Abstract
Background:
Amyotrophic lateral sclerosis (ALS) is devastating, leading to paralysis and death. Disease onset begins pre-symptomatically through spinal motor neuron (MN) axon die-back from musculature at ~47 days of age in the mutant superoxide dismutase 1 (mSOD1G93A) transgenic ALS mouse model. This period may be optimal to assess potential therapies. We previously demonstrated that post-symptomatic adipose-derived stem cell conditioned medium (ASC-CM) treatment is neuroprotective in mSOD1G93A mice. We hypothesized that early disease onset treatment could ameliorate neuromuscular junction (NMJ) disruption.
Objective:
To determine whether pre-symptom administration of ASC-CM prevents early NMJ disconnection.
Methods:
We confirmed the NMJ denervation time course in mSOD1G93A mice using co-labeling of neurofilament and post-synaptic acetylcholine receptors (AchR) by α-bungarotoxin. We determined whether ASC-CM ameliorates early NMJ loss in mSOD1G93A mice by systemically administering 200μl ASC-CM or vehicle medium daily from post-natal days 35 to 47 and quantifying intact NMJs through co-labeling of neurofilament and synaptophysin with α-bungarotoxin in gastrocnemius muscle.
Results:
Intact NMJs were significantly decreased in 47 day old mSOD1G93A mice (p < 0.05), and daily systemic ASC-CM prevented disease-induced NMJ denervation compared to vehicle treated mice (p < 0.05).
Conclusions:
Our results lay the foundation for testing the long-term neurological benefits of systemic ASC-CM therapy in the mSOD1G93A mouse model of ALS.
Keywords: Amyotrophic lateral sclerosis, ALS, neuromuscular junctions, adipose-derived stem cells, conditioned medium
1. Introduction
Motor neuron (MN) diseases are devastating, causing death of both upper MNs and lower MNs, paralysis and death. Amyotrophic lateral sclerosis (ALS) is the most common MN disease, affecting thousands of individuals at productive peaks in their lives (Brooks, Miller, Swash, & Munsat, 2000; Naganska & Matyja, 2011; Wijesekera & Leigh, 2009). Symptom onset is often subtle and easily undetected in early stages. Peripheral axonal die-back from target musculature begins pre-symptomatically, followed by MN cell death throughout the neuraxis (Chiu et al., 1995; Fischer et al., 2004; Haenggeli & Kato, 2002).
Many treatments for ALS have been tried, largely without success. Riluzole and edaravone, are the only treatments approved by the Food and Drug Administration. Riluzole exhibits limited efficacy, providing only a few months of increased lifespan (Traynor, Alexander, Corr, Frost, & Hardiman, 2003), and evaluation of edaravone is ongoing (Sawada, 2017). Most ALS therapies have targeted central nervous system (CNS) MN survival; however, if peripheral axonal die-back continues without intervention, muscle denervation and paralysis persist. As such, both central and peripheral disease components may need to be targeted simultaneously.
The stromal-vascular cell fraction of adipose tissue contains abundant adipose-derived stem cells (ASCs) (Bourin et al., 2013; Hong, Traktuev, & March, 2010). We have shown that ASCs are reparative in ischemia and other pathology via secretion of anti-apoptotic, anti-inflammatory, and pro-angiogenic growth factors and cytokines (Rehman et al., 2004). ASCs also secrete several neuroprotective growth factors (Wei, Du, et al., 2009; Wei, Zhao, et al., 2009; Zhao et al., 2009). Benefits of ASC-secreted paracrine factors have been demonstrated in multiple pre-clinical animal models including hindlimb ischemia, diabetes, acute lung injury, brain ischemia, and ALS (Fontanilla et al., 2015). In rat neonatal brain hypoxia-ischemia, ASC-CM markedly reduced ischemic tissue injury in vivo, and decreased neuron death in vitro (Wei, Du, et al., 2009; Wei, Zhao, et al., 2009). Our data have extended these pre-clinical neuroprotective findings to the evaluation of ASC-CM in mSOD1G93A mice. In this model, post-symptomatic ASC-CM therapy reduced MN apoptosis and prolonged lifespan (Fontanilla et al., 2015).
Peripheral axonal “die-back” remains a key aspect of ALS onset and progression, causing synaptic breakdown and neuromuscular junction (NMJ) denervation (Fischer et al., 2004; Frey et al., 2000). This process begins in mSOD1G93A mouse hindlimb muscles long before MN loss and symptom onset (Fischer et al., 2004; Frey et al., 2000). Despite possible long-term effects on the disease, surprisingly little research has focused on therapies targeting early NMJ denervation. Understanding how to slow or reverse this process may be critical for preventing disease progression or extending lifespan. This study aimed to confirm the time window for NMJ denervation onset and determine whether systemic ASC-CM therapy could limit this process in the mSOD1G93A mouse model of ALS.
2. Materials and methods
2.1. Animals
We utilized female mSOD1G93A ALS mice [B6SJL-Tg(SOD1*G93A)1Gur/J; Jackson Laboratories] (n = 16). Female wild-type (WT) mice served as controls (Jackson Laboratories, n = 9). For time course assessment of NMJ innervation, mSOD1G93A mice were euthanized with an overdose of ketamine/xylazine at post-natal day 35, 39, 43 and 47 (n = 3–4/timepoint). WT mice were euthanized at post-natal day 47 (n = 3). Once anesthetized, as determined by lack of response to toe pinch stimulus, transcardial perfusion was performed with 50 ml phosphate buffered saline (PBS, pH 7.4) followed by 4% paraformaldehyde in 0.1 M PBS for tissue fixation. Once perfused, hindlimb gastrocnemius muscles were dissected and post-fixed for 40 min followed by transfer to 30% sucrose solution for cryoprotection and cryosectioning. In animals used for comparing ASC-CM therapy effects on NMJ innervation, mSOD1G93A mice (n = 3–4) were treated with ASC-CM or Basal Medium Eagle (BME) and the mice were euthanized and tissue prepared as described above at post-natal day 47. All use of animals in this study followed the Guidelines for the Institutional Care and Use of Animals of Indiana University School of Medicine.
2.2. ASC-CM preparation and administration
Human subcutaneous adipose tissue samples were obtained from lipoaspiration/liposuction procedures and prepared as previously described (Gu et al., 2013; Traktuev et al., 2008; Wang et al., 2014). In brief, human adipose tissue samples were digested in collagenase type I (Worthington Biochemical) for 1 hr at 37°C, filtered with 100 μm and 70 μm filters, and centrifuged at 300 g for 5 min to separate stromal cells from adipocytes. The ASC pellet was treated with red blood cell lysis buffer for 5 min at 37°C, then centrifuged at 300 g for 5 min. The supernatant was discarded, and the cell pellet was resuspended in endothelial growth medium-2 MV (EGM-2-MV, Lonza) consisting of endothelial basal medium-2 (EBM-2), 5% fetal bovine serum (FBS), and the supplemental growth factors vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), epidermal growth factor (EGF), and insulin-like growth factor (IGF-1). ASCs (4 × 106 cells/cm2) were plated in T75 tissue culture flasks and incubated at 37°C in 5% CO2. Fresh EGM2-MV was replaced the next day. ASCs were cultured to 80% confluence before passaging and freezing. Passage 4 ASCs were seeded at 5 × 104 cells/cm2 and grown to 80% confluence, and then rinsed and each T75 flask was incubated with 10 ml of Basal Media Eagle (BME) containing 5 mM KCl for 24 hrs. Conditioned media was collected, centrifuged for 5 min to eliminate debris and then filtered through a 22μm micrometer sterile filter before freezing CM and BME in 1 ml aliquots for use in the study. Collection of human adipose tissue was approved by the Indiana University Institutional Review Board.
For treatment, mSOD1G93A mice were randomly assigned to ASC-CM or vehicle treatment groups, and daily i.p. injections of 200μl ASC-CM or vehicle (Basal Medium Eagle, BME) were administered. Injections were started post-natal day 35 and continued to day 47. During treatment, the general health and overall behavior of the mice was regularly monitored by the investigators, animal facility veterinary staff and qualified veterinarians. Daily ASC-CM injections did not cause any observable outward physical or behavior changes in the mice or weight changes compared to vehicle treated mice during the course of the study.
2.3. Tissue preparation
Following perfusion, bilateral gastrocnemius and soleus muscles were dissected and prepared for cryosectioning. Following cryoprotection, right medial gastrocnemius muscle was isolated and placed in Tissue Tek OCT medium and frozen at −80°C. Embedded frozen muscle tissue was transferred to a cryostat chamber before cryosectioning. Prepared gastrocnemius tissue was serially sectioned at 20μm for immunofluorescent labeling of neuromuscular junctions and innervation.
2.4. Microscopic imaging and NMJ analysis
In brief, 8 serial sections separated by an interval of 100 μm were air-dried on Superfrost microscope slides (Fisher Scientific), and incubated in donkey serum-based blocking buffer for 1 hr at room temperature. Next, the slides were incubated with primary antibodies against neurofilament (chicken anti-neurofilament, 1:500, Aves Inc.), which labels innervation, or synaptophysin (Alex 488-conjugated mouse anti-synaptophysin, 1:100, Millipore), which labels the neuromuscular pre-synapse, overnight at 4°C.
Slides were then washed in PBS+0.1% Triton-X100 (PBST). The slides incubated with primary neurofilament antibody were then incubated with donkey anti-chicken Alex 488-conjugated secondary antibody (Jackson Immunoresearch) and Alex 555-conjugated α-bungarotoxin (1:800, Life Technologies), which labels the post-synaptic acetylcholine receptors (AchRs) of the NMJ, for 1 hr at room temperature. The slides incubated with Alex 488-conjugated primary antibody against synaptophysin were also incubated with Alex 555-conjugated α -bungarotoxin (1:800, Life Technologies) for 1 hr at room temperature. Afterward, all slides were prepared for microscopic fluorescent imaging. Five random high-power fields per section were selected for quantification of synaptophysin/ α -bungarotoxin (4 sections) and neurofilament/ α -bungarotoxin co-labeled NMJs (4 sections). Quantification of percent intact NMJs (yellow) was determined by an overlay of α-bungarotoxin (red) and synaptophysin (green) or neurofilament (green). Denervated NMJs were defined by the presence of only α -bungarotoxin (red). Percent intact NMJs was calculated using the following formula: [number of intact NMJs (yellow) / total number of motor end plates (yellow + red)] ×100. All slides were coded by an uninvolved investigator prior to analysis. Imaging was performed on an Olympus BX40 epifluorescent microscope and NMJ quantification was accomplished using NIH ImageJ software.
2.5. Statistical analysis
Statistical analysis was accomplished via an ANOVA and the Student-Newman-Keuls post hoc test (Sokal & Rohlf, 1995) using GraphPad Prism 6.0 software (GraphPad, Inc).
3. Results
3.1. Gastrocnemius NMJ innervation significantly diminished between post-natal days 39 and 47 in mSOD1G93A mice
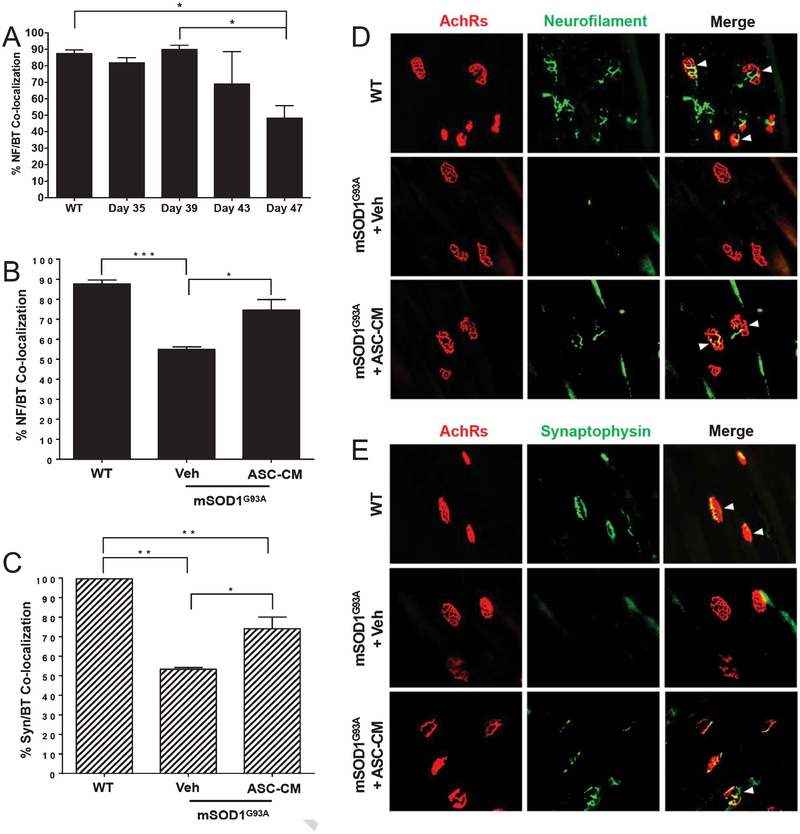
Upon quantifying intact NMJs in the medial gastrocnemius of mSOD1G93A and WT mice, we found that a significant decrease in neurofilament/α-bungarotoxin co-localized NMJs occurred between post-natal days 35 and 47 (p < 0.05) and days 39 and 47 in mSOD1G93A mice (p < 0.05, Fig. 1A). The results from this time course suggest this decrease is rapid in mSOD1G93A mice.
Fig. 1.
NMJ denervation time course and therapeutic effects of ASC-CM on NMJ loss. A) Quantification of neurofilament and α-bungarotoxin NMJs showed significant reduction in co-labeling between post-natal days 35 and 47, and post-natal days 39 and 47 (p < 0.05). B & D) At post-natal day 47, mSOD1G93A mice that were treated with daily i.p. vehicle showed significant loss of innervated NMJs as determined by neurofilament/ α -bungarotoxin co-labeling compared to WT mice (p < 0.001). mSOD1G93A mice treated with ASC-CM showed significantly more co-labeled NMJs than vehicle treated mice (p < 0.05) at this time point. C & E) Similar results were observed for pre-synaptic synaptophysin co-labeling with α -bungarotoxin. Significantly fewer co-labeled NMJs were observed in vehicle treated mSOD1G93A mice than WT mice at post-natal day 47 (p < 0.01), while more co-labeling was observed with ASC-CM treatment compared to vehicle treatment (p < 0.05). *, p < 0.05; **, p < 0.01; ****, p < 0.001.
3.2. Innervation of NMJs was preserved by ASC-CM treatment
As our data showed intact NMJs were significantly decreased by post-natal day 47, we examined the effects of daily systemic ASC-CM therapy before and through this time period in mSOD1G93A mice. When ASC-CM or BME vehicle was administered daily between post-natal days 35 and 47, ASC-CM treated mSOD1G93A mice had significantly more neurofilament/ α -bungarotoxin co-localized NMJs than vehicle treated mice, with co-localization rescued to WT levels (p < 0.05, Fig. 1B & D.) Likewise, synaptophysin-positive NMJs were significantly increased over vehicle treated mSOD1G93A mice at post-natal day 47 (p < 0.05, Fig. 1C & E). Images shown in Fig. 1D and E are representative of the reduced intact NMJs following vehicle treatment, and increased intact NMJs observed through both neurofilament and synaptophysin co-labeling with post-synaptic AchRs following ASC-CM therapy.
4. Discussion
Our findings support previous reports of pre-symptomatic mSOD1G93A mouse hindlimb muscle denervation (Fischer et al., 2004), and show that systemic ASC-CM therapy promoted intact NMJs. To our knowledge, this is the first study of its kind to demonstrate therapeutic efficacy on early disease onset in an ALS mouse model. Understanding how ASC-CM may be imparting these effects requires further investigation. Historically, therapeutic studies in ALS have emphasized motor neuron survival; however, accumulating evidence indicates ALS is non-cell autonomous in that onset and progression of the disease involves more than one cell type and various anatomical structures. Other central and peripheral components can clearly influence MN survival and disease progression (Bilsland, Nirmalananthan, Yip, Greensmith, & Duchen, 2008; Boillee et al., 2006; Clement et al., 2003; Hossaini et al., 2011; Zagami, Beart, Wallis, Nagley, & O’Shea, 2009). MN interaction with glial cells, skeletal muscle, and immune cells can influence MN death in ALS (Martin & Chang, 2012; Murdock, Bender, Segal, & Feldman, 2015; Nardo et al., 2016; Pansarasa, Rossi, Berardinelli, & Cereda, 2014). These complex and dynamic interactions likely contribute to MN loss and early axonal die-back from the NMJ.
Several mechanisms for pre-symptomatic mSOD1G93A axonal die-back have been proposed. Neuromuscular junction structure and transmission alterations are some of the earliest peripheral axonal dysfunctions in ALS (Fischer et al., 2004; Gurney et al., 1994; Rocha, Pousinha, Correia, Sebastiao, & Ribeiro, 2013). As MNs are functional during this early period, intrinsic metabolic changes could play a role in die-back. Autophagy and axonal transport dysregulation are associated with mutant SOD1-associated MN disease and synaptic integrity (Lee, Shin, Lee, & Choi, 2015). Upregulated autophagy has been shown to mitigate abnormal protein aggregation, enhance MN survival, and increase lifespan in ALS models (Lee et al., 2015; Rudnick et al., 2017), as well as possibly accelerate disease onset (Rudnick et al., 2017) which suggests that MN metabolic processes in ALS are complicated and that late and early disease stages are regulated differently.
Our research has shown that ASC-secreted trophic factors including brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) are neuroprotective (Wei, Du, et al., 2009; Kim et al., 2013). Blocking NGF prior to ASC-CM treatment in mSOD1G93A mice revealed it contributed to observed neuroprotective effects (Fontanilla et al., 2015). BDNF proved to be a key neuroprotective component of ASC-CM in experimental stroke therapy (Wei, Du, et al., 2009). This suggests trophic factors in ASC-CM act centrally through crossing the blood brain/spinal cord barrier (BSCB). As the BSCB is compromised as early as 60 days of age in mSOD1G93A mice, this would allow proteins like trophic factors into the CNS (Zhong et al., 2008). However, we did not examine peripheral tissues for therapeutic effects following late-stage treatment. Such peripheral effects are possible, as some functional improvement was observed following post-symptomatic ASC-CM ALS therapy.
The central effects of BDNF have been widely studied, though it could also directly affect NMJ integrity. BDNF acts on Trk subtype B (TrkB) receptors, which are shown to preserve adult NMJ morphology and function (Mantilla et al., 2014). In addition, TrkB activity reduction induced presynaptic terminal restructuring and motor axon disruption. Since ASC-CM contains BDNF (Wei, Du, et al., 2009) and is neuroprotective within the CNS (Wei, Du, et al., 2009; Fontanilla et al., 2015), it could activate and enhance TrkB receptor function at the NMJ, strengthening axonal innervation and NMJ functional integrity.
The present study cannot distinguish whether NMJ preservation by early ASC-CM therapy occurred through peripheral or central influence. Intraspinal transfection of MNs in mSOD1G93A mice with viral-mediated granulocyte colony stimulating factor (AAV-GCSF) reduced gastrocnemius NMJ denervation (Henriques et al., 2011), indicating that centrally targeted therapies can positively influence NMJ integrity.
In conclusion, we have confirmed the period of significant NMJ loss in mSOD1G93A mice, which aligns with prior studies. Importantly, our findings indicate ASC-CM prevents early disease pathology, which supports its use in familial ALS. These findings highlight the urgency for early identification in at-risk populations for developing ALS. A valuable time window is present long before symptoms start, and ASC-CM could potentially delay disease onset or progression. Further research is required to determine the site and mechanism of action and the influence of early benefits on long term neurological and survival outcomes.
Acknowledgments
This research was funded by an Indiana University IUCRG Pilot Grant and NIH T32 HL79995.
References
- Bilsland LG, Nirmalananthan N, Yip J, Greensmith L, & Duchen MR (2008). Expression of mutant SOD1 in astrocytes induces functional deficits in motoneuron mitochondria. Journal of Neurochemistry, 107(5), 1271–1283. doi: 10.1111/j.1471-4159.2008.05699.x [DOI] [PubMed] [Google Scholar]
- Boillee S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, … & Cleveland DW (2006). Onset and progression in inherited ALS determined by motor neurons and microglia. Science, 312(5778), 1389–1392. doi: 10.1126/science.1123511 [DOI] [PubMed] [Google Scholar]
- Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, … & Gimble JM (2013). Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy, 15(6), 641–648. doi: 10.1016/j.jcyt.2013.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks BR, Miller RG, Swash M, & Munsat TL (2000). El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis, 1(5), 293–299. [DOI] [PubMed] [Google Scholar]
- Chiu AY, Zhai P, Dal Canto MC, Peters TM, Kwon YW, Prattis SM, & Gurney ME (1995). Age-dependent penetrance of disease in a transgenic mouse model of familial amyotrophic lateral sclerosis. Molecular and Cellular Neuroscience, 6(4), 349–362. doi: 10.1006/mcne.1995.1027 [DOI] [PubMed] [Google Scholar]
- Clement AM, Nguyen MD, Roberts EA, Garcia ML, Boillee S, Rule M, … & Cleveland DW (2003). Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science, 302(5642), 113–117. doi: 10.1126/science.1086071 [DOI] [PubMed] [Google Scholar]
- Fischer LR, Culver DG, Tennant P, Davis AA, Wang M, Castellano-Sanchez A, … & Glass JD (2004). Amyotrophic lateral sclerosis is a distal axonopathy: Evidence in mice and man. Experimental Neurology, 185(2), 232–240. doi: 10.1016/j.expneurol.2003.10.004 [DOI] [PubMed] [Google Scholar]
- Fontanilla CV, Gu H, Liu Q, Zhu TZ, Zhou C, Johnstone BH, … & Du Y (2015). Adipose-derived stem cell conditioned media extends survival time of a mouse model of amyotrophic lateral sclerosis. Scientific Reports, 5, 16953. doi: 10.1038/srep16953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey D, Schneider C, Xu L, Borg J, Spooren W, & Caroni P (2000). Early and selective loss of neuromuscular synapse subtypes with low sprouting competence in motoneuron diseases. Journal of Neuroscience, 20(7), 2534–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Wang J, Du N, Tan J, Johnstone B, & Du Y (2013). Adipose stromal cells-conditioned medium blocks 6-hydroxydopamine-induced neurotoxicity and reactive oxygen species. Neuroscience Letters, 544, 15–19. doi: 10.1016/j.neulet.2013.02.057 [DOI] [PubMed] [Google Scholar]
- Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD et al. (1994). Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science, 264(5166), 1772–1775. [DOI] [PubMed] [Google Scholar]
- Haenggeli C, & Kato AC (2002). Differential vulnerability of cranial motoneurons in mouse models with motor neuron degeneration. Neuroscience Letters, 335(1), 39–43. doi: 10.1016/S0304-3940(02)01140-0 [DOI] [PubMed] [Google Scholar]
- Henriques A, Pitzer C, Dittgen T, Klugmann M, Dupuis L, & Schneider A (2011). CNS-targeted viral delivery of G-CSF in an animal model for ALS: Improved efficacy and preservation of the neuromuscular unit. Molecular Therapy, 19(2), 284–292. doi: 10.1038/mt.2010.271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SJ, Traktuev DO, & March KL (2010). Therapeutic potential of adipose-derived stem cells in vascular growth and tissue repair. Current Opinion in Organ Transplantation, 15(1), 86–91. [DOI] [PubMed] [Google Scholar]
- Hossaini M, Cardona Cano S, van Dis V, Haasdijk ED, Hoogenraad CC, Holstege JC, & Jaarsma D (2011). Spinal inhibitory interneuron pathology follows motor neuron degeneration independent of glial mutant superoxide dismutase 1 expression in SOD1-ALS mice. Journal of Neuropatholy & Experimental Neurology, 70(8), 662–677. doi: 10.1097/NEN.0b013e31822581ac [DOI] [PubMed] [Google Scholar]
- Lee JK, Shin JH, Lee JE, & Choi EJ (2015). Role of autophagy in the pathogenesis of amyotrophic lateral sclerosis. Biochimica et Biophysica Acta, 1852(11), 2517–2524. doi: 10.1016/j.bbadis.2015.08.005 [DOI] [PubMed] [Google Scholar]
- Mantilla CB, Stowe JM, Sieck DC, Ermilov LG, Greising SM, Zhang C, … & Sieck GC (2014). TrkB kinase activity maintains synaptic function and structural integrity at adult neuromuscular junctions. Journal of Applied Physiology 1985 117(8), 910–920. doi: 10.1152/japplphysiol.01386.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ, & Chang Q (2012). Inhibitory synaptic regulation of motoneurons: A new target of disease mechanisms in amyotrophic lateral sclerosis. Molecular Neurobiology, 45(1), 30–42. doi: 10.1007/s12035-011-8217-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdock BJ, Bender DE, Segal BM, & Feldman EL (2015). The dual roles of immunity in ALS: Injury overrides protection. Neurobiology of Disease, 77, 1–12. doi: 10.1016/j.nbd.2015.02.017 [DOI] [PubMed] [Google Scholar]
- Naganska E, & Matyja E (2011). Amyotrophic lateral sclerosis—looking for pathogenesis and effective therapy. Folia Neuropathologica, 49(1), 1–13. [PubMed] [Google Scholar]
- Nardo G, Trolese MC, de Vito G, Cecchi R, Riva N, Dina G, & Bendotti C (2016). Immune response in peripheral axons delays disease progression in SOD1G93A mice. Journal of Neuroinflammation, 13(1), 261. doi: 10.1186/s12974-016-0732-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pansarasa O, Rossi D, Berardinelli A, & Cereda C (2014). Amyotrophic lateral sclerosis and skeletal muscle: An update. Molecular Neurobiology, 49(2), 984–990. doi: 10.1007/s12035-013-8578-4 [DOI] [PubMed] [Google Scholar]
- Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, … & March KL (2004). Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation, 109(10), 1292–1298. doi: 10.1161/01.cir.0000121425.42966.f1 [DOI] [PubMed] [Google Scholar]
- Rocha MC, Pousinha PA, Correia AM, Sebastiao AM, & Ribeiro JA (2013). Early changes of neuromuscular transmission in the SOD1(G93A) mice model of ALS start long before motor symptoms onset. PLoS One, 8(9), e73846. doi: 10.1371/journal.pone.0073846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnick ND, Griffey CJ, Guarnieri P, Gerbino V, Wang X, Piersaint JA, … & Maniatis T (2017). Distinct roles for motor neuron autophagy early and late in the SOD1(G93A) mouse model of ALS. Proceedings of the National Academy of Sciences of the United States of America, 114(39), E8294–E8303. doi: 10.1073/pnas.1704294114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada H (2017). Clinical efficacy of edaravone for the treatment of amyotrophic lateral sclerosis. Expert Opinion on Pharmacotherapy, 18(7), 735–738. doi: 10.1080/14656566.2017.1319937 [DOI] [PubMed] [Google Scholar]
- Sokal RR, & Rohlf FJ (1995). Biometry: The principles and practice of statistics in biological research (3rd ed.), New York: W.H. Freeman. [Google Scholar]
- Traktuev DO, Merfeld-Clauss S, Li J, Kolonin M, Arap W, Pasqualini R, Johnstone BH and March KL (2008) A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circulation Research, 102, 77–85. [DOI] [PubMed] [Google Scholar]
- Traynor BJ, Alexander M, Corr B, Frost E, & Hardiman O (2003). An outcome study of riluzole in amyotrophic lateral sclerosis–a population-based study in Ireland, 1996–2000. Journal of Neurology, 250(4), 473–479. doi: 10.1007/s00415-003-1026-z [DOI] [PubMed] [Google Scholar]
- Wang X, Zhao Z, Gong J, Zhou S, Peng H, Shatara A, … & Gu H (2014). Adipose stem cells-conditioned medium blocks 6-hydroxydopamine-induced neurotoxicity via the IGF-1/PI3K/AKT pathway. Neuroscience Letters, 581, 98–102. doi: 10.1016/j.neulet.2014.08.033 [DOI] [PubMed] [Google Scholar]
- Wei X, Du Z, Zhao L, Feng D, Wei G, He Y, & Du Y (2009). IFATS collection: The conditioned media of adipose stromal cells protect against hypoxia-ischemia-induced brain damage in neonatal rats. Stem Cells, 27(2), 478–488. doi: 10.1634/stemcells.2008-0333 [DOI] [PubMed] [Google Scholar]
- Wei X, Zhao L, Zhong J, Gu H, Feng D, Johnstone BH, … & Du Y (2009). Adipose stromal cells-secreted neuroprotective media against neuronal apoptosis. Neuroscience Letters, 462(1), 76–79. doi: 10.1016/j.neulet.2009.06.054 [DOI] [PubMed] [Google Scholar]
- Wijesekera LC, & Leigh PN (2009). Amyotrophic lateral sclerosis. Orphanet Journal of Rare Diseases, 4, 3. doi: 10.1186/1750-1172-4-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagami CJ, Beart PM, Wallis N, Nagley P, & O’Shea RD (2009). Oxidative and excitotoxic insults exert differential effects on spinal motoneurons and astrocytic glutamate transporters: Implications for the role of astrogliosis in amyotrophic lateral sclerosis. Glia, 57(2), 119–135. doi: 10.1002/glia.20739 [DOI] [PubMed] [Google Scholar]
- Zhao L, Wei X, Ma Z, Feng D, Tu P, Johnstone BH, … & Du Y (2009). Adipose stromal cells-conditional medium protected glutamate-induced CGNs neuronal death by BDNF. Neuroscience Letters, 452(3), 238–240. doi: 10.1016/j.neulet.2009.01.025 [DOI] [PubMed] [Google Scholar]
- Zhong Z, Deane R, Ali Z, Parisi M, Shapovalov Y, O’Banion MK, … & Zlokovic BV (2008). ALS-causing SOD1 mutants generate vascular changes prior to motor neuron degeneration. Nature Neuroscience, 11(4), 420–422. doi: 10.1038/nn2073 [DOI] [PMC free article] [PubMed] [Google Scholar]