Abstract
In the north-central United States, the blacklegged tick (Ixodes scapularis) is currently known to vector seven human pathogens. These include five bacteria (Borrelia burgdorferi sensu stricto, Borrelia mayonii, Borrelia miyamotoi, Anaplasma phagocytophilum, Ehrlichia muris eauclairensis), one protozoan (Babesia microti) and one virus (Powassan). We sought to assess the prevalence and distribution of these pathogens in host-seeking nymphs collected throughout Minnesota, a state on the northwestern edge of the tick’s expanding range, where reported cases of I. scapularis-borne diseases have increased in incidence and geographic range over the past decade. Among the 1240 host-seeking I. scapularis nymphs that we screened from 64 sites, we detected all seven pathogens at varying frequencies. Borrelia burgdorferi s.s. was the most prevalent and geographically widespread, found in 25.24% of all nymphs tested. Anaplasma phagocytophilum and Babesia microti were also geographically widespread, but they were less prevalent than Bo. burgdorferi s.s. (detected in 6.29% and 4.68% of ticks, respectively). Spatial clusters of sites with high prevalence for these three pathogens were identified in the north-central region of the state. Prevalence was less than 1.29% for each of the remaining pathogens. Two or more pathogens were detected in 90 nymphs (7.26%); coinfections with Bo. burgdorferi s.s. and either A. phagocytophilum (51 nymphs, 4.11%) or Ba. microti (43 nymphs, 3.47%) were the most common combinations. The distribution and density of infected ticks mirrors the distribution of notifiable tick-borne diseases in Minnesota and provides information on the distribution and prevalence of recently described human pathogens.
Keywords: Lyme disease, Anaplasmosis, Babesiosis, Nymphal infection prevalence (NIP), Density of infected nymphs (DIN), Coinfection
1. Introduction
In the eastern United States, the incidence and geographic range of Ixodes scapularis-borne diseases have been increasing over the last two decades (Eisen and Eisen, 2018; Mead, 2015). Correspondingly, the known geographic distribution of I. scapularis has also increased substantially and the number of recognized I. scapularis-borne human pathogens has increased from none prior to 1970, to seven in 2016 (Eisen and Eisen, 2018; Eisen et al., 2016). Over the past decade, some of the most substantial changes in the epidemiology and ecology of I. scapularis-borne infections have been documented in the upper Midwest, particularly in Minnesota. For example, from the mid-1990s to present, the number of counties in Minnesota with established populations of I. scapularis has increased more than five-fold and investigators have described two novel pathogens (Ehrlichia muris eauclairensis and Borrelia mayonii) that appear to be restricted to the upper Midwest (Pritt et al., 2011; Robinson et al., 2015).
Lyme disease, caused by Borrelia burgdorferi sensu stricto (or less commonly by Borrelia mayonii) and transmitted by I. scapularis in the eastern United States, is the most commonly reported vector-borne disease in the United States, with more than 30,000 cases reported annually (Burgdorfer et al., 1982; Dolan et al., 2016; Pritt et al., 2016b; Schwartz et al., 2017). Minnesota is one of fourteen high-incidence states from which the majority of Lyme disease cases are reported (Mead, 2015). In addition to Lyme disease, which is the most commonly reported I. scapularis-borne disease in Minnesota, five other I. scapularis-borne diseases have been recognized in that state (Robinson et al., 2015; http://www.health.state.mn.us/divs/idepc/newsletters/dcn/sum15/lyme.html). These include anaplasmosis (Anaplasma phagocytophilum), babesiosis (Babesia microti), Powassan virus disease (Powassan virus), ehrlichiosis (Ehrlichia muris eauclairensis), and Borrelia miyamotoi disease (Birge and Sonnesyn, 2012; Johnson et al., 2011; Pritt et al., 2011; Robinson et al., 2015). Although cases of anaplasmosis and babesiosis are less frequently reported than Lyme disease, they follow a similar spatial distribution (Robinson et al., 2015). Each of the other pathogens has been detected in ticks collected in Minnesota, but little is known about their distribution or prevalence throughout the state (Birge and Sonnesyn, 2012; Hahn et al., 2017; Johnson et al., 2015, 2011; Pritt et al., 2016b, 2011; Stromdahl et al., 2014).
To complement existing epidemiological surveillance data, we assessed the acarological risk for exposure to seven I. scapularis-borne pathogens by sampling host-seeking nymphs at 81 publicly-owned sites across the tick’s current range in Minnesota. We focused on the nymphal life stage because it frequently bites humans and has been shown to be the most epidemiologically significant life stage for the most common I. scapularis-borne human infections (des Vignes et al., 2001; Falco et al., 1999; Merten and Durden, 2000; Piesman, 1993; Spielman, 1976; Telford et al., 1996). The density of infected nymphs is commonly used as a measure of acarological risk for human exposure to I. scapularis-borne diseases, particularly Lyme disease, and is often positively correlated with Lyme disease incidence (Mather et al., 1996; Pepin et al., 2012; Stafford et al., 1998). The goals of this study were to: 1) report the prevalence Bo. burgdorferi s.s., Bo. mayonii, Bo. miyamotoi, A. phagocytophilum, Ba. microti, E. muris, and Powassan virus in hostseeking nymphs collected throughout Minnesota, 2) identify spatial patterns in the distribution of pathogen-infected nymphs, and 3) map the density of host-seeking nymphs infected with each of these pathogens (DIN).
2. Materials and methods
2.1. Site selection and tick sampling
We collected ticks from 81 publicly-owned wooded sites located in 42 of Minnesota’s 87 counties as described previously (Johnson et al., 2018). To quantify the density of host-seeking I. scapularis nymphs, we sampled each site on two occasions between 31 May and 30 June 2015, with the two sampling sessions each separated by at least six days. We drag sampled 750 m2 of forest floor using a 1-m2 drag that we inspected for ticks every 15 m. For individual sites, we used the sampling date with the highest count to estimate nymphal density. For pathogen detection, we aimed to collect 50 I. scapularis nymphs from each site. If a total of ten, but less than 50 nymphs had been collected after both sampling sessions, we conducted extra sampling in the immediate vicinity of the sampling transect; ticks collected during extra sampling were not included in the density estimates, but were reported previously (Johnson et al., 2018; Fig. S1). We shipped and stored ticks in 70% ethanol and identified them to species (Coley, 2015; Durden and Keirans, 1996; Keirans and Litwak, 1989) prior to pathogen testing.
2.2. Nucleic acid extraction
We prepared nucleic acid from individual I. scapularis nymphs as previously described (Graham et al., 2018). Briefly, we prepared a 470-μl homogenate from each of 1241 nymphs, extracted nucleic acid from 200 μl homogenate, and eluted each sample in 100 μl buffer AVE using the QIAcube HT automated nucleic acid isolation system and the cador Pathogen 96 QIAcube HT kit (Qiagen, Valencia, CA). The remaining homogenate from each sample and all nucleic acid extracts were stored at 4 °C for up to 13 days, then at −80 °C. We subsequently prepared a second nucleic acid extract from 200 μl of the leftover homogenate from each specimen. With the exception of 96 samples that were stored at 4 °C for up to 2 days, all second extracts were stored at −80 °C within a few hours of extraction. As described in Graham et al. (2018), where the DNA in the first extract was of poor quality or yielded ambiguous pathogen test results, we used the second extract to complete testing for Borrelia spp., A. phagocytophilum, and Ba microti. In cases where we ran out of the first extract, we also used the second extract to complete testing for these pathogens as well as E. muris eauclairensis. We used the second set of nucleic acid extracts, which had spent limited time at 4 °C, for all Powassan virus testing.
One of the 1241 nymphs from which we extracted nucleic acid failed to yield DNA of sufficient quality and quantity for analysis. We excluded this sample from all analyses. We tested the remaining 1240 nymphs for Bo. burgdorferi s.l., Bo. mayonii, Bo. miyamotoi, A. phagocytophilum, Ba. microti, and E. muris eauclairensis, and we tested all nymphs for which there was enough remaining sample (N = 1233) for Powassan virus.
2.3. Pathogen detection
We tested each I. scapularis nymph for Bo. burgdorferi s.s., Bo. mayonii, Bo. miyamotoi, A. phagocytophilum, and Ba. microti using a published algorithm (Graham et al., 2018). Briefly, we first screened all samples using paired multiplex assays to detect two A. phagocytophilum targets (genes encoding P44 outer membrane proteins and major surface protein 4), two Ba microti targets (genes encoding secreted antigen 1 and 18S rRNA), Borrelia 16S rDNA (a pan-Borrelia target), and a segment of the Bo. burgdorferi s.s. flagellin gene also present in Bo. mayonii. With regard to Babesia and Anaplasma, our testing algorithm was designed to be B. microti-and A. phagocytophilum specific (Hojgaard et al., 2014). We have tested the Ba microti 18S primer-probe set against one Ba. odocoilei strain and found that it did not detect that strain (data not shown), and Souza et al. (2016) found that neither the Ba microti sa1 nor the Ba microti 18S primer-probe set detected other Babesia spp. The algorithm was not designed, however, to distinguish A. phagocytophilum-ha (human associated) from A. phagocytophilum-var1, a deer associated variant which has not been associated with human disease. The paired assays also included an I. scapularis actin target that allowed us to verify the presence of amplifiable DNA in each tick-derived sample. Each test reaction included 4.8 μl extract (equivalent to approximately 2% of a total tick homogenate). We previously determined that these assays consistently detected A. phagocytophilum, Ba. microti, Bo. burgdorferi s.s., Bo. mayonii, and Bo. miyamotoi at concentrations as low as 3–6 pathogen genomes per reaction. Following the initial screening, we used a duplex TaqMan real-time PCR assay targeting the Bo. burgdorferi s.s. and Bo. mayonii oligopeptide permease periplasmic A2 gene (oppA2), and a pair of TaqMan real-time PCR assays targeting the Bo. miyamotoi adenylosuccinate lyase (purB) and glycerophosphodiesterase (glpQ) genes, to detect and differentiate Bo. burgdorferi s.s., Bo. mayonii, and Bo. miyamotoi in Borrelia-positive samples. All reaction components and cycling conditions were performed as previously described (Graham et al., 2018, 2016).
Per the algorithm (Graham et al., 2018), we attempted to amplify and sequence segments of the genes encoding Clp protease subunit A (clpA) and dipeptidyl aminopeptidase (pepX) from Borrelia-positive samples that tested negative for Bo. burgdorferi s.s., Bo. mayonii, and Bo. miyamotoi in order to putatively identify the infecting Borrelia species. We used the same approach to verify the presence of Bo. burgdorferi s.s. in samples considered suspect Bo. burgdorferi positives because they returned disparate Bo. burgdorferi oppA2 and Borrelia 16S Cq values (Cq difference > 4 cycles; Graham et al., 2018). We also amplified the clpA and pepX targets to verify the presence of Bo. mayonii in PCR-positive samples that were negative for Bo. burgdorferi s.s. Because the clpA and pepX primers generate amplicons from all Bo. burgdorferi sensu lato species, we used a species-specific primer set targeting a segment of Bo. mayonii circular plasmid 26 (cp26) to verify the presence of Bo. mayonii in samples co-infected with Bo. burgdorferi s.s. We amplified, purified, sequenced, and analyzed all targets as previously described (Graham et al., 2018). We used the Basic Local Alignment Search Tool (BLAST) to identify similar sequences in GenBank. Using the clpA and pepX sequences, we also queried the PubMLST database (http://pubmlst.org/borrelia/; Margos et al., 2015) to identify similar alleles.
We screened nymphs (in duplicate) for the presence of E. muris eauclairensis using a C1000 Touch thermal cycler with a CFX96 real time system (BioRad, USA), following the protocol published in Allerdice et al. (2016) but using the QuantiTect Probe Kit from Qiagen instead of the QuantiTect Multiplex PCR kit.
We detected Powassan virus using a TaqMan RT-PCR assay targeting Powassan virus nonstructural protein 5 targeting the primers and probe described in Aliota et al. (2014). Each 10-μl RT-PCR reaction included 5 μl 2X iQ Multiplex Powermix (BioRad, USA), 0.125 μl MultiScribe Reverse Transcriptase (50 Units/μl) (ThermoFisher, USA), 300 nM each primer, 200 nM probe, and 4.7 μl sample. Each 96-well plate included a non-template control (water) and two Powassan virus (lineage II or “deer tick virus” strain CT390–1995 CT) RNA control reactions: one, with and one without reverse transcriptase. The RT-PCR cycling conditions were as follows: incubation at 50 °C for 15 min, denaturation at 95 °C for 3 min, followed by 40 cycles of 95 °C for 10 s and 60 °C for 45 s on a C1000 Touch thermal cycler with a CFX96 real time system (BioRad, USA).
2.4. Data summary and analysis
Using a maximum likelihood analysis, we estimated nymphal infection prevalence (NIP) and associated 95% confidence intervals for each pathogen at each site and for all ticks combined across all collection sites [Pooled Infection Rate Excel add-in; (Biggerstaff, 2009)]. We calculated the density of nymphs infected with each pathogen by multiplying NIP (maximum likelihood point estimate) by the density of host-seeking nymphs (DON) for each site; the DON for each site was reported previously (Johnson et al., 2018). We estimated the confidence limits for DIN by multiplying the lower and upper 95% confidence limits for NIP by DON and taking the difference. We used a generalized linear model, using the number of nymphs tested from each site as an offset, to determine if DON predicted Bo. burgdorferi s.s. NIP. We excluded one site in Anoka County because it was found to have a large amount of leverage on the model. The leverage of an observation is a measure of the distance between its independent variable i.e., density of nymphs, and the mean of the independent variable across all observations. A small change in the dependent variable i.e., infection prevalence, for a high leverage observation can change the results of a regression dramatically. We performed the analysis with and without the high-leverage observation and determined that its influence warranted its exclusion. Unless otherwise noted, we performed all statistical analyses in R (R v 3.2.1, R Foundation for Statistical Computing, Vienna, Austria), using a significance level of α = 0.05.
We used SaTScan (v 9.4.4, Kulldorff, 1997) to detect spatial clusters of sites with significantly higher or lower NIP as compared to surrounding sites for each pathogen. The spatial scan statistic imposes a circular window onto the study area; the window is centered on each sampling site in turn. For each site, the radius of the circle was allowed to vary from zero (which allowed a single site to be a cluster) to include up to 50% of the total tick population tested from all sites combined. For each site, the geographic coordinates, the number of infected ticks and the total number of ticks tested were used to calculate a spatial scan statistic using the discrete Poisson method (Kulldorff, 1997). Under this model, the expected number of positive nymphs is proportional to the population within the cluster, making it possible to identify clusters of sites of higher and lower than expected infection prevalence. We used the Gini coefficient to identify the best set of statistically significant non-overlapping clusters of high and low NIP sites (Han et al., 2016).
3. Results
3.1. Distribution and prevalence of Ixodes scapularis-borne pathogens
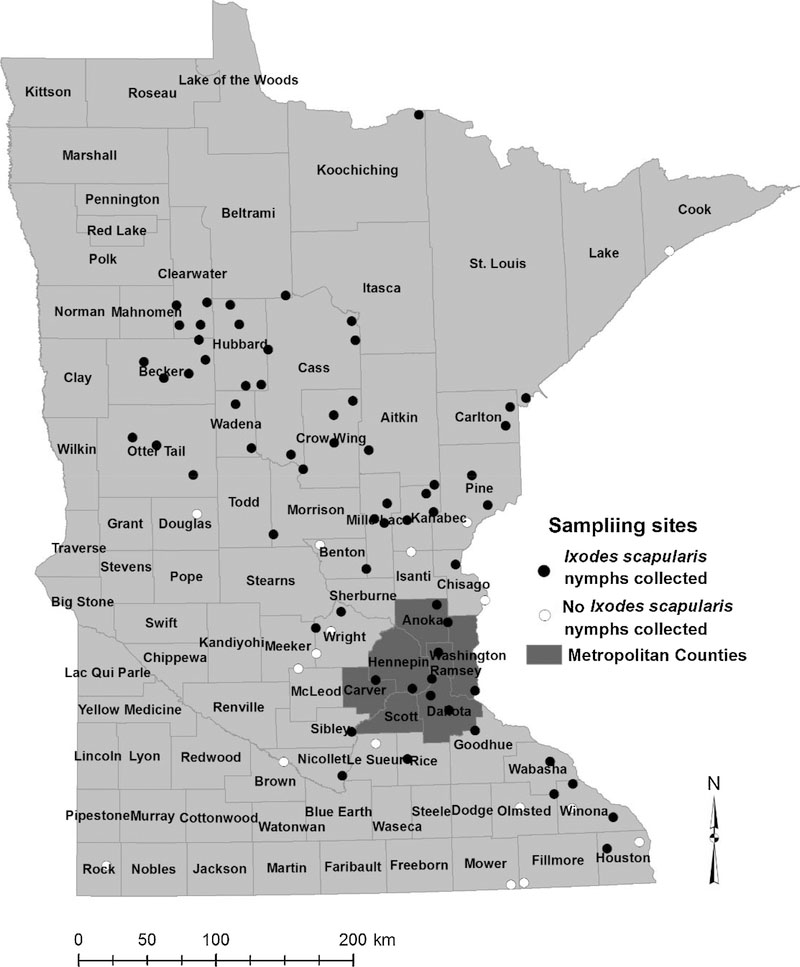
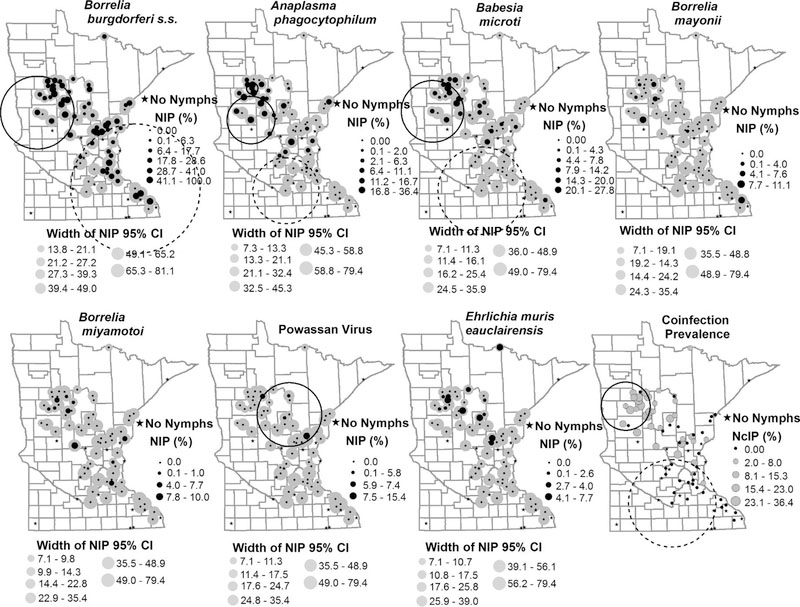
We collected at least one nymph from 64 of the total 81 sites we sampled (Fig. 1). We screened 1240 I. scapularis nymphs for the presence of the five bacterial and one protozoan pathogens and screened 1233 nymphs for Powassan virus; the median number of nymphal ticks tested from each site was 13.5 (range, 1–50; Table S1). We detected all seven pathogens, although infection prevalence was variable among pathogens (Table 1, Fig. 2). A total of 376 (30.32%; 95% CI: 27.81–32.93%) nymphs tested positive for at least one pathogen and statewide NIP ranged from as high as 25.24% (95% CI: 22.88–27.72%) for Bo. burgdorferi s.s. to as low as 0.49% (0.20–1.01%) for Powassan virus (Table 1). We did not detect any pathogens in nymphs collected from nine sites in southern Minnesota and on the western edge of the I. scapularis distribution (Fig. 2). However, the numbers of nymphs we tested from each of these sites ranged from only one to eight. Based on maximum likelihood estimation (upper 95% confidence limit), even though no pathogens were detected from these sites, NIP could have been as high as 32% where eight ticks were tested to as high as 79% for sites with a single nymph tested. Nonetheless, the likelihood of human encounters with infected nymphs is expected to be low at these sites because of the low DON. We explored the relationship between DON and NIP of Bo. burgdorferi s.s. and found no significant association (glm; P = 0.13).
Fig. 1.
Locations of 81 sites sampled for Ixodes scapularis nymphs, Minnesota, May 31–June 30, 2015.
Table 1.
Number of nymphs testing positive for each of seven pathogens, statewide nymphal infection prevalence (NIP) and associated 95% confidence intervals calculated using maximum likelihood, Minnesota, May 31–June 30, 2015.
| No. positive sitesa (%) | No. positive ticks (%)b | NIP (95% CI) | |
|---|---|---|---|
| Borrelia burgdorferi sensu stricto | 53 (83) | 313 (25.24) | 25.24 (22.88–27.72) |
| Anaplasma phagocytophilum | 28 (44) | 78 (6.29) | 6.29 (5.04–7.75) |
| Babesia microti | 27 (42) | 58 (4.68) | 4.68 (3.60–5.96) |
| Ehrlichia muris subsp. eauclairensis | 13 (20) | 16 (1.29) | 1.29 (0.77–2.04) |
| Borrelia miyamotoi | 10 (16) | 13 (1.05) | 1.05 (0.59–1.74) |
| Borrelia mayonii | 7 (11) | 8 (0.65) | 0.65 (0.30–1.22) |
| Powassan virusc | 4 (6) | 6 (0.49) | 0.48 (0.20–1.00) |
The total number of collection sites where nymphs were recovered was 64.
The total number of nymphal ticks collected from 64 sites was 1240.
The total number of ticks tested for Powassan virus lineage II was 1233.
Fig. 2.
Nymphal infection prevalence (NIP) and nymphal coinfection prevalence (NcIP) of Ixodes scapularis collected in Minnesota and tested for seven human disease causing pathogens. Site-specific estimates of NIP are represented by the black dots and the corresponding width of the 95% confidence interval is shown by the grey circles. NcIP is shown by grey circles. Site-specific estimates of NIP and corresponding 95% confidence intervals were calculated using maximum likelihood. Sites encompassed by an large circle represent clusters of sites with high NIP/NcIP (solid black line) and clusters of sites with low NIP/NcIP (dashed line) identified by SaTScan (v 9.4.4).
Five nymphs tested positive for Borrelia spp. but negative for Bo. burgdorferi s.s., Bo. mayonii, and Bo. miyamotoi. Repeated efforts to amplify the Borrelia clpA and pepX targets from one of the samples failed. We were therefore unable to identify this Borrelia to species level. We were able to amplify and sequence the clpA target from one of the four remaining samples and the pepX target from all four remaining samples. The four pepX sequences, derived from nymphs collected in Cass, Clearwater, and Wadena counties, showed 99–100% identity with Borrelia kurtenbachii strains in GenBank and ≤96% identity with available sequences from all other species. All four sequences contained pepX allele 107, an allele associated exclusively with Bo. kurtenbachii isolates in pubMLST. The clpA sequence showed 99% identity with Bo. kurtenbachii. Borrelia kurtenbachii is not known to cause human disease.
3.2. Nymphal infection prevalence of Lyme disease-causing Borrelia
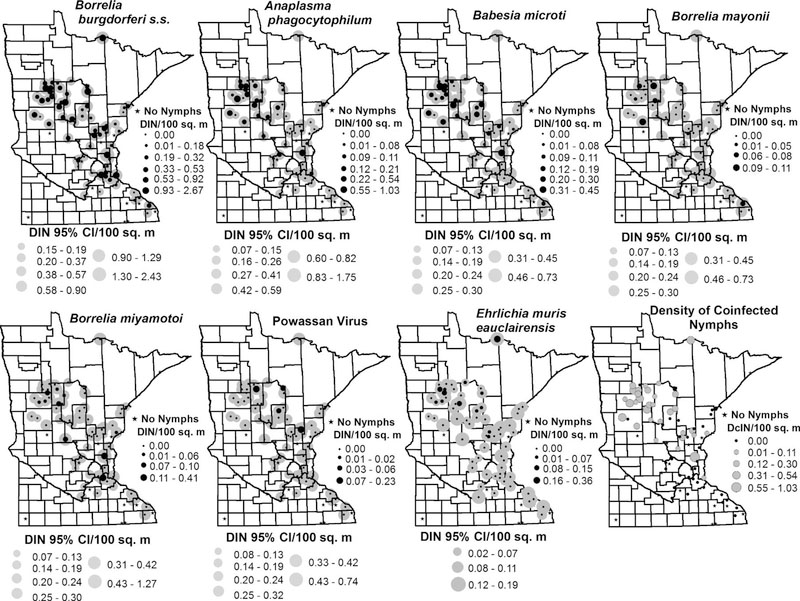
We detected Bo. burgdorferi s.s. in 313 out of 1240 nymphs collected from 53 of 64 (83%) sites where ticks were tested, yielding an overall prevalence of 25.24% (95% CI: 22.88–27.72%; Table 1). We collected infected nymphs across the state, from southeast of the Minneapolis/St. Paul metropolitan area and along the Wisconsin border, and north/northwest from the Minneapolis/St. Paul metropolitan area (Fig. 2). The density of Bo. burgdorferi s.s. infected nymphs per site ranged from 0.00 (95% CI: 0.00–0.24) to 2.67 (95% CI: 1.63–4.06) nymphs/100 m2 (Fig. 3). We identified a cluster of 17 sites with a higher than expected prevalence of Bo. burgdorferi s.s. compared with surrounding sites (P = 0.01). These sites were situated near the northwestern extent of our study sites (Fig. 2); infection prevalence ranged from 0% (95% CI: 0–49%) at one site to as high as 64% (95% CI: 35–73%) among these sites. In total, 137 of 398 (34.4%) nymphs from these sites tested positive for Bo. burgdorferi s.s. We also identified a cluster of 21 sites in the southeastern portion of the state with prevalence of Bo. burgdorferi s.s. lower than the statewide prevalence [P = 0.04; NIP range 0% (95% CI: 0–56%) to 100% (95% CI: 21–100%]. Within that cluster, 79 of 438 (18.0%) of nymphs tested positive for Bo. burgdorferi s.s. (Fig. 2).
Fig. 3.
Density of infected Ixodes scapularis nymphs (DIN=DON × NIP) and density of coinfected nymphs (DcIN=DON × NcIP) at 81 study sites sampled in Minnesota—2015. Nymphs were tested for the presence of seven human disease causing pathogens. Site-specific estimates of DIN are represented by the black dots and the corresponding width of the 95% confidence intervals are shown by the grey circles. NcIP is shown by grey circles. Site-specific estimates of NIP and corresponding 95% confidence intervals were calculated using maximum likelihood.
We detected the newly-identified Lyme disease-causing agent Bo. mayonii in 0.65% (8 out of 1240) of nymphs tested. We collected these B. mayonii-infected nymphs from seven of 64 sampling sites in the following counties: Becker, Clearwater, Houston, Hubbard, Mille Lacs, and Otter Tail (Table 1, Fig. 1). We only detected Bo. mayonii from sites where Bo. burgdorferi s.s.was also present. The statewide NIP for Bo. mayonii was 0.65% (95% CI: 0.30–1.22%). Infection prevalence with Bo. mayonii ranged from 0% (95% CI: 0–79%) to as high as 11% (95% CI: 2–44%) at one site (N = 9); however, we did not observe a density of Bo. mayonii-infected nymphs greater than 0.11 nymphs/100 m2 (95% CI: 0.02–0.43; Fig. 3). We did not detect any significant spatial clustering of sites with low or high B. mayonii NIP. However, five out of seven sites with Bo. mayonii positive nymphs were located near the high NIP Bo. burgdorferi s.s. cluster at the northwestern range of our sample sites (Fig. 2).
3.3. Nymphal infection prevalence of Anaplasma phagocytophilum
We detected A. phagocytophilum at almost half (44%; 28 out of 64) of our sampling sites (Table 1, Fig. 2). Despite showing a geographic distribution nearly as broad as Bo. burgdorferi s.s. (Fig. 2), we only detected A. phagocytophilum in 78 of 1240 nymphs (6.21%). The statewide NIP for A. phagocytophilum was 6.29% (95% CI: 5.04–7.75; Table 1). DIN was < 1 nymph/100 m2 at all sites except one site located in Anoka County, where DIN was 1.03 nymphs/100 m2 (95% CI: 0.45–2.19). Two clusters of sites with A. phagocytophilum NIP significantly higher than the surrounding sites were identified. Similar to Bo. burgdorferi s.s., both clusters, one of eight sites [P < 0.0001, NIP range: 5.7% (95% CI: 1.6–18.6) to 34.0% (95% CI: 22.2–48.3)] and the other consisting of four sites [P = 0.03, NIP range: 20.0% (95% CI: 5.7–51.0%) to 36.4% (95% CI:15.2–64.6%)], were located near the northwestern extent of our study sites (Fig. 2). The cluster of eight sites had an A. phagocytophilum infection prevalence of 18.0% (31 of 172) while infection prevalence in the cluster of four sites was 17.0% (18 of 106). A cluster of 10 sites was identified as having significantly lower than expected NIP [P = 0.02, NIP range: 0.0% (95% CI: 0.0–79.4%) to 2.0% (95% CI: 0.4–10.5%)], with only one of 171 (0.6%) ticks from these sites positive for A. phagocytophilum. On the southwestern edge of our sampling sites, low infection prevalence dominated the bulk of sampling sites where nymphs were collected (Fig. 2).
3.4. Nymphal infection prevalence of Babesia microti
We detected Ba. microti in 58 of 1240 (4.60%) nymphs from 27 of 64 sites (42%; Table 1, Fig. 2). The geographic distribution for Ba. microti was similar to that of A. phagocytophilum, with the exception of sites south of Minneapolis/St. Paul, where Ba. microti was largely absent. Statewide estimated NIP was 4.68% (95% CI: 3.60–5.96%) and ranged from 0% (95% CI: 0–79%) to 28% (95% CI: 13–51%) across sites (Fig. 2); DIN ranged from 0.00 (95% CI: 0.00–0.43) to 0.45 (95% CI: 0.20–0.97) nymphs/100 m2 (Fig. 3). Similar to the distribution of high NIP clusters for Bo. burgdorferi s.s. and A. phagocytophilum, we detected a cluster of 13 sites with high Ba. microti NIP [P < 0.0001, NIP range: 0.0% (95% CI: 0.0–49.0%) to 28.8% (95% CI: 12.5–50.9%)], near the northwestern extent of our study area (Fig. 2). Among the high prevalence sites, 11.4% (35 of 308) of ticks tested positive for Ba. microti. Similar to A. phagocytophilum, we identified a cluster of 15 sites on the south-central fringe of our sampling sites, with significantly lower than expected NIP with Ba. microti. None of the 315 nymphs tested from this low prevalence cluster were positive for Ba. microti (P < 0.0001; NIP 95% CI: 0.0–79.4%).
3.5. Nymphal infection prevalence of Borrelia miyamotoi
Thirteen nymphs (1.05%) from ten (16%) sites tested positive for Bo. miyamotoi (Table 1, Fig. 2). We detected infected nymphs in Becker, Hubbard, Clearwater, Cass, Morrison, Pine, Anoka, and Dakota counties. The statewide NIP for Bo. miyamotoi was 1.05% (95% CI: 0.59–1.74%). NIP ranged from 0% (95% CI: 0–79%) to a high of 10% (95% CI: 8–40%) at one site where one of ten nymphs tested positive. By site of collection, DIN was never greater than 0.41 nymph/100 m2 (95% CI: 0.11–1.38; Fig. 3). We did not detect any significant spatial clustering of sites with low or high Bo. miyamotoi NIP.
3.6. Nymphal infection prevalence of Ehrlichia muris eauclairensis
Sixteen out of 1240 (1.29%) I. scapularis nymphs collected from 13 of 64 sites (20%) tested positive for E. muris eauclairensis (Fig. 2). We detected positive ticks in nine counties, from sites northwest of the Minneapolis/St. Paul metropolitan area (Fig. 2). The statewide infection rate for E. muris was 1.29% (95% CI: 0.77–2.04%) and per site, the density of nymphs infected with E. muris was never higher than 0.35 nymphs/100m2 (95% CI: 2.10–16.52; Fig. 2). We did not detect any significant spatial clustering of sites with low or high NIP.
3.7. Nymphal infection prevalence of Powassan virus lineage II/deer tick virus
A total of six ticks tested positive for Powassan virus. The statewide pooled infection rate for Powassan virus was 0.49% (95% CI: 0.20–1.01%; Table 1), NIP ranged from 0% (95% CI: 00–79%) to a high of 15% (95% CI: 4–42%) at one site where two of thirteen nymphs tested positive (Fig. 2). Four of 64 sites (6%) yielded ticks that tested positive for Powassan virus; these were located in Cass, Crow Wing, Hubbard, and Kanabec counties. The density of nymphs infected with Powassan virus was never greater than 0.23 nymph/100 m2 (95% CI: 0.06–0.62; Fig. 3). Sites with elevated NIP of Powassan virus were clustered among northcentral and east central study areas [P = 0.04, NIP range: 0.0% (95% CI: 0.0–56.2%) to 15.4% (95% CI: 4.3–42.2%); Fig. 2]; six of 413 nymphs (1.5%) from this cluster tested positive. The nymphs in this study were originally collected and processed for bacterial and protozoal pathogen detection; because our methods were not optimized for viral RNA, the Powassan virus results may represent an overall underestimation of the prevalence of the virus in Minnesota.
3.8. Nymphal coinfections prevalence
We detected coinfections with two or more pathogens in ticks collected from 32 of 64 collection sites. While infections with a single pathogen were most common occurring in 30.32% (376 of 1240) of nymphs tested, coinfections with two pathogens were detected in 5.40% of nymphs and infections with > 2 pathogens were detected in 2.02% of all nymphs tested. Overall, coinfections with two or more pathogens were detected in 7.26% of nymphs tested [90 of 1240 tested (1233 nymphs were tested for Powassan virus); Table 2]. Coinfections between Bo. burgdorferi s.s. and A. phagocytophilum (49 coinfected nymphs; 3.95%) or Ba. microti (45 coinfected nymphs; 3.63%) were most common (Table 2).
Table 2.
Coinfections with two or more of the seven pathogens of Ixodes scapularis nymphs tested, Minnesota, May 31–June 30, 2015.
| Pathogensa | No. sites with coinfected ticks (N=64 sites) | No. ticks coinfected (N=1240b) |
|---|---|---|
| Bb/Aph | 15 | 25 |
| Bb/Bam | 15 | 21 |
| Bb/Aph/Bam | 10 | 19 |
| Bb/Eme | 8 | 10 |
| Bb/Bmay | 2 | 2 |
| Aph/Bam | 2 | 2 |
| Bb/Bmiy/Aph | 2 | 2 |
| Bb/Bmiy | 1 | 1 |
| Bmay/Bmiy | 1 | 1 |
| Bmiy/Bam | 1 | 1 |
| Bb/Bmay/Bam | 1 | 1 |
| Bb/Bam/POWV | 1 | 1 |
| Aph/Bam/Eme | 1 | 1 |
| Bb/Aph/Bam/Eme | 1 | 1 |
| Bb/Bmiy/Aph/Bam | 1 | 1 |
| Bb/Bmay/Aph/Bam | 1 | 1 |
Bb = Borrelia burgdorferi sensu stricto; Aph = Anaplasma phagocytophilum; Bam = Babesia microti; Eme = Ehrlichia muris eauclairensis; Bmay = Borrelia mayonii; Bmiy = Borrelia miyamotoi, POWV=Powassan virus (lineage II, deer tick virus).
1240 nymphs were tested for all pathogens except Powassan virus (N=1233).
We identified a cluster of 13 sites with coinfection prevalence higher than surrounding sites [P < 0.0001, range: 2.1% (95% CI: 0.1–9.6%) to 36.4% (95% CI: 13.2–65.8%)]. These sites were situated near the northwestern extent of our study area (Fig. 2). In total, 53 of 355 (14.9%) ticks from these sites were coinfected with two or more pathogens. We also identified a cluster of 13 sites with coinfection prevalence lower than surrounding sites [P < 0.0001, range: 0.0% (95% CI: 0.0–79.4%) to 2% (95% CI: 0.1–9.3%)]; only 1 of 315 (0.3%) nymphs tested from these sites was coinfected (Fig. 2). The distribution of sites with elevated nymphal coinfection prevalence (NcIP) was geographically similar to the distributions of sites with elevated NIP for Bo. burgdorferi s.s., A. phagocytophilum and Ba. microti (Fig. 2).
4. Discussion
We detected each of the seven human pathogens which I. scapularis is known to vector from host-seeking nymphs collected in Minnesota. Mirroring human case counts, Bo. burgdorferi s.s. was the most common pathogen detected in ticks, followed by A. phagocytophilum and Ba. microti. The distribution of densities of host-seeking nymphs infected with these pathogens was similar to the reported distribution of Lyme disease, anaplasmosis and babesiosis cases, with the majority of cases and infected ticks situated in the north-central portion of the state (Robinson et al., 2015; http://www.health.state.mn.us/divs/idepc/newsletters/dcn/sum15/lyme.html). Among the pathogens for which we have limited data on case distributions (Bo. mayonii, Bo. miyamotoi, and E. muris eauclairensis) we did not detect any statistically significant spatial distribution patterns and each was detected at low prevalence in host-seeking nymphs.
The three most commonly detected pathogens, Bo. burgdorferi s.s., A. phagocytophilum, and Ba. mictroi, had similar geographical distributions and high prevalence clusters detected in north-central counties, including Clearwater, Becker, Otter Tail, and Hubbard (Figs. 2 and 3). As a result, coinfections with these pathogens occurred at higher rates in this region compared with other parts of the state. Similar to other studies conducted in Lyme disease endemic areas, Bo. burgdorferi s.s. was the most commonly detected pathogen, found at substantially higher rates than A. phagocytophilum or Ba. microti (Telford et al., 1996; Varde et al., 1998). The white-footed mouse (Peromyscus leucopus) is a reservoir of each of these pathogens (Mather et al., 1990; Spielman et al., 1981). Both Bo. burgdorferi s.s.- and Ba. microti-infected P. leucopus can remain infectious to feeding ticks for long periods of time (Mather et al., 1990; Spielman et al., 1981; Telford et al., 1996). However, larval acquisition of Ba. microti is inefficient and transstadial survival from larvae to nymphs is low compared with Bo. burgdorferi (Dunn et al., 2014; Mather et al., 1990), explaining why Ba. microti is less common in host-seeking nymphs compared with Bo. burgdorferi s.s. Infectiousness of A. phagocytophilum in P. leucopus is transient compared with Bo. burgdorferi s.s. or Ba. microti (Levin and Ross, 2004; Stafford et al., 1999; Telford et al., 1996). As a result, the opportunity for larval ticks to acquire A. phagocytophilum is abbreviated compared with the other pathogens, likely resulting in fewer ticks acquiring infection. However, larval acquisition rates and transstadial survival from larva to nymph appear to be efficient for A. phagocytophilum (Levin and Ross, 2004). Together, these characteristics might explain why A. phagocytophilum is less prevalent than Bo. burgdorferi s.s., but similar to Ba. microti.
Prior to our study, the recently described Lyme disease-causing spirochete, Bo. mayonii, was detected in I. scapularis or was cultured from small mammals collected in three counties, Cass, Morrison, and Pine (Johnson et al., 2017; Pritt et al., 2016a, b). We report Bo. mayonii in ticks collected in six additional counties (Becker, Clearwater, Houston, Hubbard, Mille Lacs, and Otter Tail), bringing the total number of counties from which the spirochete has been reported in ticks or hosts to nine. The low infection prevalence documented in I. scapularis nymphs (0.65%) is consistent with the rarity of human Bo. mayonii infections reported to date. Pritt et al. (2016b) detected Bo. mayonii in only six of 9197 clinical samples obtained from residents of Minnesota, Wisconsin and North Dakota. Why Bo. mayonii occurs at low prevalence compared with closely-related Bo. burgdorferi s.s. is unclear, but may be related to differences in transstadial survival efficiency (Dolan et al., 2016; Piesman and Sinsky, 1988), or pathogen-host dynamics. Although Bo. mayonii has been isolated from white-footed mice (Peromyscus leucopus) and an American red squirrel (Tamiasciurus hudsonicus), reservoir competence has not yet been demonstrated for any potential natural reservoirs (Johnson et al., 2017). One study demonstrated that outbred laboratory mice are competent reservoirs that remain infectious to feeding ticks for at least a year, but infection rates in xenodiagnostic ticks were highly variable among mice and time points (Dolan et al., 2017). Additional studies are needed to identify potential natural reservoirs for Bo. mayonii and to determine the duration of infectivity and efficiency of transmission to feeding ticks.
Like Bo. mayonii, E. muris eauclairensis appears to be restricted to the upper Midwest, specifically to Minnesota and Wisconsin (Johnson et al., 2015). Based on the peak of human illness onsets in June and July, I. scapularis nymphs are assumed to be the primary bridging vector to people (Johnson et al., 2015). Despite finding no statistically significant clustering of sites with high prevalence of E. muris eauclairensis infection in host-seeking nymphs, the 13 sites that were positive for this pathogen followed a distribution similar to that of suspected human ehrlichiosis exposure sites, primarily north and northwest of the Minneapolis-St. Paul metropolitan area (Johnson et al., 2015). Reasons for low infection prevalence are unclear, but may be explained by the relatively short duration of infectivity in reservoir hosts (Lynn et al., 2017).
Our B. miyamotoi results were comparable to those of a recent study of similar design that explored the distribution of Bo. miyamotoi in I. pacificus nymphs in Mendocino County, California. Both studies detected Bo. miyamotoi at low prevalence in host-seeking nymphs, with neither study identifying any significant patterns in the spatial distribution of Bo. miyamotoi-infected ticks. In general, despite vastly different host community composition between regions, Bo. miyamotoi appears to be widespread, yet detected at low prevalence, in Ixodes spp. ticks in Europe and the United States (Crowder et al., 2014; Wagemakers et al., 2015). Although horizontal transmission is likely required to maintain the pathogen in natural cycles, there may not be strong amplifying reservoir hosts, and it is possible that transovarial transmission instead plays a major role in B. miyamotoi persistence (Lynn et al., 2018). Similar to Bo. miyamotoi, Powassan virus can be transovarially transmitted (Costero and Grayson, 1996), is widespread, and is found at low prevalence in I. scapulars (Ebel, 2010). Although we did not assess host community composition among study sites, this may explain the observed clustering of sites with higher than expected prevalence of Powassan virus in host-seeking nymphs. For example, the deer tick lineage of Powassan virus is maintained primarily in a whitefooted mouse (Peromyscus leucopus) I. scapularis enzootic cycle (Ebel, 2010). If some characteristics of the landscape make it more or less suitable to high densities of mice, POWv infection prevalence may be expected to be elevated. Although we did not assess landscape features or host communities, it is possible for instance that the landscape is much more fragmented in this portion of the state leading to higher edge densities, which have been shown to be preferred habitat for white-footed mice (Anderson et al., 2003). This portion of Minnesota is characterized by mixed vegetation of cool temperate forests and lowland and montane boreal forests (Johnson et al., 2016).
Our results reveal substantial heterogeneity in acarological risk for exposure to seven human pathogens. Patterns in the spatial distribution and prevalence of Bo. burgdorferi s.s., A. phagocytophilum, and Ba. microti are consistent with the distribution and incidence of reported cases of Lyme disease, anaplasmosis, and babesiosis in Minnesota. Our data suggest that acarological risk of exposure to I. scapularis-borne pathogens other than Bo. burgdorferi s.s. is relatively low throughout the state. Nonetheless, given the diversity of pathogens infecting host-seeking ticks and their prevalence, this study underscores the importance of prevention strategies that aim to reduce the risk of tick bites in the state of Minnesota.
Supplementary Material
Acknowledgements
We thank numerous individuals for assisting with tick collection: Franny Dorr and several student workers from the Vector-borne Disease Unit (Minnesota Department of Health); Sonia Kjos, Ramsey Hess, Melissa Tekippe, and Steve Waring (University of Minnesota, Duluth); Jeanne Minnerath, Haley Colton, and Sarah Fanning (St. Mary’s University of Minnesota); Steven Windels (Voyageurs National Park); Micah Hahn and Rebecca Clark (Centers for Disease Control and Prevention, Fort Collins, Colorado). We thank Kalanthe Horiuchi (Centers for Disease Control and Prevention, Fort Collins, Colorado) for statistical advice.
Footnotes
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ttbdis.2018.07.009.
References
- Aliota MT, Dupuis AP 2nd, Wilczek MP, Peters RJ, Ostfeld RS, Kramer LD, 2014. The prevalence of zoonotic tick-borne pathogens in Ixodes scapularis collected in the Hudson Valley, New York State. Vector Borne Zoonotic Dis. 14, 245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allerdice ME, Pritt BS, Sloan LM, Paddock CD, Karpathy SE, 2016. A real-time PCR assay for detection of the Ehrlichia muris-like agent, a newly recognized pathogen of humans in the upper Midwestern United States. Ticks Tick-Borne Dis. 7, 146–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CS, Cady AB, Meikle DB, 2003. Effects of vegetation structure and edge habitat on the density and distribution of white-footed mice (Peromyscus leucopus) in small and large forest patches. Can. J. Zool 81, 897–904. [Google Scholar]
- Biggerstaff BJ, 2009. PooledInfRate, Version 4.0: A Microsoft® Office Excel© Add-In to Compute Prevalence Estimates from Pooled Samples. CDC, Fort Collins, CO. [Google Scholar]
- Birge J, Sonnesyn S, 2012. Powassan virus encephalitis, Minnesota, USA. Emerg. Infect. Dis 18, 1669–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP, 1982. Lyme disease-a tick-borne spirochetosis? Science 216, 1317–1319. [DOI] [PubMed] [Google Scholar]
- Coley K, 2015. Identification Guide to Larval Stages of Ticks of Medical Importance in the USA. University Honors Program Thesis. Available at. Georgia Southern University; https://digitalcommons.georgiasouthern.edu/. [Google Scholar]
- Costero A, Grayson MA, 1996. Experimental transmission of Powassan virus (Flaviviridae) by Ixodes scapularis ticks (Acari: Ixodidae). Am. J. Trop. Med. Hyg. 55, 536–546. [DOI] [PubMed] [Google Scholar]
- Crowder CD, Carolan HE, Rounds MA, Honig V, Mothes B, Haag H, Nolte O, Luft BJ, Grubhoffer L, Ecker DJ, Schutzer SE, Eshoo MW, 2014. Prevalence of Borrelia miyamotoi in Ixodes ticks in Europe and the United States. Emerg. Infect. Dis 20, 1678–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- des Vignes F, Piesman J, Heffernan R, Schulze TL, Stafford KC III, Fish D, 2001. Effect of tick removal on transmission of Borrelia burgdorferi and Ehrlichia phagocytophila by Ixodes scapularis nymphs. J. Infect. Dis 183, 773–778. [DOI] [PubMed] [Google Scholar]
- Dolan MC, Hojgaard A, Hoxmeier JC, Replogle AJ, Respicio-Kingry LB, Sexton C, Williams MA, Pritt BS, Schriefer ME, Eisen L, 2016. Vector competence of the blacklegged tick, Ixodes scapularis, for the recently recognized Lyme borreliosis spirochete Candidatus Borrelia mayonii. Ticks Tick-Borne Dis. 7, 665–669. [DOI] [PubMed] [Google Scholar]
- Dolan MC, Breuner NE, Hojgaard A, Hoxmeier JC, Pilgard MA, Replogle AJ, Eisen L, 2017. Duration of Borrelia mayonii infectivity in an experimental mouse model for feeding Ixodes scapularis larvae. Ticks Tick-Borne Dis. 8, 196–200. [DOI] [PubMed] [Google Scholar]
- Dunn JM, Krause PJ, Davis S, Vannier EG, Fitzpatrick MC, Rollend L, Belperron AA, States SL, Stacey A, Bockenstedt LK, Fish D, Diuk-Wasser MA, 2014. Borrelia burgdorferi promotes the establishment of Babesia microti in the Northeastern United States. PLoS One 9, e115494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durden LA, Keirans JE, 1996. Nymphs of the Genus Ixodes (Acari: Ixodidae) of the United States: Taxonomy, Identification Key, Distribution, Hosts, and medical/veterinary Importance. Entomological Society of America, USA. [Google Scholar]
- Ebel GD, 2010. Update on Powassan virus: emergence of a North American tick-borne flavivirus. Annu. Rev. Entomol. 55, 95–110. [DOI] [PubMed] [Google Scholar]
- Eisen RJ, Eisen L, 2018. The Blacklegged Tick, Ixodes scapularis: an increasing public health concern. Trends Parasitol. 34, 295–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Eisen L, Beard CB, 2016. County-scale distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the continental United States. J. Med. Entomol 53, 349–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falco RC, McKenna DF, Daniels TJ, Nadelman RB, Nowakowski J, Fish D, Wormser GP, 1999. Temporal relation between Ixodes scapularis abundance and risk for Lyme disease associated with erythema migrans. Am. J. Epidemiol 149, 771–776. [DOI] [PubMed] [Google Scholar]
- Graham CB, Pilgard MA, Maes SE, Hojgaard A, Eisen RJ, 2016. Paired real-time PCR assays for detection of Borrelia miyamotoi in North American Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae). Ticks Tick-Borne Dis. 7, 1230–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham CB, Maes SE, Hojgaard A, Fleshman AC, Sheldon SW, Eisen RJ, 2018. A molecular algorithm to detect and differentiate human pathogens infecting Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae). Ticks Tick-Borne Dis. 9, 390–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MB, Bjork JKH, Neitzel DF, Dorr FM, Whitemarsh T, Boegler KA, Graham CB, Johnson TL, Maes SE, Eisen RJ, 2017. Evaluating acarological risk for exposure to Ixodes scapularis and Ixodes scapularis-borne pathogens in recreational and residential settings in Washington County, Minnesota. Ticks TickBorne Dis. 9, 340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Zhu L, Kulldorff M, Hostovich S, Stinchcomb DG, Tatalovich Z, Lewis DR, Feuer EJ, 2016. Using Gini coefficient to determining optimal cluster reporting sizes for spatial scan statistics. Int. J. Health Geogr 15, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojgaard A, Lukacik G, Piesman J, 2014. Detection of Borrelia burgdorferi, Anaplasma phagocytophilum and Babesia microti, with two different multiplex PCR assays. Ticks Tick-Borne Dis. 5, 349–351. [DOI] [PubMed] [Google Scholar]
- Johnson RC, Kodner C, Jarnefeld J, Eck DK, Xu Y, 2011. Agents of human anaplasmosis and Lyme disease at Camp Ripley, Minnesota. Vector-Borne Zoonotic Dis. 11, 1529–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DK, Schiffman EK, Davis JP, Neitzel DF, Sloan LM, Nicholson WL, Fritsche TR, Steward CR, Ray JA, Miller TK, Feist MA, Uphoff TS, Franson JJ, Livermore AL, Deedon AK, Theel ES, Pritt BS, 2015. Human Infection with Ehrlichia muris-like Pathogen, United States, 2007–2013. Emerg. Infect. Dis 21, 1794–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T, Bjork JKH, Neitzel DF, Dorr FM, Schiffman EK, Eisen RE, 2016. Habitat suitability model for the distribution of Ixodes scapularis (Acari: Ixodidae) in Minnesota. J. Med. Entomol 53, 598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TL, Graham CB, Hojgaard A, Breuner NE, Maes SE, Boegler KA, Replogle AJ, Kingry LC, Petersen JM, Eisen L, Eisen RJ, 2017. Isolation of the Lyme disease spirochete Borrelia mayonii from naturally infected rodents in Minnesota. J. Med. Entomol 54, 1088–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TL, Boegler KA, Clark RJ, Delorey MJ, Bjork JKH, Dorr FM, Schiffman EK, Neitzel DF, Monaghan AJ, Eisen RJ, 2018. An acarological risk model predicting the density and distribution of host-seeking Ixodes scapularis nymphs in Minnesota. Am. J. Top. Med. Hyg 98, 1671–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirans JE, Litwak TR, 1989. Pictorial key to the adults of hard ticks, family Ixodidae (Ixodida: Ixodoidea), east of the Mississippi River. J. Med. Entomol 26, 435–448. [DOI] [PubMed] [Google Scholar]
- Kulldorff M, 1997. Bernoulli, discrete Poisson and continuous Poisson models. Commun. Stat. Theor. Methods 1481–1496. [Google Scholar]
- Levin ML, Ross DE, 2004. Acquisition of different isolates of Anaplasma phagocytophilum by Ixodes scapularis from a model animal. Vector-Borne Zoonotic Dis. 4, 53–59. [DOI] [PubMed] [Google Scholar]
- Lynn GE, Oliver JD, Cornax I, O’Sullivan MG, Munderloh UG, 2017. Experimental evaluation of Peromyscus leucopus as a reservoir host of the Ehrlichia muris-like agent. Parasit. Vectors 10, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn GE, Graham CB, Horiuchi K, Eisen L, Johnson TL, Lane RS, Eisen RJ, 2018. Prevalence and geographic distribution of Borrelia miyamotoi in host-seeking Ixodes pacificus (Acari: Ixodidae) nymphs in Mendocino County, California. J. Med. Entomol, tjx258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margos G, Binder K, Dzaferovic E, Hizo-Teufel C, Sing A, Wildner M, Fingerle V, Jolley KA, 2015. PubMLST.org-the new home for the Borrelia MLSA database. Ticks Tick-Borne Dis. 6, 869. [DOI] [PubMed] [Google Scholar]
- Mather TN, Telford SR 3rd, Moore SI, Spielman A, 1990. Borrelia burgdorferi and Babesia microti: efficiency of transmission from reservoirs to vector ticks (Ixodes dammini). Exp. Parasitol 70, 55–61. [DOI] [PubMed] [Google Scholar]
- Mather TN, Nicholson MC, Donnelly EF, Matyas BT, 1996. Entomologic index for human risk of Lyme disease. Am. J. Epidemiol 144, 1066–1069. [DOI] [PubMed] [Google Scholar]
- Mead PS, 2015. Epidemiology of Lyme disease. Infect. Dis. Clin. North Am 29, 187–210. [DOI] [PubMed] [Google Scholar]
- Merten HA, Durden LA, 2000. A state-by-state survey of ticks recorded from humans in the United States. J. Vector Ecol 25, 102–113. [PubMed] [Google Scholar]
- Pepin KM, Eisen RJ, Mead PS, Piesman J, Fish D, Hoen AG, Barbour AG, Hamer S, Diuk-Wasser MA, 2012. Geographic variation in the relationship between human Lyme disease incidence and density of infected host-seeking Ixodes scapularis nymphs in the Eastern United States. Am. J. Trop. Med. Hyg 86, 1062–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piesman J, 1993. Dynamics of Borrelia burgdorferi transmission by nymphal Ixodes dammini ticks. J. Infect. Dis 167, 1082–1085. [DOI] [PubMed] [Google Scholar]
- Piesman J, Sinsky RJ, 1988. Ability to Ixodes scapularis, Dermacentor variabilis, and Amblyomma americanum (Acari: Ixodidae) to acquire, maintain, and transmit Lyme disease spirochetes (Borrelia burgdorferi). J. Med. Entomol 25, 336–339. [DOI] [PubMed] [Google Scholar]
- Pritt BS, Sloan LM, Johnson DK, Munderloh UG, Paskewitz SM, McElroy KM, McFadden JD, Binnicker MJ, Neitzel DF, Liu G, Nicholson WL, Nelson CM, Franson JJ, Martin SA, Cunningham SA, Steward CR, Bogumill K, Bjoorgaard ME, Davis JP, McQuiston JH, Warshauer DM, Wilhelm MP, Patel E, Trivedi VA, Eremeeva ME, 2011. Emergence of a new pathogenic Ehrlichia species, Wisconsin and Minnesota, 2009. N. Engl. J. Med 365, 422–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritt BS, Mead PS, Johnson DK, Neitzel DF, Respicio-Kingry LB, Davis JP, Schiffman E, Sloan LM, Schriefer ME, Replogle AJ, Paskewitz SM, Ray JA, Bjork J, Steward CR, Deedon A, Lee X, Kingry LC, Miller TK, Feist MA, Theel ES, Patel R, Irish CL, Petersen JM, 2016a. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect. Dis 16, 556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritt BS, Respicio-Kingry LB, Sloan LM, Schriefer ME, Replogle AJ, Bjork J, Liu G, Kingry LC, Mead PS, Neitzel DF, Schiffman E, Johnson DKH, Davis JP, Paskewitz SM, Boxrud D, Deedon A, Lee X, Miller TK, Feist MA, Steward CR, Theel ES, Patel R, Irish CL, Petersen JM, 2016b. Borrelia mayonii sp. nov., a member of the Borrelia burgdorferi sensu lato complex, detected in patients and ticks in the upper midwestern United States. Int. J. Syst. Evol. Microbiol 66, 4878–4880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SJ, Neitzel DF, Moen RA, Craft ME, Hamilton KE, Johnson LB, Mulla DJ, Munderloh UG, Redig PT, Smith KE, Turner CL, Umber JK, Pelican KM, 2015. Disease risk in a dynamic environment: the spread of tick-borne pathogens in Minnesota, USA. Ecohealth 12, 152–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz AM, Hinckley AF, Mead PS, Hook SA, Kugeler KJ, 2017. Surveillance for Lyme disease - United States, 2008–2015. MMWR Surveill. Summ 66, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza SS, Bishop HS, Sprinkle P, Qvarnstrom Y, 2016. Comparison of Babesia microti real-time polymerase chain reaction assays for confirmatory diagnosis of babesiosis. Am. J. Trop. Med. Hyg 65, 1413–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielman A, 1976. Human babesiosis on Nantucket Island: transmission by nymphal Ixodes ticks. Am. J. Trop. Med. Hyg 25, 784–787. [DOI] [PubMed] [Google Scholar]
- Spielman A, Etkind P, Piesman J, Ruebush TK 2nd, Juranek DD, Jacobs MS, 1981. Reservoir hosts of human babesiosis on Nantucket Island. Am. J. Trop. Med. Hyg 30, 560–565. [DOI] [PubMed] [Google Scholar]
- Stafford KC, Cartter ML, Magnarelli LA, Ertel S-H, Mshar PA, 1998. Temporal correlations between tick abundance and prevalence of ticks infected with Borrelia burgdorferi and increasing incidence of Lyme disease. J. Clin. Microbiol 36, 1240–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford KC 3rd, Massung RF, Magnarelli LA, Ijdo JW, Anderson JF, 1999. Infection with agents of human granulocytic ehrlichiosis, lyme disease, and babesiosis in wild white-footed mice (Peromyscus leucopus) in Connecticut. J. Clin. Microbiol 37, 2887–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromdahl E, Hamer S, Jenkins S, Sloan L, Williamson P, Foster E, Nadolny R, Elkins C, Vince M, Pritt B, 2014. Comparison of phenology and pathogen prevalence, including infection with the Ehrlichia muris-like (EML) agent, of Ixodes scapularis removed from soldiers in the midwestern and the northeastern United States over a 15 year period (1997–2012). Parasit. Vectors 7, 553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telford SR 3rd, Dawson JE, Katavolos P, Warner CK, Kolbert CP, Persing DH, 1996. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc. Natl. Acad. Sci 93, 6209–6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varde S, Beckley J, Schwartz I, 1998. Prevalence of tick-borne pathogens in Ixodes scapularis in a rural New Jersey County. Emerg. Infect. Dis 4, 97–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagemakers A, Staarink PJ, Sprong H, Hovius JW, 2015. Borrelia miyamotoi: a widespread tick-borne relapsing fever spirochete. Trends Parasitol. 31, 260–269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.