Abstract
Two randomized, double‐blind, placebo‐controlled studies demonstrated responses (≥50 000/μL) to fostamatinib in adults with long‐standing immune thrombocytopenia (ITP). The long‐term safety and efficacy of fostamatinib were evaluated in a follow‐on, open‐label extension (OLE) study. Patients received double‐blind fostamatinib in the randomized trials, and responders continued the same dose, 100 to 150 mg BID, in the OLE study. Nonresponders received 100 mg BID for 4 weeks and could escalate to 150 mg BID at week 4. Endpoints included stable response, platelet count ≥50 000/μL at 4/6 biweekly (randomized trials) or 2/3 monthly visits (OLE), and overall response, ≥1 platelet count ≥50 000/μL during weeks 1 to 12. A total of 146 patients received fostamatinib including 123 in the OLE study. Median treatment duration was 6.7 months. Baseline median ITP duration was 8 years and median platelet count was 16 000/μL; prior treatments included thrombopoietic (TPO) agents (47%), splenectomy (35%), and rituximab (32%). Twenty‐seven (18%) patients achieved a stable response with median duration of >28 months and a median platelet count of 89 000/μL. Sixty‐four (44%) patients achieved an overall response (including stable responders) with a median platelet count of 63 000/μL and a median response duration of >28 months. Twenty‐four of 71 (34%) patients who had failed TPO agents achieved overall responses to fostamatinib. The most common adverse events (AEs) were diarrhea, hypertension, nausea, epistaxis, and abnormal liver function tests. Most AEs were mild/moderate and resolved or were managed with dose reduction, dose interruption, and/or secondary medication. Almost half of the patients achieved an overall response, and most of these maintained their responses for >2 years. No new or increased frequency of AEs was seen at up to 31 months of treatment.
1. INTRODUCTION
Immune thrombocytopenia (ITP) is an acquired autoimmune bleeding disorder with a prevalence of approximately 60 000 adults in the United States1, 2, 3 and an estimated incidence of 26.8 cases per million persons in Northern Europe, suggesting that the annual global incidence is over 200 000.4 When platelet counts are low, bleeding of varying degrees of severity may occur from mucosal bleeding to intracranial hemorrhage.5, 6, 7
ITP is primarily caused by autoantibodies to platelets, which accelerate phagocytosis and destruction of platelets by macrophages in the spleen and also inhibit platelet production.8, 9, 10 The binding of the Fc region of antiplatelet autoantibodies to Fc‐gamma receptors on macrophages activates the spleen tyrosine kinase (SYK) signaling pathway involved in the cytoskeletal rearrangements that initiate phagocytosis.11, 12, 13, 14 Therefore, inhibition of SYK has been a therapeutic target for treatment of ITP, and a reduction in antibody‐mediated platelet destruction has been demonstrated in rodent models of ITP and human studies.15, 16
A variety of different treatments can be given to increase the platelet count as needed to alleviate symptoms and stop/prevent bleeding in patients with ITP. Treatments designed to achieve a rapid‐onset substantial increase in the platelet count include high‐dose corticosteroids, intravenous immunoglobulins (IVIg), and IV anti‐RhD immunoglobulin (anti‐D). Most patients use these first‐line treatments and respond initially but relapse. Although splenectomy often has a curative effect, it is unsuccessful in approximately 33% of patients,17 and there is a trend to avoid splenectomy in favor of available effective medical treatments.18, 19, 20 Therefore, current approaches have focused on second‐line treatments, which were designed to maintain hemostatic platelet counts over weeks, months, and years, for example, thrombopoietic (TPO) agents, rituximab, immunosuppressives, and recently fostamatinib, a SYK inhibitor.
Rituximab was initially thought to provide curative effects in up to 50% of patients but more recent information suggests only a 21%‐30% “cure rate,” primarily in women within 1 year of the onset of ITP.21, 22, 23, 24 The TPO agents are effective long‐term agents in up to 60% of patients, but other therapies are needed for most patients who either do not achieve adequate platelet counts or who respond but cannot discontinue their medication.25, 26, 27 Fostamatinib is a potent SYK inhibitor.16 A phase 2 study demonstrated significant improvement in platelet counts in 8 of 16 patients with chronic ITP.15 Subsequently, two identical, 24‐week, randomized, double‐blind, placebo‐controlled, phase 3 multicenter studies demonstrated stable responses in 17% of patients on fostamatinib versus 2% of patients on placebo (P = 0.007) and clinically meaningful overall platelet responses (≥50 000/μL) in 43% of patients on fostamatinib versus 14% on placebo (P = 0.0006).28 The phase 3 data led to the FDA (US Food and Drug Administration) approval of fostamatinib for treatment of adults with chronic ITP in April 2018.
After patients completed the phase 3 studies, the long‐term safety and efficacy of fostamatinib were evaluated in a long‐term, multicenter, open‐label extension (OLE) study. Patients could enroll in the OLE study after completing either the 24‐week randomized studies or ≥12 weeks of double‐blind treatment and discontinuing due to lack of response. The OLE study allowed patients who had responded to fostamatinib in the two randomized trials to continue treatment and allowed patients initially randomized to placebo to experience fostamatinib. Since the randomized studies were double‐blind, patients receiving but not responding to fostamatinib were also frequently entered in the OLE study. This article is the first report on the long‐term safety and efficacy of fostamatinib in patients with ITP.
2. METHODS
The randomized studies, FIT1 (NCT02076399) and FIT2 (NCT02076412), enrolled adults with ITP from July 2014 to August 2016 at 58 centers in North America, Australia, and Europe. The ongoing long‐term, OLE study, FIT3 (NCT02077192), began enrolling adults with ITP in October 2014. The data cutoff date of this interim analysis is April 14, 2017. The randomized studies were approved by independent ethics committees at participating centers and performed in accordance with the Declaration of Helsinki. All patients provided written informed consent.
2.1. Patients
All patients first had to be enrolled in one of the randomized, placebo‐controlled, phase 3 studies (FIT1 or FIT2) and meet enrollment criteria for those studies.28 Key enrollment criteria included being adults with primary ITP for at least 3 months and having received at least one ITP treatment prior to the randomized studies. To enter the OLE study, patients had to either have completed the full 24 weeks of treatment in the previous study (FIT1 or FIT2) or have discontinued the previous study due to lack of efficacy after completing at least 12 weeks of double‐blind treatment including at least 4 weeks at 150 mg BID of study drug (active medication or placebo). Because patients and physicians remained blinded through and after the randomized trials, a number of nonresponders who had been on fostamatinib entered the OLE study hoping that they had been on placebo and might respond to open‐label active drug.
2.2. Treatment
Patients started treatment in the randomized studies with fostamatinib at 100 mg BID and could increase the dose after 4 weeks or later to 150 mg BID, depending on platelet count and tolerability. Patients who had a platelet count ≥50 000/μL at the time of rollover to the OLE study received fostamatinib at the same dose and regimen in the OLE study; one patient continued 100 mg daily. Patients who entered the OLE study as nonresponders in the randomized trials received fostamatinib 100 mg BID. The dose could be increased to 150 mg BID at week 4 or later if their platelet count was still <50 000/μL and if fostamatinib was well tolerated. The dose could be reduced to 150 mg QD, 100 mg BID, or 100 mg QD if a dose‐limiting adverse event (AE) occurred.
Patients were allowed one concomitant ITP medication at a stable dose (corticosteroids at <20 mg prednisone equivalent per day, azathioprine, or danazol). Rescue therapies such as increased dosing of concomitant ITP therapy, IVIg, anti‐D, steroids, or platelet transfusion were allowed. The concomitant medications ideally would be tapered as possible in the OLE study.
2.3. Endpoints
Patients were evaluated every 2 weeks in the randomized studies and no less often than monthly for 18 months and then bimonthly for up to 5 years in the OLE study. Overall response (post hoc) was defined as 1 or more platelet counts ≥50 000/μL (without the use of rescue medication) within 3 months of starting fostamatinib including in the OLE study. The primary endpoint of stable response was defined as: in the randomized studies, a platelet count ≥50 000/μL at ≥4 of 6 biweekly visits during weeks 14 to 24 or, for patients initiating fostamatinib in the OLE, ≥1 platelet count ≥50 000/μL in the first 3 months followed by platelet counts ≥50 000/μL at the subsequent two of three monthly visits without use of rescue medication. Duration of first response was defined as the time from the first visit with a platelet count of ≥50 000/μL until use of rescue medication or the first of two consecutive visits (at least 4 weeks apart) with platelet counts of <30 000/μL (unless otherwise noted). AEs were collected and graded at each office visit.
2.4. Statistical analysis
The duration of stable and overall responses was analyzed using the Kaplan‐Meier method, and the Kaplan‐Meier estimate of the median duration of the first response was calculated with the 95% CI for the median. A within‐subject comparison of fostamatinib and placebo was conducted in patients who had been randomized to placebo in the randomized studies and subsequently initiated fostamatinib in the OLE study whereby each subject served as their own control; these data were analyzed using a two‐sided McNemar's test with a significance level of 0.05. Descriptive statistics were used to describe other endpoints and safety assessments.
3. RESULTS
A total of 146 patients received fostamatinib in the three FIT studies (including all but four of the placebo patients in the two randomized trials): these patients represent the fostamatinib exposure population. Of those, 123 were enrolled in the OLE study (FIT3), including: (a) 79 of the 102 patients who initially received fostamatinib in the randomized, double‐blind, placebo‐controlled, phase 3 studies (FIT1 and FIT2), and (b) 44 of the 48 patients who received placebo in the randomized studies and initially received fostamatinib in the OLE study. At data cutoff date (April 14, 2017), 49 of the overall 146 (34%) patients were continuing treatment in the OLE study and 97 (66%) patients had discontinued treatment (Figure 1). Reasons for discontinuation of fostamatinib included lack of response (31% of the 146 patients), AEs (16%), completed the prior randomized studies and did not enter the OLE study (2%), failed screening for the OLE study (1%), withdrawal by subject (5%), investigator decision (2%), noncompliance (1%), lost to follow up (1%), and those listed as “other” (8%). The median duration of fostamatinib treatment was 6.7 months (range <1‐31 months ongoing) with 98 (67%) patients on fostamatinib treatment for ≥6 months.
Figure 1.
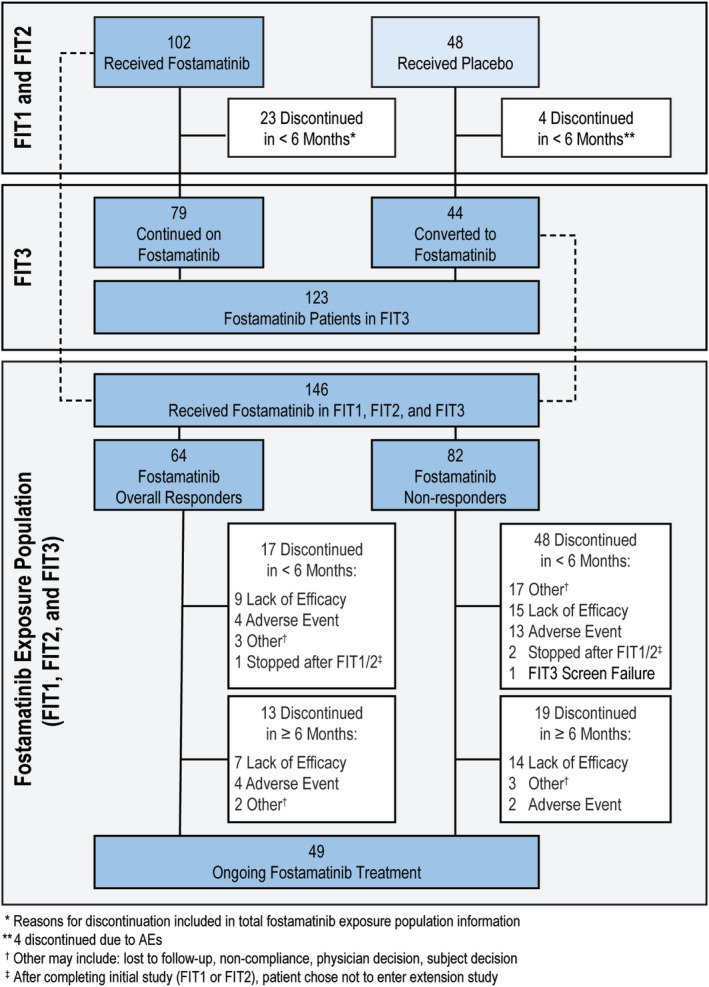
Disposition
Demographics and baseline characteristics are summarized in Table 1; the values are those from the randomized studies. The patient's median age at baseline was 53 years, 60% were females, and 93% were Caucasian. Ninety‐three percent of patients had chronic ITP; 7% had persistent ITP, with progression to chronic ITP during the randomized and later OLE studies. Median duration from ITP diagnosis was 8 years, the median platelet count was 16 000/μL, and patients had a median of 3 (range 1‐13) of unique prior medications for ITP. The majority of patients received 150 mg BID of fostamatinib. Compliance with treatment (assessed by pill counts) was good with an estimated median of only 6 of missed doses per patient during treatment.
Table 1.
Patient demographics and baseline characteristics
| All patients (n = 146) | |
|---|---|
| Age, median (range), years | 53 (20‐88) |
| Sex (%) | |
| Female | 60% |
| Male | 40% |
| Race (%) | |
| White | 93% |
| Asian | 3% |
| Black/African American | 3% |
| Other | 1% |
| ITP classification (%) | |
| Persistent (duration <1 y) | 7% |
| Chronic (≥1 y) | 93% |
| Duration of ITP, median (range), years | 8.4 (<1–53) |
| Baseline platelet count, median, /μL (range) | 16 000 |
| Number of unique prior medications, median (range) | 3 (1‐13) |
| Prior treatments (%) | |
| Corticosteroids | 92% |
| IVIg or IV anti‐D | 53% |
| Thrombopoietic agents | 47% |
| Immunosuppressants | 43% |
| Splenectomy | 35% |
| Rituximab | 32% |
| Danazol | 19% |
| Chemotherapy | 10% |
| Other (Dapsone) | 9% |
| Concomitant ITP‐related medications (%) | |
| Corticosteroids | 53% |
| Immunoglobulins | 25% |
| Platelets | 6% |
| Immunosuppressants | 5% |
| Vitamin K and other hemostatics | 2% |
| Anabolic steroids | 1% |
An overall platelet response was achieved by 64 of 146 (44%) patients (Figure 2A), including 43 patients from the randomized studies and 21 (48%) of the 44 patients first exposed to fostamatinib in the OLE study. These patients maintained the first overall response, including stable responders, while on therapy for a median duration of >28 months (<1 to >28 months); see Supporting Information Figure S1A. In patients with an overall response, the median platelet count for all post‐baseline visits was 63 000/μL (14 500‐277 000) (Figure 2B). Of the 69 patients with an insufficient response to TPO agents prior to entering these studies, 24 (35%) had an overall response to fostamatinib and 14 of the 24 maintained platelet counts consistently over 30 000/μL (Figure 2A).
Figure 2.
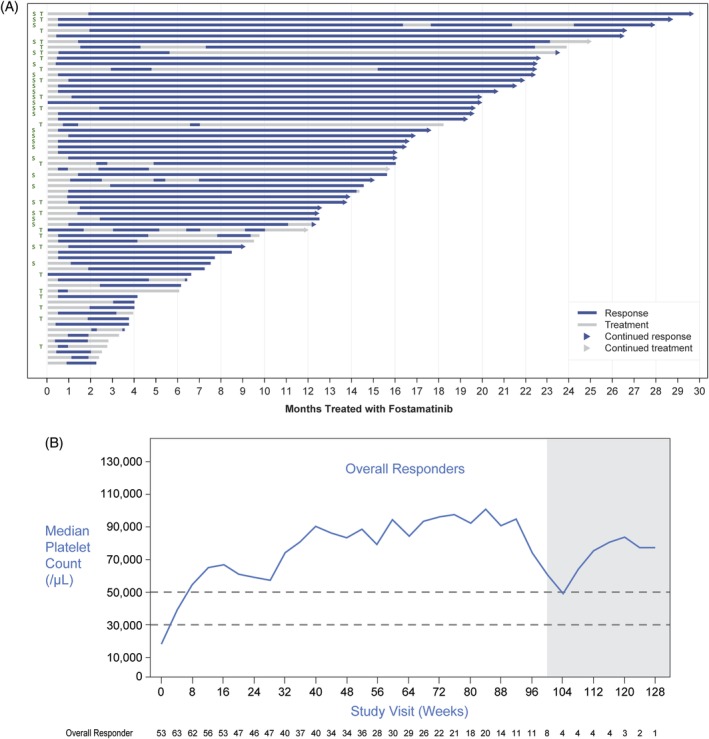
A, Duration of response in overall responders. Arrow at the end of each lane indicates continuation of response and/or treatment. S, stable responder; T, failed prior therapy with thrombopoietin receptor agonist. Note: End of response defined as two platelet counts <30 000/μL at least 4 weeks apart (or use of rescue therapy). B, Median platelet levels over time in overall responders including stable responders. Shaded area indicates timepoints with less than 10 patients contributing data
Seventeen patients (17%) receiving fostamatinib achieved a stable response, the primary endpoint in the randomized studies (Bussel 2018). Ten (23%) of 44 patients who received placebo in the randomized studies achieved a stable response to fostamatinib in the OLE study including the one placebo patient who had responded in the randomized studies. Overall, they comprise 27 stable responders among 146 patients (18%) exposed to fostamatinib (Supporting Information Figure S2). The Kaplan‐Meier estimated median duration of first stable response from time of first fostamatinib exposure had not been reached at >28 months as 17 of 27 (63%) patients continued to respond to fostamatinib (range 5‐>28 months) (Supporting Information S1B). The median platelet count in patients with a stable response for all post‐baseline visits was 89 000/μL (22 350‐277 000/μL), and 22/27 (81%) stable responders virtually always had platelet counts >50 000/μL during treatment in the OLE (Supporting Information Figures S2 and S3).
Patients who achieved an overall response but were not stable responders did not do as well as the stable responders but generally maintained platelet counts above 30 000/μL (Figure 2). Overall responders who were not stable responders had occasional platelet count fluctuations to below 30 000/μL but then regained their response.
There were 15 patients who remained on treatment at the data cutoff date even though they did not achieve a platelet count of 50 000/μL in the first 12 weeks. They were therefore not overall responders but remained on treatment because they derived clinical benefit from treatment. After the initial 12‐week treatment period, 10 of these 15 patients achieved platelet counts above 50 000/μL, and the other 5 patients had peak levels of 16 000/μL to 45 000/μL.
3.1. Safety
AEs were reported in 86% of the 146 patients exposed to fostamatinib during the randomized studies and the OLE study. The most common AEs were diarrhea, hypertension, nausea, and epistaxis; increased alanine aminotransferase (ALT) was seen in 10% of patients (Table 2). Most AEs were moderate (41% of patients) or mild (21% of patients) in severity. No new or more frequent toxicities or intolerabilities were detected with prolonged use of fostamatinib during the OLE study.
Table 2.
The most commonly reported adverse events (≥5% of patients) during fostamatinib treatment in the phase 3 studies and OLE study
| Percent of patients (n = 146) | ||||
|---|---|---|---|---|
| Mild | Moderate | Severe | Total | |
| Diarrhea | 18 | 16 | 1 | 35 |
| Hypertension | 10 | 10 | 1 | 21 |
| Nausea | 17 | 2 | 0 | 19 |
| Epistaxis | 11 | 6 | 0 | 17 |
| Petechiae | 10 | 4 | 1 | 15 |
| Headache | 9 | 4 | 0 | 13 |
| Dizziness | 9 | 1 | 1 | 11 |
| Upper RTI | 7 | 3 | 0 | 10 |
| ALT increased | 6 | 4 | 0 | 10 |
| Vomiting | 8 | 0 | 0 | 8 |
| Abdominal pain | 3 | 2 | 0 | 6 |
| Nasopharyngitis | 6 | 0 | 0 | 6 |
| Cough | 4 | 1 | 0 | 6 |
| Fatigue | 8 | 1 | 0 | 9 |
| Dyspnea | 3 | 1 | 1 | 6 |
| Rash | 6 | 0 | 0 | 6 |
| Chest pain (noncardiac) | 3 | 2 | 1 | 6 |
| AST increased | 4 | 3 | 0 | 7 |
| Neutropenia | 2 | 3 | 1 | 6 |
| Thrombocytopenia | 0 | 1 | 5 | 6 |
| Contusion | 6 | 1 | 1 | 8 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; RTI, respiratory tract infection.
Severe AEs were reported in 34 (23%) patients, were judged to be related to study drug in 11 (8%), and occurred in 1 patient each except for thrombocytopenia in 7 patients; bile duct stone in 2, diarrhea in 2 patients, dyspnea in 2 patients, platelet count decreased in 2 patients, pneumonia in 2 patients, and nephrolithiasis in 2 patients. All severe AEs resolved except 1 case of platelet count decreased and 1 case of thrombocytopenia.
Serious AEs were reported in 38 (26%) patients, were judged to be related to study drug in 8 (6%), and occurred in one patient each except for thrombocytopenia (seven patients), epistaxis (five patients), diarrhea (two patients), gastrointestinal hemorrhage (two patients), pneumonia (two patients), and petechiae (two patients). One patient had a mild transient ischemic attack that resolved spontaneously and was unlikely related to study drug; no other thrombotic events were reported. Three patients had an AE resulting in death (pneumonia, sepsis, and plasma cell myeloma), judged by the investigator to not be related to treatment. One death due to bilateral lobar pneumonia occurred 3 weeks after the last dose of fostamatinib). Dose interruption occurred in 23% patients (diarrhea and ALT increased in five patients each; hypertension in three patients; abdominal pain, influenza‐like illness, and epistaxis in two patients each; and other events in one patient each). Alternatively, secondary medication was used to manage AEs; the majority resolved over time. An increase or abnormality in any liver function test (LFT) occurred in 24 patients (16%), which was mild to moderate in all but two patients, both of whom recovered following drug interruption (transaminases increased) or dose reduction (hepatic enzymes increased). Both of the severe LFT increases were considered by the investigator as unlikely to be related to treatment. Other LFT increases or abnormalities were judged by the investigator to be possibly or probably treatment related in 17 (14.0%) patients and led to a dose reduction in 3% of patients. No patients met Hy's law criteria for liver injury. Discontinuation of fostamatinib due to AEs occurred in 16% of patients, including 2 of the 27 patients who achieved a stable response. These AEs included diarrhea in six patients, neutropenia in three patients, pneumonia in two patients, and other events in one patient each.
4. DISCUSSION
This report describes the long‐term safety and efficacy of fostamatinib in patients who previously participated in the two randomized, double‐blind, placebo‐controlled, phase 3 studies28 (Figure 1) along with those who subsequently enrolled in the ongoing OLE study. The patients entering the OLE study had a long duration of ITP (median 8 years) and a substantial ITP treatment history (median of three treatments) prior to enrollment in the fostamatinib randomized studies. Forty‐three patients on fostamatinib who had responded to blinded fostamatinib in the randomized studies continued fostamatinib at the same dose without interruption; the placebo responder in FIT2 also enrolled in the OLE study and received fostamatinib. The OLE study also enrolled “nonresponders” from the phase 3 studies of two types. One type included patients who had experienced a treatment benefit, for example, a platelet increase (but not to >50 000/μL within 12 weeks), with less bleeding, and wished to continue treatment. The second type included patients who had not benefited in the randomized studies but wanted a chance to initiate fostamatinib in the OLE study (not knowing whether the study drug they had received in the randomized trial was fostamatinib or placebo). This report describes the overall experience of all of these patients from the beginning of their exposure to fostamatinib to the cutoff of the interim analysis of the ongoing OLE study.
An overall platelet response, defined as at least one platelet count of ≥50 000/μL by week 12, was achieved by 64 of 146 (44%) patients including patients who had a stable response, which is similar to the randomized studies (43/102 or 43%).28 The median platelet count in overall responders was 63 250/μL across all on‐treatment visits with the median maintained at or above 50 000/μL at each visit. The median duration of the first response has not been reached as of the data cutoff date, and exceeds 28 months for both stable and overall responses (Supporting Information Figure S1); however, the stable responders maintained their responses longer and at a higher platelet level than was seen in the overall responders (Figure 2B and Supporting Information Figure S3).
Stable responses were observed in 27 patients with a median first‐response duration of >28 months with 17 patients still responding to fostamatinib at data cutoff. Ten of the 44 (23%) patients who initiated fostamatinib in the OLE study after taking placebo in the randomized trials had a stable response rate, which is similar to the 18% reported for the randomized studies.28 To remain a stable responder after achieving a prespecified number of platelet counts ≥50 000/μL, these patients had to maintain platelet counts above 30 000/μL without rescue therapy; over 80% of stable responders continued to maintain almost all of their counts >50 000/μL (Supporting Information Figure S2). The median platelet count in stable responders was 89 000/μL at all scheduled post‐baseline visits and above 50 000/μL at each visit.
Forty‐nine patients continued their treatment as of data cutoff: 23 stable responders, 11 overall responders who were not stable responders, and 15 patients who were not overall responders. The latter could have had platelet counts as high as 49 000/μL during their first 12 weeks on fostamatinib; 10 of these 15 patients achieved platelet counts above 50 000/μL after the initial 12 weeks, and five remained below 50 000/μL. These patients continued treatment due to benefits such as increased platelet counts over baseline, decreased bleeding, avoiding a need for other treatments, and/or other potential benefits not assessed in this study.
In most responders, stable and overall responses were sustained by ongoing treatment for periods of up to and greater than 2 years. In an earlier phase 2 study, two patients continued treatment with fostamatinib for more than 7 years, one of whom is ongoing after 11 years with maintained efficacy without new toxicities.15 These findings suggest that patients can be treated with fostamatinib for extended periods of time without loss of response or development of new or more frequent side effects. The rates of stable and overall responses seen in patients who had received placebo in the randomized trials and then received fostamatinib in the OLE were similar to the rates seen in patients who received fostamatinib in the randomized studies.
AEs in the OLE study were similar to those observed in the randomized studies with the caveat that some of the patients from the randomized studies who could not tolerate fostamatinib or had side effects requiring discontinuation of treatment did not participate in the OLE study.28 The frequency of AEs in the OLE study alone (75%) was similar to the frequency reported in the randomized studies (83% with fostamatinib and 75% with placebo), notwithstanding the extended drug exposure (median of 5.9 months in the OLE study and 2.8 months in the randomized studies).28 The majority of AEs were mild to moderate and could be managed with medication, dose reduction, or interruption, or, in a small number of cases, including 2 of 27 stable responders, discontinuation of treatment.
The FDA approved fostamatinib for patients with “an insufficient response to a previous treatment”; thus, fostamatinib may be used as a second line (or later) therapy for ITP, and, since licensure, clinicians have utilized fostamatinib as a second‐line therapy as well as subsequent lines of therapy for ITP.29 Practitioners will need to determine the best treatment sequence for each of their patients. While the response rate based on the primary endpoint (platelet counts of ≥50 000/μL at four of six visits during weeks 14‐24) was modest, the endpoint itself was a difficult one to achieve because it required success with most of multiple consecutive platelet counts and the patient group enrolled may have been the most difficult to treat; this is due to not only longer duration of ITP and the entry requirement of three platelet counts below 30 000/μL (rather than just one) but also this was the first large‐scale trial in ITP in which previous use of TPO agents was common.26, 27, 30 Furthermore, more than one‐third of patients who had failed previous TPO agents responded to fostamatinib; the difference in effect mechanism between fostamatinib (inhibiting platelet destruction) and TPO agents (stimulating platelet production) may explain this relatively high rate of overall response to fostamatinib. In the randomized trials of fostamatinib, patients with persistent ITP (3‐12 months) had an overall response rate of 75% (six of eight patients).28 Thus, patients with earlier stage disease may be more likely to have a good response to fostamatinib. A subgroup analysis of demographic and disease characteristics of patients in the randomized trials showed that responses occurred in all subgroups categorized by age, sex, prior therapy (splenectomy, rituximab, TPO‐RA), baseline platelet count <15 000/μL, presence or absence of platelet antibodies, and duration of ITP,28 showing that even patients with chronic, difficult to treat, ITP responded. Further studies to determine which patients are most likely to respond to fostamatinib in ITP are warranted.
Limitations of any open‐label study such as the OLE study include the lack of a control group. However, the 44 placebo patients who only initiated fostamatinib in the OLE study served as a validation cohort for the findings of the phase 3 studies and responded similarly to those in the phase 2 study.15 These studies do not facilitate comparison of fostamatinib to other treatments for ITP due to variations in endpoints, the timing of visits, and baseline characteristics of the patient population and severity; 47% of patients in the randomized trials had not responded to TPO agents. The OLE study did not analyze responses by patient characteristics and did not measure antiplatelet antibodies; positivity of the latter was preliminarily associated with response to fostamatinib, which is consistent with the effect mechanism.28 Patients were not followed after discontinuing fostamatinib. Therefore, no data were collected relative to whether or not fostamatinib has curative effects nor to what treatments patients failing fostamatinib would be most likely to respond.
In conclusion, the OLE study showed durability of stable and overall platelet responses, with the median ongoing at over 28 months. Patients continue to be treated and followed. Further analysis of clinical and biologic factors associated with treatment response is needed as well as analysis of real‐world data now that this treatment is available in the United States and potentially will be available in other countries in the near future. One advantage of fostamatinib over other treatments may be its antithrombotic effect; this also remains to be determined based on further clinical trials.16, 31, 32
CONFLICT OF INTEREST
JB has received research support from Rigel, Novartis, and Amgen, and has participated on advisory boards for Rigel, Novartis, Amgen, Momenta, and Protalex. DMA has received research support from Novartis, Amgen, and Bristol Meyers Squibb, and has been a consultant for Rigel, Amgen, Novartis, and UCB. MB has participated in speakers' bureaus for Rigel, Incyte, and Abbvie. NC has participated in a consultancy or advisory committee for Amgen and Novartis. JM has received research funding from Rigel. HZ, ST and AMD are employees of Rigel Pharmaceuticals.
Supporting information
Figure S1. Kaplan–Meier estimate of the median duration of (A) first overall response including stable response and (B) first stable response
Figure S2. Duration of response in stable responders. Arrow at the end of each lane indicates continuation of treatment. Note: End of response defined as two platelet counts <50 000/μL at least 4 weeks apart (or use of rescue therapy)
Figure S3. Median platelet levels over time in stable responders. Shaded area indicates timepoints with less than 10 patients contributing data
ACKNOWLEDGMENTS
We thank the patients, study investigators, nurses, and coordinators for their participation in the study. The study was funded by Rigel Pharmaceuticals, Inc. Editorial support was provided by Leslie Todd and funded by Rigel Pharmaceuticals.
Bussel JB, Arnold DM, Boxer MA, et al. Long‐term fostamatinib treatment of adults with immune thrombocytopenia during the phase 3 clinical trial program. Am J Hematol. 2019;94:546–553. 10.1002/ajh.25444
Funding information Rigel Pharmaceuticals, Inc
REFERENCES
- 1. Frederiksen H, Schmidt K. The incidence of idiopathic thrombocytopenic purpura in adults increases with age. Blood. 1999;94:909‐913. [PubMed] [Google Scholar]
- 2. Terrell DR, Beebe LA, Vesely SK, Neas BR, Segal JB, George JN. The incidence of immune thrombocytopenic purpura in children and adults: a critical review of published reports. Am J Hematol. 2010;85:174‐180. [DOI] [PubMed] [Google Scholar]
- 3. Segal JB, Powe NR. Prevalence of immune thrombocytopenia: analyses of administrative data. J Thromb Haemost. 2006;4:2377‐2383. [DOI] [PubMed] [Google Scholar]
- 4. Michel M. Immune thrombocytopenic purpura: epidemiology and implications for patients. Eur J Haematol Suppl. 2009;71:3‐7. [DOI] [PubMed] [Google Scholar]
- 5. Cohen YC, Djulbegovic B, Shamai‐Lubovitz O, Mozes B. The bleeding risk and natural history of idiopathic thrombocytopenic purpura in patients with persistent low platelet counts. Arch Intern Med. 2000;160:1630‐1638. [DOI] [PubMed] [Google Scholar]
- 6. Lee MS, Kim WC. Intracranial hemorrhage associated with idiopathic thrombocytopenic purpura: report of seven patients and a meta‐analysis. Neurology. 1998;50:1160‐1163. [DOI] [PubMed] [Google Scholar]
- 7. Picozzi VJ, Roeske WR, Creger WP. Fate of therapy failures in adult idiopathic thrombocytopenic purpura. Am J Med. 1980;69:690‐694. [DOI] [PubMed] [Google Scholar]
- 8. Cines DB, Blanchette VB. Immune thrombocytopenia purpura. N Engl J Med. 2002;346(13):995‐1008. [DOI] [PubMed] [Google Scholar]
- 9. Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386‐2393. [DOI] [PubMed] [Google Scholar]
- 10. Zufferey A, Kapur R, Semple J. Pathogenesis and therapeutic mechanisms in immune thrombocytopenia (ITP). J Clin Med. 2017;6(2):pii: E16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Crowley MT, Costello PS, Fitzer‐Attas CJ, et al. A critical role for Syk in signal transduction and phagocytosis mediated by Fcy receptors on macrophages. J Exp Med. 1997;186(7):1027‐1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mócsai A, Ruland J, Tybulewicz VL. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat Rev Immunol. 2010;10(6):387‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Takada Y, Aggarwal BB. TNF activates Syk protein tyrosine kinase leading to TNF‐induced MAPK activation, NF‐kappaB activation, and apoptosis. J Immunol. 2004;173(2):1066‐1077. [DOI] [PubMed] [Google Scholar]
- 14. Ozaki N, Suzuki S, Ishida M, et al. Syk‐dependent signaling pathways in neutrophils and macrophages are indispensable in the pathogenesis of anti‐collagen antibody‐induced arthritis. Int Immunol. 2012;24(9):539‐550. [DOI] [PubMed] [Google Scholar]
- 15. Podolanczuk A, Lazarus AH, Crow AR, Grossbard E, Bussel JB. Of mice and men: an open‐label pilot study for treatment of immune thrombocytopenic purpura by an inhibitor of Syk. Blood. 2009;113(14):3154‐3160. [DOI] [PubMed] [Google Scholar]
- 16.(a) Braselmann S, Taylor V, Zhao H, et al. R406, an orally available spleen tyrosine kinase inhibitor blocks fc receptor signaling and reduces immune complex‐mediated inflammation. J Pharmacol Exp Ther. 2006;319(3):998‐1008. [DOI] [PubMed] [Google Scholar]; (b) Erratum: Correction to R406, an orally available spleen tyrosine kinase inhibitor blocks fc receptor signaling and reduces immune complex‐mediated inflammation . J Pharmacol Exp Ther. 2013;345(2):326. [DOI] [PubMed] [Google Scholar]
- 17. Vianelli N, Palandri F, Polverelli N, et al. Splenectomy as a curative treatment for immune thrombocytopenia: a retrospective analysis of 233 patients with a minimum follow up of 10 years. Haematologica. 2013;98(6):875‐880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chaturvedi S, Arnold DM, McCrae KR. Splenectomy for immune thrombocytopenia: down but not out. Blood. 2018;131(11):1172‐1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rodeghiero F. A critical appraisal of the evidence for the role of splenectomy in adults and children with ITP. Br J Haematol. 2018;181(2):183‐195. [DOI] [PubMed] [Google Scholar]
- 20. Boyle S, White RH, Brunson A, Wun T. Splenectomy and the incidence of venous thromboembolism and sepsis in patients with immune thrombocytopenia. Blood. 2013;121(23):4782‐4790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Patel VL, Mahevas M, Lee SY, et al. Outcome at 5 years following response to rituximab therapy in children and adults with immune thrombocytopenia (ITP). Blood. 2012;119(25):5989‐5995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zaja F, Volpetti S, Chiozzotto M, et al. Long‐term follow‐up analysis after rituximab salvage therapy in adult patients with immune thrombocytopenia. Am J Hematol. 2012;87(9):886‐889. [DOI] [PubMed] [Google Scholar]
- 23. Chapin J, Lee CS, Zhang H, Zehnder JL, Bussel JB. Gender and duration of disease differentiate responses to rituximab‐dexamethasone therapy in adults with immune thrombocytopenia. Am J Hematol. 2016;91(9):907‐911. [DOI] [PubMed] [Google Scholar]
- 24. Marangon M, Vianelli N, Palandri F, et al. Rituximab in immune thrombocytopenia: gender, age, and response as predictors of long‐term response. Eur J Haematol. 2017;98(4):371‐377. [DOI] [PubMed] [Google Scholar]
- 25.(a) Bussel JB, Kuter DJ, George JN, et al. AMG 531, a thrombopoiesis‐stimulating protein, for chronic ITP. N Engl J Med. 2006;355(16):1672‐1681. [DOI] [PubMed] [Google Scholar]; (b) Erratum: Correction to AMG 531, a thrombopoiesis‐stimulating protein, for chronic ITP . N Engl J Med. 2006;355(19):2054. [DOI] [PubMed] [Google Scholar]
- 26. Bussel JB, Cheng G, Saleh MN, et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N Engl J Med. 2007;357(22):2237‐2247. [DOI] [PubMed] [Google Scholar]
- 27. Bussel JB, Provan D, Shamsi T, Cheng G, Psaila B, Kovaleva L. Effect of eltrombopag on platelet counts and bleeding during treatment of chronic idiopathic thrombocytopenic purpura: a randomised, double‐blind, placebo‐controlled trial. Lancet. 2009;373(9664):641‐648. [DOI] [PubMed] [Google Scholar]
- 28. Bussel J, Arnold DM, Grossbard E, et al. Fostamatinib for the treatment of adult persistent and chronic immune thrombocytopenia: results of two phase 3, randomized, placebo‐controlled trials. Am J Hematol. 2018;93:921‐930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Burke D. News Release. Rigel Website. https://ir.rigel.com/news-releases/news-release-details/rigel-pharmaceuticals-provides-business-update. Accessed February 5, 2019.
- 30. Kuter DJ, Bussel JB, Lyons RM, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double‐blind randomized controlled trial. Lancet. 2008;371:395‐403. [DOI] [PubMed] [Google Scholar]
- 31. van Eeuwijk JMM, Stegner D, Lamb DJ, et al. The novel oral Syk inhibitor, Bl1002494, protects mice from arterial thrombosis and thromboinflammatory brain infarction. Arterioscler Thromb Vasc Biol. 2016;36:1247‐1253. [DOI] [PubMed] [Google Scholar]
- 32. Andre P, Morooka T, Sim D, et al. Critical role for Syk in responses to vascular injury. Blood. 2011;118(18):5000‐5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Kaplan–Meier estimate of the median duration of (A) first overall response including stable response and (B) first stable response
Figure S2. Duration of response in stable responders. Arrow at the end of each lane indicates continuation of treatment. Note: End of response defined as two platelet counts <50 000/μL at least 4 weeks apart (or use of rescue therapy)
Figure S3. Median platelet levels over time in stable responders. Shaded area indicates timepoints with less than 10 patients contributing data


