Abstract
Problem
During normal pregnancy, delicate crosstalk is established between fetus‐derived trophoblasts and maternal immune cells to ensure maternal‐fetal tolerance and successful placentation. Dysfunction in these interactions has been highly linked to certain pregnancy complications.
Method of study
Naïve CD4+T cells were cultivated with or without 1st trimester derived trophoblast cell line HTR8/SVneo cells in the absence or presence of T helper 17 (Th17) or regulatory (Treg)cell‐inducing differentiation conditions. After 5 days of co‐culture, HTR8/SVneo cells and CD4+T cells were harvested and analyzed using flow cytometry.
Results
CD4+T cells exposed to HTR8/SVneo cells showed enhanced induction of CD4+Foxp3+Treg cells with strong expression of TGF‐β1 and inhibitory molecules (cytotoxic T lymphocyte‐associated protein‐4 [CTLA‐4], T‐cell immunoglobulin mucin‐3 [Tim‐3], and programmed cell death‐1 [PD‐1]). Though not effecting Th17 differentiation, exposure to HTR8/SVneo cells promoted increased expression of proliferative and apoptotic markers on Th17 cells. Co‐culture with Th0 cells, or differentiated Th17 or Treg cells, down‐regulated Caspase‐3 and MMP‐9 (but not MMP‐2) expression in HTR8/SVneo cells, while promoting Ki67 expression.
Conclusions
HTR8/SVneo cells regulated maternal CD4+T‐cell differentiation, resulting in the expansion of immunosuppressive Treg cells, while CD4+T cells might promote the growth, and control the invasiveness of HTR8/SVneo cells. Thus, a bidirectional regulatory loop might exist between trophoblasts and maternal immune cell subsets, thereby promoting harmonious maternal‐fetal crosstalk.
Keywords: HTR8/SVneo cells, pregnancy, Th17 cells, Treg cells, trophoblasts
1. INTRODUCTION
The establishment of maternal‐fetal tolerance and successful placentation are key events during early pregnancy. Under normal conditions, the maternal immune system accepts the semi‐allogeneic fetus, which protects both mother and fetus against infection. Disruption of this immune balance, however, causes the placenta and fetus to be attacked as a foreign organ transplant, resulting in pregnancy failure.1 Extravillous trophoblasts (EVT), the main cell type involved in the placentation process, invade the underlying decidua, dissolve the extracellular matrix (ECM), and migrate into the uterine spiral arteriolar walls, remodeling the uterine vasculature. Inadequate EVT invasion has been closely associated with several pregnancy‐associated diseases, including recurrent pregnancy loss (RPL), pre‐eclampsia (PE), and gestational trophoblastic diseases.2
T‐cell subsets, especially CD4+ helper T (Th) cells, play a pivotal role in successful pregnancy.3 Driven by a set of transcriptional regulators and cytokines, naive CD4+T cells are able to differentiate into distinct subsets, including Th1, Th2, Th17, and Treg cells.4 A polarization toward Th2 bias in the maternal immune response has long been considered the main mechanism of tolerance induction toward the fetus.5 According to recent studies, the balance between regulatory (Treg) and T helper 17 (Th17) cells is also important in the maintenance of normal pregnancy, whereas the shift in the Th17/Treg ratio toward Th17 cells has been proposed as a cause for several pregnancy‐associated diseases, such as RPL, PE, and gestational diabetes mellitus.6, 7, 8
Decidual immune cells (DICs) not only regulate the maternal immune response to promote fetal semi‐allograft tolerance but also mediate the implantation and trophoblast invasion.9, 10 At the same time, trophoblasts mediate interactions between the fetus and mother for the exchange of nutrients, gases, waste products, as well as for the regulation of immune tolerance.11 EVTs are potential candidates for educating maternal immune cells to generate a tolerant microenvironment at the maternal‐fetal interface. At present, studies regarding the interaction between trophoblasts and DICs have shown that trophoblast has the unique ability of instructing DICs to develop a regulatory phenotype for fetal tolerance.12, 13, 14, 15 However, the regulatory effect of trophoblasts on CD4+T‐cell differentiation, especially on Th17/Treg differentiation, remains poorly understood. Knowledge regarding the influence of Th17/Treg differentiation on the biological behaviors of trophoblasts is also limited.
Based on the aforementioned observations, we assumed that trophoblasts might affect Th17/Treg cell differentiation from naive CD4+T cells, leading to the induction of Treg cell expansion at the maternal‐fetal interface. In turn, differentiated CD4+T cells might affect the biological functions of trophoblasts. Based on this hypothesis, we investigated the effect of trophoblasts on Th17/Treg cell differentiation from naive CD4+T cells and trophoblast phenotypic markers of function modulated by Th17/Treg cells generated in vitro using the immortalized human first‐trimester EVT cell line HTR8/SVneo,16 which has been widely used as a substitute for human primary trophoblasts.
2. MATERIALS AND METHODS
2.1. Human samples
This study was approved by the Human Research Ethics Committee of the Obstetrics and Gynecology Hospital, Fudan University. All participants signed a written informed consent form. Heparinized peripheral blood was obtained from pregnant women who had one or more previous normal pregnancies without any miscarriage, excluding those diagnosed with endocrine diseases, tumor, infection, etc (n = 18, mean age [years]: 29.7 ± 0.6, range: 25‐35). Samples were immediately collected for isolation of peripheral blood mononuclear cells (PBMCs).
2.2. Isolation of human cells
PBMCs were isolated from peripheral blood samples of pregnant women using Ficoll (Huajing, China) density gradient centrifugation. Naïve CD4+T cells were isolated through magnetic affinity cell sorting using the Naïve CD4+T Isolation Kit II (MiltenyiBiotec, Germany). An antibody cocktail (biotin‐conjugated monoclonal antibodies against CD8, CD14, CD15, CD16, CD19, CD25, CD34, CD36, CD45RO, CD56, CD123, TCRγ/δ, HLA‐DR, and Glycophorin A) and anti‐biotin microbeads were added to PBMCs following the manufacturer's instructions. The suspension of cells (naïve CD4+T cells) was recovered in a new tube, while magnetically labeled unwanted cells remained bound to the original tube through the magnetic field. The obtained cells were fluorescently stained for CD45RA and CD4. Approximately 3 × 106 naïve CD4+T cells with a purity of around 99% were obtained from each woman. Cells were extensively washed and resuspended in RPMI 1640 (HyClone, USA) supplemented with 10% fetal bovine serum (FBS, Gibco, USA), 100 U/mL penicillin, 100 μg/mL streptomycin, and 1 μg/mL amphotericin B (Sangon Biotech, China).
2.3. In vitro differentiation of Th17 and Treg cells
Naïve CD4 T cells were cultured in precoated plates with anti‐CD3 (5 μg/mL, Clone OKT‐3, BioLegend, USA) + anti‐CD28 (1 μg/mL, Clone CD28.2,BioLegend, USA) antibodies and maintained in media supplemented with IL‐2 (10 ng/mL, PeproTech, USA) (Group Th0). Recombinant IL‐6 (50 ng/mL, PeproTech, USA), recombinant TGF‐β1 (10 ng/mL, PeproTech, USA), anti‐IFN‐γ mAb (10 μg/mL, Clone B27, BioLegend, USA), and anti‐IL‐4 mAb (10 μg/mL, Clone 8D4‐8, BioLegend, USA) were added to generate Th17 cells (Group Th17). Recombinant TGF‐β1 (50 ng/mL, PeproTech, USA), anti‐IL‐6 mAb (10 μg/mL, Clone MQ2‐13A5, BioLegend, USA), anti‐IFN‐γ mAb (10 μg/mL, Clone B27, BioLegend, USA), and anti‐IL‐4 mAb (10 μg/mL, Clone 8D4‐8, BioLegend, USA) were added to generate Treg cells (Group Treg). Media were changed every 48 hours. This protocol was based on previous publications.17, 18 For intracellular cytokine analysis, brefeldin A (10 mg/mL, BioLegend, USA), phorbol 12‐myrstate 13‐acetate (PMA)(50 ng/mL, BioLegend, USA), and ionomycin (1 μg/mL, BioLegend, USA) were added 4 hours before the end of the culture. The cells were then harvested, stained, and analyzed through flow cytometry. After 5 days of culture, 8.3 ± 1.7% of CD4+IL‐17A+ cells and 13.2 ± 1.7% of CD4+Foxp3+ cells were obtained.
2.4. Co‐culture of CD4+T and HTR8/SVneo cells
HTR8/SVneo cells (2 × 105) were initially cultured in 6‐well flat‐bottom plates in RPMI 1640 medium supplemented with 10% FBS the day before co‐culture with naïve CD4+T cells. When the adherent HTR8/SVneo cells reached 50%, 4 × 105 Th0, Th17, and Treg cells (according to the different differentiation agents mentioned previously) were seeded on the top of the HTR8/SVneo layer for 5 days in media comprising different differentiation agents. After induction, the number of CD4+T cells in each well was approximately 2 × 106. While in the co‐culture system, the cell number was approximately double in this amount. To exclude the effect of HTR8/SVneo cell proliferation on CD4+T cells, HTR8/SVneo cells were pre‐treated with mitomycin C for 30 min (10 mM) in some wells. For intracellular cytokine analysis of CD4+T cells, brefeldin A (10 mg/mL, BioLegend, USA), PMA (50 ng/mL, BioLegend, USA), and ionomycin (1 μg/mL, BioLegend, USA) were added 4 hours before the end of the culture. HTR8/SVneo cells and CD4+T cells were then harvested, stained, and analyzed through flow cytometry. After culture, the proportion of live cells (assessed by the 7‐AAD− cells) was around 90%.
2.5. Flow cytometry
Cell surface molecular expression and intracellular cytokine production were evaluated using flow cytometry. AlexaeFluor® 488‐conjugated anti‐human Foxp3 or MMP‐9; FITC‐conjugated anti‐human CD4; PE‐conjugated anti‐human CD45RA, MMP‐2, T‐cell immunoglobulin mucin‐3 (Tim‐3), or TGF‐β1; PE/CY7‐conjugated anti‐human IL‐10, CD45 or IL‐17A; PerCP‐conjugated anti‐human CD4; AlexaeFluor® 647‐conjugated anti‐human Caspase‐3, APC‐conjugated anti‐human CTLA‐4; Brilliant Violet 421‐conjugated anti‐human Ki‐67 or IL‐17a; Brilliant Violet 510‐conjugated anti‐human CD45 or PD‐1 antibodies (BioLegend, USA) were used. Details regarding Ab are presented in Table S1. For intracellular staining, cells were fixed and permeabilized using the Fix/Perm kit (BioLegend, USA). Flow cytometry was performed on a Beckman‐Coulter CyAn ADP cytometer and analyzed with FlowJo software (Tree Star, USA).
2.6. Statistical analysis
Normally distributed variables were presented as means and SD. ANOVA was used to evaluate differences in normally distributed variables with homogeneity of variance among groups. Variables with skewed distribution were described using median and inter‐quartile range. The Kruskal‐wallis test was used to assess the difference in variables with skewed distribution among groups. The Bonferroni multiple comparisons test was performed for variables that showed a significant difference with Kruskal‐Wallis test. A P value of <0.05 was considered significant. For variables with a P value less than 0.05 following ANOVA, the post hoc Dunnett t test was performed to determine differences between each group. All analyses were carried out using the GraphPad Prism 5 software (GraphPad, San Diego, CA).
3. RESULTS
3.1. Trophoblasts contribute to Treg cell differentiation from maternal Naïve CD4+T cells
To examine whether trophoblasts influence the differentiation of Th17/Treg cells, Naïve CD4+T cells from PBMCs of pregnant women were cultivated with or without human EVT cell line HTR‐8/SVneo cells in the absence or presence of Th17‐/Treg‐inducing differentiation conditions (described in the Methods section). As depicted in Figure 1A, A mix of differentiated Th17 cells (8.3 ± 1.7% of CD4+IL‐17A+ cells) and non‐differentiated CD4+T cells was obtained under Th17 induction. IL‐17 expression in Treg‐polarized cells was approximately 1.7 ± 0.2% (Figure S1). HTR8/SVneo cells had no effect on Th17 cell differentiation as the proportion of CD4+IL‐17A+ cells did not vary after co‐culture with HTR8 cells.
Figure 1.
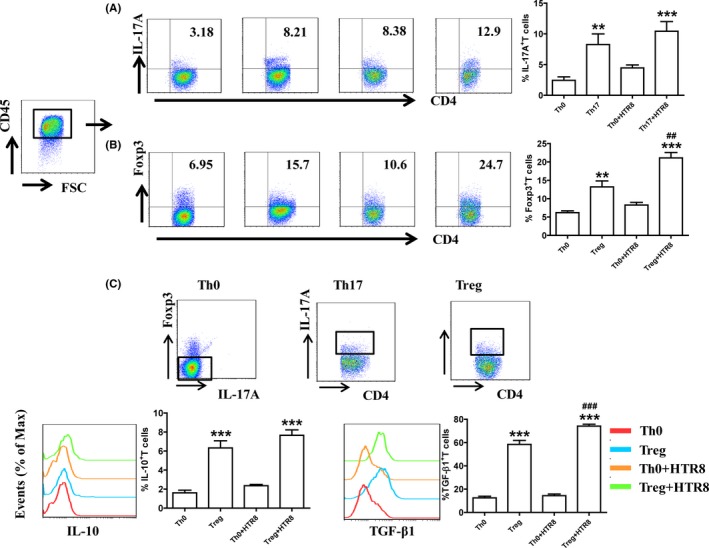
HTR8/SVneo cells contributed to the differentiation of Treg cells from maternal naïve CD4+T cells. (A) Maternal naïve CD4+T cells were differentiated in the presence of IL‐2 (Th0) or IL‐2 + TGF‐β1 + IL‐6 + anti‐IFN‐γ mAb + anti‐IL‐4 mAb (Th17) for 5 days. In some wells, CD4+T cells were co‐cultured with HTR8/SVneo cells. IL‐17A expression in T cells was analyzed through flow cytometry. (B and C) Maternal naïve CD4+T cells were differentiated in the presence of IL‐2 (Th0) or IL‐2 + TGF‐β1 + anti‐IL‐6 mAb + anti‐IFN‐γ mAb + anti‐IL‐4 mAb (Treg) for 5 days. In some wells, CD4+T cells were co‐cultured with HTR8/SVneo cells. Foxp‐3 expression and IL‐10 and TGF‐β1 production in CD4+T cells were analyzed through flow cytometry. **P < 0.01, ***P < 0.001, compared to the Th0 group. ##P < 0.01, ###P < 0.001, compared to Treg group. Data are represented as the mean ± SD, n = 18. Flow cytometry plots are representative of three independent experiments
Treg cells were differentiated as described in the Methods section. As shown in Figure 1B,C, 13.2 ± 1.7% of differentiated Treg cells (CD4+Foxp3+ cells) were obtained accompanied by up‐regulation of IL‐10 and TGF‐β1 expression. The proportion of CD4+Foxp3+ cells and the expression of TGF‐β1 increased after co‐culture with HTR8/SVneo cells. Foxp3 expression in Th17‐polarized cells was approximately 3.5 ± 0.2% (Figure S1). To exclude the effect of HTR8/SVneo cell proliferation on CD4+T cells, we used mitomycin C to inhibit the proliferation of HTR8/SVneo cells and found that HTR8/SVneo cell proliferation did not affect CD4+T‐cell differentiation (Figure S2). These results support the fact that HTR8/SVneo cells increase the frequency of Treg cells after in vitro differentiation.
3.2. Trophoblasts regulate the function of differentiated Th17/Treg cells
To directly assess whether HTR8/SVneo cells regulated the biological functions of Th17/Treg cells generated in vitro, CD4+T‐cell apoptosis and proliferation were analyzed. As shown in Figure 2A, apoptosis (assessed by the expression of Caspase‐3) of generated Th17 cells was higher than that of Th0 cells but lower than that of Th17 cells co‐cultured with HTR8/SVneo cells. Meanwhile, Treg cell apoptosis decreased after co‐culture with HTR8/SVneo cells. HTR8/SVneo cells also increased the proliferation (assessed by the expression of Ki‐67) of Th17 cell, but had no effect on Treg cell proliferation (Figure 2B). Taken together, these data indicated that HTR8/SVneo cells promoted the renewal of Th17 cells and inhibited the apoptosis of Treg cells.
Figure 2.
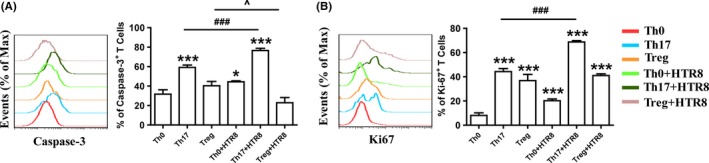
HTR8/SVneo cells regulated the apoptosis and proliferation of differentiated T cells. Maternal naïve CD4+T cells were differentiated in the presence of IL‐2 (Th0), IL‐2 + TGF‐β1 + IL‐6 + anti‐IFN‐γ mAb + anti‐IL‐4 mAb (Th17) or IL‐2 + TGF‐β1 + anti‐IL‐6 mAb + anti‐IFN‐γ mAb + anti‐IL‐4 mAb (Treg) for 5 days. In some wells, T cells were co‐cultured with HTR8/SVneo cells. Caspase‐3 (A) and Ki‐67 (B) expression in CD4+T cells was analyzed through flow cytometry.*P < 0.05, ***P < 0.001, compared to Th0 group. ###P < 0.001, compared to Th17 group. ^P < 0.05, compared to Treg group. Data are represented as the mean ± SD, n = 18. Flow cytometry plots are representative of three independent experiments
Inhibitory receptors on immune cells, such as CTLA‐4, Tim‐3, and PD‐1 regulate the T cell–mediated immune response and have been proposed as functional markers of specific T‐cell subsets.19, 20 Next, we analyzed CTLA‐4, Tim‐3, and PD‐1 expression in in vitro‐generated Th17/Treg cells cultivated with or without HTR8 cells. As shown in Figure 3, compared to Th0 cells, Th17 cells expressed higher levels of Tim‐3 and PD‐1, while Treg cells expressed more CTLA‐4, Tim‐3, and PD‐1. HTR8/SVneo cells increased the expression of all inhibitory receptors in Treg cells, but had no influence on their expression in Th17 cells.
Figure 3.
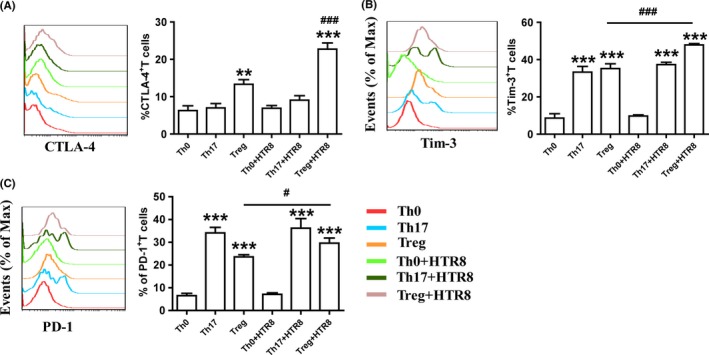
HTR8/SVneo cells promoted the expression of inhibitory receptors in differentiated Treg cells. Maternal naïve CD4+T cells were differentiated in the presence of Th0‐, Th17‐, or Treg‐inducing differentiation agents for 5 days. In some wells, CD4+T cells were co‐cultured with HTR8/SVneo cells. Cytotoxic T lymphocyte‐associated protein‐4 (CTLA‐4) (A), Tim‐3 (B), and programmed cell death‐1 (PD‐1) (C) expression in CD4+T cells was analyzed through flow cytometry. **P < 0.01, ***P < 0.001, compared to Th0 group. #P < 0.05, ###P < 0.001, compared to Treg group. Data are represented as the mean ± SD, n = 18. Flow cytometry plots are representative of three independent experiments
3.3. CD4+T cells modulate the biological behaviors of trophoblast
We then studied whether in vitro‐generated Th17/Treg cells regulated the biological behaviors of HTR8/SVneo cells. After co‐culture with Th0, Th17, or Treg cell, HTR8/SVneo cell apoptosis (assessed using Caspaase‐3+ cells) was inhibited, while HTR8/SVneo cell proliferation (assessed using the Ki‐67+ cells) was promoted (Figure 4A,B). MMP‐2 and MMP‐9 are two important gelatinases involved in ECM remodeling during trophoblast invasion.21 The effects of CD4+T cells on MMP‐2 and MMP‐9 expression were examined using flow cytometry. As shown in Figure 4C,D, CD4+T cells decreased MMP‐9 production in HTR8/SVneo cells, but had no effect on MMP‐2 expression.
Figure 4.
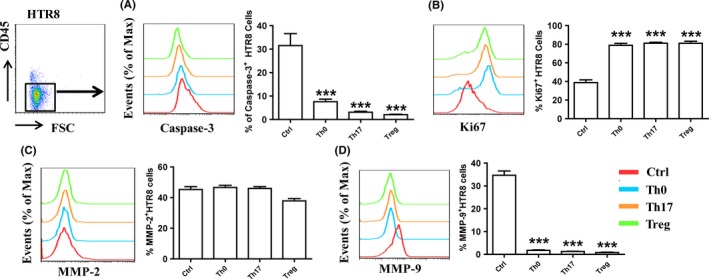
Differentiated CD4+T cells modulated the biological functions of HTR8/SVneo cells. HTR8/SVneo cells were co‐cultured with Th0, Th17 and Treg cells (with the indicated agents) for 5 days. Caspase‐3 (A), Ki‐67 (B), MMP‐2 (C) and MMP‐9 (D) expression in HTR8/SVneo cells was analyzed through flow cytometry.***P < 0.001, compared to the Ctrl group. Data are represented as the mean ± SD. Flow cytometry plots are representative of three independent experiments
4. DISCUSSION
A successful pregnancy relies on the maternal immune system's tolerance toward the semi‐allogeneic fetus and sufficient placental formation. As such, a complex and reciprocal interaction between the maternal immune system and fetal trophoblasts is required to maintain normal pregnancy. The present study utilized the well‐characterized human first‐trimester EVT cell line HTR8/SVneo to demonstrate the notable role of these cells in regulating CD4+T‐cell differentiation, resulting in the expansion of immunosuppressive Treg cells. Meanwhile, CD4+T cells also modulated the biological functions of HTR8/SVneo cells. To our knowledge, this study presented novel findings suggesting a unique maternal‐fetal co‐culture system could be used to regulate the Th17/Treg ratio and that a positive regulatory loop might exist between trophoblasts and maternal immune cell subsets, promoting the harmonious maternal‐fetal crosstalk.
Upon encountering antigens presented by antigen presenting cells or being driven by a set of cytokines, naive CD4+T cells are able to differentiate into distinct subsets, including Th1, Th2, Th17, and Treg cells.22 Th2 bias at the maternal‐fetal interface has long been considered as the main mechanism of tolerance induction toward the fetus.5 Th17 cells play a critical role in inducing of inflammation, while abnormal Th17 cell levels have been associated with the pathogenesis of autoimmune diseases.23 Treg cells express anti‐inflammatory cytokines such as IL‐10 and TGF‐β1, which dampen an excessively effective immune response.24 Studies have proposed that alterations in the balance between Th17 and Treg cells may be associated with the development of RSA.25, 26, 27
Although an appropriate pro‐inflammatory response is a prerequisite for successful implantation and defense against pathogens, multiple regulatory mechanisms are also required to balance maternal immune cell‐trophoblast interaction throughout gestation, which will ultimately determine the fate of pregnancy. Specifically, the fine balance between Th17 and Treg cells mediates maternal tolerance to the fetus.7 In the present study, though HTR8/SVneo cells promoted Th17 cell proliferation,they also up‐regulated the expression of apoptotic marker in Th17 cells. HTR8/SVneo cell‐conditioned CD4+T cells showed enhanced induction of the CD4+Foxp3+Treg population with potent TGF‐β1 production and inhibited Treg cell apoptosis, suggesting that the interaction between EVTs and immune cells might contribute to the in situ generation of a set of tolerogenic Treg cells and regulate the Th17/Treg ratio to help maintain normal pregnancy. While Svensson et al have shown that trophoblasts induce functionally suppressive Treg cells,15 the present study used an alternative procedure wherein direct cellular interactions and the ability to further polarize Treg cells were evaluated. Of course, further in vitro experiments using primary trophoblasts and in vivo functional and pathological studies are needed to understand the interaction between trophoblasts and maternal CD4+T cells.
Inhibitory molecules such as CTLA‐4, Tim‐3, or PD‐1, play a role in regulating Th17 and Treg cell function to maintain a balanced immune response.28, 29, 30, 31 For example, Tim‐3 has been associated with a shift in the balance from Th17 and Treg cells to Treg dominance.30 Moreover, PD‐1 and CTLA‐4 are critical in the suppressive activity of Treg cells,32, 33 while PD‐1+Tim‐3+Treg cells exhibit higher effector function.34 HTR8/SVneo cells, as well as primary trophoblasts but not decidual stromal cells (data not shown) or OVC1 (an ovarian cancer cell line), up‐regulated CTLA‐4, Tim‐3, and PD‐1 expression in Treg cells along with TGF‐β1 production, indicating that fetal‐derived trophoblasts were pivotal inducers of maternal immune adaptation. HLA‐G, which is selectively expressed in cytotrophoblasts and invasive EVTs and also expressed in HTR8/SVneo cells,35, 36 was responsible for the training function of Trophblast (data not shown). Moreover, given that HTR8/SVneo cells could promote CD4+T‐cell activation (assessed through CD69 expression, data not shown), we determined that HTR8/SVneo cells at least partially instructed the CD4+T‐cell phenotype by activating these cells through HLA‐G.
With its high proliferative ability, invasive properties, and capacity to avoid immune attack, the human placenta has been regarded as a pseudo‐malignant structure in which a subpopulation of placental trophoblasts erode the uterus, its underlying stroma and local blood vessels to achieve adequate maternal‐fetal exchange of molecules. However, unlike tissue invasion by malignant tumor cells, trophoblast invasion to the uterus is temporally and locally controlled by hormones, cytokines, and functional cells at the maternal‐fetal interface.34 Accordingly, inadequate EVT growth and invasion have been closely associated with several pregnancy‐associated diseases.2 Like tumor invasion, MMPs are major regulators of tissue remodeling during pregnancy, with gelatinases MMP‐2 and MMP‐9 being involved in trophoblast invasion to the spiral arteries. In cultured trophoblasts, suppression of MMP‐9 expression inhibits their invasive capability.37 After co‐culture with Th0 cells, or differentiated Th17 or Treg cells, Caspase‐3 and MMP‐9 (but not MMP‐2) expression in HTR8/SVneo cells was down‐regulated, while Ki67 expression was promoted, suggesting that CD4+T cells promoted the growth of trophoblasts and controlled the invasiveness of human trophoblasts. MMP‐2 is the main gelatinase involved in the function of early first‐trimester trophoblast (6‐8 weeks). In the late first trimester (8‐12 weeks), however, both MMP‐2 and MMP‐9 participate in trophoblast invasion.38 Accordingly, our findings show that CD4+T cells down‐regulated MMP‐9 but not MMP‐2 expression in HTR8/SVneo cells. As such, the interaction between CD4+T cells and trophoblast might play a role in the prevention of excessive trophoblast invasion in the late first trimester of pregnancy.
Though Th17 cells had been reported to participate in pregnancy‐related pathologies,39 our study and others40 showed that Th17 cells played important roles in regulating trophoblast function. Recent findings have also indicated that not all Th17 cells are pro‐inflammatory given that Th17 subpopulations with diverse functions may exist.41 More investigations are needed to explore whether non‐pathogenic Th17 subsets and pro‐inflammatory Th17 subpopulations co‐exist at the maternal‐fetal interface, which subsets are involved in the functional regulation of trophoblast, and which mechanisms are used to modulate Th17 subsets during pregnancy.
Questions remain regarding the validity of using immortalized cell lines to represent the in vivo environment,15, 42 though HTR8/SVneo cells have been proven effective for recapitulating key aspects of EVTs.43 Nonetheless, the interaction between primary trophoblasts and naïve CD4+T cells and the related mechanisms still require further study. Moreover, further ELISA assay and flow cytometry sorting technology would be needed to determine cytokine production by different T‐cell subsets. Despite these limitations, the results presented in the present study undoubtedly demonstrated that trophoblasts might influence the differentiation of naïve CD4+T cells, while CD4+T cells could in turn modulate the biological functions of trophoblasts, resulting in Treg expansion and adequate trophoblast invasion during pregnancy.
CONFLICT OF INTEREST
The authors disclose no conflict of interests.
Supporting information
Wang S, Qian J, Sun F, et al. Bidirectional regulation between 1st trimester HTR8/SVneo trophoblast cells and in vitro differentiated Th17/Treg cells suggest a fetal‐maternal regulatory loop in human pregnancy. Am J Reprod Immunol. 2019;81:e13106 10.1111/aji.13106
Funding information
This work was supported by grant from the Nature Science Foundation from National Nature Science Foundation of China (NSFC) (31700799 to SCW, 81630036, 91542116 and 31570920 to MRD), the National Basic Research Program of China (2015CB943300 to DJL and MRD), the National Key R&D Program of China (2017YFC1001403 to DJL and MRD), the Program of Shanghai Academic/Technology Research Leader (17XD1400900 to MRD), the Innovation‐oriented Science and Technology Grant from NHC Key Lab of Reproduction Regulation (CX2017‐2 to MRD), the Shanghai Sailing Program (17YF1411600 to SCW), the Training Program for Young Talents of Shanghai Health System (2018YQ07 to SCW), Shanghai Chenguang Program (18CG09 to SCW), Development Fund of Shanghai Talents (2018110 to SCW) and the Introduction Project of Suzhou Clinical Medicine Expert Team (grant SZYJTD201708 to DJL).
Songcun Wang and Jingfeng Qian contributed equally to this manuscript.
Contributor Information
Meirong Du, Email: dmrlq1973@sina.cn.
Dajin Li, Email: djli@shmu.edu.cn.
REFERENCES
- 1. Arck PC, Hecher K. Fetomaternal immune cross‐talk and its consequences for maternal and offspring's health. Nat Med. 2013;19(5):548‐556. [DOI] [PubMed] [Google Scholar]
- 2. Al‐Khan A, Bulmer JN, Chantraine F, et al. IFPA Meeting 2012 Workshop Report III: trophoblast deportation, gestational trophoblastic disease, placental insufficiency and fetal growth restriction, trophoblast over‐invasion and accreta‐related pathologies, placental thrombosis and fibrinolysis. Placenta. 2013;34(Suppl):S11‐S16. [DOI] [PubMed] [Google Scholar]
- 3. Figueiredo AS, Schumacher A. The T helper type 17/regulatory T cell paradigm in pregnancy. Immunology. 2016;148(1):13‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zeng W, Liu Z, Liu X, et al. Distinct Transcriptional and alternative splicing signatures of decidual CD4(+) T cells in early human pregnancy. Front Immunol. 2017;8:682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chaouat G, Ledee‐Bataille N, Dubanchet S, et al. Reproductive immunology 2003: reassessing the Th1/Th2 paradigm? Immunol Lett. 2004;92(3):207‐214. [DOI] [PubMed] [Google Scholar]
- 6. Sheu A, Chan Y, Ferguson A, et al. A proinflammatory CD4(+) T cell phenotype in gestational diabetes mellitus. Diabetologia. 2018;61(7):1633‐1643. [DOI] [PubMed] [Google Scholar]
- 7. Muyayalo KP, Li ZH, Mor G, Liao AH. Modulatory effect of intravenous immunoglobulin on Th17/Treg cell balance in women with unexplained recurrent spontaneous abortion. Am J Reprod Immunol. 2018;80(4):e13018. [DOI] [PubMed] [Google Scholar]
- 8. Hosseini A, Dolati S, Hashemi V, Abdollahpour‐Alitappeh M, Yousefi M. regulatory T and T helper 17 cells: their roles in preeclampsia. J Cell Physiol. 2018;233(9):6561‐6573. [DOI] [PubMed] [Google Scholar]
- 9. Laskarin G, Medancic SS, Redzovic A, Duric D, Rukavina D. Specific decidual CD14(+) cells hamper cognate NK cell proliferation and cytolytic mediator expression after mucin 1 treatment in vitro. J Reprod Immunol. 2012;95(1–2):36‐45. [DOI] [PubMed] [Google Scholar]
- 10. Blois SM, Klapp BF, Barrientos G. Decidualization and angiogenesis in early pregnancy: unravelling the functions of DC and NK cells. J Reprod Immunol. 2011;88(2):86‐92. [DOI] [PubMed] [Google Scholar]
- 11. Huppertz B, Weiss G, Moser G. Trophoblast invasion and oxygenation of the placenta: measurements versus presumptions. J Reprod Immunol. 2014;101–102:74‐79. [DOI] [PubMed] [Google Scholar]
- 12. Du MR, Guo PF, Piao HL, et al. Embryonic trophoblasts induce decidual regulatory T cell differentiation and maternal‐fetal tolerance through thymic stromal lymphopoietin instructing dendritic cells. J Immunol. 2014;192(4):1502‐1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang SC, Li YH, Piao HL, et al. PD‐1 and Tim‐3 pathways are associated with regulatory CD8+ T‐cell function in decidua and maintenance of normal pregnancy. Cell Death Dis. 2015;6:e1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang S, Zhu X, Xu Y, et al. Programmed cell death‐1 (PD‐1) and T‐cell immunoglobulin mucin‐3 (Tim‐3) regulate CD4+ T cells to induce Type 2 helper T cell (Th2) bias at the maternal‐fetal interface. Hum Reprod. 2016;31(4):700‐711. [DOI] [PubMed] [Google Scholar]
- 15. Svensson‐Arvelund J, Mehta RB, Lindau R, et al. The human fetal placenta promotes tolerance against the semiallogeneic fetus by inducing regulatory T cells and homeostatic M2 macrophages. J Immunol. 2015;194(4):1534‐1544. [DOI] [PubMed] [Google Scholar]
- 16. Graham CH, Hawley TS, Hawley RC, et al. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res. 1993;206(2):204‐211. [DOI] [PubMed] [Google Scholar]
- 17. Sekiya T, Kashiwagi I, Yoshida R, et al. Nr4a receptors are essential for thymic regulatory T cell development and immune homeostasis. Nat Immunol. 2013;14(3):230‐237. [DOI] [PubMed] [Google Scholar]
- 18. Sekiya T, Yoshimura A. In vitro Th differentiation protocol. Methods Mol Biol. 2016;1344:183‐191. [DOI] [PubMed] [Google Scholar]
- 19. Wykes MN, Lewin SR. Immune checkpoint blockade in infectious diseases. Nat Rev Immunol. 2018;18(2):91‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kudo M. Immuno‐oncology in hepatocellular carcinoma: 2017 update. Oncology‐Basel. 2017;93(Suppl 1):147‐159. [DOI] [PubMed] [Google Scholar]
- 21. Zhang H, Hou L, Li CM, Zhang WY. The chemokine CXCL6 restricts human trophoblast cell migration and invasion by suppressing MMP‐2 activity in the first trimester. Hum Reprod. 2013;28(9):2350‐2362. [DOI] [PubMed] [Google Scholar]
- 22. Liao W, Lin JX, Leonard WJ. IL‐2 family cytokines: new insights into the complex roles of IL‐2 as a broad regulator of T helper cell differentiation. Curr Opin Immunol. 2011;23(5):598‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Crome SQ, Wang AY, Levings MK. Translational mini‐review series on Th17 cells: function and regulation of human T helper 17 cells in health and disease. Clin Exp Immunol. 2010;159(2):109‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jutel M, Akdis M, Budak F, et al. IL‐10 and TGF‐beta cooperate in the regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy. Eur J Immunol. 2003;33(5):1205‐1214. [DOI] [PubMed] [Google Scholar]
- 25. Sereshki N, Gharagozloo M, Ostadi V, et al. Variations in T‐helper 17 and regulatory T cells during the menstrual cycle in peripheral blood of women with recurrent spontaneous abortion. Int J Fertil Steril. 2014;8(1):59‐66. [PMC free article] [PubMed] [Google Scholar]
- 26. Sha J, Liu F, Zhai J, Liu X, Zhang Q, Zhang B. Alteration of Th17 and Foxp3(+) regulatory T cells in patients with unexplained recurrent spontaneous abortion before and after the therapy of hCG combined with immunoglobulin. Exp Ther Med. 2017;14(2):1114‐1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wu L, Luo L, Zhang Y, et al. Alteration of Th17 and Treg cells in patients with unexplained recurrent spontaneous abortion before and after lymphocyte immunization therapy. Reprod Biol Endocrin. 2014;12:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cooksley H, Riva A, Katzarov K, et al. Differential expression of immune inhibitory checkpoint signatures on antiviral and inflammatory T cell populations in chronic hepatitis B. J Interferon Cytokine Res. 2018;38(7):273‐282. [DOI] [PubMed] [Google Scholar]
- 29. Zhao J, Lin B, Deng H, et al. Decreased expression of TIM‐3 on Th17 cells associated with ophthalmopathy in patients with graves' disease. Curr Mol Med. 2018;18(2):83‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wei CB, Tao K, Jiang R, et al. Quercetin protects mouse liver against triptolide‐induced hepatic injury by restoring Th17/Treg balance through Tim‐3 and TLR4‐MyD88‐NF‐kappaB pathway. Int Immunopharmacol. 2017;53:73‐82. [DOI] [PubMed] [Google Scholar]
- 31. Zhang Y, Liu Z, Tian M, et al. The altered PD‐1/PD‐L1 pathway delivers the 'one‐two punch' effects to promote the Treg/Th17 imbalance in pre‐eclampsia. Cell Mol Immunol. 2018;15(7):710‐723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liao H, Peng X, Gan L, et al. Protective regulatory T cell immune response induced by intranasal immunization with the live‐attenuated pneumococcal vaccine SPY1 via the transforming growth factor‐beta1‐smad2/3 pathway. Front Immunol. 2018;9:1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kawai K, Uchiyama M, Hester J, Wood K, Issa F. Regulatory T cells for tolerance. Hum Immunol. 2018;79(5):294‐303. [DOI] [PubMed] [Google Scholar]
- 34. Gupta S, Thornley TB, Gao W, et al. Allograft rejection is restrained by short‐lived TIM‐3+PD‐1+Foxp3+ Tregs. J Clin Invest. 2012;122(7):2395‐2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kovats S, Main E, Librach C, Stubblebine M, Fisher S, DeMars R. A class I antigen, HLA‐G, expressed in human trophoblasts. Science. 1990;248(4952):220‐223. [DOI] [PubMed] [Google Scholar]
- 36. Trinh QD, Izumi Y, Komine‐Aizawa S, et al. H3N2 influenza A virus replicates in immortalized human first trimester trophoblast cell lines and induces their rapid apoptosis. Am J Reprod Immunol. 2009;62(3):139‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yu Y, Wang L, Liu T, Guan H. MicroRNA‐204 suppresses trophoblast‐like cell invasion by targeting matrix metalloproteinase‐9. Biochem Biophys Res Commun. 2015;463(3):285‐291. [DOI] [PubMed] [Google Scholar]
- 38. Staun‐Ram E, Goldman S, Gabarin D, Shalev E. Expression and importance of matrix metalloproteinase 2 and 9 (MMP‐2 and ‐9) in human trophoblast invasion. Reprod Biol Endocrinol. 2004;2:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fu B, Tian Z, Wei H. TH17 cells in human recurrent pregnancy loss and pre‐eclampsia. Cell Mol Immunol. 2014;11(6):564‐570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wu HX, Jin LP, Xu B, Liang SS, Li DJ. Decidual stromal cells recruit Th17 cells into decidua to promote proliferation and invasion of human trophoblast cells by secreting IL‐17. Cell Mol Immunol. 2014;11(3):253‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee Y, Awasthi A, Yosef N, et al. Induction and molecular signature of pathogenic TH17 cells. Nat Immunol. 2012;13(10):991‐999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Orendi K, Kivity V, Sammar M, et al. Placental and trophoblastic in vitro models to study preventive and therapeutic agents for preeclampsia. Placenta. 2011;32(Suppl):S49‐54. [DOI] [PubMed] [Google Scholar]
- 43. Shan N, Zhang X, Xiao X, et al. Laminin alpha4 (LAMA4) expression promotes trophoblast cell invasion, migration, and angiogenesis, and is lowered in preeclamptic placentas. Placenta. 2015;36(8):809‐820. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


