Abstract
The copper(I) catalyzed alkyne–azide cycloaddition (CuAAC), a click reaction, is one of the most powerful catalytic reactions developed during the last two decades. Conducting CuAAC enantioselectively would add a third dimension to this reaction and would enable the direct synthesis of α-chiral triazoles. Doing so is demanding because the two precursors have linear geometries, and the triazole product is a flat heterocycle. Designing a chiral catalyst is further complicated by the complex mechanism of CuAAC. We report an enantio-selective CuAAC (E-CuAAC), enabled by dynamic kinetic resolution (DKR). The E-CuAAC is high yielding and affords up to 99:1 er. The E-CuAAC can directly generate α-chiral triazoles in a complex molecular environment.
The copper(I) catalyzed alkyne–azide cycloaddition (CuAAC) has transformed many aspects of modern chemical synthesis since it was first reported contemporaneously by Meldal, Sharpless, and co-workers.1,2 The CuAAC reaction is robust, mild, high yielding, and chemo-orthogonal.3–5 Applications for CuAAC have permeated and transformed numerous fields including chemical biology, material science, polymer chemistry, and medicinal chemistry.5 Triazoles, formed by CuAAC, are now common peptidomimetics and pharmaceutical building blocks.6 With the tremendous utility of CuAAC, a versatile catalyst that could impart enantioselectivity to the process would likely find numerous applications, especially as examples of α-chiral triazoles are emerging in active biological agents. 7–11
Facilitating an E-CuAAC reaction presents several fundamental challenges. First, CuAAC uses an alkyne and an azide and forms a triazole (Figure 1a). Alkynes and azides have a linear geometry and the resulting triazole is a sp2 hybridized heterocycle. No new stereogenic centers are formed in most CuAAC reactions. Therefore, E-CuAAC requires the transmission of stereochemical information beyond the forming triazole. Second, an E-CuAAC reaction must outcompete the facile background CuAAC reaction.12 This is nontrivial because the CuAAC reaction is an extremely efficient process that proceeds by a complex and dynamic reaction mechanism.13,14 Herein, we report that E-CuAAC can be enabled by dynamic kinetic resolution (>95% yield and up to 99:1 er).
Figure 1.
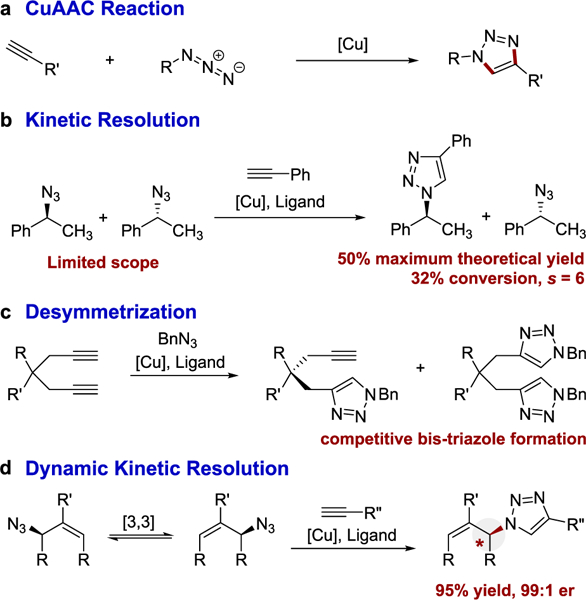
CuAAC reaction and approaches to E-CuAAC.
Fokin and Finn originally reported attempts at an E-CuAAC through a kinetic resolution (Figure 1b).15 The results were significantly limited in scope and proceeded with only a modest selectivity (selectivity factor s up to 6). These early results implied a two-fold problem with E-CuAAC. First, the back-ground CuAAC, in the absence of a chiral ligand, is fast and must be outcompeted or suppressed. Second, the catalyst’s ligand environment must be able to sense remote stereochemical information. Furthermore, even in the ideal sense, kinetic resolution proceeds with a maximum theoretical yield of 50%.16 Others have attempted to use bis-alkynes or bis-azides for E-CuAAC by desymmetrization (Figure 1c).15,17–24 Reactions based on this approach are likewise limited in scope and occur with modest chemoselectivity due to the competitive formation of bis-triazoles.
Our group has an interest in using dynamic kinetic resolution (DKR)25–29 to enable enantioselective synthetic methods. A DKR couples a pathway for racemization to an enantioselective functionalization. Methods based on DKR use racemic starting material and can result in both high yield and high enantioselectivity. Our lab envisioned using allylic azides in DKR because allylic azides spontaneously rearrange.30 The rearrangement complicates using these intermediates, but a few inspirational reports described successfully trapping allylic azides. 31–35 We hypothesized that allylic azides could be used to enable an E-CuAAC reaction (Figure 1d). Herein, we report a DKR enabled E-CuAAC that proceeds in yields exceeding 95%, with er up to 99:1, which is compatible with a complex molecular environment.
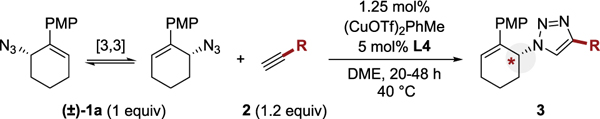
This study began with allylic azide 1a and tert-butyl propiolate (2a, Table 1). We observed minimal enantioselectivity with copper iodide (entry 1). Changing to a cationic copper(I) precatalyst had a notable impact on both the rate and enantioselectivity of the reaction (entry 2). A collection of ligands were screened that included bidentate and tridentate phosphorus and nitrogen ligands (entries 2–9 and Supporting Information). On the basis of these initial results, aryl-PYBOX ligands appeared to be particularly effective (entry 4 and 5). We hypothesized that increasing the ligand loading would slow the background click reaction by saturating the copper center. An increase in ligand loading enhanced the observed er to 88:12 (entry 10). Increasing the temperature had a notable positive effect (entry 11), which resulted in a quantitative yield (>98%) and high enantioselectivity (>99:1 er). The increased temperature likely increased the relative rate of racemization via a sigmatropic pathway.36 Other copper precatalysts were not as effective (entries 12 and 13), and the conditions outlined in entry 11 were selected as being optimal.
Table 1.
Optimization of E-CuAAC by DKRa
 | |||||
|---|---|---|---|---|---|
| Entry | [Cu] source | Ligand | Temp (°C) | Yield (%) | er |
| 1 | CuI | L1 (2.5%) | rt | 80 | 57:43 |
| 2 | (CuOTf)2PhMe | L1 (2.5%) | rt | >98 | 60:40 |
| 3 | (CuOTf)2FhMe | L2 (2.5%) | rt | 95 | 53:47 |
| 4 | (CuOTf)2PhMe | L3 (2.5%) | rt | 93 | 86:14 |
| 5 | (CuOTf)2PhMe | L4 (2.5%) | rt | 80 | 76:24 |
| 6 | (CuOTf)2PhMe | L5 (2.5%) | rt | 58 | 51:49 |
| 7 | (CuOTf)2PhMe | L6 (2.5%) | rt | 87 | 52:48 |
| 8 | (CuOTf)2PhMe | L7 (2.5%) | rt | 65 | 52:48 |
| 9 | (CuOTf)2PhMe | L8 (2.5%) | rt | >98 | 52:48 |
| 10 | (CuOTf)2PhMe | L4 (5.0%) | rt | 83 | 88:12 |
| 11 | (CuOTf)2PhMe | L4 (5.0%) | 40 | >98 | 99:1 |
| 12 | Cu(MeCN)4PF6 | L4 (5.0%) | 40 | 73 | 90:10 |
| 13 | Cu(MeCN)4BF4 | L4 (5.0%) | 40 | 82 | 91:9 |
Reactions conducted with allylic azide la (0.1 mmol), alkyne 2a (0.12 mmol), in dimethoxyethane (0.2 M), with 2.5 mol% [Cu] and either 2.5 mol% or 5 mol% ligand. Yields based on 1H NMR analysis using 1,3,5-trimethoxybenzene as an internal standard. Chiral HPLC was used to determine er. All yield and er values reflect the average of duplicate trials. See Supporting Information for full details.
We conducted a nonlinear experiment by determining the effect of varying the ligand’s enantiopurity on the enantiomeric excess of the product.37 It has been reported that E-CuAAC by desymmetrization can proceed with a positive19,24 or negative17 nonlinear effect. A negative nonlinear effect was observed for this E-CuAAC reaction (see Supporting Information). The PYBOX scaffold may promote dimerization, which is consistent with crystallographic data on Cu(I)-PYBOX complexes.38,39
The scope of our E-CuAAC reaction was investigated with respect to the alkyne (Table 2). Using the azide as the limiting reagent, the model substrate 3a was isolated in 95% yield and >99:1 er. The scope of the alkyne was quite broad. Electron rich and electron deficient aryl alkynes could participate in the E-CuAAC (3b–3j). A crystal of product 3h was suitable for diffraction analysis, which unambiguously assigned the absolute configuration of the product generated from the (S,S)-PYBOX/Cu catalyst as having the (R)-configuration (see Supporting Information). The configuration of the other products were assigned based on analogy to product 3h. An ortho-substituted arene provided an acceptable yield in high er (3j). An alkyne containing an aliphatic (3k), heterocycle (3l), protected alcohol (3m), ketone (3n), and cyclopropyl (3o) group could all be used in E-CuAAC.
Table 2.
Scope of DKR E-CuAAC with Respect to Alkyne 2
 |
|---|
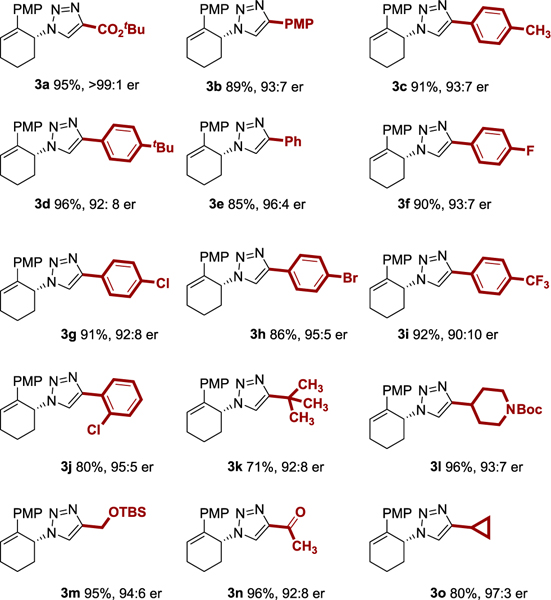 |
Yields are reported for isolated and purified products. Enantiomeric ratio was determined by chiral HPLC. Yield and er values reflect the average of duplicate trials. See Supporting Information for details.
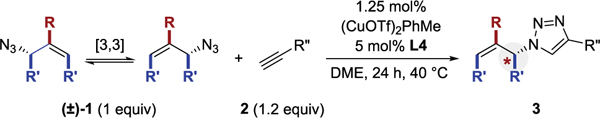
The azide component was varied (Table 3). The cyclohexyl-ring could be contracted (3p), expanded (3q), or modified (3r–3x). In all of these cases, both the yield and enantioselectivity remained acceptably high. The 2-aryl group is not required (3y and 3z) and an acyclic substrate was tolerated (3aa).
Table 3.
Scope of DKR E-CuAAC with Respect to Azide 1
 |
|---|
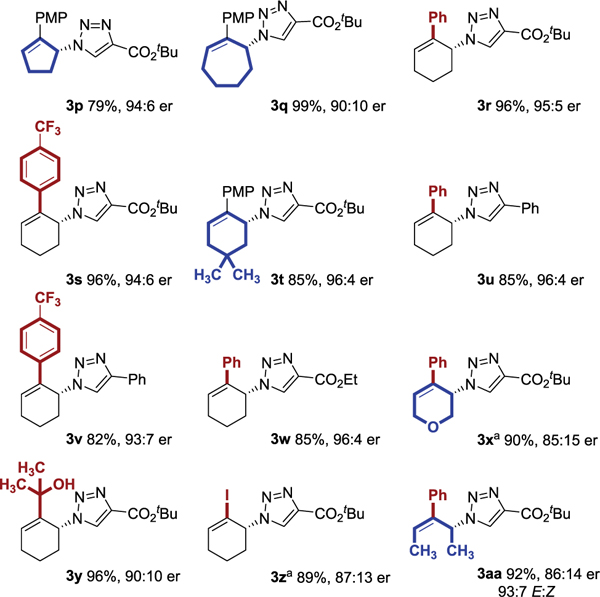 |
Yields are reported for isolated and purified products. Enantiomeric ratio was determined by chiral HPLC. Yield and er values reflect the average of duplicate trials.
Reaction used alkyne as limiting reagent. See Supporting Information for details.
To further demonstrate the features of this E-CuAAC reaction, we conducted the reaction with (R)-1-phenyl-2-propyn-1-ol (4, Scheme 1). Both enantiomers of the ligand were used to test for matched/mismatched behavior related to double diastereoselectivity. Changing the ligand’s stereochemistry reversed the diastereoselectivity (products 5a and 5b), indicating a robust catalyst.
Scheme 1.
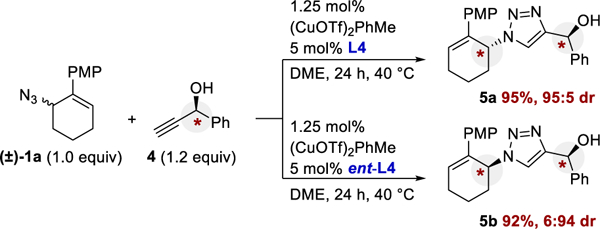
Test for Matched/Mismatched Behavior
Several additional examples demonstrate that the E-CuAAC is viable in a complex molecular setting (Table 4). Derivatives of vitamin E (6a), gibberellic acid (6b), esterone (6c), glucose (6d), mycophenolate mofetil (6e), and moexipril (6f) could all be successfully “clicked” by E-CuAAC in near perfect yield and excellent selectivity. This illustrates that E-CuAAC is capable of generating α-chiral triazoles in the presence of densely functionalized molecules with robust stereochemical fidelity.
Table 4.
DKR E-CuAAC in Complex Molecular Setting
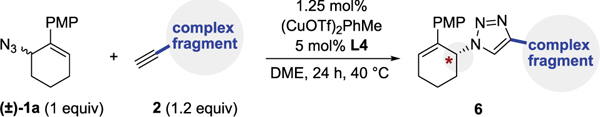 |
|---|
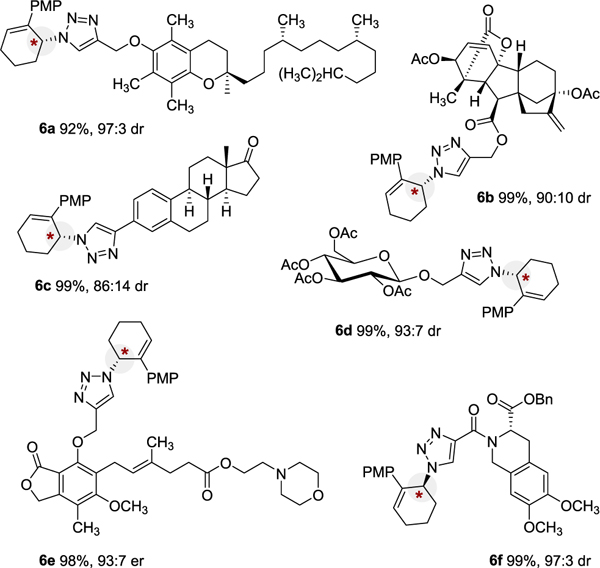 |
Yields are reported for isolated and purified products. Diastereomeric ratio or enantiomeric ratio was determined by HPLC or 1H NMR. Yield, er, and dr values reflect the average of duplicate trials. Substrate 6f was prepared using (R,R)-4-Cl-Ph-PYBOX ligand (ent-L4). See Supporting Information for full details.
We report an effective system for the enantioselective copper(I) catalyzed alkyne–azide cycloaddition (E-CuAAC) “click” reaction that is enabled by the dynamic kinetic resolution of allylic azides. A negative nonlinear effect was observed in this system. The reaction proceeds in high yield and high selectivity. The scope of this process is broad and the reaction can proceed in a complex molecular environment.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the University of Minnesota and The American Chemical Society’s Petroleum Research Fund (PRF #56505- DNI1) for financial support. This research was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award No. R35GM124718.
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.9b01091.
Experimental procedures and data (PDF)
Crystallographic data (CIF)
The authors declare no competing financial interest.
REFERENCES
- (1).Tornøe CW; Christensen C; Meldal M Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides. J. Org. Chem. 2002, 67, 3057–3064. [DOI] [PubMed] [Google Scholar]
- (2).Rostovtsev VV; Green LG; Fokin VV; Sharpless KB A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem., Int. Ed. 2002, 41, 2596–2599. [DOI] [PubMed] [Google Scholar]
- (3).Hein JE; Fokin VV Copper-Catalyzed Azide-Alkyne Cycloaddition (CuAAC) and beyond: New Reactivity of Copper(I) Acetylides. Chem. Soc. Rev. 2010, 39, 1302–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Meldal M; Tornøe CW Cu-Catalyzed Azide–Alkyne Cycloaddition. Chem. Rev. 2008, 108, 2952–3015. [DOI] [PubMed] [Google Scholar]
- (5).Finn MG; Fokin VV Click Chemistry: Function Follows Form. Chem. Soc. Rev. 2010, 39, 1231–1232. [DOI] [PubMed] [Google Scholar]
- (6).Pedersen DS; Abell A 1,2,3-Triazoles in Peptidomimetic Chemistry. Eur. J. Org. Chem. 2011, 2011, 2399–2411. [Google Scholar]
- (7).Wood WJL; Patterson AW; Tsuruoka H; Jain RK; Ellman JA Substrate Activity Screening: A Fragment-Based Method for the Rapid Identification of Nonpeptidic Protease Inhibitors. J. Am. Chem. Soc. 2005, 127, 15521–15527. [DOI] [PubMed] [Google Scholar]
- (8).Lee T; Cho M; Ko SY; Youn HJ; Dong JB; Cho WJ; Kang CY; Kim S Synthesis and Evaluation of 1,2,3-Triazole Containing Analogues of the Immunostimulant α-GalCer. J. Med. Chem. 2007, 50, 585–589. [DOI] [PubMed] [Google Scholar]
- (9).Divakaran A; Talluri SK; Ayoub AM; Mishra N; Cui H; Widen JC; Berndt N; Zhu J-Y; Carlson AS; Topczewski JJ; et al. Molecular Basis for the N-Terminal Bromodomain and Extra Terminal (BET) Family Selectivity of a Dual Kinase-Bromodomain Inhibitor. J. Med. Chem. 2018, 61, 9316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Rezaei Z; Khabnadideh S; Pakshir K; Hossaini Z; Amiri F; Assadpour E Design, Synthesis, and Antifungal Activity of Triazole and Benzotriazole Derivatives. Eur. J. Med. Chem. 2009, 44, 3064–3067. [DOI] [PubMed] [Google Scholar]
- (11).Berthold D; Breit B Chemo-, Regio-, and Enantioselective Rhodium-Catalyzed Allylation of Triazoles with Internal Alkynes and Terminal Allenes. Org. Lett. 2018, 20, 598–601. [DOI] [PubMed] [Google Scholar]
- (12).Berrisford DJ; Bolm C; Sharpless KB Ligand-Accelerated Catalysis. Angew. Chem., Int. Ed. Engl. 1995, 34, 1059–1070. [Google Scholar]
- (13).Worrell BT; Malik JA; Fokin VV Direct Evidence of a Dinuclear Copper Intermediate in Cu(I)-Catalyzed Azide-Alkyne Cycloadditions. Science 2013, 340, 457–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Ziegler MS; Lakshmi KV; Tilley TD Dicopper Cu(I)Cu(I) and Cu(I)Cu(II) Complexes in Copper-Catalyzed Azide–Alkyne Cycloaddition. J. Am. Chem. Soc. 2017, 139, 5378–5386. [DOI] [PubMed] [Google Scholar]
- (15).Meng JC; Fokin VV; Finn MG Kinetic Resolution by Copper-Catalyzed Azide-Alkyne Cycloaddition. Tetrahedron Lett. 2005, 46, 4543–4546. [Google Scholar]
- (16).Keith JM; Larrow JF; Jacobsen EN Practical Considerations in Kinetic Resolution Reactions. Adv. Synth. Catal. 2001, 343, 5–26. [Google Scholar]
- (17).Zhou F; Tan C; Tang J; Zhang YY; Gao WM; Wu HH; Yu YH; Zhou J Asymmetric Copper(I)-Catalyzed Azide-Alkyne Cycloaddition to Quaternary Oxindoles. J. Am. Chem. Soc. 2013, 135, 10994–10997. [DOI] [PubMed] [Google Scholar]
- (18).Brittain WDG; Buckley BR; Fossey JS Asymmetric Copper-Catalyzed Azide-Alkyne Cycloadditions. ACS Catal 2016, 6, 3629–3636. [Google Scholar]
- (19).Osako T; Uozumi Y Mechanistic Insights into Copper-Catalyzed Azide–Alkyne Cycloaddition (CuAAC): Observation of Asymmetric Amplification. Synlett 2015, 26, 1475–1479. [Google Scholar]
- (20).Chen M-Y; Zheng X; Li C; Tao S; Zhan-Jiang Z; Jian C; Yu-Ming C; Li-Wen X Catalytic Asymmetric Huisgen Alkyne-Azide Cycloaddition of Bisalkynes by Copper(I) Nanoparticles. ChemCatChem 2018, 10,280–286. [Google Scholar]
- (21).Osako T; Uozumi Y Enantioposition-Selective Copper-Catalyzed Azide–Alkyne Cycloaddition for Construction of Chiral Biaryl Derivatives. Org. Lett. 2014, 16, 5866. [DOI] [PubMed] [Google Scholar]
- (22).Brittain WDG; Buckley BR; Fossey JS Kinetic Resolution of Alkyne-Substituted Quaternary Oxindoles via Copper Catalysed Azide-Alkyne Cycloadditions. Chem. Commun. 2015, 51, 17217–17220. [DOI] [PubMed] [Google Scholar]
- (23).Song T; Li L; Zhou W; Zheng Z-J; Deng Y; Xu Z; Xu L-W Enantioselective Copper-Catalyzed Azide–Alkyne Click Cyclo-addition to Desymmetrization of Maleimide-Based Bis(Alkynes). Chem. - Eur. J 2015, 21, 554–558. [DOI] [PubMed] [Google Scholar]
- (24).Chen M-Y; Xu Z; Chen L; Song T; Zheng Z-J; Cao J; Cui Y-M; Xu L-W Catalytic Asymmetric Huisgen Alkyne–Azide Cycloaddition of Bisalkynes by Copper(I) Nanoparticles. ChemCatChem 2018, 10,280–286. [Google Scholar]
- (25).Verho O; Bäckvall JE Chemoenzymatic Dynamic Kinetic Resolution: A Powerful Tool for the Preparation of Enantiomerically Pure Alcohols and Amines. J. Am. Chem. Soc. 2015, 137, 3996–4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Bhat V; Welin ER; Guo X; Stoltz BM Advances in Stereoconvergent Catalysis from 2005 to 2015: Transition-Metal-Mediated Stereoablative Reactions, Dynamic Kinetic Resolutions, and Dynamic Kinetic Asymmetric Transformations. Chem. Rev. 2017, 117, 4528–4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Pellissier H Recent Developments in Dynamic Kinetic Resolution. Tetrahedron 2008, 64, 3769–3802. [Google Scholar]
- (28).Huerta F; Minidis A; et al. Racemisation in Asymmetric Synthesis. Dynamic Kinetic Resolution and Related Processes in Enzyme and Metal Catalysis. Chem. Soc. Rev. 2001, 30, 321–331. [Google Scholar]
- (29).Breit B; Hilpert L; Sieger S; Haydl A Palladium- and Rhodium-Catalyzed Dynamic Kinetic Resolution of Racemic Internal Allenes Towards Chiral Pyrazoles. Angew. Chem., Int. Ed. 2019, 58, 3378–3381. [DOI] [PubMed] [Google Scholar]
- (30).Gagneux A; Winstein S; Young WG Rearrangement of Allyl Azides. J. Am. Chem. Soc. 1960, 82, 5956–5957. [Google Scholar]
- (31).Feldman AK; Colasson BB; Sharpless KB; Fokin VV The Allylic Azide Rearrangement: Achieving Selectivity. J. Am. Chem. Soc. 2005, 127, 13444–13445. [DOI] [PubMed] [Google Scholar]
- (32).Liu R; Gutierrez O; Tantillo DJ; Aubé J Stereocontrol in a Combined Allylic Azide Rearrangement and Intramolecular Schmidt Reaction. J. Am. Chem. Soc. 2012, 134, 6528–6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Ott AA; Goshey CS; Topczewski JJ Dynamic Kinetic Resolution of Allylic Azides via Asymmetric Dihydroxylation. J. Am. Chem. Soc. 2017, 139, 7737–7740. [DOI] [PubMed] [Google Scholar]
- (34).Porter MR; Shaker RM; Calcanas C; Topczewski JJ Stereoselective Dynamic Cyclization of Allylic Azides: Synthesis of Tetralins, Chromanes, and Tetrahydroquinolines. J. Am. Chem. Soc. 2018, 140, 1211–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Vekariya RH; Liu R; Aubée J A Concomitant Allylic Azide Rearrangement/Intramolecular Azide-Alkyne Cycloaddition Sequence. Org. Lett. 2014, 16, 1844–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Ott AA; Packard MH; Ortuño MA; Johnson A; Suding VP; Cramer CJ; Topczewski JJ Evidence for a Sigmatropic and an Ionic Pathway in the Winstein Rearrangement. J. Org. Chem. 2018, 83, 8214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Girard C; Kagan HB Nonlinear Effects in Asymmetric Synthesis and Stereoselective Reactions: Ten Years of Investigation. Angew. Chem., Int. Ed. 1998, 37, 2922–2959. [DOI] [PubMed] [Google Scholar]
- (38).Díez J; Gamasa MP; Panera M Tetra-, Di-, and Mononuclear Copper(I) Complexes Containing (S,S)-IPr-Pybox and (R,R)-Ph-Pybox Ligands. Inorg. Chem. 2006, 45, 10043–10045. [DOI] [PubMed] [Google Scholar]
- (39).Nakajima K; Shibata M; Nishibayashi Y Copper-Catalyzed Enantioselective Propargylic Etherification of Propargylic Esters with Alcohols. J.Am. Chem. Soc. 2015, 137, 2472–2475. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


