Abstract
Aim
To assess the long‐term clinical benefits of early combination treatment with vildagliptin‐metformin vs. standard‐of‐care, metformin monotherapy in the ongoing VERIFY study.
Methods
We randomized 2001 participants with multi‐ethnic background, aged 18–70 years, having HbA1c levels 48–58 mmol/mol (6.5–7.5%) and BMI 22–40 kg/m2. Baseline data included HbA1c, fasting plasma glucose and homeostasis model β‐cell and insulin sensitivity. Standardized meal‐tests, insulin secretion rate relative to glucose, and oral glucose insulin sensitivity were assessed in a subpopulation.
Results
Out of 4524 screened, data were collected from the 2001 eligible participants (53% women) across Europe (52.4%), Latin America (26.8%), Asia (17.2%), South Africa (3.1%) and Australia (0.5%). The median (interquartile range) disease duration was 3.4 (0.9, 10.2) months; mean (±SD) age 54.3±9.4 years; weight 85.5±17.5 kg and BMI 31.1±4.7 kg/m2. Baseline HbA1c was 52±3 mmol/mol (6.9±0.3%), fasting plasma glucose 7.5±1.5 mmol/l and the median (interquartile range) of fasting insulin was 109 (75–160) mU/l. Homeostasis model β‐cell and insulin sensitivity values were 84% (60, 116) and 46% (31, 68), respectively. In those undertaking meal‐tests, insulin secretion rate relative to glucose was 28±12 pmol/min/m2/mmol/l and oral glucose insulin sensitivity was 353±57 ml/min/m2.
Conclusions
Our current, multi‐ethnic, newly diagnosed VERIFY population reflects a characteristic presence of early insulin resistance in participants with increased demand for insulin associated with obesity. The VERIFY study will provide unique evidence in characterizing therapeutic intervention in a diverse population with hyperglycaemia, focusing on durability of early glycaemic control.
What's new?
The VERIFY study is the first study to assess the long‐term clinical benefits of early combination treatment with a dipeptidyl peptidase‐4 inhibitor (vildagliptin)‐metformin vs. standard‐of‐care metformin monotherapy in people newly diagnosed with Type 2 diabetes.
This report describes the baseline characteristics of a newly diagnosed population with Type 2 diabetes from a diverse geographical and ethnic background, demonstrating a classic profile of presence of early insulin resistance associated with elevated BMI as a surrogate for obesity.
The study anticipates generating unique evidence on the progression of β‐cell function, insulin resistance, early complications of diabetes, and effect on health status upon treatment with early vildagliptin‐metformin combination.
What's new?
The VERIFY study is the first study to assess the long‐term clinical benefits of early combination treatment with a dipeptidyl peptidase‐4 inhibitor (vildagliptin)‐metformin vs. standard‐of‐care metformin monotherapy in people newly diagnosed with Type 2 diabetes.
This report describes the baseline characteristics of a newly diagnosed population with Type 2 diabetes from a diverse geographical and ethnic background, demonstrating a classic profile of presence of early insulin resistance associated with elevated BMI as a surrogate for obesity.
The study anticipates generating unique evidence on the progression of β‐cell function, insulin resistance, early complications of diabetes, and effect on health status upon treatment with early vildagliptin‐metformin combination.
Introduction
There is debate about the optimum early pharmacological treatment of diabetes, although most authorities recommend metformin 1. Beyond metformin it is usual to add a second therapy, but often this intensification occurs late, long after good glycaemic control is lost 2. Second line agents include dipeptidyl peptidase‐4 (DPP‐4) inhibitors, which are good candidates for early combination therapy 1. DPP‐4 inhibitors improve glucose homeostasis synergistically with metformin even in mild hyperglycaemia, without the adverse effects of weight gain and hypoglycaemia 3, 4.
VERIFY (Vildagliptin Efficacy in combination with metfoRmIn For earlY treatment of Type 2 diabetes) is an ongoing, 5‐year, multinational, multi‐ethnic study being conducted in 254 centres across 34 countries (Appendix: Table A1). We aimed to investigate, for the first time, the long‐term benefits of early treatment intensification with a DPP‐4 inhibitor (vildagliptin)‐metformin combination over standard‐of‐care metformin monotherapy in maintaining durable glycaemic control in people with newly diagnosed Type 2 diabetes.
In contrast to many cardiovascular outcome studies, we aimed to recruit a population reflecting the typical characteristics of newly diagnosed people living with diabetes worldwide.
Methods
Study design
The study design has been described in detail elsewhere 5. Briefly, the VERIFY trial (NCT01528254) is an ongoing randomized, double‐blind, parallel‐group study consisting of a screening visit, a 3‐week metformin‐alone run‐in period, and a 5‐year treatment period during which the treatment is consecutively intensified, when clinically indicated at the investigators’ discretion. Durability of glycaemic control, time to insulin initiation, changes in β‐cell function and insulin sensitivity have been assessed over time.
The study protocol was approved by the Institutional Review Boards, Independent Ethics Committees and Competent Health Authorities in accordance with European Community Directive 2001/20/EC or as per national and international regulatory requirements in participating countries.
Study population
Participants aged 18–70 years, newly diagnosed with Type 2 diabetes (≤24 months) as per local diagnostic criteria, having centrally confirmed HbA1c levels between 48 mmol/mol (6.5%) and 58 mmol/mol (7.5%), and BMI 22–40 kg/m2, were included in the study 5. Individuals undergoing anti‐diabetes treatment (except for short‐term metformin) within 3 months prior to screening, or using any weight‐loss medications were excluded, as were pregnant or breastfeeding women, and those with chronic liver disease or ongoing congestive heart failure [New York Heart Association (NYHA) III or IV].
Study assessments
Baseline measurements were obtained at the screening visit, or at the next visit prior to initiation of metformin up‐titration. The primary efficacy assessments include HbA1c measurements to determine the time to initial treatment failure and the rate of loss in glycaemic control over time. Participants visit the study site every 13 weeks for 5 years to comply with the study procedures 5. Laboratory samples are collected at each visit and analysed. Vital signs, electrocardiogram, body weight, haematology and biochemistry, fasting lipid profile and triglycerides, liver and renal function tests, urinalysis and adverse events are the key safety assessments. Major adverse cardiovascular events are independently adjudicated (exploratory endpoint) and an independent data safety committee monitors an unblinded periodic review of all safety data.
In a large subpopulation (n=462), standardized and locally adapted, annual meal‐tests are performed for assessment of plasma glucose levels, insulin, and C‐peptide concentrations. Indices of β‐cell function (insulin secretion rate relative to glucose and homeostasis model assessment of β‐cell function (HOMA‐ß)), insulin sensitivity (oral glucose sensitivity index), and insulin resistance (HOMA‐% sensitivity) are calculated 6, 7.
Statistical analysis
Blinded baseline demographics and key glycaemic variables were analysed descriptively and summarized for all randomized participants. Categorical variables including age, gender and BMI were summarized with frequency and percentage, whereas continuous variables including duration of disease and HbA1c were summarized with mean ±SD.
Results
Recruitment of participants
Recruitment for the VERIFY trial started in March 2012 and randomization was completed in April 2014. A total of 2001 people, newly diagnosed with mild hyperglycaemia, were randomized out of the 4524 screened. The major reason for screening failure was an HbA1c value outside the protocol‐defined, centrally assessed range of 48–58 mmol/mol (6.5–7.5%). A total of 66 participants were classified as run‐in failures because of metformin‐intolerance prior to up‐titration to the lowest targeted dose of 1000 mg/day. Details of participants’ dispositions are shown in Figure 1.
Figure 1.
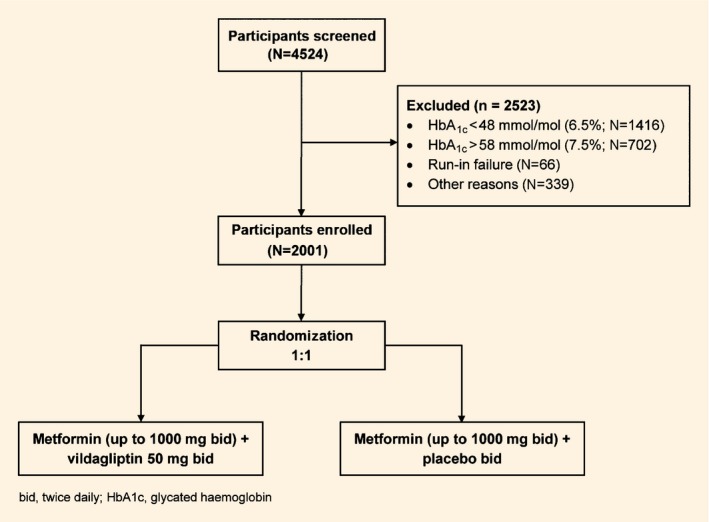
Disposition of participants screened in the VERIFY trial
The geographical distribution of participants enrolled for this trial was: Europe (52.4%), Latin America (26.8%), Asia (17.2%), South Africa (3.1%) and Australia (0.5%).
Baseline characteristics
Overall demographics and baseline characteristics of participants are presented in Table 1.
Table 1.
Demographics and baseline characteristics of participants
| Variable | Total |
|---|---|
| Patient population, n | 2001 |
| Women, n (%) | 1060 (53.0) |
| Age, years | |
| Median (IQR) | 55 (48, 62) |
| Race, n (%) | |
| White European | 1217 (60.8) |
| Black | 49 (2.4) |
| Asian | 373 (18.6) |
| Native American | 210 (10.5) |
| Other | 152 (7.6) |
| Duration of Type 2 diabetes, months | |
| Median (IQR) | 3.4 (0.9, 10.3) |
| HbA1c, mmol/mol (%) | 52±3 (6.9±0.3) |
| FPG, mmol/l | 7.5±1.5 |
| Fasting insulin, median (IQR) (mU/L) | 109 (75–160) |
| HOMA‐%β, median (IQR) (%) | 84 (60, 116) |
| HOMA‐%sensitivity, median (IQR) (%) | 46 (31, 68) |
| BMI, kg/m2 | 31.1±4.7 |
| Pulse rate, bpm | 72.8±9.3 |
| Systolic BP, mmHg | 132.3±14.4 |
| Diastolic BP, mmHg | 80.6±8.6 |
| HDL cholesterol, mmol/l | 1.3±0.3 |
| LDL cholesterol, mmol/l | 2.9±0.9 |
| Triglycerides, mmol/l | 1.9±1.0 |
| UALCRR, mg/mmol | |
| Median (Min–Max) | 1.0 (0.1–262.3) |
| GFR (MDRD), mL/min/1.73m2 | 87.4±18.5 |
| History of diabetes and complications*, n (%) | |
| Proliferative retinopathy | 1 (0.0) |
| Non‐proliferative retinopathy | 11 (0.5) |
| Nephropathy | 26 (1.3) |
| Neuropathy | 116 (5.8) |
| Foot ulcers | 5 (0.2) |
| Metformin daily dose, mg | 1597.3±396.5 |
| Most common metformin dose; 2000 mg, n (%) | 796 (39.8) |
*Retinopathy and neuropathy were assessed according to the local protocols. BMI, body mass index; BP, blood pressure; FPG, fasting plasma glucose; GFR, glomerular filtration rate; HbA1c, glycated haemoglobin; HDL, high‐density lipoprotein; HOMA‐%β, homeostatic model assessment‐%β; HOMA‐%sensitivity, homeostatic model assessment‐%sensitivity; IQR, interquartile range; LDL, low‐density lipoprotein; MDRD, modification of diet in renal disease; SOC, system organ class; UALCRR, urinary micro albumin/creatinine ratio; ± indicates standard deviation (SD).
The median (interquartile range) age of participants was 55 (48, 62) years, baseline HbA1c 52±3 mmol/mol (corresponding to 6.9±0.3%), fasting plasma glucose 7.5±1.5 mmol/l, and median (interquartile range) duration of diabetes 3.4 (0.9,10.2) months. Overall, men and women were often enrolled equally in the study despite some country‐level differences. The mean baseline GFR was 87.4±18.5 ml/min/1.73m2. Overall, 14.5% of the study population were smoking at baseline. Presence of early microvascular complications were reported in 8% of the participants enrolled.
At baseline the median (interquartile range) of fasting insulin was 109 (75–160) mU/l, and HOMA‐ß and HOMA‐% sensitivity values were 84% (60, 116) and 46% (31, 68), respectively. In the subset of participants (n=462) undertaking meal‐tests, 2‐hour plasma glucose values were 9.3±2.8 mmol/l, insulin secretion rate relative to glucose was 28±12 pmol/min/m2/mmol/l, and oral glucose sensitivity index value was 353±57 ml/min/m2. Table 2 shows the variability of the meal‐test measurements by geographic distribution.
Table 2.
2‐hour meal‐test data by variables and geographical distribution
| Variable | Europe | Latin America | Asia* | South Africa |
|---|---|---|---|---|
| Distribution, n (%) | 267 (57.8) | 152 (32.9) | 32 (6.9) | 11 (2.4) |
| Plasma glucose (mmol/l) Median (Min–Max) | 9.3 (4.0–16.5) | 7.9 (4.2–24.0) | 10.4 (6.4–15.1) | 9.8 (5.6–17.1) |
| Insulin (pmol/l) Median (Min–Max) | 58.9 (3.5–286.6) | 55.7 (7.6–404.5) | 97.8 (20.7–435.6) | – |
| C‐peptide (nmol/l) Median (Min–Max) | 1.9 (0.4–5.7) | 1.8 (0.3–4.8) | 2.1 (0.5–5.0) | – |
*values for Asia exclude India.
Discussion
The VERIFY study cohort explores a newly diagnosed population with Type 2 diabetes with mild hyperglycaemia who have the potential for preservation of their β‐cell function, and for achieving a long‐term durable response to early therapy.
One principal goal of treating newly diagnosed drug‐naive individuals is to achieve glycaemic control approaching normoglycaemia 8. This trial explores the concept that optimization of therapy, in this case with an early vildagliptin‐metformin combination, could overcome β‐cell functional deterioration and thereby extend the durability of treatment over time.
Previous intervention studies on initial combination therapy have recruited participants with baseline HbA1c levels ≥64 mmol/mol (≥8.0%) 9, 10, 11, 12, 13, 14, 15. Additionally, A Diabetes Outcome Progression Trial (ADOPT) 16 and Diabetes Prevention Program (DPP) 17 reported limited baseline variables with populations having a higher range of baseline HbA1c. By contrast, the VERIFY trial will assess the durability of glycaemic response in individuals recruited at, or close to, diagnosis and with near‐normal HbA1c. The data show a 16.5% median decrease in β‐cell function but marked reduction in insulin sensitivity to 46%. Insulin resistance is the reciprocal of the sensitivity, so those recruited have an insulin resistance that is double that found in people without diabetes.
Data obtained from the meal‐test substudy are reflective of regional variations observed in plasma glucose, C‐peptide, and insulin concentrations, which may prove important in the subgroup analysis of β‐cell failure. Previously published data 18, 19 demonstrated variations in postprandial glucose response, fasting insulin, and C‐peptide concentrations between various ethnic groups. Such regional differences in the inter‐relationships of early signs of increased insulin resistance (reduced sensitivity) and reduced β‐cell function would be important to both document and interpret for optimized clinical decision making.
Long‐term clinical trials normally pose a big challenge with low study participant retention. Evaluating the durability of treatment prospectively necessitates retention throughout the duration of the study. The VERIFY trial has an active retention programme, tailored to the needs of individuals, but over time the study is also carrying out innovative, relational real‐time data monitoring to improve the retention rates.
The presence of baseline microvascular complications, including proliferative and non‐proliferative retinopathy, nephropathy, neuropathy, and foot ulcer conditions, demonstrates the asymptomatic nature of Type 2 diabetes and early onset of foundation for its complications, emphasizing the importance of early treatment interventions to prevent or slow down the disease progression prior to advent of further diabetic complications.
The major strength of the VERIFY trial is the selection of a geographically distributed diverse, multi‐ethnic population and long‐term duration of 5 years for all the participants, ensuring the generalizability of the trial results and providing guidance in clinical decision making for the increasing number of people with newly diagnosed Type 2 diabetes. The enrolled participants display a classic profile of presence of early insulin resistance associated with elevated BMI as a surrogate for obesity. The study anticipates the generation of unique evidence for many geographical areas with limited or no prior epidemiological or other data on β‐cell function, insulin resistance, early complications of diabetes, and effect on health status upon treatment with a DPP‐4 inhibitor‐metformin combination. The study is currently underway and will report in 2019.
Funding sources
This work was supported by Novartis Pharma AG.
Competing interests
D.R.M. has served on advisory boards or as a consultant for Novo Nordisk, GlaxoSmithKline, Novartis, Eli Lilly, Sanofi‐Aventis, Janssen and Servier; receives current research support from Janssen; and has given lectures for Novo Nordisk, Servier, Sanofi‐Aventis, Eli Lilly, Novartis, Janssen and Aché Laboratories. P.M.P. and P.P. are employed by and own stocks in Novartis. J.E.F. was an employee of Novartis Pharmaceuticals Corporation at the time of manuscript development. M.S. received speaker's honoraria and consulting fees from Novartis, Novo Nordisk, AstraZeneca, Aegerion, Eli Lilly and Company, Boehringer Ingelheim. S.D.P. serves or has served on advisory boards for AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, GlaxoSmithKline, Hanmi Pharmaceuticals, Intarcia, Janssen Pharmaceutics, Merck Sharp & Dohme Ltd, Novartis, Novo Nordisk, Sanofi, Servier and Takeda; serves or has served on the speakers’ bureau for AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Janssen Pharmaceutics, Merck Sharp & Dohme Ltd, Novartis, Novo Nordisk, Sanofi and Takeda; and has received research support from Boehringer Ingelheim, Merck Sharp & Dohme Ltd and Novartis.
Acknowledgements
The authors would like to thank Dr. Wolfgang Kothny, Novartis Pharma AG, Basel, Switzerland for his contribution and scientific advice during the study design and initiation phase. The authors would also like to thank Rangan Gupta and Amit Kumar Garg for editorial assistance, collation, and incorporation of comments from all authors, conducted in accordance with Good Publication Practice guidelines (http://www.ismpp.org/gpp3).
Table A1 Trial investigators and sites
| Site number | Principal investigator | Institution |
|---|---|---|
| 1 | Silvia Gorban de Lapertosa | Centro Universitario de Investigaciones en Farmacologia Clin, Corrientes, Argentina |
| 2 | Diego Aizemberg | Centro Medico Viamonte, Buenos Aires, Argentina |
| 3 | Ines Bartolacci | Instituto Privado De Investigaciones Clinicas De Cordoba, Cordoba, Argentina |
| 4 | Silvia Orio | IMOBA, CABA, Capital Federal, Argentina |
| 5 | Federico Perez Manghi | CINME, CABA, Buenos Aires, Argentina |
| 6 | Laura Maffei | Consultorios Medicos (Investigacion Clinica Aplicada SRL), CABA, Buenos Aires, Argentina |
| 7 | Jorge Aiub | Grupo Medico Alem, San Isidro, Buenos Aires, Argentina |
| 8 | Paula Kavalieros | Woy Woy General Practice, Woy Woy, NSW, Australia |
| 9 | Hans Blom | Vale Medical Practice, Brookvale, NSW, Australia |
| 10 | Adrian Kenny | Morayfield Medical Centre, Morayfield, QLD, Australia |
| 11 | Rudolf Prager | Krankenhaus der Stadt Wien Hietzing‐Lainz, Wien, Austria |
| 12 | Alexandra Kautzky‐Willer | Univ. Klinik fuer Innere Medizin III, AKH Wien, Wien, Austria |
| 13 | Maria Zanella | Universidade Federal de Saeo Paulo, Sao Paulo, SP, Brazil |
| 14 | Carolina Chrisman | Núcleo de Medicina Integrada, Mogi das Cruzes, Brazil |
| 15 | Freddy Eliaschewitz | Centro de Pesquisa Clínica Ltda, Sao Paulo, SP, Brazil |
| 16 | Joao Felicio | Hospital Universitário João de Barros Barreto, Belem, PA, Brazil |
| 17 | Jorge Gross | Centro de Pesquisas em Diabetes, Porto Alegre, RS, Brazil |
| 18 | Joao Borges | Centro de Pesquisa Clinica do Brasil, Brasilia, DF, Brazil |
| 19 | Maria Jose Cerqueira | Instituto de Ensino e Pesquisa Clínica do Ceará, Fortaleza, CE, Brazil |
| 20 | Miguel Nasser Hissa | Centro de Pesquisas em Diabetes e Doenças Endrócrino‐Metaból, Fortaleza, CE, Brazil |
| 21 | Sergio Cunha Vencio | Instituto de Ciências Farmacêuticas, Goiania, GO, Brazil |
| 22 | Edgard Niclewicz | Hospital Nossa Senhora das Gracas, Curitiba, PR, Brazil |
| 23 | Joao Salles | Irmandade da Santa Casa de Misericordia de Sao Paulo, Sao Paulo, SP, Brazil |
| 24 | Rosa Santos | Hospital das Clinicas da Faculdade de Medicina da USP, Sao Paulo, SP, Brazil |
| 25 | Galina Dakovska | USHATE”Akad. Ivan Penchev”, Sofia, Bulgaria |
| 26 | Ivona Daskalova | MMA‐MHAT‐ Sofia, Sofia, Bulgaria |
| 27 | Zdravko Kamenov | UMHAT Alexandrovska, Sofia, Bulgaria |
| 28 | Stefka Vladeva | UMHAT Kaspela, Plovdiv, Bulgaria |
| 29 | Nataliya Temelkova | Alexandrovska University Hospital, Dermaology & Venerology, Sofia, Bulgaria |
| 30 | Natalia Veleva | DCC XII, Sofia, Bulgaria |
| 31 | Maria Lucheva | MHAT D‐r Hristo Stambolski EOOD, Kazanlak, Bulgaria |
| 32 | Emilia Apostolova | MHAT Bratan Shukerov, Smolian, Bulgaria |
| 33 | Dotska Minkova | MHAT Razgrad, Razgrad, Bulgaria |
| 34 | Rositsa Shumkova | MHAT Dr. Tota Venkova AD, Cardiology Department, Gabrovo, Bulgaria |
| 35 | Tsvetodara Kuneva | DCC 1 Russe EOOD, Ruse, Bulgaria |
| 36 | Jaime Ibarra | Centro de Diabetes Cardiovascular del Caribe, Barranquilla, Colombia |
| 37 | Hernan Yupanqui | DEXADIAB, Bogotá, Colombia |
| 38 | Arturo Orduz | Fundacion Hospital Infantil Universitario de San Jose, Bogota, Cundinamarca, Colombia |
| 39 | Fernando Manzur | Centro de Diagnostico Cardiológico, Cartagena, Bolivar, Colombia |
| 40 | Jose Luis Accini Mendoza | IPS Centro Cientifico Asistencial, Barranquilla, Colombia |
| 41 | Jan Gerle | Medica JM S.R.O., Praha, Czech Republic |
| 42 | Tomas Spousta | Diabetologicka ambulance Ostrava, Ostrava, Czech Republic |
| 43 | Jan Vorisek | Diabetologicka ambulance MUDr. Jan Vrkoc S.R.O., Moravska Ostrava, Czech Republic |
| 44 | Sarka Kopecka | DIACENTRUM Brandys n.L. s.r.o, Brandys Nad Labem, Brandys Nad Labem |
| 45 | Katarina Halciakova | Diabetologicka ambulance, Prague 5, Czech Republic |
| 46 | Miloslava Komrskova | Diabetologicka, interni ambulance, Pisek, Czech Republic |
| 47 | Casimiro Velazco | Instituto de Endocrinologia, Nutricion y Osteoporosis, Santo Domingo, Republica Dominicana |
| 48 | Dolores Mejia | Hospital General Plaza de la Salud, Santo Domingo, Republica Dominicana |
| 49 | Juan Vargas | Hospiten Santo Domingo, Santo Domingo, Republica Dominicana |
| 50 | Svea Rosenthal | Rosenthal Family Doctors Centre, Tallinn, Estonia |
| 51 | Mirjam Turkson | Pirita Family Doctor's Centre, Tallinn, Estonia |
| 52 | Kristi Otsmaa | OU Kodudoktori PAK Sinu Arst, Tallinn, Estonia |
| 53 | Kaja Martsin | Mustamae Health Centre, Tallinn, Estonia |
| 54 | Mai Stern | Saku Health Care Center, Saku, Estonia |
| 55 | Juri Linros | Keravan terveyskeskus, Kerava, Finland |
| 56 | Karita Sadeharju | Seinajoen Seudun Terveyskeskus, Seinajoki, Finland |
| 57 | Jyrki Makela | Mehilainen Lahti, Lahti, Finland |
| 58 | Paivi Matsi | Kouvolan terveysasema, Kouvola, Finland |
| 59 | Anneli Hametvaara | Terveystalo Tampere, Tampere, Finland |
| 60 | Susanna Pihlman | Pohjois‐Karjala projekti‐saatio, Joensuu, Finland |
| 61 | Matti Kuusela | Kokkolan Laakarikeskus,, Kokkola, Finland |
| 62 | Sirkka Keinanen‐Kiukaanniemi | Oulun Diankonissalaitos, Oulu, Finland |
| 63 | Thomas Behnke | Zentrum für Klinische Forschung Neuwied (ZKSN), Neuwied, Germany |
| 64 | Michael Eggeling | Aerztehaus Schulstr. 165 Dres. Eggeling, Koch, Wollny, Kamp‐Lintfort, Germany |
| 65 | Stefan Goelz | Praxis Dr. Goelz, Esslingen am Neckar, Germany |
| 66 | Hans‐Peter Kempe | Gemeinschaftspraxis Dres. Stemler u. Kempe, Ludwigshafen, Germany |
| 67 | Gerhard Klausmann | Gemeinschaftspraxis Dr. Klausmann/Dr. Welslau, Aschaffenburg, Germany |
| 68 | Uwe Kleinecke‐Pohl | Praxis Dr. Kleinecke‐Pohl / Zentrum für Klinische Forschung, Koeln, Germany |
| 69 | Michael Morcos | Stoffwechselzentrum Rhein‐Pfalz, Mannheim, Germany |
| 70 | Thorsten Rau | Praxis Dr. Rau, Essen, Germany |
| 71 | Joachim Sauter | Praxis Dr. Sauter, Wangen, Germany |
| 72 | Alexander Segner | Praxis Dr. Segner, St. Ingbert – Oberwuerzbach, Germany |
| 73 | Joerg Simon | Praxis Dr. med. Joerg Simon, Fulda, Germany |
| 74 | Marc Haeffner | Praxis Dr. Haeffner / Steinmaier, Viernheim, Germany |
| 75 | Dietrich Tews | Diabeteszentrum Dr. Tews, Gelnhausen, Germany |
| 76 | Martin Grundner | Praxis Dr. Grundner / Dr. Hintze, Hainstadt, Hainburg, Germany |
| 77 | Michael Roden | Deutsches Diabetes Zentrum / Heinrich‐Heine‐Universitaet, Duesseldorf, Germany |
| 78 | Tobias Ohde | Ambulantes Diabeteszentrum Essen Nord, Essen, Germany |
| 79 | Markolf Hanefeld | GWT‐TUD mbH, Studienzentrum Prof. Hanefeld, Dresden, Germany |
| 80 | Sergio Bran | Clínica Dr. Sergio Bran, Guatemala City, Guatemala, Mexico |
| 81 | Clara Chang | Clinica Dra Clara Chang,, Guatemala City, Mexico |
| 82 | Lorena Garcia | Centro Clínico Reumatológico, Guatemala City, Guatemala, Mexico |
| 83 | Luis Ramirez | Clínica Dr. Luis Ramirez 2, Guatemala City, Guatemala, Mexico |
| 84 | Narda Guerrero | Centro de Investigacion Clinica, Guatemala City, Guatemala, Mexico |
| 85 | Juan Moreira | Centro de Investigacion Dr. Moreira clinica, Mexico |
| 86 | Flor Ranchos | Centro de Investigacion Dra. Flor de Maria Ranchos, Guatemala City, Guatemala, Mexico |
| 87 | Risa Ozaki | Medicine & Therapeutics,The Chinese University of Hong Kong, HongKong |
| 88 | Chiu‐Chi Tsang | Alice Ho Mui Ling Netherole Hosptial, Hong Kong |
| 89 | Michelle Wong | Shau Kei Wan Jockey Club GOPC, Hong Kong |
| 90 | Robert Takacs | Szent Gyorgyi Albert Klinikai Kozpont, Szeged, Hungary |
| 91 | Albert Szocs | Szocs Depot Eu Szolg Kft, Budapest, Hungary |
| 92 | Janos Penzes | Haziorvosi Rendelo Csongrad, Csongrad, Hungary |
| 93 | Laszlo Futo | Markhot Ferenc Korhaz, Eger, Hungary |
| 94 | Zsuzsanna Kerenyi | Toth Ilona Eu Szolgalat, Budapest, Hungary |
| 95 | Tamas Oroszlan | Zala Megyei Korhaz, Zalaegerszeg, Hungary |
| 96 | Margit Mileder | Veszprém Megyei Csolnoky Ferenc Kórház Nonprofit Zrt., Veszprem, Hungary |
| 97 | Gizella Pap | Kalocsai Szent Kereszt Korhaz, Kalocsa, Hungary |
| 98 | Kasthuri Alagiasingachar Srinivasan | Bangalore Diabetes Centre, Bangalore, Karnataka, India |
| 99 | Mala Dharmalingam | Bangalore Endrocrinology Diabetes Research Center, Bangalore, Karnataka, India |
| 100 | Sudhir Bhandari | Bhandari Clinic & Research Center, Jaipur, Rajasthan, India |
| 101 | Uday Phadke | Hormones and Diabetes Care Clinic, Pune, Maharashtra, India |
| 102 | Rakesh Kumar Maliram Parikh | Diamed Clinical Research Services Pvt. Limited, Jaipur, Rajasthan, India |
| 103 | A. Ramachandran | Dr.A.Ramachandran's Diabetes Hospital, Chennai, Tamil Nadu, India |
| 104 | Anil Bhansali | Post Graduate Institute of Medical Education & Research, Chandigarh, India |
| 105 | C. S. Yajnik | KEM Hospital, Pune, Maharashtra, India |
| 106 | Vishwanathan Mohan | Dr. V. Mohan's Diabetes Specialities Centre, Chennai, Tamil Nadu, India |
| 107 | Arun Chankramath Somasekharan | Amritha Institute of Medical Sciences (AIMS), Kochi, Kerala, India |
| 108 | Satish Agarwal | Indraprastha Apollo Hospital, New Delhi, India |
| 109 | Ganapathi Bantwal | St. John's National Academy of Health Sciences, Bangalore, Karnataka, India |
| 110 | Sunil M Jain | TOTALL Diabetes Hormone Institute, Indore, Madhya Pradesh, India |
| 111 | Julio Wainstein | The E Wolfson Medical Center, Tel Giborim, Holon, Israel |
| 112 | Mohammed Sabbah | Research Unit, Diabetes and Lipids Department, Linn MC, Heifa, Israel |
| 113 | Taiba Zornitzky | Kaplan Medical Center, Rehovot, Israel |
| 114 | Victor Vishlitzky | Meir Sapir Medical Center, Kfar‐Saba, Israel |
| 115 | Anat Tsur | Clalit Health Services management, Jerusalem, Israel |
| 116 | Faiad Adawi | Ziv MC, Sefad, Israel |
| 117 | Raed Alami | Saint Joseph Hospital, Jerusalem, Israel |
| 118 | Piermarco Piatti | Ospedale San Raffaele IRCCS S R L, Milano, MI, Italy |
| 119 | Maurizio Tiziano Bevilacqua | ASST Fatebenefratelli Sacco Ospedale Luigi Sacco, Milano, MI, Italy |
| 120 | Nicola Lucio Liberato | Az.Ospedaliera della Prov.di Pavia Ospedale C. Mira, Casorate Primo, PV, Italy |
| 121 | Marianna Maranghi | A O Policlinico Umberto I Universita La Sapienza, Roma, RM, Italy |
| 122 | Antimo Aiello | Presidio Ospedaliero A. Cardarelli ‐ ASREM Az.San.Reg.Molise, Campobasso, CB, Italy |
| 123 | Davide Lauro | Fondaz.Policlin.Tor Vergata‐Univ. degli Studi Tor Vergata, Rome, RM, Italy |
| 124 | Paola Ponzani | Stab Osp La Colletta Presidio Ospedal Unico ASL 3 Genovese, Arenzano, GE, Italy |
| 125 | Paolo Desenzani | ASST degli Spedali Civili Brescia‐Pres.Osped.di Montichiari, Montichiari, BS, Italy |
| 126 | Kunho Yoon | The Catholic University of Korea Seoul St Marys Hospital, Seoul, South Korea |
| 127 | Hyuksang Kwon | The Catholic University of Korea Yeouido St. Mary's Hospital, Seoul, South Korea |
| 128 | Jongmin Lee | The Catholic University of Korea Daejeon St.Mary's hospital, Daejeon, South Korea |
| 129 | Sungdae Moon | Incheon St. Mary's hospital The Catholic University of Kore, Incheon, South Korea |
| 130 | Soonjib Yoo | The Catholic University of Korea, Bucheon St.Mary Hospital, Bucheon, Gyeonggi‐do, South Korea |
| 131 | Yubae Ahn | The Catholic University of Korea St. Vincent's Hospital, Suwon, Gyeonggi‐do, South Korea |
| 132 | Taeseo Sohn | Catholic University of Korea Uijeongbu St. Mary's Hospital, Uijeongbu‐Si, Gyeonggi‐do, South Korea |
| 133 | Sangah Chang | The Catholic University of Korea St. Paul's Hospital, Seoul, South Korea |
| 134 | Jelena Sokolova | Daugavpils Regional Hospital LTD, Daugavpils, Latvia |
| 135 | Ilze Lagzdina | ap SANUS, Liepaja, Latvia |
| 136 | Dace Teterovska | Dr. Teterovska's Private Practice in Endocrinology, Ogre, Latvia |
| 137 | Valdis Pirags | P Stradina Clinical University Hospital, Riga, Latvia |
| 138 | Inga Rezgale | Pulss 5 Medical Centre, Riga, Latvia |
| 139 | Inta Leitane | SIA Rigas veselibas centrs Tornakalns branch, Riga, Latvia |
| 140 | Valda Stalte | VSV Centrs, Talsi, Latvia |
| 141 | Sigita Pastare | Zemgales Diabetes Centre, Jelgava, Latvia |
| 142 | Laila Kudule | Riga Outpatient Clinic “Dziednieciba”, Riga, Latvia |
| 143 | Ruta Eglite | General Practice “R.Eglites Doktorats”, Kuldiga, Latvia |
| 144 | Agne Abraitiene | Vilnius University Hospital Santariskiu Klinikos, Vilnius, Lithuania |
| 145 | Vaidotas Urbanavicius | Private Endocrinology Clinic, Vilnius, Lithuania |
| 146 | Jurate Lasiene | Hospital of Lithuanian University of Health Sciences Kaunas, Kaunas, Lithuania |
| 147 | Lina Radzeviciene | Kaunas Dainavos Outpatient Clinic, Kaunas, Lithuania |
| 148 | Egle Urbanaviciene | Kaunas Silainiai Outpatient Clinic, Kaunas, Lithuania |
| 149 | Kristina Baltramonaitiene (Aglinskiene) | Kristavita UAB, Jonava, Lithuania |
| 150 | Ab Aziz Al‐Safi Ismail | Hospital Universiti Sains Malaysia, Kota Bahru, Kelantan, Malaysia |
| 151 | Ee Ming Khoo | University Malaya Medical Centre, Kuala Lumpur, Malaysia |
| 152 | Nor Azmi Kamaruddin | Hospital Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia |
| 153 | Leobardo Sauque | Instituto de Diabetes, Obesidad y Nutricion S.C., Cuernavaca, Morelos, Mexico |
| 154 | Leobardo Sauque | Instituto de Diabetes, Obesidad y Nutricion S.C., Cuautla, Morelos, Mexico |
| 155 | Sergio Hernandez | Instituto Nacional de Ciencias Medicas y Nutricion Salvador, Distrito Federal |
| 156 | Guadalupe Morales | Centro de Diabetes Durango, Durango, Mexico |
| 157 | Enrique Morales | Centro de Investigación Cardiometabólica Ags, Aguascalientes, Mexico |
| 158 | Jorge Aldrete | Paracelsus, S.A. de C.V., México, Distrito Federal |
| 159 | Guillermo Fanghanel | Clinica integral del paciente diabetic, Ciudad De Mexico, Distrito Federal |
| 160 | Manuel Aguilera | Centro de Investigacion Biomedica y Farmaceutica, Mexico D.F, Distrito Federal |
| 161 | Juan Villagordoa | Centro de Estudios Clinicos de Queretaro S.C., Querétaro, Mexico |
| 162 | Eli Heggen | Oslo Universitetssykehus HF, Ullevål, Oslo, Norway |
| 163 | Jorn Gronert | Flattum legesenter, Hønefoss, Norway |
| 164 | Asad Uzzaman | Fet Legesenter AS, Fetsund, Norway |
| 165 | Lars‐Erik Fikke | Enebakk legesenter, Enebakk, Norway |
| 166 | Rolf Johansen | Spikkestadlegene, Spikkestad, Norway |
| 167 | Marilyn Donato | CEDITER, Panama City, Panamá |
| 168 | Pablo Fletcher | Private Clinic Dr. Pablo Fletcher, Panama City, Panamá |
| 169 | Giselle Rodriguez | PAMRI Panama City, Panamá |
| 170 | Angelica Valdivia | Clinica Geriatrica del Ejercito, Chorrillos, Lima, Peru |
| 171 | Cesar Delgado | Instituto Delgado de Investigacion Medica, Arequipa, Peru |
| 172 | Jose Solis | Hospital Nacional Arzobispo Loayza, Cercado de Lima, Lima, Peru |
| 173 | Miguel Pinto | Hospital Nacional Cayetano Heredia, San Martin de Porres, Lima, Peru |
| 174 | Luis More | Consultorio de Endocrinologia, San Isidro, Lima, Peru |
| 175 | Luis Camacho | Clinica Peruano Americana, Trujillo, La Libertad, Peru |
| 176 | Luis Zapata | Casa de Diabetes & Nutricion, Magdalena, Lima, Peru |
| 177 | Ma Concepcion Marcelo | Cardinal Santos Medical Center, San Juan City, Philippines |
| 178 | Cecilia Jimeno | San Juan de Dios Educational Foundation Inc. Hospital, Pasay City, Philippines |
| 179 | Elizabeth Catindig | Institute for Studies on Diabetes Foundation Inc, Marikina, Metro Manila, Philippines |
| 180 | Tomas Lazatin, Jr | Quirino Memorial Medical Center, Quezon City, Metro Manila, Philippines |
| 181 | Roberto Mirasol | Rizal Medical Center, Pasig City, Philippines |
| 182 | Rhea Severina Comia | Amang Rodriguez Memorial Medical Center (ARMMC), Marikina City, Philippines |
| 183 | Malgorzata Rozycka‐Grudniewicz | NZOZ Specjalista Sp.z.o.o, Kutno, Poland |
| 184 | Ewa Krzyzagorska | Praktyka Lekarska Ewa Krzyzagorska, Poznan, Poland |
| 185 | Maria Modzelewska | NZOZ DIABMED, Poznan, Poland |
| 186 | Janusz Gumprecht | Gabinet Prywatny Prof. Janusz Gumprecht, Zabrze, Poland |
| 187 | Piotr Napora | Centrum Badan Klinicznych Piotr Napora Lekarze Sp.p., Wroclaw, Poland |
| 188 | Dorota Pisarczyk‐Wiza | GAJA Poradnie Lekarskie Maciej Wiza, Poznan, Poland |
| 189 | Amorin Popa | Emergency County Hospital Oradea, Oradea, Jud. Bihor, Romania |
| 190 | Mihaela Popoviciu | Medical Practice srl, Oradea, Jud. Bihor, Romania |
| 191 | Mihaela Voitec | Ambulatory of Institute of Nutrition Diseases and Diabetes, Bucharest, Romania |
| 192 | Adriana Dumitrescu | Medical Centre “Sanatatea ta”, Bucharest, Romania |
| 193 | Cornelia Zetu | Institute of Nutrition Diseases and Diabetes “N. Paulescu”, Bucharest, Romania |
| 194 | Bogdan Popa | Spitalul Judetean de Urgenta Ploiesti, Ploiesti, Jud. Prahova, Romania |
| 195 | Lavinia Ionutiu | Centrul Medical Sf. Stefan SRL, Timisoara, Romania |
| 196 | Diana Alpenidze | Out‐patient City Clinic #117, St‐Petersburg, Russia |
| 197 | Valeria Esip | Consultation and Diagnostic Centre #85, St‐Petersburg, Russia |
| 198 | Sergey Martsevich | State Research Centre for Preventive Medicine, Moscow, Russia |
| 199 | Galina Reshedko | Smolensk State Medical Academy of Roszdrav, Smolensk, Russia |
| 200 | Ruslan Sardinov | Institute of Experimental Medicine, St‐ Petersburg, Russia |
| 201 | Sergey Shustov | Military Medical Academy n.a.S.M Kirov, St‐Petersburg, Russia |
| 202 | Yury Shvarts | Saratov State Medical University of Roszdrav, Saratov, Russia |
| 203 | Natalia Vezikova | Baranovs Republican Hospital, Petrozavodsk, Russia |
| 204 | Sergey Yakushin | Ryazan State Medical University n.a.Pavlov, Ryazan, Russia |
| 205 | Olga Zanozina | N.A.Semashko's Regional Clinical Hospital of N.Novgorod, N.Novgorod, Russia |
| 206 | Marina Sergeeva‐Kondrachenko | Penza Regional clinical hospital n/a Burdenko, Penza, Russia |
| 207 | Viera Donicova | Human‐Care S.R.O., Kosice, Slovakia |
| 208 | Katarina Belesova | Lumedic S.R.O., Kosice, Slovakia |
| 209 | Maria Slovenska | Vnútorné lekárstvo, diabetológia, poruchy látkovej premeny a, Kosice, Slovakia |
| 210 | Dana Solcova | DIADAN S.R.O., Ambulancia s odborným zameraním vnút.lekarstv, Kosice, Slovakia |
| 211 | Dalibor Sosovec | DIAB S.R.O., Roznava, Slovakia |
| 212 | Dasa Skripova | ARETEUS S.R.O. Diabetologicka ambulancia, Trebisov, Slovakia |
| 213 | Marek Macko | Diabetol S.R.O., Presov, Slovakia |
| 214 | Livia Tomasova | IN‐DIA S.R.O., Lucenec, Slovakia |
| 215 | Drahoslava Kanderkova | MUDr. Kanderková S.R.O., Namestovo, Slovakia |
| 216 | Ingrid Buganova | MEDIVASA s.r.o., Diabetologia, Zilina, Slovakia |
| 217 | Anna Vargova | DIA‐KONTROL S.R.O., Levice, Slovakia |
| 218 | Ladislav Pavlik | DIA MEDICO S.R.O., Sala, Slovakia |
| 219 | Miriam Teplanova | FUNKYSTUFF S.R.O., Nové Zámky, Slovakia |
| 220 | Jozef Strba | Endiant S.R.O., Sered, Slovakia |
| 221 | Adriana Ilavska | MEDISPEKTRUM s r o, Bratislava, Slovakia |
| 222 | Milan Behuncik | Zeleznicne zdravotnictvo, S.R.O. Kosice, Slovakia |
| 223 | Martina Merciakova | MEDI‐DIA S.R.O., Diabetologicka ambulancia, Sabinov, Slovakia |
| 224 | Denisa Spodniakova | DIASTYLE S.R.O. Interna‐diabetologicka ambulancia, Banska Bystrica, Slovakia |
| 225 | Iveta Kurcova | DIA Zilina S.R.O., Diabetologicka a interna ambulancia, Zilina, Slovakia |
| 226 | Olga Benusova | BENROD S.R.O., diabetologicka ambulancia, Sturovo, Slovakia |
| 227 | Aslam Amod | Suite 215, Durban, South Africa |
| 228 | Magda Conradie | Department of Endocrinology, Cape Town, South Africa |
| 229 | Deepak Lakha | 1644 Starling Street, Johannesburg, South Africa |
| 230 | J Kok | Cardiology Clinical Research, Alberton, South Africa |
| 231 | Hemant Makan | Private Practice, Gauteng, South Africa |
| 232 | S Pillay | Suite C5 Seadoon Mall, Durban, South Africa |
| 233 | Tasneem Vally | Synexus SA Watermeyer Clinical Research, Pretoria, South Africa |
| 234 | Akbar Mahomed | Dr A A Mahomed Medical Centre, Pretoria, South Africa |
| 235 | Luthando Adams | LCS Clinical Research Unit, Johannesburg, South Africa |
| 236 | Xavier Cos Claramunt | CAP Sant Marti de Provençals, Barcelona, Spain |
| 237 | Carles Brotons Cuixart | CAP SARDENYA, Barcelona, Spain |
| 238 | Jordi Ingla | CAP Santa Coloma, Santa Coloma de Gramanet, Barcelona, Spain |
| 239 | Manel Mata | CAP La Mina, Sant Adria del Besos, Barcelona, Spain |
| 240 | Wayne Huey‐Herng Sheu | Taichung Veterans General Hospital, Taichung, Taiwan |
| 241 | Jui‐Hung Sun | Chang Gung Memorial Hospital Linkou, Lin‐Kou, Taiwan |
| 242 | Yi‐Jen Hung | Tri‐Service General Hospital, Taipei, Taiwan |
| 243 | Dee Pei | Cardinal Tien Hospital, Hsin‐tien, Taiwan |
| 244 | Nevin Dinccag | Istanbul University Istanbul Medical Faculty, Istanbul, Turkey |
| 245 | Mehmet Buyukbese | Sutcu Imam University Medical Faculty, Kahramanmaras, Turkey |
| 246 | Muyesser Sayki Arslan | S.B. Yildirim Beyazit Training and Research Hospital, Diskapi / Ankara, Turkey |
| 247 | Ramazan Sari | Akdeniz University Medical Faculty, Antalya, Turkey |
| 248 | Fusun Saygili | Ege University Medical Faculty, Izmir, Turkey |
| 249 | Abdurrahman Comlekci | Dokuz Eylul University Medical Faculty, Izmir, Turkey |
| 250 | Senay Topsakal | Pamukkale University Medical Faculty, Kinikli / Denizli, Turkey |
| 251 | Hasan Kudat | Istanbul University Istanbul Medical Faculty, Istanbul, Turkey |
| 252 | Murat Sert | Cukurova University Medical Faculty, Adana, Turkey |
| 253 | Yagiz Uresin | Istanbul University Istanbul Medical Faculty, Istanbul, Turkey |
| 254 | Zerrin Yigit | Istanbul University Cardiology Institute, Istanbul, Turkey |
Diabet. Med. 36: 505–513 (2019)
(Clinical Trials Registry No.: NCT 01528254)
References
- 1. Zinman B. Initial combination therapy for type 2 diabetes mellitus: is it ready for prime time? Am J Med 2011; 124: S19–S34. [DOI] [PubMed] [Google Scholar]
- 2. Khunti K, Wolden ML, Thorsted BL, Andersen M, Davies MJ. Clinical inertia in people with type 2 diabetes: A retrospective cohort study of more than 80,000 people. Diabetes Care 2013; 36: 3411–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Matthews DR, Dejager S, Ahren B, Fonseca V, Ferrannini E, Couturier A et al Vildagliptin add‐on to metformin produces similar efficacy and reduced hypoglycaemic risk compared with glimepiride, with no weight gain: results from a 2‐year study. Diabetes Obes Metab 2010; 12: 780–789. [DOI] [PubMed] [Google Scholar]
- 4. Bosi E, Dotta F, Jia Y, Goodman M. Vildagliptin plus metformin combination therapy provides superior glycaemic control to individual monotherapy in treatment‐naive patients with type 2 diabetes mellitus. Diabetes Obes Metab 2009; 11: 506–515. [DOI] [PubMed] [Google Scholar]
- 5. Del Prato S, Foley JE, Kothny W, Kozlovski P, Stumvoll M, Paldánius PM et al Study to determine the durability of glycaemic control with early treatment with a vildagliptin‐metformin combination regimen vs. standard‐of‐care metformin monotherapy‐the VERIFY trial: a randomized double‐blind trial. Diabet Med 2014; 31: 1178–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pratley RE, Schweizer A, Rosenstock J, Foley JE, Banerji MA, Pi‐Sunyer FX et al Robust improvements in fasting and prandial measures of beta‐cell function with vildagliptin in drug‐naïve patients: analysis of pooled vildagliptin monotherapy database. Diabetes Obes Metab 2008; 10: 931–938. [DOI] [PubMed] [Google Scholar]
- 7. Hill NR, Levy JC, Matthews DR. Expansion of the homeostasis model assessment of beta‐cell function and insulin resistance to enable clinical trial outcome modeling through the interactive adjustment of physiology and treatment effects: iHOMA2. Diabetes Care 2013; 36: 2324–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA et al Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm ‐ 2017 executive summary. Endocr Pract 2017; 23: 207–238. [DOI] [PubMed] [Google Scholar]
- 9. Abdul‐Ghani MA, Puckett C, Triplitt C, Maggs D, Adams J, Cersosimo E et al Initial combination therapy with metformin, pioglitazone and exenatide is more effective than sequential add‐on therapy in subjects with new‐onset diabetes. Results from the Efficacy and Durability of Initial Combination Therapy for Type 2 Diabetes (EDICT): a randomized trial. Diabetes Obes Metab 2015; 17: 268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pratley RE, Fleck P, Wilson C. Efficacy and safety of initial combination therapy with alogliptin plus metformin versus either as monotherapy in drug‐naïve patients with type 2 diabetes: a randomized, double‐blind, 6‐month study. Diabetes Obes Metab 2014; 16: 613–621. [DOI] [PubMed] [Google Scholar]
- 11. Phung OJ, Sobieraj DM, Engel SS, Rajpathak SN. Early combination therapy for the treatment of type 2 diabetes mellitus: systematic review and meta‐analysis. Diabetes Obes Metab 2014; 16: 410–417. [DOI] [PubMed] [Google Scholar]
- 12. Haak T, Meinicke T, Jones R, Weber S, von Eynatten M, Woerle HJ. Initial combination of linagliptin and metformin in patients with type 2 diabetes: efficacy and safety in a randomised, double‐blind 1‐year extension study. Int J Clin Pract 2013; 67: 1283–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Williams‐Herman D, Xu L, Teng R, Golm GT, Johnson J, Davies MJ et al Effect of initial combination therapy with sitagliptin and metformin on β‐cell function in patients with type 2 diabetes. Diabetes Obes Metab 2012; 14: 67–76. [DOI] [PubMed] [Google Scholar]
- 14. Reasner C, Olansky L, Seck TL, Williams‐Herman DE, Chen M, Terranella L et al The effect of initial therapy with the fixed‐dose combination of sitagliptin and metformin compared with metformin monotherapy in patients with type 2 diabetes mellitus. Diabetes Obes Metab 2011; 13: 644–652. [DOI] [PubMed] [Google Scholar]
- 15. Mirasol RC, Pathan MF, Chawla M, Kim TH, Cooke K, Hours‐Zesiger P et al INITIAL combination therapy with vildagliptin/metformin in drug‐naïve Asian T2DM patients: influence of age, BMI and co‐morbidities in a real‐world setting. PO‐774. Poster presented at the 53rd European Association for the Study of Diabetes Annual Meeting, 11–15 September 2017, Lisbon, Portugal.
- 16. Viberti G, Kahn Se, Greene DA, Herman WH, Zinman B, Holman RR et al A diabetes outcome progression trial (ADOPT): an international multicenter study of the comparative efficacy of rosiglitazone, glyburide, and metformin in recently diagnosed type 2 diabetes. Diabetes Care 2002; 25: 1737–1743. [DOI] [PubMed] [Google Scholar]
- 17. Diabetes Prevention Program (DPP) Research Group . Hypertension, insulin, and proinsulin in participants with impaired glucose tolerance. Hypertension 2002; 40: 679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tan VM, Lee YS, Venkataraman K, Khoo EY, Tai ES, Chong YS et al Ethnic differences in insulin sensitivity and beta‐cell function among Asian men. Nutr Diabetes 2015; 5: e173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harris MI, Cowie CC, Gu K, Francis ME, Flegal K, Eberhardt MS. Higher fasting insulin but lower fasting C‐peptide levels in African Americans in the US population. Diabetes Metab Res Rev 2002; 18: 149–155. [DOI] [PubMed] [Google Scholar]


