Summary:
The use of human induced pluripotent stem (iPS) cells would be of great value for a variety of applications involving drug development studies. Several reports have been published on the differentiation of human iPS cells into hepatocyte-like cells; however, the cells were insufficient for application in drug metabolism studies. In this study, we aimed to establish effective methods for differentiation of human iPS cells into hepatocytes. Two human iPS cell lines were differentiated by addition of activin A, dimethyl sulfoxide, hepatocyte growth factor, oncostatin M, and dexamethasone. The differentiated cells expressed hepatocyte markers and drug-metabolizing enzymes, revealing that the human iPS cells were differentiated into hepatocyte-like cells. Expression of CYP3A4 and UGT1A1 mRNAs increased with treatment with typical inducers of the enzymes, and the response of the cells against the inducers was similar to that of human hepatocytes. Furthermore, the drug-metabolizing activity of CYP3A4, as monitored by testosterone 6/S-hydroxylase activity, was elevated by these inducers. In conclusion, we established methods for differentiation of hepatocyte-like cells expressing drug metabolizing activity from human iPS cells. The hepatocyte-like cells derived from human iPS cells will be useful for drug metabolism studies.
Keywords: iPS, differentiation, hepatocyte, drug-metabolizing enzyme, CYP
Introduction
Induced pluripotent stem (iPS) cells have been generated directly from fibroblast cells by the expression of defined reprogramming factors (OCT3/4, SOX2, KLF4, and c-MYC).1–4) Recent studies have shown that iPS cells are comparable to embryonic stem (ES) cells including multilineage differentiation potential and intensive proliferation in vitro.5) iPS cell-derived hepatocytes could provide a valuable model system for novel pharmaceutical drug discovery assays, investigation of drug metabolism, and prediction of liver toxicity.5,6) However, efficient generation of highly differentiated hepatocytes from iPS cells has not yet been established without transduction of transcription factors in addition to treatment with optimal growth factors.
Cytochrome P450 (CYP) proteins display differential expression levels according to the developmental stages of the liver. CYP3A7 is a major enzyme in the human fetal and newborn liver,7,8) whereas CYP3A4 is expressed throughout development and finally accounts for about 30% of the amount of total CYP in the human adult liver.9,10) The human fetal and adult liver expression levels of CYP3A enzymes are increased by exposure to a variety of drugs,11–13) which in turn leads to accelerated metabolism and concomitant use of the drugs. Several studies have examined the differentiation of ES or iPS cells into hepatocyte-like cells,14–21) and these cells expressed hepatic markers such as albumin (ALB), α-1 antitrypsin, and hepatocyte nuclear factor (HNF) 4α. However, the activities of drug-metabolizing enzymes have not been detected in the ES or iPS cell-derived hepatocytes using simple methods. Thus, the ES or iPS cell-derived hepatocytes do not entirely recapitulate mature liver function.
We recently established an efficient method to differentiate ES cells into hepatocyte-like cells.22,23) In the present study, iPS cell-derived hepatocyte-like cells exhibited a sequential pattern of expression of hepatic markers and drug-metabolizing enzymes. Furthermore, drug-metabolizing activity was induced by treatment with drugs, similar to what is observed in human liver and hepatocytes.
Materials and Methods
Materials:
Umezawa et al. established 2 human iPS cell lines (Fetch, NIHS0604 and Lollipop, JCRB1336), derived from the embryonic human lung fibroblast cell line MCR-5. HepG2 cells were obtained from the RIKEN Cell Bank (Tsukuba, Japan). Mouse embryonic fibroblasts (MEF) were obtained from Oriental Yeast (Tokyo, Japan). BD Matrigel Matrix Growth Factor Reduced (Matrigel) was obtained from BD Biosciences (Bedford, MA). Dulbecco’s modified Eagle’s medium (DMEM), and DMEM and Ham’s nutrient mixture F-12 (DMEM/F12) were obtained from Sigma-Aldrich (St. Louis, MO). KnockOut Serum Replacement (KSR), KnockOut DMEM (KO-DMEM), Roswell Park Memorial Institute (RPMI) + GlutaMax medium, GlutaMax, minimal essential medium nonessential amino acids (MEM NEAA), and SuperScript III were obtained from Invitrogen Life Technologies (Carlsbad, CA). Fetal bovine serum (FBS) was obtained from Hyclone (Waltham, MA). Basic fibroblast growth factor (bFGF) and activin A were obtained from PeproTech (Rocky Hill, NJ). Modified Lanford medium was obtained from Charles River (Tokyo, Japan). Accutase was obtained from MS TechnoSystems (Osaka, Japan). Hepatocyte growth factor (HGF) was obtained from R&D Systems (Minneapolis, MN). RNeasy Mini Kit was obtained from Qiagen (Valencia, CA). Oncostatin M (OSM), dexa-methasone (DEX), omeprazole (OME), rifampicin (RIF), dimethyl sulfoxide (DMSO), and human normal adult liver total RNA, derived from a 64-year-old male donor, were obtained from Wako Pure Chemicals (Osaka, Japan). SYBR Green real-time polymerase chain reaction (PCR) Master Mix was obtained from Takara Bio (Otsu, Japan). Collagen Type I (collagen I)-coated microplate was obtained from Asahi Glass (Chiba, Japan). Dissociation solution for human ES/iPS cells was obtained from ReproCELL Incorporated (Tokyo, Japan). All other reagents used were of the highest quality available.
Human iPS cell culture:
Undifferentiated human iPS cells were maintained on a feeder layer of mitomycin C-treated MEF on a gelatin-coated dish in a human iPS medium at 37°C in humidified air with 5% CO2. The human iPS medium consisted of DMEM/F12 supplemented with 20% KSR, 2mM l-glutamine, 1% MEM NEAA, 0.1 mM 2-mercaptoethanol, and 5ng/mL bFGF. The medium was changed daily. Human iPS cell colonies composed of closely packed cells were split approximately every 3 to 4 days by incubation in a dissociation solution for 5 min at 37°C and passaged onto new mitomycin C-treated MEF.
Differentiation of human iPS cells into hepatocyte-like cells:
Differentiation was initiated when the human iPS cells reached a confluence level of approximately 70%. The human iPS cells were cultured with RPMI + GlutaMax medium containing 0.5% inactivated FBS and 100 ng/mL activin A. Three days later, the culture medium was switched to the RPMI + GlutaMax medium containing 2% KSR and 100 ng/mL activin A for 2 days. After induction of differentiation, the cells were dissociated by the addition of Accutase for 5 min at 37°C and passaged onto 24-well plates coated with collagen I or a thin layer Matrigel (dilute Matrigel 1:30 with cold human iPS medium) and cultured in KO-DMEM containing 20% KSR, 1% GlutaMax, 1% MEM NEAA, 0.1 mM 2-mercaptoethanol, and 1% DMSO for 7 days. The cells were subsequently cultured in modified Lanford medium containing 10 ng/mL HGF, 20 ng/mL OSM, and 100 nM DEX for 9 days. The cells were then cultured in modified Lanford medium for 4 days (Fig. 1).
Fig. 1. Differentiation into hepatocytes from 2 human iPS cell lines.
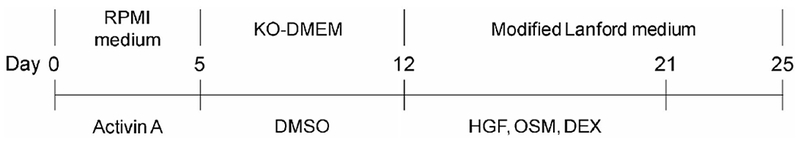
Two human iPS cell lines (Fetch and Lollipop) were differentiated into endoderm cells by addition of 100 ng/mL activin A for 5 days, and then into hepatocytes by the addition of 1% DMSO for 7 days. The hepatocytes were then matured by the addition of 10 ng/mL HGF, 20 ng/mL OSM, and 10−7 M DEX for 9 days. For the final 4 days, the cells were cultured in modified Lanford medium alone, without HGF, OSM, or DEX.
Drug treatments:
To clarify the effects of inducers on the expression of CYPs, the cells were treated with 40 μM RIF, 50 μM OME, or 100 μM DEX as described previously22–25) for the final 72 h of the differentiation protocol. The compounds were dissolved in DMSO, which was added to the culture medium at a final concentration of 0.1%.
RNA extraction and reverse transcription reaction:
An RNeasy Mini Kit was used to extract total RNA from the cells according to the manufacturer’s instructions. First-strand cDNA was generated from 2–4 μg of total RNA. Reverse transcription (RT) reaction was performed using SuperScript III, according to the manufacturer’s instructions.
Real-time RT-PCR analysis:
For determination of expression levels, mRNAs were analyzed using SYBR Green real-time quantitative RT-PCR. Relative mRNA expression levels in each sample were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression levels. All PCR procedures were performed using the ABI 7300 Fast System SDS software version 1.3.1 (Applied Biosystems, Foster City, CA), according to each manufacturer’s instructions. PCR was performed using diluted cDNA template in a 25-μL reaction mixture containing 0.3 μM of each primer and 12.5 μL of SYBR Green real-time PCR Master Mix. The primers used are summarized in Table 1.
Table 1.
Sequences of primers for real-time RT-PCR analysis
| Gene names | Forward primer sequences (5′- −3′) | Reverse primer sequences (5′- −3′) |
|---|---|---|
| HNF4α | GAGCTGCAGATCGATGACAA | TACTGGCGGTCGTTGATGTA |
| ALB | GAGCTTTTTGAGCAGCTTGG | GGTTCAGGACCACGGATAGA |
| AFP | AGCTTGGTGGTGGATGAAAC | TCTGCAATGACAGCCTCAAG |
| CYP1A2 | CTTTGACAAGAACAGTGTCCG | AGTGTCCAGCTCCTTCTGGAT |
| CYP1B1 | ACCAGGTATCCTGATGTGCAGAC | AGGTGTTGGCAGTGGTGGCATGAG |
| CYP2C9 | GAACACCAAGAATCGATGGACA | TCAGCAGGAGAAGGAGAGCATA |
| CYP3A4 | CTGTGTGTTTCCAAGAGAAGTTAC | TGCATCAATTTCCTCCTGCAG |
| CYP3A5 | CTCTCTGTTTCCAAAAGATACC | TGAAGATTATTGACTGGGCTG |
| CYP3A7 | AGATTTAATCCATTAGATCCATTCG | AGGCGACCTTCTTTTATCTG |
| UGT1A1 | CAGCAGAGGGGACATGAAAT | ACGCTGCAGGAAAGAATCAT |
| GAPDH | GAGTCAACGGATTTGGTCGT | GACAAGCTTCCCGTTCTCAG |
Assay for testosterone 6β-hydroxylase activity:
Human iPS cell-derived hepatocyte-like cells were cultured with modified Lanford medium containing 100 μM testosterone for 6h at 37°C. After incubation, the reaction medium was collected into the tubes with 1.25 mL of ethyl acetate, and 10 μL of 1 μM ethoxyresorufin was added to the medium as an internal standard. The tube was vortexed and centrifuged. Organic phase aliquots (1 mL) were transferred to microcentrifuge tubes. Ethyl acetate was evaporated under nitrogen gas, and the samples were dissolved with 100 μL of a mixture of 10 mM ammonium acetate (98%) and methanol (2%) containing 0.1% formic acid. Metabolites were analyzed using liquid chromatography coupled with tandem mass spectrometry according to the method reported previously.26)
cDNA microarray analysis:
An RNeasy Mini column (Qiagen) was used to extract and purify RNA (10 μg). The RNA was labeled using a SuperScript Indirect cDNA Labeling Kit (Invitrogen) and Cy™3 or Cy™5 Mono-Reactive Dye (GE Healthcare, Little Chalfont, UK). The dye-coupled cDNAs were purified using a MiniElute PCR purification kit (Qiagen) and hybridized to an Agilent 44 K human 60-mer oligo microarray (Agilent Technologies, Santa Clara, CA) according to Agilent instructions. An Agilent microarray scanner (Agilent Technologies) was used to wash, dry, and scan the slides. A Genespring GX software package (Agilent Technologies) was used to process and analyze the data. Ingenuity IPA software (Ingenuity Systems Redwood City, CA) was used to perform pathway analysis.
Results
Expression of hepatic marker gene:
In the present study, we succeeded in differentiating human iPS cells into hepatocyte-like cells that exhibited drug metabolizing activity. Activin A was used to differentiate two human iPS cell lines, Fetch and Lollipop, into endoderm cells.27) The endoderm cells were then induced to hepatocytes by the addition of DMSO.28) The hepatocytes were subsequently matured by the addition of HGF, OSM, and DEX (Fig. 1).29–31) Collagen I or Matrigel was used as extracellular matrix in the hepatic differentiation of the human iPS cells. The expression of mRNAs encoding typical hepatic marker proteins in the cells differentiated from both human iPS cell lines was compared with those in HepG2 cells, human adult liver, and primary hepatocytes (Fig. 2). HNF4α, ALB, and α-fetoprotein (AFP) mRNAs were detected in the cells differentiated from human iPS cells. The expression levels of these mRNAs in the cells differentiated from the two iPS cell lines were similar and the cells cultured on Matrigel were relatively higher than those cultured on collagen I. The expression levels of HNF4α and ALB mRNAs in the cells differentiated from iPS cells were similar to those in hepatocytes. However, the expression of AFP mRNA, a marker protein for an immature liver,32) in the cells was almost identical to that of the HepG2 cells, and was markedly higher than the expression in human adult liver and hepatocytes.
Fig. 2. Expression levels of liver marker protein mRNAs.
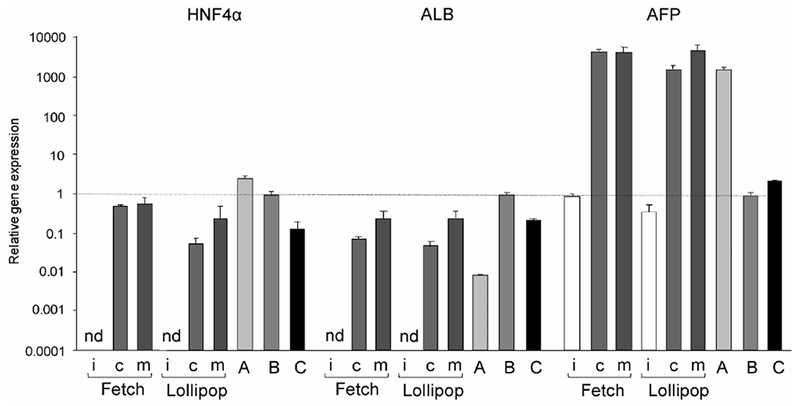
The expression levels of HNF4α, ALB, and AFP mRNAs in undifferentiated human iPS cells (i) and hepatocyte-like cells differentiated from two human iPS cell lines (Fetch and Lollipop) were analyzed using real-time PCR. Collagen I (c) or Matrigel (m) was used for the differentiation as the extracellular matrix. A, B, and C represent HepG2 cells, human adult liver, and hepatocytes, respectively, as positive controls. Each bar represents the mean ± SD from triplicate experiments. Values were normalized to the level of GAPDH mRNA. The graph represents the relative gene expression level when the level in the liver was taken as 1. nd, not detected.
Expression of CYP and UGT gene:
Figure 3 shows the expression of CYP mRNAs in the cells differentiated into hepatocytes from iPS cells. The majority of the CYPs involved in drug metabolism display tissue-specific expression in the liver and small intestine.33) Of these, the mRNA encoding CYP1A2, a liver-specific CYP,34) was not detected in the cells differentiated into hepatocytes. On the other hand, three CYP3A mRNAs were detected in the cells, but their expression levels were approximately 100–1,000 times lower in the cells differentiated into hepatocytes than in liver and hepatocytes, and were similar to that in HepG2 cells. It is of interest that CYP2C9 mRNA was detected in the cells differentiated into hepatocytes but not in HepG2 cells, although the expression level was lower than that of the liver and hepatocytes. The expression of CYP1B1 mRNA, which is not a liver-specific CYP, was observed in all cells used in this experiment at similar levels. The expression profiles for these CYPs were not largely different between the two cell lines; however, the expression levels of CYP mRNAs tended to be higher in the cells cultured on Matrigel than in those cultured on collagen I. To determine the expression of phase I and II enzymes, the differentiated cells were compared with adult or fetal hepatocytes using microarray analysis. The expression levels of mRNAs encoding the phase II enzymes, uridine diphosphate glucuronosyltransferase (UGT) 2A3, UGT2B4, and UGT2B7, were high in the differentiated cells. In contrast, the expression levels of majority of the phase I enzymes in the differentiated cells were lower than those in adult hepatocytes (Fig. 4).
Fig. 3. Expression levels of CYP mRNAs.
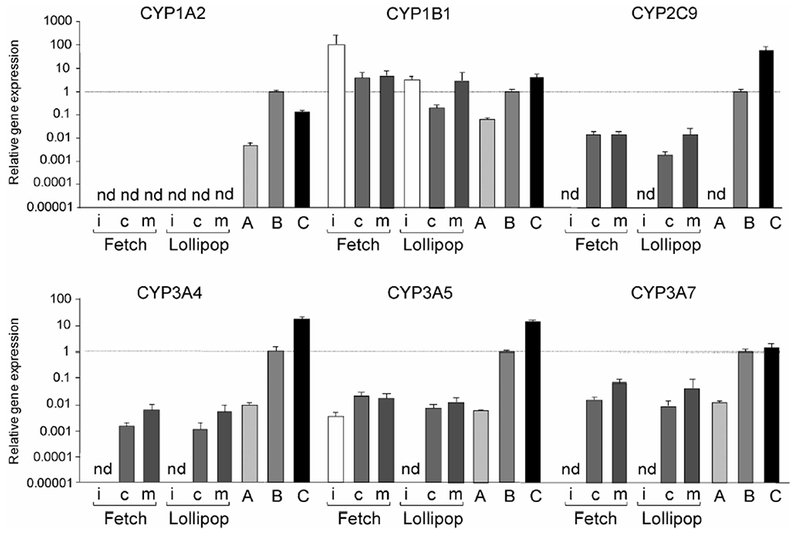
The expression levels of CYP1A2, CYP1B1, CYP2C9, CYP3A4, CYP3A5, and CYP3A7 mRNAs in undifferentiated human iPS cells (i) and hepatocyte-like cells differentiated from 2 human iPS cell lines (Fetch and Lollipop) were analyzed using real-time PCR. Collagen I (c) or Matrigel (m) was used for the differentiation as the extracellular matrix. A, B, and C represent HepG2 cells, human adult liver, and hepatocytes, respectively, as positive controls. Each bar represents the mean ± SD from triplicate experiments. Values were normalized to the levels of GAPDH mRNA. The graphs represent the relative gene expression level when the level in the liver was taken as 1. nd, not detected.
Fig. 4. Microarray analysis of phase I and II enzymes.
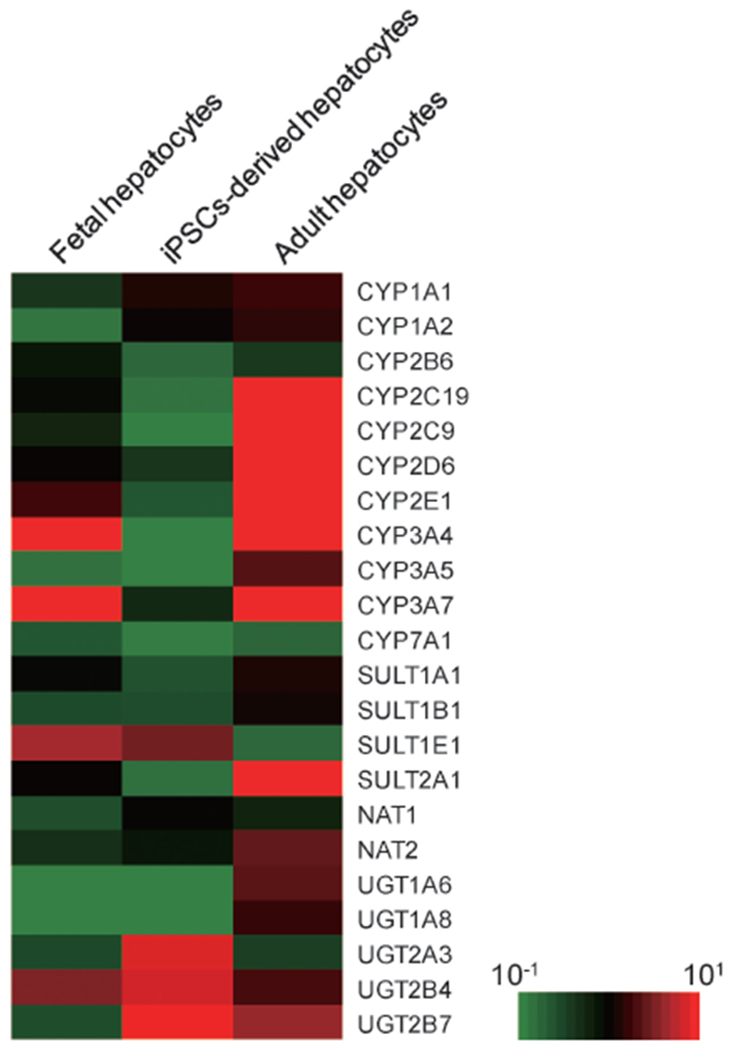
Human iPS cells (Fetch) were differentiated into hepatocyte-like cells. After differentiation, total mRNA was extracted from the cells. The expression levels of phase I and II enzymes were analyzed by microarray analysis as described in Materials and Methods. The expression levels of fetal liver cells, hepatocyte-like cells differentiated from human iPS cells, and adult hepatocytes are presented in the left, center, and right columns, respectively.
Inducibility of CYP3A enzymes and drug metabolizing activity:
A specific property of some drug-metabolizing enzymes is the induction of their expression by treatment with drugs. Figure 5 presents the effects of inducers on expression of CYP3A enzymes and UGT1A1. In the cells differentiated into hepatocytes from human iPS cells on collagen I, expression of CYP3A4 mRNA increased 2.5- and 3-fold with treatment with DEX and RIF, respectively, although expressions of CYP3A5 and CYP3A7 mRNAs were not markedly increased. When the human iPS cells were cultured on Matrigel, marked induction of CYP3A enzymes was not observed. On the other hand, UGT1A1 mRNAwas strongly induced by treatment with OME in the cells differentiated into hepatocytes. The induced level was higher in the cells cultured on Matrigel (50-fold induction) than on collagen I (22-fold induction). UGT1A1 is a typical drug-metabolizing enzyme that is strongly induced by polycyclic aromatic hydrocarbons and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) through activation of aryl hydrocarbon receptor (AhR).35) OME is also known as an AhR activator.36)
Fig. 5. Effects of drugs on expression of CYP3A enzymes and UGT1A1 mRNAs in the hepatocyte-like cells.
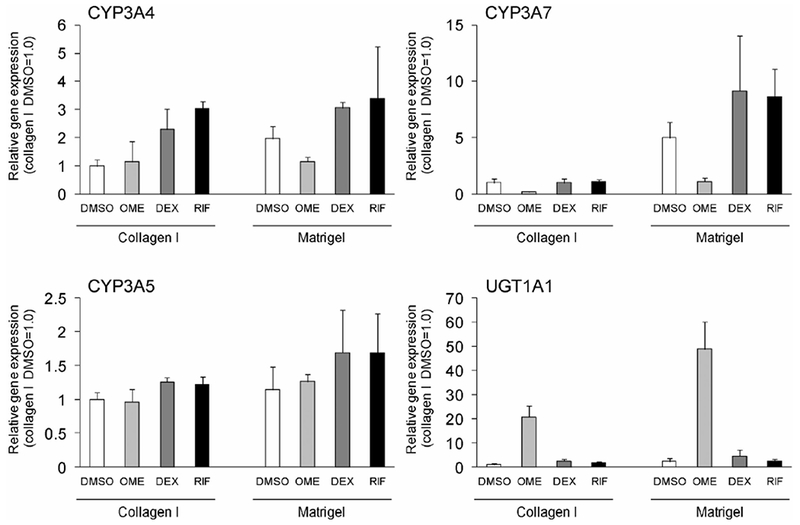
The cells differentiated from human iPS cells (Fetch) were treated with OME, DEX, and RIF for 72 h. The total mRNA was extracted from the cells. The expression of CYP3A and UGT1A1 mRNAs were analyzed by microarray analysis as described in Materials and Methods. Each bar represents the mean ± SD from triplicate experiments. Values were normalized to the levels of GAPDH mRNA. The graphs represent the relative gene expression level when the levels in the hepatocyte-like cells using collagen I and DMSO were assigned a value of 1.
Based on the induction of CYP3A4 mRNA expression, we measured testosterone 6β-hydroxylase activity, which is catalyzed by CYP3A enzymes. As shown in Figure 6, testosterone 6β-hydroxylase activity was induced 2.3- and 2.7-fold by treatment with DEX and RIF, respectively, in the cells cultured on collagen I. The activity in the cells cultured on Matrigel was approximately 2.5-fold higher than that of the cells cultured on collagen I in the control (DMSO) and was markedly induced by treatment with RIF.
Fig. 6. Effects of drugs on testosterone 6β-hydroxylase activity in hepatocyte-like cells.
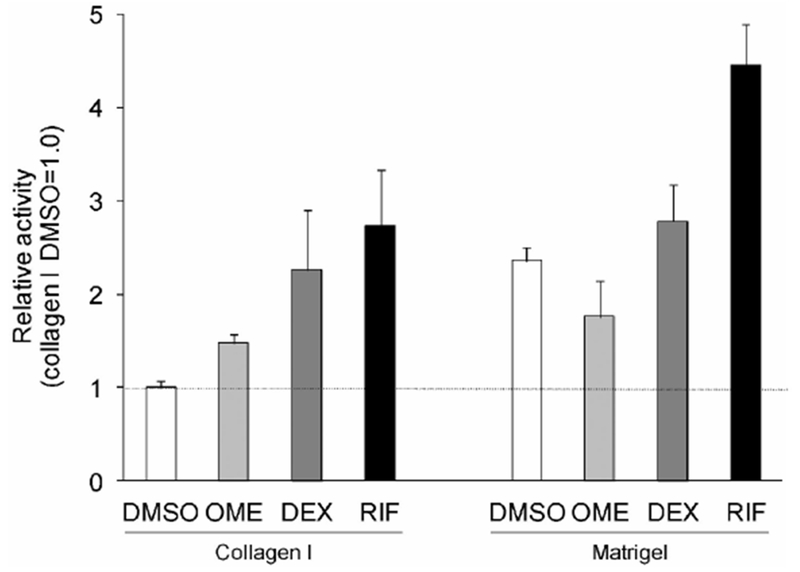
The cells differentiated from human iPS cells (Fetch) were treated with OME, DEX, and RIF for 72 h and then cultured with the medium containing testosterone for 6h. 6β-Hydroxytestosterone was analyzed using liquid chromatography coupled with tandem mass spectrometry as described in Materials and Methods. Each bar represents the mean ± SD from triplicate experiments. The graphs represent the relative activity ratios when the value in the hepatocyte-like cells using collagen I and DMSO were assigned a value of 1.
Discussion
In the present study, we established methods for the differentiation of human iPS cells into functional hepatocytes as determined by constitutive and induced expression of CYP3A4 mRNA. Several groups have previously reported the differentiation of human iPS cells into hepatocytes. Song et al. reported that human iPS cells can be induced to differentiate into hepatocyte-like cells by using six growth factors and supplements.17) In addition, Si-Tayeb et al. reported that human iPS cells can be induced to efficiently differentiate into hepatocytes by using five growth factors and supplements under hypoxic conditions.15) Another study by Takayama et al. described the use of a highly efficient method for the generation of functional hepatocytes from human iPS cells by sequential transduction of the sex determining region Y box 17 (SOX17), hematopoietically expressed homeobox (HHEX) and HNF4α or forkhead box A2 (FOXA2), and HNF1α.18,19) Hepatic differentiation methods (e.g., 3D coculture20) and hollow fiber21)) have also been used. However, highly efficient methods are complicated and extremely expensive. Because of the facile approaches that utilized only humoral factors, the responsiveness including CYP activity of differentiated human iPS cells-derived hepatocytes to inducers was unclear.
In the present study, a three-step protocol using three growth factors and two small-molecule compounds was used to differentiate human iPS cells into hepatocytes. The human iPS cells were differentiated into endoderm cells by the addition of 100 ng/mL activin A27) and were subsequently differentiated into hepatocytes by the addition of 1% DMSO.28) The hepatocytes were matured by the addition of 10 ng/mL HGF,28) 20 ng/mL OSM,28) and 10−7 M DEX.30) Activin A is necessary for the differentiation of human iPS cells into endoderm cells.27) Few studies have used DMSO, bone morphogenetic protein 4 (BMP4), FGF2, FGF7 or FGF10 for differentiation of human iPS cells into hepatic progenitor cells from human stem cells.15,17,28) In addition, we used these single factors alone or in combinations, but no increase in differentiation efficiency was observed (data not shown). Further, we selected DMSO, which is structurally stable and inexpensive compared with recombinant proteins such as the cytokines described above. DMSO has been known to maintain the functions of adult hepatocytes in vitro37) and was used to differentiate HepaRG cells.38) The hepatic progenitor cells were matured by addition of HGF, OSM, and DEX, necessary for maturation of hepatocytes.29–31)
After 25 days of differentiation, the mRNAs encoding hepatocyte markers, such as ALB, AFP, and drug-metabolizing enzymes, were expressed in the cells differentiated from human iPS cells; the expression of these mRNA were increased using Matrigel. In a previous report, Matrigel led to high expression of liver-enriched transcription factors such as HNF4α39) and laminin, a main component of Matrigel, increased ALB mRNA level and ALB secretion.40) The result in the present study is consistent with these findings. The expression level of CYP2C9 mRNA in the cells differentiated from human iPS cells was higher than that in HepG2 cells, although lower than that in hepatocytes. This result suggested that the cells are similar to hepatocytes rather than HepG2 cells, a hepatocellular carcinoma cell line. In addition, mRNA expression levels of CYP3A4 and UGT1A1 in the cells were increased by each inducer and presented responsiveness similar to that of the hepatocytes. These results indicate that human iPS cells were differentiated into hepatocyte-like cells under the present conditions. Furthermore, the cells showed testosterone 6β-hydroxylase activity that was mediated by CYP3A4, and the activity increased with addition of RIF or DEX. CYP3As, in particular CYP3A4, are strongly induced by treatment with RIF and DEX in livers.41) These data indicated that functional hepatocyte-like cells had been differentiated from human iPS cells. Induction of the CYP3A4 and UGT1A1 genes is mediated through the activation of pregnane X receptor (PXR)/constitutive androstane receptor (CAR) and AhR, respectively.42) AhR and CAR were clearly detected, but PXR was not detected in the hepatocyte-like cells (data not shown). Thus, the CYP3A4 induction observed in this study may have been dependent on CAR and glucocorticoid receptor activation by RIF and DEX, respectively. Furthermore, induction of mRNAs encoding CYP1A2 as well as CYP1A1 was observed in hepatocyte-like cells treated with TCDD, although CYP1A2 mRNA was not detected in untreated hepatocyte-like cells (data not shown). The expression level of CYP3A7 mRNA was reduced when the cells were treated with OME. However, the reason for this remains unknown. Krusekopf et al. reported that CYP3A4 in HepG2 cells was induced by OME, whereas CYP3A5, CYP3A7, and CYP3A43 were unaffected or even slightly downregulated by OME.43) They suggested that the expression of CYP3A4 is differently regulated from that of CYP3A5, CYP3A7, and CYP3A43 depending on the inducer. Expression of the CYP3A subfamily might be differently regulated by OME.
In fetal liver, it is known that the expression level of CYPs is extremely low compared with that in a mature liver.44) In the hepatocyte-like cells differentiated from human iPS cells, the expression levels of HNF6 and CCAAT-enhancer-binding protein alpha (CEBPα), transcriptional factors involved in the differentiation into mature liver cells, were low (data not shown). From the results of microarrays, the expression levels of phase II enzymes were relatively high in the differentiated cells. In contrast, the expression levels of the phase I enzymes were low, which demonstrated that the human iPS cell-derived hepatocyte-like cells were still immature. These results may suggest that the hepatocyte-like cells from human iPS cells were differentiated at least in part to fetal liver-like cells. Therefore, the differentiated cells may be useful for evaluation in a human fetal liver model.
Multiple iPS cell lines were differentiated into hepatocytes using this method, although there were differences in the differentiation ability. There is a variation of differentiation propensity among multiple human ES/iPS cell lines45,46) because of the method of stem cell generation, culture conditions, and genetic backgrounds of the donor cells. In future, it is necessary to optimize a differentiation method adapted to each cell line, and to select a cell line that is easy to differentiate into cells of interest.
In conclusion, the three-step protocol and the limited number of differentiation factors used in this study constituted an efficient method for differentiation of human iPS cells into hepatocyte-like cells that showed drug metabolizing activity and induction of CYP3A4 by RIF. However, the expression levels of drug-metabolizing enzymes were very low compared with those in mature livers. Further studies are needed to increase the expression of drug-metabolizing enzymes produced under the conditions of our method.
Acknowledgments
This work was supported, in part, by Grants-in-Aid from the Japan Society for the Promotion of Science (23390036), by Research on Publicly Essential Drugs and Medical Devices from Japan Health Sciences Foundation (KHB1011 and KHB1208), and by a National Grant-in-Aid from Japanese Ministry of Health, Labour and Welfare (H22-003).
Footnotes
Full text of this paper is available at http://www.jstage.jst.go.jp/browse/dmpk
References
- 1).Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW and Daley GQ: Reprogramming of human somatic cells to pluripotency with defined factors. Nature, 451: 141–146 (2008). [DOI] [PubMed] [Google Scholar]
- 2).Lowry WE, Richter L, Yachechko R, Pyle AD, Tchieu J, Sridharan R, Clark AT and Plath K: Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc. Natl. Acad. Sci. USA, 105: 2883–2888 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K and Yamanaka S: Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell, 131: 861–872 (2007). [DOI] [PubMed] [Google Scholar]
- 4).Takahashi K and Yamanaka S: Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 126: 663–676 (2006). [DOI] [PubMed] [Google Scholar]
- 5).Ochiya T, Yamamoto Y and Banas A: Commitment of stem cells into functional hepatocytes. Differentiation, 79: 65–73 (2010). [DOI] [PubMed] [Google Scholar]
- 6).Hannoun Z, Filippi C, Sullivan G, Hay DC and Iredale JP: Hepatic endoderm differentiation from human embryonic stem cells. Curr. Stem Cell Res. Ther, 5: 233–244 (2010). [DOI] [PubMed] [Google Scholar]
- 7).Lacroix D, Sonnier M, Moncion A, Cheron G and Cresteil T: Expression of CYP3A in the human liver—Evidence that the shift between CYP3A7 and CYP3A4 occurs immediately after birth. Eur. J. Biochem, 247: 625–634 (1997). [DOI] [PubMed] [Google Scholar]
- 8).Stevens JC, Hines RN, Gu C, Koukouritaki SB, Manro JR, Tandler PJ and Zaya MJ: Developmental Expression of the Major Human Hepatic CYP3A Enzymes. J. Pharmacol. Exp. Ther, 307: 573–582 (2003). [DOI] [PubMed] [Google Scholar]
- 9).Shimada T, Yamazaki H, Mimura M, Inui Y and Guengerich FP: Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. J. Pharmacol. Exp. Ther, 270: 414–423 (1994). [PubMed] [Google Scholar]
- 10).Wienkers LC and Health TG: Predicting in vivo drug interactions from in vitro drug discovery data. Nat. Rev. Drug Discov, 4: 825–833 (2005). [DOI] [PubMed] [Google Scholar]
- 11).Li AP, Rasmussen A, Xu L and Kaminski DL: Rifampicin induction of lidocaine metabolism in cultured human hepatocytes. J. Pharmacol. Exp. Ther, 274: 673–677 (1995). [PubMed] [Google Scholar]
- 12).Kocarek TA, Schuetz EG, Strom SC, Fisher RA and Guzelian PS: Comparative analysis of cytochrome P4503A induction in primary cultures of rat, rabbit, and human hepatocytes. Drug Metab. Dispos, 23: 415–421 (1995). [PubMed] [Google Scholar]
- 13).Silva JM, Morin PE, Day SH, Kennedy BP, Payette P, Rushmore T, Yergey JA and Nicoll-Griffith DA: Refinement of an in vitro cell model for cytochrome P450 induction. Drug Metab. Dispos, 26: 490–96 (1998). [PubMed] [Google Scholar]
- 14).Cai J, Zhao Y, Liu Y, Ye F, Song Z, Qin H, Meng S, Chen Y, Zhou R, et al. : Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology, 45: 1229–1239 (2007). [DOI] [PubMed] [Google Scholar]
- 15).Si-Tayeb K, Noto FK, Nagaoka M, Li J, Battle MA, Duris C, North PE, Dalton S and Duncan SA: Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology, 51: 297–305 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Touboul T, Hannan NR, Corbineau S, Martinez A, Martinet C, Branchereau S, Mainot S, Strick-Marchand H, Pedersen R, et al. : Generation of functional hepatocytes from human embryonic stem cells under chemically defined conditions that recapitulate liver development. Hepatology, 51: 1754–1765 (2010). [DOI] [PubMed] [Google Scholar]
- 17).Song Z, Cai J, Liu Y, Zhao D, Yong J, Duo S, Song X, Guo Y, Zhao Y, et al. : Efficient generation of hepatocyte-like cells from human induced pluripotent stem cells. Cell Res, 19: 1233–1242 (2009). [DOI] [PubMed] [Google Scholar]
- 18).Takayama K, Inamura M, Kawabata K, Katayama K, Higuchi M, Tashiro K, Nonaka A, Sakurai F, Hayakawa T, et al. : Efficient generation of functional hepatocytes from human embryonic stem cells and induced pluripotent stem cells by HNF4a transduction. Mol. Ther, 20: 127–137 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Takayama K, Inamura M, Kawabata K, Sugawara M, Kikuchi K, Higuchi M, Nagamoto Y, Watanabe H, Tashiro K, et al. : Generation of metabolically functioning hepatocytes from human pluripotent stem cells by FOXA2 and HNF1a transduction. J. Hepatol, 57: 628–636 (2012). [DOI] [PubMed] [Google Scholar]
- 20).Nagamoto Y, Tashiro K, Takayama K, Ohashi K, Kawabata K, Sakurai F, Tachibana M, Hayakawa T, Furue MK, et al. : The promotion of hepatic maturation of human pluripotent stem cells in 3D co-culture using type I collagen and Swiss 3T3 cell sheets. Biomaterials, 33: 4526–4534 (2012). [DOI] [PubMed] [Google Scholar]
- 21).Amimoto N, Mizumoto H, Nakazawa K, Ijima H, Funatsu K and Kajiwara T: Hepatic differentiation of mouse embryonic stem cells and induced pluripotent stem cells during organoid formation in hollow fibers. Tissue Eng. Part A, 2011(17): 2071–2078 (2011). [DOI] [PubMed] [Google Scholar]
- 22).Tsuchiya H, Matsunaga T, Aikawa K, Kamada N, Nakamura K, Ichikawa H, Sasaki K and Ohmori S: Evaluation ofhuman embryonic stem cell-derived hepatocyte-like cells for detection of CYP1A inducers. Drug Metab. Pharmacokinet, 27: 598–604 (2012). [DOI] [PubMed] [Google Scholar]
- 23).Momose Y, Matsunaga T, Murai K, Takezawa T and Ohmori S: Differentiation of monkey embryonic stem cells into hepatocytes and mRNA expression of cytochrome P450 enzymes responsible for drug metabolism: comparison of embryoid body formation conditions and matrices. Biol. Pharm. Bull, 32: 619–626 (2009). [DOI] [PubMed] [Google Scholar]
- 24).Maruyama M, Matsunaga T, Harada E and Ohmori S: Comparison of basal gene expression and induction ofCYP3As in HepG2 and human fetal liver cells. Biol. Pharm. Bull, 30: 2091–2097 (2007). [DOI] [PubMed] [Google Scholar]
- 25).Nishimura M, Koeda A, Suganuma Y, Suzuki E, Shimizu T, Nakayama M, Satoh T, Narimatsu S and Naito S: Comparison of inducibility of CYP1A and CYP3A mRNAs by prototypical inducers in primary cultures of human, cynomolgus monkey, and rat hepatocytes. Drug Metab. Pharmacokinet, 22: 178–186 (2007). [DOI] [PubMed] [Google Scholar]
- 26).Maezawa K, Matsunaga T, Takezawa T, Kanai M, Ohira S and Ohmori S: Cytochrome P450 3As gene expression and testosterone 6β-hydroxylase activity in human fetal membranes and placenta at full term. Biol. Pharm. Bull, 33: 249–254 (2010). [DOI] [PubMed] [Google Scholar]
- 27).D’Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E and Baetge EE: Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat. Biotechnol, 23: 1534–1541 (2005). [DOI] [PubMed] [Google Scholar]
- 28).Hay DC, Zhao D, Fletcher J, Hewitt ZA, McLean D, Urruticoechea-Uriguen A, Black JR, Elcombe C, Ross JA, et al. : Efficient differentiation of hepatocytes from human embryonic stem cells exhibiting markers recapitulating liver development in vivo. Stem Cells, 26: 894–902 (2008). [DOI] [PubMed] [Google Scholar]
- 29).Kamiya A, Kinoshita T, Ito Y, Matsui T, Morikawa Y, Senba E, Nakashima K, Taga T, Yoshida K, et al. : Fetal liver development requires a paracrine action of oncostatin M through the gp130 signal transducer. EMBO J, 18: 2127–2136 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Kamiya A, Kinoshita T and Miyajima A: Oncostatin M and hepatocyte growth factor induce hepatic maturation via distinct signaling pathways. FEBS Lett, 492: 90–94 (2001). [DOI] [PubMed] [Google Scholar]
- 31).Suzuki A, Iwama A, Miyashita H, Nakauchi H and Taniguchi H: Role for growth factors and extracellular matrix in controlling differentiation of prospectively isolated hepatic stem cells. Development, 130: 2513–2524 (2003). [DOI] [PubMed] [Google Scholar]
- 32).Chou JY: Regulators of fetal liver differentiation in vitro. Arch. Biochem. Biophys, 263: 378–386 (1988). [DOI] [PubMed] [Google Scholar]
- 33).Jochheim A, Hillemann T, Kania G, Scharf J, Attaran M, Manns MP, Wobus AM and Ott M: Quantitative gene expression profiling reveals a fetal hepatic phenotype of murine ES-derived hepatocytes. Int. J. Dev. Biol, 48: 23–29 (2004). [DOI] [PubMed] [Google Scholar]
- 34).Kondraganti SR, Jiang W and Moorthy B: Differential regulation of expression of hepatic and pulmonary cytochrome P4501A enzymes by 3-methylcholanthrene in mice lacking the CYP1A2 gene. J. Pharmacol. Exp. Ther, 303: 945–951 (2002). [DOI] [PubMed] [Google Scholar]
- 35).Bock KW: Functions and transcriptional regulation of adult human hepatic UDP-glucuronosyl-transferases (UGTs): mechanisms responsible for interindividual variation of UGT levels. Biochem. Pharmacol, 80: 771–777 (2010). [DOI] [PubMed] [Google Scholar]
- 36).Quattrochi LC and Tukey RH: Nuclear uptake of the Ah (dioxin) receptor in response to omeprazole: transcriptional activation of the human CYP1A1 gene. Mol. Pharmacol, 43: 504–508 (1993). [PubMed] [Google Scholar]
- 37).Kamiya A, Kojima N, Kinoshita T, Sakai Y and Miyaijma A: Maturation of fetal hepatocytes in vitro by extracellular matrices and oncostatin M: induction of tryptophan oxygenase. Hepatology, 35: 1351–1359 (2002). [DOI] [PubMed] [Google Scholar]
- 38).Cerec V, Glaise D, Garnier D, Morosan S, Turlin B, Drenou B, Gripon P, Kremsdorf D, Guguen-Guillouzo C, et al. : Transdifferentiation of hepatocyte-like cells from the human hepatoma HepaRG cell line through bipotent progenitor. Hepatology, 45: 957–967 (2007). [DOI] [PubMed] [Google Scholar]
- 39).Oda H, Nozawa K, Hitomi Y and Kakinuma A: Laminin-rich extracellular matrix maintains high level of hepatocyte nuclear factor 4 in rat hepatocyte culture. Biochem. Biophys. Res. Commun, 212: 800–805 (1995). [DOI] [PubMed] [Google Scholar]
- 40).Caron JM: Induction of albumin gene transcription in hepatocytes by extracellular matrix proteins. Mol. Cell. Biol, 10: 1239–1243 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Gibson GG, Plant NJ, Swales KE, Ayrton A and El-Sankary W: Receptor-dependent transcriptional activation of cytochrome P4503A genes: induction mechanisms, species differences and interindividual variation in man. Xenobiotica, 32: 165–206 (2002). [DOI] [PubMed] [Google Scholar]
- 42).Sinz M, Wallace G and Sahi J: Current industrial practices in assessing CYP450 enzyme induction: preclinical and clinical. AAPS J, 10: 391–400 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43).Krusekopf S, Roots I and Kleeberg U: Differential drug-induced mRNA expression ofhuman CYP3A4 compared to CYP3A5, CYP3A7 and CYP3A43. Eur. J. Pharmacol, 466: 7–12 (2003). [DOI] [PubMed] [Google Scholar]
- 44).Ring JA, Ghabrial H, Ching MS, Smallwood RA and Morgan DJ: Fetal hepatic drug elimination. Pharmacol. Ther, 84: 429–445 (1999). [DOI] [PubMed] [Google Scholar]
- 45).Osafune K, Caron L, Borowiak M, Martinez RJ, Fitz-Gerald CS, Sato Y, Cowan CA, Chien KR and Melton DA: Marked differences in differentiation propensity among human embryonic stem cell lines. Nat. Biotechnol, 26: 313–315 (2008). [DOI] [PubMed] [Google Scholar]
- 46).Kajiwara M, Aoi T, Okita K, Takahashi R, Inoue H, Takayama N, Endo H, Eto K, Toguchida J, et al. : Donor-dependent variations in hepatic differentiation from human-induced pluripotent stem cells. Proc. Natl. Acad. Sci. USA, 109: 12538–12543 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]


