, et al. Pilot study of liraglutide effects in non‐alcoholic steatohepatitis and non‐alcoholic fatty liver disease with glucose intolerance in Japanese patients (LEAN‐J). Hepatol Res 2015;45:269‐278. [DOI] [PubMed] [Google Scholar]
- 25. Cuthbertson DJ, Irwin A, Gardner CJ, Daousi C, Purewal T, Furlong N, et al. Improved glycaemia correlates with liver fat reduction in obese, type 2 diabetes, patients given glucagon‐like peptide‐1 (GLP‐1) receptor agonists. PLoS ONE 2012;7:e50117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lynch L, Hogan AE, Duquette D, Lester C, Banks A, LeClair K, et al. iNKT cells induce FGF21 for thermogenesis and are required for maximal weight loss in GLP1 therapy. Cell Metab 2016;24:510‐519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu F, Lin B, Zheng X, Chen Z, Cao H, Xu H, et al. GLP‐1 receptor agonist promotes brown remodelling in mouse white adipose tissue through SIRT1. Diabetologia 2016;59:1059‐1069. [DOI] [PubMed] [Google Scholar]
- 28. Zang L, Liu Y, Geng J, Luo Y, Bian F, Lv X, et al. Efficacy and safety of liraglutide versus sitagliptin, both in combination with metformin, in Chinese patients with type 2 diabetes: A 26‐week, open‐label, randomized, active comparator clinical trial. Diabetes Obes Metab 2016;18:803‐811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Polyzos SA, Toulis KA, Goulis DG, Zavos C, Kountouras J. Serum total adiponectin in nonalcoholic fatty liver disease: A systematic review and meta‐analysis. Metabolism 2011;60:313‐312. [DOI] [PubMed] [Google Scholar]
- 30. Genc H, Dogru T, Kara M, et al. Association of plasma visfatin with hepatic and systemic inflammation in nonalcoholic fatty liver disease. Ann Hepatol 2013;12:548‐555. [PubMed] [Google Scholar]
- 31. Zhang P, Ge Z, Wang H, Feng W, Sun X, Chu X, et al. Prolactin improves hepatic steatosis via CD36 pathway. J Hepatol 2018;68:1247‐1255. [DOI] [PubMed] [Google Scholar]
- 32. Hosui A, Tatsumi T, Hikita H, Saito Y, Hiramatsu N, Tsujii M, et al. Signal transducer and activator of transcription 5 plays a crucial role in hepaticlipid metabolism through regulation of CD36 expression. Hepatol Res 2017;47:813‐825. [DOI] [PubMed] [Google Scholar]
- 33. Russell‐Jones D, Vaag A, Schmitz O, Sethi BK, Lalic N, Antic S, et al. Liraglutide effect and action in diabetes 5 (LEAD‐5) met+SU Study Group. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD‐5 met+SU): A randomised controlled trial. Diabetologia 2009;52:2046‐2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zinman B, Gerich J, Buse JB, Lewin A, Schwartz S, Raskin P, et al. Efficacy and safety of the human glucagon‐like peptide‐1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD‐4 Met+TZD). Diabetes Care 2009;32:1224‐1230. [DOI] [PMC free article] [PubMed] [Google Scholar]


