Abstract
1. Osthole, a coumarin compound from plants, is a promising agent for the treatment of metabolic diseases, including hyperglycemia, fatty liver, and cancers. Studies indicate that the peroxisome proliferator-activated receptors (PPAR) α and γ are involved in the pharmacological effects of osthole. The in vitro and in vivo metabolism of osthole and its biological activity are not completely understood.
2. In this study, ultra-performance chromatography electrospray ionization quadrupole time-of-flight mass spectrometry (UPLC–ESI–QTOFMS)-based metabolomics was used to determine the metabolic pathway of osthole and its influence on the levels of endogenous metabolites. Forty-one osthole metabolites, including 23 novel metabolites, were identified and structurally elucidated from its metabolism in vitro and in vivo. Recombinant cytochrome P450s (CYPs) screening showed that CYP3A4 and CYP3A5 were the primary enzymes contributing to osthole metabolism.
3. More importantly, osthole was able to decrease the levels of lysophosphatidylethanolamine (LPE) and lysophosphatidylcholine (LPC) in the plasma, which explains in part its modulatory effects on metabolic diseases.
4. This study gives the insights about the metabolic pathways of osthole in vivo, including hydroxylation, glucuronidation, and sulfation. Furthermore, the levels of the lipids regulated by osthole indicated its potential effects on adipogenesis. These data contribute to the understanding of the disposition and pharmacological activity of osthole in vivo.
Keywords: Lipid, metabolomics, metabolic disease, osthole, time-of-flight mass spectrometry, ultra-performance liquid chromatography
Introduction
Natural coumarin compounds have been studied extensively since some are known to protect against the metabolic diseases, including hyperlipidemia and hypertension (Katsori & Hadjipavlou-Litina, 2014). Scopoletin, fraxin, and esculin belong to the coumarin family, and show inhibitory effects on hypertension and hyperlipidemia (Ojewole & Adesina, 1983; Tsukamoto et al., 1985; Venugopala et al., 2013). The coumarin osthole is widely distributed in Cnidium moonieri (L.) Cussion and Angelica pubescens. Accumulating evidence demonstrate that osthole shows protective effects on various metabolic diseases, including hyperglycemia (Liang et al., 2009), alcohol and non-alcohol fatty liver diseases (Qi et al., 2011; Song et al., 2006), and cancers (Jarzab et al., 2014; Wen et al., 2015). Several studies suggested that the effects of osthole on metabolic diseases may be associated with activation of peroxisome proliferator-activated receptors (PPAR) α and γ (Qi et al., 2016; Zhao et al., 2014). The metabolism of osthole has been reported (Lv et al., 2012b; Yuan et al., 2009), however, its metabolic map in vivo still remains unclear. The structure-activity analyze of osthole (the structure is provided in Figure 8) was explored, revealing that the 3-methyl-2-butenyl-group at position-8 and the methoxy-group at position-7 in the chemical structure of osthole were essential for the inhibition of concanavalin-induced liver toxicity (Okamoto et al., 2005). Three synthetic osthole derivatives, 7-ethoxy-8-(3-methyl-2-butenyl) coumarin, 8-allyl-7-methoxy-4-methyl-6-nitrocoumarin, and 8-allyl-7-methoxy-3-nitroquinoline, shown higher protective effects on the liver damage than osthole (Okamoto et al., 2007). To date, there are limited studies to determine which cytochrome P450s (CYPs) are responsible for the formation of osthole metabolites.
Figure 8.
Metabolic map of osthole. Reaction types: 1: 7-demethylation; 2: dehydrogenation; 3: 3,4-epoxide; 4: hydroxylation; 5: aldehylation; 6: oxidation; 7: hydrogenation; 8: hydration; 9: glucuronidation; 10: sulfation. Metabolites marked with asterisk (*) represent isomers were observed.
Extensive studies have demonstrated that ultraperformance chromatography electrospray ionization quadrupole time-of-flight mass spectrometry (UPLC–ESI–QTOFMS)-based metabolomics is a powerful tool for the comprehensive profiling of xenobiotic metabolites. It was applied to determine the metabolic behaviors of many clinical drugs, including acetaminophen (Chen et al., 2008a), procainamide (Li et al., 2012), and gefitinib (Liu et al., 2015), yielding new insights on their efficacy and side-effects. The pharmacological activities and the potential action target also could be revealed by metabolomics; for example fenofibrate (Patterson et al., 2009) and tempol (Li et al., 2013b). Using this powerful technology, the metabolic maps of many natural compounds and endogenous compounds have been described, such as arecoline (Giri et al., 2007), noscapine (Fang et al., 2012), and melatonin (Li et al., 2013a).
In this study, a metabolomic approach was used to investigate the metabolic behavior of osthole. The aims of the present study were (i) to elucidate the metabolic pathways of osthole in the mouse; (ii) to identify the drug-metabolizing enzymes involved in osthole metabolism; and (iii) to evaluate the biological activity of osthole based on its regulation of endogenous metabolites. Forty-one osthole metabolites were identified in this study, including 23 novel metabolites. Osthole undergoes hydroxylation, hydrogenation, demethylation, dehydrogenation, glucuronidation, and sulfation. It was revealed that CYP3A4 and CYP3A5 were the primary enzymes contributing to osthole metabolism. The levels of several lysophosphatidylethanolamines (LPE) and lysophosphatidylcholines (LPC) in the plasma could be decreased by osthole, suggesting its potential benefit effects of osthole on adipogenesis. Overall, this study exemplifies the value of LC–MS-based metabolomics for the simultaneous evaluation of drug metabolism and its effect on host metabolism.
Materials and methods
Chemicals and reagents
Osthole was obtained from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). NADPH, chlorpropamide, and formic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA). Mouse liver microsomes (MLM) and human liver microsomes (HLM) were provided by Bioreclamationivt Inc. (Hicksville, NY, USA). Xenotech, LLC (Kansas City, KS, USA) provided the recombinant CYPs. All other reagents (e.g. acetonitrile and water) were of the highest grade commercially available.
In vitro phase I metabolism of osthole
Microsomal incubations were carried out in phosphate-buffered saline solution (PBS, pH = 7.4), containing 50 μM osthole, 0.5 mg/ml MLM or 2 pmol/ml of each cDNA-expressed CYPs (Bactosome control, CYP1A1, 1A2, 1B1, 2A6, 2B6, 2C19, 2C8, 2C9, 2D6, 2E1, 3A4, 3A5, and 4A11) in a final volume of 180 μl. After a 5 min pre-incubation at 37 °C with shaking at 800 rpm, the reaction was initiated by adding 20 μl of 10 mM NADPH (dissolved in PBS). After 40 min incubation at 37 °C with shaking at 800 rpm, the reaction was stopped by adding 200 μl of ice-cold acetonitrile. The solution was centrifuged at 15,000 rpm for 10 min following vortexing for 1 min. A 5 μl aliquot of the supernatant was injected into a UPLC–ESI–QTOFMS system for analysis. Incubations were conducted in duplicate for cDNA-expressed CYPs, and triplicate for MLM and HLM incubation experiments.
In vivo metabolism of osthole
In this study, 6- to 8-week-old male Kunming mice (25–30 g) provided by Shanghai laboratory animal center (SLAC), China were used to investigate osthole metabolism in vivo. The mice were maintained under a standard 12 h dark/light cycle with water and chow provided ad libitum. The mice were treated with osthole in corn oil at a dose of 40 mg/kg body weight by intragastric (i.g.) and intraperitoneal (i.p.) administration. All the experimental mice were housed separately in metabolic cages for 24 h. Control mice were treated with corn oil alone. Urine and feces were collected for a period of 0–24 h, and blood samples harvested 3 h and 24 h after administration of osthole. The plasma samples were prepared from the blood by centrifugation at 3500 rpm for 5 min. All samples were stored at −80 °C until analysis. Handling was in accordance with study protocols approved by the Institutional Animal Care and Use Committee of the Kunming Institute of Botany, Chinese Academy of Sciences.
Sample preparation
Briefly, plasma samples were prepared through mixing the 10 μl plasma with 190 μl 67% aqueous acetonitrile containing 5 μM chlorpropamide. Urinary samples were prepared by mixing 20 μl of urine with 180 μl 50% aqueous acetonitrile containing 5 μM chlorpropamide. Both the plasma samples and urine samples were separately centrifuged at 15,000 rpm for 20 min. Twenty milligram of feces were mixed with 200 μl of 50% aqueous acetonitrile containing 5 μM chlorpropamide. After shaking at room temperature for 20 min, the samples were centrifuged at 15,000 rpm for 20 min to obtain fecal content extract supernatants. Subsequently, 100 μl of supernatant was transferred to a new Eppendorf vial and diluted with 200 μl of 50% aqueous acetonitrile. After centrifugation at 15,000 rpm for 20 min, a 5 μl aliquot of the supernatant was injected into the UPLC–ESI–QTOFMS system.
UPLC–ESI–QTOFMS analysis
The separation of osthole and its metabolites was achieved using a UPLC system containing a 1290 Autosampler and 1290 Quat Pump (Aglient, Santa Clara, CA, USA) equipped with XDB-C18 column (2.1 × 100mm, 1.8 μM). Column temperature was maintained at 45 °C throughout the run. The flow rate was 0.3 ml/min with a gradient ranging from 5% to 95% acetonitrile containing 0.01% formic acid in 16 min run. The mass signals of ion were collected in both positive and negative mode by the electrospray ionization 6530 QTOFMS (Agilent, Santa Clara, CA, USA). Nitrogen was applied as both drying gas (9 l/min) and the collision gas. The drying gas temperature was set at 350 °C and nebulizer pressure was kept at 35 psi. Capillary voltage was set at 3.5 kV.
Multivariate data analysis
Chromatographic and spectral data of samples were acquired by MassHunter Workstation data Acquisition software (Aglient, Santa Clara, CA, USA) and processed by Mass Profinder and Mass Profiler Professional software (Aglient, Santa Clara, CA, USA) to generate a multivariate data matrix. Then, the corresponding data matrix was further analyzed by SIMCA-P + 13.0 software (Umetrics, Kinnelon, NJ, USA) as described previously (Patterson et al., 2008). Different from unsupervised principal component analysis (PCA), partial least squares-discriminate analysis (PLS-DA), orthogonal projection to latent structures-discriminant analysis (OPLS-DA) was used to identify the major latent variables in the data matrix. Potential metabolites of osthole were identified by analyzing the ions contributing to the separation of the experimental group and control group in the S-plot.
Identification of osthole metabolites and endogenous metabolites
The identification and confirmation of osthole metabolites and endogenous metabolites were performed according the previous approaches (Li et al., 2013b, 2014, 2015). Seven golden rules were applied to calculate the mass error based on the elemental compositions of each metabolite (Kind & Fiehn, 2007). After the putative osthole metabolites in the mouse urine and microsomal incubations were determined by OPLS-DA analysis, tandem MS of selected metabolites was performed in the targeted mode with a default isolation width of 0.01 m/z obtained by ramping collision energies from 5 to 15 V. The chemical structures of osthole metabolites were elucidated by interpretation of their MS/MS fragmentation patterns compared to that of osthole. The METLIN database was searched to match the changed endogenous metabolites. To confirm metabolites identity, the MS/MS fragmentation patterns of the putative endogenous metabolite were compared with that of an authentic compound.
Data analysis
Statistical analysis was conducted using Prism v. 6 (GraphPad Software, San Diego, CA, USA). p Value less than 0.05 was considered significant. The experimental data were presented as mean ± SD.
Results
Metabolomic profiling of osthole metabolites in mice
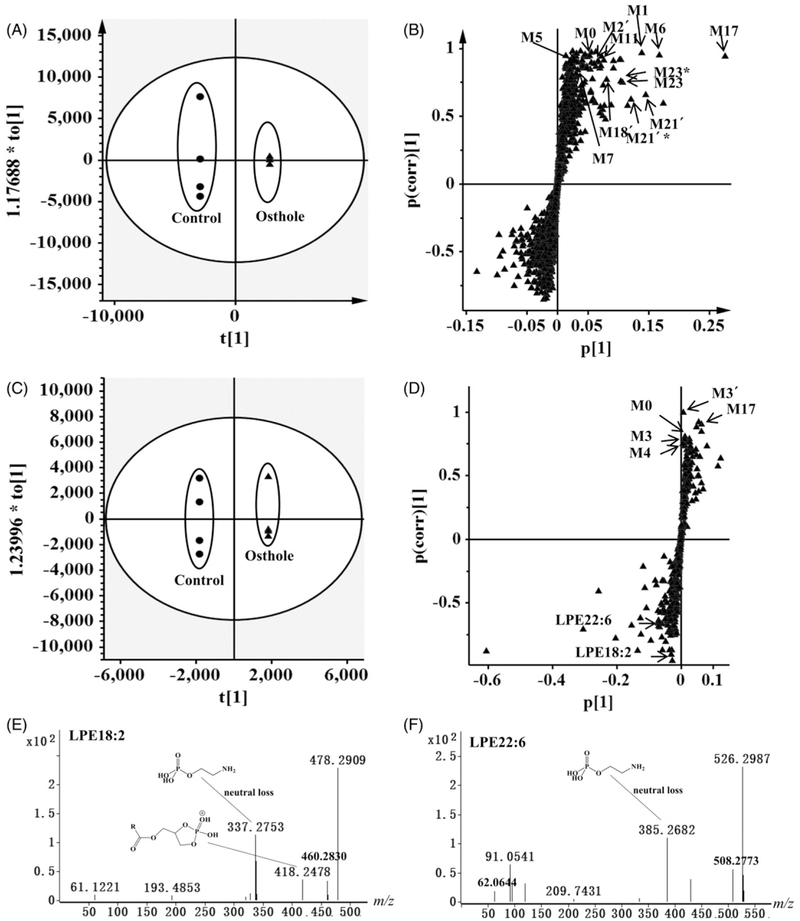
UPLC–ESI–QTOFMS coupled with PCA, PLS-DA, and OPLS-DA modeling was used to examine the differences between osthole treatment group and control groups. The osthole treatment and control group could be well separated by these models. To specifically identify the osthole metabolites, the OPLS model was applied to distinguish osthole metabolites from the endogenous metabolites in urine, plasma, and feces (Figure 1A, C; Figure S1A, S1C). Examination of an S-plot of the mouse samples revealed the ions from osthole metabolites. Many ions were elevated in the urine, plasma and feces sample after treatment with osthole as evident in the top right of the S-plot (Figure 1B, D; Figure S1B, D). A comparison of the increased ions in the urine, plasma, or feces samples revealed many ions with similar mass-to-charge (m/z) ratios and retention times. Combined with the trend plots of ions in the control and treatment groups, these ions were detected only in the treatment group and putatively identified as osthole metabolites and their fragments (Figure S2A-D). Overall, 34 osthole metabolites were identified in mouse urine (Table 1), of which metabolites M1, M2, M2′, M6, M17, M23, M24, M26, and M28 were main excreted metabolites (Figure 2A, B). In mouse feces, 25 osthole metabolites were identified (M1–M17′, M18′, and M19) (Table 1), of which metabolites M1, M2′, M3, M3′, M5, M6′, and M17 were main excreted metabolites (Figure S3). No phase II metabolites were detected in feces. A total of 8 osthole metabolites (M3, M3′, M4, M17, M23, M24, M25′, and M26) and unreacted osthole were observed in plasma following the treatment with osthole (Figure 2C, D). Among these metabolites, 23 (M7, M8, M10, M11, M13–M17′, M19, M22–M25′, M27–M30) were found in the present study and represented as novel metabolites that have never been reported.
Figure 1.
Metabolomic analysis of osthole metabolites in mouse urine and plasma. Analysis of control and osthole-treated mice urine by OPLS-DA score plots (A) and S-plot (B). Analysis of control and osthole-treated mouse plasma in the positive mode in OPLS-DA score plots (C) and S-plot (D) (●, control-treated mice in score plots; ▴, osthole-treated mice in score plots). Urine from mice was collected continuously over the 24 h and plasma was collected at 3 h after osthole treatment (40 mg/kg, i.g.). In score plots, the t[1] and to[1] values represent the score of each sample in principal component 1 and 2, respectively. In the S-plots, the X-axis represents the relative abundance of ions and the Y-axis represents the correction of each ion to the model. These S-plots represent the relationship between ions in relation to the first and second components present in the OPLS-DA score plot. Metabolites are labeled in S-plots. *, M + Na. (E) Tandem MS of LPE18:2. (F) Tandem MS of LPE22:6. MS/MS fragmentation was conducted with collision energy at 10 eV.
Table 1.
Summary of osthole metabolites produced in vivo and in vitro metabolism.
| Metabolite ID | Rt (min) | Observed (m/z) | Mass error (ppm) | Predicted molecular formula | Ion mode | Source |
|---|---|---|---|---|---|---|
| M0 | 9.3 | 245.1193 | 9.0 | C15H16O3 | Positive | Urine, plasma, feces, MLM, HLM |
| M1 | 7.4 | 231.1019 | 1.7 | C14H14O3 | Positive | Urine, feces, MLM, HLM |
| M2 | 5.3 | 229.0863 | 1.7 | C14H12O3 | Positive | Urine, feces, MLM, HLM |
| M2′ | 5.5 | 229.0864 | 2.2 | C14H12O3 | Positive | Urine, feces, MLM, HLM |
| M3 | 6.6 | 243.1023 | 2.9 | C15H14O3 | Positive | Urine, plasma, feces, MLM, HLM |
| M3′ | 6.7 | 243.1023 | 3.3 | C15H14O3 | Positive | Urine, plasma, feces, MLM, HLM |
| M4 | 7.3 | 261.1131 | 3.4 | C15H16O4 | Positive | Urine, plasma, feces, MLM, HLM |
| M5 | 8.7 | 261.1140 | 6.9 | C15H16O4 | Positive | Urine, feces, MLM |
| M6 | 6.3 | 259.0969 | 1.5 | C15H14O4 | Positive | Urine, feces, MLM, HLM |
| M6′ | 7.4 | 259.0969 | 1.5 | C15H14O4 | Positive | Feces, MLM, HLM |
| M7a | 6.0 | 247.0962 | −1.2 | C14H14O4 | Positive | Urine, feces, MLM, HLM |
| M8a | 5.2 | 277.1083 | 4.7 | C15H16O5 | Positive | Urine, feces, MLM, HLM |
| M9 | 5.6 | 277.1070 | 0 | C15H16O5 | Positive | Feces, MLM, HLM |
| M9′ | 5.7 | 277.1074 | 1.4 | C15H16O5 | Positive | Feces, MLM, HLM |
| M10a | 6.0 | 277.1083 | 4.7 | C15H16O5 | Positive | Feces, MLM |
| M11a | 6.9 | 277.1080 | 3.6 | C15H16O5 | Positive | Urine, feces, MLM, HLM |
| M12 | 5.6 | 249.1122 | 0.0 | C14H16O4 | Positive | Urine, feces, MLM, HLM |
| M13a | 6.9 | 249.1137 | 6.0 | C14H16O4 | Positive | Feces, MLM |
| M14a | 6.2 | 251.1284 | 2.4 | C14H18O4 | Positive | Feces, MLM, HLM |
| M14′a | 6.4 | 251.1293 | 5.6 | C14H18O4 | Positive | Feces, MLM, HLM |
| M15a | 7.8 | 251.1293 | 6.0 | C14H18O4 | Positive | Urine, feces, MLM, HLM |
| M16a | 6.0 | 245.0820 | 4.9 | C14H12O4 | Positive | Urine, feces, MLM, HLM |
| M17a | 6.7 | 275.0929 | 5.5 | C15H14O5 | Positive | Urine, plasma, feces, MLM, HLM |
| M17′a | 7.0 | 275.0924 | 3.6 | C15H14O5 | Positive | Urine, feces, MLM, HLM |
| M18 | 5.3 | 247.0946 | −7.7 | C14H14O4 | Positive | Urine |
| M18′ | 5.6 | 247.0958 | −2.8 | C14H14O4 | Positive | Urine, feces |
| M19a | 6.5 | 279.1233 | 2.1 | C15H18O5 | Positive | Urine, feces |
| M20 | 5.2 | 405.1222 | 7.6 | C20H22O9 | Negative | Urine |
| M21 | 5.3 | 435.1303 | 2.1 | C21H24O10 | Negative | Urine |
| M21′ | 5.4 | 435.1294 | 0.5 | C21H24O10 | Negative | Urine |
| M22a | 5.4 | 433.1103 | −8.5 | C21H22O10 | Negative | Urine |
| M23a | 4.9 | 451.1249 | 0.4 | C27H32O17 | Negative | Plasma, urine |
| M24a | 5.5 | 449.1075 | −3.1 | C21H22O11 | Negative | Plasma, urine |
| M25a | 5.3 | 425.1452 | −0.2 | C20H26O10 | Negative | Urine |
| M25′a | 5.6 | 425.1438 | −3.5 | C20H26O10 | Negative | Plasma, urine |
| M26 | 6.2 | 309.0431 | −2.3 | C14H14O6S | Negative | Plasma, urine |
| M27a | 7.1 | 339.0551 | 2.1 | C15H16O7S | Negative | Urine |
| M28a | 4.6 | 325.0395 | 2.5 | C14H14O7S | Negative | Urine |
| M28′a | 4.8 | 325.0388 | 0.3 | C14H14O7S | Negative | Urine |
| M29a | 4.6 | 355.0493 | 0.0 | C15H16O8S | Negative | Urine |
| M29′a | 4.9 | 355.0497 | 1.1 | C15H16O8S | Negative | Urine |
| M30a | 5.0 | 323.0246 | 4.6 | C14H12O7S | Negative | Urine |
Novel metabolites identified in this study.
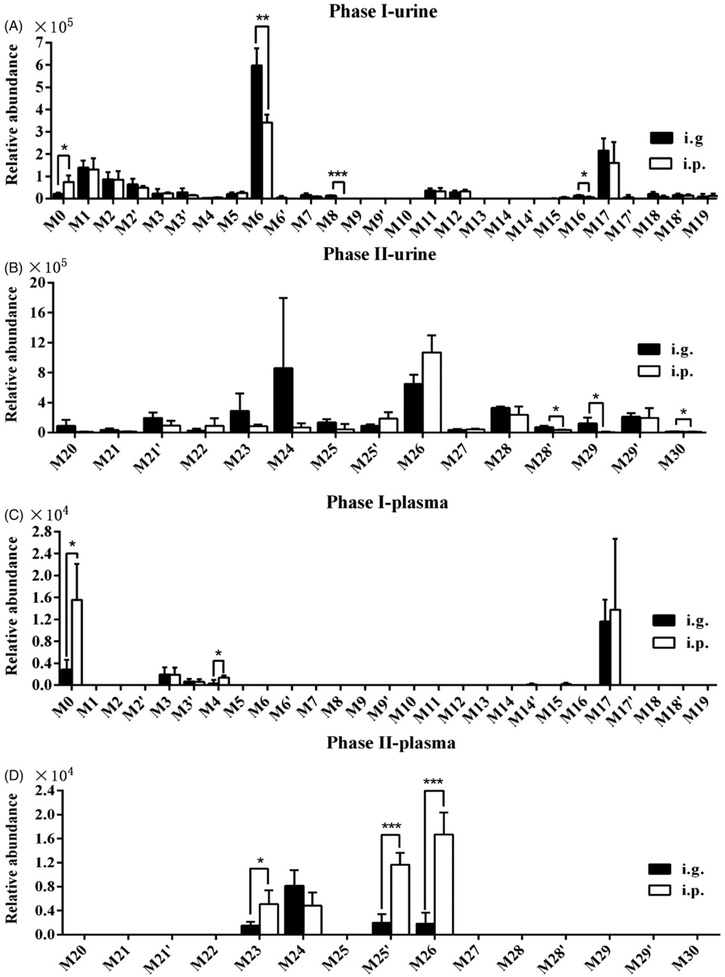
Figure 2.
Comparison of phase I and II metabolites of osthole in mice. (A) Relative abundance of phase I metabolites in urine. (B) Relative abundance of phase II metabolites in urine. (C) Relative abundance of phase I metabolites in plasma. (D) Relative abundance of phase II metabolites in plasma. Urine from mice was collected continuously over 24 h and plasma was collected at 3 h after osthole treatment (40 mg/kg, i.g. and i.p.). The relative quantification was conducted based on the peak areas of ion counts. Statistical analysis was performed using two-tailed Student’s t-test and ANOVA (n = 4 in each group). *P < 0.05; **P < 0.01, and ***P < 0.001.
Identification and structural elucidation of phase I metabolites of osthole
Initial analysis of mouse samples by OPLS revealed some potential osthole metabolites and their fragments in the S-plot. The chemical compositions and structures of these metabolites were identified based on accurate mass measurements and MS/MS fragmentography. Overall, osthole (M0) and 41 metabolites were identified in urine, plasma, and fecal samples. Eighteen metabolites (M1–M6′, M9, M9′, M12, M18, M18′, M2O–M21′, and M26) were known metabolites, and 23 metabolites (M7, M8, M10, M11, M13–M17′, M19, M22–M25′, and M27–M30) were novel metabolites. The chemical compositions, retention times of osthole metabolites are listed in Table 1, and the representative MS/MS spectra of phase I and II metabolites are presented in Figures 3 and 4. The fragmentations of osthole metabolites are present in Table S1 and S2. The major phase I metabolic pathways of osthole were 7-demethylation, dehydrogenation, hydroxylation, hydrogenation, hydration and 3,4-epoxide.
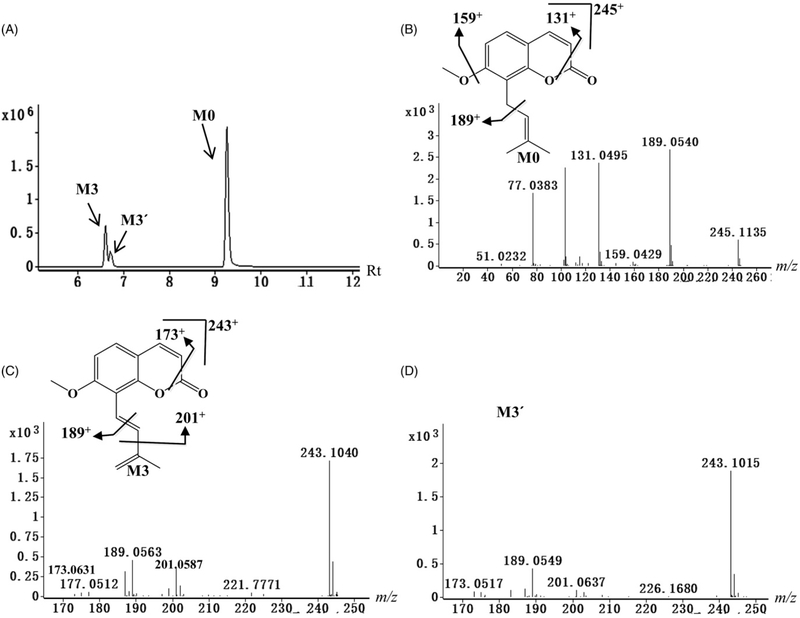
Figure 3.
Identification of phase I metabolites of osthole. (A) Chromatograms of metabolites M0, M3 and M3′. (B) Tandem MS and chemical structure of M0. (C) Tandem MS and chemical structure of M3. (D) Tandem MS of M3′. M3 and M3′ were the cis–trans isoform. MS/MS fragmentation was conducted with collision energy at 5 eV.
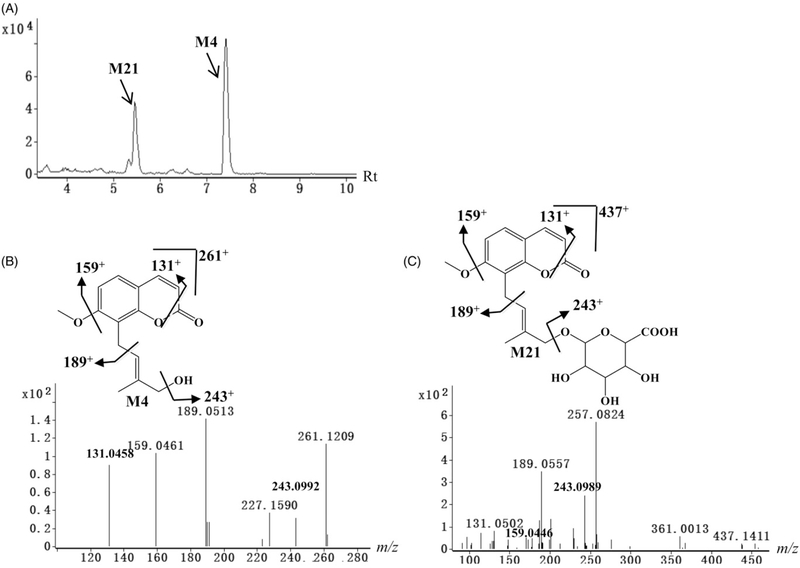
Figure 4.
Identification of phase II metabolites of osthole. (A) Chromatograms of M4 and M21. (B) Tandem MS and chemical structure of M4. (C) Tandem MS and chemical structure of M21. The phase II metabolite and its deconjugated metabolite show the similar fragmentation mode. MS/MS fragmentation was conducted with collision energy at 5 eV.
M0 was calculated as C15H16O3 based on the accurate mass measurement m/z 245.1193+. The main MS/MS fragmentation ions of osthole were 189, 159, and 131, corresponding to the elimination of the carbon chain, methoxy group, and carbonyl group in Figure 3B.
Neutral losses of 56 (C4H8) suggested the existence of 3-methyl-2-butenyl-group in metabolites M1 and M5. Metabolites M1 and M5 were calculated as C14H14O3 and C15H16O4 based on the accurate masses of m/z 231.1019+ and 261.1140+, respectively. Compared with the [M + H]+ ion (m/z 245.1193+) of osthole, M1 was lacked 14 Da (CH2) as compared with osthole and M4 was 16 Da (O) higher than osthole. According to the MS/MS fragments shown in Table S1, both metabolites were determined as 7-demethyl and hydroxyl metabolites of osthole.
Neutral losses of 54 (C4H6) or 42 (C3H6) suggested the existence of a 3-methyl-1,3-dibutenyl group in metabolites M2, M2′, M3, M3′, M6, M6′, M7, and M16, suggesting that these metabolites were a dehydrogenation product of the carbon chain. Metabolites M3 and M3′ were calculated as C15H14O3 based on the accurate masses of m/z 243.1023+, and lacked 2 Da (H2) as compared with osthole, thus indicating that they were dehydrogenation products of osthole. Metabolites M2 and M2′ matching the molecular formula C14H12O3 based on the accurate mass m/z 229.0863+, were proposed as the 7-demethylation product of M3 and M3′ based on the MS/MS fragmentations (Figure 3C, D). Metabolite M7 was deduced as C14H14O4 based on the accurate mass of m/z 247.0962+, and higher by 18 Da (H2O) than M2 and M2′. The product ion at m/z 193 of M7 was also higher by 18 Da than the product ion of M2 and M2′ at m/z 175 (Table S1), indicating that it was the hydration product of M2 or M2′. Metabolites M6 and M6′ were deduced as C15H14O4 based on the accurate mass measurement m/z 259.0969+ and higher by 16 Da (O) than M3 and M3′. The product ions at m/z 217 and 189 of M6 and M6′ were higher by 16 Da than the product ions of M3 and M3′ at m/z 201 and 173 (Table S1), indicating they were the oxidation product of M3 and M3′. Metabolite M16 was deduced as C14H12O4 based on the accurate mass measurement m/z 245.0840+, and was 16 Da (O) higher than M2 and M2′. The product ions at m/z 217 and 175 of M16 were 16 Da higher than the product ions of M2 and M2′ at m/z 201 and 159 (Table S1), indicating it was the oxidation product of M2 or M2′.
Neutral losses of 72 (C4H8O or H2O + C4H6) or 60 (H2O + C3H6) suggested the existence of a hydroxylated 3-methyl-2-butenyl group in metabolites M4, M8, M9, M9′, M10, M11, and M13. Metabolite M4 was calculated as C15H16O4 based on the accurate mass of m/z 261.1131+, and higher by 16 Da (O) than osthole, indicating that it was the carbon chain hydroxylation product of osthole based on the MS/MS fragmentations (Table S1). Metabolite M13 was deduced as C14H16O4 based on the accurate mass measurement m/z 249.1137+, indicating that it was the 7-demethylation and hydrogenation product of M4 based on the MS/MS fragmentations in Table S1. Metabolites M8, M9, M9′, M10, and M11 were deduced as C15H16O5, and higher by 32 Da (O2) than osthole, indicating they were the dihydroxylation product of osthole. The other MS/MS fragmental ions of M8, M9, M9′, M10, and M11 were interpreted in Table S1.
Neutral losses of 18 (H2O) and 46 (CH2O2) suggested the existence of carboxyl groups in metabolites M12 and M15, and they were carboxylation products of osthole after a 3,4-epoxide reaction. The other MS/MS fragmental ions of M12 and M15 are interpreted in Table S1. Metabolites M14 and M14′ were deduced as C14H18O4 based on the accurate mass of m/z 251.1284+, and were 2 Da (H2) higher than M13, indicating they were the hydrogenation products on the carbon chain of M13 based on the MS/MS fragmentations in Table S1. Metabolites M17 and M17′ exhibited the molecular ion at m/z 275.0929+, which gave a match for C15H14O5. The product ion at m/z 177 of M17 and M17′ was the same as the product ion of M9 and M9′ (Table S1), indicating that they were further dehydrogenation products of M9 and M9′. Metabolites M18 and M18′ were deduced as C14H14O4 based on the accurate mass of m/z 247.0946+, and were 16 Da (O) higher than M1. The product ion at m/z 175 of M18 and M18′ was the same as the product ion found in M1 (Table S1), indicating that M18 and M18′ were the carbon chain hydroxylation products of M1. Metabolite M19 was deduced as C15H18O5 based on the accurate mass of m/z 279.1221+, and was 2 Da (H2) higher than M11. The product ion at m/z 261 of M19 was also 2 Da higher than product ion of M11 at m/z 259 (Table S1), indicating M19 was the hydrogenated product of M11.
Identification of phase II metabolites of osthole
Metabolites M20–M30 were identified as conjugated metabolites of osthole (Table 1). All the phase II metabolites showed good responses in the negative ion mode (Table S2), and only partial glucuronic acid conjugate products could be detected in the positive ion mode (Table S1). After elimination of the glucuronic acid group (C6H10O7) in the negative ion mode, metabolites M20, M21, M22, M23, M24, and M25 yielded fragment ions at m/z 229, 259, 257, 275, 273, and 249 that were similar to the masses of M1, M4, M6, M9, M17, and M14, respectively (Table S2). The typical MS/MS fragmentations of M4 and M21 are provided in Figure 4B, C. These data suggested that metabolites M20–M25 were glucuronic acid-conjugated products. Similarly, after elimination of the sulfuric acid group (SO3) in the negative ion mode, metabolites M26, M27, M28, M29, and M30 gave fragment ions at m/z 229, 259, 245, 275, and 243 that were similar to the masses of M1, M4, M7, M9, and M16, respectively (Table S2). These data suggested that metabolites M26–M30 were sulfuric acid-conjugated products.
Comparison of osthole metabolism between oral and intraperitoneal administration
Overall, 34 phase I and II metabolites of osthole were identified in mouse urine after treatment with osthole, and 25 and 8 metabolites of osthole were identified in feces and plasma, respectively (Table 1). The levels of metabolites in mouse urine and plasma are presented in Figure 2. In urine, the excretion of osthole and its metabolites (M6, M8, M16, M28′, M29, and M30) following i.g. administration was more than after i.p. administration (p<0.05) (Figure 2A, B). The levels of osthole and its metabolites (M4, M23, M25′, and M26) were higher in plasmas following i.p. administration as compared with i.g. administration (p<0.05) (Figure 2C, D). The higher excretion of unmetabolized osthole in feces was also detected following i.g. administration as compared to i.p. administration, although no significant difference was found (Figure S3).
Comparison of in vitro metabolism of osthole by human and mouse liver microsomes
The osthole metabolites were analyzed by metabolomics using UPLC–ESI–QTOFMS after osthole was incubated with microsomes. OPLS-DA scores plot of the osthole treatment group and control group indicated two clusters (Figure 5A). The S-plot (Figure 5B) generated from OPLS-DA displayed the differential ions which contributed to the separation of two groups. Combined with the trend plots of ions in the experimental and control groups (Figure 5C-F), the topranking ions were putatively identified as osthole metabolites. The potential metabolites of osthole are marked in the S-plot (Figure 5B).
Figure 5.
Metabolomic analysis of MLM incubations with osthole. Separation of metabolites derived from MLM incubated with osthole in the positive mode by OPLS-DA score plots (A) and loading S-plots (B). In the score plots, the t[1] and to[1] values represent the score of each sample in principal component 1 and 2, respectively. In the S-plots, the X-axis represents the relative abundance of ions and the Y-axis represents the correction of each ion to the model. These S-plots represent the relationship between ions in relation to the first and second components present in the OPLS-DA score plot. Metabolites are labeled in S-plots. *, M + Na; **, M + K. (C) Trend plot of M3 in MLMs with and without osthole. (D) Trend plot of M3′ in MLMs with and without osthole. (E) Trend plot of M4 in MLMs with and without osthole. (F) Trend plot of M6′ in MLMs with and without osthole (●, control-treated MLMs; ▴, osthole-treated MLMs). The absence of NADPH in the microsome system was incubated as control groups in the figure.
A total of 23 osthole metabolites (M1–M17′) were identified in the incubations of osthole with MLM and HML (Table 1), including 11 novel metabolites (M7, M8, M10, M11, and M13–M17′). It was noted that osthole could be transformed into metabolites with cis- and trans-isomers, such as M3 and M3′ (Table 1, Figure 3C, D). However, due to the similar MS/MS fragments, the current mass spectrometry technology could not determine the absolute configuration of these cis- and trans-isomers (Figure 3C, D).
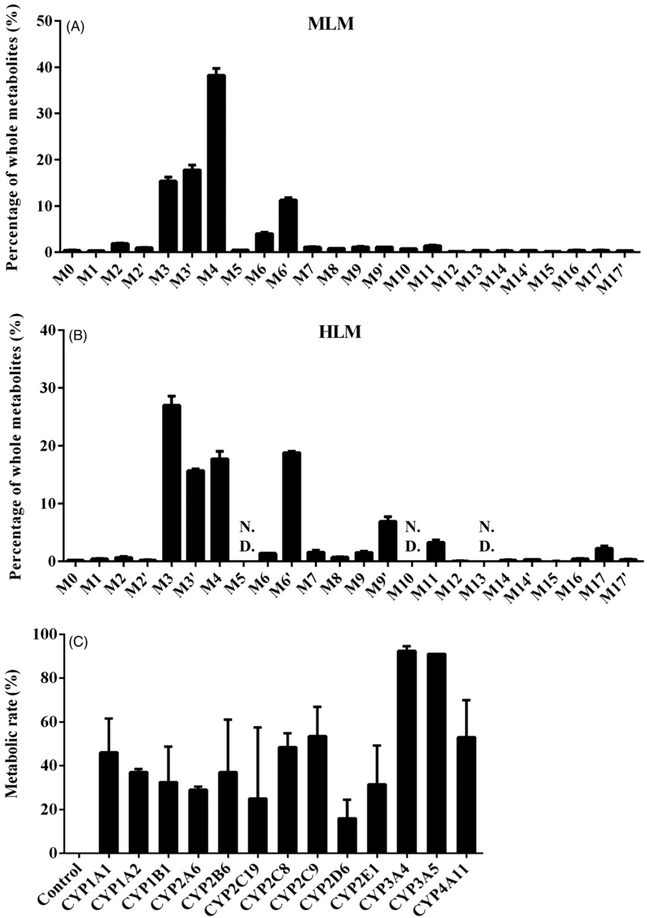
Metabolites M3, M3′, M4, M6, and M6′ were the major metabolites in the MLM incubations, while M3, M3′, M4, M6′, M9′, and M11 were major metabolites in the HLM incubations (Figure 6A, B). The percentage of hydroxylation metabolite M4 was decreased and dihydroxylation metabolites M9′ and M11 increased in the HLM incubations compared to MLM incubations (Figure 6A, B). Additionally, metabolites M5, M10, and M13 were not detected in the HLM incubations, but detected in the MLM incubations. The relative abundances of these metabolites in the MLM and HLM incubations are shown in Figure 6A, B.
Figure 6.
In vitro metabolism of osthole by recombinant CYPs. (A) Relative abundance of metabolites from MLM incubations with osthole. (B) Relative abundance of metabolites from HLM incubations with osthole. The overall abundance of metabolites was set as 100% in each sample. The data are expressed as mean ± SD (n = 3). (C) Metabolic rates of each recombinant CYP for osthole. The data are expressed as mean ± SD (n = 2). The residual amount of unreacted osthole after adding CYPs was collected. We specified the amount of unreacted osthole in control CYPs as the whole. Metabolic rate was calculated by the following formula: metabolic rate (%) = [1 – (the residual amount of osthole in each CYPs/the residual amount of osthole in control CYPs)] × 100%.
Screening the CYPs involved in the metabolism of osthole
CYPs are responsible for the transformation of more than 60% of currently marketed drugs (Hu et al., 2015). No study reported which CYPs are involved in the generation of osthole metabolites. In this study, the CYPs were screened by use of different human cDNA-expressed CYPs including the control, CYP1A1, 1A2, 1B1, 2A6, 2B6, 2C19, 2C8, 2C9, 2D6, 2E1, 3A4, 3A5, and 4A11. CYP1A1, 2C8, 2C9, 3A4, 3A5, and 4A11 were identified as the major forms contributing to the metabolism of osthole (metabolic rate >45%) (Figure 6C). Multiple enzymes were responsible for the formation of metabolite M1, M2, M2′, M3, M3′, M6, and M6′ (Table 2). CYP1A1, 3A4, and 3A5 were the major enzymes that responsible for the formation of monohydroxylated osthole metabolites (M4 and M5) and dihydroxylated osthole metabolites (M8, M9, M9′, M10 and M11) (Table 2). The formation of hydrogenated osthole metabolites (M14 and M14′) was mainly mediated by CYP3A4 and CYP3A5 (Table 2). CYP2D6, 2E1, 3A4, and 3A5 were the primary enzymes involved in the formation of the 3,4-epoxide products M15 (Table 2).
Table 2.
Role of CYPs in the formation of osthole metabolites.
| M1 | M2 | M2′ | M3 | M3′ | M4 | M5 | M6 | M6′ | M8 | M9 | M9′ | M10 | M11 | M14 | M14′ | M15 | M17 | M17′ | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | – | – | – | – | – | 0.3 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| CYP1A1 | 9.1 | 9.9 | 11.0 | 3.4 | 32.6 | 3.1 | 63.8 | 36.9 | 4.2 | 3.6 | – | – | 100 | 3.3 | – | – | – | – | – |
| CYP1A2 | 18.0 | 55.6 | 11.6 | 2.7 | 0.9 | 0.3 | 2.6 | 33.5 | 24 | – | – | – | – | – | – | – | – | 6.7 | 84.2 |
| CYP1B1 | 0.4 | 0.8 | 10 | 2.6 | 0.7 | 0.6 | – | – | 0.4 | – | – | – | – | – | – | – | – | – | – |
| CYP2A6 | 1.4 | – | – | 1 | 4.4 | 0.6 | 7.6 | 13.1 | 2.2 | – | – | – | – | – | – | – | – | – | – |
| CYP2B6 | 41.4 | 6.1 | 24.9 | 18.4 | 15.8 | 0.3 | 4 | 16.5 | 7.6 | 26.6 | – | – | – | – | – | – | – | 39.5 | – |
| CYP2C19 | 8 | 1.6 | 0.9 | 6.1 | 1.7 | 0.3 | 10.1 | – | 1.8 | – | – | – | – | – | – | – | – | – | – |
| CYP2C8 | 2.5 | – | 2.2 | 6.3 | 1.5 | 1.4 | – | – | 1.1 | – | – | – | – | – | – | – | – | – | – |
| CYP2C9 | 3.4 | 0.9 | 5.3 | 0.5 | 0.5 | 0.2 | 7.2 | – | 0.6 | – | – | – | – | – | – | – | – | – | – |
| CYP2D6 | 3.4 | 2.1 | – | 19.4 | 4 | 1.6 | 4.7 | – | 3.8 | – | – | – | – | – | – | – | 32.1 | – | – |
| CYP2E1 | 8.6 | – | 11.4 | 0.1 | 0.2 | 0.2 | – | – | 0.3 | – | – | – | – | – | – | – | 17 | – | – |
| CYP3A4 | 1.5 | 4.9 | – | 12.8 | 10.8 | 42.2 | – | – | 22.7 | 48.1 | 76.6 | 64.6 | – | 62.8 | 42.5 | 85.3 | 16.0 | 43.5 | 12.2 |
| CYP3A5 | 2.3 | 18.2 | 14.3 | 26.7 | 26.9 | 48.8 | – | – | 31.2 | 21.7 | 23.4 | 35.4 | – | 33.8 | 57.5 | 14.7 | 34.9 | 10.3 | 3.6 |
| CYP4A11 | – | – | 8.4 | – | – | 0.2 | – | – | 0.1 | – | – | – | – | – | – | – | – | – | – |
cDNA-expressed CYPs (control, CYP1A1, 1A2, 1B1, 2A6, 2B6, 2C19, 2C8, 2C9, 2D6, 2E1, 3A4, 3A5, and 4A11) were used to determine the role of in individual CYP in osthole metabolism. All samples were analyzed by UPLC-ESI-QTOFMS. The total peak areas of each metabolite of osthole from all the CYPs were set as 100%. All data are expressed as mean (n = 2).
Evaluation of the biological activity of osthole
Earlier studies reported the protective effects of osthole on various metabolic diseases (Qi et al., 2011; Song et al., 2006). Here, the levels of endogenous metabolites that are affected by osthole treatment were examined. As shown in Figure 1D, two top decreased ions, 478.2909+ and 526.2955+, were found under the bottom of the third quadrant of the S-plot. Chemical formula calculations showed that two ions were corresponded to C23H44NO7P and C27H44NO7P. The MS/MS fragmentation demonstrated that the ions 478.2909+ (Rt = 9.116) and 526.2955+ (Rt =9.083) were [M + H]+ ions of LPE18:2 and LPE22:6 (Figure 1E, F). Considering that LPEs and LPCs are closely associated with metabolic syndrome (Garcia-Fontana et al., 2016; Li et al., 2014), the levels of LPEs and LPCs in the plasma were analyzed using targeted metabolomics. LPCs and LPEs have one acyl chain that is linked to either the sn-1 or sn-2 position of glycerol, which produce the isomer, such as LPC16:0 and LPC16:0′ (Okudaira et al., 2014). The levels of three LPEs (LPE18:2, LPE20:4, and LPE22:6) and LPC20:3 were significantly reduced by osthole after 3 h i.g. treatment (p<0.05) (Figure S4). The levels of four LPEs (LPE18:2′, LPE20:4, LPE20:4′, and LPE22:6) were significantly decreased after 3 h i.p. treatment of osthole (p<0.05) (Figure S4).
After a 24 h treatment by i.g. with osthole, the levels of two LPEs (LPE16:0, and LPE18:2) and five LPCs (LPC16:1, LPC18:1, LPC18:2, LPC20:3, and LPC20:4) were significantly lower than the control group (Figure 7). Similarly, after a 24 h i.p. treatment with osthole, the levels of three LPEs (LPE18:2, LPE20:4, and LPE22:6) and five LPCs (LPC18:1, LPC18:1′, LPC18:2, LPC20:3, and LPC20:4) were significantly diminished (p<0.05) (Figure 7). These results indicated that both i.g. and i.p. treatment of osthole could cause the decrease in plasma LPEs and LPCs levels.
Figure 7.
Osthole regulates the lipid metabolism at 24 h after treatment. (A) Relative abundance of LPEs in plasma at 24 h after osthole treatment. (B) Relative abundance of LPCs in plasma at 24 h after osthole treatment. LPEs and LPCs have one acyl chain that is linked to either the sn-1 or sn-2 position of glycerol, which produce the isomer, such as LPC16:0 and LPC16:0′. Statistical analysis was performed using two-tailed student’s t-test and ANOVA (n = 4 in each group). *P < 0.05, and **P < 0.01 compared with control mice.
Discussion
Using high-resolution UPLC–ESI–QTOFMS combined with OPLS analysis, 41 metabolites of osthole, including 23 novel metabolites, were identified and further structurally elucidated by MS/MS fragmentography. By using this strategy, a series of hydroxylation, hydrogenation, demethylation, dehydrogenation, glucuronidation, and sulfation metabolites of osthole were identified in vivo and in vitro. Another interesting observation from this study is that two types of lipids LPEs and LPCs were decreased following treatment of osthole. Metabolomic analysis can simultaneously determine the metabolic map of osthole and elucidate its biological activity through its regulation on the endogenous metabolites. The metabolic pathways of osthole are summarized in Figure 8.
Although in vitro and in vivo metabolism of osthole in mice was previously reported (Lv et al., 2012a,b), a comprehensive profile of osthole metabolism has not been investigated. Results from the current metabolomic analysis provided a detailed view of the potential biotransformation pathway of osthole. The hydroxylation, hydrogenation, demethylation, dehydrogenation, glucuronidation, and sulfation of osthole generated 41 osthole metabolites, of which 23 metabolites (M7, M8, M10, M11, M13–M17′, M19, M22–M25′, and M27–M30) were novel metabolites. Overall, 34 osthole metabolites from phase I and II metabolism were identified in mice urine of which 7-demethylated-osthole (M1, M2, and M2′), dehydrogenated-osthole (M2, M2′, M6, and M17), hydroxylated-osthole (M6 and M17), glucuronic acid- and sulfuric acid-conjugates (M23, M24, M26, and M28) were the primary metabolites in mouse urine (Figure 2A, B). In mouse feces, metabolites M1, M2′, M3, M3′, M5, M6′, and M17 were mainly excreted, while no phase II metabolites were found in feces (Figure S3). The major circulating metabolites were M0, M3, M3′, M17 and the phase II metabolites M23, M24, M25′, and M26 (Figure 2C, D). It was reported that inhibitors of CYP3A4, 2D6, and 2C9 could inhibit the partial metabolism of osthole in liver microsomal incubations (Hu et al., 2015). However, which CYPs are responsible for the formation of osthole metabolites remains unclear. In this study, using recombinant CYPs indicated that the CYP3A4, 2C9, 1A1, 2C8, 3A5, and 4A11 were the major isoenzymes responsible for the formation of osthole metabolites (Figure 6C).
Coumarin 3,4-epoxide formation is extensive in mice (Born et al., 2003; Lewis et al., 2006). Osthole also had coumarin-structure, so we anticipated it also undergone 3,4-epoxidation. In our studies, we found M12 and M15 in osthole metabolites, which were carboxylation product of osthole after 3,4-epoxide reaction (Figure 8). By using CYPs, we found CYP2D6, 2E1, 3A4 and 3A5 were primary enzymes involved in the formation of M15 (Table 2), which was consistent with previous study who found that CYP2E1 and 3A4 were primary enzymes involved in the formation of 3,4-epoxidation in coumarin (Lewis et al., 2006).
We also found lots of hydroxylation (M4 and M5) and dihydroxylation (M8–M11) metabolites of osthole in our studies. By using CYPs, we found that CYP1A1, 3A4 and 3A5 were major enzymes participation in the oxidation reaction (Table 2), which was consistent with previous study who found that CYP3A4 was primary enzyme involved in the formation of 3-hydroxylation metabolites in coumarin (Lewis et al., 2006).
Osthole is metabolized by both phase I and phase II pathways. Phase II metabolism is critical for the drug detoxification, and glucuronidation and sulfation are the most important and widespread conjugation pathways. Previous studies found that the 7-hydroxy group in coumarin is prone to form glucuronic acid and sulfuric acid conjugated products (Duffy & O’Kennedy, 1998), but the reports about phase II conjugates of osthole are limited. In the present study, eight glucuronic acid-conjugated products (M20–M25′) and seven sulfuric acid-conjugated products (M26–M30) were found of which 11 were novel including M22–M25′ and M27–M30. Their structures were elucidated using MS/MS fragmentation patterns by comparison to the phase I metabolites. Glucuronic acid-conjugated products are produced by UDP-glucuronosyltransferases (UGT) (Duffy & O’kennedy, 1998), and these products are more water-soluble, less toxic and more readily excreted than the parent compound (de Leon, 2003). Sulfuric acid-conjugated products are formed by sulfotransferases. These products increase the water solubility and their renal excretion, but generally decrease the biological action of parent compound (Duffy & O’Kennedy, 1998). Furthermore, metabolites M6′, M9, M9′, M10, M13, M14, and M14′ were not found in urine compared with the in vitro MLM experiments, probably due to the formation of glucuronic acid and sulfuric acid conjugate products, including M22, M23, M25, M25′, M29, and M29′ (Figure 8).
The concentration of almost all osthole metabolites in urine were much higher than that in plasma (Wang et al., 2017), such as metabolite M6 (Figure 2). This phenomenon was in agree with the report of Wang et al. (2016), who found that the esculin (a coumarin derivative) metabolites in rat urine were much higher than that in plasma. In our studies, metabolite M6 apparently so dominant in urine yet barely perceptible in plasma (Figure 2). These results might indicate most of the osthole metabolites were potent to excrete from mice (Wang et al., 2017). Beside with liver, these CYPs are also expressed in intestine and kidney. The high abundance of M6 in urine suggests that the CYPs in the intestine and kidney also involve in the metabolism of osthole.
As we all know, the majority of urine was water (above 95%), and the feces were mainly emerged as solid form (Qiu et al., 2017). Therefore, the metabolites present in mouse urine were more polar than that present in feces. Metabolite M6 was more polar than M5, and we could found the rule from the RT in Table 1 and its conjugated system in Figure 8. Therefore, metabolite M6 seems the predominant excreted phase I metabolites in mouse urine (Figure 2A); metabolite M5 seems the predominant excreted phase I metabolites in mouse feces (Figure S3). The differences might come from their polarity, and more M6 was absorbed through enterohepatic circulation and excreted via kidneys into urine.
Metabolites M6 and M1 were the main excreted phase I metabolites in mouse urine. The excretion of metabolites M6 and M1 followed the order: M6 > M1 (Figure 2A). The excretion of the phase II metabolites of M6 and M1 followed the order: SO3-M1 (M26) > Glu-M1 (M20) ≈ Glu-M6 (M22) > undetected SO3-M6 (Figure 2B). Therefore, metabolites M1 was more liable to be eliminate in the form of phase II metabolites, and more liable to conjugation with sulfuric acid and glucuronic acid than M6. This might because the hydroxyl in M6 could form quinoid conjugated system, and this structure was stable. Furthermore, metabolites M1 was more liable to conjugation with sulfuric acid (M26) than glucuronic acid (M20) (Figure 2B).
CYPs was one of the most important enzyme families involved in the metabolism of xenobiotics. CYPs 1–3 were responsible for 70–80% of metabolism of clinically used drugs (Olsen et al., 2015), so we evaluated the contribution of some CYPs 1 to 4 to the metabolism of osthole (Table 2). CYP3A4 functioned as the most important human drug-metabolizing enzyme that cleared over a half of all administered pharmaceuticals, which could catalyze diverse oxidative reactions in addition to traditional hydroxylation reactions. This phenomenon was possible due to a large and malleable action site of CYP3A4, capable of accommodating substrates varying in size and chemical nature, as well as its ability to catalyze diverse chemical reactions such as O- and N-dealkylation, alkyl carbon and aromatic ring hydroxylation and epoxidation (Olsen et al., 2015). Our studies also found that CYP3A4 and 3A5 had a greater role on the formation of the osthole metabolites than other CYPs (Figure 6C), and they could promote the formation of nearly all the metabolites except M6 and M10 (Table 2). In the meanwhile, CYP2D6 had a relatively small role on the formation of the osthole metabolites (Figure 6C), and it could only promote the formation of metabolites M1, M2, M3–M5, M6′ and M15 (Table 2).
Previous studies indicated that osthole was able to decrease cholesterol and triglycerides in the liver of stroke-prone spontaneously hypertensive rats, however, it did not affect the levels of total lipids, glucose and apolipoproteins in the serum (Ogawa et al., 2007). In the current study, UPLC–ESI–QTOFMS combined with OPLS analysis revealed that the treatment with osthole significantly decreased the levels of LPE18:2 and LPE22:6 in the serum as early as 3 h after administration. The anomalous high levels of LPEs are related to certain disease, including hyperlipidemia and atherosclerosis (Chen et al., 2008b). Further targeted metabolomic analysis revealed that levels of a series of LPEs and LPCs could be reduced following osthole treatment, including LPE18:2, LPE20:4, LPE22:6, LPC16:1, LPC18:1, LPC18:2, LPC20:3, and LPC20:4. One possible mechanism is that osthole could activate PPARα and PPARγ. Earlier studies revealed that osthole decreased the levels of lipids by modulating the expression of lipogenic genes through activation of PPARα and PPARγ, including the diacylglycerol acyltransferase, 3-hydroxy-3-methylglutaryl-CoA reductase and cholesterol-7α-hydroxylase (Qi et al., 2011; Sun et al., 2010). PPARα plays a pivotal role in lipid metabolism by increasing high-density lipoprotein levels and decreasing serum free fatty acids and triglycerides (Mandard et al., 2004), and PPARγ has a critical role in glucose metabolism and fatty acid storage by coordinating the expression of genes involved in adipogenesis and lipid metabolism (Tontonoz & Spiegelman, 2008). Although previous studies suggested that osthole might be a dual agonist of PPARα and PPARγ (Qi et al., 2016; Zhao et al., 2014), further studies are needed to support this contention. Another potential mechanism is that osthole modulates the levels of the cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) (Teng et al., 1994; Zhang et al., 2015). cAMP signaling was shown to significantly regulate lipid metabolism, and dysregulation of hepatic cAMP levels plays a critical role in alcohol-induced steatosis (Avila et al., 2016). The exact mechanism by which osthole modulates cellular metabolism and metabolic diseases requires additional experimentation.
Hepatic disease was closely related to lipid metabolism disorder (Beyoglu & Idle, 2013). Therefore, the 3-methyl-2-butenyl-group at position-8 and the methoxy-group at position-7 in the chemical structure of osthole might be essential for its lipid-lowing activity because these group shown protective effects on hepatic diseases (Okamoto et al., 2005).
It was reported that bioavailability was 15.6% at a dose of 20 mg/kg body weight after oral administration of osthole (Yun et al., 2013). In this study, the differences of osthole metabolism between i.g. and i.p. administration were further investigated. Due to the mean peak concentration of osthole at about 3 h after oral administration to the rats (Yun et al., 2013), the levels of osthole and its metabolites in the plasma were determined at 3 h in this study. The results indicate that the phase I and II metabolites of osthole were significantly higher in plasmas at 3 h following i.p. administration as compared to the i.g. administration route (Figure 2C, D). In contrast to the levels of osthole and its metabolites in the plasma, the excretions of osthole metabolites in urine and osthole in feces 24 h following i.g. administration were higher than after i.p. administration (Figure 2A, B; Figure S3). The lower levels of partial LPEs and LPCs in the plasma following i.p. administration were observed in 24 h compared to i.g. administration of osthole, although there were no significant differences between i.p. and i.g. administration (Figure 7). These data suggest that the dose routes will affect the metabolism of osthole and its effect on lipids.
Conclusions
In summary, UPLC–ESI–QTOFMS-based metabolomics was used to determine the metabolic behavior of osthole, including comprehensive defining of the metabolites and endogenous metabolites that were altered after osthole treatment. Drug metabolite profiling of osthole-treated mice indicated that osthole undergoes several metabolic pathways in vivo, including the hydroxylation, hydrogenation, demethylation, dehydrogenation, glucuronidation, and sulfation. A total of 41 osthole metabolites were characterized and structurally elucidated, of which 23 were novel metabolites. Recombinant CYPs screening showed that CYP3A4 and CYP3A5 were the primary enzymes contributing to the metabolism of osthole. A comprehensive metabolic map of osthole was generated through use of metabolomics. Furthermore, changes in endogenous metabolites indicated the potential benefit effects of osthole on adipogenesis.
Supplementary Material
Acknowledgments
Funding
This work was supported by the Thousand Young Talents Program of China, National Natural Science Foundation of China (81360509), and the State Key Laboratory of Phytochemistry and Plant Resources in West China (52Y67A9211Z1).
Footnotes
Declaration of interest
The authors report no declarations of interest.
References
- Avila DV, Barker DF, Zhang J, et al. (2016). Dysregulation of hepatic cAMP levels via altered Pde4b expression plays a critical role in alcohol-induced steatosis. J Pathol 240:96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyoglu D, Idle JR. (2013). The metabolomic window into hepatobiliary disease. J Hepatol 59:842–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born SL, Api AM, Ford RA, et al. (2003). Comparative metabolism and kinetics of coumarin in mice and rats. Food Chem Toxicol 41:247–58. [DOI] [PubMed] [Google Scholar]
- Chen C, Krausz KW, Idle JR, et al. (2008a). Identification of novel toxicity-associated metabolites by metabolomics and mass isotopomer analysis of acetaminophen metabolism in wild-type and Cyp2e1-null mice. J Biol Chem 283:4543–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Shah YM, Morimura K, et al. (2008b). Metabolomics reveals that hepatic stearoyl-CoA desaturase 1 downregulation exacerbates inflammation and acute colitis. Cell Metab 7:135–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon J (2003). Glucuronidation enzymes, genes and psychiatry. Int J Neuropsychopharmacol 6:57–72. [DOI] [PubMed] [Google Scholar]
- Duffy CF, O’kennedy R. (1998). Determination of 7-hydroxycoumarin and its glucuronide and sulphate conjugates in liver slice incubates by capillary zone electrophoresis. J Pharm Biomed Anal 17:1279–84. [DOI] [PubMed] [Google Scholar]
- Fang ZZ, Krausz KW, Li F, et al. (2012). Metabolic map and bioactivation of the anti-tumour drug noscapine. Br J Pharmacol 167:1271–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Fontana B, Morales-Santana S, Navarro CD, et al. (2016). Metabolomic profile related to cardiovascular disease in patients with type 2 diabetes mellitus: a pilot study. Talanta 148:135–43. [DOI] [PubMed] [Google Scholar]
- Giri S, Krausz KW, Idle JR, et al. (2007). The metabolomics of (+/−)-arecoline 1-oxide in the mouse and its formation by human flavin-containing monooxygenases. Biochem Pharmacol 73:561–73. [DOI] [PubMed] [Google Scholar]
- Hu X, Huang W, Yang Y. (2015). Cytochrome P450 isoenzymes in rat and human liver microsomes associate with the metabolism of total coumarins in Fructus Cnidii. Eur J Drug Metab Pharmacokinet 40:373–7. [DOI] [PubMed] [Google Scholar]
- Jarzab A, Grabarska A, Kielbus M, et al. (2014). Osthole induces apoptosis, suppresses cell-cycle progression and proliferation of cancer cells. Anticancer Res 34:6473–80. [PubMed] [Google Scholar]
- Katsori AM, Hadjipavlou-Litina D. (2014). Coumarin derivatives: an undated patent review (2012–2014). Expert Opin Ther Patents 24:1323–1347. [DOI] [PubMed] [Google Scholar]
- Kind T, Fiehn O. (2007). Seven golden rules for heuristic filtering of molecular formulas obtained by accurate mass spectrometry. BMC Bioinformatics 8:105. doi: 10.1186/1471-2105-8-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DF, Ito Y, Lake BG. (2006). Metabolism of coumarin by human P450s: a molecular modelling study. Toxicol Vitro 20:256–64. [DOI] [PubMed] [Google Scholar]
- Li C, Li G, Tan DX, et al. (2013a). A novel enzyme-dependent melatonin metabolite in humans. J Pineal Res 54:100–6. [DOI] [PubMed] [Google Scholar]
- Li F, Jiang C, Larsen MC, et al. (2014). Lipidomics reveals a link between CYP1B1 and SCD1 in promoting obesity. J Proteome Res 13:2679–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Pang X, Krausz KW, et al. (2013b). Stable isotope- and mass spectrometry-based metabolomics as tools in drug metabolism: a study expanding tempol pharmacology. J Proteome Res 12:1369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Patterson AD, Krausz KW, et al. (2012). Metabolomics reveals the metabolic map of procainamide in humans and mice. Biochem Pharmacol 83:1435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Yang XW, Krausz KW, et al. (2015). Modulation of colon cancer by nutmeg. J Proteome Res 14:1937–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang HJ, Suk FM, Wang CK, et al. (2009). Osthole, a potential antidiabetic agent, alleviates hyperglycemia in db/db mice. Chem Biol Interact 181:309–15. [DOI] [PubMed] [Google Scholar]
- Liu X, Lu Y, Guan X, et al. (2015). Metabolomics reveals the formation of aldehydes and minimum in gefitinib metabolism. Biochem Pharmacol 97:111–21. [DOI] [PubMed] [Google Scholar]
- Lv X, Wang CY, Hou J, et al. (2012a). Isolation and identification of metabolites of osthole in rats. Xenobiotica 42:1120–7. [DOI] [PubMed] [Google Scholar]
- Lv X, Xin XL, Deng S, et al. (2012b). Biotransformation of osthole by Mucor spinosus. Process Biochem 47:2542–6. [Google Scholar]
- Mandard S, Muller M, Kersten S. (2004). Peroxisome proliferator-activated receptor alpha target genes. Cell Mol Life Sci 61:393–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa H, Sasai N, Kamisako T, et al. (2007). Effects of osthol on blood pressure and lipid metabolism in stroke-prone spontaneously hypertensive rats. J Ethnopharmacol 112:26–31. [DOI] [PubMed] [Google Scholar]
- Ojewole JAO, Adesina SK. (1983). Cardiovascular and neuromuscular actions of scopoletin from fruit of tetrapleura tetraptera. Planta Med 49:99–102. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Kobayashi T, Yoshida S. (2005). Chemical aspects of coumarin compounds for the prevention of hepatocellular carcinomas. Curr Med Chem Anticancer Agents 5:47–51. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Kobayashi T, Yoshida S. (2007). Synthetic derivatives of osthole for the prevention of hepatitis. Med Chem 3:35–44. [DOI] [PubMed] [Google Scholar]
- Okudaira M, Inoue A, Shuto A, et al. (2014). Separation and quantification of 2-acyl-1-lysophospholipids and 1-acyl-2-lysophospholipids in biological samples by LC-MS/MS. J Lipid Res 55:2178–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen L, Oostenbrink C, Jorgensen FS. (2015). Prediction of cytochrome P450 mediated metabolism. Adv Drug Deliv Rev 86:61–71. [DOI] [PubMed] [Google Scholar]
- Patterson AD, Li H, Eichler GS, et al. (2008). UPLC-ESI-TOFMS-based metabolomics and gene expression dynamics inspector self-organizing metabolomic maps as tools for understanding the cellular response to ionizing radiation. Anal Chem 80:665–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson AD, Slanar O, Krausz KW, et al. (2009). Human urinary metabolomic profile of PPARα induced fatty acid beta-oxidation. J Proteome Res 8:4293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Z, Xue J, Zhang Y, et al. (2011). Osthole ameliorates insulin resistance by increment of adiponectin release in high-fat and high-sucrose-induced fatty liver rats. Planta Med 77:231–5. [DOI] [PubMed] [Google Scholar]
- Qi ZG, Zhao X, Zhong W, et al. (2016). Osthole improves glucose and lipid metabolism via modulation of PPARα/γ-mediated target gene expression in liver, adipose tissue, and skeletal muscle in fatty liver rats. Pharm Biol 54:882–8. [DOI] [PubMed] [Google Scholar]
- Qiu Z, Yu J, Dai Y, et al. (2017). A simple LC-MS/MS method for the simultaneous quantification of resveratrol and its major phase II metabolites: assessment of their urinary and biliary excretions in rats. J Chromatogr B 1048:85–93. [DOI] [PubMed] [Google Scholar]
- Song F, Xie ML, Zhu LJ, et al. (2006). Experimental study of osthole on treatment of hyperlipidemic and alcoholic fatty liver in animals. World J Gastroenterol 12:4359–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F, Xie ML, Xue J, et al. (2010). Osthol regulates hepatic PPAR α-mediated lipogenic gene expression in alcoholic fatty liver murine. Phytomedicine 17:669–73. [DOI] [PubMed] [Google Scholar]
- Teng C, Lin C, Ko F, et al. (1994). The relaxant action of osthole isolated from Angelica pubescens in guinea-pig trachea. Naunyn Schmiedebergs Arch Pharmacol 349:202–8. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Spiegelman BM. (2008). Fat and beyond: the diverse biology of PPARγ. Annu Rev Biochem 77:289–312. [DOI] [PubMed] [Google Scholar]
- Tsukamoto H, Hisada S, Nishibe S. (1985). Coumarins from bark of Fxaxinus japonica and F. mandshurica var. japonica. Chem Pharm Bull 33:4069–73. [DOI] [PubMed] [Google Scholar]
- Venugopala KN, Rashmi V, Odhav B. (2013). Review on natural coumarin lead compounds for their pharmacological activity. Biomed Res Int 2013:963248. doi: 10.1155/2013/963248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhu W, Qiu J, et al. (2017). Enantioselective metabolism and enantiomerization of benalaxyl in mice. Chemosphere 169:308–15. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhao M, Ou Y, et al. (2016). Metabolic profile of esculin in rats by ultra high performance liquid chromatography combined with Fourier transform ion cyclotron resonance mass spectrometry. J Chromatogr B 1020:120–8. [DOI] [PubMed] [Google Scholar]
- Wen YC, Lee WJ, Tan P, et al. (2015). By inhibiting snail signaling and miR-23a-3p, osthole suppresses the EMT-mediated metastatic ability in prostate cancer. Oncotarget 6:21120–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z, Xu H, Wang K, et al. (2009). Determination of osthol and its metabolites in a phase I reaction system and the Caco-2 cell model by HPLC-UV and LC-MS/MS. J Pharm Biomed Anal 49:1226–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun F, Kang A, Shan J, et al. (2013). A rapid and sensitive LC-MS/MS method for the determination of osthole in rat plasma: application to pharmacokinetic study. Biomed Chromatogr 27:676–80. [DOI] [PubMed] [Google Scholar]
- Zhang ZR, Leung WN, Cheung HY, et al. (2015). Osthole: a review on its bioactivities, pharmacological properties, and potential as alternative medicine. Evid Based Complement Alternat Med 2015:919616. doi: 10.1155/2015/919616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Xue J, Wang XL, et al. (2014). Involvement of hepatic peroxisome proliferator-activated receptor α/γ in the therapeutic effect of osthole on high-fat and high-sucrose-induced steatohepatitis in rats. Int Immunopharmacol 22:176–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.