Abstract
Several studies have identified the aryl hydrocarbon receptor (AhR) as a negative regulator of the innate and adaptive immune responses. However, the molecular mechanisms by which this transcription factor exerts such modulatory effects are not well understood. Interaction between AhR and RelA/p65 has previously been reported. RelA/p65 is the major NFκB subunit that plays a critical role in immune responses to infection. The aim of the present study was to determine whether the activation of AhR disrupted RelA/p65 signaling in mouse peritoneal macrophages by decreasing its half-life. The data demonstrate that the activation of AhR by TCDD and β-naphthoflavone (β-NF) decreased protein levels of the pro-inflammatory cytokines TNF-α, IL-6 and IL-12 after macrophage activation with LPS/IFNγ. In an AhR-dependent manner, TCDD treatment induces RelA/p65 ubiquitination and proteosomal degradation, an effect dependent on AhR transcriptional activity. Activation of AhR also induced lysosome-like membrane structure formation in mouse peritoneal macrophages and RelA/p65 lysosome-dependent degradation. In conclusion, these results demonstrate that AhR activation promotes RelA/p65 protein degradation through the ubiquitin proteasome system, as well as through the lysosomes, resulting in decreased pro-inflammatory cytokine levels in mouse peritoneal macrophages.
Keywords: Aryl hydrocarbon receptor, Macrophage, Ubiquitination, RelA/p65 inflammatory cytokines
1. Introduction
The aryl hydrocarbon receptor (AhR) is a ligand-dependent transcription factor and a member of the bHLH-PAS (basic-helix-loop-helix, Per-Arnt-Sim) superfamily. Upon binding to its ligands, AhR translocates from the cytoplasm to the nucleus where it binds to xenobiotic response elements (XREs) located in the promoter of its target genes, such as cytochrome P450s (CYP1A1, CYP1A2, CYP1B1), NAD(P)H quinone oxidoreductase, and UDP-glucuronosyl-transferase 6 [1]. In addition to its role in xenobiotic metabolism, AhR has been implicated in several cellular processes, including liver development [2], neurogenesis [3], cholesterol and glucose metabolism [4,5], cell proliferation and apoptosis [6], and in the homeostasis of the immune system [7-9]. It was shown that AhR modulates the adaptive and innate immune responses and has been implicated in the differentiation of IL-17-producing helper T cells and Treg cells [10]. AhR might act as a negative regulator of the immune system according to several studies. Ahr-null mouse lymphocytes produce more IFNγ and IL-12 compared to wild-type (WT) lymphocytes [11]. Moreover, after IFNγ and LPS activation, Ahr-null mouse macrophages produce higher levels of IL-1β, IL-6, IL-12, and TNF-α than do WT macrophages [12,13]. The latter might explain why Ahr-null mice are more susceptible to endotoxic shock induced by LPS [12] and exhibit an exacerbated immune response against Leishmania major and Toxoplasma gondii infections [14,15]. However, the molecular mechanisms by which AhR modulates macrophage pro-inflammatory cytokine levels are not well understood.
Rapid activation of an acute inflammatory response in macrophages is essential for innate immunity and host defense. Acute macrophage activation is mediated by receptors that sense microbial products and activate inflammatory signaling pathways such as Toll-like receptors (TLRs) [16]. In particular, TLR4 activation leads to the production of cytokines and other inflammatory mediators, including IL-1, IL-6, TNF-α, IL-12 and IFNs [17]. TLR4 signaling occurs via the activation of nuclear factor kappa light-chain-enhancer of activated B cells (NF-κB), in particular RelA/p65 [18], which is typically involved in the inflammatory response. It was shown that Ahr-null alveolar cells present increased NF-κB activity compared to WT cells [19]. Moreover, interaction between NF-κB and AhR was observed. In particular, the NF-κB subunit RelB was found to be physically associated with AhR in U937 macrophage [20], while Tian and collaborators demonstrated a physical association between AhR and RelA/p65 in Hepa1c1c7 cells treated with TCDD [21].
The above information suggests a crosstalk between NFκB and AhR pathways that may explain the negative modulation exerted by the AhR on the levels of inflammatory cytokines. On the other hand, we have shown that AhR activation decrease p53 and c-Fos levels by inducing their ubiquitination and proteosomal degradation [22,23]. The foregoing leads us to propose that the AhR promote the degradation of RelA/p65 in a similar way as it does with p53 and c-Fos.
Therefore, the goal of the present study was to evaluate whether the activation of AhR disrupted RelA/p65 signaling by decreasing the RelA/p65 protein levels and/or nuclear internalization in mouse macrophages.
2. Material and methods
2.1. Materials
Fetal bovine serum (FBS) was purchased from Hyclone (Logan, UT, USA). RPMI-1640 media, L-glutamine, non-essential amino acids, antibiotics/antimycotics, and sodium pyruvate were purchased from Invitrogen Life Technologies (Carlsbad, CA, USA). 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) was obtained from AccuStandard (New Haven, CT, USA). β-Naphthoflavone (βNF), lipopolysaccharides (LPS), MG132 (Z-Leu-Leu-Leu-al), actinomycin D (Act D), dimethyl sulfoxide (DMSO), chloroquine (CHL), cycloheximide (CHX) and bovine serum albumin (BSA) were purchased from Sigma–Aldrich (St. Louis, MO, USA), and recombinant mouse IFNγ was obtained from BD Pharmingen (San Jose, CA, USA).
2.2. Animals
The previously described Ahr-null mice [7] were genotyped by polymerase chain reaction (PCR). Briefly, genomic DNA was extracted from the tail tip using an extraction buffer containing 1.6 μg of proteinase K, 1 M Tris-HCl, pH 8.5, 1 M NaCl, 0.5 M EDTA and 20% SDS. To generate a 450-bp fragment (corresponding to the WT) or a 1.5-kb fragment (corresponding to the Ahr-null), the following primers were used: forward: 5′-GGCTAGCGTGCGGGTTTCTC-3′ and reverse: 5′-CTA GAACGGCACTAGGTAGGTCAG-3′. The PCR reaction conditions were as follows: 3 min of denaturation at 94 °C, 30 cycles of 94 °C for 1 min, 60 °C for 1 min, and 72 °C for 2.5 min, followed by 72 °C for 10 min for the final extension. The resulting PCR products were subjected to electrophoresis in a 2% agarose gel containing ethidium bromide and visualized by UV trans-illumination. WT littermates were used as control mice. C57BL/6 mice were housed in a pathogen-free facility, fed with autoclaved Purina rodent chow (St. Louis, MO, USA), and provided with water ad libitum. All animal studies were performed according to the Guide for the Care and Use of Laboratory Animals, as adopted and promulgated by the U.S. National Institutes of Health and the Mexican Regulation of Animal Care and Maintenance (NOM-062-ZOO-1999, 2001).
2.3. Cell culture and stimulation
Resident macrophages were harvested from the peritoneal cavity of 10- to 13-week-old female mice. Briefly, 10 mL of ice-cold sterile PBS pH 7.4 was injected into the peritoneal cavity, and the area massaged for 5 min. Cells were collected from five to six mice, pooled, and collected by centrifugation at 2000 rpm for 10 min at 4 °C and then washed twice. Cell viability was assessed by Trypan blue exclusion. Macrophages were adjusted to 1 × 106 cell/mL in RPMI-1640 media supplemented with 10% FBS, 2mM l-glutamine, 0.5% non-essential amino acids, 0.5% sodium pyruvate, 100 U/mL penicillin, and 100 μg/mL streptomycin. One milliliter of the cell suspension was transferred into a 12-well polystyrene tissue culture plate and incubated for 2 h at 37 °C in humidified air containing 5% CO2. The suspended cells were washed twice with RPMI-1640 media, and the remaining adherent macrophages were replenished with supplemented RPMI-1640 media. Macrophages were activated by adding LPS and IFNγ (1 μg/mL and 20 ng/mL, respectively, 30 min) and treated with βNF (1 μM), TCDD (10 nM) or CHL (10 μM) at the indicated times. DMSO was used as a vehicle (< 0.1%, v/v).
2.4. Cytokine quantification
After the macrophage stimulation and treatments, the supernatants were collected and secretion of TNF-α and IL-6 (OPtEIA ELISA KIT BD, San Jose, CA, USA) and secretion of IL-12/IL-23 (p40) (ELISA MAX Deluxe sets, BioLegend, San Diego, CA, USA) were determined by enzyme-linked immunosorbent assays (ELISAs), according to the manufacturer's instructions.
2.5. Western blotting
Cultured cells were lysed in 400 μL of buffer containing 50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA pH 7.4, 1% NP-40, 1% sodium deoxycholate, 10 mM NaF, 10 mM Na3VO4, 20 mM glycerol phosphate and minicomplete protease inhibitor cocktail (1 tablet/10 mL, Roche, Mannheim, Germany). The protein concentrations were determined using the Bradford reaction (Bio-Rad, Hercules, CA, USA). Aliquots (50 μg) were solubilized in sample buffer (60 mM Tris–HCl, pH 6.8, 2% sodium dodecyl sulfate (SDS), 20% glycerol, 2% mercaptoethanol, and 0.001% bromophenol blue) and subjected to 10% SDS-polyacrylamide gel electrophoresis at 100 V for 3 h. The proteins were transferred to a nitrocellulose membrane using a mini trans-blot device (Bio-Rad, Hercules, CA, USA) at a constant voltage of 100 V for 4h in transfer buffer (48 mM Tris–HCl, 39 mM glycine, pH 8.3, 20% methanol). Following the transfer, the membranes were blocked overnight at 4 °C in the presence of 2% nonfat dry milk and 0.5% bovine serum albumin (BSA) in blocking buffer (25 mM Tris–HCl, pH 7.5, 150 mM NaCl) and subsequently incubated at 4 °C overnight with anti-RelA/p65 (1:500, Cat: sc-372, Santa Cruz Biotechnology, CA, USA) or anti-phospho-RelA/p65 (Ser536) (1:4000, Cat: 3033, Cell Signaling Technology, Danvers MA, USA) or anti-actin (1:1000, Cat: sc-1616, Zymed, San Francisco, CA, USA) antibodies diluted in buffer (25 mM Tris–HCl at pH 7.5, 150 mM NaCl, 0.1% Tween-20, 0.05% nonfat dry milk, 0.05% BSA). After washing, the membranes were incubated with their corresponding horseradish peroxidase (HRP)-conjugated secondary antibodies: HRP-goat anti-rabbit IgG (1:2000, Cat: 81–6120, Thermo Fisher Scientific, Inc., Massachusetts, USA) or HRP-rabbit anti-goat IgG (1:2000, Cat: 31402, Thermo Fisher Scientific, Inc., Massachusetts, USA) for 3 h at 4 °C. The membranes were washed in TBS, and the immunoreactive proteins were detected using an ECL Western blotting detection kit (Millipore, Billerica, MA, USA). The integrated optical density of the bands was quantified by densitometry using ImageJ Software.
2.6. RelA/p65 ubiquitination assay
The capture of ubiquitinated proteins from peritoneal macrophage cultures was performed using tandem ubiquitin binding entities (TUBEs), as previously described [24]. Briefly, cell extracts were obtained using 400 μL of lysis buffer (50 mM sodium fluoride, 5 mM sodium pyrophosphate, 10 mM glycerol-2-phosphate, 0.2% Nonidet-P40, 2mM EDTA, 20 mM Na2HPO4, 20 mM NaH2PO4, 1 mM PMSF, and protease inhibitor cocktail Minicomplete [Roche Diagnostic, Indianapolis, IN, USA] at pH 7.5) complemented with 100 μg of TUBEs, previously obtained as described [25]. Subsequently, the lysates were centrifuged at 13,000 rpm for 10 min at 4 °C, and an aliquot of the supernatant corresponding to the input fraction was collected (50 μL). Next, 100 μL of glutathione agarose beads (Sigma–Aldrich, St. Louis, MO, USA) and 5 μL of 0.1 M DTT were added and incubated with slow agitation at 4 °C overnight. The samples were centrifuged at 12,000 rpm for 5 min at 4 °C, and an aliquot corresponding to the flow through fraction was collected. Finally, the pellet obtained in the previous step, corresponding to the bound fraction, was washed 3 times with 1 mL of 0.05% Tween-20. The fractions were loaded onto an SDS-PAGE gel, and RelA/p65 was detected by Western blotting, as described in Section 2.5.
2.7. Confocal microscopy
Peritoneal macrophages (5 × 105) were seeded into 35-mm dishes with glass coverslips. After 24 h, the cells were treated with LPS/IFNγ (1 μg/mL and 20 ng/mL, respectively) for 30 min or were pre-treated with 10 nM TCDD for 12 h before LPS/IFNγ followed by fixation with 3.5% paraformaldehyde in PBS for 2 h at 4 °C. The fixed cells were permeabilized with 0.05% Triton X-100 in PBS for 5 min and blocked for 1 h with 5% BSA at room temperature (RT). The cells were then incubated for 2 h with anti-phospho-RelA/p65 (Ser536) (1:50, Cat: 3033, Cell Signaling Technology, Danvers MA, USA) in 5% BSA. The primary antibody was detected with FITC-labeled goat anti-rabbit antibodies (1:100, Cat: 65; 6111, Thermo Fisher Scientific, Inc., Massachusetts, USA). F-actin was stained with rhodamine–phalloidin (Cat: R415, Thermo Fisher Scientific, Inc., Massachusetts, USA) for 15 min at RT and then mounted with Vectashield mounting medium with 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Inc., Burlingame, CA, USA). Images were captured with a Leica TCS SP8 confocal microscope (Leica Microsystems, Wetzlar, Germany).
2.8. Statistical analysis
The results are presented as the mean values ± the standard deviation (S.D.). The statistical significance of the data was evaluated using Student’s t-test. In all cases, the differences between groups were considered to be statistically significant when the p-value was < 0.05.
3. Results
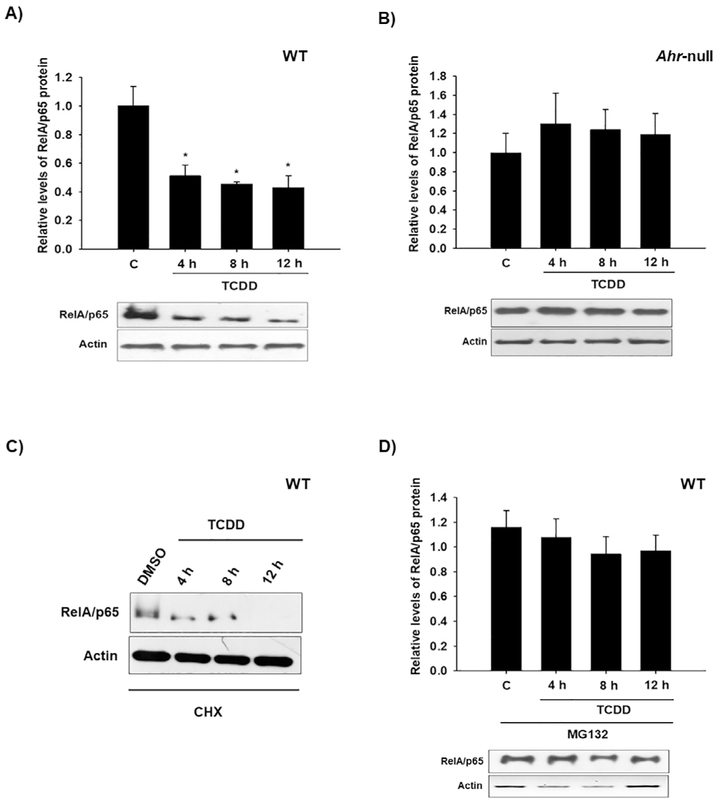
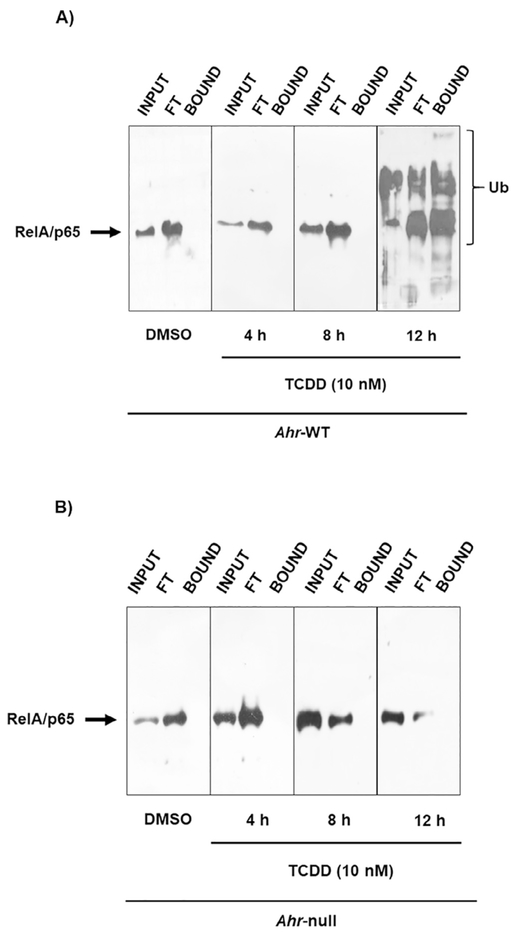
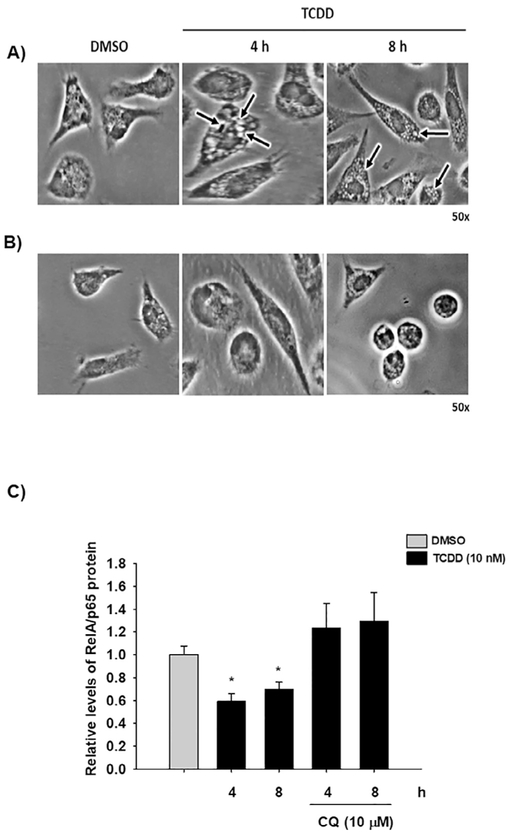
Several research groups have demonstrated the involvement of AhR in cytokine expression in macrophages [12,13,26]. However, the nature of such regulation is not yet well understood. The goal of the present study was to determine the mechanism by which AhR modulates cytokine levels. The effect of AhR activation by treatment with βNF and TCDD on cytokine protein levels was determined in WT macrophages. Both AhR ligands decreased LPS/IFNγ-induced TNF-α, IL-6. IL-12 protein levels were only decreased by TCDD treatment (Fig. 1). In contrast, similar treatments failed to block the LPS/IFNγ-induced pro-inflammatory cytokines in Ahr-null macrophages (Fig. 2). These results, together with previous data, establish the negative action of AhR on pro-inflammatory cytokine levels [11,14,26]. TNFα, IL-6, and IL-12 gene expression is under p65/p50 regulation, also known as RelA/p65 [27], which is the most frequently activated form of NF-κB in TLR signaling. Therefore, the effect of TCDD on RelA/p65 protein levels was evaluated. Peritoneal macrophages treated with TCDD resulted in decreased RelA/p65 protein levels. This effect was observed after 4 h of treatment, and was maintained for 12 h (Fig. 3A). In contrast, TCDD failed to decrease RelA/p65 protein levels when Ahr-null peritoneal macrophages were used (Fig. 3B). TCDD induced RelA/p65 degradation, though it was incomplete, this is most likely because RelA/p65 present a dynamic protein turnover, with a half-life of around 6 h [28]. Therefore, we investigated whether the inhibition of de novo protein synthesis exacerbates RelA/p65 protein degradation after AhR activation. Peritoneal macrophages were treated with 10 nM TCDD for 4, 8, and 12 h, followed by cycloheximide treatment (10 μg/mL) for 6 h before RelA/p65 protein levels were determined. Treatment with TCDD significantly decreased RelA/p65 levels at 4 h. At the later time points, RelA/p65 signal was no longer detected (Fig. 3C). Approximately 80–90% of proteins undergo proteasome degradation [29]. Thus, the role of proteasome 26S on RelA/p65 degradation was evaluated. MG132, an inhibitor of the catalytic core of the proteasome, blocks the TCDD-dependent degradation of RelA/p65, suggesting that AhR activation promotes the degradation of this transcription factor by the 26S proteasome (Fig. 3D). Since the identification of proteins by the 26S proteasome requires prior ubiquitination, the effect of TCDD on the ubiquitination of RelA/p65 from WT and Ahr-null macrophages was determined. TCDD treatment for 12 h resulted in an increase in RelA/p65 ubiquitinated forms in macrophages from WT mice (Fig. 4A). In contrast, this effect was not observed when Ahr-null macrophages were treated with TCDD, indicating that TCDD-induced ubiquitination is also AhR dependent (Fig. 4B).
Fig. 1.
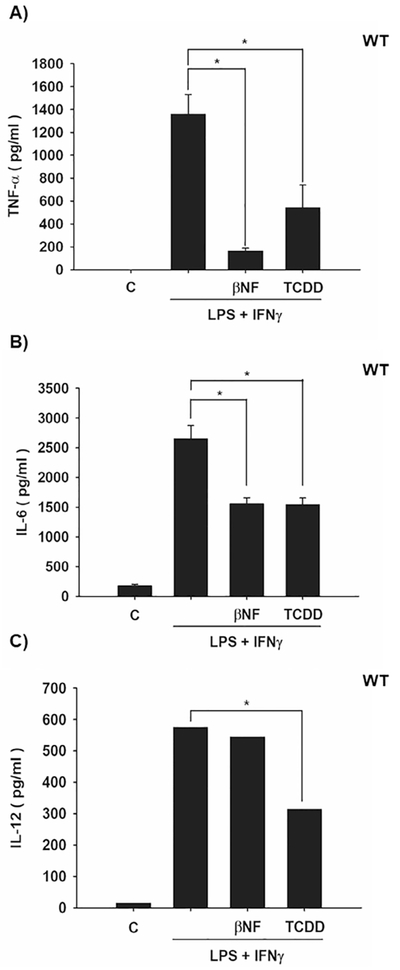
Effect of βNF and TCDD on pro-inflammatory cytokine levels in WT mouse macrophages. Peritoneal macrophages (1 × 106 cells/mL) from WT mice were activated with LPS and IFNγ together (1 μg/mL and 20 ng/mL, respectively) and treated with 1 μM βNF or 10 nM TCDD. After 12 h, TNF-α (A), IL-6 (B), and IL-12 (C) protein levels from supernatant cultures were determined by ELISA. DMSO was used as a control. The results are expressed as the mean ± S.D. of three independent experiments. *p < 0.05.
Fig. 2.
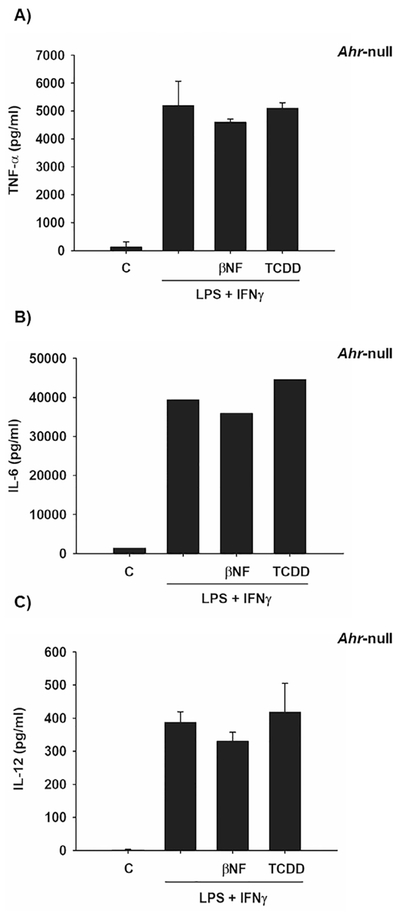
Effect of βNF and TCDD on pro-inflammatory cytokine levels in Ahr-null mouse macrophages. Peritoneal macrophages (1 × 106 cells/mL) from Ahr-null mice were activated with LPS and IFNγ together (1 μg/mL and 20 ng/mL, respectively) and treated with1 μM βNF or 10 nM TCDD. After 12 h, TNF-α (A), IL-6 (B), and IL-12 (C) protein levels from supernatant cultures were determined by ELISA. DMSO was used as a control. The results are expressed as the mean ± S.D. of three independent experiments.
Fig. 3.
TCDD induces RelA/p65 26S proteasome-dependent degradation. Peritoneal macrophages (1 × 106 cells/mL) from WT (A) or Ahr-null (B) mice were treated with 10 nM TCDD. C) WT peritoneal macrophages were treated with cycloheximide (CHX, 10 μg/mL) for 6 h to inhibit the de novo protein synthesis and harvested at the indicated times. D) WT peritoneal macrophages were co-treated with 10 nM TCDD and 25 μM MG132 at the indicated times. RelA/p65 protein levels were determined by Western blot. Representative blots and densitometric scanning of the blots are shown. DMSO was used as a control and β-actin as a loading control. The data are presented as the mean ± S.D. of three independent experiments. *p < 0.05, control vs. treatments.
Fig. 4.
AhR activation promotes RelA/p65 ubiquitination. Peritoneal macrophages (1 × 106 cells/mL) from WT (A) and Ahr-null (B) mice were treated with 10 nM TCDD for 4, 8 and 12 h, and ubiquitinated proteins were captured using TUBEs, as described in the Material and Methods section. RelA/p65 was evaluated by Western blot. DMSO was used as a control. Representative blots of each fraction (Input, Flowthrough and Bound) corresponding to three independent experiments are shown.
We previously found that AhR promotes p53 and c-fos ubiquitination and proteasome degradation through induction of the Ubch7 gene, encoding an E2 ubiquitin conjugating enzyme [22,23]. Therefore, the role of AhR transcriptional activity on RelA/p65 ubiquitination was investigated. WT peritoneal macrophages were co-treated with actinomycin D (a transcription inhibitor) and TCDD, and RelA/p65 protein and ubiquitin levels were then determined. Under this treatment scheme, TCDD failed to promote RelA/p65 ubiquitination and degradation (Fig. 5A and B). These results suggest that AhR transcriptional activity is required for RelA/p65 ubiquitination.
Fig. 5.
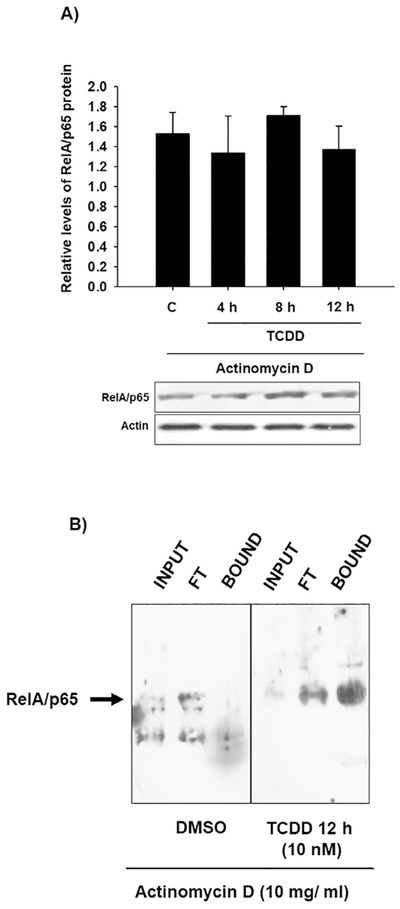
RelA/p65 protein ubiquitination and degradation induced by TCDD depend on the transcriptional activity. Peritoneal macrophages (1 × 106 cells/mL) from WT mice were pretreated with 10 mg/mL actinomycin-D for 10 min, followed by treatment with 10 nM TCDD at the indicated times. RelA/p65 protein (A) and ubiquitinated levels (B) were determined using TUBEs and Western blot. Representative blots and densitometric scanning of the blots are shown. DMSO was used as a control and βactin as a loading control. The data are presented as the mean ± S.D. of three independent experiments.
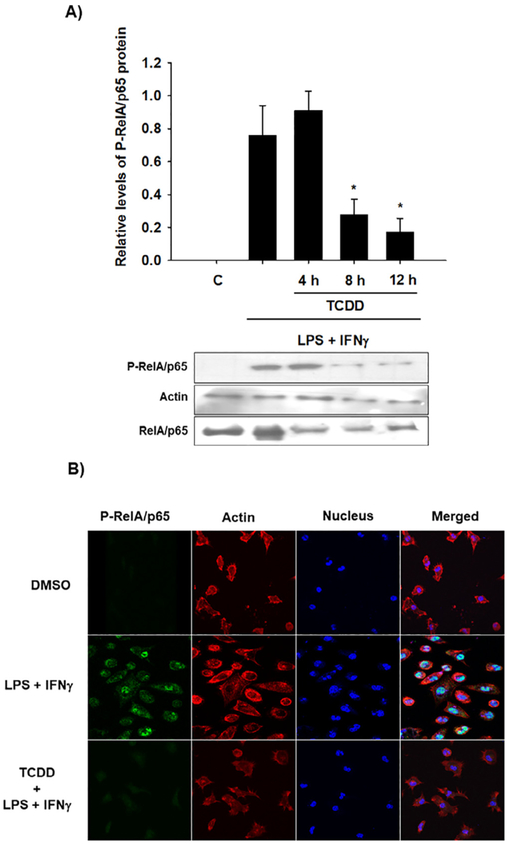
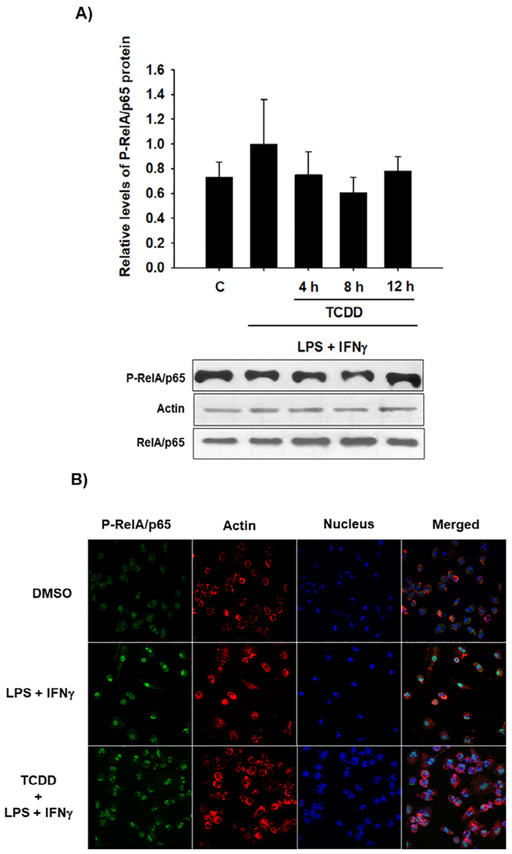
Given that the activation of the AhR partially promotes the degradation of RelA/p65, it was determined whether this decrease is sufficient to affect the levels of active RelA/p65. For it, peritoneal macrophages from WT mice were treated with TCDD before activating the cells with LPS and IFNγ. The levels of phosphorylated RelA/p65 protein (P-RelA/p65) were determined by Western blotting. P-RelA/p65 protein levels were diminished by TCDD after 8 h and 12 h of treatment (Fig. 6A). Accordingly, confocal images confirmed the activation of RelA/p65 after LPS and IFNγ treatment as revealed by its nuclear internalization. Similar to the Western blot results, treatment with TCDD decreased the P-RelA/p65 signal (Fig. 6B). In contrast to these results, TCDD treatment failed to decrease the P-RelA/p65 levels when Ahr-null macrophages were used (Fig. 7), confirming the AhR dependence of this effect. Interestingly, the Ahr-null macrophages presented P-RelA/p65 signal in control conditions without stimulus, suggesting that the absence of AhR promotes RelA/p65 phosphorylation.
Fig. 6.
TCDD promotes P-RelA/p65 degradation in WT mouse macrophages. Peritoneal macrophages (1 × 106 cells/mL) from WT mice were pretreated with 10 nM TCDD at the indicated times and activated with LPS/IFNγ (1 μg/mL and 20 ng/mL, respectively) for 30 min. Control cultures were activated with LPS/IFNγ. A) P-RelA/p65 and RelA/p65 were determined by Western blot. Representative blots and densitometric scanning of the blots are shown. β-Actin was used as a loading control. The data are presented as the mean ± S.D. of three independent experiments. *p < 0.05, control vs. treatments. B) Representative confocal microscopy images of P-RelA/p65 are shown after macrophage activation only or after 12 h of TCDD pretreatment and LPS/IFNγ. P-RelA/p65, actin, and DAPI were visualized as green, red, and blue, respectively. DMSO was used as a control. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 7.
TCDD failed to promote P-RelA/p65 degradation in Ahr-null mouse macrophages. Peritoneal macrophages (1 × 106 cells/mL) from Ahr-null mice were pretreated with 10 nM TCDD at the indicated times and activated with LPS/IFNγ (1 μg/mL and 20 ng/mL, respectively) for 30 min. Control cultures were activated with LPS/IFNγ. A) P-RelA/p65 and RelA/p65 were determined by Western blot. Representative blots and densitometric scanning of the blots are shown, β-actin was used as a loading control. The data are presented as the mean ± S.D. of three independent experiments. B) Representative confocal microscopy images of P-RelA/p65 are shown after macrophage activation only or after 12 h of TCDD pretreatment and LPS/IFNγ. P-RelA/p65, β-actin, and DAPI were visualized as green, red, and blue, respectively. DMSO was used as a control. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
TCDD treatment promotes RelA/p65 degradation after 4 h (Fig. 3A). However, ubiquitination was observed only after 12 h, which suggests an alternative degradation pathway. Autophagy, an evolutionarily conserved lysosome-dependent system in eukaryotes, also plays a role in protein turnover. During autophagy, the targeted proteins are delivered into double-membrane autophagosomes for lysosomal degradation. Therefore, we investigated whether this degradation system was involved in the TCDD-dependent RelA/p65 protein degradation at the early time points. TCDD treatment at 4 h and 8 h induced the formation of lysosome-like membrane structures (Fig. 8A). Washing the cell cultures resulted in a decrease in the number of vacuoles, a property of autophagosomes [30] (Fig. 8B). Additionally, treatment with chloroquine, a lysosome inhibitor, blocked the RelA/p65 protein degradation induced by TCDD (Fig. 8C). Taken together, these results suggest that TCDD also induces lysosome-dependent RelA/p65 protein degradation.
Fig. 8.
TCDD induces RelA/p65 lysosome-dependent degradation. Peritoneal macrophages (1 × 106 cells/mL) from WT mice were visualized by phase contrast microscopy after 10 nM TCDD treatment (A) and after cell cultures were washed and cultured for another 12 h in fresh control media (B). The arrows point to vacuoles. C) Peritoneal macrophages (1 × 106 cells/mL) from WT mice were treated with 10 nM TCDD or were co-treated with 10 nM TCDD and 10 μM chloroquine (CQ) at the indicated times, and RelA/p65 protein levels were determined by Western blot. Densitometric scanning of the blots are shown. DMSO was used as a control and β-actin as a loading control. The results are expressed as the mean ± S.D. of three independent experiments. *p < 0.05, control vs. treatments.
4. Discussion
Macrophages play a significant role in immunity by controlling the secretion of multiple cytokines, which normally act in a protective manner. However, these same cytokines are associated with many acute and chronic inflammatory diseases. Several studies have established that AhR acts as a negative regulator of the immune response by inhibiting the expression of inflammatory cytokines [12,13], but the mechanism is not well understood. Therefore, the goal of the present study was to investigate whether AhR regulates cytokine expression in peritoneal mouse macrophages by modifying the RelA/p65 protein levels.
Initially, the protein levels of pro-inflammatory cytokines in peritoneal macrophages of WT mice were measured. The induction of TNF-α, IL-6 and IL-12 after LPS and IFNγstimulation was inhibited by activation of AhR. In contrast, βNF and TCDD failed to inhibit the pro-inflammatory cytokine induction when Ahr-null macrophages were used. Moreover, after LPS and IFNγ stimulation, Ahr-null macrophages presented up to 3.8 and 16 times more TNF-α and IL-6, respectively, than WT macrophages. These data are consistent with previous results showing that after 24 h of LPS treatment, Ahr-null macrophages produce higher TNF-α, IL-6 and IL-12 protein levels compared to WT cells [12] and confirm the negative regulatory function of AhR over the pro-inflammatory cytokine levels.
Others proposed that AhR negatively regulates pro-inflammatory cytokines by suppressing NF-κB transcriptional activity through interaction with STAT1 [12]. Alternatively, AhR might act by promoting RelA/p65 protein degradation. We previously reported that activation of AhR mediates the ubiquitination and proteasome degradation of p53 and c-Fos by inducing the expression of UbcH7 (also known as Ube2L3 or UbcM4), an E2 ubiquitin enzyme [22,23]. Treatment of WT macrophages with the AhR agonist TCDD resulted in the ubiquitination of RelA/p65, together with a decrease in protein level. In contrast, TCDD failed to induce these effects when Ahr-null macrophages were used. Additionally, inhibition of the 26S proteasome blocked the TCDD-in-duced RelA/p65 protein degradation. Taken together, these data indicate that activation of AhR promotes RelA/p65 protein ubiquitination and degradation by the ubiquitin proteasome system (UPS).
Inhibition of transcription abrogates RelA/p65 protein ubiquitination and degradation mediated by AhR, indicating that the transcriptional activity of AhR may be required. These data also suggest that, as in the case of c-Fos and p53, AhR might induce the expression of an ubiquitin enzyme involved in RelA/p65 ubiquitination. RelA/p65 is ubiquitinated for proteasome degradation in the cytosol by PPARγ [31-33]. It can also be tagged with ubiquitin in the nucleus by the SOCS1/COMMD1 complex [34-36], PDLIM2 [37] and UbcH3 [28]. Therefore, overexpression of one or more of these ubiquitinating enzymes can lead to an increase in the degradation of RelA/p65 via UPS. Considering the above, we are currently investigating whether the expression of any of these genes is under the control of AhR.
Recently, it was shown that AhR is part of the cullin 4B ubiquitin ligase complex, involve in estrogen receptor α and androgen receptor degradation [38]. Therefore, in addition to the possibility of inducing the expression of ubiquitinating proteins, the AhR might promote the degradation of RelA/p65 through being part of a ubiquitinating complex.
TCDD promotes RelA/p65 ubiquitination after 12 h of treatment. However, a decrease in RelA/p65 protein levels was observed after 4 h of treatment, indicating the potential involvement of another degradation pathway, different from UPS. Autophagy describes a group of at least three evolutionary conserved cellular degradation processes in eukaryotes that deliver cytoplasmic constituents for lysosomal degradation [39]. Among them, macroautophagy is characterized by de novo formation of a double membrane engulfed vesicle (autophagosomes) which then fuses with lysosomes for the degradation of its cargo [40]. TCDD treatment also results in the reversible formation of vacuoles in peritoneal macrophages after 4 h, and inhibition of lysosomal activity abrogates the RelA/p65 protein degradation induced by TCDD after 4 and 8 h of treatment, indicating that TCDD also promotes RelA/p65 lysosomal degradation. These results are supported by recent studies showing that RelA/p65 is also degraded by macrophagy [41,42] and that NF-κB activation represses autophagy [43].
The AhR-induced RelA/p65 proteasomal degradation, as was expected, affects the level of activated RelA/p65, as revealed by Western blot and confocal microscopy imaging. LPS and IFNγ activated macrophages presented a 60% and 90% decrease in P-RelA/p65 levels after 8 and 12 h, respectively, of TCDD pretreatment, when compared to controls. Interestingly, confocal microscopy analysis also showed that Ahr-null macrophages presented a signal for P-RelA/p65 under control conditions, suggesting that absence of AhR promotes the phosphorylation of RelA/p65. This observation is in agreement with a previous report showing that non-activated Ahr-null macrophages presented higher levels of pro-inflammatory cytokines compared with WT cells [13]. The reason for this is unknown. However, an explanation could be found in the role that the AhR interacting protein (AIP) plays in regulation of the NF-κB signaling pathway. Recently, it was shown in T cells that through its interaction with CARMA1, AIP enhances the formation of the CARMA1-BCL10-MALT1 complex (CBM), promoting NFκB activation [44]. In macrophages, the CBM complex is essential for dectin-sky-induced NFκB activation [45]. Therefore, the absence of AhR might result in increased free AIP, favoring its interaction with CARM1, with its consequent increase in inflammatory cytokines. Alternatively, AhR might block the NFκB signaling pathway through its interaction with IKK α as Kurita and collaborators recently showed [46]. Therefore, the absence of AhR would promote a greater availability of IKKα, which in turn favors NF-κB activation.
In conclusion, AhR activation promotes RelA/p65 protein degradation through the UPS, as well as through lysosomes, resulting in a decreased pro-inflammatory cytokine level in mouse macrophages. To our knowledge, this is the first report that establishes a relationship between AhR, RelA/p65 ubiquitination and lysosomal degradation. More studies are needed to identify the putative ubiquitin enzymes under the regulation of the AhR that are involved in RelA/p65 ubiquitination and to determine whether the absence of AhR would promote a greater availability of IKKα and free AIP together with NF-κB signaling activation in macrophages.
Acknowledgement
This work was supported by CONACYT grant 153377.
References
- [1].Rowlands JC, Gustafsson JA, Aryl hydrocarbon receptor-mediated signal transduction, Crit. Rev. Toxicol 27 (1997) 109–134. [DOI] [PubMed] [Google Scholar]
- [2].Lahvis GP, Lindell SL, Thomas RS, McCuskey RS, Murphy C, Glover E, et al. , Portosystemic shunting and persistent fetal vascular structures in aryl hydrocarbon receptor-deficient mice, PNAS 97 (2000) 10442–10447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Latchney SE, Hein AM, O'Banion MK, DiCicco-Bloom E, Opanashuk LA, Deletion or activation of the aryl hydrocarbon receptor alters adult hippocampal neurogenesis and contextual fear memory, J. Neurochem 125 (2013) 430–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sato S, Shirakawa H, Tomita S, Ohsaki Y, Haketa K, Tooi O, et al. , Low-dose dioxins alter gene expression related to cholesterol biosynthesis, lipogenesis, and glucose metabolism through the aryl hydrocarbon receptor-mediated pathway in mouse liver, Toxicol. Appl. Pharmacol 229 (2008) 10–19. [DOI] [PubMed] [Google Scholar]
- [5].Reyes-Hernandez OD, Mejia-Garcia A, Sanchez-Ocampo EM, Castro-Munozledo F, Hernandez-Munoz R, Elizondo G, Aromatic hydrocarbons upregulate glyceraldehyde-3-phosphate dehydrogenase and induce changes in actin cytoskeleton. Role of the aryl hydrocarbon receptor (AhR), Toxicology 266 (2009) 30–37. [DOI] [PubMed] [Google Scholar]
- [6].Elizondo G, Fernandez-Salguero P, Sheikh MS, Kim GY, Fornace AJ, Lee KS, et al. , Altered cell cycle control at the G(2)/M phases in aryl hydrocarbon receptor-null embryo fibroblast, Mol. Pharmacol 57 (2000) 1056–1063. [PubMed] [Google Scholar]
- [7].Fernandez-Salguero P, Pineau T, Hilbert DM, McPhail T, Lee SS, Kimura S, et al. , Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor, Science 268 (1995) 722–726. [DOI] [PubMed] [Google Scholar]
- [8].Esser C, The immune phenotype of AhR null mouse mutants: not a simple mirror of xenobiotic receptor over-activation, Biochem. Pharmacol 77 (2009) 597–607. [DOI] [PubMed] [Google Scholar]
- [9].Kerkvliet NI, AHR-mediated immunomodulation: the role of altered gene transcription, Biochem. Pharmacol 77 (2009) 746–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, et al. , Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor, Nature 453 (2008) 65–71. [DOI] [PubMed] [Google Scholar]
- [11].Rodriguez-Sosa M, Elizondo G, Lopez-Duran RM, Rivera I, Gonzalez FJ, Vega L, Over-production of IFN-gamma and IL-12 in AhR-null mice, FEBS Lett. 579 (2005) 6403–6410. [DOI] [PubMed] [Google Scholar]
- [12].Kimura A, Naka T, Nakahama T, Chinen I, Masuda K, Nohara K, et al. , Aryl hydrocarbon receptor in combination with Stat1 regulates LPS-induced inflammatory responses, J. Exp. Med 206 (2009) 2027–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Climaco-Arvizu S, Dominguez-Acosta O, Cabanas-Cortes MA, Rodriguez-Sosa M, Gonzalez FJ, Vega L, et al. , Aryl hydrocarbon receptor influences nitric oxide and arginine production and alters M1/M2 macrophage polarization, Life Sci. 155 (2016) 76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Elizondo G, Rodriguez-Sosa M, Estrada-Muniz E, Gonzalez FJ, Vega L, Deletion of the aryl hydrocarbon receptor enhances the inflammatory response to Leishmania major infection, Int. J. Biol. Sci 7 (2011) 1220–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sanchez Y, Rosado Jde D, Vega L, Elizondo G, Estrada-Muniz E, Saavedra R, et al. , The unexpected role for the aryl hydrocarbon receptor on susceptibility to experimental toxoplasmosis, J. Biomed. Biotechnol 2010 (2010) 505694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Takeuchi O, Akira S, Pattern recognition receptors and inflammation, Cell 140 (2010) 805–820. [DOI] [PubMed] [Google Scholar]
- [17].Schaub B, Bellou A, Gibbons FK, Velasco G, Campo M, He H, et al. , TLR2 and TLR4 stimulation differentially induce cytokine secretion in human neonatal, adult, and murine mononuclear cells, J. Interferon Cytokine Res 24 (2004) 543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fitzgerald KA, Rowe DC, Barnes BJ, Caffrey DR, Visintin A, Latz E, et al. , LPS-TLR4 signaling to IRF-3/7 and NF-kappaB involves the toll adapters TRAM and TRIF, J. Exp. Med 198 (2003) 1043–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Thatcher TH, Maggirwar SB, Baglole CJ, Lakatos HF, Gasiewicz TA, Phipps RP, et al. , Aryl hydrocarbon receptor-deficient mice develop heightened inflammatory responses to cigarette smoke and endotoxin associated with rapid loss of the nuclear factor-kappaB component RelB, Am. J. Pathol 170 (2007) 855–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Vogel CF, Sciullo E, Li W, Wong P, Lazennec G, Matsumura F, RelB, a new partner of aryl hydrocarbon receptor-mediated transcription, Mol. Endocrinol 21 (2007) 2941–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tian Y, Ke S, Denison MS, Rabson AB, Gallo MA, Ah receptor and NF-kappaB interactions, a potential mechanism for dioxin toxicity, J. Biol. Chem 274 (1999) 510–515. [DOI] [PubMed] [Google Scholar]
- [22].Reyes-Hernandez OD, Mejia-Garcia A, Sanchez-Ocampo EM, Cabanas-Cortes MA, Ramirez P, Chavez-Gonzalez L, et al. , Ube2l3 gene expression is modulated by activation of the aryl hydrocarbon receptor: implications for p53 ubiquitination, Biochem. Pharmacol 80 (2010) 932–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mejia-Garcia A, Gonzalez-Barbosa E, Martinez-Guzman C, Torres-Ramos MA, Rodriguez MS, Guzman-Leon S, et al. , Activation of AHR mediates the ubiquitination and proteasome degradation of c-Fos through the induction of Ubcm4 gene expression, Toxicology 337 (2015) 47–57. [DOI] [PubMed] [Google Scholar]
- [24].Hjerpe R, Aillet F, Lopitz-Otsoa F, Lang V, England P, Rodriguez MS, Efficient protection and isolation of ubiquitylated proteins using tandem ubiquitin-binding entities, EMBO Rep. 10 (2009) 1250–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Aillet F, Lopitz-Otsoa F, Hjerpe R, Torres-Ramos M, Lang V, Rodriguez MS, Isolation of ubiquitylated proteins using tandem ubiquitin-binding entities, Methods Mol. Biol 832 (2012) 173–183. [DOI] [PubMed] [Google Scholar]
- [26].Sekine H, Mimura J, Oshima M, Okawa H, Kanno J, Igarashi K, et al. , Hypersensitivity of aryl hydrocarbon receptor-deficient mice to lipopolysaccharide-induced septic shock, Mol. Cell. Biol 29 (2009) 6391–6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hayden MS, West AP, Ghosh S, NF-kappaB and the immune response, Oncogene 25 (2006) 6758–6780. [DOI] [PubMed] [Google Scholar]
- [28].Hou Y, Zhang Z, Xu Q, Wang H, Xu Y, Chen K, Inhibitor of growth 4 induces NFkappaB/p65 ubiquitin-dependent degradation, Oncogene 33 (2014) 1997–2003. [DOI] [PubMed] [Google Scholar]
- [29].Salomons FA, Acs K, Dantuma NP, Illuminating the ubiquitin/proteasome system, Exp. Cell Res. 316 (2010) 1289–1295. [DOI] [PubMed] [Google Scholar]
- [30].Kirisako T, Ichimura Y, Okada H, Kabeya Y, Mizushima N, Yoshimori T, et al. , The reversible modification regulates the membrane-binding state of Apg8/Aut7 essential for autophagy and the cytoplasm to vacuole targeting pathway, J. Cell Biol 151 (2000) 263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chung SW, Kang BY, Kim SH, Pak YK, Cho D, Trinchieri G, et al. , Oxidized low density lipoprotein inhibits interleukin-12 production in lipopolysaccharide-activated mouse macrophages via direct interactions between peroxisome proliferator-activated receptor-gamma and nuclear factor-kappa B, J. Biol. Chem 275 (2000) 32681–32687. [DOI] [PubMed] [Google Scholar]
- [32].Kelly D, Campbell JI, King TP, Grant G, Jansson EA, Coutts AG, et al. , Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-gamma and RelA, Nat. Immunol 5 (2004) 104–112. [DOI] [PubMed] [Google Scholar]
- [33].Hou Y, Moreau F, Chadee K, PPARgamma is an E3 ligase that induces the degradation of NFkappaB/p65, Nat. Commun 3 (2012) 1300. [DOI] [PubMed] [Google Scholar]
- [34].Burstein E, Hoberg JE, Wilkinson AS, Rumble JM, Csomos RA, Komarck CM, et al. , COMMD proteins, a novel family of structural and functional homologs of MURR1, J. Biol. Chem 280 (2005) 22222–22232. [DOI] [PubMed] [Google Scholar]
- [35].Strebovsky J, Walker P, Lang R, Dalpke AH, Suppressor of cytokine signaling 1 (SOCS1) limits NFkappaB signaling by decreasing p65 stability within the cell nucleus, FASEB J. 25 (2011) 863–874. [DOI] [PubMed] [Google Scholar]
- [36].Maine GN, Burstein E, COMMD proteins: COMMing to the scene, Cell. Mol. Life Sci 64 (2007) 1997–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Tanaka T, Grusby MJ, Kaisho T, PDLIM2-mediated termination of transcription factor NF-kappaB activation by intranuclear sequestration and degradation of the p65 subunit, Nat. Immunol 8 (2007) 584–591. [DOI] [PubMed] [Google Scholar]
- [38].Ohtake F, Baba A, Takada I, Okada M, Iwasaki K, Miki H, et al. , Dioxin receptor is a ligand-dependent E3 ubiquitin ligase, Nature 446 (2007) 562–566. [DOI] [PubMed] [Google Scholar]
- [39].Mizushima N, Klionsky DJ, Protein turnover via autophagy: implications for metabolism, Annu. Rev. Nutr 27 (2007) 19–40. [DOI] [PubMed] [Google Scholar]
- [40].Munz C, Macroautophagy during innate immune activation, Front. Microbiol 2 (2011) 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chang CP, Su YC, Hu CW, Lei HY, TLR2-dependent selective autophagy regulates NF-kappaB lysosomal degradation in hepatoma-derived M2 macrophage differentiation, Cell Death Differ. 20 (2013) 515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Tang J, Zhan MN, Yin QQ, Zhou CX, Wang CL, Wo LL, et al. , Impaired p65 degradation by decreased chaperone-mediated autophagy activity facilitates epithelial-to-mesenchymal transition, Oncogenesis 6 (2017) e387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Djavaheri-Mergny M, Amelotti M, Mathieu J, Besancon F, Bauvy C, Souquere S, et al. , NF-kappaB activation represses tumor necrosis factor-alpha-induced autophagy, J. Biol. Chem 281 (2006) 30373–30382. [DOI] [PubMed] [Google Scholar]
- [44].Schimmack G, Eitelhuber AC, Vincendeau M, Demski K, Shinohara H, Kurosaki T, et al. , AIP augments CARMA1-BCL10-MALT1 complex formation to facilitate NF-kappaB signaling upon T cell activation, Cell Commun. Signal 12 (2014) 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hara H, Saito T, CARD9 versus CARMA1 in innate and adaptive immunity, Trends Immunol. 30 (2009) 234–242. [DOI] [PubMed] [Google Scholar]
- [46].Kurita H, Schnekenburger M, Ovesen JL, Xia Y, Puga A, The Ah receptor recruits IKKalpha to its target binding motifs to phosphorylate serine-10 in histone H3 required for transcriptional activation, Toxicol. Sci 139 (2014) 121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]