Abstract
Objective:
In recent years there has been increasing clinical and empirical interest in neurocognitive functioning in eating disorders (EDs), which has resulted in numerous quantitative and qualitative reviews. However, there has yet to be a comprehensive synthesis or critical review of this literature to identify future directions to advance the field in this area. Therefore the aim of this systematic review of systematic reviews was to (1) characterize the existing literature on neurocognitive functioning in EDs based on recent reviews (i.e., published since 2010), (2) describe related limitations, and (3) suggest avenues for future research to address gaps in the current literature.
Method:
Electronic databases were queried for reviews of neurocognitive domains (i.e., inhibitory control, decision making, central coherence, set-shifting, working memory, and attention bias) in EDs, which identified 28 systematic and meta-analytic reviews.
Results:
Broadly, the literature indicates deficits across these neurocognitive domains in EDs, though heterogeneity was noted in the magnitude of these effects, which varied to some extent across ED subtypes, sample characteristics, and methodological approaches.
Discussion:
While these reviews have generally suggested varying patterns of neurocognitive deficits across EDs, there remain critical limitations regarding the methodological quality of these studies (e.g., the lack of prospective designs, consideration of confounding influences, or examination of interrelationships between neurocognitive domains and relationships between neurocognition and other relevant behavioral constructs). Specifically, we outline ten key areas that are imperative to address in future research in this area in order to move our field forward.
Keywords: anorexia nervosa, bulimia nervosa, binge eating disorder, neurocognition, executive functioning
1. Introduction
Neurocognitive deficits have been broadly implicated as potential transdiagnostic mechanisms contributing to psychopathology (e.g., Goschke, 2014), and in recent years a wealth of research has emerged investigating underlying neurocognitive processes that may contribute to the etiology and/or maintenance of eating disorders (EDs). Executive functioning (also termed cognitive control) is a multidimensional construct referring to “top-down” processes that allow individuals to adapt information processing and behaviors according to their goals (Diamond, 2013). Moreover, cognitive control processes are inherent components of self-regulation and are essential for adaptive emotional, social, and physical functioning (Hofmann, Schmeichel, & Baddeley, 2012). Executive functioning can be further classified into several subdomains that are also broadly related to cognitive control, including inhibition and interference control (i.e., cognitive and behavioral inhibitory control, selective attention), working memory, and cognitive flexibility (Diamond, 2013).
While early ED studies focused on general cognitive deficits, a large body of subsequent research has examined specific facets of neurocognitive functioning hypothesized to contribute to ED psychopathology, specifically inhibitory control, decision making, central coherence, cognitive flexibility, attention bias, and working memory. A number of prior quantitative and qualitative reviews have synthesized functioning of these domains in EDs, though most recent reviews have focused on a single diagnostic category or neurocognitive domain, and thus far there has not been a comprehensive synthesis or critical review of this emergent literature. Systematic reviews of reviews offer a viable way of summarizing evidence and appraising the quality of reviews of primary studies that span multiple topics, methodologies, and samples (Smith, Deva, Begley, & Clarke, 2011). This methodology has been applied in several other fields and across healthcare interventions (e.g., de Vet, de Ridder, & de Wit, 2010; Greaves et al., 2011; Lamming et al., 2017; Lemmens, Oenema, Knut, & Brug, 2008; Maniglio, 2009) to synthesize evidence, resolve inconsistencies in findings, and evaluate methodological limitations. Systematic reviews of reviews therefore allow researchers and clinicians to form conclusions and develop new lines of investigation based on the most accurate and comprehensive depiction of a body of literature.
Given the plethora and diversity of reviews of neurocognitive functioning in EDs, such a review of this literature will not only provide a comprehensive assessment of the empirical evidence across various neurocognitive domains and ED diagnoses, but also help to identify the extent to which deficits are common to EDs or specific to a particular diagnosis, the limitations of the existing research, and future directions to advance our field in understanding potential neurocognitive mechanisms underlying ED psychopathology. Therefore the aim of this review of reviews was to (1) broadly characterize the literature to date on neurocognitive functioning (i.e., inhibitory control, decision making, central coherence, set-shifting, working memory, attention bias) in EDs by conducting a systematic review of recent systematic reviews (i.e., 2010 or later), (2) describe limitations of existing research, and (3) suggest avenues for future research to address gaps in the current literature.
2. Method
Review Selection
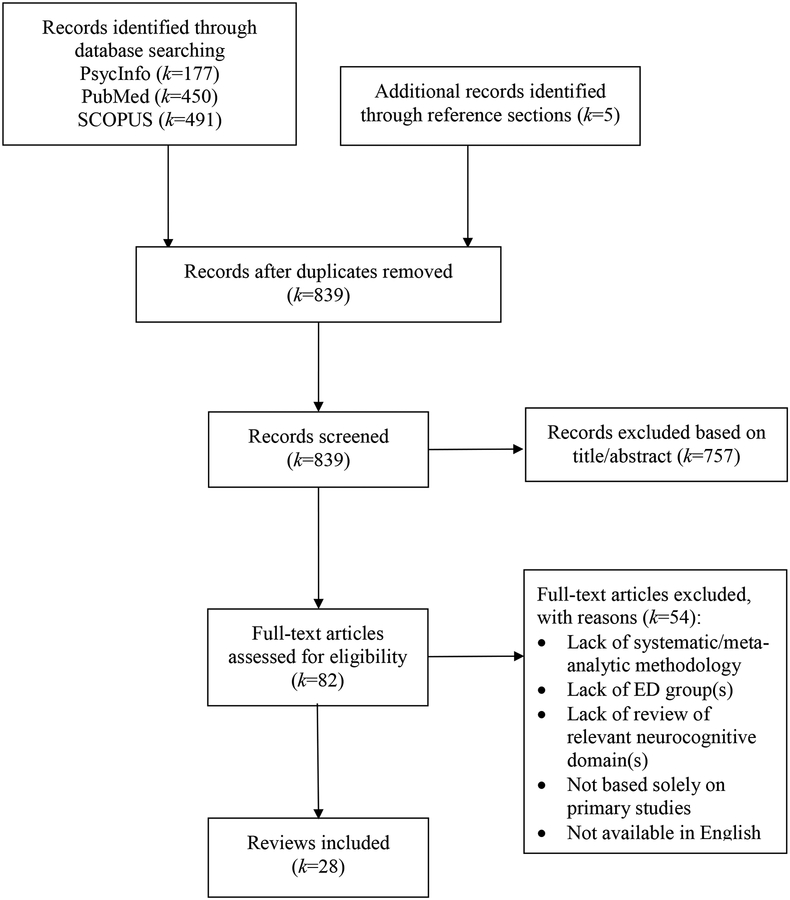
Methodology for this review was informed by recommended guidelines for systematic reviews of systematic reviews (Smith et al., 2011). To identify relevant reviews, PsycInfo (“*” allows for identification of terms with the same stem but have multiple endings), SCOPUS (limited to psychology and/or neuroscience subject areas), and PubMed electronic databases were queried using the following search terms: [“cognitive” or “inhibition” or “inhibitory” or “attention” or “working memory” or “central coherence” or “set shifting” or “set-shifting” or “flexibility” or “decision” or “decision making” or “executive” or “neurocognitive” or “neurocog*” or “neuropsychology” or “neuropsych*” or “risk taking”] in conjunction with [“eating disorder” or “disordered eating” or “anorexia” or “anorexic” or “bulimia” or “bulimic” or “binge”] in the title and/or abstract. Articles were included if a systematic or meta-analytic review was conducted that included a summary of studies examining one of the aforementioned neurocognitive domains in one or more ED diagnostic groups (i.e., anorexia nervosa [AN], bulimia nervosa [BN], and/or binge eating disorder [BED]). These diagnoses were selected given these are the ED groups in which neurocognitive functioning has been most commonly studied in recent years. Reviews were limited to those published in English between 2010 and March 2018 and which included a summary of primary studies using neurocognitive tasks to assess one or more of the domains of interest. The review selection process is summarized in Figure 1; authors K.S. and S.W. independently evaluated full-text articles for inclusion and resolved any discrepancies via discussion.
Figure 1.
PRISMA flow diagram of study selection.
Quality Assessment
Authors K.S. and S.W. independently evaluated the methodological quality of each review by assessing characteristics outlined in the Assessment of Multiple Systematic Reviews (AMSTAR) tool (Shea et al., 2009), which is a validated measure to assess the quality of systematic reviews. Authors agreed upon modified wording of some items on the AMSTAR items to improve clarity in completing ratings; a description of items is available in Supplementary Table 1. One point was assigned for each “yes” response to items on the AMSTAR measure, which were summed to create a composite score for each review. Total scores for meta-analyses and systematic reviews range from 0–11 and 0–9, respectively, with higher scores reflecting higher methodological quality. Inter-rater reliability for items was excellent (kappa=.94). Discrepancies were resolved between raters before calculating total scores.
Data Extraction and Synthesis
A checklist was developed to systematically extract and summarize key characteristics and results from each review, including descriptive information (e.g., sample characteristics, study designs, and tasks), task-based results regarding neurocognitive functioning such as effect sizes for meta-analyses, and relevant covariates and/or moderators (e.g., ED severity, duration of illness, BMI, co-occurring psychopathology). After reviews were identified for inclusion, each of the five authors synthesized findings from one to two of the five neurocognitive domains according to the agreed upon guidelines.
3. Results
This search yielded a total of 28 review articles, including 13 meta-analyses. Of these, 12 reviewed set-shifting; 11 reviewed decision making; 9 reviewed inhibitory control; 9 reviewed central coherence; 8 reviewed attention bias; and 5 reviewed working memory. The mean quality score for meta-analyses was 6.54 (SD=1.98; Range: 3–9) out of 11, and the mean score for systematic reviews was 3.47 (SD=1.19; Range: 1–5) out of 9.
Inhibitory Control
Broadly, inhibitory control refers to a range of processes that reflect the ability to suppress or interrupt responses (Bari & Robbins, 2013), and previous research has suggested that EDs lie on an inhibition-disinhibition spectrum, with binge eating type EDs characterized by deficient inhibitory control, and restricting EDs (i.e., AN-restricting subtype [AN-R]) characterized by excessive inhibitory control (e.g., Wierenga et al., 2014a). Inhibitory control has been further delineated into subdomains of cognitive and behavioral (motor) response inhibition. Cognitive inhibition refers to the ability to filter out irrelevant stimuli from working memory and is often assessed in EDs by measures of interference control such as the Stroop task. Behavioral inhibition refers to the ability to withhold an inappropriate/unwanted motor response, which can be separated into proactive and reactive forms of inhibition. Proactive inhibition reflects inhibition of a response that has not been initiated (i.e., action restraint), and has generally been assessed in EDs using the Go/No-Go task. This task requires participants to respond to a frequently presented target and to inhibit responses to a less frequently presented non-target, and is typically indexed by commission errors (i.e., failures to inhibit). Reactive inhibition is the ability to stop an already initiated behavioral response (i.e., action cancellation), and has most commonly been assessed in EDs with the Stop Signal Task (SST). In the SST, participants are instructed to withhold a prepotent response when a “stop” signal is presented after the appearance of a target stimulus, with slower stop signal reaction time (SSRT) indexing poorer inhibitory control.
Nine systematic reviews including three meta-analyses were identified in this domain (see Table 1 for study characteristics and results). The majority of research focused on comparisons of adults with binge-type EDs with normal weight or obese control groups, and all were cross-sectional in design. Based on the largest review to date (Wu et al. 2013), converging evidence suggests that bulimic-spectrum EDs (i.e., AN binge-purge subtype [AN-BP], BN, BED) are associated with inhibitory control deficits across a range of measures (e.g., Go/No-Go, SST) compared to healthy controls (HC), g=−.32, with AN-BP associated with the greatest magnitude of deficits (g=−.91). However, evidence appears more inconsistent when based solely on the SST, as five out of eight studies using the SST in a range of ED diagnoses found no differences in reaction time compared to HC, which may suggest that reactive behavioral inhibition is less strongly associated with ED psychopathology than proactive inhibition (Bartholdy et al., 2016). Although few studies have included individuals with AN-R or differentiated between AN subtypes, results thus far have not demonstrated consistent differences in inhibitory control functioning between AN subtypes (Bartholdy et al., 2016).
Table 1.
Summary of reviews of inhibitory control in EDs (2010–2018)
| Review | Method | No. of included studies (k) | Age | ED diagnoses included | Non-ED comparison group(s) | Method(s) of assessment | Stimuli | Quality score1 | Key findings and effect sizes |
|---|---|---|---|---|---|---|---|---|---|
| Bartholdy et al. (2016) | Systematic review | 8 | Adults (k=7) and adolescents (k=1) | AN-R, AN-BP, ANrec, BN, BED, EDNOS | HC | SST | N.S. | 5/9 |
|
| Giel et al. (2017) | Systematic review | 7 | Adults (k=6) and adolescents (k=1) | BED, OB/BED | OBC, NWC | Go/No-go, SST, anti-saccade tasks | Food-specific and neutral | 4/9 |
|
| Jáuregui-Lobera (2013) | Systematic review | N.S. | Adults | BN, EDNOS-BN | HC | Go/No-go | Food-specific and neutral | 4/9 |
|
| Kittel et al. (2015) | Systematic review | 8 | N.S. | BED | OBC and NWC | Go/No-go, SST, Stroop, R5CSRT, CCPT | Food/body-related and neutral | 3/9 |
|
| Lavagnino et al. (2016) | Systematic review & meta-analysis | 7 | Adults | OB/BED | OBC and NWC | SST, Stroop, Go/No-Go2 | Food-specific and neutral | 7/11 |
|
| Schag et al. (2013) | Systematic review | 2 | Adults | OB/BED | OBC and NWC | Go/No-go | Food/body-related and neutral | 3/9 |
|
| Van den Eynde et al. (2011) | Systematic review | 16 | Adults and adolescents | BN, BED | HC | Stroop, Go/No-Go, MFFT, HSCT, modified ELF | Neutral | 5/9 |
|
| Wu et al. (2013) | Systematic review and meta-analysis | 36 | Adult (k=34) and adolescent (k=2); Neutral stimuli: M=26.4 yrs, 16–40; ED stimuli: M=25.5 yrs; 19–27) | AN-BP, BN, BED | HC | SST, Go/No-Go, Stroop, MFFT, HSCT, SSIT, ELF | Food/body-related and neutral | 9/11 |
|
| Zakzanis et al. (2010) | Meta-analysis | AN:6 BN: 5 |
AN: 23.60±3.68 yrs
(14–29) BN: 23.74±2.70 yrs (19–28) |
AN, BN | HC | SST, Go/No-Go, Stroop | N.S. | 4/11 |
|
Note. ED=eating disorder; IC=inhibitory control; AN=anorexia nervosa; ANrec=Recovered anorexia nervosa; AN-R=AN-restricting subtype; AN-BP=AN binge-purge subtype; AN-P=AN purging subtype; BN=bulimia nervosa; BED=binge eating disorder; EDNOS=eating disorder not otherwise specified; HC=healthy control; OBC=obese control; OWC=overweight control; NWC=normal weight control; N.S.=not specified; SST=stop signal task; SSRT= stop signal reaction time; MRT= mean reaction time; HSCT=Hayling Sentence Completion Test; MFFT=Matching Familiar Figure Test; SSIT=Simon Spatial Incompatibility Task; ELF=Excluded Letter Frequency test.
Quality scores for systematic reviews are based on a total possible score of 9; scores for meta-analyses are based on a total possible score of 11.
Studies assessing inhibitory control using the delay discounting task were excluded from the summary.
In addition, greater inhibitory control deficits have been observed with disorder-relevant (i.e., food or weight/shape-related) stimuli in BN (Wu et al., 2013) and BED (Giel et al., 2017; Kittel et al., 2015), and several studies found no difference between BED and obese or normal weight control groups using non-disorder relevant stimuli (Kittel et al., 2015). Thus, it is possible that BED is associated with ED-specific rather than general inhibitory control impairments. It is also not clear whether BED status is associated with additional inhibitory control deficits beyond what is seen in obesity, as suggested by one meta-analysis that found no additive influence of BED beyond obesity (Lavagnino et al., 2016). While most reviews focused on comparisons of task performance between ED and control groups, Bartholdy et al. (2016) also demonstrated positive associations between SSRT and ED symptom severity and BMI in BED, but not AN or BN groups; only one study was noted to have examined relationships between inhibitory control, age of onset, and illness duration. Lastly, no reviews reported assessment or analysis of the influence of co-occurring psychopathology.
Set-shifting
Set-shifting, also termed cognitive flexibility, refers to the ability to shift thoughts or actions according to situational demands, which is integral to the self-regulation of behavior (Lezak, 1995). For instance, individuals may exhibit perceptual deficits in scanning or shifting perceptual sets, conceptual deficits that manifest in rigid approaches to understanding and problem-solving, and response deficits that are reflected by stereotyped, perseverative behaviors and difficulties switching behaviors to adapt to changing demands (Lezak, 1995). Poor set-shifting has been posited as a neurocognitive endophenotype of AN that may contribute to rigid and compulsive behaviors (Holliday, Tchanturia, Landau, Collier, & Treasure, 2005). A variety of measures have been used to assess set-shifting that require participants to switch from between previously learned strategies that are no longer effective to new rule sets. In EDs, set-shifting is most commonly assessed by the Wisconsin Card Sorting Test (WCST; Heaton, Chelune, Curtiss, Kay, & Talley, 1993), as indexed by perseverative errors, and the Trail Making Task (TMT; Reitan, 1992), as indexed by task completion time.
There have been 12 systematic reviews including 6 meta-analyses that have focused on set-shifting (see Table 2). All reviews summarized cross-sectional studies of children and adults, which compared EDs to HC groups. In the most comprehensive meta-analysis of set-shifting to date, Wu et al. (2014) found evidence for poor set-shifting in all EDs compared to HC, with medium effect sizes (g=−.51–53) in AN-R, BN, and BED, but not in AN-BP (g=−.18). Consistent with these findings, other reviews have found evidence for deficits in set-shifting in adults with AN (Westwood et al., 2016) and that set-shifting deficits in AN remain after controlling for depressive symptoms (Abbate-Daga et al., 2015). Notably, one meta-analytic review failed to find significant differences in set-shifting in children and adolescents with AN compared to age-matched controls using the TMT and WCST (Lang et al., 2014), and another meta-analysis did not find evidence of deficient set-shifting in BN (Zakzanis et al., 2010); however, these inconsistent findings may be related to the fact that Lang et al. and Zakzanis et al. included fewer studies compared to Wu et al. (k=64).
Table 2.
Summary of reviews of set-shifting in EDs (2010–2018)
| Review | Method | No. of included studies (k) | Age | ED diagnoses included | Non-ED comparison group(s) | Method(s) of assessment | Stimuli | Quality score1 | Key findings and effect sizes |
|---|---|---|---|---|---|---|---|---|---|
| Abbate-Daga et al. (2015) | Systematic review | 16 | Adolescents and adults | AN | HC | WCST, TMT A and B, Brixton Test | Neutral | 4/9 |
|
| Hirst et al. (2017) | Meta-analysis | 28 | Adults and adolescents | AN; BN | HC | WCST, TMT, Brixton | Neutral | 7/11 |
|
| Jáuregui-Lobera (2013) | Systematic review | 57 | Adults and adolescents | AN; BN | HC | TMT | Neutral | 4/9 |
|
| Kittel, Brauhardt, & Hilbert (2015) | Systematic review | 4 | Adults | BED | OBC, OWC, NWC | TMT, Rule Shift Cards Test, WCST | Neutral | 3/9 |
|
| Lang, Stahl, Espie, Treasure, Tchanturia (2014) | Systematic review and meta-analysis | 9 | Children and adolescents | AN | HC | WCST, TMT, Brixton Task, IED, Color-Word Interference Test, Verbal Fluency Test, Groton–Maze Learning Task, Switching of Attention Test, The Category Learning Task, Visual Set-Shifting Task | Neutral | 5/11 |
|
| Reville, O’Connor, & Frampton (2016) | Systematic review | 9 | Adults and adolescents | AN | HC | WCST | Neutral | 5/9 |
|
| Stedal, Frampton,Landro, & Lask, (2012) | Meta-analysis | 11 | Adults and adolescents | AN | HC | TMT | Neutral | 5/11 |
|
| Van Autreve & Vervaet (2015) | Systematic review | 8 | Adults and adolescents | AN | HC | WCST, TMT, Brixton Task, Intra/Extra Dimensional Set Shift Subtest, Verbal Fluency Test, Haptic Illusion, Picture Set Test, Cat Bat Test, Adapted Task Switching Paradigm | Neutral | 3/9 |
|
| Van den Eynde et al. (2011) | Systematic review | 6 (card sorting paradigm); 6 (TMT); 2 (Haptic Illusion); 1 (CATBAT; Picture Set Test) | Adults and adolescents | BN; BED | HC | WCST, TMT Brixton, Haptic Illusion task, Cat Bat, Picture Set Test | Neutral | 5/9 |
|
| Westwood, Stahl, Mandy, & Tchanturia (2016) | Meta-analysis | 22 | Adults, children, and adolescents | AN | HC, ASD | WCST | Neutral | 8/11 |
|
| Wu et al. (2014) | Meta-analysis | 64 | Adults and adolescents | AN; BN; BED | HC | Probabilistic Object Reversal Task, Rule Shift
Cards Test, Berg’s Card Sorting Test, Brixton Task, Cognitive-behavioural flexibility task, Category Learning Test, Uznadze haptic illusion test, Intra-Dimensional/Extra-Dimensional, Nelson’s Card Sorting Test, Picture Set Test, Set-Shifting Test, TMT, Verbal Fluency Test, WCST, Weigl’s Sorting Test. |
Neutral | 9/11 |
|
| Zakzanis et al (2010) | Meta-analysis | 15 (AN); 6 (BN) | Adults | AN; BN | HC | Brixton Task, Controlled Oral Word Association
Test, TMT-B, WCST, Weigl’s sorting |
Neutral | 4/11 |
|
Note. ED=eating disorder; AN=anorexia nervosa; AN-R=AN-restricting subtype; AN-BP=AN binge-purge subtype; AN-P=AN purging subtype; BN=bulimia nervosa; BED=binge eating disorder; EDNOS=eating disorder not otherwise specified; HC=healthy control; OBC=obese control; OWC=overweight control; NWC=normal weight control; ASD=Autism Spectrum Disorder group; WCST=Wisconsin Card Sorting Test; Trail Making Task; IED= Intra/Extra Dimensional Set Shift test.
Quality scores for systematic reviews are based on a total possible score of 9; scores for meta-analyses are based on a total possible score of 11.
Wu et al. (2014) compared set shifting abilities across the range of ED diagnoses and reported that AN, BN, and BED did not significantly differ from one another in set-shifting. However, in Voon’s (2015) review, one study (i.e., Aloi et al., 2015) found that BED was associated with greater set-shifting impairments compared to AN. Research has yielded mixed findings regarding differences between AN subtypes. Wu et al. (2014) found evidence for deficits in set-shifting in AN-R compared to controls, but not AN-BP. However, in a systematic review of eight studies comparing AN-R and AN-BP in set-shifting, Van Autreve and Vervaet (2015) noted that in two of the studies AN-BP performed worse than AN-R, while in the other six studies, there were no differences. In summary, current evidence tends to provide little support for set shifting differences among ED diagnostic groups.
The observed deficits may vary somewhat depending on the specific set-shifting task that is used, as well as participant age. The magnitude of effect sizes has varied across different set-shifting tasks among individuals with EDs (Wu et al., 2014). Effect sizes for the most frequently used tasks (i.e., TMT and WCST) were small to medium (TMT: g =−.41; WCST: g=−.53). However, the effect sizes for less frequently used tasks varied more, with a large effect size for the Uznadze task (g=−1.02) and a smaller effect size for the Brixton task (g=–0.43). While effect sizes were comparable across several tasks in both adolescent and adult samples with AN (Wu et al., 2014), a separate meta-analysis of studies by Westwood et al. (2016) focused on the WCST (2016) reported that effect sizes for deficits in set-shifting in AN were greater in adults compared to children and adolescents. Conclusions currently cannot be made regarding differences in set-shifting between adolescents and adults with EDs other than AN, as only one study investigated set-shifting in adolescents with BN (Darcy et al., 2012), and found no significant differences. To date, no study has evaluated adolescents with BED in terms of set shifting abilities (Wu et al., 2014).
Central Coherence
Central coherence is characterized by the degree of focus on details in processing information and the global integration of such information (Frith, 1989). Several cognitive tasks are thought to assess central coherence, including the Group Embedded Figures Test (EFT/GEFT; Witkin et al., 2002), the Rey-Osterrieth Complex Figure Test (ROFT; Osterrieth et al., 1944), the Object Assembly task (OA; Wechsler et al., 1981), the Overlapping Figures Test (OFT; Della Salla et al., 1995), and the Fragmented Pictures Task (FPT; Snodgrass and Vanderwart, 1980). Each of these measures engages participants in a complex series of tasks regarding shape detection, drawing presented shapes, time to complete puzzles, or other components, which together reflect an emphasis on detail oriented information processing versus broad and global processing of information. In the case of the ROFT, an overall central coherence index (CCI) can be calculated in which higher scores represent a more global processing strategy.
Weak central coherence is considered a potential etiologic or maintenance factor for EDs due to the alleged propensity for persons with EDs to excessively focus on individual details (e.g. body weight) as opposed to broader concepts (e.g. broader sense of self), which in turn influence behavior. The fundamental idea that eating disordered individuals, particularly those with AN, display poor central coherence with over-attention to detail and reduced global processing abilities was generally supported across the seven systematic reviews and three meta-analyses included in this review. As summarized in Table 3, the majority of the studies focused on between-group comparisons of adults with AN versus HC. The largest meta-analysis available (Lang et al., 2014) reveals that across several measures of central coherence (e.g., ROFT, GEFT, OA) individuals with AN displayed poorer central coherence than HC (d=-0.58 to - 0.63). The effect sizes for comparisons of individuals with BN to HC were more variable across measures, but often significant (d=−0.28 to −0.84). There was no evidence of significant differences between AN-R and AN-BP subtypes in central coherence (VanAutreve, and Vervaet, 2015). Furthermore, another systematic review found little evidence that differences between AN and HC could be accounted for by levels of depression (Abbate-Daga et al., 2015). Currently there are few studies of central coherence in BED.
Table 3.
Summary of reviews of central coherence in EDs (2010–2018)
| Review | Method | No. of included studies (k) | Age | ED diagnoses included | Non-ED comparison group(s) | Method(s) of assessment | Stimuli | Quality score1 | Key findings and effect sizes |
|---|---|---|---|---|---|---|---|---|---|
| Abbate-Daga et al. (2015) | Systematic review | 20 | Adults | AN | HC | ROFT | N.S. | 4/9 |
|
| Hirst et al. (2017) | Meta-analysis | 3 | Adults and adolescents | AN | HC | ROFT | N.S. | 7/11 |
|
| Lang & Tchanturia (2014) | Systematic review | 7 | Adults and adolescents | AN | HC | ROFT, OA, OFT |
N.S. | 4/9 |
|
| Lang et al. (2014) | Systematic review and meta-analysis | 19 | Adults | AN, BN, ED-rec, AN-rec | HC | ROFT, OA, OFT, FPT, GEFT |
N.S. | 5/11 |
|
| Madsen et al. (2013) | Systematic review | 26 | Adults and adolescents | AN | HC | ROFT, GEFT, MFFT |
N.S. | 2/9 |
|
| Reville et al. (2016) | Systematic review | 1 | N.S. | AN | HC, non-affected Sisters |
N.S. | N.S. | 5/9 |
|
| Stedal et al. (2012) | Meta-analysis | 9 | N.S. | AN | HC | ROFT | N.S. | 5/11 |
|
| Van Autreve & Vervaet (2015) | Systematic review | 4 | Adults | AN-R AN-BP |
HC | MFFT, FPT, OA, ROFT, GEFT, BDT |
N.S. | 3/9 |
|
| Van den Eynde et al. (2011) | Systematic review | 1 | Adults | BN | HC | ROFT, GEFT, BDT |
N.S. | 5/9 |
|
Note. ED=eating disorder; AN= Anorexia Nervosa; BN= Bulimia Nervosa; AN-R= Anorexia Nervosa-Restricting Type; AN-BP= Anorexia Nervosa-Binge Purge Type; ED-rec=recovered from ED; AN-rec=recovered from AN; HC= Healthy Controls; ROFT= Rey Osterreith Complex Figures Test; OA= Object Assembly; OFT= Overlapping Figures Test; FPT= Fragmented Pictures Test; GEFT= Group Embedded Figures Test; MFFT= Matching Familiar Figure Test; BDT= Block Design Test.
Quality scores for systematic reviews are based on a total possible score of 9; scores for meta-analyses are based on a total possible score of 11.
Attention Bias
Attention bias refers to a tendency to overfocus on environmental sources of information that are considered to be disorder relevant. The two most commonly employed behavioral methods of assessing attention bias in EDs are the modified Stroop task (Stroop, 1935) and variants of the dot-probe task (MacLeod, Mathews, & Tata, 1986). The modified Stroop interference task assesses the time taken to name the color of a written word when the word itself is either neutral (e.g. “hat”) or disorder salient (e.g., “fat”). An attention bias is inferred if the presence of a disorder-salient word increases the time taken to name the color of the word. In the dot-probe task, pairs of stimuli (e.g., a food- or body-related image and a neutral image) are presented, followed by a dot (the probe) at one of the two locations previously occupied by an image. Participants are then required to make a speeded response to the probe. An attention bias is demonstrated when response times (RTs) are reduced for trials in which the probe appears at the location of the disorder relevant stimulus, or, conversely, increased when the probe appears at a different location, suggesting that attention was automatically shifted to the location of the disorder-relevant stimulus (Posner, 1980).
Attention bias is relevant to EDs because these disorders are characterized by an overvaluation of and preoccupation with weight, shape, and food (American Psychiatric Association, 2013). In particular, it has been suggested that excessive concern with disorder-specific (i.e., body- and food-related) stimuli may maintain ED symptoms by directing limited cognitive resources towards disorder-salient stimuli, thereby interfering with tasks and distorting how the environment is perceived and interpreted (Blechert, Ansorge, & Tuschen-Caffier, 2010; Vitousek & Orimoto, 1993). Eight systematic reviews including two meta-analyses were identified in this domain (see Table 4). The majority of studies focused on comparisons of adults with AN, BN, and BED with normal weight or HC. One meta-analysis by Brooks and colleagues (2011) that examined attention bias in AN and BN versus HC, reported consistent evidence for an attention bias towards food words in the Stroop task (k=12; for similar results, see also Jáuregui-Lobera, 2013 and Kittel et al., 2015), with overall effect sizes in the small to medium range (AN d=.38; BN d=.43). Similarly, a systematic review in binge-type EDs (BN, AN-B/P, BED; Stojek et al., 2018) found consistent evidence of biases towards food stimuli in binge-type EDs without compensatory behaviors, though more inconsistent evidence in BN (Stojek et al., 2018; for similar mixed results, see Van den Eynde et al, 2011). However, a more consistent pattern in BN was found for Stroop studies using weight/shape and threat stimuli, which reported a larger attention bias in BN versus controls (Stojek et al., 2018). A meta-analysis of dot-probe task performance (k=4) revealed a large attention bias towards negative shape-related stimuli (d=.80) and away from positive eating and shape-related stimuli (d=−.83) in AN and BN versus controls (Aspen et al., 2013; see also Brooks et al., 2011).
Table 4.
Summary of reviews of attention bias in EDs (2010–2018)
| Review | Method | No. of included studies (k) | Age | ED diagnoses included | Non-ED comparison group(s) | Method(s) of assessment | Stimuli | Quality score1 | Key findings and effect sizes |
|---|---|---|---|---|---|---|---|---|---|
| Aspen (2013) | Systematic review and meta-analysis | 4 | Adults | AN, BN, BED, EDNOS | HC |
DP | Body/eating, positive/negative | 3/11 |
|
| Brooks et al. (2011) | Meta-analysis | 17 | Adults | AN, BN | HC | Stroop, DP, DT | Food words, high/low calorie food images | 7/11 |
|
| Giel et al. (2017) | Systematic review | 4 | Adults (k=3) and adolescents (k=1) | BED, OB/BED | OBC, NWC | RSVP, spatial cuing, free viewing | Food-specific and neutral | 4/9 |
|
| Jáuregui-Lobera (2013) | Systematic review | 8 | Adults | AN, BN, BED | HC | Stroop, Flankers task |
Words-“fat” and “thin” vs. neutral, Food-specific and neutral | 4/9 |
|
| Kittel et al. (2015) | Systematic review | 4 | Adults | BED | OBC and NWC | Stroop, free viewing | Food/ body-related and neutral |
3/9 |
|
| Reville et al (2016) | Systematic review | 5 | Adults | AN | HC | Free viewing (eye tracking) | Body, eating stimuli, faces-angry and neutral | 5/9 |
|
| Stojek et al (2018) | Systematic review | 50 | Adults and adolescents | BED, BN (clinical and sub-clinical), AN-B/P | HC, sub-clinical/recovered BN | Stroop, DP, Visual cuing, Visual Search, free viewing | Weight/shape, food, threat words/images | 3/9 |
|
| Van den Eynde et al. (2011) | Systematic review | 16 | Adults and adolescents | BN, BED | HC | Stroop | Neutral | 5/9 |
|
Note. ED=eating disorder; AN=anorexia nervosa; ANrec=Recovered anorexia nervosa; AN-R=AN-restricting subtype; AN-BP=AN binge-purge subtype; AN-P=AN purging subtype; BN=bulimia nervosa; BED=binge eating disorder; EDNOS=eating disorder not otherwise specified; HC=healthy control; OBC=obese control; OWC=overweight control; NWC=normal weight control; N.S.=not specified; AB=attention bias; ES=emotional Stroop; DP=dot-probe; DT=distracter tasks; RSVP= rapid serial visual presentation task.
Quality scores for systematic reviews are based on a total possible score of 9; scores for meta-analyses are based on a total possible score of 11.
Finally, several systematic reviews included studies that measured attention bias in EDs by tracking eye movements during viewing of disorder-salient versus neutral stimuli (Giel et al., 2017; Kittel et al., 2015; Reville, O’Connor, & Frampton, 2016; Stojek et al., 2018). Although fairly heterogeneous overall, results suggest that women who binge eat may have difficulties disengaging from food cues, whereas women with BN exhibit both an attention bias towards and avoidance of food images versus neutral stimuli. Additionally, limited evidence suggests that women with BN may have difficulty disengaging from low-BMI images of other women and tend to avoid fixating on images of high-BMI people. Taken together, there is fairly reliable evidence for an attention bias in women with EDs compared to HC. However, research suggests that the presence of an attention bias, and whether this is primarily characterized by hyper-vigilance or avoidance tendencies, likely depends on a combination of stimulus timing, type of stimulus, and ED diagnosis (Aspen et al., 2013).
Working Memory
Working memory functioning refers to the ability to hold and work with information in mind and use such information to guide behavior (Lezak, 1995). Working memory, which can be subtyped into verbal and non-verbal (visual-spatial) components, is centrally important in a range of cognitive processes, including incorporating new information into plans and actions (updating), considering alternatives, seeing connections between separate elements, and integrating perceptual input (Diamond, 2013). While a variety of tasks may be used to assess working memory, commonly noted measures in EDs included the spatial and digit span tasks and the N-back task.
Four systematic reviews and one meta-analysis were located that included working memory task results in EDs, with the majority of studies comparing AN to HC (see Table 5). Limited data were available regarding study characteristics, and some reviewers did not include details regarding tasks or stimuli. An earlier review by Zakzanis and colleagues (2010) found that AN groups evidenced deficits in working memory functioning compared to HC (d=−.35), though a more recent review of 16 studies found inconsistent results across studies comparing working memory functioning in AN to HC (Brooks, 2017). There were fewer studies assessing BN and BED groups, though evidence suggested small or non-significant differences in visual and verbal working memory between BN and HC (Van den Eynde et al., 2011; Zakzanis et al., 2010: d=−.12). Results were inconsistent with respect to visual and verbal working memory in BED compared to obese controls in the presence of neutral stimuli (Kittel et al., 2015; Van den Eynde et al., 2011), though it was noted that more interference on the N-back was seen in BED compared to overweight controls in the context of food-related stimuli relative to neutral stimuli (Kittel et al., 2015).
Table 5.
Summary of reviews of working memory functioning in EDs (2010–2018)
| Review | Method | No. of included studies (k) | Age | ED diagnoses included | Non-ED comparison group(s) | Method(s) of assessment | Stimuli | Quality score1 | Key findings and effect sizes |
|---|---|---|---|---|---|---|---|---|---|
| Brooks et al. (2017) | Systematic review | 16 | N.S. | AN, ANrec | HC | N.S. | N.S. | 2/9 |
|
| Kittel et al. (2015) | Systematic review | 3 | N.S. | BED | OBC | Digit span test, spatial span test, N-back with lures | Food-specific and neutral | 3/9 |
|
| Reville et al. (2016) | Systematic review | 1 | N.S. | AN | HC | N.S. | N.S. | 5/9 |
|
| Van den Eynde et al. (2011) | Systematic review | 3 | N.S. | BN, BED | HC | Digit-span and block-span tests | Neutral | 5/9 |
|
| Zakzanis et al. (2010) | Meta-analysis | 12 | AN: 23.60±3.68 yrs
(14–29) BN: 23.74±2.70 yrs (19–28) |
AN, BN | HC | Block
span, CANTAB-spatial-span, CDR-quality-of-working-memory, CFT-20-number-sequencing, Digit span, Supraspan |
Neutral | 4/11 |
|
Note. ED=eating disorder; WM=working memory; AN=anorexia nervosa; ANrec=Recovered anorexia nervosa; BN=bulimia nervosa; BED=binge eating disorder; EDNOS=eating disorder not otherwise specified; HC=healthy control; OBC=obese control; N.S.=not specified; CANTAB=Computer Automated Neuropsychological Test Battery; CDR=Computerized Drug Research Battery; CFT=Culture Fair Test.
Quality scores for systematic reviews are based on a total possible score of 9; scores for meta-analyses are based on a total possible score of 11.
The majority of studies discussed were cross-sectional group comparisons of currently ill patients with EDs, though three studies examined (1) individuals with AN before and after treatment and (2) individuals recovered from AN compared to controls, with findings indicating improvements in working memory after treatment and in recovered states (Brooks et al., 2017). In AN, working memory functioning was noted to be related to duration of illness, but was not consistently correlated with other measures of ED symptoms (Brooks et al., 2017). Taken together, thus far there is some evidence suggesting working memory deficits across EDs, yet there is substantial heterogeneity across studies with respect to the magnitude of deficits, types of measures, and stimuli, and few studies have examined the potential influence of other relevant factors such as illness duration, symptom severity, or co-occurring psychopathology.
Decision Making
Decision making is a construct involving multiple processes including stimulus appraisal, action selection and execution, outcome evaluation, and preference formation (Ernst & Paulus, 2005). Two tasks that have been most commonly employed to examine decision making in EDs are the Iowa Gambling Task (IGT; Bechara, Damasio, Damasio, & Anderson, 1994) and the Game of Dice Task (GDT; Brand et al., 2007). In the IGT, participants complete a series of trials involving selection of a card from one of four decks, two of which are disadvantageous (i.e., result in higher levels of net loss) and two of which are advantageous (i.e., result in lower levels of net loss). The IGT net score, calculated as the number of advantageous choices minus the number of disadvantageous choices, represents the index of decision making. In the GDT, decision making is indexed by the number of safe choices minus the number of risky choices.
Another common approach to investigating decision making in EDs has been the use of temporal or delay discounting tasks (see Odum, 2011), which are thought to assesses the capacity to delay gratification/receipt of reward. The general procedure involves participants completing a series of trials involving a selection between a larger reward provided following a variable delay versus a smaller reward provided immediately. Choosing the larger reward with a delay is considered reduced delay discounting, while picking the smaller reward with no delay is considered increased delay discounting. Monetary stimuli are most commonly employed in the task, although ED-related stimuli have been used as well. Performance on the task is most commonly reported as the discounting parameter k (see Odum, 2011).
Given that EDs are characterized by inconsistencies in goals and actions (e.g., engaging in binge eating despite a desire to lose or maintain weight) or persistence of actions despite evidence supporting the need for a modification (e.g., continued caloric restriction in the context of seriously low weight), decision making has been investigated across the range of ED psychopathology. Eleven systematic review and meta-analyses were identified in this domain, with the majority focused on comparisons of adults currently ill with an ED versus controls (see Table 6). All but two of these studies addressed AN, with BN and BED represented similarly across a smaller number of studies compared to AN. Comparison groups varied depending on the ED diagnosis of primary interest, with overweight or obese controls often included in comparisons focused on BED.
Table 6.
Summary of reviews of decision making in EDs (2010–2018)
| Review | Method | No. of included studies (k) | Age | ED diagnoses included | Non-ED comparison group(s) | Method(s) of assessment | Stimuli | Quality score1 | Key findings and effect sizes |
|---|---|---|---|---|---|---|---|---|---|
| Abbate-Daga et al. (2015) | Systematic Review | 15 | Adults | AN | HC | IGT | Neutral | 4/9 |
|
| Guillaume et al. (2015) | Meta-analysis | 23 | AN: 25.9±6.5 BN: 25.49±5.2 BED: 38.3±10.8 |
AN (ill, recovered, subtypes) BN, BED | HC | IGT (primary) | Neutral | 7/11 |
|
| Hirst et al. (2017) | Meta-analysis | 3 | Adults and adolescents | AN, BN | HC | IGT | Neutral | 7/11 |
|
| Jáuregui-Lobera (2013) | Systematic review | N.S. | N.S. | AN | HC | IGT | Neutral | 4/9 |
|
| Kittel et al. (2015) | Systematic review | 5 | Adults | BED | OBC and NWC | IGT; GDT, DDT | Food/ body-related and neutral |
3/9 |
|
| McClelland et al. (2016) | Systematic review | 10 | Adults and adolescents | AN, BN, BED/LOC | HC | TD Tasks | Disorder-related and neutral | 4/9 |
|
| Reville et al. (2016) | Systematic review | 9 | Adults and adolescents | AN | HC | IGT (primary) | Neutral | 5/9 |
|
| Stojek & MacKillop (2017) | Systematic Review | 8 | Youth and Adults | BED, AN | OBC and NWC | DDT | Disorder-related and neutral | 1/9 |
|
| Van den Eynde et al. (2011) | Systematic review | 7 | Adults and adolescents | BN, BED | HC | IGT; GDT, DDT | Neutral | 5/9 |
|
| Wu et al. (2016) | Systematic review and meta-analysis | 50 |
Adults and adolescents | AN, BN, BED (k=44); recovered AN (k=6) | NWC, HC | IGT; GDT; DDT; BART, CARROT, MGT, IWT, PMRT, PRT | Non-food reward (k=46); food reward (k=4) | 9/11 |
|
| Zakzanis et al. (2010) | Meta-Analysis | 4 | Adults and adolescents | AN, BN | HC | IGT, GDT | Neutral | 4/11 |
|
Note. ED=eating disorder; AN=anorexia nervosa; BN=bulimia nervosa; BED=binge eating disorder; EDNOS=eating disorder not otherwise specified; HC=healthy control; OBC=obese control; NWC=normal weight control; N.S.=not specified; IGT=Iowa Gambling Task; GDT=Game of Dice Task; DDT=Delay Discounting Task; BART=Balloon Analogue Risk Task; CARROT=Card Arranging Reward Responsiveness Objective Test; MGT=Monetary Guessing Task; IWT=Implicit Wanting Task; PMRT=Premature Responding Task; PRT=Progressive Ratio Task.
Quality scores for systematic reviews are based on a total possible score of 9; scores for meta-analyses are based on a total possible score of 11.
Based on the reviews that focused primarily on studies using the IGT and GDT, converging evidence suggests that individuals ill with AN or BN demonstrate impairments compared to HC. In particular, meta-analyses evidenced significant effects that were medium to large (AN: gs=.62-.74, ds=.57-.85). Evidence for BED was more mixed, and dependent on the comparison group. Although a significant large effect (g=1.26) was found for BED in one meta-analysis (Guillaume et al., 2015), the comparison condition was HC, while other reviews included overweight or obese control conditions and reported inconsistent findings. Notably, there were fewer reviews that focused on temporal/delay discounting, and the pattern of findings for these studies was more heterogeneous. Despite some evidence of potentially reduced discounting in AN (i.e., greater ability to delay reward), the reviews noted inconsistencies in the findings and the small existing literature. Similarly, while there was some evidence of greater discounting in BED (i.e., reduced ability to delay reward), the results were inconsistent depending on the weight-status of the comparison group (i.e., significant differences were less common with overweight or obese comparison groups). In the largest review to date (including studies utilizing the IGT, GDT, DDT, and other relevant tasks), Wu et al. (2016) reported a significant medium effect size for studies using non-food reward stimuli in tasks in comparing individuals with an ED versus controls (g=.49); in contrast, the corresponding comparison for studies using food-related stimuli in tasks (although based on a much smaller number of studies) was not significant. With regard to specific ED diagnoses, Wu et al. reported significant medium effects for AN (g=.61) and BN (g=.44) for studies using non-food reward stimuli in tasks, but the corresponding effect was nonsignificant for BED (g=.17).
Finally, a number of reviews addressed possible heterogeneity in effects. For instance, Wu et al., (2016) found significant effects for studies with adults, but not adolescents, although only three studies had adolescent ED samples. Wu et al. also reported significant variability of effect sizes across tasks. Notably, effect sizes for the two mostly commonly applied tasks (i.e., IGT and GDT) were significant, with a medium effect size found for studies using the IGT (g=−.51) and a small effect size (g=−.26) found for studies using the GDT. With regard to possible AN subtype differences, inconsistent findings were reported across reviews, although findings were generally consistent in suggesting non-significant differences across AN and BN. Further, although addressed in only a few reviews, evidence suggests that the impairments in those currently ill with an ED are either reduced or absent in samples that are recovered from an ED, suggesting that the deficits are related to aspects of the illness state (e.g., malnutrition).
4. Discussion
Despite the expanding number of studies on neurocognitive functioning in EDs, significant methodological issues have emerged across this literature. In this section, we will address a number of these issues, which together limit the conclusions that can be drawn from this body of research, as well as highlight important areas to address in future research. It is also important to consider that the quality ratings applied in the present review of reviews reflect additional methodological limitations in the way data were extracted and synthesized, which highlights areas to address in future systematic reviews of these domains. Specifically, reviews did not consistently employ duplicate study selection and data extraction procedures, consider unpublished “grey” literature, provide adequate information regarding excluded studies, or systematically assess and consider methodological quality of primary studies to inform conclusions, which together may introduce bias and contribute to discrepancies across this literature.
Ten Key Areas to Address in Future Research
Characterizing neurocognitive functioning across ED diagnoses.
A limited number of studies have examined neurocognitive functioning across the full spectrum of ED psychopathology. While existing research on inhibitory control has primarily focused on EDs characterized by binge eating, the literature on set-shifting and central coherence research has generally focused on AN samples, and attention bias and decision making studies have been more equally distributed across ED diagnoses. Furthermore, findings thus far have not demonstrated consistent differences between ED diagnoses in the extent of these deficits. While some researchers have suggested neurocognitive endophenotypes may exist across disease states in AN based on set-shifting and central coherence (e.g., Holliday et al., 2005; Lopez et al., 2009; Kanakam & Treasure, 2013), recent research has challenged such conclusions (Talbot, Hay, Buckett, & Touyz, 2015), and evidence remains inconclusive regarding the presence of such endophenotypes in BN or BED. It also has yet to be determined whether neurocognitive task performance can accurately characterize symptom heterogeneity across EDs during acute illness, such as whether restricting-type and bulimic-spectrum EDs lie on an inhibition-disinhibition spectrum. Thus, future research in larger, heterogenous ED samples across illness stages is needed to address theses issues.
Possible confounding influences in research design.
The majority of research has not taken into account possible confounds that may influence neurocognitive functioning. Most notably, studies have not utilized psychiatric controls, and few have co-varied for non-ED psychopathology (e.g., depression, anxiety), which precludes examination of the specificity of observed deficits. Given the well-documented finding that altered functioning in many of these neurocognitive domains are associated with psychopathology (e.g., Snyder, Miyake, & Hankin, 2015), it is not clear whether the observed deficits are specific to EDs versus broader transdiagnostic factors. In addition, few data exist regarding the degree to which malnutrition and weight status influence neurocognitive functioning in EDs. Relatedly, extant evidence is inconclusive as to whether BED status exacerbates inhibitory control and decision making deficits in the context of overweight or obesity, and studies of BED have not consistently accounted for weight status. Furthermore, many studies do not report whether or not participants were receiving psychotropic medications during the study, which could influence performance on neurocognitive tasks.
It is also possible that deficits (e.g., set-shifting, decision making) are associated with duration of illness and/or age, though little research has addressed these issues or their relevance to risk and maintenance models. For instance, age is particularly important to consider given that EDs typically onset during adolescence and young adulthood (Schmidt et al., 2016). Adolescence is an especially important period for neurodevelopment, particularly in terms of frontal regions involved in executive functions and frontolimbic connectivity underlying evaluation of, and responses to, risk and reward, as well as emotion regulation (Steinberg, 2005). Furthermore, while the course of adolescence is generally characterized by improvements in executive functions (Crone, 2009), their development continues into late adolescence and occurs alongside physical, social, and emotional changes, which in turn modulate cognitive processes (Crone, 2009; Steinberg, 2005). Consequently, during adolescence there are potential risks for imbalances between motivational drives and cognitive control, which may promote self-regulation difficulties and various forms of psychopathology (Steinberg, 2005). Therefore, executive functioning deficits will be imperative to consider in ED risk models. For instance, Kaye and colleagues’ (2009) previously outlined a developmental model of AN describing the multiple influences of temperament, neurobiological factors, and psychosocial processes that potentiate AN onset. In sum, additional work is needed that assesses relevant covariates, examines neurocognitive processes as risk factors across the spectrum of ED psychopathology, and the extent to which such deficits interact with other relevant developmental factors during adolescence (e.g., sociocultural influences, emotional functioning) to potentiate the development of EDs versus other forms of psychopathology.
Tasks and procedural stimuli.
Another methodological limitation of this literature pertains to task purity and variability, as well as the nature of stimuli. While the domains discussed in this review are multi-dimensional constructs, studies have typically only used one task to assess a given neurocognitive domain. Furthermore, some tasks are more complex than others and tap multiple cognitive capabilities (e.g., Stroop, WCST), and the nature of certain tasks complicates the determination of which processes underlie the observed effects. There is also wide variability in the type of tasks used across studies, as well as inconsistent reporting of task indices.
With respect to stimuli, increasing evidence suggests that some deficits are particularly salient in the context of disorder-specific stimuli, which also may differ according to ED diagnosis. For example, based on existing data from BN and BED samples, it appears inhibitory control deficits are most pronounced, or only observed, in the presence of disorder-specific stimuli (i.e., food/shape/weight-related), and it is unclear whether AB are specific to ED-relevant stimulus categories (e.g., body images), or, alternately, whether they reflect more general processing differences. Thus it remains to be determined whether impairments in such domains are context-specific or more extensive in nature. Taken together, careful consideration of task specificity, standardized approaches to reporting results, and systematic comparisons of stimuli categories are needed.
Neurocognitive functioning: a stable trait or variable state?
To date, most studies have utilized cross-sectional designs and have conceptualized these areas of neurocognitive functioning as trait-like constructs. Emerging ecological momentary assessment (EMA) research suggests some domains such as inhibitory control vary within persons without EDs (e.g., Powell, McMinn, & Allan, 2017), and thus could be state-dependent, and recent progress has been made in adapting cognitive tasks for mobile assessment (e.g., Moore, Swendsen, & Depp, 2017; Sliwinski et al., 2018). In EDs, it is yet unclear to what extent these neurocognitive abilities fluctuate within persons and how state neurocognitive deficits may maintain ED behaviors. Incorporating neurocognitive assessments in EMA protocols could provide insights into whether deficits reflect state or trait phenomena, identify momentary antecedents and consequences (e.g., affect, restraint, ED behaviors) of fluctuations in cognitive functioning, as well as explore between-person differences in cognitive functioning.
In addition to the need for intensive longitudinal designs (i.e., EMA) to examine momentary processes, the lack of longitudinal studies precludes inferences regarding directionality as to whether neurocognitive deficits are a risk factor for, or consequence of EDs. Extant evidence regarding the persistence of neurocognitive deficits into recovery remains inconsistent and inconclusive. Thus, longitudinal research is needed to clarify whether specific neurocognitive deficits reflect endophenotypes that begin prior to the illness and persist after recovery, start with the onset of the illness and improve with symptom reduction, or might be best viewed as scars of the illness that begin with the onset of the illness, but persist after recovery. Given the methodological difficulties associated with longitudinal studies, one promising option to address such obstacles going forward is by harnessing the advantages of ambulatory and web-based neurocognitive assessment methods that are more easily accessible to participants (e.g., Hansen, Haferstrom, Brunner, Lehn, & Håberg, 2015; Morrison, Simone, Ng, & Hardy, 2015; Sliwinski et al., 2018).
Neurocognitive interventions.
A substantial body of research in other areas of psychopathology has demonstrated potential for cognitive training approaches (e.g., Koster, Hoorelbeke, Onraedt, Owens, & Derakshan, 2017; Linetzky, Pergamin-Hight, Pine, & Bar-Haim, 2015; Wykes, Huddy, Cellard, McGurk, & Czobor, 2011). Despite the broad pattern of deficits noted in this review there is comparatively limited research in EDs focused on cognitive training and examining the effectiveness of such treatments. While emerging theory and empirical evidence suggests various neurocognitive processes may be modifiable through interventions (e.g., Boutelle, Monreal, Strong, & Amir, 2016; Eichen, Matheson, Appleton-Knapp, & Boutelle, 2017; Jones et al., 2016; Koffarnus, Jarmolowicz, Mueller, & Bickel, 2013; Tchanturia et al., 2014), much of this research in EDs is preliminary and has been limited to small samples. For example, initial findings suggest that attention bias modification (ABM) may lead to improvements in binge eating and other ED symptoms (Boutelle et al., 2016), and a recent meta-analysis demonstrated that single-session inhibitory control training (ICT) led to significant decreases in food and alcohol choices or consumption in a laboratory study (Jones et al., 2016). With respect to interventions targeting cognitive flexibility, one systematic review of cognitive remediation therapy for AN (CRT-AN) concluded that CRT-AN led to improvements in set-shifting, though some studies found that neurocognitive improvements were not significantly greater than those observed in other treatment groups (Tchanturia et al., 2014), which suggests that additional work is needed to clarify the specificity of effects observed in CRT-AN. Investigations should also examine whether such interventions are best applied as a stand-alone intervention or an augment to existing treatments, and evaluate possible mechanisms of change. Thus far, studies also have yet to determine whether improving specific neurocognitive abilities translates into behavioral changes in daily life, which would be well-suited to address with EMA. In sum, while initial cognitive training interventions have shown promise in targeting and improving functioning in some domains, there is a need for larger samples and longer follow-up periods to assess the durability and generalizability of effects, as well as the optimal therapeutic doses of training.
Relationships between neurocognitive domains.
Notably, studies have typically evaluated specific neurocognitive domains in isolation from others and have not considered how associations or interactions between neurocognitive processes may relate to ED symptomatology. All of the domains discussed in the present review fall under a larger “umbrella” of executive functioning, which are multifaceted and interrelated domains that together influence goal-directed behavior and self-regulation (Diamond, 2013). For example, evidence from basic vision science research has shown that holding a particular object or feature in working memory biases attention towards matching objects in the environment (e.g., Soto, Heinke, Humphreys, & Blanco, 2005). This raises the possibility that attention bias towards disorder salient information in EDs may be mediated by the storage of ED-relevant (e.g., shape or food-related) information in working memory; in turn, preoccupation with food, shape- or weight-related cognitions could increase attention towards similar cues in the environment in a self-perpetuating cycle (Higgs, 2016). Thus, in the context of EDs, deficient cognitive inhibition may serve to increase processing of salient emotional or ED-related information in working memory and lead to subsequent negative affect. Similarly, weak central coherence and difficulties with set-shifting (i.e., cognitive inflexibility) may be related to and/or exacerbate attention bias, such that individuals with high levels of attention bias may have difficulties shifting attention away from ED-related stimuli. With respect to behavioral inhibition, difficulties holding task-relevant information in working memory may also interfere with one’s ability to inhibit impulsive or prepotent responses. Taken together, further study is needed to elucidate how neurocognitive domains may interact with each other, as deficits in different domains may have interactive or synergistic effects that potentiate ED symptoms.
Relationships between neurocognitive domains and other relevant constructs.
Limited data exists regarding how these neurocognitive domains may interact with other factors known to influence eating behaviors (e.g., reward sensitivity, dietary restraint, affect). As one example, negative affectivity and emotion dysregulation have been implicated in the onset and maintenance of ED behaviors across diagnoses (e.g., Lavender et al., 2015; Stice, 2001), and outside of EDs, substantial research has demonstrated that neurocognitive processes are related to emotion in a variety of ways (Okon-Singer et al., 2015). However, these relationships have yet to be elucidated in EDs. As outlined in a well-supported model of cognitive control of emotion, neurocognitive processes play a role in each stage of emotion generation and regulation via the processes of perception, attention deployment, stimulus appraisal, and response modulation (Ochsner et al., 2012). In addition, the multidimensional model of emotion regulation (Gratz & Roemer, 2004) outlines several dimensions of emotion regulation that are implicitly related to neurocognitive processes (e.g., inhibitory control). Within these frameworks, attention and cognitive control functions are particularly relevant constructs, all of which exhibit altered functioning in EDs based on the aforementioned evidence.
For example, regarding attention bias, the ability to effectively modulate attention is closely tied to emotion regulation and cognitive control processes, specifically the ability to filter or gate information in working memory. Attentional focus is determined by a combination of automatic, bottom-up, stimulus-driven (i.e., exogenous) and deliberate (i.e., endogenous) attention processes (Egeth & Yantis, 1997). Emotionally salient cues and information are thought to “grab” exogenous attention and are thus more likely to enter working memory; conversely, emotional states guide attention towards mood-congruent cues. Once in working memory, emotional information “hijacks” endogenous attention and interferes with cognitive control processes. In turn, difficulties with working memory filtering result in increased processing of emotionally salient information, and subsequent elevation or maintenance of negative affect (Okon-Singer et al., 2015). Additionally, while attentional processes may be involved in the generation and intensification of emotion, deliberate attention deployment or redirection also has a regulating effect on emotion, in that shifting attention away from emotional cues can alter one’s emotional response by reducing processing of such information (Okon-Singer et al., 2015).
In addition to attention bias, the ability to maintain regulatory goals and inhibit irrelevant stimuli (i.e., cognitive inhibition) in working memory is also related to the ability to modulate input from emotional systems and engage in emotion regulation strategies (e.g., cognitive re-appraisal; Rolls, 2013). Further, response inhibition is a salient component of the emotion regulation dimensions involving behavioral control in the context of negative emotion (Gratz & Roemer, 2004), whereas cognitive flexibility (related to set shifting) is important to the flexible and situationally appropriate application of emotion regulation strategies (Gratz & Roemer, 2004). While we highlighted the need for assessment and analysis of emotion-cognition relationships to provide an example for future research directions, we also believe that it will be important for future studies to consider interactions between cognitive control and other domains that are relevant to EDs, particularly habit and reward processes, in light of growing neurobiological evidence implicating these processes (Frank, 2015; Steinglass & Walsh, 2016; Wierenga et al., 2014a).
Integrative assessment and analytic approaches.
In light of the aforementioned issues, future research must adopt innovative, multimethod assessment approaches to clarify findings, lend insights into mechanisms underlying neurocognitive deficits in EDs, and elucidate the functional significance of these deficits. For example, intensive longitudinal designs that integrate momentary assessments of cognition, affect, and behavior would be ideally suited to elucidate within-person mechanisms that potentiate ED behaviors. With respect to neuroimaging research, promising directions for future research may include examining neural activation during neurocognitive tasks across distinct conditions (e.g., neutral versus negative affect induction), as well as the adoption of network perspectives and analytic approaches that can evaluate temporally related activations both within and between functionally related brain regions (e.g., dynamic functional connectivity). It will also be necessary to assess whether neurocognitive deficits and functional brain abnormalities assessed in laboratory settings predict behavioral phenomena in daily life, as recently demonstrated by integrated EMA-fMRI research by Fischer and colleagues (2017).
Notably, task performance may not always serve as accurate indicators of aberrant neural functioning, as some research has reported differences in brain activation despite a lack of behavioral differences in task performance (e.g., Oberndorfer et al., 2011; Wierenga et al., 2014b), which may contribute to divergent findings in the literature. In addition, some have suggested that while deficits in some domains (e.g., decision making) may be observed across EDs, the specific neural mechanisms underlying altered task performance may differ across diagnoses (e.g., Guillame et al., 2015). Thus, further functional neuroimaging studies domains may provide meaningful information that is not possible to obtain from behavioral data alone, and may elucidate the extent to which neural circuitry underlying various tasks and domains converge.
Transdiagnostic and dimensional perspectives.
The majority of existing relevant research has assessed neurocognitive functioning between categories based on ED diagnosis or weight status. Consistent with the objectives of the National Institute of Mental Health Research Domain Criteria (RDoC) initiative, assessing ED psychopathology in dimensional terms (e.g., bulimic-spectrum) could help clarify whether deficits are specific to diagnostic categories, or rather correspond to types and levels of particular symptoms. Within EDs, studying mixed ED samples and employing empirical classification approaches (e.g., latent profile analysis) could also elucidate possible distinct neurocognitive subtypes across EDs and/or patterns of neurocognitive deficits that may differentiate broad symptom patterns, such as bulimic-spectrum and restricting-type EDs.
Notably, an extensive body of research has documented executive functioning problems across psychopathology (Goschke, 2014; McTeague, Goodkind, & Etkin, 2016; Snyder et al., 2015), yet this body of research has not received sufficient consideration in studies of neurocognition in EDs. As previously described, emotional processes are known to influence neurocognitive functioning, and notably, EDs commonly co-occur with mood and anxiety disorders (Hudson, Hiripi, Pope, & Kessler, 2007). Given that few studies in the existing neurocognitive task literature have controlled for other psychiatric symptoms, it is unclear to what degree the observed neurocognitive deficits are related to common impairments across psychopathology or specific to EDs. Thus, transdiagnostic studies that include samples of both ED groups and psychiatric controls could begin to address this question and help to understand common and specific neurocognitive impairments across psychopathology.
Integrative theory.
Despite a large body of research indicating neurocognitive deficits in EDs and across psychopathology, there are relatively few well-articulated and integrative models of how neurocognitive processes interact with other processes to influence EDs, and none have considered the role of co-occurring psychopathology. Aspen et al. (2013) proposed a model in which cognitive vulnerabilities (i.e., genetic predispositions, executive functioning deficits, and cognitive inefficiencies) predispose individuals to exhibit attention bias in the context of ED-relevant stimuli, which in turn leads to negative affect and ED behaviors. Another recent review synthesized literature across neuroimaging, neurocognitive, and animal studies in BED to provide a cohesive account of the possible neuropathophysiology of this disorder (Kessler, Hutson, Herman, & Potenza, 2016). In brief, this model suggested that BED is characterized by alterations in circuits implicated in inhibitory control, reward and motivation, and habitual behavior; however, the authors also note several other domains to examine in future studies, including neurobiological investigations of the role of stress and emotion regulation in BED (Kessler et al., 2016). Taken together, both developing and empirically testing such integrative theories would be important directions for future research in EDs, as doing so could lead to more parsimonious models and mechanism-focused treatments.
Conclusions
In summary, there is an extensive and ever-growing body of literature examining neurocognitive functioning in EDs, as reflected by the publication of 28 identified review articles since 2010 alone. While these reviews have broadly suggested EDs are associated with a range of neurocognitive deficits, there remain critical limitations regarding the methodological quality and design of these studies and reviews, most of which have relied on cross-sectional comparisons of ED samples to HC. Although several of these limitations and methodological issues have been noted in prior work (e.g., ecological validity, prospective assessment, influence of emotion), these issues remain unaddressed in the majority of studies conducted. Together these limitations preclude our ability to make definitive conclusions regarding the role of neurocognitive deficits in the etiology and maintenance of EDs, or to move our field forward in the development of integrative models and more effective interventions. In the present review, we have identified and described ten areas that we believe are imperative to address in future research, which will serve to provide a more nuanced understanding of the nature and specificity of neurocognitive deficits in EDs.
Supplementary Material
References
- Abbate-Daga G, Buzzichelli S, Marzola E, Aloi M, Amianto F, & Fassino S (2015). Does Depression Matter in Neuropsychological Performances in Anorexia Nervosa? A Descriptive Review. International Journal of Eating Disorders, 48, 736–745. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, Va: American Psychiatric Publishing. [Google Scholar]
- Asmaro D, Jaspers-Fayer F, Sramko V, Taake I, Carolan P, & Liotti M (2012). Spatiotemporal dynamics of the hedonic processing of chocolate images in individuals with and without trait chocolate craving. Appetite, 58(3), 790–799. 10.1016/j.appet.2012.01.030 [DOI] [PubMed] [Google Scholar]
- Aspen V, Darcy A, & Lock J (2013). A Review of Attention Biases in Women with Eating Disorders. Cognition & Emotion, 27(5), 820–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balodis IM, Molina ND, Kober H, Worhunsky PD, White MA, Grilo CM, & Potenza MN (2013). Divergent neural substrates of inhibitory control in binge eating disorder relative to other manifestations of obesity. Obesity, 21(2), 367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, & Robbins TW (2013). Inhibition and impulsivity: Behavioral and neural basis of response control. Progress in Neurobiology, 108, 44–79. [DOI] [PubMed] [Google Scholar]
- Bartholdy S, Dalton B, O’Daly OG, Campbell IC, & Schmidt U (2016). A systematic review of the relationship between eating, weight and inhibitory control using the stop signal task. Neuroscience & Biobehavioral Reviews, 64, 35–62. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, & Anderson SW (1994). Insensitivity to future consequences following damage to human prefrontal cortex. Cognition, 50, 7–15. [DOI] [PubMed] [Google Scholar]
- Blechert J, Feige B, Joos A, Zeeck A, & Tuschen-Caffier B (2011). Electrocortical processing of food and emotional pictures in anorexia nervosa and bulimia nervosa. Psychosomatic Medicine, 73, 415–421. [DOI] [PubMed] [Google Scholar]
- Boutelle KN, Monreal T, Strong DR, & Amir N (2016). An open trial evaluating an attention bias modification program for overweight adults who binge eat. Journal of Behavior Therapy and Experimental Psychiatry, 52, 138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand M, Franke-Sivert C, Jacoby GE, Markowitsch HJ, & Tuschen-Caffier B (2007). Neuropsychological correlates of decision making in patients with bulimia nervosa. Neuropsychology, 21, 742–750. [DOI] [PubMed] [Google Scholar]
- Brooks S (2016). A debate on working memory and cognitive control: Can we learn about the treatment of substance use disorders from the neural correlates of anorexia nervosa? BMC Psychiatry, 16(1), 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks S, Prince A, Stahl D, Campbell IC, & Treasure J (2011). A systematic review and meta-analysis of cognitive bias to food stimuli in people with disordered eating behaviour. Clinical Psychology Review, 31(1), 37–51. [DOI] [PubMed] [Google Scholar]
- Castro-Fornieles J, Caldú X, Andrés-Perpiñá S, Lázaro L, Bargalló N, Falcón C, … & Junqué C (2010). A cross-sectional and follow-up functional MRI study with a working memory task in adolescent anorexia nervosa. Neuropsychologia, 48(14), 4111–4116. [DOI] [PubMed] [Google Scholar]
- Crone EA (2009). Executive functions in adolescence: inferences from brain and behavior. Developmental Science, 12(6), 825–830. [DOI] [PubMed] [Google Scholar]
- Della Sala S, Laiacona M, Trivelli C, & Spinnler H (1995). Poppelreuter-Ghent’s overlapping figures test: its sensitivity to age, and its clinical use. Archives of Clinical Neuropsychology, 10(6), 511–534. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, & Postle BR (2015). The cognitive neuroscience of working memory. Annual Review of Psychology, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A (2013). Executive functions. Annual review of psychology, 64, 135–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeth HE, & Yantis S (1997). Visual attention: Control, representation, and time course. Annual Review of Psychology, 48(1), 269–297. [DOI] [PubMed] [Google Scholar]
- Eichen DM, Matheson BE, Appleton-Knapp SL, & Boutelle KN (2017). Neurocognitive treatments for eating disorders and obesity. Current Psychiatry Reports, 19(9), 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekeland AG, Bowes A, & Flottorp S (2010). Effectiveness of telemedicine: a systematic review of reviews. International Journal of Medical Informatics, 79(11), 736–771. [DOI] [PubMed] [Google Scholar]
- Eneva KT, Arlt JM, Yiu A, Murray SM, & Chen EY (2017). Assessment of executive functioning in binge-eating disorder independent of weight status. International Journal of Eating Disorders, 50(8), 942–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, & Paulus MP (2005). Neurobiology of decision making: a selective review from a neurocognitive and clinical perspective. Biological Psychiatry, 58, 597–604. [DOI] [PubMed] [Google Scholar]
- Etkin A (2009). Functional neuroanatomy of anxiety: a neural circuit perspective In Behavioral neurobiology of anxiety and its treatment (pp. 251–277). Springer Berlin; Heidelberg. [DOI] [PubMed] [Google Scholar]
- Frank GK (2015). Recent advances in neuroimaging to model eating disorder neurobiology. Current Psychiatry Reports, 17(4), 22. [DOI] [PubMed] [Google Scholar]
- Frank GK, & Kaye WH (2012). Current status of functional imaging in eating disorders. International Journal of Eating Disorders, 45(6), 723–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederich HC, Wu M, Simon JJ, & Herzog W (2013). Neurocircuit function in eating disorders. International Journal of Eating Disorders, 46, 425–432. [DOI] [PubMed] [Google Scholar]
- Frith U (1989). Autism: Explaining the enigma. Oxford, UK: Blackwell. [Google Scholar]
- Goschke T (2014). Dysfunctions of decision-making and cognitive control as transdiagnostic mechanisms of mental disorders: advances, gaps, and needs in current research. International journal of methods in psychiatric research, 23(S1), 41–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz KL, & Roemer L (2004). Multidimensional assessment of emotion regulation and dysregulation: Development, factor structure, and initial validation of the difficulties in emotion regulation scale. Journal of Psychopathology and Behavioral Assessment, 26(1), 41–54. [Google Scholar]
- Greaves CJ, Sheppard KE, Abraham C, Hardeman W, Roden M, Evans PH, & Schwarz P (2011). Systematic review of reviews of intervention components associated with increased effectiveness in dietary and physical activity interventions. BMC Public Health, 11(1), 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillaume S, Gorwood P, Jollant F, Van den Eynde F, Courtet P, & Richard-Devantoy S (2015). Impaired decision-making in symptomatic anorexia and bulimia nervosa patients: a meta-analysis. Psychological Medicine, 45, 3377–3391. [DOI] [PubMed] [Google Scholar]
- Hansen TI, Haferstrom ECD, Brunner JF, Lehn H, & Håberg AK (2015). Initial validation of a web-based self-administered neuropsychological test battery for older adults and seniors. Journal of Clinical and Experimental Neuropsychology, 37(6), 581–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison A, Tchanturia K, & Treasure J (2011). Measuring state trait properties of detail processing and global integration ability in eating disorders. The World Journal of Biological Psychiatry, 12(6), 462–472. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Chelune GJ, Curtiss G, Kay GG, & Talley JL (1993). Wisconsin card sorting test. Psychological Assessment Resources. [Google Scholar]
- Higgs S (2016). Cognitive processing of food rewards. Appetite, 104, 10–17. [DOI] [PubMed] [Google Scholar]
- Hofmann W, Schmeichel BJ, & Baddeley AD (2012). Executive functions and self-regulation. Trends in Cognitive Sciences, 16(3), 174–180. [DOI] [PubMed] [Google Scholar]
- Holliday J, Tchanturia K, Landau S, Collier D, & Treasure J (2005). Is impaired set-shifting an endophenotype of anorexia nervosa? American Journal of Psychiatry, 162, 2269–2275. [DOI] [PubMed] [Google Scholar]
- Hudson JI, Hiripi E, Pope HG, & Kessler RC (2007). The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biological psychiatry, 61(3), 348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel M, Klein M, Pruessner J, Thaler L, Spilka M, Efanov S, … & Van den Eynde F (2015). N-back task performance and corresponding brain-activation patterns in women with restrictive and bulimic eating-disorder variants: preliminary findings. Psychiatry Research: Neuroimaging, 232(1), 84–91. [DOI] [PubMed] [Google Scholar]
- Jáuregui-Lobera I (2014). Executive functions in anorexia nervosa. Nutricion Hospitalaria, 29, 500–507. [DOI] [PubMed] [Google Scholar]
- Jones A, Di Lemma LC, Robinson E, Christiansen P, Nolan S, Tudur-Smith C, & Field M (2016). Inhibitory control training for appetitive behaviour change: A meta-analytic investigation of mechanisms of action and moderators of effectiveness. Appetite, 97, 16–28. [DOI] [PubMed] [Google Scholar]
- Kanakam N, & Treasure J (2013) A review of cognitive neuropsychiatry in the taxonomy of eating disorders: State, trait, or genetic? Cognitive Neuropsychiatry, 18, 83–114. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Fudge JL, & Paulus M (2009). New insights into symptoms and neurocircuit function of anorexia nervosa. Nature Reviews Neuroscience, 10(8), 573. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Wierenga CE, Bailer UF, Simmons AN, & Bischoff-Grethe A (2013). Nothing tastes as good as skinny feels: the neurobiology of anorexia nervosa. Trends in Neurosciences, 36(2), 110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keel PK, & Brown TA (2010). Update on course and outcome in eating disorders. International Journal of Eating Disorders, 43(3), 195–204. [DOI] [PubMed] [Google Scholar]
- Kessler RM, Hutson PH, Herman BK, & Potenza MN (2016). The neurobiological basis of binge-eating disorder. Neuroscience & Biobehavioral Reviews, 63, 223–238. [DOI] [PubMed] [Google Scholar]
- Kittel R, Brauhardt A, & Hilbert A (2015). Cognitive and emotional functioning in binge-eating disorder: A systematic review. International Journal of Eating Disorders, 48(6), 535–554. [DOI] [PubMed] [Google Scholar]
- Koffarnus MN, Jarmolowicz DP, Mueller ET, & Bickel WK (2013). Changing delay discounting in the light of the competing neurobehavioral decision systems theory: a review. Journal of the Experimental Analysis of Behavior, 99, 32–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster EH, Hoorelbeke K, Onraedt T, Owens M, & Derakshan N (2017). Cognitive control interventions for depression: a systematic review of findings from training studies. Clinical Psychology Review, 53, 79–92. [DOI] [PubMed] [Google Scholar]
- Lamming L, Pears S, Mason D, Morton K, Bijker M, Sutton S, & Hardeman W (2017). What do we know about brief interventions for physical activity that could be delivered in primary care consultations? A systematic review of reviews. Preventive Medicine, 99, 152–163. [DOI] [PubMed] [Google Scholar]
- Lang K, Lopez C, Stahl D, Tchanturia K, & Treasure J (2014). Central coherence in eating disorders: An updated systematic review and meta-analysis. The World Journal of Biological Psychiatry, 15(8), 586–598. [DOI] [PubMed] [Google Scholar]
- Lang K, Stahl D, Espie J, Treasure J, & Tchanturia K (2014). Set shifting in children and adolescents with anorexia nervosa: An exploratory systematic review and meta-analysis. International Journal of Eating Disorders, 47, 394–399. [DOI] [PubMed] [Google Scholar]
- Lang K, & Tchanturia K (2014). A systematic review of central coherence in young people with anorexia nervosa. Journal of Child and Adolescent Behavior, 2(140), 2. [Google Scholar]
- Lavagnino L, Arnone D, Cao B, Soares JC, & Selvaraj S (2016). Inhibitory control in obesity and binge eating disorder: A systematic review and meta-analysis of neurocognitive and neuroimaging studies. Neuroscience & Biobehavioral Reviews, 68, 714–726. [DOI] [PubMed] [Google Scholar]
- Lavender JM, Wonderlich SA, Engel SG, Gordon KH, Kaye WH, & Mitchell JE (2015). Dimensions of emotion dysregulation in anorexia nervosa and bulimia nervosa: A conceptual review of the empirical literature. Clinical Psychology Review, 40, 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmens V, Oenema A, Knut IK, & Brug J (2008). Effectiveness of smoking cessation interventions among adults: a systematic review of reviews. European Journal of Cancer Prevention, 17(6), 535–544. [DOI] [PubMed] [Google Scholar]
- Linetzky M, Pergamin-Hight L, Pine DS, & Bar-Haim Y (2015). Quantitative evaluation of the clinical efficacy of attention bias modification treatment for anxiety disorders. Depression and Anxiety, 32(6), 383–391. [DOI] [PubMed] [Google Scholar]
- Lock J, Garrett A, Beenhakker J, & Reiss AL (2011). Aberrant brain activation during a response inhibition task in adolescent eating disorder subtypes. American Journal of Psychiatry, 168(1), 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez C, Tchanturia K, Stahl D, & Treasure J (2008). Central coherence in eating disorders: a systematic review. Psychological Medicine, 38(10), 1393–1404. [DOI] [PubMed] [Google Scholar]
- Lopez C, Tchanturia K, Stahl D, & Treasure J (2009). Weak central coherence in eating disorders: a step towards looking for an endophenotype of eating disorders. Journal of Clinical and Experimental Neuropsychology, 31(1), 117–125. [DOI] [PubMed] [Google Scholar]
- McClelland J, Dalton B, Kekic M, Bartholdy S, Campbell IC, & Schmidt U (2016). A systematic review of temporal discounting in eating disorders and obesity: Behavioural and neuroimaging findings. Neuroscience and Biobehavioral Reviews, 71, 506–528. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Mathews A, & Tata P (1986). Attentional bias in emotional disorders. Journal of Abnormal Psychology, 95(1), 15–20. [DOI] [PubMed] [Google Scholar]
- McTeague LM, Goodkind MS, & Etkin A (2016). Transdiagnostic impairment of cognitive control in mental illness. Journal of Psychiatric Research, 83, 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniglio R (2009). The impact of child sexual abuse on health: A systematic review of reviews. Clinical Psychology Review, 29(7), 647–657. [DOI] [PubMed] [Google Scholar]
- Miyake Y, Okamoto Y, Onoda K, Kurosaki M, Shirao N, Okamoto Y, & Yamawaki S (2010). Brain activation during the perception of distorted body images in eating disorders. Psychiatry Research: Neuroimaging, 181(3), 183–192. [DOI] [PubMed] [Google Scholar]
- Monzon BM, Hay P, Foroughi N, & Touyz S (2016). White matter alterations in anorexia nervosa: A systematic review of diffusion tensor imaging studies. World Journal of Psychiatry, 6(1), 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RC, Swendsen J, & Depp CA (2017). Applications for self-administered mobile cognitive assessments in clinical research: A systematic review. International Journal of Methods in Psychiatric Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison GE, Simone CM, Ng NF, & Hardy JL (2015). Reliability and validity of the NeuroCognitive Performance Test, a web-based neuropsychological assessment. Frontiers in Psychology, 6, 1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, & Buhle JT (2012). Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences, 1251(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odum AL (2011). Delay Discounting: I’m a k, you’re a k. Journal of the Experimental Analysis of Behavior, 96, 427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okon-Singer H, Hendler T, Pessoa L, & Shackman AJ (2015). The neurobiology of emotion–cognition interactions: fundamental questions and strategies for future research. Frontiers in Human Neuroscience, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson JK, Nordin S, Sequeira H, & Polich J (2008). Affective picture processing: An integrative review of ERP findings. Biological Psychology, 77(3), 247–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterrieth P (1944). The test of copying a complex figure: A contribution to the study of perception and memory. Arch Psych, 30, 206– 356. [Google Scholar]
- Posner MI (1980). Orienting of attention. Quarterly Journal of Experimental Psychology, 32, 3–25. [DOI] [PubMed] [Google Scholar]
- Powell DJ, McMinn D, & Allan JL (2017). Does real time variability in inhibitory control drive snacking behavior? An intensive longitudinal study. Health Psychology, 36(4), 356. [DOI] [PubMed] [Google Scholar]
- Pruis TA, Keel PK, & Janowsky JS (2012). Recovery from anorexia nervosa includes neural compensation for negative body image. International Journal of Eating Disorders, 45(8), 919–931. [DOI] [PubMed] [Google Scholar]
- Reitan RM (1992). Trail Making Test: Manual for administration and scoring. Reitan Neuropsychology Laboratory. [Google Scholar]
- Reville MC, O’Connor L, Frampton I (2016). Literature review of cognitive neuroscience and anorexia nervosa. Current Psychiatry Reports, 18, 18. [DOI] [PubMed] [Google Scholar]
- Roberts ME, Tchanturia K, & Treasure JL (2013). Is attention to detail a similarly strong candidate endophenotype for anorexia nervosa and bulimia nervosa? The World Journal of Biological Psychiatry, 14(6), 452–463. [DOI] [PubMed] [Google Scholar]
- Rolls ET (2013). A biased activation theory of the cognitive and attentional modulation of emotion. Frontiers in Human Neuroscience, 7, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt U, Adan R, Böhm I, Campbell IC, Dingemans A, Ehrlich S, … & Himmerich H (2016). Eating disorders: the big issue. The Lancet Psychiatry, 3(4), 313–315. [DOI] [PubMed] [Google Scholar]
- Shea BJ, Hamel C, Wells GA, Bouter LM, Kristjansson E, Grimshaw J, … & Boers M (2009). AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. Journal of Clinical Eepidemiology, 62(10), 1013–1020. [DOI] [PubMed] [Google Scholar]
- Sliwinski MJ, Mogle JA, Hyun J, Munoz E, Smyth JM, & Lipton RB (2018). Reliability and validity of ambulatory cognitive assessments. Assessment, 25(1), 14–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass JG, & Vanderwart M (1980). A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. Journal of Experimental Psychology: Human Learning and Memory, 6(2), 174–215. [DOI] [PubMed] [Google Scholar]
- Snyder HR, Miyake A, & Hankin BL (2015). Advancing understanding of executive function impairments and psychopathology: bridging the gap between clinical and cognitive approaches. Frontiers in Psychology, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto D, Heinke D, Humphreys GW, & Blanco MJ (2005). Early, involuntary top-down guidance of attention from working memory. Journal of Experimental Psychology: Human Perception and Performance, 31(2), 248. [DOI] [PubMed] [Google Scholar]
- Steinberg L (2005). Cognitive and affective development in adolescence. Trends in Cognitive Sciences, 9(2), 69–74. [DOI] [PubMed] [Google Scholar]
- Steinglass JE, & Walsh BT (2016). Neurobiological model of the persistence of anorexia nervosa. Journal of Eating Disorders, 4(1), 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward T, Menchón JM, Jiménez-Murcia S, Soriano-Mas C, & Fernández-Aranda F (2017). Neural network alterations across eating disorders: a narrative review of fMRI studies. Current Neuropharmacology. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E (2001). A prospective test of the dual-pathway model of bulimic pathology: mediating effects of dieting and negative affect. Journal of Abnormal Psychology, 110(1), 124. [DOI] [PubMed] [Google Scholar]
- Stice E, & Shaw H (2017). Eating disorders: Insights from imaging and behavioral approaches to treatment. Journal of Psychopharmacology. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Stojek MMK, & MacKillop J (2017) Relative reinforcing value of food and delayed reward discounting in obesity and disordered eating: A systematic review. Clinical Psychology Review, 55, 1–11. [DOI] [PubMed] [Google Scholar]
- Stroop J. r. (1935). Studies of interference in serial verbal reactions. Journal of Experimental Psychology, 18, 643–662. [Google Scholar]
- Svaldi J, Schmitz F, Trentowska M, Tuschen-Caffier B, Berking M, & Naumann E (2014). Cognitive interference and a food-related memory bias in binge eating disorder. Appetite, 72, 28–36. [DOI] [PubMed] [Google Scholar]
- Talbot A, Hay P, Buckett G, & Touyz S (2015). Cognitive deficits as an endophenotype for anorexia nervosa: An accepted fact or a need for re-examination? International Journal of Eating Disorders, 48(1), 15–25. [DOI] [PubMed] [Google Scholar]
- Tchanturia K, Lounes N, & Holttum S (2014). Cognitive remediation in anorexia nervosa and related conditions: a systematic review. European Eating Disorders Review, 22, 454–462. [DOI] [PubMed] [Google Scholar]
- Tenconi E, Santonastaso P, Degortes D, Bosello R, Titton F, Mapelli D, & Favaro A (2010). Set-shifting abilities, central coherence, and handedness in anorexia nervosa patients, their unaffected siblings and healthy controls: exploring putative endophenotypes. The World Journal of Biological Psychiatry, 11(6), 813–823. [DOI] [PubMed] [Google Scholar]
- Val-Laillet D, Aarts E, Weber B, Ferrari M, Quaresima V, Stoeckel LE, … & Stice E (2015). Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity. NeuroImage: Clinical, 8, 1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Autreve S, & Vervaet M (2015). Are There differences in central coherence and set shifting across the subtypes of anorexia nervosa? A systematic review. The Journal of Nervous and Mental Disease, 203, 774–780. [DOI] [PubMed] [Google Scholar]
- Van den Eynde F, Guillaume S, Broadbent H, Stahl D, Campbell I, Schmidt U, & Tchanturia K (2011). Neurocognition in bulimic eating disorders: A systematic review. Acta Psychiatrica Scandinavica, 124(2), 120–140. [DOI] [PubMed] [Google Scholar]
- Voon V (2015). Cognitive biases in binge eating disorder: The hijacking of decision making. CNS Spectrums, 20(06), 566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1981). WAIS-R manual: Wechsler adult intelligence scale-revised. New York: Psychological Corporation. [Google Scholar]
- Weider S, Indredavik MS, Lydersen S, & Hestad K (2015). Neuropsychological function in patients with anorexia nervosa or bulimia nervosa. International Journal of Eating Disorders, 48(4), 397–405. [DOI] [PubMed] [Google Scholar]
- Westwood H, Stahl D, Mandy W, & Tchanturia K (2016). The set-shifting profiles of anorexia nervosa and autism spectrum disorder using the Wisconsin Card Sorting Test: a systematic review and meta-analysis. Psychological Medicine, 46, 1809–1827. [DOI] [PubMed] [Google Scholar]
- Wierenga CE, Ely A, Bischoff-Grethe A, Bailer UF, Simmons AN, & Kaye WH (2014a). Are extremes of consumption in eating disorders related to an altered balance between reward and inhibition? Frontiers in Behavioral Neuroscience, 8, 410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga CE, Bischoff-Grethe A, Melrose AJ, Grenesko-Stevens E, Irvine Z, Wagner A, … & Kaye WH (2014b). Altered BOLD response during inhibitory and error processing in adolescents with anorexia nervosa. PloS one, 9(3), e92017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildes JE, & Marcus MD (2013). Incorporating dimensions into the classification of eating disorders: three models and their implications for research and clinical practice. International Journal of Eating Disorders, 46(5), 396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin HA (2002). Group embedded figures test. California: Mind garden. [Google Scholar]
- Wolz I, Fagundo AB, Treasure J, & Fernández-Aranda F (2015). The Processing of Food Stimuli in Abnormal Eating: A Systematic Review of Electrophysiology. European Eating Disorders Review, 23(4), 251–261. 10.1002/erv.2366 [DOI] [PubMed] [Google Scholar]
- Wu M, Brockmeyer T, Hartmann M, Skunde M, Herzog W, & Friederich HC (2014). Set-shifting ability across the spectrum of eating disorders and in overweight and obesity: a systematic review and meta-analysis. Psychological Medicine, 44, 3365–3385. [DOI] [PubMed] [Google Scholar]
- Wu M, Brockmeyer T, Hartmann M, Skunde M, Herzog W, & Friederich HC (2016). Reward-related decision making in eating and weight disorders: A systematic review and meta-analysis of the evidence from neuropsychological studies. Neuroscience and Biobehavioral Reviews, 61, 177–196. [DOI] [PubMed] [Google Scholar]
- Wu M, Hartmann M, Skunde M, Herzog W, & Friederich H (2013). Inhibitory control in bulimic-type eating disorders: A systematic review and meta-analysis. PloS One, 8(12), e83412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykes T, Huddy V, Cellard C, McGurk SR, & Czobor P (2011). A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. American Journal of Psychiatry, 168(5), 472–485. [DOI] [PubMed] [Google Scholar]
- Zakzanis KK, Campbell Z, & Polsinelli A (2010). Quantitative evidence for distinct cognitive impairment in anorexia nervosa and bulimia nervosa. Journal of Neuropsychology, 4(1), 89–106. [DOI] [PubMed] [Google Scholar]
- Zastrow A, Kaiser S, Stippich C, Walther S, Herzog W, Tchanturia K, … & Friederich HC (2009). Neural correlates of impaired cognitive-behavioral flexibility in anorexia nervosa. American Journal of Psychiatry, 166, 608–616. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.