Abstract
Objectives:
The aim of this study was to estimate the frequency of chromosomal abnormalities and establish the association with clinical of factors such as secondary sexual characters and gonad development in primary amenorrhea (PA).
Study Design:
The study was carried out in a large cohort of PA. The chromosomal aberrations were correlated with secondary sexual characters and anatomical abnormalities.
Materials and Methods:
The data of 490 cases of PA were collected retrospectively. The chromosomal preparations were done from the peripheral blood and subjected to giemsa-trypsin-giemsa banding and karyotyped according to the International System of Human Cytogenetic Nomenclature 2013. The fluorescence in situ hybridization was carried out using centromeric and whole painting probes for X and Y chromosome.
Statistical Analysis:
Statistical analysis of the data was performed using online version of social science statistics software.
Results:
A high frequency of abnormal uterus (81.9%) and ovaries (86.7%) were detected in our study. A total of 121 (24.7%) cases were identified with abnormal karyotype. The numerical chromosomal abnormalities were identified in 53 (43.8%) cases while structural abnormalities were identified in 32 (26.4%) cases. The XY karyotype was detected in 29.8% females with PA. The PA individuals with anatomical abnormalities (84.3%) had a high frequency (24.6%) of chromosomal aberrations.
Conclusions:
The present study concluded that cytogenetics plays an important role in precise diagnosis which helps in the management of PA. The cytogenetic analysis should be carried out to know the genetic basis of PA.
KEYWORDS: Chromosomal abnormalities, karyotype, mosaicism, primary amenorrhea, secondary sexual characters, ultrasonography
INTRODUCTION
Primary amenorrhea (PA) is defined as no menstruation by the age of 14 in the absence of growth or development of secondary sexual characters, as also no menstruation by the age of 16 regardless of the presence of normal growth and development with the appearance of secondary sexual characters.[1] According to World Health Organization studies, 15% of the population are infertile and amenorrhea is the sixth largest major cause of female infertility and 2%–5% of all women affected with PA are in childbearing age.[2]
The normal menstruation is regulated by the pituitary gland which is controlled by hypothalamus. While defining amenorrhea along with menstruation, secondary sexual characters such as pubic and axillary hair and breast development are given due importance. The estrogen is an ovarian hormone which causes duct growth in the breasts resulting in breast enlargement at puberty in girls and is also responsible for the development of other secondary sexual characters. Depending on these anatomical and physiological principles of menstruation, the various etiological factors of amenorrhea can be compartmentalized. Overall, it is estimated that endocrine disorders cause PA in approximately 40% of the cases, while the remaining 60% having developmental (genetic or structural) origins.[3] Frequency of chromosomal abnormalities in PA ranges from 16% to 64%.[4] In our study, the types of chromosomal abnormalities were analyzed and correlated with secondary sexual character and anatomical abnormalities.
MATERIALS AND METHODS
The study was carried out retrospectively on four hundred and ninety (490) individuals with PA referred from 2005 to 2015 to our cytogenetic laboratory. After informed written consent from the patients, the clinical examination was done to determine the provisional diagnosis. All necessary clinical details such as age, height, secondary sexual characters (breast, pubic, and axillary hair development), and radiological details were recorded in the case record sheet. The study protocols were approved by Institutional Ethics Committee.
Chromosomal preparations were obtained from the peripheral blood cultures briefly; 0.5 ml of peripheral blood was added to RPMI 1640 medium (9 ml), supplemented with fetal calf serum (1 ml), L-glutamine (0.1 ml), and antibiotics (penicillin and streptomycin) and stimulated with phytohemagglutinin incubated at 37°C for 72 h; then, the culture was treated with a hypotonic solution (0.075M KCL) and fixed with Carnoy's fixative (Methanol: Acetic acid 3:1).[5] The cell pellets were diluted and dropped on prechilled slides. The chromosomal preparations were subjected to giemsa-trypsin-giemsa (GTG) banding.[6] The chromosomal analysis was carried out from 50 well-spreaded and good-banded metaphases under Nikon 90i microscope, and images were captured with charge-coupled device camera and karyotyped according to International System of Human Cytogenetic Nomenclature 2013.[7] Fluorescence in situ hybridization (FISH) was carried out by standard method using centromeric and whole chromosome painting probes for X and Y chromosome (Vysis, Abbott Molecular Inc., Des Plaines, IL, USA).[8] The statistical analysis of the data was performed using online version of social science statistics software (www.socscistatistics.com).
RESULTS
The age of the patients with PA was ranged between 10 and 34 years, and the mean age was 19.20 ± 37 years. The clinical abnormality observed in individuals with PA is presented in Figure 1. A high frequency of abnormal uterus (81.9%) and ovaries (86.7%) were observed in our study. The observed frequency of clinical features including short stature, absence of pubic and axillary hairs, and breast development (<tanner Stage 3) were 45.4%, 66.1%, and 78.5% respectively. The cytogenetic study revealed chromosomal abnormalities in 121 (24.7%) of 490 PA cases [Table 1]. The types of chromosomal abnormality detected in our study were monosomy X, iso (Xq), and dic (X) [Figure 2]. In our series, the frequency of the numerical chromosomal changes (43.8%) was found to be high as compared to the structural aberrations (26.4%). A high (29.8%) incidence of 46, XY karyotype was detected in females with PA. The X chromosome mosaicism was identified in 24 (19.8%) cases of PA.
Figure 1.
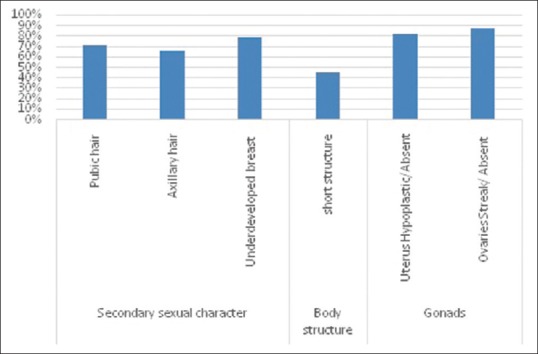
Clinical abnormality frequency in 490 cases with primary amenorrhea
Table 1.
Frequency and type of chromosomal abnormalities in primary amenorrhea
| Cytogenetic category | Karyotype | n (%) |
|---|---|---|
| Normal | 46, XX | 369 (75.3) |
| Chromosomal abnormality | 121 (24.7) | |
| 1. Numerical abnormalities | 53 (43.8) | |
| a. Pure Turner’s syndrome | (45, X) | 28 (23.2) |
| b. Trisomy X | 47, XXX | 1 (0.8) |
| Mosaicism of X | - | 24 (19.8) |
| 45, X/46, XX | 17 (14.0) | |
| 45, X/46, XY | 3 (2.4) | |
| 46, XX/47, XXX | 3 (2.4) | |
| 45, X/46, XX/47, XXX | 1 (0.8) | |
| 2. Structural abnormalities | - | 32 (26.4) |
| a. Deletion Xq | 46, X, del (Xq) | 10 (8.3) |
| b. Isochromosome | 46, X, iso (Xq) | 9 (7.4) |
| c. Translocation | 46, X, t (X; A) | 4 (3.3) |
| d. Idic | 46, X, idic (X) p | 1 (0.8) |
| e. Marker chromosome | 46, X+marker | 4 (3.3) |
| f. Inversion 9 | 46, XX, inv (9) 46, XY | 4 (3.3) |
| 3. Male karyotype | 46, XY | 36 (29.8) |
Figure 2.
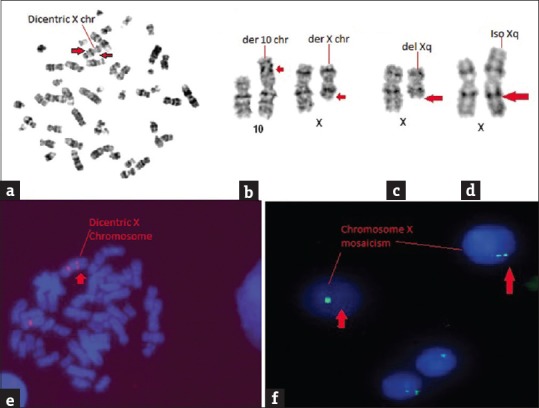
Types of X chromosome abnormalities. (a) Metaphase showing dicentric X chromosome, (b) Partial karyotype of t (10; X), (c) Partial karyotype of del (Xq), (d) Partial karyotype of iso (Xq), (e) FISH showing dicentric X chromosome using centromeric probe, (f) X chromosome mosaicism in interphase cells by fluorescence in situ hybridization
The correlation of cytogenetic abnormalities with the height of the patients is presented in Table 2. Of 121 chromosomally abnormally PA individuals, 45.4% had a short stature. A high frequency of short stature was noticed in individuals with monosomy X (89.3%) and structural abnormalities of X (71.9%). The data on secondary sexual characters and anatomical features in PA are presented in Table 3. The pubic and axillary hair was absent or sparse in 66.1% of cytogenetically abnormal cases of PA. A high frequency of the absence of pubic hair or axillary hair was noticed in cases of monosomy X (100%) and structural anomalies (62.5%) of PA cases [Table 3a]. The clinical examination of breast revealed a high frequency (78.5%) of underdeveloped (<3 tanner stage) breast in PA cases [Table 3b]. The ultrasonography examination revealed a significantly high frequency of anatomical anomalies i.e. hypoplastic or absence of uterus (81.9%) and streak or absence of ovaries (86.7%) [Table 4].
Table 2.
Association of cytogenetic abnormality and heights of the patient in primary amenorrhea cases
| Serial number | Cytogenetic abnormality | Age (years), mean±SD | Height (cm) |
P (Chi-square test) Level of significance <0.05 | |
|---|---|---|---|---|---|
| <150 (%) | 150 and above (%) | ||||
| 1 | 45, X (28) | 17.04±3.43 | 25 (89.3) | 3 (10.7) | 0.0002 (significant) |
| 2 | Mosaicism X (24) | 19.71±6.54 | 7 (29.2) | 17 (70.8) | |
| 3 | Trisomy X (1) | 17±0.00 | 00 | 1 (100) | |
| 4 | Structural abnormality (32) | 19.84±4.57 | 23 (71.9) | 9 (28.1) | |
| 5 | Male karyotype (36) | 20.28±7.72 | 00 | 36 (100) | |
| Total | 121 | 55 (45.4) | 66 (54.6) | ||
SD=Standard deviation
Table 3a.
Association of cytogenetic abnormality and growth of pubic and axillary hair in primary amenorrhea
| Serial number | Cytogenetic abnormality | Pubic and axillary hair |
||
|---|---|---|---|---|
| Present (%) | Absent/sparse (%) | P (Chi-square test) Level of significance <0.05 | ||
| 1 | 45, X (28) | 0 | 28 (100) | 0.460 (Insignificant) |
| 2 | Mosaicism X (24) | 13 (54.2) | 11 (45.8) | |
| 3 | Trisomy X (1) | 0 | 1 (100) | |
| 4 | Structural abnormality (32) | 12 (37.5) | 20 (62.5) | |
| 5 | Male karyotype (36) | 16 (44.4) | 20 (55.5) | |
| Total | 121 | 41 (33.9) | 80 (66.1) | |
Table 3b.
Association of cytogenetic abnormality and breast development in primary amenorrhea
| Serial number | Cytogenetic abnormality | Breast tanner stage (<3 stage) (%) | Breast tanner stage (>3 stage) (%) | P (Chi-square test) Level of significance <0.05 |
|---|---|---|---|---|
| 1 | 45, X (28) | 28 (100) | 0 (96.4) | 0.928 (Insignificant) |
| 2 | Mosaicism X (24) | 17 (70.9) | 7 (29.1) | |
| 3 | Trisomy X (1) | 00 | 1 (100) | |
| 4 | Structural abnormality (32) | 23 (71.8) | 9 (28.1) | |
| 5 | Male karyotype (36) | 27 (75) | 9 (25) | |
| Total | 121 | 95 (78.5) | 26 (21.5) |
Table 4.
Association of cytogenetic abnormality and anatomical development in primary amenorrhea
| Serial number | Cytogenetic abnormality | Uterus |
Ovary |
||||
|---|---|---|---|---|---|---|---|
| Normal (%) | Hypoplastic/absent (%) | P (Chi-square test) Level of significance <0.05 | Normal (%) | Streak/absent (%) | P (Chi square test) Level of significance <0.05 | ||
| 1 | 45, X (28) | 1 (3.6) | 27 (96.4) | 0.009 (significant) | 0 | 28 (100) | 0.002 (significant) |
| 2 | Mosaicism X (24) | 6 (25) | 18 (75) | 4 (16.7) | 20 (83.3) | ||
| 3 | Trisomy X (1) | 0 | 1 (100) | 0 | 1 | ||
| 4 | Structural abnormality (32) | 11 (34.3) | 21 (65.6) | 11 (34.4) | 21 (65.6) | ||
| 5 | Male karyotype (36) | 4 (11.1) | 32 (88.9) | 1 (2.8) | 35 (97.2) | ||
| Total | 121 | 22 (18.1) | 99 (81.9) | 16 (13.3) | 105 (86.7) | ||
DISCUSSION
PA occurs due to several factors including hormonal imbalance, anatomical abnormalities, genetic factors, and environmental factors. Depending on these various etiological factors, amenorrhea can be compartmentalized into those related to the outflow tract (congenital malformation or receptor insensitivity), the ovary (abnormal or absent germ cells and abnormal folliculogenesis), the anterior pituitary (disrupted gonadotropin production or secretion), and the central nervous system (disrupted hypothalamic factor affecting pituitary signaling). However, chromosomal aberrations, especially sex chromosome play an important role in PA. The chromosomal aberration frequency reported to be ranging from 14% to 60% in PA cases.[3,4,9,10,11,12,13,14,15,16] The overall frequency (24.7%) of chromosome aberrations observed in our study is similar to those reported from different parts of the world [Tables 1 and 5]. As one-fourth of PA cases have chromosome aberrations, cytogenetic evaluation is essential for the diagnosis of PA. Our study highlights the importance of the chromosomal analysis in PA cases. In our cohort the correlation of chromosomal abnormalities with clinical presentation suggests that the numerical (80%) and structural chromosome aberrations (70%) influence the clinical presentation (height, secondary sexual characters, and anatomical abnormalities) [Tables 2–4]. Hence, clinical presentation should be considered as indication of sex chromosome involvement in PA. Monosomy X is commonly seen in Turner syndrome. However, all the cases of Turner's syndrome may not be present with classical clinical features. Short stature, poor secondary sexual characters, and anatomical abnormalities along with PA should be considered for the cytogenetic evaluation. In these cases, germ cells, which trigger early puberty and enhance pubertal growth, start to diminish in the end of first trimester of intrauterine life. Consequently only 10%–15% of affected patients could achieve menarche at expected time point.[17,18,19,20,21,22,23,24] The patients who achieved menarche do not maintain it for long time due to gonadal dysgenesis.[25,26,27] However, time of gonadal dysgenesis vary due to difference in affected region of homologous chromosome.
Table 5.
Chromosomal aberration frequency reported from various studies
| Zone | Study | Authors | Number of cases | Percentage abnormality reported |
|---|---|---|---|---|
| South Asia | India | Roy and Banerjee[11] | 60 | 38 (63.5) |
| Lakshimi Kalpana and Satyanarayana[13] | 70 | 20 (28.57) | ||
| Mondal et al.[14] | 72 | 24 (33.34) | ||
| Rajangam and Nanjappa[3] | 620 | 162 (26.13) | ||
| Hariharan et al.[16] | 51 | 26 (50.8) | ||
| Vijayalakshmi et al.[17] | 140 | 39 (27.85) | ||
| Merin et al.[18] | 246 | 36 (14.64) | ||
| Amin SV et al.[19] | 98 | 20 (20.5) | ||
| Ghosh et al.[20] | 150 | 36 (24) | ||
| Malaysia | Ten et al.[10] | 117 | 36 (30.8) | |
| Pakistan | Rizwan and Abbasi[21] | 19 | 5 (26.32) | |
| Thailand | Tanmahasamut et al.[22] | 295 | 59 (20) | |
| Western Asia | Turkey | Temoçin et al.[12] | 68 | 18 (26.5) |
| Iran | Safaei et al.[23] | 220 | 44 (20) | |
| East Asia | Honkong, PRC | Wong and Lam[4] | 237 | 58 (24.5) |
| South Africa | South Africa | van Niekerk et al.[9] | 77 | 21 (21.7) |
| Egypt | El-Dahtory[24] | 223 | 46 (20.63) | |
| North America | Mexico | Cortés-Gutiérrez et al.[15] | 187 | 78 (41.72) |
Some cases of PA remain undiagnosed due to undetected sex chromosome mosaicism. In our study, 24 (19%) females with PA had sex chromosome (45, X/46, XX, 45, X/46, XY, 46, XX/47, XXX) mosaicism [Table 1]. These cases are difficult to diagnose as clinical presentation is not indicative for chromosomal analysis. Hence, combination of GTG banding and FISH analysis using X and Y chromosome probes is important to identify undetected mosaicism PA. The cases with sex chromosome mosaicism can be well managed with hormonal therapy as they also had normal cell lineage. Hence, our study strongly suggests the application of FISH investigation in cases of X chromosome mosaicism.
The 46, XY karyotype also has been reported in females with PA. In our cohort, a high frequency (29.8%) of 46, XY karyotype is detected in females with PA which is contradictory to previous published literature from India.[11,13,14,16,28] In our study, the frequency of 46, XY karyotype is high compared to reported literature. Although the reason for the high incidence is not known, the application of FISH could be the one of the factors to detect Y chromosome in PA cases. However, the presence of Y chromosome should be confirmed by molecular cytogenetic technique.[17,18,19,21,22,23,24] At the same time, these cases need to be further assessed for any mutation of SRY and SF1 gene and also in the other genes which are responsible for male karyotype in phenotypic female with PA.[28,29,30,31,32] Gonads developed in such cases do not secrete hormones and should be removed at the time of diagnosis.
CONCLUSIONS
The study highlights the importance of cytogenetic investigation in the precise diagnosis and appropriate utilization of FISH in the PA. The X chromosome mosaicism is unnoticed in females with PA as they are phenotypically normal. The interphase FISH should be carried out to rule out the mosaicism. As the genetic factors are limited to chromosome abnormalities, the copy number variations may be playing a role in PA. These variations need to be correlated with clinical abnormalities for better understanding of genetic basis of PA. This will also help PA patient in marital counseling and the future family planning.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
Authors thank residents of Department of Endocrinology, KEM Hospital, Mumbai. Authors also thank technical staff of Department of Cytogenetics. The study was carried out from Institutional core grant.
REFERENCES
- 1.Speroff L, Fritz MA, editors. Amenorrhea: Clinical Gynaecologic Endocrinology and Infertility. 7th ed. Philadelphia: Lippincott Williams; 2005. pp. 401–64. [Google Scholar]
- 2.Hayden CJ. Primary amenorrhea: Investigation and treatment. Obstetrics, Gynaecology and Reproductive Medicine. 2007;17:199–204. [Google Scholar]
- 3.Rajangam S, Nanjappa L. Cytogenetic studies in amenorrhea. Saudi Med J. 2007;28:187–92. [PubMed] [Google Scholar]
- 4.Wong MS, Lam ST. Cytogenetic analysis of patients with primary and secondary amenorrhoea in Hong Kong: Retrospective study. Hong Kong Med J. 2005;11:267–72. [PubMed] [Google Scholar]
- 5.Moorhead PS, Nowell PC, Mellman WJ, Battips DM, Hungerford DA. Chromosome preparations of leukocytes cultured from human peripheral blood. Exp Cell Res. 1960;20:613–6. doi: 10.1016/0014-4827(60)90138-5. [DOI] [PubMed] [Google Scholar]
- 6.Seabright M. A rapid banding technique for human chromosomes. Lancet. 1971;2:971–2. doi: 10.1016/s0140-6736(71)90287-x. [DOI] [PubMed] [Google Scholar]
- 7.Simons A, Shaffer LG, Hastings RJ. Cytogenetic nomenclature: Changes in the ISCN 2013 compared to the 2009 edition. Cytogenet Genome Res. 2013;141:1–6. doi: 10.1159/000353118. [DOI] [PubMed] [Google Scholar]
- 8.Buckle VJ, Rack K. Fluorescent in situ hybridization. In: Davies KE, editor. Human Genetic Diseases: A Practical Approach. Oxford: IRL Press; 1993. pp. 59–80. [Google Scholar]
- 9.van Niekerk WA. Chromosomes and the gynecologist. Am J Obstet Gynecol. 1978;130:862–75. doi: 10.1016/0002-9378(78)90263-6. [DOI] [PubMed] [Google Scholar]
- 10.Ten SK, Chin YM, Noor PJ, Hassan K. Cytogenetic studies in women with primary amenorrhea. Singapore Med J. 1990;31:355–9. [PubMed] [Google Scholar]
- 11.Roy AK, Banerjee D. Cytogenetic study of primary amenorrhoea. J Indian Med Assoc. 1995;93:291–2. [PubMed] [Google Scholar]
- 12.Temoçin K, Vardar MA, Süleymanova D, Ozer E, Tanriverdi N, Demirhan O, et al. Results of cytogenetic investigation in adolescent patients with primary or secondary amenorrhea. J Pediatr Adolesc Gynecol. 1997;10:86–8. doi: 10.1016/s1083-3188(97)70057-3. [DOI] [PubMed] [Google Scholar]
- 13.Lakshimi Kalpana V, Satyanarayana M. Cytogenetic analysis of primary amenorrhea cases. Ind J Hum Genet. 1997;3:81–91. [Google Scholar]
- 14.Mondal SK, Guha D, Banerjee D, Sinha SK. Study of primary amenorrhoea with special reference to cytogenetic evaluation. Indian J Pathol Microbiol. 2002;45:155–9. [PubMed] [Google Scholar]
- 15.Cortés-Gutiérrez EI, Dávila-Rodríguez MI, Vargas-Villarreal J, Cerda-Flores RM. Prevalence of chromosomal aberrations in Mexican women with primary amenorrhoea. Reprod Biomed Online. 2007;15:463–7. doi: 10.1016/s1472-6483(10)60374-4. [DOI] [PubMed] [Google Scholar]
- 16.Hariharan S, Sheeja VR, Santhi S, Vani S, Sreeja L, Ankathil R. Chromosome analysis in patients with primary amenorrhea. Proceeding 19th Kerala Science Congress. 2007:331–2. [Google Scholar]
- 17.Vijayalakshmi J, Koshy T, Kaur H, Andrea F, Selvi R, Parvathi D, et al. Cytogenetic analysis of patients with primary amenorrhea. Int J Hum Genet. 2010;10:71–6. [Google Scholar]
- 18.Merin T, Rema D, Preetha T, Amuda S, Jayalakshamma J, Mary M. Amenorrhoea: Cytogenetic studies and beyond. Am J Mol Cell Biol. 2012;1:25–32. [Google Scholar]
- 19.Amin SV, Rai L, Palpandi P, Kumaran A. Ever intriguing 'primary amenorrhea'- an audit. Int J Reprod Contracept Obstet Gynecol. 2014;3:1090–6. [Google Scholar]
- 20.Ghosh S, Roy S, Pal P, Dutta A, Halder A. Cytogenetic analysis of patients with primary amenorrhea in Eastern India. J Obstet Gynaecol. 2018;38:270–5. doi: 10.1080/01443615.2017.1353595. [DOI] [PubMed] [Google Scholar]
- 21.Rizwan N, Abbasi RM. Primary amenorrhea and the outcome of treatment at Liaquat university hospital. J Liaquat Univ Med Health Sci. 2008;7:110–4. [Google Scholar]
- 22.Tanmahasamut P, Rattanachaiyanont M, Dangrat C, Indhavivadhana S, Angsuwattana S, Techatraisak K, et al. Causes of primary amenorrhea: A report of 295 cases in thailand. J Obstet Gynaecol Res. 2012;38:297–301. doi: 10.1111/j.1447-0756.2011.01677.x. [DOI] [PubMed] [Google Scholar]
- 23.Safaei A, Vasei M, Hossein AH. Cytogenetic analysis of patients with primary amenorrhea in Southwest of Iran. Iran J Pathol. 2010;5:121–5. [Google Scholar]
- 24.El-Dahtory F. Chromosomal abnormalities and hormonal disorders of primary amenorrhea patients in Egypt. Indian J Hum Genet. 2012;18:183–6. doi: 10.4103/0971-6866.100758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hovatta O. Ovarian function and in vitro fertilization (IVF) in turner syndrome. Pediatr Endocrinol Rev. 2012;9(Suppl 2):713–7. [PubMed] [Google Scholar]
- 26.Cabanes L, Chalas C, Christin-Maitre S, Donadille B, Felten ML, Gaxotte V, et al. Turner syndrome and pregnancy: Clinical practice. Recommendations for the management of patients with turner syndrome before and during pregnancy. Eur J Obstet Gynecol Reprod Biol. 2010;152:18–24. doi: 10.1016/j.ejogrb.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 27.Reindollar RH, Novak M, Tho SP, McDonough PG. Adult-onset amenorrhea: A study of 262 patients. Am J Obstet Gynecol. 1986;155:531–43. doi: 10.1016/0002-9378(86)90274-7. [DOI] [PubMed] [Google Scholar]
- 28.Naz M, Khanum S, Niaz A, Fatima U. Frequency of disturbance of hormonal profile (LH to FSH ratio) in girls of age group 14-18 years with primary amenorrhea. J Univ Med Dent Coll. 2013;4:30–5. [Google Scholar]
- 29.Paliwal P, Sharma A, Birla S, Kriplani A, Khadgawat R, Sharma A, et al. Identification of novel SRY mutations and SF1 (NR5A1) changes in patients with pure gonadal dysgenesis and 46, XY karyotype. Mol Hum Reprod. 2011;17:372–8. doi: 10.1093/molehr/gar002. [DOI] [PubMed] [Google Scholar]
- 30.Manuel M, Katayama PK, Jones HW., Jr The age of occurrence of gonadal tumors in intersex patients with a Y chromosome. Am J Obstet Gynecol. 1976;124:293–300. doi: 10.1016/0002-9378(76)90160-5. [DOI] [PubMed] [Google Scholar]
- 31.Kara N, Tural S, Elbistan M, Karakus N, Guven D, Kocak I. Cytogenetic findings of patients with amenorrhea in Turkish population. Int J Hum Genet. 2012;12:87–92. [Google Scholar]
- 32.Joseph A, Thomas IM. Cytogenetic investigations in 150 cases with complaints of sterility or primary amenorrhea. Hum Genet. 1982;61:105–9. doi: 10.1007/BF00274197. [DOI] [PubMed] [Google Scholar]


