Summary
Primary mediastinal B‐cell lymphoma (PMBCL) is a distinct disease closely related to classical nodular sclerosing Hodgkin lymphoma. Conventional diagnostic paradigms utilising clinical, morphological and immunophenotypical features can be challenging due to overlapping features with other B‐cell lymphomas. Reliable diagnostic and prognostic biomarkers that are applicable to the conventional diagnostic laboratory are largely lacking. Nuclear factor kappa B (NF‐κB) and Janus kinase/signal transducers and activators of transcription (JAK‐STAT) signalling pathways are characteristically dysregulated in PMBCL and implicated in several aspects of disease pathogenesis, and the latter pathway in host immune evasion. The tumour microenvironment is manipulated by PMBCL tumours to avoid T‐cell mediated destruction via strategies that include loss of tumour cell antigenicity, T‐cell exhaustion and activation of suppressive T‐regulatory cells. R‐CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisolone) and DA‐EPOCH‐R (dose‐adjusted etoposide, prednisolone, vincristine, cyclophosphamide, doxorubicin, rituximab) are the most common first‐line immunochemotherapy regimens. End of treatment positron emission tomography scans are the recommended imaging modality and are being evaluated to stratify patients for radiotherapy. Relapsed/refractory disease has a relatively poor outcome despite salvage immunochemotherapy and subsequent autologous stem cell transplantation. Novel therapies are therefore being developed for treatment‐resistant disease, targeting aberrant cellular signalling and immune evasion.
Keywords: non‐Hodgkin lymphoma, tumour immunotherapy, haematological oncology, tumour biology, malignant lymphomas
Primary mediastinal B‐cell lymphoma (PMBCL) is an aggressive B‐cell lymphoma that represents 2–3% of non‐Hodgkin lymphoma (NHL) cases. It was considered a subtype of diffuse large B‐cell lymphoma (DLBCL) but, due to distinct clinicopathological features, it was acknowledged as a discrete entity in the 2008 World Health Organization (WHO) diagnostic criteria (Campo et al, 2011). This distinction from other DLBCL subtypes has been confirmed at a molecular level using gene expression profiling (Rosenwald et al, 2003; Manso et al, 2017). In fact, PMBCL is more akin to nodular sclerosing Hodgkin lymphoma (NScHL) than DLBCL. Both arise in the mediastinum from thymic B‐cells, affect a young patient cohort and share similar clinicopathological and genetic features (Rosenwald et al, 2003). There is an intermediate between PMBCL and NScHL, known as mediastinal grey zone lymphoma (MGZL). Distinguishing these overlapping diseases can be challenging but is necessary due to their differing treatment strategies. Despite highly curative immunochemotherapy regimens, 10–30% PMBCL patients have relapsed/refractory (RR) disease and require salvage therapies, which do not offer satisfactory outcomes. Consequently, novel therapeutic agents are being developed although with limited efficacy in PMBCL to date.
Clinical features, diagnosis and prognosis
PMBCL typically affects women (3:1 ratio) in their third or fourth decade of life. This gender preponderance is only seen in Caucasians; the incidence is similar in African‐Americans (Liu et al, 2016). PMBCL typically presents with compressive symptoms from the large mediastinal mass, such as cough or breathlessness. Superior vena cava obstruction is present in 25–30% of patients at diagnosis (Zinzani et al, 2002). Initial tumour progression tends to be localised although at relapse the disease can disseminate widely. Central nervous system (CNS) and bone marrow involvement are uncommon at presentation, 9% and 5% respectively (Bishop et al, 1999; Zinzani et al, 2002). In the rituximab‐era the 5‐year overall survival (OS) is estimated at between 79% and 97%, superior to that seen in de novo DLBCL (Dunleavy et al, 2013; Soumerai et al, 2014; Jackson et al, 2016).
It has been proposed that PMBCL originates from germinal or post‐germinal centres due to hypermutation in BCL6 and immunoglobulin (Ig) heavy chain variable region (VH) genes, which are markers of B‐cell transit through the germinal centre (Pileri et al, 2003; Csernus et al, 2004). As thymic B‐cells have been shown to have a similar spectrum of mutations they are believed to be the cell of origin (Csernus et al, 2004). The bulky anterior mediastinal tumour comprises large clear B‐cells often with compartmentalised fibrosis (Harris et al, 1994). Multinucleated Reed‐Sternberg‐like cells can also rarely be seen. The malignant cells strongly express B‐cell antigens, such as CD20, but have weak/variable expression of CD30, which can hinder differentiation from NScHL, and usually lack surface immunoglobulins (Pileri et al, 2003). De novo DLBCL shares many of the same antigens as PMBCL, making a differential diagnosis challenging.
MGZL is defined in the WHO classification as B‐cell lymphoma, unclassifiable, with features intermediate between DLBCL and classical Hodgkin lymphoma (cHL) (Swerdlow et al, 2017). A diagnosis of MGZL might therefore be made if the tumour has clinical, morphological and/or immunophenotypical features intermediate between cHL, PMBCL and DLBCL (Table 1). MGZL characteristically has a range of cytological appearances that can resemble any of these three related diseases, including more than one in the same tumour. The cytological appearance may be discordant with the immunophenotype, although this alone should not lead to a MGZL diagnosis (Swerdlow et al, 2017). Like PMBCL, MGZL presents with symptoms of localised compression and is more common in women, with a median age of 30 years (Sarkozy et al, 2017; Swerdlow et al, 2017).
Table 1.
Differences between supradiaphragmatic‐cHL, MGZL, PMBCL and DLBCL
| Supradiaphragmatic‐cHL | MGZL | PMBCL | DLBCL | |
|---|---|---|---|---|
| Gender predominance | Female>male | Female>male | Female>male | Male≥female |
| Median age (years) | Bimodal age distribution | 30 | 35 | 65 |
| Typical presentation | A painless mass often in the neck or incidental finding of a mediastinal mass. | Symptoms of localised mediastinal compression. | Symptoms of localised mediastinal compression. | One or more rapidly enlarging nodal or extranodal masses. |
| Morphological features | Mononuclear Hodgkin cells and multinuclear Reed‐Sternberg cells in a reactive infiltrate. Four histological subtypes: nodular sclerosing, lymphocyte‐rich, mixed cellularity and lymphocyte‐depleted. | Heterogenous appearances. Large cells, high cell density with areas of necrosis. |
Medium to large cells with clear cytoplasm. Compartmentalised fibrosis is often seen. |
Medium to large cells. Broad or fine bands of sclerosis may be seen. There are three common variants: centroblastic, immunoblastic and anaplastic. |
| Immunophenotypical features |
Reduced expression of B‐cell antigens. Absent surface immunoglobulin. Transcription factors OCT2 and BOB1 usually not expressed, PAX5 is weak or negative. CD30 positive. CD15 usually expressed. EBV positive in 40%. |
Heterogenous immunophenotype. Variable expression of B‐cell antigens. Absent surface immunoglobulin. Transcription factors PAX5, OCT2, BOB1 usually expressed. CD30 usually expressed. |
Strong expression of B‐cell antigens, such as CD20. Absent surface immunoglobulin. Transcription factors PAX5, OCT2, BOB1 expressed. Weak and variable CD30 expression. 70% express CD23, MAL, PDL‐1 and PDL‐2. |
Strong expression of B‐cell antigens such as CD20. Surface immunoglobulin typically present. Transcription factors PAX5, OCT2, BOB1 usually expressed. CD30 rarely expressed. EBV positive in 5%. |
| 5‐year overall survival | 85% (Shanbhag & Ambinder, 2018) | 74% (Wilson et al, 2014) | 79–97% | 65% |
cHL, classical Hodgkin lymphoma; DLBCL, diffuse large B‐cell lymphoma; EBV, Epstein–Barr virus; MGZL, mediastinal grey zone lymphoma; PMBCL, primary mediastinal B‐cell lymphoma.
Data directly comparing sensitivities and specificities of PMBCL immunohistochemical biomarkers have been reported, although many are not available in routine clinical practice. Biomarkers with high positive predictive value (PPV) include CD23 (98%), p63 (96%), BOB.1 (94%) and CD79a (90%) with some recommending CD79a, BOB.1 and cyclin E (100% PPV in cHL) as the most useful in differentiating between cHL and PMBCL (Hoeller et al, 2010). MAL, a lipid raft‐associated protein found in a relatively large proportion of PMBCL (70% cases), has been proposed as a useful marker to differentiate PMBCL from DLBCL, where it is expressed in only 3% of cases (Copie‐Bergman et al, 2002). Dorfman et al (2012) also found CD200 to have a superior sensitivity (94%) and equivalent specificity (93%) to other markers, including MAL and CD23.
Gene expression profiling may play an integral part in future diagnostic paradigms as it has been shown to accurately diagnose 80% of PMBCL cases (Scott et al, 2014). PDCD1LG2 (PD‐L2) RNA in situ hybridisation has also been investigated as an alternative to immunohistochemistry in PMBCL and showed sensitivity of 72% and specificity of 92% over DLBCL (Wang & Cook, 2018). Recently, the development and validation of a 58‐gene expression assay (Lymph3Cx) applicable to formalin‐fixed paraffin‐embedded tissue to distinguish between PMBCL and DLBCL has been described, with a 3·8% misclassification rate compared to conventional clinicopathological diagnostics (Mottok et al, 2018). As there is no single biomarker applicable to the diagnostic laboratory, PMBCL diagnosis remains challenging and necessitates combined evaluation of the above clinical, morphological and immunophenotypical features.
Unlike other lymphomas, prognostic biomarkers are largely lacking in PMBCL. Early identification of high‐risk patients enables focus on novel therapies and would spare low‐risk patients from unnecessary intensive therapy. Clinical predictors of poor outcome include pleural or pericardial effusion and high International Prognostic Index score (Aoki et al, 2013) but there is no PMBCL‐specific prognostic index. The International Extranodal Lymphoma Study Group (IELSG)‐26 study showed end of treatment (EOT) [18F]fluorodeoxyglucose positron emission tomography (FDG‐PET) predicted patient outcome in PMBCL (Martelli et al, 2014) and is subsequently being investigated as a tool to stratify patients for radiotherapy (RT) (IELSG‐37; NCT01599559).
Pathogenesis of PMBCL
Dysregulation of nuclear factor kappa B (NF‐κB) signalling
The NF‐κB pathway is instrumental in the development, survival and activation of B‐lymphocytes, and dysregulated signalling has been implicated in many B‐cell malignancies, including PMBCL (Sasaki & Iwai, 2015). The NF‐κB pathway is activated through distinct signalling axes. The canonical (classical) pathway is instigated when growth factors or cytokines, such as tumour necrosis factor (TNF), binds its receptor, initiating a signalling cascade culminating in activation of inhibitor of κB kinase subunit beta (IKKβ) (Hayden & Ghosh, 2012). The non‐canonical (alternative) pathway instead utilises IKKα, also triggered via TNF receptor stimulation (Sun, 2011). Gene expression profiling studies have demonstrated over‐expression of TNF family members, and TRAF1 in PMBCL and in cHL (Savage et al, 2003). In cHL, and presumably in PMBCL, this hyperactivation induces downstream anti‐apoptotic genes (e.g. BCL2 family members BCL2L1 [Bcl‐xL] and BCL2A1 [Bfl‐1/A1]), activation of caspases (e.g. caspase‐3) and transcription of cell cycle regulators (e.g. cyclins D1 and D2) resulting in malignant proliferation (Hinz et al, 2001).
There are several lines of evidence that implicate the NF‐κB pathway in PMBCL. Treatment of a PMBCL cell line with a small molecule IKB kinase inhibitor resulted in cell cytotoxicity, even at low doses, indicating dependence of this lymphoma on NF‐κB for survival (Lam et al, 2005). Other aberrations in this pathway include REL, a member of the NF‐κB transcription factor family, which was shown to be frequently amplified in PMBCL, cHL and DLBCL (Bea et al, 2005). Over 75% of PMBCL primary tumours and cell lines in one study were found to have chromosomal gains and gene amplifications in REL, and although this did not correlate with increased transcription nor protein expression, there was a significant association with nuclear REL expression consistent with pathway activation (Weniger et al, 2007). Moreover, amplifications in the NF‐κB regulators BCL10 and MALT1 have been reported where these gene products form a multimeric signalling complex to mediate pathway activation (Wessendorf et al, 2007).
Inactivation of negative feedback mechanisms that normally restrain NF‐κB activity also account for constitutive NF‐κB activation. A20, encoded by TNFAIP3 is a ubiquitin‐modifying enzyme that inhibits NF‐κB signalling downstream of TNF receptor engagement. The IKK complex and NF‐κB activation is reliant on Lys63 polyubiquitination of RIP1, a kinase that is recruited to the receptor upon TNF stimulation. A20 replaces Lys63 ubiquitins from RIP1 with Lys48 polyubiquitins, a switch that results in RIP1 proteasomal degradation and subsequent NF‐κB downregulation (Wertz et al, 2004). Loss‐of‐function nonsense and frameshift mutations in TNFAIP3 have been found in 36% of PMBCL cell lines and primary cases resulting in unarrested NF‐κB activation (Schmitz et al, 2009).
Dysregulation of Janus kinase/signal transducers and activators of transcription (JAK‐STAT) signalling
In normal cells, JAK‐STAT is tightly controlled to prevent unscheduled gene regulation and inappropriate biological responses. In malignant cells this system is altered, leading to constitutive JAK‐STAT‐dependent gene expression, including several key gene products required to initiate and/or maintain malignant transformation (Bowman et al, 2000). Target genes activated by this pathway contribute to oncogene activation, tumour suppressor de‐activation, abnormal cell proliferation, tumour growth and metastasis. Peptide ligands (e.g. cytokines) binding to transmembrane receptors initiate the pathway leading to receptor dimerization and cross‐phosphorylation of JAK kinases. In turn, this hyperphosphorylates STAT molecules, which translocate to the nucleus and act as DNA‐binding transcription factors, inducing expression of target genes (Aaronson & Horvath, 2002).
STAT6 is activated by tyrosine phosphorylation in response to interleukin (IL)4 or IL13 and plays a prominent role in modulating the immune system (Goenka & Kaplan, 2011). Recurrent point mutations in STAT6 DNA binding domain have been reported in 36% of PMBCL cases (Ritz et al, 2009) with a hotspot mutation affecting the amino acid p.419D. This lesion hyperactivates the IL4‐JAK‐STAT6 axis, as evidenced by elevated expression of STAT6 target genes (Yildiz et al, 2015). Silencing activated STAT6 in the PMBCL‐derived cell line MedB‐1 showed decreased Bcl‐xL (BCL2L1) expression and cell survival (Ritz et al, 2008). Upstream of this signalling axis, gain‐of‐function mutations in IL4R have been reported in 24% of PMBCL primary samples and in 100% of PMBCL cell lines, which led to ligand‐independent phosphorylation of STAT6 and STAT5 (Viganò et al, 2018). Most mutations were single nucleotide variants that affected residue p.242I in the transmembrane domain of IL4R, indicating a hotspot lesion. Expression of mutant IL4R in a mouse xenotransplantation model conferred growth advantage in vivo. Downstream of constitutive JAK‐STAT activation, the mutant upregulated the B‐cell specific antigen CD23 and the tumour‐promoting chemokine CCL17. Interestingly, once secreted to the tumour microenvironment (TME), CCL17 attracts CCR4+ tumour‐promoting cells, such as CD4+ T‐cells and T‐regulatory cells (Imai et al, 1999; Iellem et al, 2001). This immunocompromised setting presumably allows PMBCL tumours to acquire an immune escape phenotype. The same study revealed multiple concurrent mutations affecting the JAK‐STAT pathway in primary tumours, demonstrating potential synergistic/additive effects of these aberrations in PMBCL pathogenesis.
Given that activated STAT proteins accumulate in the nucleus to drive target gene transcription, it is critical to tightly regulate the duration and strength of activation to “dampen” the pathway when unneeded. Termination of signalling is achieved by negative regulatory factors, including suppressors of cytokine signalling (SOCS) and protein tyrosine phosphatases (PTPs) (Levy & Darnell, 2002). SOCS protein family members share a common SH2 domain that contains an upstream kinase inhibitory region that has been shown to bind the tyrosine phosphate activation loop of JAK proteins to attenuate kinase activity (Yasukawa et al, 1999). Loss‐of‐function mutations in SOCS1 spanning all domains, consisting of indels and missense mutations, leading to premature peptide abort and JAK‐STAT pathway de‐regulation have been reported in B‐cell lymphomas (Mottok et al, 2009). The occurrence of mutations in PMBCL is 45% and bi‐allelic deletions concurrent with delayed degradation of de novo JAK2, hyperphosphorylation of JAK2/STAT5 in PMBCL cell lines have also been reported. Furthermore, restoration of wild type SOCS1 in these cell lines repressed CCND1, induced RB1 and activated caspase‐3, indicating an increase in the apoptotic cell fraction (Melzner et al, 2005; Ritz et al, 2008).
The prototype of protein tyrosine phosphatases, PTP1B (encoded by PTPN1) also mitigates JAK‐STAT activity in its role of dephosphorylating active kinases. Somatic PTPN1 mutations have been found in PMBCL cases (22%) and cell lines (33%) (Gunawardana et al, 2014). Mutations leading to premature protein truncations and amino acid substitutions were deleterious to phosphatase activity and resulted in sustained activation of JAK‐STAT. Chromosomal rearrangements involving the classical lymphoma oncogenes BCL6 and MYC are atypical events in PMBCL (Savage et al, 2003), however amplifications in both genes have been reported by genomic profiling (Wessendorf et al, 2007). Intriguingly, shRNA‐mediated PTPN1 silencing led to overexpression of BCL6 and MYC, indicative of tissue specificity of the phosphatase.
Genes encoding components of JAK‐STAT are often over‐expressed in PMBCL including JAK2, STAT1 and IL13RA2 (Savage et al, 2003). JAK2 mutations are well described and implicated in myeloproliferative disorders but largely absent in lymphoid malignancies. However, JAK2 genomic copy number amplifications at chromosome 9p24.1 are characteristic of Hodgkin lymphoma (HL) and PMBCL (seen in 63% of PMBCL cases) and induce cell proliferation via JAK2/STAT1 signalling (Joos et al, 2000). Mice challenged with derived cell lines of both diseases bearing amplified JAK2 and treated with JAK2 inhibitors exhibited decreased tumour growth and intratumoural p‐STAT3 levels (Hao et al, 2014). Despite the attenuation of tumourigenesis following JAK2 inhibition seen in vivo, the precise mechanism of JAK2 activation as a direct result of copy number aberrations remains unclear. Notably, JAK2 amplification was associated with upregulation of the programmed death ligands PD‐L1 (CD274) and PD‐L2 (PDC1LG2) (Green et al, 2010), demonstrating a link between the JAK‐STAT pathway and tumour immune evasion.
The PMBCL tumour microenvironment
Contemporary tumorigenesis models have diverged from being centred on the description of accumulating genetic changes and signalling alterations in malignant cells towards the dynamic interactions between cancer cells and the surrounding stroma, termed the tumour microenvironment (TME). The TME is recognised as a critical element for tumour development and progression, and a measurable parameter of response to treatment (Barry et al, 2018). Malignant cells are conferred a selected growth advantage when they successfully evade the immune system and prosper as the prevailing cell (Wang et al, 2017). Therefore, manipulating the balance between immune responsiveness and self‐tolerance is essential to avoid T‐cell mediated destruction and to thrive in an immunocompromised setting. In this section, we review microenvironment‐related strategies used by PMBCL tumours to sculpt their reactive milieu to survive host immunity (Fig 1).
Figure 1.
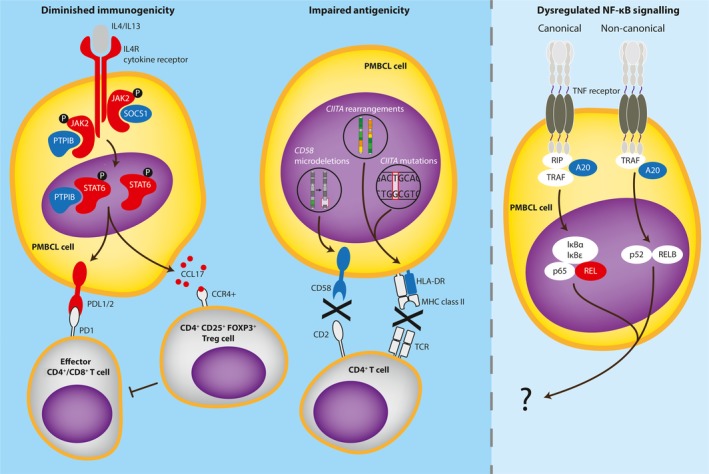
Dysregulated immune response in the primary mediastinal B‐cell lymphoma (PMBCL) tumour microenvironment. Activating (red) genetic lesions/copy number gains and inactivating (blue) gene mutations in components of the JAK‐STAT signalling pathway diminish tumour immunogenicity via upregulation of programmed death ligands (PDLs) and C‐C motif chemokine ligand 17 (CCL17). Microdeletions in CD58 and mutations/structural rearrangements in class II major histocompatibility complex transactivator (CIITA) impair tumour antigenicity via downregulation of conjugate formation and major histocompatibility complex (MHC) class II, respectively. These immune escape strategies lead to T‐cell exhaustion, activation of suppressive T‐regulatory cells (Treg) and crippled immune surveillance. The impact of genetic aberrations in components of the nuclear factor kappa B (NF‐ĸB) signalling pathway on the PMBCL tumour microenvironment is not known.
Loss of antigenicity
Major histocompatibility complex (MHC) molecules display neoantigenic peptides to the T‐cell receptor (TCR) to initiate the adaptive immune response. The integrity of this process is dependent on the ability of the malignant B cell to present antigen to a T cell in the context of a peptide‐MHC complex. Tumours which acquire defects in antigen presentation or lose MHC expression will be resistant to immune‐mediated elimination by tumour‐specific T‐cells, resulting in impaired activation of CD4+ (MHC‐II recognition) and CD8+ T‐cells (MHC‐I recognition). Downregulation of MHC‐II are defining immunophenotypes in many subtypes of lymphoma (Rosenwald et al, 2002; Rimsza et al, 2006; Diepstra et al, 2007) and similarly in PMBCL, substantial loss of HLA‐DR has been reported, with 12% of cases showing complete loss of protein. Inferior patient survival in PMBCL correlated with incremental decreases in MHC‐II expression (Roberts et al, 2006). This loss may partly be explained by aberrations in the MHC‐II master transcriptional regulator, CIITA. Genetic alterations in CIITA is a defining feature in PMBCL with 70% cases affected via coding sequence mutations, deletions and chromosomal translocations. Most mutations were caused by activation‐induced (cytosine) deaminase (AID)‐mediated aberrant somatic hypermutation that downregulated MHC‐II surface expression (Mottok et al, 2015). Interestingly, CIITA gene fusions resulted in upregulation of PD‐L1 and PD‐L2 (Twa et al, 2014).
T‐cell anergy
PD‐L1 and PD‐L2 engage their cognate receptor PD‐1 to modulate effector T‐cell function and to induce peripheral tolerance. Many lymphomas, including PMBCL, exploit the PD‐L/PD‐1 axis to suppress the antitumor response (Gatalica et al, 2015; Keane et al, 2015; Vari et al, 2018). PD‐L1/2 overexpressing malignant cells increase the co‐inhibitory pathways leading to hypo‐responsive T‐cells known as T‐cell anergy (Wang et al, 2018). PD‐L1/2 expression is aberrant in malignant B cells through a combination of somatically acquired copy‐number gains and chromosomal rearrangements (Chong et al, 2016). CD274 (previously termed PD‐L1) and PDCD1LG2 are located on chromosome 9p24.1. This common cytoband is shared with JAK2 and is concurrently amplified, as discussed previously. By fluorescence in situ hybridization and chromosome break‐apart analysis, amplification of this locus was highest in PMBCL (29% in 125 cases) compared to other B‐cell lymphomas, and frequently and specifically rearranged in 20% of cases, respectively as compared with other lymphomas (Twa et al, 2014). Fourteen structural rearrangement events involving PD‐L1 and 19 involving PD‐L2 have been described. Both amplifications and structural rearrangements resulted in increased transcript and protein levels of PD‐L1 and PD‐L2 (Shi et al, 2014; Twa et al, 2014; Chong et al, 2016). Co‐culture of a B‐cell line expressing the PMBCL PDCD1LG2‐IGHV7‐81 arrangement with Jurkat T‐cells significantly decreased the early T‐cell activation marker CD69, reinforcing the paradigm that juxtaposition with IGH super‐enhancers influences many aspects of malignancy, including the TME.
CD2 is a member of the immunoglobulin supergene family and is expressed primarily on T and NK cells, and binds ligands expressed on antigen‐presenting cells and malignant B cells, mainly CD58 (also known as LFA‐3) (Bierer et al, 1988). The binding between CD2 and CD58 stabilises the joining of the T cell to the malignant B cell so that the two cells form a conjugate. Once closely associated, the TCR scans various peptide‐loaded MHC combinations to initiate intracellular signalling necessary for T‐cell activation. In vitro, neutralizing antibodies directed against CD2 or CD58 prevents conjugate formation and decreases T‐cell activity (Mak & Saunders, 2006). The CD58 locus in PMBCL was found to be targeted by mono‐ and bi‐allelic microdeletions in 3 of 4 cell lines and in 5 primary tissues interrogated, resulting in conspicuous silencing of CD58 gene expression (Dai et al, 2015). Although functional studies in PMBCL are lacking, reconstitution of CD58 in a null DLBCL cell line increased NK‐cell mediated cytolysis (Challa‐Malladi et al, 2011). Furthermore, in DLBCL, HL and in transformed follicular lymphoma (FL), mutations of CD58 have been found to co‐occur frequently with B2M mutations suggesting complementary mechanisms to establish immune privilege (Challa‐Malladi et al, 2011; Pasqualucci et al, 2014; Abdul Razak et al, 2016).
Regulatory T‐cell activation
In malignancies, the TME and host immune suppression are frequently dictated by regulatory T‐cell (Treg) function. This cell subset accounts for 4–10% of all peripheral CD4+ cells and is responsible for maintenance of autoantigen tolerance and regulation of the immune response by suppression of effector cells. These CD4+CD25+ cells intracellularly express the forkhead transcription factor, FOXP3 which is essential for Treg development and function (Ohkura et al, 2013).
As mentioned earlier, IL4R mutations in PMBCL have been shown to induce the tumour‐promoting chemokine, CCL17 (also known as TARC) both in vitro and in vivo. Moreover, 36% of PMBCL cases showed elevated protein levels (Viganò et al, 2018). Once secreted to the TME, CCL17 binds with high affinity to CCR4 receptors on T cells, including Treg cells (Ghia et al, 2001). In line with this, immunohistochemical analysis of 48 PMBCL tumours showed that a high (but variable) proportion of the tumour‐infiltrating CD4+CD25+ T‐cell subset expresses FOXP3, one of the highest increases among other lymphomas. However, unlike in FL, DLBCL and HL, no influence on patient survival was found (Tzankov et al, 2008). CCL17 is also highly expressed by Reed‐Sternberg cells and is detectable in HL patient serum (Jones et al, 2013). As it correlates with disease staging, it has been used as a HL biomarker for predicting and monitoring response and detection of relapse (Plattel et al, 2012; Jones et al, 2013). The role of CCL17 as a disease response biomarker in PMBCL remains to be established.
Despite these encouraging findings, the role of Tregs in PMBCL immunity remains poorly understood. Further investigations in larger cohorts and functional studies replicating the TME are needed to precisely delineate immune evasion and tumour progression associated with this subpopulation of suppressive T‐cells.
Current first‐line treatment
Currently, there is no established standard of care in PMBCL due to the paucity of patients and its recent recognition as a distinct disease. Data used to guide management is somewhat limited to retrospective studies or analysis of PMBCL subgroups within prospective DLBCL trials; Fig 2 outlines a range of management options that are currently available or under evaluation. The major controversies that still exist revolve around balancing maximum cure against minimum long‐term toxicity in this young patient population. Successful first‐line treatment is ever more important as cure rates for RR disease are poor.
Figure 2.
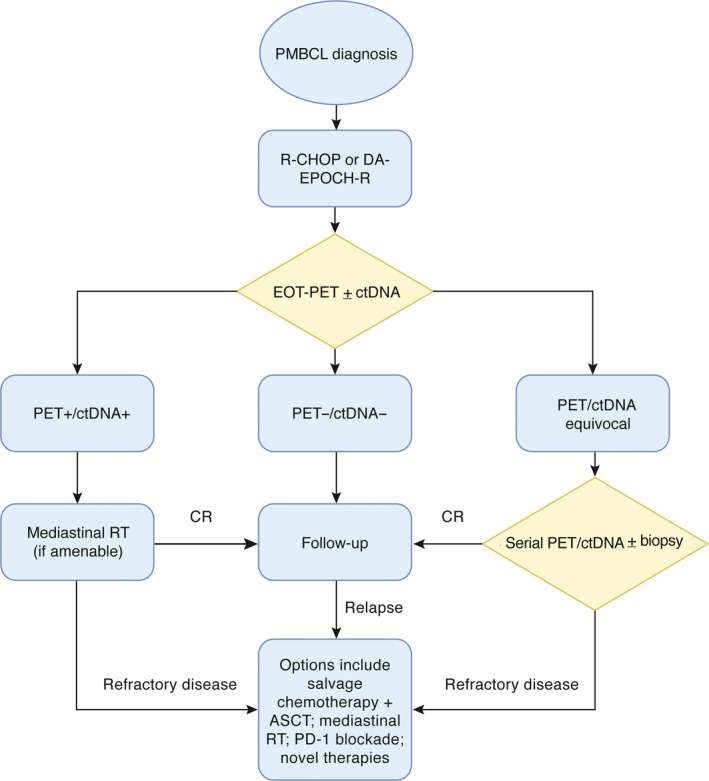
Potential therapeutic options in PMBCL. Prospective studies to establish optimal first and subsequent line treatment and monitoring strategies are currently ongoing. This schema outlines a range of options that are either currently available or under evaluation (such as ctDNA). ASCT, autologous stem cell transplant; CR, complete response; ctDNA, circulating tumour DNA; DA‐EPOCH‐R, dose‐adjusted etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin, plus rituximab; EOT, end of treatment; PD‐1, programmed cell death 1 (also termed PDCD1); PET, positron emission tomography; PMBCL, primary mediastinal B‐cell lymphoma; R‐CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, prednisolone; RT, radiotherapy.
Historically, as it was considered a subtype of DLBCL, CHOP (cyclophosphamide, doxorubicin, vincristine, prednisolone) was the usual first‐line treatment regimen for PMBCL. The addition of rituximab (R‐CHOP) improved 3‐year event‐free survival (EFS) compared to CHOP (78% vs. 52%; P = 0·012) with equivalent 3‐year overall survival (OS) (88·5% vs. 78·2%; P = 0·158) in a subgroup analysis of 87 PMBCL patients in a DLBCL trial (Rieger et al, 2011). Alternative regimens include MACOP‐B (methotrexate, doxorubicin, cyclophosphamide, prednisolone, bleomycin) or VACOP‐B (etoposide, doxorubicin, cyclophosphamide, prednisolone, bleomycin). MACOP‐B‐like regimens have shown similar complete response (CR) rates of 51% (142/277) compared to 49% (50/105) in CHOP‐like regimens, but better predicted 10‐year OS of 71% vs. 44% respectively (P < 0·001) (Zinzani et al, 2002). Since the addition of rituximab to CHOP, however, the advantage is no longer clear. In a retrospective study comparing 153 patients receiving V/MACOP‐B, CHOP and R‐CHOP, the only significant difference in survival was between V/MACOP‐B and CHOP (P = 0·016); therefore R‐CHOP and V/MACOP‐B are considered equivalent (Savage et al, 2006). Of note, addition of rituximab to V/MACOP‐B has been trialled but with no obvious benefit (Zinzani et al, 2009).
More recent evidence suggests that dose‐intensive regimens, such as DA‐EPOCH‐R (dose‐adjusted etoposide, prednisolone, vincristine, cyclophosphamide, doxorubicin, rituximab) have either comparable or superior outcomes to R‐CHOP. In a phase 2 trial, 51 PMBCL patients who received DA‐EPOCH‐R demonstrated EFS of 93% at a median follow‐up of 63 months [95% confidence interval (CI) 81–98%] with overall survival of 97% (95% CI 81–99%) (Dunleavy et al, 2013). Of significant interest, only 2/51 (4%) of the patients in this trial required consolidative RT, indicating that remission can be achieved without RT. A retrospective study in 2017 supported these results when it reported on 156 adults and children with PMBCL who received DA‐EPOCH‐R. The estimated 3‐year EFS was 85·9% (95% CI 80·3–91·5%) and OS 95·4% (95% CI 91·8–99·0%); there were no differences in outcomes between adults and children (Giulino‐Roth et al, 2017). In this study, 14·9% patients received RT. A 2016 subgroup analysis of PMBCL patients in the UK National Cancer Research Institute R‐CHOP‐14 versus 21 trial resulted in a 5‐year OS (83·8%) that fell within the 95% CI range for the Dunleavy et al DA‐EPOCH‐R trial results (Gleeson et al, 2016); of interest this study showed a non‐significant improvement in outcome with R‐CHOP‐14 compared to R‐CHOP‐21 but this requires further investigation. In paediatric patients there is conflicting data regarding DA‐EPOCH‐R in PMBCL. The Inter‐B‐NHL Ritux 2010 study investigated DA‐EPOCH‐R in 47 paediatric PMBCL patients, resulting in a 2‐year EFS of 69% (95% CI 52–82%) and OS of 82% (95% CI 67–91%), which were no better than previous PMBCL studies (Burke et al, 2017), suggesting that DA‐EPOCH‐R is not superior to conventional treatment in children.
In considering the outcomes with DA‐EPOCH‐R it is important to note that dose‐intensive regimens come with significant associated toxicities, despite potential lower RT use. In a phase 3 R‐CHOP versus DA‐EPOCH‐R trial in DLBCL there were higher treatment cessation rates due to adverse events (AE) with DA‐EPOCH‐R (5·6% vs. 1·7% in R‐CHOP arm) (Wilson et al, 2016). The safety profile reported in the prospective DA‐EPOCH‐R trial in PMBCL patients however appears better than that above (Dunleavy et al, 2013). Long‐term toxicities are also expected with DA‐EPOCH‐R given the high cumulative anthracycline dose. In one retrospective study with a short follow‐up interval 13·1% patients had a cardiac abnormality (Giulino‐Roth et al, 2017). There is unfortunately no randomised trial to date comparing R‐CHOP and DA‐EPOCH‐R from which to draw a conclusion for PMBCL patients.
Some therapies previously reserved for RR disease, such as brentuximab vedotin, are now being investigated as part of first‐line regimens as they may have better safety profiles and efficacy. Up‐front autologous stem cell transplant (ASCT) has been investigated in PMBCL with no change in OS compared to first‐line chemotherapy (Hamlin et al, 2005).
FDG‐PET
FDG‐PET is the recommended modality for EOT imaging in aggressive lymphoma. Various methods of FDG interpretation have emerged, including the qualitative Deauville score comparing FDG uptake to the liver, typically used in clinical practice, along with more quantitative methods such as standardised uptake value (SUV) and total lesion glycolysis (TLG), which are still primarily used in research studies (Ziai et al, 2016). Following the IELSG‐26 study a negative EOT‐PET in PMBCL is considered one with uptake less than or equal to the liver (Deauville 1–3), indicating complete metabolic response to immunochemotherapy. In this prospective study using these criteria, a negative EOT‐PET scan predicted a 5‐year PFS of 99% compared to 68% in a positive scan (P < 0·001) and 5‐year OS of 100% vs. 83% respectively (P < 0·001) (Martelli et al, 2014). There was also a difference between Deauville 4 and 5 positive scans: the rate of RR disease in the Deauville 4 group was 5/24 compared to 6/10 with Deauville 5. Many positive EOT‐PET scans are falsely positive, thought to be due to residual inflammation in the mediastinum post‐treatment. This is illustrated by EOT‐PET's high negative predictive value but poor PPV, 100% and 17% respectively in the DA‐EPOCH‐R study (Dunleavy et al, 2013). To confirm RR disease in this study patients underwent serial PET imaging ± biopsy; three patients underwent biopsy and only one was positive for residual tumour.
Newer techniques are enhancing the prognostic ability of FDG‐PET. TLG, which combines assessment of tumour volume and metabolism, has been shown to be an excellent prognostic tool: low TLG at diagnosis was associated with 100% 5‐year OS compared to 80% with high TLG (P = 0·0001) and PFS of 99% and 64% respectively (P < 0·0001) (Ceriani et al, 2015). More recently, the heterogeneity of FDG uptake calculated by the area under the curve on a cumulative SUV histogram (AUC‐CSH) has been developed as another method of interpreting FDG with a strong predictive value. Amongst 103 PMBCL patients, the most heterogenous FDG uptake at baseline correlated with poorer outcomes, such as 5‐year PFS of 73% vs. 94% in less heterogenous distributions (Meignan & Cottereau, 2018). In this study both TLG and AUC‐CSH were shown to be independent prognostic markers of PFS in PMBCL.
Use of radiotherapy
PMBCL is a radiosensitive disease and RT continues to play an important but controversial role in treatment. Lymphoma survivors have high incidences of secondary cancers due to chemotherapy and RT, estimated at around 1% per year in HL (Dores et al, 2002), as well as increased risk of coronary artery disease, valvular disease and heart failure attributed to anthracyclines and RT.
A retrospective analysis of 426 patients in the National Cancer Database suggested superior 5‐year OS for patients receiving RT after multiagent chemotherapy compared to those who did not (83% vs. 93% respectively) (Jackson et al, 2016). Other evidence indicates that the improved outcomes with RT are more marked in those with partial response (PR), classified as Deauville 4–5 on PET but improved disease from baseline. In one retrospective study, the addition of consolidative RT to MACOP‐B transformed 55/59 (93%) of patients with PR to CR (Zinzani et al, 2001). This improvement in partial responders is supported by another retrospective study including patients treated with both CHOP‐like regimens and MACOP‐B (Zinzani et al, 2002). These studies both assessed response using CT scanning, in the era before routine FDG‐PET.
Although there is evidence for RT in those with residual disease following R‐CHOP or V/MACOP‐B, the need for RT in patients who have CR is uncertain. A negative EOT‐PET has been successfully used to detect patients who can safely forego RT following R‐CHOP (Savage et al, 2012) and R‐MACOP‐B (Zinzani et al, 2015). Both retrospective studies demonstrated no difference in outcome for PET‐negative patients who did not receive RT compared to PET‐positive patients that did, suggesting that RT is not needed for PET‐negative patients. Fortunately, a randomised trial is in progress to conclusively answer this question (IELSG‐37): patients who have a negative EOT‐PET following rituximab‐containing immunochemotherapy will be randomised to either mediastinal radiation or close observation.
With the favourable outcomes of dose intensive chemotherapy, RT could be avoided in most patients. A prospective trial using dose‐intense R‐CHOP/ICE (ifosfamide, carboplatin, etoposide ± rituximab) in 54 PMBCL patients with mostly bulky or extra‐nodal presentation demonstrated estimated 3‐year OS of 88% and PFS of 78% without using RT (Moskowitz et al, 2010). Arguably, an additional benefit of this trial was that withholding RT first‐line allowed second‐line use of salvage auto‐transplant with mediastinal radiation. The prospective DA‐EPOCH‐R study (Dunleavy et al, 2013) also did not include RT in first‐line treatment. An updated analysis showed that of 25 PET‐positive patients, 5 (20%) had evidence of residual disease or progression, notably more commonly in the Deauville 5 group (4/8) compared to the Deauville 4 group (1/17) (Melani et al, 2018). Only one EOT‐PET negative patient relapsed after 320 days.
The pragmatic approach to the patient with the positive EOT‐PET scan is a watch and wait approach involving serial PET scans and/or biopsy with its associated risks (Giulino‐Roth, 2018). Already utilised in DLBCL (Rossi et al, 2017), plasma circulating tumour DNA (ctDNA) could obviate the need for tissue diagnosis in future.
Relapsed/refractory disease
Despite having more favourable outcomes to initial therapy than DLBCL, 10–30% of PMBCL patients have primary refractory or relapsed disease and the outcomes are poor (Aoki et al, 2015). Relapse usually occurs within 12 months, is more likely to be widespread and can involve the CNS. Late relapses are very uncommon. Once RR, the 5‐year PFS is around 27% (Aoki et al, 2015).
As reported in the DA‐EPOCH‐R prospective study, if the patient has localised disease and is RT‐naïve then RT alone can be curative (Dunleavy et al, 2013). The usual alternative is salvage immunochemotherapy followed by high‐dose chemotherapy and ASCT, as in DLBCL (Aoki et al, 2015; Kuruvilla et al, 2008). Salvage immunochemotherapy regimens include R‐DHAP (rituximab, dexamethasone, cytarabine, cisplatin), R‐ICE (rituximab, ifosfamide, carboplatin and etoposide) (Gisselbrecht et al, 2010) or the potentially less toxic R‐GDP (rituximab, gemcitabine, dexamethasone, cisplatin) (Crump et al, 2014). Outcomes depend greatly on the response to the salvage chemotherapy regimen (Sehn et al, 1998) and consequently, poor response precludes ASCT. In one retrospective study only 22% of 37 RR PMBCL patients responded sufficiently to proceed to ASCT despite utility of second‐ and third‐line salvage chemotherapy, in contrast with 50% DLBCL patients (n = 143) (Kuruvilla et al, 2008); of note, rituximab was not routinely used at the time of many of these patients’ treatment. Due to this poor response the 2‐year OS for all RR patients was only 15% compared to 34% in DLBCL, P = 0·018. However, those that proceeded to ASCT had similar outcomes to DLBCL patients with 2‐year post‐ASCT OS at 67% compared to 53% in DLBCL (P = 0·78) and PFS of 57% vs. 36% respectively (P = 0·64). These positive outcomes post‐ASCT were confirmed in a subsequent multicentre retrospective review. Of 44 RR PMBCL patients undergoing ASCT the 4‐year OS was 70% and PFS 61% (Aoki et al, 2015). Despite its curative potential the role of allogenic stem cell transplant (allo‐SCT) in RR PMBCL is unclear due to limited data. A recent retrospective study however suggests that it could be an appropriate treatment option in selected patients (Herrera et al, 2018).
The most promising target of immune checkpoint blockade to date is PD‐1, including agents such as pembrolizumab and nivolumab. Data supporting the use of pembrolizumab in PMBCL has led to US Food and Drug Administration (FDA) approval for its use in RR PMBCL patients. The phase 1b KEYNOTE‐013 trial enrolled 21 RR PMBCL patients who received pembrolizumab. The ORR was 48% (10/21; 95% CI 26–70%) with CR of 33% (7/21). AEs were experienced by 61% patients, mostly grade 1 or 2, and none led to treatment cessation (Zinzani et al, 2017a; Armand et al, 2018). There is a subsequent ongoing phase 2 trial KEYNOTE 170 using pembrolizumab in RR PMBCL patients. Interim data presented show that in 53 enrolled PMBCL patients the ORR was 45% (24/53; 95% CI 32–60%) and CR 13% (7/53) (Zinzani et al, 2017b; Armand et al, 2018). The combination of chemotherapy with PD‐1 blockade may have advantages. There is an ongoing trial treating RR PMBCL patients with GVD (gemcitabine, vinorelbine and doxorubicin) chemotherapy plus SHR‐1210 (anti‐PD‐1) with or without decitabine priming. Initial results were encouraging in a small number of patients (Zhang et al, 2018).
Novel therapeutic approaches
The key to realising good outcomes for RR PMBCL patients is achieving better responses to salvage immunochemotherapy, allowing subsequent ASCT. The focus has therefore shifted towards novel therapies because conventional salvage immunochemotherapy regimens deliver unsatisfactory results (Table 2). The latest molecular advances in PMBCL have revealed new therapeutic targets.
Table 2.
Trials investigating novel therapies in relapsed/refractory PMBCL
| Study | Study type | Treatment | Patient cohort | n | Outcome |
|---|---|---|---|---|---|
| Studies with a distinct PMBCL analysis | |||||
| Jacobsen et al (2015) | Single‐arm multicentre phase 2 trial; subset analysis | Brentuximab | ECOG PS 0‐2 | 6 | ORR 17% (1 CR) |
| Zinzani et al (2017c) | Single‐arm multicentre phase 2 trial | Brentuximab | ECOG PS 0‐1 | 15 | ORR 13·3% (2 PR) |
| Zinzani et al (2017a); Armand et al (2018) | Single‐arm multicentre phase 1b trial; subset analysis | Pembrolizumab | ECOG PS 0‐1 | 21 | ORR 48% (7 CR, 3 PR) |
| Studies with PMBCL included in cohort analysis | |||||
| Neelapu et al (2017) | Single‐arm multicentre phase 1–2 trial; subset analysis | CD19 targeted CAR‐T cells: KTE‐C19 (axicabtagene ciloleucel) |
RR PMBCL and transformed FL ECOG PS 0‐1 |
24 (8 PMBCL) | ORR 85% (CR 70%) |
| Armand et al (2013) | Single‐arm multicentre phase 2 trial | Pidilizumab |
RR PMBCL, DLBCL and transformed indolent lymphoma ECOG PS 0‐1 |
66 (4 PMBCL) | ORR 51% (CR 34%) |
CAR, chimeric antigen receptor; CR, complete response; DLBCL, diffuse large B‐cell lymphoma; ECOG PS, Eastern Cooperative Oncology Group performance status; FL, follicular lymphoma; ORR, overall response rate; PMBCL, primary mediastinal B‐cell lymphoma; PR, partial response; RR, relapsed/refractory.
The JAK‐STAT pathway is a rational target for therapeutic intervention given its role in lymphomagenesis. To date JAK/STAT inhibitors trialled against lymphoma in vivo are the JAK2‐specific inhibitors pacritinib and ruxolitinib, both in small numbers with trials still in progress. Pacritinib was investigated in RR lymphoma patients including HL and NHL; 17/31 (55%) patients with assessable imaging showed a reduction in tumour volume, ranging from 4% to 70% (Younes et al, 2012). Ruxolitinib has undergone a pilot phase 2 trial in RR HL (n = 14) and PMBCL patients (n = 6). All PMBCL patients progressed rapidly after the first or second cycle (Kim et al, 2016). STAT3 inhibitors are in preclinical development (Zhao et al, 2018). Ibrutinib, a Bruton tyrosine kinase inhibitor implicated in the NF‐κB pathway, has also been trialled in combination with R‐ICE in RR DLBCL, including 4 PMBCL patients. Amongst the 4 PMBCL patients there was an ORR of 100%, all demonstrating PR (Sauter et al, 2018).
Antibody‐drug conjugates deliver cytotoxic drugs via monoclonal antobodies to malignant cells expressing specific antigens, for example brentuximab vedotin (BV) which is selective for CD30. BV is routinely used in RR HL and there is recent evidence that as a front‐line treatment with chemotherapy it offers at least equivalent efficacy and less toxicity than current first‐line regimens in advanced HL (Connors & Radford, 2018). A multicentre phase 2 trial investigated BV in CD30‐positive RR PMBCL patients. Of 15 recruited patients the ORR was 13·3% (2 patients with PR, 1 with SD and 12 with PD) (Zinzani et al, 2017c). As in HL, BV has been trialled as an adjunct to first‐line R‐CHP (vincristine omitted to reduce risk of peripheral neuropathy) in CD30‐positive PMBCL, DLBCL and grey zone lymphoma. Interim data has been published, but full data is available only in abstract form at the time of writing. Twenty‐three treatment‐naïve PMBCL patients were treated with BV plus R‐CHP, resulting in a 1‐year PFS of 87%, CI 57–97% (Svoboda et al, 2017).
T cells that have been genetically engineered to express a chimeric antigen receptor (CAR) are an exciting prospect in fighting haematological malignancy. The CAR is a TCR engineered to bind specific antigens on tumour cells and in doing so cause focussed activation of the T cell. CD19 is the usual targeted antigen in haematological malignancy as it is ubiquitous on B cells. The major concern with this pioneering therapy is the possibility of severe toxicity due to high cytokine levels, such as cytokine release syndrome (CRS) and neurotoxicity, termed CAR‐T‐cell‐related‐encephalopathy syndrome (CRES). Moreover CAR‐T therapy can cause on‐target/off‐tumour recognition where healthy B cells are an unintended target, leading to B‐cell aplasia (Neelapu et al, 2018). An ongoing phase 1–2 multicentre trial is investigating Abxicatragene Ciloleucel (axi‐cel, KTE‐C19), an autologous anti‐CD19 CAR‐T treatment, in RR aggressive NHL patients (ZUMA‐1) and its results have led to FDA and European Commission approval for RR DLBCL and PMBCL patients. Initial data published included a total of 101 patients with RR DLBCL, PMBCL (n = 8) and transformed FL treated with axi‐cel. Among all patients at 1 year the ORR was 82% with a CR of 58% (Neelapu et al, 2017). Among the combined PMBCL/FL sub‐group the ORR was 85% with CR of 70%. Ninety‐three percent of patients experienced CRS and 64% had neurotoxicity although both were largely reversible. Around 43% of patients required tocilizumab, an anti‐IL6R monoclonal antibody, to treat these events with no obvious detriment to CAR‐T response. Initial real‐world results using axi‐cel CAR‐T therapy in RR B‐cell lymphomas, including PMBCL (8% of patients), are comparable to the ZUMA‐1 trial at the 30‐day follow‐up. Of 112 evaluable patients the ORR was 79% and CR 50%, with equivalent safety data (Nastoupil et al, 2018). There is not yet any data regarding other CAR‐T treatments in PMBCL.
CAR‐T therapy may be enhanced by other novel therapies, such as anti‐PD‐1 antibodies, shown by preclinical evidence of their synergy (John et al, 2013). There is one reported case of a RR DLBCL patient who progressed on anti‐CD19 CAR‐T treatment and was given pembrolizumab with a subsequent clinical improvement. Interestingly, the highest percentage of CAR19+ T‐cells was seen in the 48 h following pembrolizumab (Chong et al, 2017). There is an ensuing phase 1–2 trial of pembrolizumab for DLBCL patients who are failing to respond to anti‐CD19 CAR‐T therapy (NCT02650999).
We must await the full data of the above novel therapy trials to appreciate long‐term outcomes and delayed toxicities. There are many trials planned or in progress that include PMBCL patients (Table 3). As an uncommon disease, PMBCL will benefit from trials in related haematological malignancies as well as ‘basket‐trials’ where patients are chosen by genetic mutation rather than malignancy type. Opportunities arising from studies in other high‐grade B‐cell lymphoma include maintenance lenalidomide, which has been explored in DLBCL (Thieblemont et al, 2016); and ibrutinib plus lenalidomide and rituximab which has shown promising results in RR DLBCL (Goy et al, 2016). Tazemetostat, targeting EZH2 mutations causing aberrant histone methylation, is also undergoing investigation in a phase 1–2 trial in RR solid tumours and B‐cell lymphomas, including PMBCL, with optimistic first results (Italiano et al, 2018). Although the status of EZH2 has not been reported in PMBCL, it is known to be highly expressed in germinal centre B‐cell (GCB) lymphomas (Béguelin et al, 2013). Another innovative therapy is bispecific T‐cell engager (BiTE) antibodies, that can bind two different antigens simultaneously; PMBCL data is awaited. Blockade of other co‐inhibitory immune receptors that may be future therapeutic targets include Lymphocyte activation gene 3 (LAG3), T‐cell immunoglobulin‐3 (TIM3) and T‐cell immunoglobulin and ITIM domain (TIGIT) (Anderson et al, 2016) as well as killer cell immunoglobulin‐like receptor (KIR) and V‐domain immunoglobulin suppressor of T‐cell activation (VISTA) (Vick & Mahadevan, 2016).
Table 3.
Ongoing clinical trials involving PMBCL patients
| Clinicaltrials.gov identifier | Study type | Treatment | Patient cohort | Target recruitment | Estimated completion date |
|---|---|---|---|---|---|
| NCT03346642 | Randomised two‐arm single centre phase 1–2 trial | GVD and SHR‐1210 (anti‐PD‐1 antibody) with or without decitabine priming |
RR PMBCL ECOG PS 0‐2 |
30 | October 2018 |
| NCT00078949 | Randomised two‐arm multicentre phase 3 trial | R‐GDP versus R‐DHAP salvage chemotherapy followed by ASCT with or without maintenance rituximab |
RR aggressive NHL ECOG PS 0‐3 |
619 | December 2018 |
| NCT02568553 | Single‐arm multicentre phase 1 trial | Lenalidomide and blinatumomab |
RR NHL ECOG PS 0‐2 |
36 | December 2018 |
| NCT03038672 | Randomised cross‐over single centre phase 2 trial | Nivolumab with or without varlilumab |
RR aggressive B‐cell NHL ECOG PS 0‐1 |
106 | December 2019 |
| NCT02950220 | Single‐arm single centre phase 1/1b trial | Pembrolizumab and ibrutinib |
RR NHL ECOG PS 0‐2 |
58 | December 2019 |
| NCT03484819 | Single‐arm phase 2 trial | Copanlisib and nivolumab |
RR DLBCL and PMBCL ECOG PS 0‐2 |
99 | August 2020 |
| NCT02576990 | Single‐arm phase 2 trial | Pembrolizumab |
RR PMBCL and Richter ECOG PS 0‐1 |
80 | September 2020 |
| NCT02631044 | Non‐randomised two‐arm multicentre phase 1 trial | CD19 targeted CAR‐T cells: JCAR017 (lisocabtagene maraleucel) single dose versus two dose schedule |
RR B‐cell NHL ECOG PS 0‐1 |
274 | December 2020 |
| NCT03601819 | Single‐arm single centre phase 1b trial | Pacritinib |
RR lymphoproliferative disorders ECOG PS 0‐2 |
26 | September 2021 |
| NCT02747732 | Single‐arm multicentre phase 2 trial | Ibrutinib and bendamustine and rituximab |
RR aggressive B‐cell NHL ECOG PS 0‐2 |
72 | October 2021 |
| NCT01599559 | Randomised two‐arm multicentre phase 3 trial | Rituximab combined with any anthracycline‐containing chemotherapy regimen followed by mediastinal RT or no RT |
New diagnosis PMBCL Fit to receive chemotherapy and radiotherapy with curative intent |
540 | May 2025 |
| NCT03625037 | Dose‐escalation multicentre phase 1–2 trial | GEN3013 (anti‐CD3 anti‐CD20 bispecific Ab) | RR B‐cell NHL | 110 | December 2025 |
| NCT02348216 | Single‐arm multicentre phase 1–2 trial | CD19 targeted CAR‐T cells: KTE‐C19 (axicabtagene ciloleucel) |
RR aggressive NHL ECOG PS 0‐1 |
200 | March 2032 |
ASCT, autologous stem cell transplantation; CAR, chimeric antigen receptor; DLBCL, diffuse large B‐cell lymphoma; ECOG PS, Eastern Cooperative Oncology Group performance status; GVD, gemcitabine, vinorelbine, doxorubicin; NHL, non‐Hodgkin lymphoma; PMBCL, primary mediastinal B‐cell lymphoma; R‐DHAP, rituximab, dexamethasone, cytarabine, cisplatin; R‐GDP, rituximab, gemcitabine, dexamethasone, cisplatin; RR, relapsed/refractory; RT, radiotherapy.
Concluding remarks
As a discrete entity, PMBCL warrants a distinct management approach, which is challenging due to the lack of prospective studies. Pragmatic diagnostic techniques applicable to the routine laboratory are required to distinguish PMBCL from its related diseases. Comparison of R‐CHOP and DA‐EPOCH‐R in a randomised trial would guide optimal front‐line immunochemotherapy. In this young patient cohort, long‐term toxicity from RT should be considered carefully. A PET‐guided approach could limit the use of RT, potentially in conjunction with ctDNA given the high false positive rate of EOT‐PET. To enable patients with RR disease to proceed to ASCT, improved responses to salvage therapies are needed; the role of small‐molecules and immune modulators in salvage regimens needs clarification. Greater understanding of the TME and immune evasion mechanisms will probably underpin the development of new therapeutic targets.
Author contributions
C. L. conceived Fig 2 and wrote the first draft of the manuscript in consultation with all authors. C. K. and M. K. G. edited the clinical content. J. G. conceived Fig 1 and edited the scientific content. All authors provided intellectual input and critically reviewed the manuscript.
Acknowledgements
The authors would like to thank Sandra Brosda for generating Fig 1 in this review.
References
- Aaronson, D.S. & Horvath, C.M. (2002) A road map for those who don't know JAK‐STAT. Science, 296, 1653–1655. [DOI] [PubMed] [Google Scholar]
- Abdul Razak, F.R. , Diepstra, A. , Visser, L. & van den Berg, A. (2016) CD58 mutations are common in Hodgkin lymphoma cell lines and loss of CD58 expression in tumor cells occurs in Hodgkin lymphoma patients who relapse. Genes & Immunology, 17, 363–366. [DOI] [PubMed] [Google Scholar]
- Anderson, A.C. , Joller, N. & Kuchroo, V.K. (2016) Lag‐3, Tim‐3, and TIGIT: co‐inhibitory receptors with specialized functions in immune regulation. Immunity, 44, 989–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki, T. , Izutsu, K. , Suzuki, R. , Nakaseko, C. , Arima, H. , Shimada, K. , Sasaki, M. , Takizawa, J. , Mitani, K. , Igarashi, T. , Maeda, Y. , Ishida, F. , Niitsu, N. , Ohmachi, K. , Takasaki, H. , Nakamura, N. , Kinoshita, T. , Nakamura, S. & Ogura, M. (2013) Novel prognostic model of primary mediastinal large B‐cell lymphoma (PMBL): a multicenter cooperative retrospective study in Japan. Blood, 122, 638. [Google Scholar]
- Aoki, T. , Shimada, K. , Suzuki, R. , Izutsu, K. , Tomita, A. , Maeda, Y. , Takizawa, J. , Mitani, K. , Igarashi, T. , Sakai, K. , Miyazaki, K. , Mihara, K. , Ohmachi, K. , Nakamura, N. , Takasaki, H. , Kiyoi, H. , Nakamura, S. , Kinoshita, T. & Ogura, M. (2015) High‐dose chemotherapy followed by autologous stem cell transplantation for relapsed/refractory primary mediastinal large B‐cell lymphoma. Blood Cancer Journal, 5, e372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armand, P. , Nagler, A. , Weller, E.A. , Devine, S.M. , Avigan, D.E. , Chen, Y.B. , Kaminski, M.S. , Holland, H.K. , Winter, J.N. , Mason, J.R. , Fay, J.W. , Rizzieri, D.A. , Hosing, C.M. , Ball, E.D. , Uberti, J.P. , Lazarus, H.M. , Mapara, M.Y. , Gregory, S.A. , Timmerman, J.M. , Andorsky, D. , Or, R. , Waller, E.K. , Rotem‐Yehudar, R. & Gordon, L.I. (2013) Disabling immune tolerance by programmed death‐1 blockade with pidilizumab after autologous hematopoietic stem‐cell transplantation for diffuse large B‐cell lymphoma: results of an international phase II trial. Journal of Clinical Oncology, 31, 4199–4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armand, P. , Rodig, S. , Melnichenko, V. , Thieblemont, C. , Bouabdallah, K. , Tumyan, G. , Özcan, M. , Portino, S. , Fogliatto, L. , Caballero, D. , Walewski, J. , Gulbas, Z. , Vincent, V. , Christian, B. , Perini, G.F. , Salles, G.A. , Svoboda, J. , Zain, J. , Patel, S. , Chen, P.‐H. , Ligon, A. , Ouyang, J. , Neuberg, D. , Redd, R. , Chatterjee, A. , Orlowski, R. , Balakumaran, A. , Shipp, M. & Zinzani, P.L. (2018) Pembrolizumab in patients with relapsed or refractory primary mediastinal large B‐cell lymphoma (PMBCL): data from the keynote‐013 and keynote‐170 studies. Blood, 132(Suppl. 1), 228. [Google Scholar]
- Barry, K.C. , Hsu, J. , Broz, M.L. , Cueto, F.J. , Binnewies, M. , Combes, A.J. , Nelson, A.E. , Loo, K. , Kumar, R. , Rosenblum, M.D. , Alvarado, M.D. , Wolf, D.M. , Bogunovic, D. , Bhardwaj, N. , Daud, A.I. , Ha, P.K. , Ryan, W.R. , Pollack, J.L. , Samad, B. , Asthana, S. , Chan, V. & Krummel, M.F. (2018) A natural killer‐dendritic cell axis defines checkpoint therapy‐responsive tumor microenvironments. Nature Medicine, 24, 1178–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bea, S. , Zettl, A. , Wright, G. , Salaverria, I. , Jehn, P. , Moreno, V. , Burek, C. , Ott, G. , Puig, X. , Yang, L. , Lopez‐Guillermo, A. , Chan, W.C. , Greiner, T.C. , Weisenburger, D.D. , Armitage, J.O. , Gascoyne, R.D. , Connors, J.M. , Grogan, T.M. , Braziel, R. , Fisher, R.I. , Smeland, E.B. , Kvaloy, S. , Holte, H. , Delabie, J. , Simon, R. , Powell, J. , Wilson, W.H. , Jaffe, E.S. , Montserrat, E. , Muller‐Hermelink, H.K. , Staudt, L.M. , Campo, E. & Rosenwald, A. (2005) Diffuse large B‐cell lymphoma subgroups have distinct genetic profiles that influence tumor biology and improve gene‐expression‐based survival prediction. Blood, 106, 3183–3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béguelin, W. , Popovic, R. , Teater, M. , Jiang, Y. , Bunting, K.L. , Rosen, M. , Shen, H. , Yang, S.N. , Wang, L. , Ezponda, T. , Martinez‐Garcia, E. , Zhang, H. , Zheng, Y. , Verma, S.K. , McCabe, M.T. , Ott, H.M. , Van Aller, G.S. , Kruger, R.G. , Liu, Y. , McHugh, C.F. , Scott, D.W. , Chung, Y.R. , Kelleher, N. , Shaknovich, R. , Creasy, C.L. , Gascoyne, R.D. , Wong, K.K. , Cerchietti, L. , Levine, R.L. , Abdel‐Wahab, O. , Licht, J.D. , Elemento, O. & Melnick, A.M. (2013) EZH2 is required for germinal center formation and somatic EZH2 mutations promote lymphoid transformation. Cancer Cell, 23, 677–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierer, B.E. , Peterson, A. , Gorga, J.C. , Herrmann, S.H. & Burakoff, S.J. (1988) Synergistic T cell activation via the physiological ligands for CD2 and the T cell receptor. The Journal of Experimental Medicine, 168, 1145–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop, P.C. , Wilson, W.H. , Pearson, D. , Janik, J. , Jaffe, E.S. & Elwood, P.C. (1999) CNS involvement in primary mediastinal large B‐cell lymphoma. Journal of Clinical Oncology, 17, 2479–2485. [DOI] [PubMed] [Google Scholar]
- Bowman, T. , Garcia, R. , Turkson, J. & Jove, R. (2000) STATs in oncogenesis. Oncogene, 19, 2474–2488. [DOI] [PubMed] [Google Scholar]
- Burke, A. , Gross, T. , Pillon, M. , Minard‐colin, V. , Delgado, R. , Zsíros, J. , Uyttebroeck, A. , Michon, J. , Bollard, C. , Csoka, M. , Barkauska, D. , Wheatley, K. , Aupérin, A. & Patte, C. (2017) Results of inter‐B‐NHL ritux 2010 ‐ phase II study of DA‐EPOCH‐R for children and adolescents with primary mediastinal large B‐cell lymphoma (PMLBL) on behalf of European Intergroup for Childhood Non Hodgkin's Lymphoma (EICNHL) and Children's Oncology Group (COG). Blood, 130(Suppl. 1), 4124. [Google Scholar]
- Campo, E. , Swerdlow, S.H. , Harris, N.L. , Pileri, S. , Stein, H. & Jaffe, E.S. (2011) The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood, 117, 5019–5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceriani, L. , Martelli, M. , Zinzani, P.L. , Ferreri, A.J. , Botto, B. , Stelitano, C. , Gotti, M. , Cabras, M.G. , Rigacci, L. , Gargantini, L. , Merli, F. , Pinotti, G. , Mannina, D. , Luminari, S. , Stathis, A. , Russo, E. , Cavalli, F. , Giovanella, L. , Johnson, P.W. & Zucca, E. (2015) Utility of baseline 18FDG‐PET/CT functional parameters in defining prognosis of primary mediastinal (thymic) large B‐cell lymphoma. Blood, 126, 950–956. [DOI] [PubMed] [Google Scholar]
- Challa‐Malladi, M. , Lieu, Y.K. , Califano, O. , Holmes, A.B. , Bhagat, G. , Murty, V.V. , Dominguez‐Sola, D. , Pasqualucci, L. & Dalla‐Favera, R. (2011) Combined genetic inactivation of β2‐microglobulin and CD58 reveals frequent escape from immune recognition in diffuse large B cell lymphoma. Cancer Cell, 20, 728–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong, L.C. , Twa, D.D. , Mottok, A. , Ben‐Neriah, S. , Woolcock, B.W. , Zhao, Y. , Savage, K.J. , Marra, M.A. , Scott, D.W. , Gascoyne, R.D. , Morin, R.D. , Mungall, A.J. & Steidl, C. (2016) Comprehensive characterization of programmed death ligand structural rearrangements in B‐cell non‐Hodgkin lymphomas. Blood, 128, 1206–1213. [DOI] [PubMed] [Google Scholar]
- Chong, E.A. , Melenhorst, J.J. , Lacey, S.F. , Ambrose, D.E. , Gonzalez, V. , Levine, B.L. , June, C.H. & Schuster, S.J. (2017) PD‐1 blockade modulates chimeric antigen receptor (CAR)‐modified T cells: refueling the CAR. Blood, 129, 1039–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors, J.M. & Radford, J.A. (2018) Brentuximab vedotin for stage III or IV Hodgkin's lymphoma. New England Journal of Medicine, 378, 1560–1561. [DOI] [PubMed] [Google Scholar]
- Copie‐Bergman, C. , Plonquet, A. , Alonso, M.A. , Boulland, M.L. , Marquet, J. , Divine, M. , Möller, P. , Leroy, K. & Gaulard, P. (2002) MAL expression in lymphoid cells: further evidence for MAL as a distinct molecular marker of primary mediastinal large B‐cell lymphomas. Modern Pathology, 15, 1172–1180. [DOI] [PubMed] [Google Scholar]
- Crump, M. , Kuruvilla, J. , Couban, S. , MacDonald, D.A. , Kukreti, V. , Kouroukis, C.T. , Rubinger, M. , Buckstein, R. , Imrie, K.R. , Federico, M. , Di Renzo, N. , Howson‐Jan, K. , Baetz, T. , Kaizer, L. , Voralia, M. , Olney, H.J. , Turner, A.R. , Sussman, J. , Hay, A.E. , Djurfeldt, M.S. , Meyer, R.M. , Chen, B.E. & Shepherd, L.E. (2014) Randomized comparison of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin chemotherapy before autologous stem‐cell transplantation for relapsed and refractory aggressive lymphomas: NCIC‐CTG LY.12. Journal of Clinical Oncology, 32, 3490–3496. [DOI] [PubMed] [Google Scholar]
- Csernus, B. , Timár, B. , Fülöp, Z. , Bognár, A. , Szepesi, A. , László, T. , Jáksó, P. , Warnke, R. , Kopper, L. & Matolcsy, A. (2004) Mutational analysis of IgVH and BCL‐6 genes suggests thymic B‐cells origin of mediastinal (thymic) B‐cell lymphoma. Leukemia & Lymphoma, 45, 2105–2110. [DOI] [PubMed] [Google Scholar]
- Dai, H. , Ehrentraut, S. , Nagel, S. , Eberth, S. , Pommerenke, C. , Dirks, W.G. , Geffers, R. , Kalavalapalli, S. , Kaufmann, M. , Meyer, C. , Faehnrich, S. , Chen, S. , Drexler, H.G. & MacLeod, R.A. (2015) Genomic landscape of primary mediastinal B‐cell lymphoma cell lines. PLoS ONE, 10, e0139663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diepstra, A. , van Imhoff, G.W. , Karim‐Kos, H.E. , van den Berg, A. , te Meerman, G.J. , Niens, M. , Nolte, I.M. , Bastiaannet, E. , Schaapveld, M. , Vellenga, E. & Poppema, S. (2007) HLA class II expression by Hodgkin Reed‐Sternberg cells is an independent prognostic factor in classical Hodgkin's lymphoma. Journal of Clinical Oncology, 25, 3101–3108. [DOI] [PubMed] [Google Scholar]
- Dores, G.M. , Metayer, C. , Curtis, R.E. , Lynch, C.F. , Clarke, E.A. , Glimelius, B. , Storm, H. , Pukkala, E. , van Leeuwen, F.E. , Holowaty, E.J. , Andersson, M. , Wiklund, T. , Joensuu, T. , van't Veer, M.B. , Stovall, M. , Gospodarowicz, M. & Travis, L.B. (2002) Second malignant neoplasms among long‐term survivors of Hodgkin's disease: a population‐based evaluation over 25 years. Journal of Clinical Oncology, 20, 3484–3494. [DOI] [PubMed] [Google Scholar]
- Dorfman, D.M. , Shahsafaei, A. & Alonso, M.A. (2012) Utility of CD200 immunostaining in the diagnosis of primary mediastinal large B cell lymphoma: comparison with MAL, CD23, and other markers. Modern Pathology, 25, 1637–1643. [DOI] [PubMed] [Google Scholar]
- Dunleavy, K. , Pittaluga, S. , Maeda, L.S. , Advani, R. , Chen, C.C. , Hessler, J. , Steinberg, S.M. , Grant, C. , Wright, G. , Varma, G. , Staudt, L.M. , Jaffe, E.S. & Wilson, W.H. (2013) Dose‐adjusted EPOCH‐rituximab therapy in primary mediastinal B‐cell lymphoma. New England Journal of Medicine, 368, 1408–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatalica, Z. , Bilalovic, N. , Vranic, S. , Arguello, D. , Reddy, S. & Ghosh, N. (2015) PD‐L1 and PD1 expression in lymphomas. Blood, 126, 3899. [Google Scholar]
- Ghia, P. , Transidico, P. , Veiga, J.P. , Schaniel, C. , Sallusto, F. , Matsushima, K. , Sallan, S.E. , Rolink, A.G. , Mantovani, A. , Nadler, L.M. & Cardoso, A.A. (2001) Chemoattractants MDC and TARC are secreted by malignant B‐cell precursors following CD40 ligation and support the migration of leukemia‐specific T cells. Blood, 98, 533–540. [DOI] [PubMed] [Google Scholar]
- Gisselbrecht, C. , Glass, B. , Mounier, N. , Singh Gill, D. , Linch, D.C. , Trneny, M. , Bosly, A. , Ketterer, N. , Shpilberg, O. , Hagberg, H. , Ma, D. , Brière, J. , Moskowitz, C.H. & Schmitz, N. (2010) Salvage regimens with autologous transplantation for relapsed large B‐cell lymphoma in the rituximab era. Journal of Clinical Oncology, 28, 4184–4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulino‐Roth, L. (2018) How I treat primary mediastinal B‐cell lymphoma. Blood, 132, 782–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulino‐Roth, L. , O'Donohue, T. , Chen, Z. , Bartlett, N.L. , LaCasce, A. , Martin‐Doyle, W. , Barth, M.J. , Davies, K. , Blum, K.A. , Christian, B. , Casulo, C. , Smith, S.M. , Godfrey, J. , Termuhlen, A. , Oberley, M.J. , Alexander, S. , Weitzman, S. , Appel, B. , Mizukawa, B. , Svoboda, J. , Afify, Z. , Pauly, M. , Dave, H. , Gardner, R. , Stephens, D.M. , Zeitler, W.A. , Forlenza, C. , Levine, J. , Williams, M.E. , Sima, J.L. , Bollard, C.M. & Leonard, J.P. (2017) Outcomes of adults and children with primary mediastinal B‐cell lymphoma treated with dose‐adjusted EPOCH‐R. British Journal of Haematology, 179, 739–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson, M. , Hawkes, E.A. , Cunningham, D. , Chadwick, N. , Counsell, N. , Lawrie, A. , Jack, A. , Smith, P. , Mouncey, P. , Pocock, C. , Ardeshna, K.M. , Radford, J. , McMillan, A. , Davies, J. , Turner, D. , Kruger, A. , Johnson, P.W. , Gambell, J. & Linch, D. (2016) Rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone (R‐CHOP) in the management of primary mediastinal B‐cell lymphoma: a subgroup analysis of the UK NCRI R‐CHOP 14 versus 21 trial. British Journal of Haematology, 175, 668–672. [DOI] [PubMed] [Google Scholar]
- Goenka, S. & Kaplan, M.H. (2011) Transcriptional regulation by STAT6. Immunologic Research, 50, 87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goy, A. , Ramchandren, R. , Ghosh, N. , Munoz, J. , Morgan, D. , Dang, N. , Knapp, M. , Delioukina, M. , Kingsley, E. , Tran, A. , Ping, J. , Beaupre, D. , Neuenburg, J. & Ruan, J. (2016) A multicenter open‐label, phase 1b/2 study of ibrutinib in combination with lenalidomide and rituximab in patients with relapsed or refractory (R/R) diffuse Large B‐cell lymphoma (DLBCL). Blood, 128, 473.27268088 [Google Scholar]
- Green, M.R. , Monti, S. , Rodig, S.J. , Juszczynski, P. , Currie, T. , O'Donnell, E. , Chapuy, B. , Takeyama, K. , Neuberg, D. , Golub, T.R. , Kutok, J.L. & Shipp, M.A. (2010) Integrative analysis reveals selective 9p24.1 amplification, increased PD‐1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B‐cell lymphoma. Blood, 116, 3268–3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardana, J. , Chan, F.C. , Telenius, A. , Woolcock, B. , Kridel, R. , Tan, K.L. , Ben‐Neriah, S. , Mottok, A. , Lim, R.S. , Boyle, M. , Rogic, S. , Rimsza, L.M. , Guiter, C. , Leroy, K. , Gaulard, P. , Haioun, C. , Marra, M.A. , Savage, K.J. , Connors, J.M. , Shah, S.P. , Gascoyne, R.D. & Steidl, C. (2014) Recurrent somatic mutations of PTPN1 in primary mediastinal B cell lymphoma and Hodgkin lymphoma. Nature Genetics, 46, 329–335. [DOI] [PubMed] [Google Scholar]
- Hamlin, P.A. , Portlock, C.S. , Straus, D.J. , Noy, A. , Singer, A. , Horwitz, S.M. , Oconnor, O.A. , Yahalom, J. , Zelenetz, A.D. & Moskowitz, C.H. (2005) Primary mediastinal large B‐cell lymphoma: optimal therapy and prognostic factor analysis in 141 consecutive patients treated at Memorial Sloan Kettering from 1980 to 1999. British Journal of Haematology, 130, 691–699. [DOI] [PubMed] [Google Scholar]
- Hao, Y. , Chapuy, B. , Monti, S. , Sun, H.H. , Rodig, S.J. & Shipp, M.A. (2014) Selective JAK2 inhibition specifically decreases Hodgkin lymphoma and mediastinal large B‐cell lymphoma growth in vitro and in vivo. Clinical Cancer Research, 20, 2674–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, N.L. , Jaffe, E.S. , Stein, H. , Banks, P.M. , Chan, J.K. , Cleary, M.L. , Delsol, G. , De Wolf‐Peeters, C. , Falini, B. & Gatter, K.C. (1994) A revised European‐American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood, 84, 1361–1392. [PubMed] [Google Scholar]
- Hayden, M.S. & Ghosh, S. (2012) NF‐κB, the first quarter‐century: remarkable progress and outstanding questions. Genes & Development, 26, 203–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera, A. , Chen, L. , Khajavian, S. , Chase, M.L. , Darrah, J. , Maloney, D.G. , Ho, V.T. , Soiffer, R.J. , Antin, J.H. , Forman, S.J. , Nademanee, A.P. , Chen, Y.‐B. , Armand, P. & Shadman, M. (2018) Allogeneic stem cell transplantation provides durable remission in patients with primary mediastinal large B‐cell lymphoma. Blood, 132(Suppl. 1), 2177. [DOI] [PubMed] [Google Scholar]
- Hinz, M. , Löser, P. , Mathas, S. , Krappmann, D. , Dörken, B. & Scheidereit, C. (2001) Constitutive NF‐kappaB maintains high expression of a characteristic gene network, including CD40, CD86, and a set of antiapoptotic genes in Hodgkin/Reed‐Sternberg cells. Blood, 97, 2798–2807. [DOI] [PubMed] [Google Scholar]
- Hoeller, S. , Zihler, D. , Zlobec, I. , Obermann, E.C. , Pileri, S.A. , Dirnhofer, S. & Tzankov, A. (2010) BOB.1, CD79a and cyclin E are the most appropriate markers to discriminate classical Hodgkin's lymphoma from primary mediastinal large B‐cell lymphoma. Histopathology, 56, 217–228. [DOI] [PubMed] [Google Scholar]
- Iellem, A. , Mariani, M. , Lang, R. , Recalde, H. , Panina‐Bordignon, P. , Sinigaglia, F. & D'Ambrosio, D. (2001) Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. Journal of Experimental Medicine, 194, 847–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai, T. , Nagira, M. , Takagi, S. , Kakizaki, M. , Nishimura, M. , Wang, J. , Gray, P.W. , Matsushima, K. & Yoshie, O. (1999) Selective recruitment of CCR4‐bearing Th2 cells toward antigen‐presenting cells by the CC chemokines thymus and activation‐regulated chemokine and macrophage‐derived chemokine. International Immunology, 11, 81–88. [DOI] [PubMed] [Google Scholar]
- Italiano, A. , Soria, J.C. , Toulmonde, M. , Michot, J.M. , Lucchesi, C. , Varga, A. , Coindre, J.M. , Blakemore, S.J. , Clawson, A. , Suttle, B. , McDonald, A.A. , Woodruff, M. , Ribich, S. , Hedrick, E. , Keilhack, H. , Thomson, B. , Owa, T. , Copeland, R.A. , Ho, P.T.C. & Ribrag, V. (2018) Tazemetostat, an EZH2 inhibitor, in relapsed or refractory B‐cell non‐Hodgkin lymphoma and advanced solid tumours: a first‐in‐human, open‐label, phase 1 study. The Lancet Oncology, 19, 649–659. [DOI] [PubMed] [Google Scholar]
- Jackson, M.W. , Rusthoven, C.G. , Jones, B.L. , Kamdar, M. & Rabinovitch, R. (2016) Improved survival with combined modality therapy in the modern era for primary mediastinal B‐cell lymphoma. American Journal of Hematology, 91, 476–480. [DOI] [PubMed] [Google Scholar]
- Jacobsen, E.D. , Sharman, J.P. , Oki, Y. , Advani, R.H. , Winter, J.N. , Bello, C.M. , Spitzer, G. , Palanca‐Wessels, M.C. , Kennedy, D.A. , Levine, P. , Yang, J. & Bartlett, N.L. (2015) Brentuximab vedotin demonstrates objective responses in a phase 2 study of relapsed/refractory DLBCL with variable CD30 expression. Blood, 125, 1394–1402. [DOI] [PubMed] [Google Scholar]
- John, L.B. , Devaud, C. , Duong, C.P. , Yong, C.S. , Beavis, P.A. , Haynes, N.M. , Chow, M.T. , Smyth, M.J. , Kershaw, M.H. & Darcy, P.K. (2013) Anti‐PD‐1 antibody therapy potently enhances the eradication of established tumors by gene‐modified T cells. Clinical Cancer Research, 19, 5636–5646. [DOI] [PubMed] [Google Scholar]
- Jones, K. , Vari, F. , Keane, C. , Crooks, P. , Nourse, J.P. , Seymour, L.A. , Gottlieb, D. , Ritchie, D. , Gill, D. & Gandhi, M.K. (2013) Serum CD163 and TARC as disease response biomarkers in classical Hodgkin lymphoma. Clinical Cancer Research, 19, 731–742. [DOI] [PubMed] [Google Scholar]
- Joos, S. , Küpper, M. , Ohl, S. , von Bonin, F. , Mechtersheimer, G. , Bentz, M. , Marynen, P. , Möller, P. , Pfreundschuh, M. , Trümper, L. & Lichter, P. (2000) Genomic imbalances including amplification of the tyrosine kinase gene JAK2 in CD30+ Hodgkin cells. Cancer Research, 60, 549–552. [PubMed] [Google Scholar]
- Keane, C. , Vari, F. , Hertzberg, M. , Cao, K.A. , Green, M.R. , Han, E. , Seymour, J.F. , Hicks, R.J. , Gill, D. , Crooks, P. , Gould, C. , Jones, K. , Griffiths, L.R. , Talaulikar, D. , Jain, S. , Tobin, J. & Gandhi, M.K. (2015) Ratios of T‐cell immune effectors and checkpoint molecules as prognostic biomarkers in diffuse large B‐cell lymphoma: a population‐based study. The Lancet Haematology, 2, e445–e455. [DOI] [PubMed] [Google Scholar]
- Kim, S.J. , Kang, H.J. , Dong‐Yeop, S. , Lee, H.S. , Oh, S.Y. , Shin, H.‐J. , Yoon, D.H. , Hong, J.Y. , Kong, J.H. , Sakamoto, K. , Ko, Y.H. , Takeuchi, K. , Suh, C. & Kim, W.S. (2016) The efficacy of JAK2 inhibitor in heavily pretreated classical Hodgkin lymphoma: a prospective pilot study of ruxolitinib in relapsed or refractory classical Hodgkin lymphoma and primary mediastinal large B‐cell lymphoma. Blood, 128, 1820. [Google Scholar]
- Kuruvilla, J. , Pintilie, M. , Tsang, R. , Nagy, T. , Keating, A. & Crump, M. (2008) Salvage chemotherapy and autologous stem cell transplantation are inferior for relapsed or refractory primary mediastinal large B‐cell lymphoma compared with diffuse large B‐cell lymphoma. Leukemia & Lymphoma, 49, 1329–1336. [DOI] [PubMed] [Google Scholar]
- Lam, L.T. , Davis, R.E. , Pierce, J. , Hepperle, M. , Xu, Y. , Hottelet, M. , Nong, Y. , Wen, D. , Adams, J. , Dang, L. & Staudt, L.M. (2005) Small molecule inhibitors of IkappaB kinase are selectively toxic for subgroups of diffuse large B‐cell lymphoma defined by gene expression profiling. Clinical Cancer Research, 11, 28–40. [PubMed] [Google Scholar]
- Levy, D.E. & Darnell, J.E. (2002) Stats: transcriptional control and biological impact. Nature Reviews Molecular Cell Biology, 3, 651–662. [DOI] [PubMed] [Google Scholar]
- Liu, P.P. , Wang, K.F. , Xia, Y. , Bi, X.W. , Sun, P. , Wang, Y. , Li, Z.M. & Jiang, W.Q. (2016) Racial patterns of patients with primary mediastinal large B‐cell lymphoma: SEER analysis. Medicine (Baltimore), 95, e4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak, T. & Saunders, M. (2006) The Immune Response. Elsevier, Amsterdam. [Google Scholar]
- Manso, B.A. , Wenzl, K. , Asmann, Y.W. , Maurer, M.J. , Manske, M. , Yang, Z.Z. , Slager, S.L. , Nowakowski, G.S. , Ansell, S.M. , Witzig, T.E. , Feldman, A.L. , Rimsza, L. , Link, B. , Cerhan, J.R. & Novak, A.J. (2017) Whole‐exome analysis reveals novel somatic genomic alterations associated with cell of origin in diffuse large B‐cell lymphoma. Blood Cancer Journal, 7, e553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelli, M. , Ceriani, L. , Zucca, E. , Zinzani, P.L. , Ferreri, A.J. , Vitolo, U. , Stelitano, C. , Brusamolino, E. , Cabras, M.G. , Rigacci, L. , Balzarotti, M. , Salvi, F. , Montoto, S. , Lopez‐Guillermo, A. , Finolezzi, E. , Pileri, S.A. , Davies, A. , Cavalli, F. , Giovanella, L. & Johnson, P.W. (2014) [18F]fluorodeoxyglucose positron emission tomography predicts survival after chemoimmunotherapy for primary mediastinal large B‐cell lymphoma: results of the International Extranodal Lymphoma Study Group IELSG‐26 Study. Journal of Clinical Oncology, 32, 1769–1775. [DOI] [PubMed] [Google Scholar]
- Meignan, M. & Cottereau, A.S. (2018) FDG‐PET in PMBCL: which heterogeneity? Blood, 132, 117–118. [DOI] [PubMed] [Google Scholar]
- Melani, C. , Advani, R. , Roschewski, M. , Walters, K.M. , Chen, C.C. , Baratto, L. , Ahlman, M.A. , Miljkovic, M.D. , Steinberg, S.M. , Lam, J. , Shovlin, M. , Dunleavy, K. , Pittaluga, S. , Jaffe, E.S. & Wilson, W.H. (2018) End‐of‐treatment and serial PET imaging in primary mediastinal B‐cell lymphoma following dose‐adjusted EPOCH‐R: a paradigm shift in clinical decision making. Haematologica, 103, 1337–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzner, I. , Bucur, A.J. , Brüderlein, S. , Dorsch, K. , Hasel, C. , Barth, T.F. , Leithäuser, F. & Möller, P. (2005) Biallelic mutation of SOCS‐1 impairs JAK2 degradation and sustains phospho‐JAK2 action in the MedB‐1 mediastinal lymphoma line. Blood, 105, 2535–2542. [DOI] [PubMed] [Google Scholar]
- Moskowitz, C. , Hamlin, P.A. Jr , Maragulia, J. , Meikle, J. & Zelenetz, A. (2010) Sequential dose‐dense RCHOP followed by ICE consolidation (MSKCC protocol 01–142) without radiotherapy for patients with primary mediastinal large B cell lymphoma. Blood, 116, 420. [Google Scholar]
- Mottok, A. , Renné, C. , Seifert, M. , Oppermann, E. , Bechstein, W. , Hansmann, M.L. , Küppers, R. & Bräuninger, A. (2009) Inactivating SOCS1 mutations are caused by aberrant somatic hypermutation and restricted to a subset of B‐cell lymphoma entities. Blood, 114, 4503–4506. [DOI] [PubMed] [Google Scholar]
- Mottok, A. , Woolcock, B. , Chan, F.C. , Tong, K.M. , Chong, L. , Farinha, P. , Telenius, A. , Chavez, E. , Ramchandani, S. , Drake, M. , Boyle, M. , Ben‐Neriah, S. , Scott, D.W. , Rimsza, L.M. , Siebert, R. , Gascoyne, R.D. & Steidl, C. (2015) Genomic alterations in CIITA are frequent in primary mediastinal large B cell lymphoma and are associated with diminished MHC class II expression. Cell Reports, 13, 1418–1431. [DOI] [PubMed] [Google Scholar]
- Mottok, A. , Wright, G. , Rosenwald, A. , Ott, G. , Ramsower, C. , Campo, E. , Braziel, R.M. , Delabie, J. , Weisenburger, D.D. , Song, J.Y. , Chan, W.C. , Cook, J.R. , Fu, K. , Greiner, T. , Smeland, E. , Holte, H. , Savage, K.J. , Glinsmann‐Gibson, B.J. , Gascoyne, R.D. , Staudt, L.M. , Jaffe, E.S. , Connors, J.M. , Scott, D.W. , Steidl, C. & Rimsza, L.M. (2018) Molecular classification of primary mediastinal large B‐cell lymphoma using routinely available tissue specimens. Blood, 132, 2401–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nastoupil, L.J. , Jain, M.D. , Spiegel, J.Y. , Ghobadi, A. , Lin, Y. , Dahiya, S. , Lunning, M.A. , Lekakis, L.A.J. , Reagan, P.M. , Oluwole, O.O. , McGuirk, J.P. , Deol, A. , Sehgal, A.R. , Goy, A. , Hill, B.T. , Andreadis, C. , Munoz, J. , Westin, J.R. , Chavez, J.C. , Cashen, A.F. , Bennani, N.N. , Rapoport, A.P. , Vose, J.M. , Miklos, D.B. , Neelapu, S.S. & Locke, F.L. (2018) Axicabtagene ciloleucel (Axi‐cel) CD19 chimeric antigen receptor (CAR) T‐cell therapy for relapsed/refractory large B‐cell lymphoma: real world experience. Blood, 132(Suppl. 1), 91. [Google Scholar]
- Neelapu, S.S. , Locke, F.L. , Bartlett, N.L. , Lekakis, L.J. , Miklos, D.B. , Jacobson, C.A. , Braunschweig, I. , Oluwole, O.O. , Siddiqi, T. , Lin, Y. , Timmerman, J.M. , Stiff, P.J. , Friedberg, J.W. , Flinn, I.W. , Goy, A. , Hill, B.T. , Smith, M.R. , Deol, A. , Farooq, U. , McSweeney, P. , Munoz, J. , Avivi, I. , Castro, J.E. , Westin, J.R. , Chavez, J.C. , Ghobadi, A. , Komanduri, K.V. , Levy, R. , Jacobsen, E.D. , Witzig, T.E. , Reagan, P. , Bot, A. , Rossi, J. , Navale, L. , Jiang, Y. , Aycock, J. , Elias, M. , Chang, D. , Wiezorek, J. & Go, W.Y. (2017) Axicabtagene ciloleucel CAR T‐cell therapy in refractory large B‐cell lymphoma. New England Journal of Medicine, 377, 2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neelapu, S.S. , Tummala, S. , Kebriaei, P. , Wierda, W. , Gutierrez, C. , Locke, F.L. , Komanduri, K.V. , Lin, Y. , Jain, N. , Daver, N. , Westin, J. , Gulbis, A.M. , Loghin, M.E. , de Groot, J.F. , Adkins, S. , Davis, S.E. , Rezvani, K. , Hwu, P. & Shpall, E.J. (2018) Chimeric antigen receptor T‐cell therapy ‐ assessment and management of toxicities. Nature Reviews Clinical Oncology, 15, 47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkura, N. , Kitagawa, Y. & Sakaguchi, S. (2013) Development and maintenance of regulatory T cells. Immunity, 38, 414–423. [DOI] [PubMed] [Google Scholar]
- Pasqualucci, L. , Khiabanian, H. , Fangazio, M. , Vasishtha, M. , Messina, M. , Holmes, A.B. , Ouillette, P. , Trifonov, V. , Rossi, D. , Tabbò, F. , Ponzoni, M. , Chadburn, A. , Murty, V.V. , Bhagat, G. , Gaidano, G. , Inghirami, G. , Malek, S.N. , Rabadan, R. & Dalla‐Favera, R. (2014) Genetics of follicular lymphoma transformation. Cell Reports, 6, 130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pileri, S.A. , Zinzani, P.L. , Gaidano, G. , Falini, B. , Gaulard, P. , Zucca, E. , Sabattini, E. , Ascani, S. , Rossi, M. & Cavalli, F. ; International Extranodal Lymphoma Study Group . (2003) Pathobiology of primary mediastinal B‐cell lymphoma. Leukemia & Lymphoma, 44 (Suppl. 3), S21–S26. [DOI] [PubMed] [Google Scholar]
- Plattel, W.J. , van den Berg, A. , Visser, L. , van der Graaf, A.M. , Pruim, J. , Vos, H. , Hepkema, B. , Diepstra, A. & van Imhoff, G.W. (2012) Plasma thymus and activation‐regulated chemokine as an early response marker in classical Hodgkin's lymphoma. Haematologica, 97, 410–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger, M. , Osterborg, A. , Pettengell, R. , White, D. , Gill, D. , Walewski, J. , Kuhnt, E. , Loeffler, M. , Pfreundschuh, M. & Ho, A.D. (2011) Primary mediastinal B‐cell lymphoma treated with CHOP‐like chemotherapy with or without rituximab: results of the Mabthera International Trial Group study. Annals of Oncology, 22, 664–670. [DOI] [PubMed] [Google Scholar]
- Rimsza, L.M. , Roberts, R.A. , Campo, E. , Grogan, T.M. , Bea, S. , Salaverria, I. , Zettl, A. , Rosenwald, A. , Ott, G. , Muller‐Hermelink, H.K. , Delabie, J. , Fisher, R.I. , Unger, J.M. , Leblanc, M. , Staudt, L.M. , Jaffe, E.S. , Gascoyne, R.D. , Chan, W.C. , Weisenburger, D.D. , Greiner, T. , Braziel, R.M. & Miller, T.P. (2006) Loss of major histocompatibility class II expression in non‐immune‐privileged site diffuse large B‐cell lymphoma is highly coordinated and not due to chromosomal deletions. Blood, 107, 1101–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz, O. , Guiter, C. , Dorsch, K. , Dusanter‐Fourt, I. , Wegener, S. , Jouault, H. , Gaulard, P. , Castellano, F. , Möller, P. & Leroy, K. (2008) STAT6 activity is regulated by SOCS‐1 and modulates BCL‐XL expression in primary mediastinal B‐cell lymphoma. Leukemia, 22, 2106–2110. [DOI] [PubMed] [Google Scholar]
- Ritz, O. , Guiter, C. , Castellano, F. , Dorsch, K. , Melzner, J. , Jais, J.P. , Dubois, G. , Gaulard, P. , Möller, P. & Leroy, K. (2009) Recurrent mutations of the STAT6 DNA binding domain in primary mediastinal B‐cell lymphoma. Blood, 114, 1236–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, R.A. , Wright, G. , Rosenwald, A.R. , Jaramillo, M.A. , Grogan, T.M. , Miller, T.P. , Frutiger, Y. , Chan, W.C. , Gascoyne, R.D. , Ott, G. , Muller‐Hermelink, H.K. , Staudt, L.M. & Rimsza, L.M. (2006) Loss of major histocompatibility class II gene and protein expression in primary mediastinal large B‐cell lymphoma is highly coordinated and related to poor patient survival. Blood, 108, 311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwald, A. , Wright, G. , Chan, W.C. , Connors, J.M. , Campo, E. , Fisher, R.I. , Gascoyne, R.D. , Muller‐Hermelink, H.K. , Smeland, E.B. , Giltnane, J.M. , Hurt, E.M. , Zhao, H. , Averett, L. , Yang, L. , Wilson, W.H. , Jaffe, E.S. , Simon, R. , Klausner, R.D. , Powell, J. , Duffey, P.L. , Longo, D.L. , Greiner, T.C. , Weisenburger, D.D. , Sanger, W.G. , Dave, B.J. , Lynch, J.C. , Vose, J. , Armitage, J.O. , Montserrat, E. , López‐Guillermo, A. , Grogan, T.M. , Miller, T.P. , LeBlanc, M. , Ott, G. , Kvaloy, S. , Delabie, J. , Holte, H. , Krajci, P. , Stokke, T. & Staudt, L.M.P. (2002) The use of molecular profiling to predict survival after chemotherapy for diffuse large‐B‐cell lymphoma. New England Journal of Medicine, 346, 1937–1947. [DOI] [PubMed] [Google Scholar]
- Rosenwald, A. , Wright, G. , Leroy, K. , Yu, X. , Gaulard, P. , Gascoyne, R.D. , Chan, W.C. , Zhao, T. , Haioun, C. , Greiner, T.C. , Weisenburger, D.D. , Lynch, J.C. , Vose, J. , Armitage, J.O. , Smeland, E.B. , Kvaloy, S. , Holte, H. , Delabie, J. , Campo, E. , Montserrat, E. , Lopez‐Guillermo, A. , Ott, G. , Muller‐Hermelink, H.K. , Connors, J.M. , Braziel, R. , Grogan, T.M. , Fisher, R.I. , Miller, T.P. , LeBlanc, M. , Chiorazzi, M. , Zhao, H. , Yang, L. , Powell, J. , Wilson, W.H. , Jaffe, E.S. , Simon, R. , Klausner, R.D. & Staudt, L.M. (2003) Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. Journal of Experimental Medicine, 198, 851–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi, D. , Diop, F. , Spaccarotella, E. , Monti, S. , Zanni, M. , Rasi, S. , Deambrogi, C. , Spina, V. , Bruscaggin, A. , Favini, C. , Serra, R. , Ramponi, A. , Boldorini, R. , Foà, R. & Gaidano, G. (2017) Diffuse large B‐cell lymphoma genotyping on the liquid biopsy. Blood, 129, 1947–1957. [DOI] [PubMed] [Google Scholar]
- Sarkozy, C. , Molina, T. , Ghesquières, H. , Michallet, A.S. , Dupuis, J. , Damotte, D. , Morsschauser, F. , Parrens, M. , Martin, L. , Dartigues, P. , Stamatoullas, A. , Hirsch, P. , Fabiani, B. , Bouabdallah, K. , da Silva, M.G. , Maerevoet, M. , Laurent, C. , Coiffier, B. , Salles, G. & Traverse‐Glehen, A. (2017) Mediastinal gray zone lymphoma: clinico‐pathological characteristics and outcomes of 99 patients from the Lymphoma Study Association. Haematologica, 102, 150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki, Y. & Iwai, K. (2015) Roles of the NF‐κB pathway in B‐lymphocyte biology. Current Topics in Microbiology and Immunology, 393, 177–209. [DOI] [PubMed] [Google Scholar]
- Sauter, C.S. , Matasar, M.J. , Schoder, H. , Devlin, S.M. , Drullinsky, P. , Gerecitano, J. , Kumar, A. , Noy, A. , Palomba, M.L. , Portlock, C.S. , Straus, D.J. , Zelenetz, A.D. , McCall, S.J. , Miller, S.T. , Courtien, A.I. , Younes, A. & Moskowitz, C.H. (2018) A phase 1 study of ibrutinib in combination with R‐ICE in patients with relapsed or primary refractory DLBCL. Blood, 131, 1805–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage, K.J. , Monti, S. , Kutok, J.L. , Cattoretti, G. , Neuberg, D. , De Leval, L. , Kurtin, P. , Dal Cin, P. , Ladd, C. , Feuerhake, F. , Aguiar, R.C. , Li, S. , Salles, G. , Berger, F. , Jing, W. , Pinkus, G.S. , Habermann, T. , Dalla‐Favera, R. , Harris, N.L. , Aster, J.C. , Golub, T.R. & Shipp, M.A. (2003) The molecular signature of mediastinal large B‐cell lymphoma differs from that of other diffuse large B‐cell lymphomas and shares features with classical Hodgkin lymphoma. Blood, 102, 3871–3879. [DOI] [PubMed] [Google Scholar]
- Savage, K.J. , Al‐Rajhi, N. , Voss, N. , Paltiel, C. , Klasa, R. , Gascoyne, R.D. & Connors, J.M. (2006) Favorable outcome of primary mediastinal large B‐cell lymphoma in a single institution: the British Columbia experience. Annals of Oncology, 17, 123–130. [DOI] [PubMed] [Google Scholar]
- Savage, K. , Yenson, P. , Shenkier, T. , Klasa, R. , Villa, D. , Goktepe, O. , Steidl, C. , Slack, G. , Gascoyne, R. , Connors, J. & Sehn, L. (2012) The outcome of primary mediastinal large B‐cell lymphoma (PMBCL) in the R‐CHOP treatment era. Blood, 120, 303.22596259 [Google Scholar]
- Schmitz, R. , Hansmann, M.L. , Bohle, V. , Martin‐Subero, J.I. , Hartmann, S. , Mechtersheimer, G. , Klapper, W. , Vater, I. , Giefing, M. , Gesk, S. , Stanelle, J. , Siebert, R. & Küppers, R. (2009) TNFAIP3 (A20) is a tumor suppressor gene in Hodgkin lymphoma and primary mediastinal B cell lymphoma. Journal of Experimental Medicine, 206, 981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, D. , Wright, G. , Williams, M. , Lih, J. , Jaffe, E. , Rosenwald, A. , Campo, E. , Chan, W. , Connors, J. , Smeland, E. , Braziel, R. , Ott, G. , Delabie, I. , Weisenburger, D. , Cook, J. , Greiner, T. , Fu, K. , Walsh, W. , Gascoyne, R. , Staudt, L. & Rimsza, L. (2014) Accurate diagnosis of aggressive B cell non‐Hodgkin lymphomas using gene expression profiling of formalin‐fixed, paraffin‐embedded tissues. Blood, 124, 3016.25150293 [Google Scholar]
- Sehn, L.H. , Antin, J.H. , Shulman, L.N. , Mauch, P. , Elias, A. , Kadin, M.E. & Wheeler, C. (1998) Primary diffuse large B‐cell lymphoma of the mediastinum: outcome following high‐dose chemotherapy and autologous hematopoietic cell transplantation. Blood, 91, 717–723. [PubMed] [Google Scholar]
- Shanbhag, S. & Ambinder, R. F. (2018) Hodgkin lymphoma: a review and update on recent progress. CA: A Cancer Journal for Clinicians, 68, 116–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, M. , Roemer, M.G. , Chapuy, B. , Liao, X. , Sun, H. , Pinkus, G.S. , Shipp, M.A. , Freeman, G.J. & Rodig, S.J. (2014) Expression of programmed cell death 1 ligand 2 (PD‐L2) is a distinguishing feature of primary mediastinal (thymic) large B‐cell lymphoma and associated with PDCD1LG2 copy gain. American Journal of Surgical Pathology, 38, 1715–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumerai, J.D. , Hellmann, M.D. , Feng, Y. , Sohani, A.R. , Toomey, C.E. , Barnes, J.A. , Takvorian, R.W. , Neuberg, D. , Hochberg, E.P. & Abramson, J.S. (2014) Treatment of primary mediastinal B‐cell lymphoma with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone is associated with a high rate of primary refractory disease. Leukemia & Lymphoma, 55, 538–543. [DOI] [PubMed] [Google Scholar]
- Sun, S.C. (2011) Non‐canonical NF‐kappa B signaling pathway. Cell Research, 21, 71–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda, J. , Landsburg, D. , Nasta, S. , Barta, S. , Khan, N. , Fung, H. , Tan, C. , Filicko‐O'Hara, J. , Gaballa, S. , Strelec, L. , Nagle, S. , Bair, S. , Mitnick, S. , Waite, T. , Sargent, R. , Bogusz, A. , Sahin, Z. , Mato, A. & Schuster, S. (2017) Brentuximab vedotin with R‐CHP chemotherapy as frontline treatment for patients with CD30 positive primary mediastinal large B‐cell, diffuse large B‐cell, and grey zone lymphomas: results of a phase I/II multisite trial. Blood, 130, 191. [Google Scholar]
- Swerdlow, S. , Campo, E. , Harris, N. , Jaffe, E. , Pileri, S. , Stein, H. , Thiele, J. A. , Hasserjian, D. , Le Beau, R. , Orazi, M. & Reiner, A.S. (2017). WHO Classification of Tumours of Haematopoietic and Lymphoid Tissue, Revised 4th Edition, International Agency for Research on Cancer (IARC), Lyon. [Google Scholar]
- Thieblemont, C. , Tilly, H. , Gomez da Silva, M. , Casasnovas, R.‐O. , Fruchart, C. , Morschhauser, F. , Haioun, C. , Lazarovici, J. , Grosicki, S. , Perrot, A. , Trotman, J. , Sebban, C. , Caballero, D. , Greil, R. , Van Eygen, K. , Briere, J. , Cabecadas, J. , Salles, G. , Gaulard, P. , Bosly, A. & Coiffier, B. (2016) First analysis of an international double‐blind randomized phase III study of lenalidomide maintenance in elderly patients with DLBCL treated with R‐CHOP in first line, the REMARC study from LYSA. Blood, 128, 471. [Google Scholar]
- Twa, D.D. , Chan, F.C. , Ben‐Neriah, S. , Woolcock, B.W. , Mottok, A. , Tan, K.L. , Slack, G.W. , Gunawardana, J. , Lim, R.S. , McPherson, A.W. , Kridel, R. , Telenius, A. , Scott, D.W. , Savage, K.J. , Shah, S.P. , Gascoyne, R.D. & Steidl, C. (2014) Genomic rearrangements involving programmed death ligands are recurrent in primary mediastinal large B‐cell lymphoma. Blood, 123, 2062–2065. [DOI] [PubMed] [Google Scholar]
- Tzankov, A. , Meier, C. , Hirschmann, P. , Went, P. , Pileri, S.A. & Dirnhofer, S. (2008) Correlation of high numbers of intratumoral FOXP3+ regulatory T cells with improved survival in germinal center‐like diffuse large B‐cell lymphoma, follicular lymphoma and classical Hodgkin's lymphoma. Haematologica, 93, 193–200. [DOI] [PubMed] [Google Scholar]
- Vari, F. , Arpon, D. , Keane, C. , Hertzberg, M.S. , Talaulikar, D. , Jain, S. , Cui, Q. , Han, E. , Tobin, J. , Bird, R. , Cross, D. , Hernandez, A. , Gould, C. , Birch, S. & Gandhi, M.K. (2018) Immune evasion via PD‐1/PD‐L1 on NK cells and monocyte/macrophages is more prominent in Hodgkin lymphoma than DLBCL. Blood, 131, 1809–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick, E. & Mahadevan, D. (2016) Programming the immune checkpoint to treat hematologic malignancies. Expert Opinion on Investigational Drugs, 25, 755–770. [DOI] [PubMed] [Google Scholar]
- Viganò, E. , Gunawardana, J. , Mottok, A. , Van Tol, T. , Mak, K. , Chan, F.C. , Chong, L. , Chavez, E. , Woolcock, B. , Takata, K. , Twa, D. , Shulha, H.P. , Telenius, A. , Kutovaya, O. , Hung, S.S. , Healy, S. , Ben‐Neriah, S. , Leroy, K. , Gaulard, P. , Diepstra, A. , Kridel, R. , Savage, K.J. , Rimsza, L. , Gascoyne, R. & Steidl, C. (2018) Somatic IL4R mutations in primary mediastinal large B‐cell lymphoma lead to constitutive JAK‐STAT signaling activation. Blood, 131, 2036–2046. [DOI] [PubMed] [Google Scholar]
- Wang, Z. & Cook, J.R. (2018) PDCD1LG2 (PD‐L2) RNA in situ hybridization is a sensitive, specific, and practical marker of primary mediastinal large B‐cell lymphoma. British Journal of Haematology, 181, 564–566. [DOI] [PubMed] [Google Scholar]
- Wang, M. , Zhao, J. , Zhang, L. , Wei, F. , Lian, Y. , Wu, Y. , Gong, Z. , Zhang, S. , Zhou, J. , Cao, K. , Li, X. , Xiong, W. , Li, G. , Zeng, Z. & Guo, C. (2017) Role of tumor microenvironment in tumorigenesis. Journal of Cancer, 8, 761–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Wu, L. , Tian, C. & Zhang, Y. (2018) PD‐1‐PD‐L1 immune‐checkpoint blockade in malignant lymphomas. Annals of Hematology, 97, 229–237. [DOI] [PubMed] [Google Scholar]
- Weniger, M.A. , Gesk, S. , Ehrlich, S. , Martin‐Subero, J.I. , Dyer, M.J. , Siebert, R. , Möller, P. & Barth, T.F. (2007) Gains of REL in primary mediastinal B‐cell lymphoma coincide with nuclear accumulation of REL protein. Genes Chromosomes and Cancer, 46, 406–415. [DOI] [PubMed] [Google Scholar]
- Wertz, I.E. , O'Rourke, K.M. , Zhou, H. , Eby, M. , Aravind, L. , Seshagiri, S. , Wu, P. , Wiesmann, C. , Baker, R. , Boone, D.L. , Ma, A. , Koonin, E.V. & Dixit, V.M. (2004) De‐ubiquitination and ubiquitin ligase domains of A20 downregulate NF‐kappaB signalling. Nature, 430, 694–699. [DOI] [PubMed] [Google Scholar]
- Wessendorf, S. , Barth, T.F. , Viardot, A. , Mueller, A. , Kestler, H.A. , Kohlhammer, H. , Lichter, P. , Bentz, M. , Döhner, H. , Möller, P. & Schwaenen, C. (2007) Further delineation of chromosomal consensus regions in primary mediastinal B‐cell lymphomas: an analysis of 37 tumor samples using high‐resolution genomic profiling (array‐CGH). Leukemia, 21, 2463–2469. [DOI] [PubMed] [Google Scholar]
- Wilson, W.H. , Pittaluga, S. , Nicolae, A. , Camphausen, K. , Shovlin, M. , Steinberg, S.M. , Roschewski, M. , Staudt, L.M. , Jaffe, E.S. & Dunleavy, K. (2014) A prospective study of mediastinal gray‐zone lymphoma. Blood, 124, 1563–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, W. , Sin‐Ho, J. , Pitcher, B. , Hsi, E. , Friedberg, J. , Cheson, B. , Bartlett, N. , Smith, S. , Wagner Johnston, N. , Kahl, B. , Staudt, L. , Blum, K. , Abramson, J. , Press, O. , Fisher, R. , Richards, K. , Schoder, H. , Chang, J. , Zelenetz, A. & Leonard, J. (2016) Phase III randomized study of R‐CHOP versus DA‐EPOCH‐R and molecular analysis of untreated diffuse large B‐cell lymphoma: CALGB/alliance 50303. Blood, 128, 469. [Google Scholar]
- Yasukawa, H. , Misawa, H. , Sakamoto, H. , Masuhara, M. , Sasaki, A. , Wakioka, T. , Ohtsuka, S. , Imaizumi, T. , Matsuda, T. , Ihle, J.N. & Yoshimura, A. (1999) The JAK‐binding protein JAB inhibits Janus tyrosine kinase activity through binding in the activation loop. The EMBO Journal, 18, 1309–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz, M. , Li, H. , Bernard, D. , Amin, N.A. , Ouillette, P. , Jones, S. , Saiya‐Cork, K. , Parkin, B. , Jacobi, K. , Shedden, K. , Wang, S. , Chang, A.E. , Kaminski, M.S. & Malek, S.N. (2015) Activating STAT6 mutations in follicular lymphoma. Blood, 125, 668–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younes, A. , Romaguera, J. , Fanale, M. , McLaughlin, P. , Hagemeister, F. , Copeland, A. , Neelapu, S. , Kwak, L. , Shah, J. , de Castro Faria, S. , Hart, S. , Wood, J. , Jayaraman, R. , Ethirajulu, K. & Zhu, J. (2012) Phase I study of a novel oral Janus kinase 2 inhibitor, SB1518, in patients with relapsed lymphoma: evidence of clinical and biologic activity in multiple lymphoma subtypes. Journal of Clinical Oncology, 30, 4161–4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W. , Liu, Y. , Mei, Q. , Shen, L. , Yang, Q. & WeiDong, H. (2018) Safety and efficacy of GVD and anti‐PD‐1 (SHR‐1210) regimen with or without low‐dose decitabine priming for refractory bulky and aggressive primary mediastinal large B‐cell lymphoma. Journal of Clinical Oncology, 36(no. 15_suppl), 7556–7556. [Google Scholar]
- Zhao, X. , Zhang, Z. , Moreira, D. , Su, Y.L. , Won, H. , Adamus, T. , Dong, Z. , Liang, Y. , Yin, H.H. , Swiderski, P. , Pillai, R.K. , Kwak, L. , Forman, S. & Kortylewski, M. (2018) B cell lymphoma immunotherapy using TLR9‐targeted oligonucleotide STAT3 inhibitors. Molecular Therapy, 26, 695–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziai, P. , Hayeri, M.R. , Salei, A. , Salavati, A. , Houshmand, S. , Alavi, A. & Teytelboym, O.M. (2016) Role of optimal quantification of FDG PET imaging in the clinical practice of radiology. Radiographics, 36, 481–496. [DOI] [PubMed] [Google Scholar]
- Zinzani, P.L. , Martelli, M. , Bendandi, M. , De Renzo, A. , Zaccaria, A. , Pavone, E. , Bocchia, M. , Falini, B. , Gobbi, M. , Gherlinzoni, F. , Stefoni, V. , Tani, M. & Tura, S. (2001) Primary mediastinal large B‐cell lymphoma with sclerosis: a clinical study of 89 patients treated with MACOP‐B chemotherapy and radiation therapy. Haematologica, 86, 187–191. [PubMed] [Google Scholar]
- Zinzani, P.L. , Martelli, M. , Bertini, M. , Gianni, A.M. , Devizzi, L. , Federico, M. , Pangalis, G. , Michels, J. , Zucca, E. , Cantonetti, M. , Cortelazzo, S. , Wotherspoon, A. , Ferreri, A.J. , Zaja, F. , Lauria, F. , De Renzo, A. , Liberati, M.A. , Falini, B. , Balzarotti, M. , Calderoni, A. , Zaccaria, A. , Gentilini, P. , Fattori, P.P. , Pavone, E. , Angelopoulou, M.K. , Alinari, L. , Brugiatelli, M. , Di Renzo, N. , Bonifazi, F. , Pileri, S.A. & Cavalli, F. (2002) Induction chemotherapy strategies for primary mediastinal large B‐cell lymphoma with sclerosis: a retrospective multinational study on 426 previously untreated patients. Haematologica, 87, 1258–1264. [PubMed] [Google Scholar]
- Zinzani, P. , Stefoni, V. , Finolezzi, E. , Brusamolino, E. , Cabras, M. , Chiappella, A. , Salvi, F. , Rossi, A. , Broccoli, A. & Martelli, M. (2009) Rituximab combined with MACOP‐B or VACOP‐B and radiation therapy in primary mediastinal large B‐cell lymphoma: a retrospective study. Clinical Lymphoma Myeloma and Leukemia, 9, 381–385. [DOI] [PubMed] [Google Scholar]
- Zinzani, P.L. , Broccoli, A. , Casadei, B. , Stefoni, V. , Pellegrini, C. , Gandolfi, L. , Maglie, R. , Argnani, L. , Pileri, S. & Fanti, S. (2015) The role of rituximab and positron emission tomography in the treatment of primary mediastinal large B‐cell lymphoma: experience on 74 patients. Hematological Oncology, 33, 145–150. [DOI] [PubMed] [Google Scholar]
- Zinzani, P.L. , Ribrag, V. , Moskowitz, C.H. , Michot, J.M. , Kuruvilla, J. , Balakumaran, A. , Zhang, Y. , Chlosta, S. , Shipp, M.A. & Armand, P. (2017a) Safety and tolerability of pembrolizumab in patients with relapsed/refractory primary mediastinal large B‐cell lymphoma. Blood, 130, 267–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinzani, P. , Thieblemont, C. , Melnichenko, V. , Bouabdallah, K. , Walewski, J. , Majlis, A. , Fogliatto, L. , Caballero, D. , Christian, B. , Gulbas, Z. , Özcan, M. , Salles, G. , Shipp, M. , Chatterjee, A. , Orlowski, R. , Balakumaran, A. & Armand, P. (2017b) Efficacy and safety of pembrolizumab in relapsed/refractory primary mediastinal large B‐cell lymphoma (rrPMBCL): updated analysis of the keynote‐170 phase 2 trial. Blood, 130, 2833. [DOI] [PubMed] [Google Scholar]
- Zinzani, P.L. , Pellegrini, C. , Chiappella, A. , Di Rocco, A. , Salvi, F. , Cabras, M.G. , Argnani, L. & Stefoni, V. (2017c) Brentuximab vedotin in relapsed primary mediastinal large B‐cell lymphoma: results from a phase 2 clinical trial. Blood, 129, 2328–2330. [DOI] [PubMed] [Google Scholar]


