We thank the authors of the letter for sharing their views on this article. We pursued the publishing of these data in an attempt to clearly define and further characterize patients with a monthly response rate of 100% to galcanezumab. The purpose of this article was to dispel the very notion that a significant number of patients had 100% response across the entire treatment period, which the authors of the Letter to the Editor raised. The title is meant to reflect the article content related to the characterization of patients with episodic migraine who achieved 100% monthly response (ie, 100% reduction from baseline in monthly migraine headache days) to galcanezumab treatment. The use of the terms “response rate” and “responder rates” is standard terminology used in clinical trials/clinical trial guidelines for migraine preventive therapies (http://www.ihs-headache.org/binary_data/158_clinical-trials-controlled-trials-of-drugs-in-migraine-3rd-ed-cha.pdf). Whether speaking of ≥50%, ≥75%, or 100% reduction of migraine headache days, the terms “response rate” and “responder rates” are common and appropriate terminology to use.
We disagree with the suggestion by the authors’ Letter to the Editor that the month‐by‐month analysis was unconventional or the results presented, which included the odds ratios, were misleading. The primary and secondary outcomes for these trials, such as the number of monthly migraine headache days and the proportions of patients with ≥50%, ≥75%, or 100% response, were measured monthly (at each visit). These outcomes capture the patients’ response during any given month between 2 separate doses. In this paper, we presented the observed as well as the model‐estimated number and proportions of patients with 100% response at each month (Supplemental Table S1 and Fig. 1, respectively) from a generalized linear mixed effects model estimate. This well‐accepted model is a robust method of analyzing longitudinal data in clinical trials and is basically a mixed‐models repeated measures version of a logistic regression (a standard analysis approach for binary outcomes for which odds ratios are readily available). The primary analysis approach of looking at the average across months yields a standard measure of monthly response seen by patients across the entire dosing month period. Such an estimate is neither biased toward higher response seen in the later months of treatment nor overly penalized by the lower responses observed in the earlier months of treatment. What is represented is the estimated proportions of patients who are expected to have a 100% response (ie, 0 migraine headache days) on an average month (averaged across all visits) and is not the same as the proportions of patients with 100% response in any 1 out of the 6 months. The proportions of patients with at least 1 month with 100% response is approximately 40%. In addition to presenting the raw month‐by‐month numbers, we also present odds ratios of 100% response for treatment compared to placebo, which is a standard output for logistical regression analyses. There was no intention to mislead the readers, but rather to provide results as is standard for these types of data. Therefore, we do not agree with the authors’ suggestion that the outcome measured is of “very questionable worth.” To the contrary, although the new analyses in the manuscript are post hoc (and were indicated as such), and should be interpreted as such, we believe that they provide clinicians with better insight into what patients might expect. We feel it is more relevant for a clinician to inform a patient with episodic migraine that in 2 clinical trials, a given percentage of patients had at least 1, 2, 3, or more months of migraine freedom than a population‐level assessment of 100% response rates in an average month.
Figure 1.
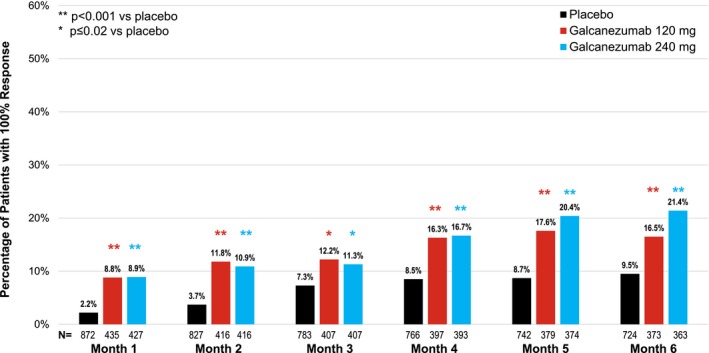
Patients with 100% response by month. N = sample size for treatment group.
In conclusion, we do feel that a possibility of 100% response in any given month with galcanezumab treatment may be very significant to patients. With 14% of patients on galcanezumab treatment achieving 100% response vs 6% on placebo, the numbers needed to treat (NNT) to have 1 additional person with a 100% monthly response would be 12 to 13 (specifically 12.5). This is much more realistic than an NNT = 125, which would represent 6 consecutive months of migraine freedom from the onset of treatment. We sincerely believe that this article provides further characterization that should allow clinicians to set appropriate expectations regarding the efficacy of galcanezumab in patients with episodic migraine. We appreciate the authors of the letter for providing us the opportunity to clarify.
Supporting information
Conflict of Interest: Noah Rosen has received research support from Allergan, Axon Optics, and Theranica, and is on the advisory board for Allergan, Teva, Eli Lilly, Supernus, and Promius. He serves as consultant for Curelator and also on the speakers’ bureau for Allergan. Eric Pearlman, Dustin Ruff, and Kathleen Day are employees of Eli Lilly and Company, and/or one of its subsidiaries, Indianapolis, IN, USA. Abraham Jim Nagy has received research support from Alder, Allergan, Lilly, and Teva, and is on the advisory board for Amgen, Lilly, Pernix, Supernus, Teva, and Upsher‐Smith. He serves as a consultant for Xenon, Zosana, and Impel, and also on the speakers’ bureau for Amgen, Avanir, Electrocore, and Teva.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


